Indole-Based and Cyclopentenylindole-Based Analogues Containing Fluorine Group as Potential 18F-Labeled Positron Emission Tomography (PET) G-Protein Coupled Receptor 44 (GPR44) Tracers
Abstract
1. Introduction
1.1. Molecular Imaging of Inflammation in Cancer
1.2. G-Protein Coupled Receptor 44 (GPR44) in Cancer-Related Inflammation as Well as Inflammation-Induced Cancer
1.3. GPR44 and Fluorine-18 PET Tracers
2. Indole-Based Potential GPR44 Analogues
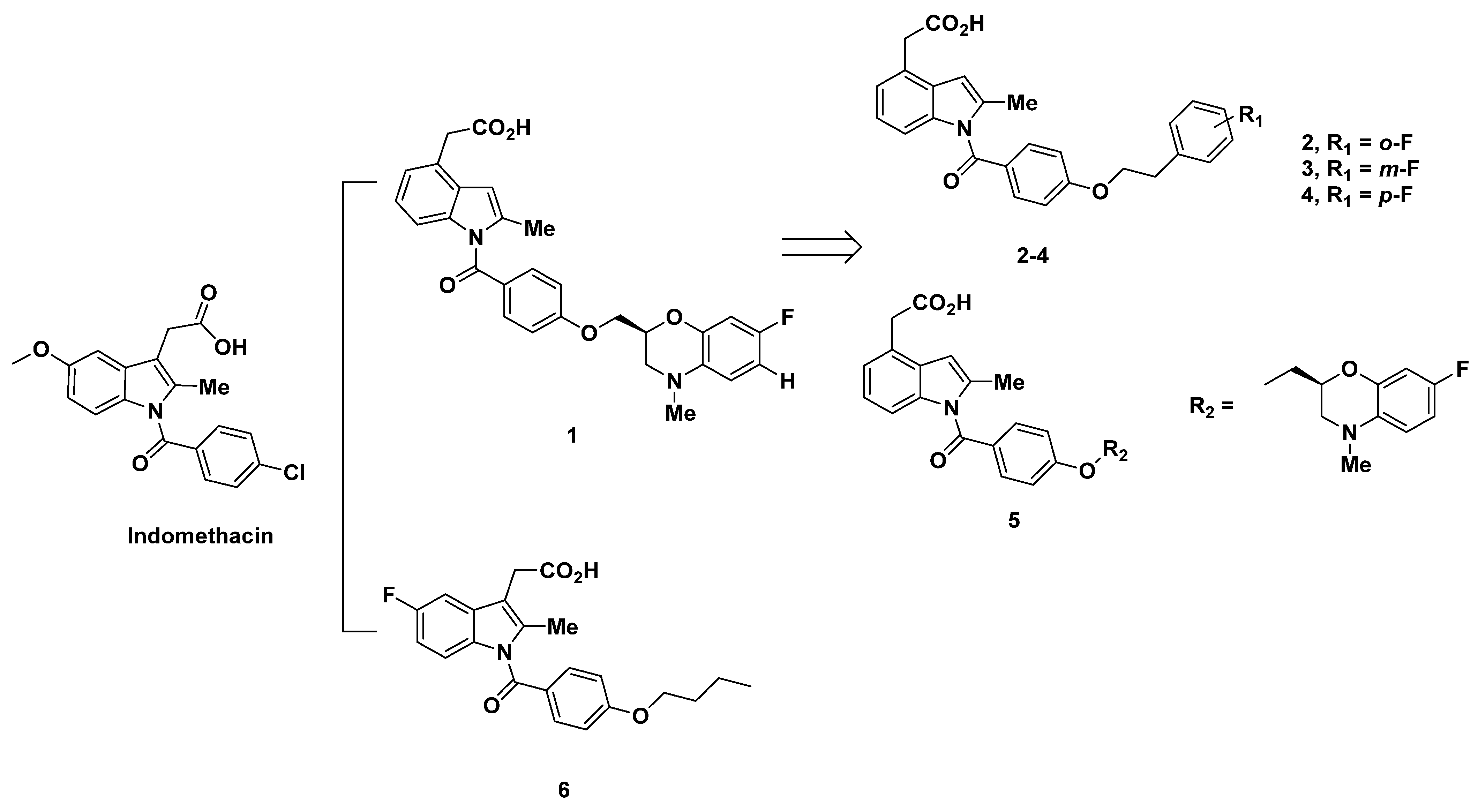
2.1. Ono Pharmaceutical Co Analogues
2.1.1. Ono Pharmaceutical Co Analogues in 2004–2005
2.1.2. Recommendations
2.1.3. Ono Pharmaceutical Co Analogues in 2011
2.1.4. Recommendations
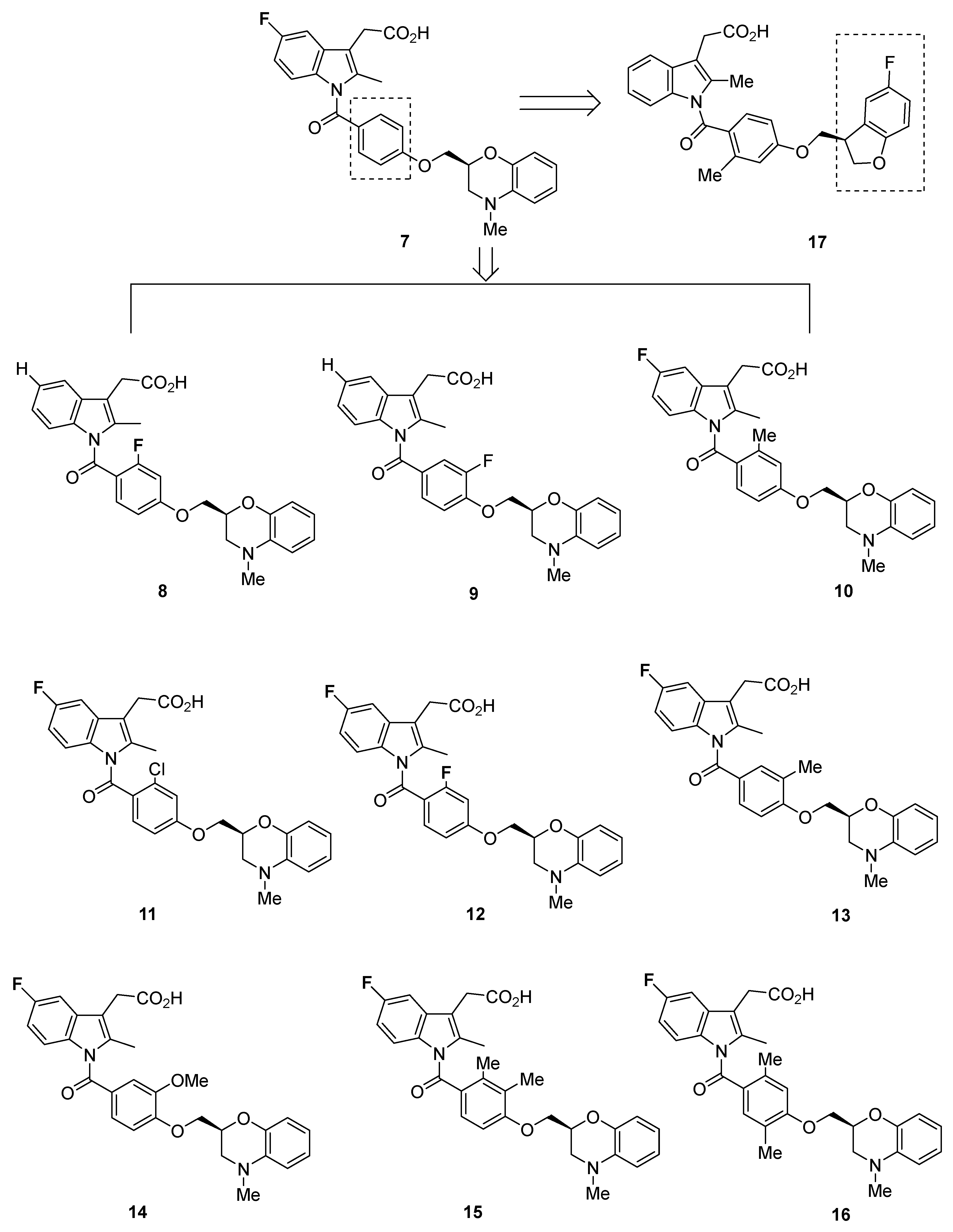
2.2. Oxagen Analogues
2.2.1. Oxagen Analogues in 2005 and 2007
2.2.2. Recommendations
2.2.3. Oxagen Analogues in 2010
2.2.4. Recommendations
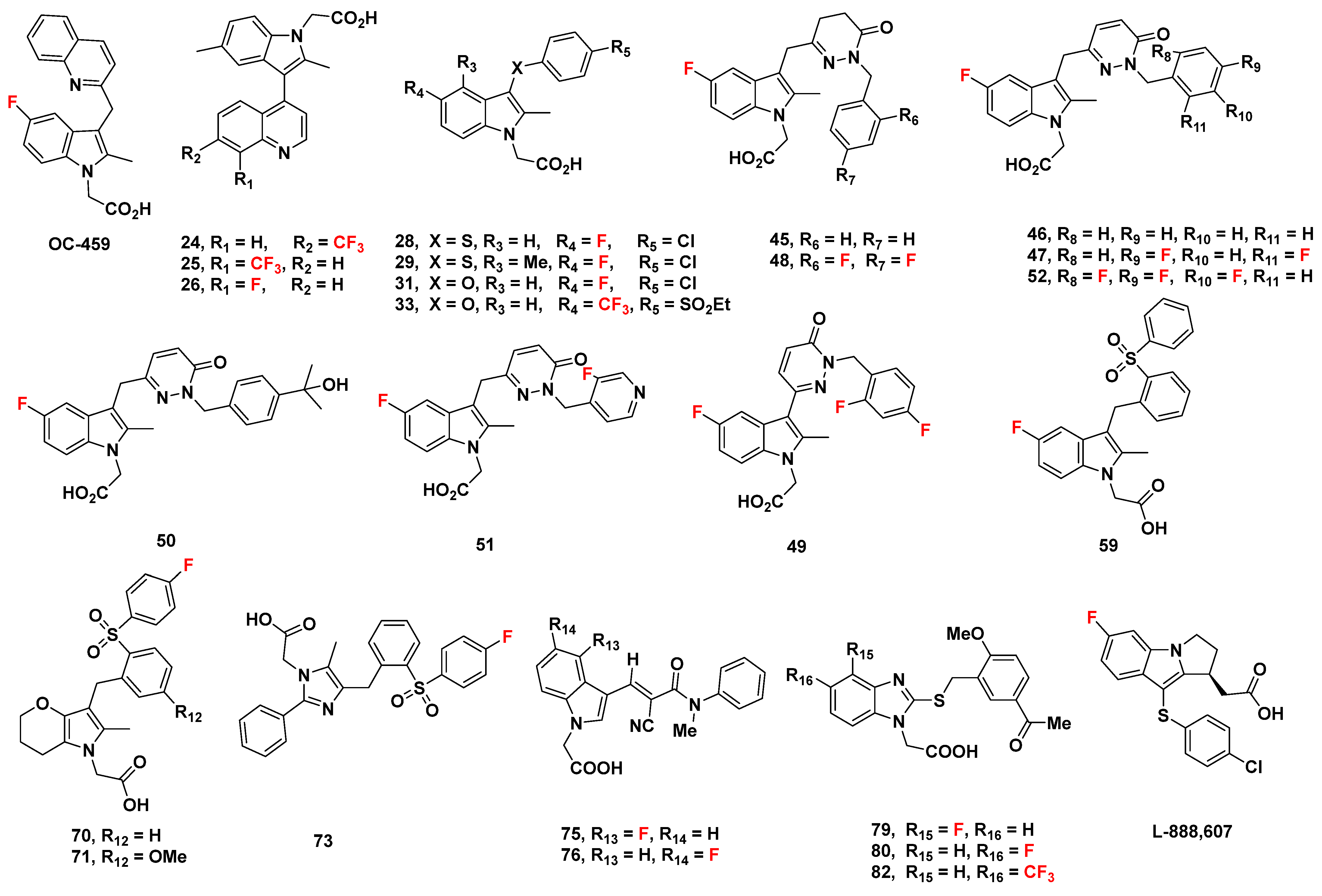
2.2.5. OC-459 (OC000459/Timapiprant)
2.2.6. Recommendations
2.3. AstraZeneca Analogues
Recommendations
2.4. AstraZeneca Analogues
Recommendations
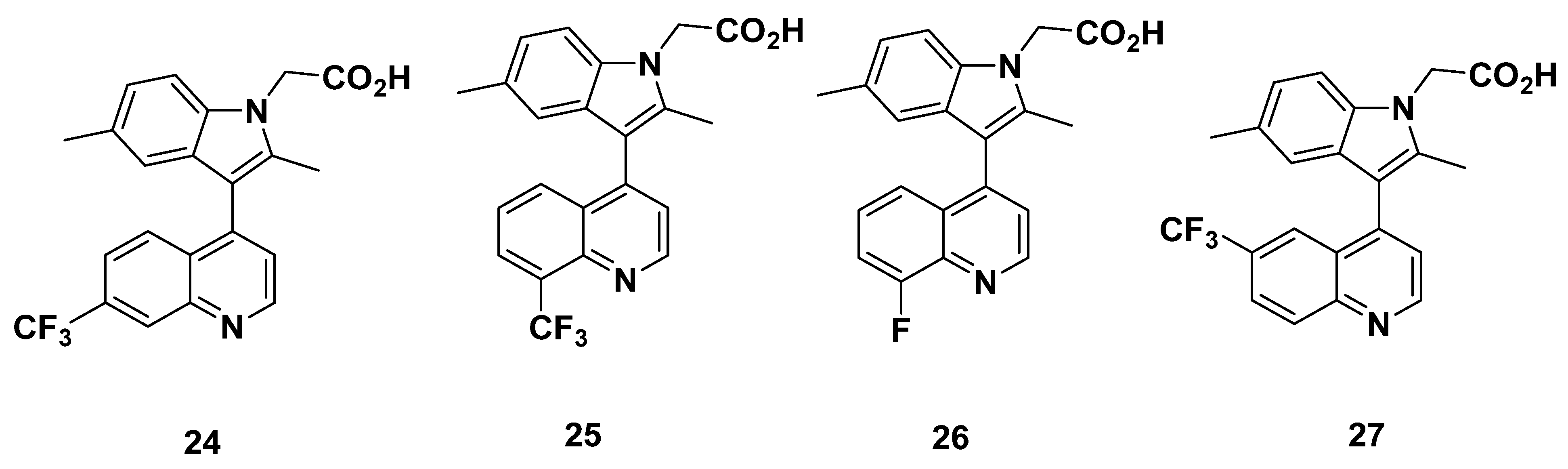
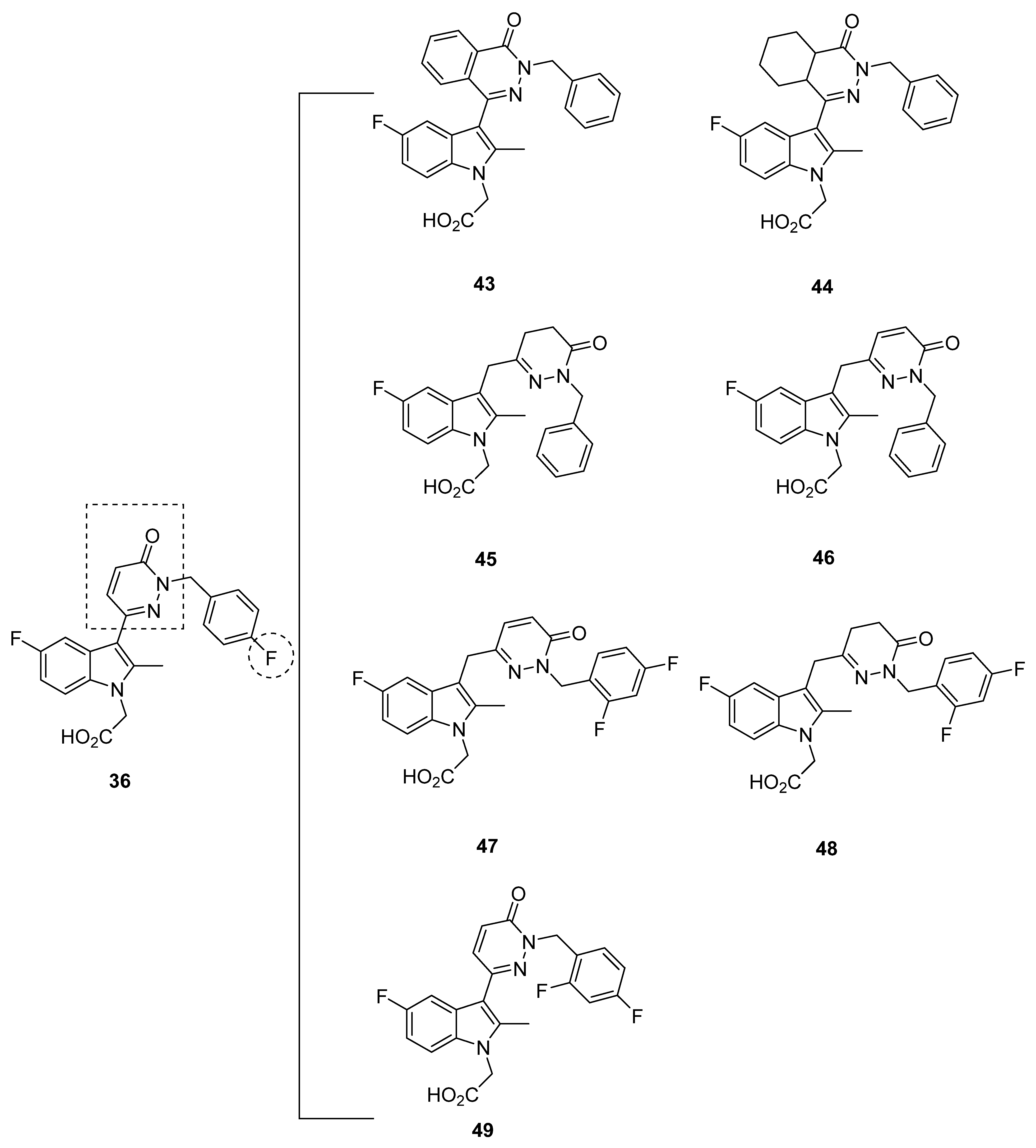
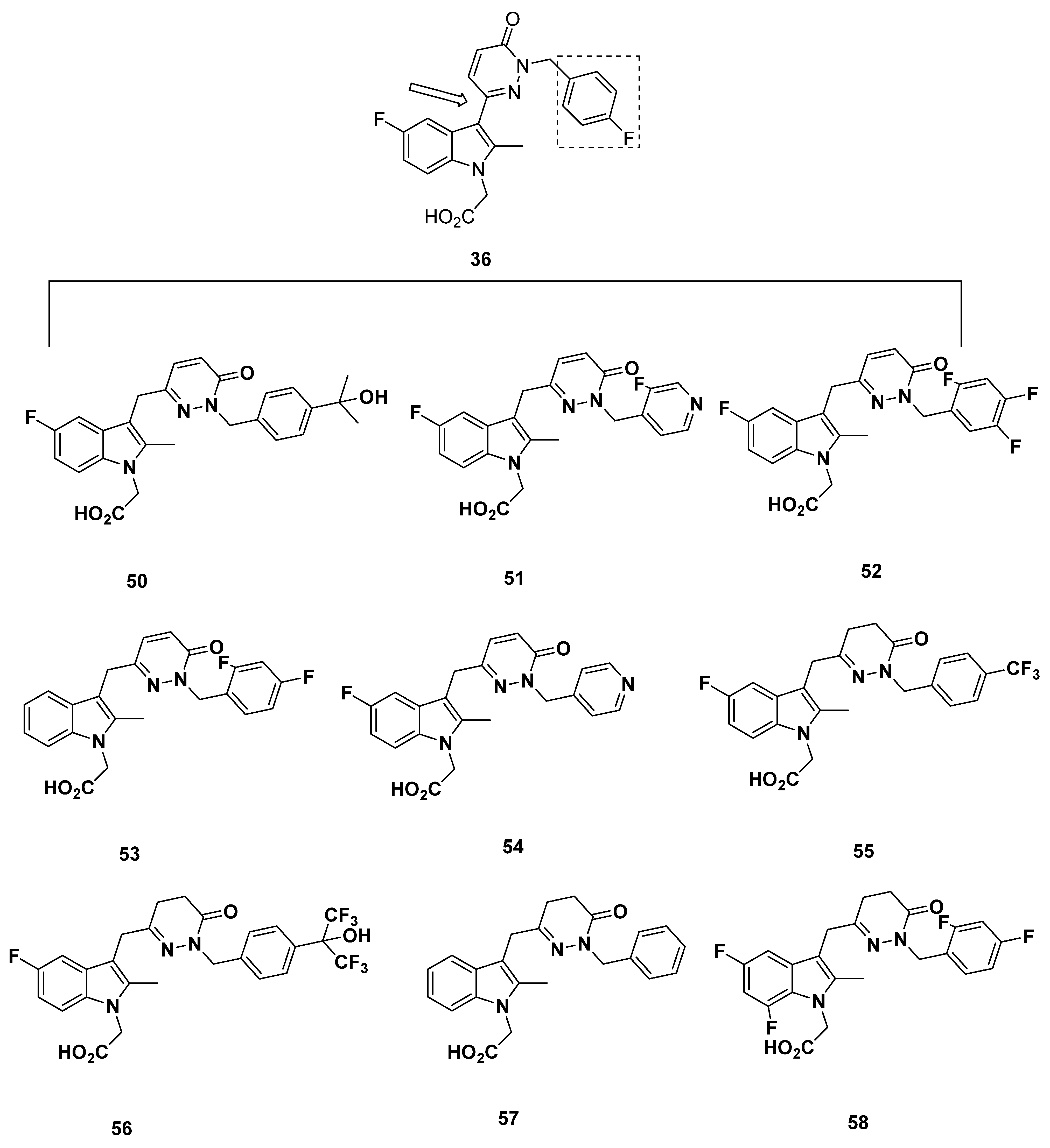
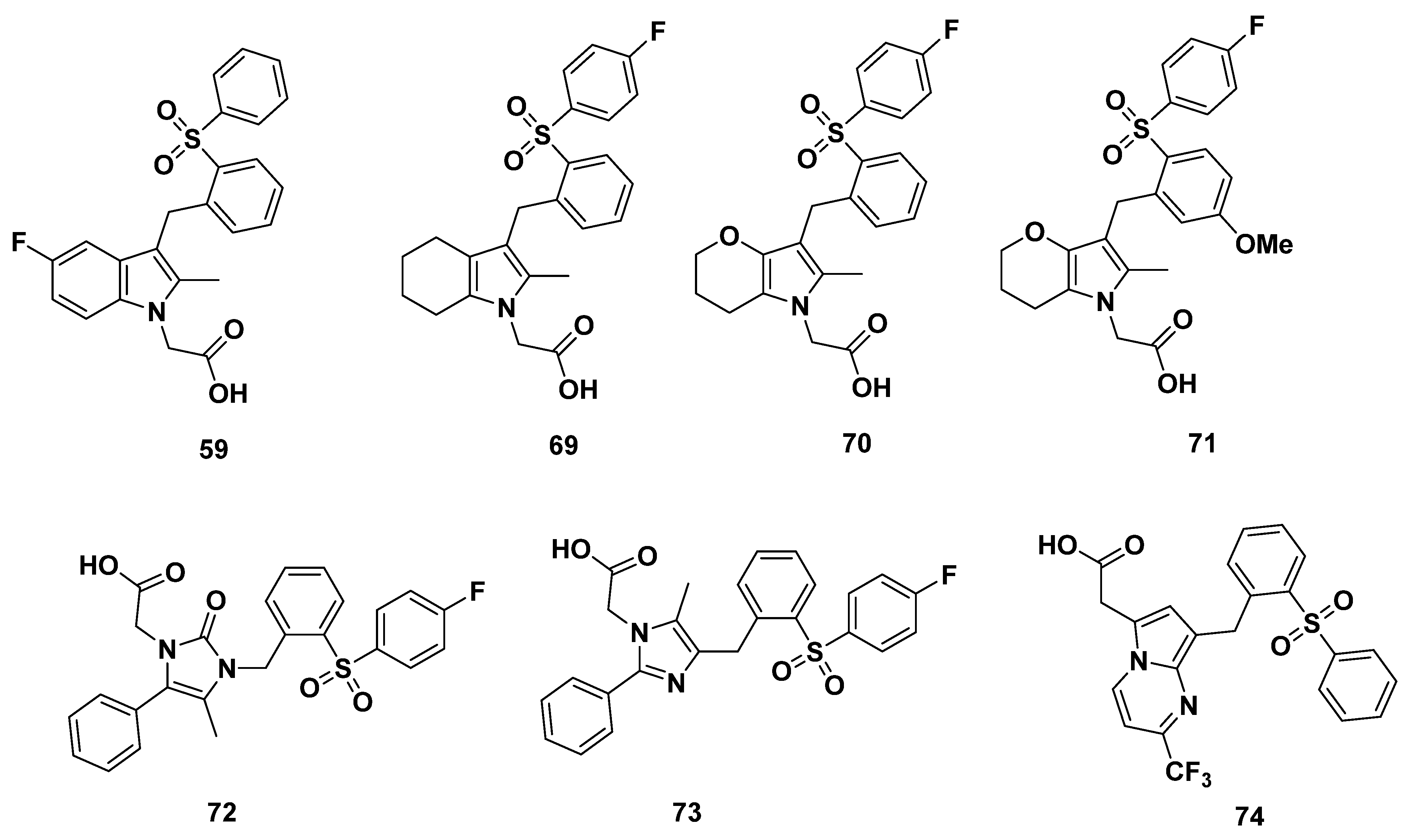
2.5. Pfizer Analogues
2.5.1. Development of Analogues
2.5.2. Mathematical Modeling
2.5.3. Recommendations
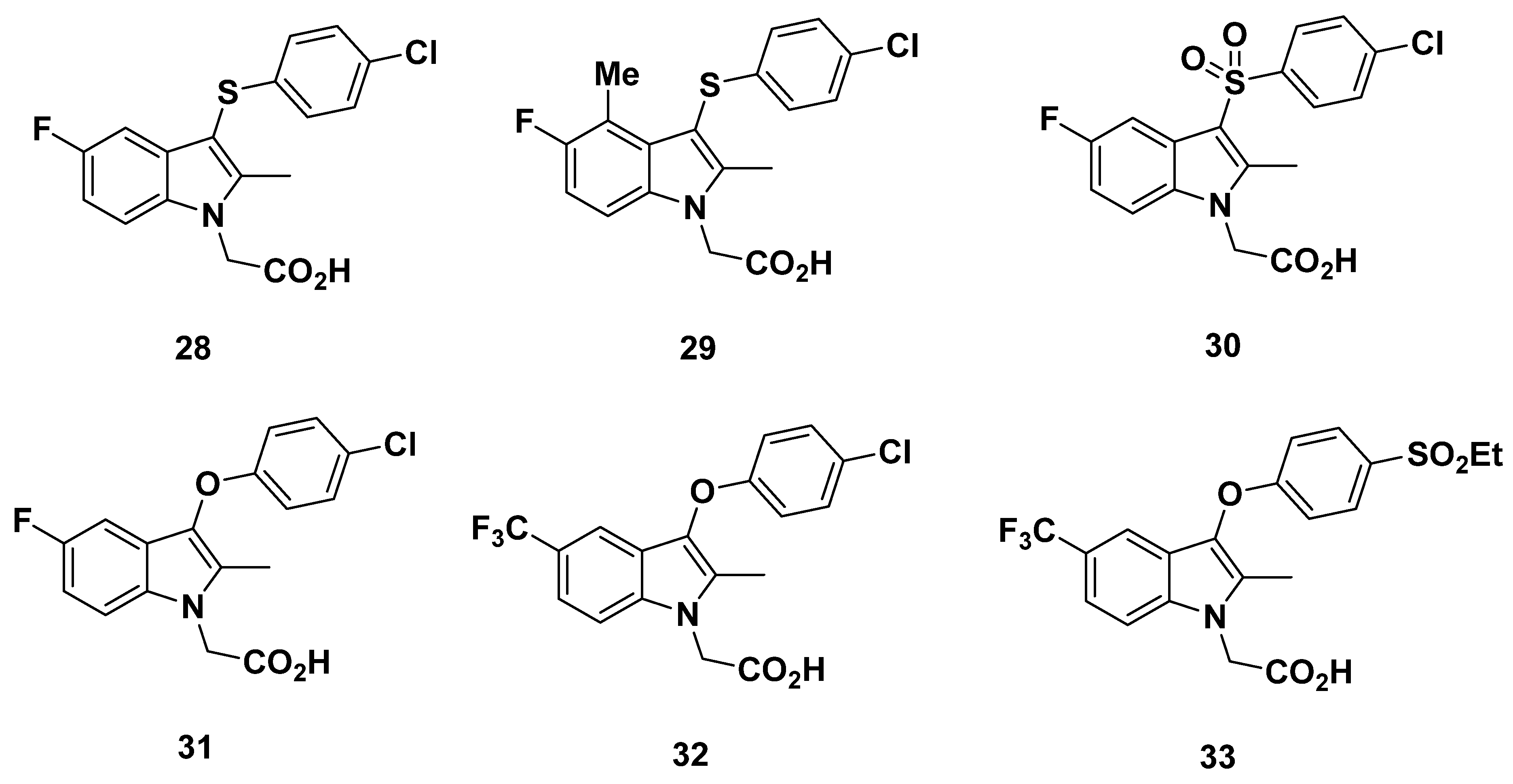
2.6. Almirall Analogues
2.6.1. Almirall Analogues in 2013
2.6.2. Recommendations
2.6.3. Almirall Analogues in 2016
2.6.4. Recommendation
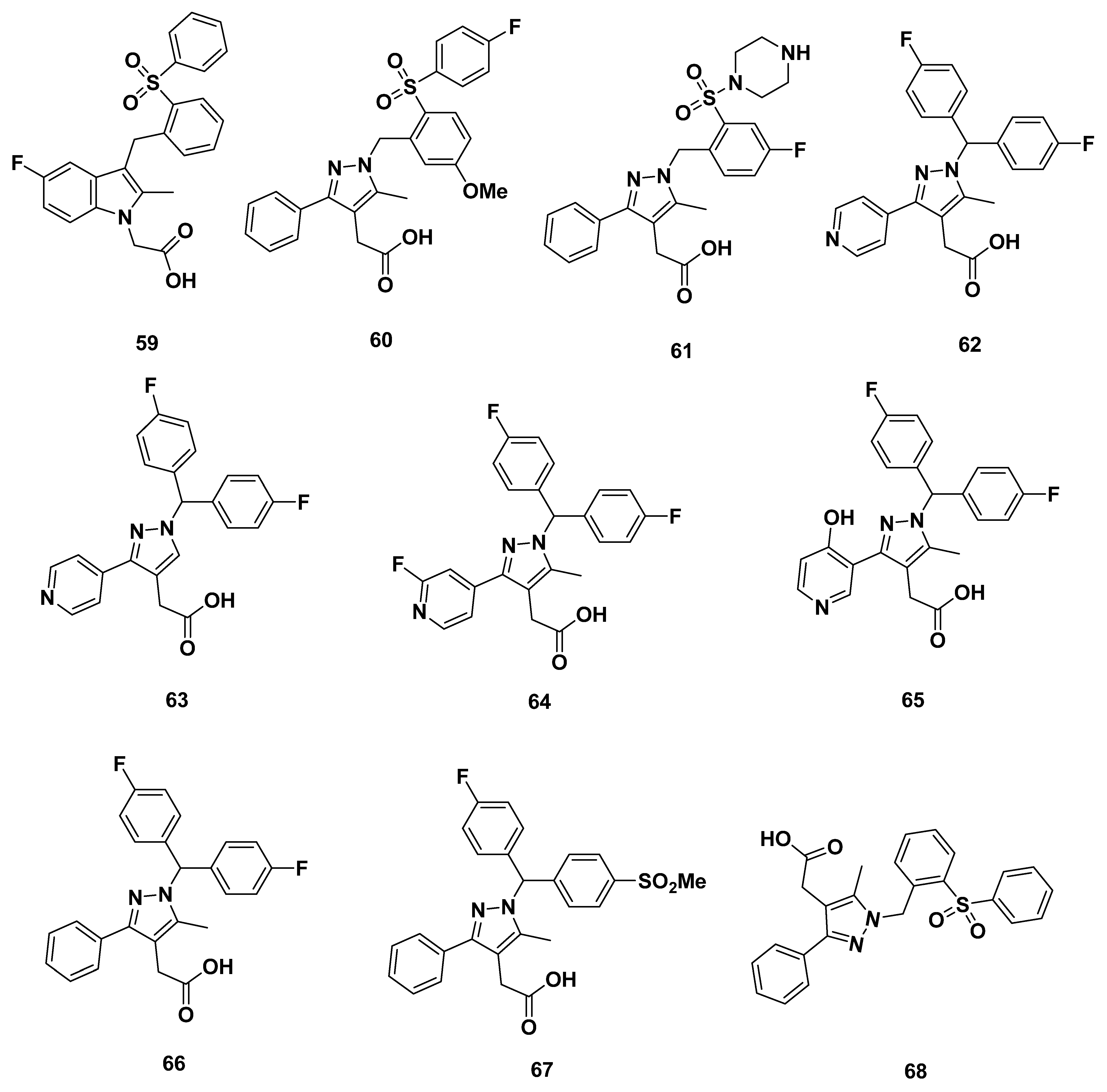
2.7. Actelion Analogues
Recommendations
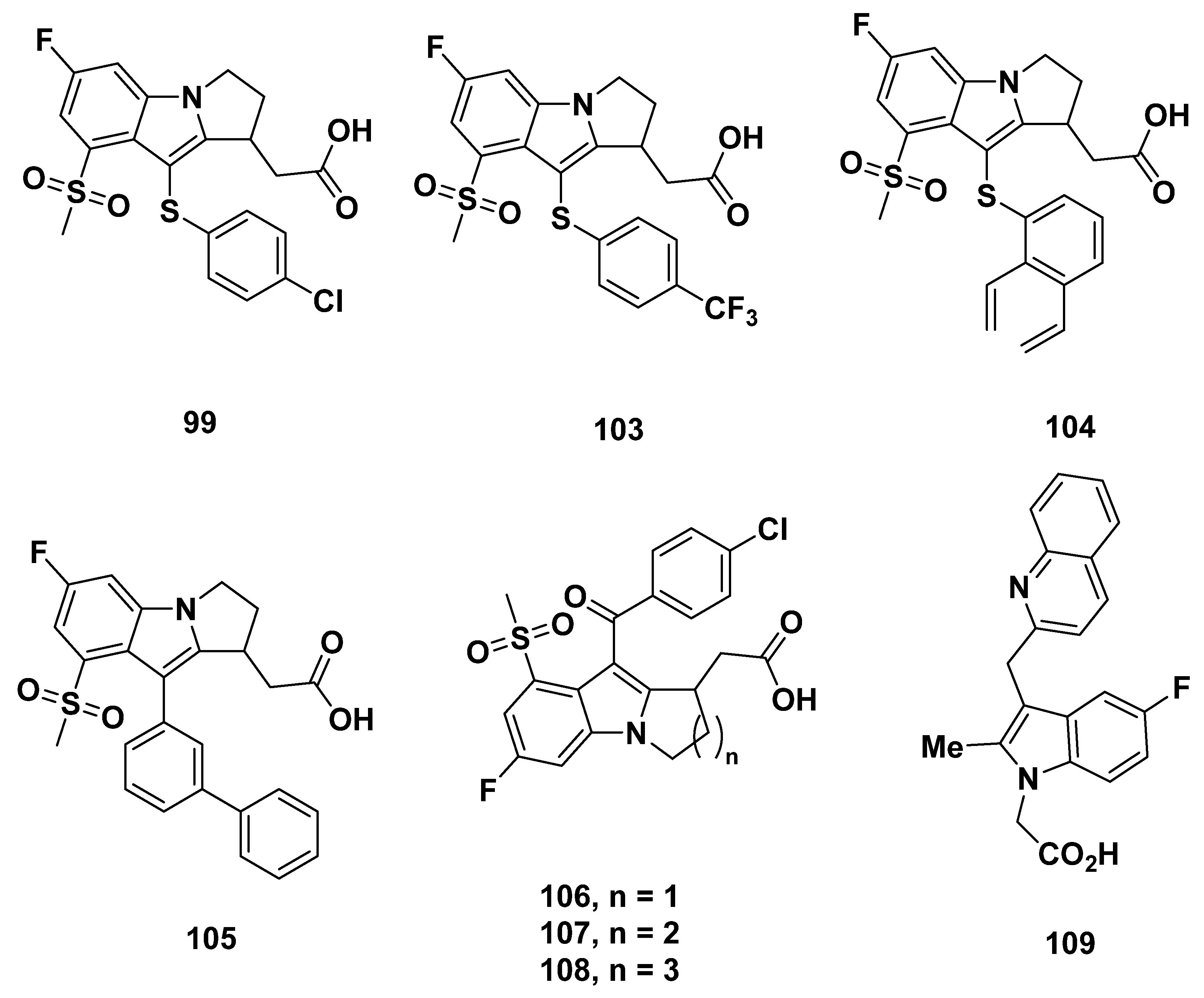
3. Cyclopentenylindole-Based Potential GPR44 Analogues
3.1. Actimis Analogues
Recommendations
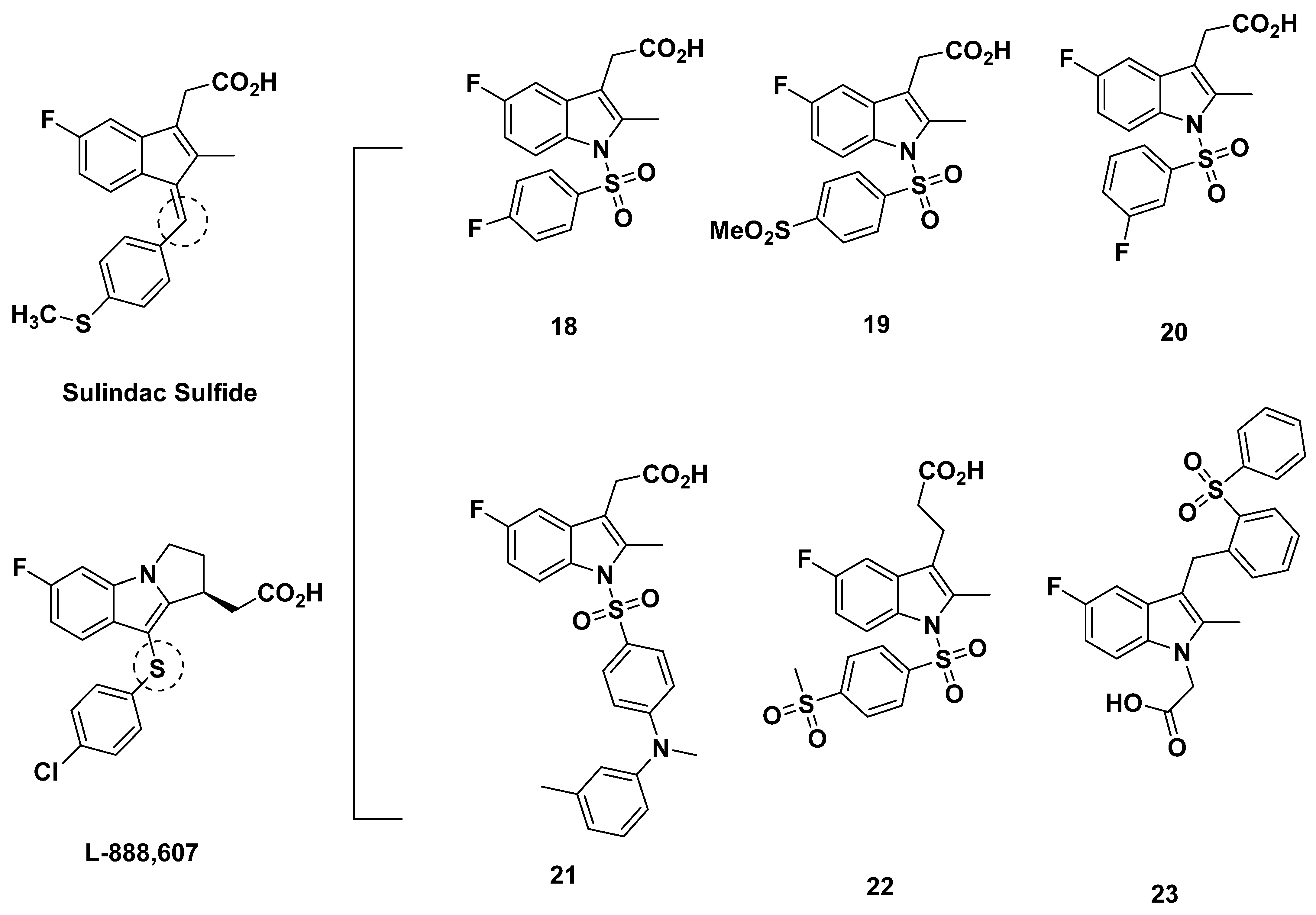
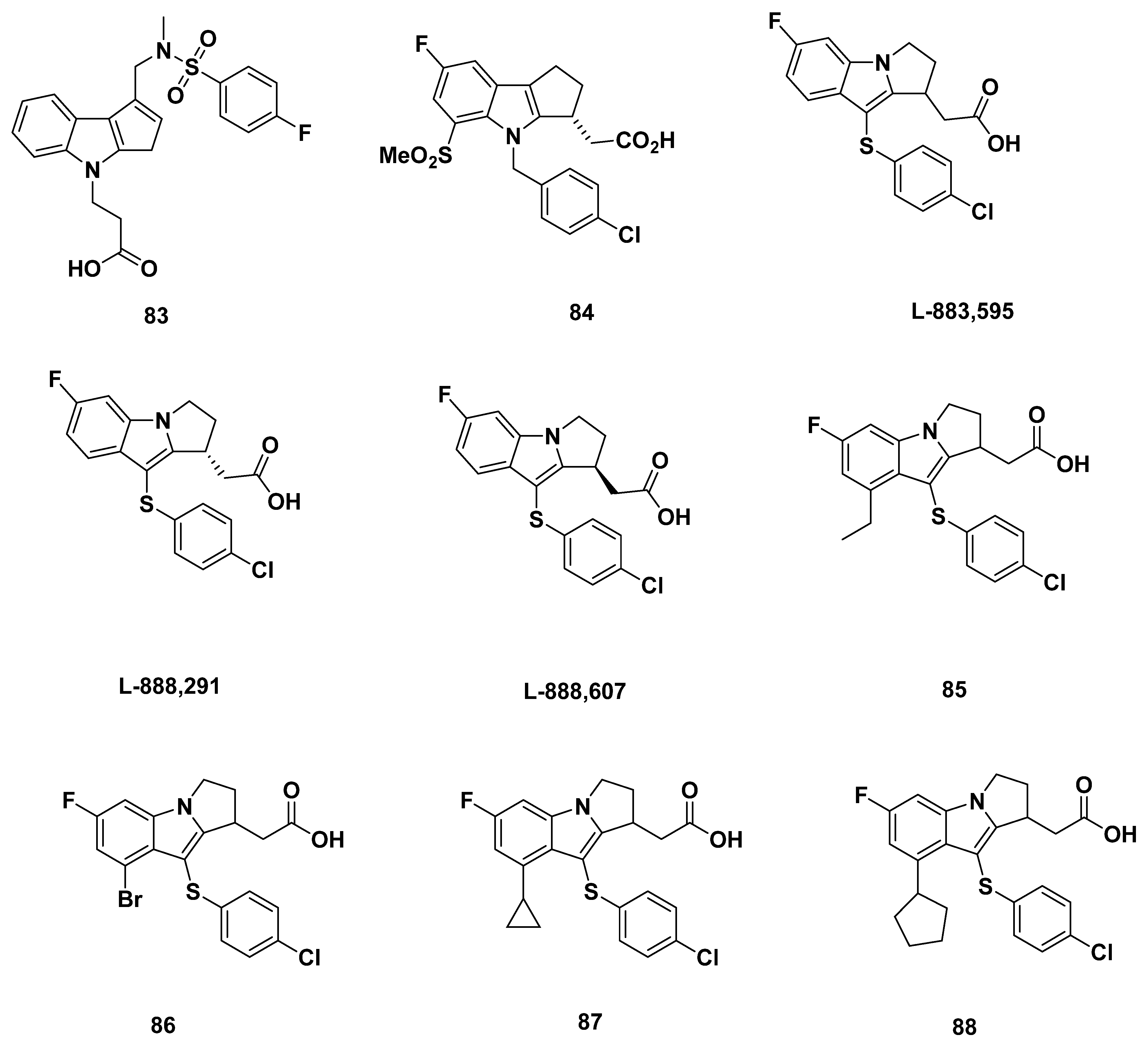
3.2. Merck Analogue
3.2.1. Merck Analogue in 2005
3.2.2. Recommendations
3.2.3. L-888,607, L-883,595, and L-888,291
3.2.4. Recommendations
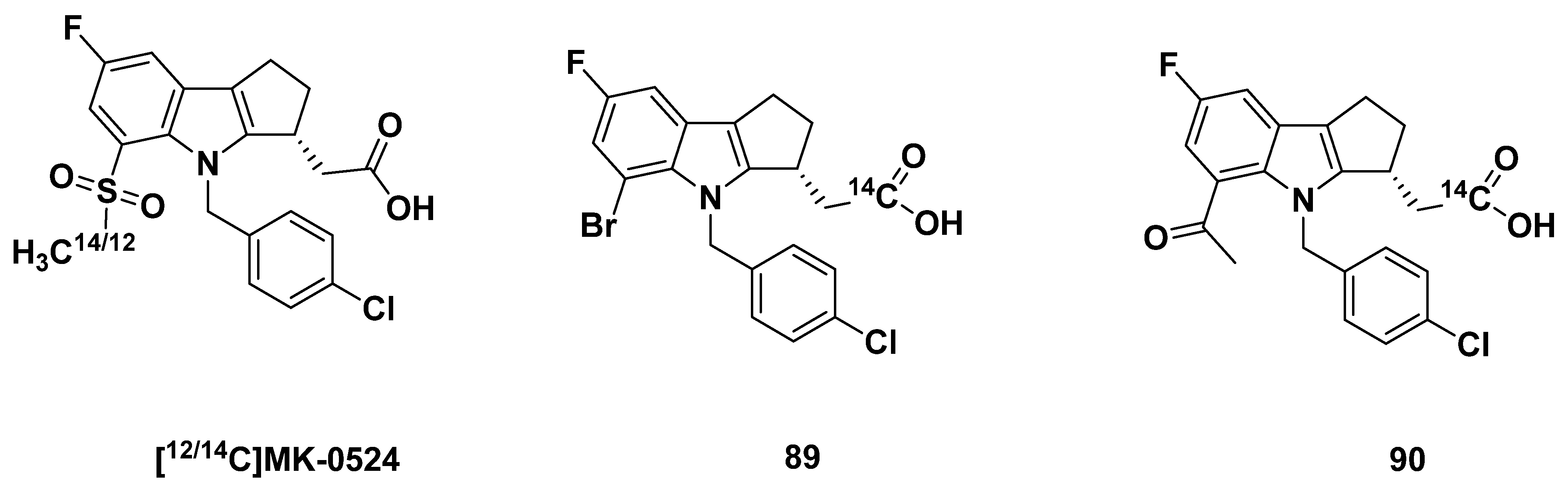
3.2.5. MK-0524
3.2.6. Recommendations
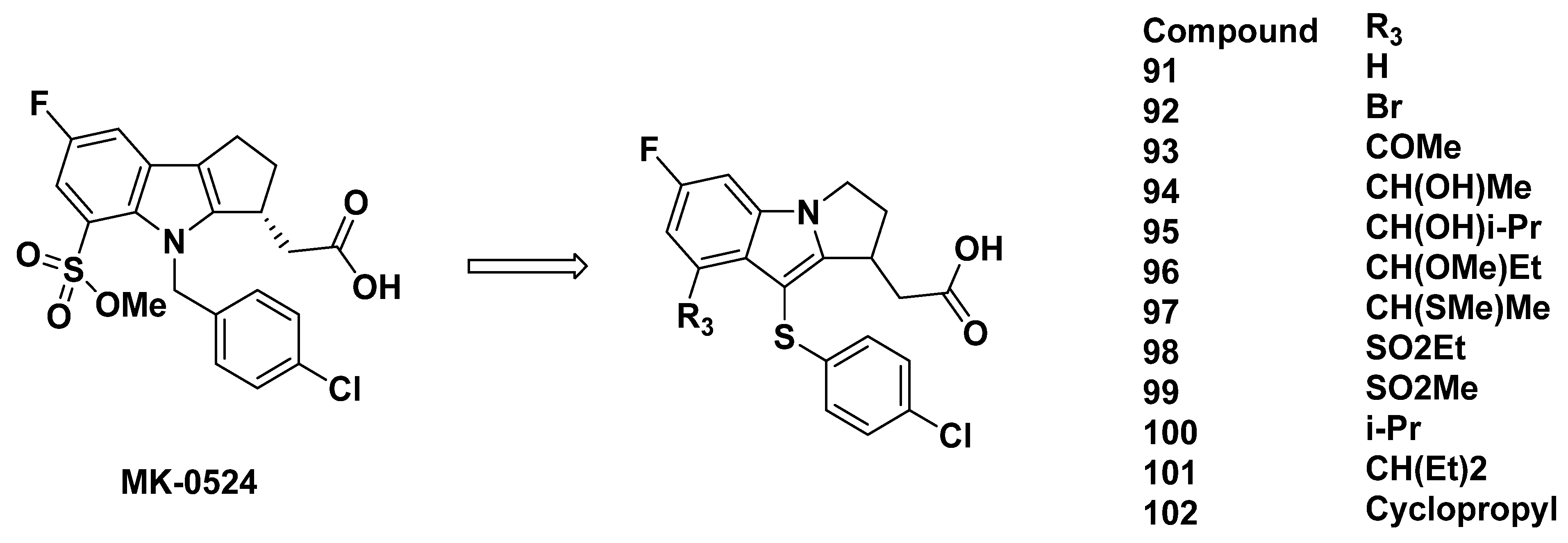
3.2.7. Merck Analogues in 2007
3.2.8. Recommendations
3.2.9. Merck Analogues in 2008
3.2.10. Recommendations
3.3. Astellas Analogues in 2008
Recommendations
4. Potential Fluorination Methods
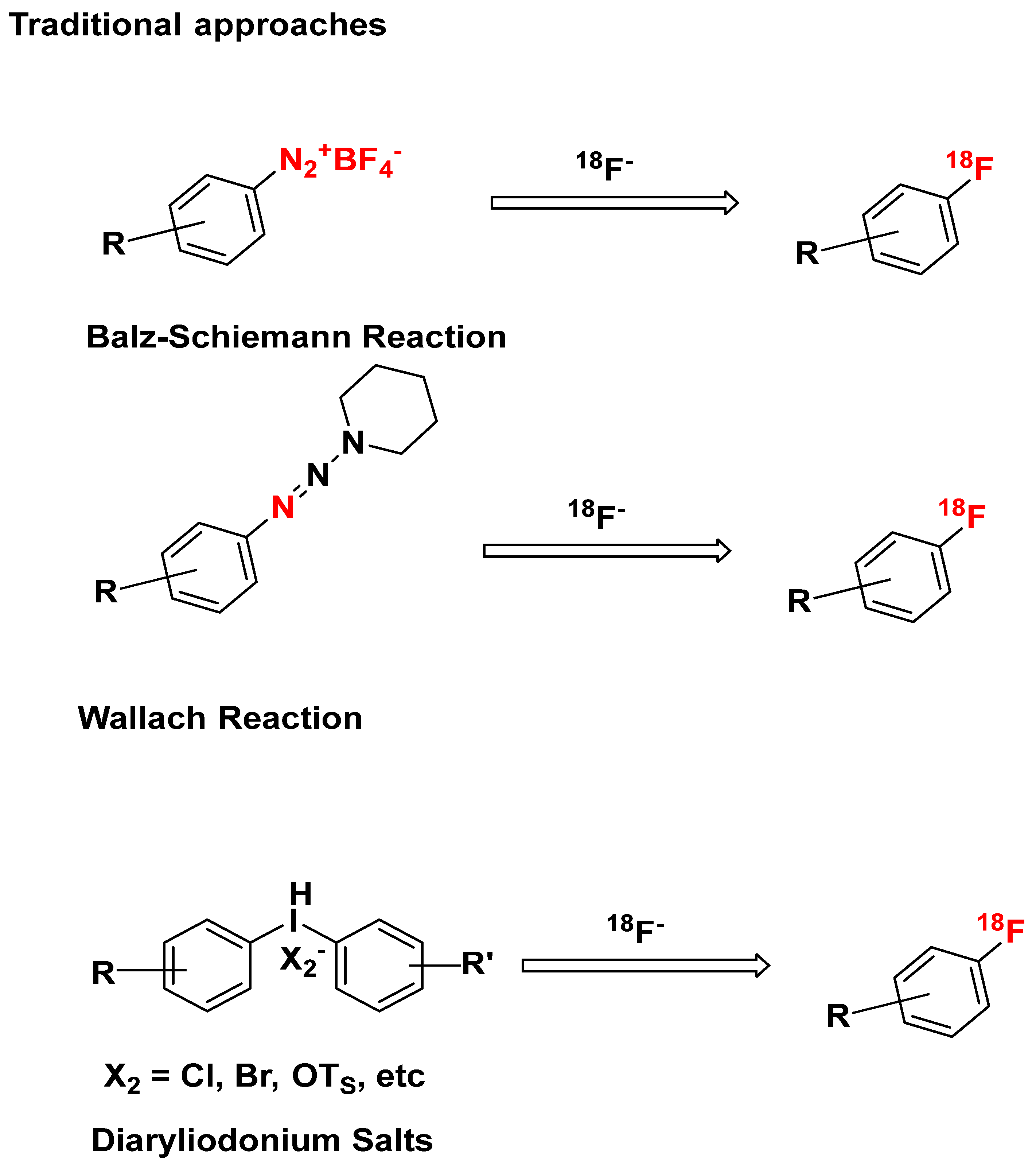
4.1. Concerted Nucleophilic Aromatic Substitution (CSNAr) with 18F-
4.2. Other Traditional Approaches
4.3. Novel 18F-Labeling Strategies
4.4. [18F] Trifluoromethylation (Compounds 24, 25, 33, and 82)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Sever, R.; Brugge, J.S. Signal transduction in cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a006098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Harjunpaa, H.; Asens, M.L.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Maehara, T. Discovery of anti-inflammatory role of prostaglandin D2. J. Vet. Med. Sci. 2016, 78, 1643–1647. [Google Scholar] [CrossRef]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Jandl, K.; Heinemann, A. The therapeutic potential of CRTH2/DP2 beyond allergy and asthma. Prostaglandins Other Lipid Mediat. 2017, 133, 42–48. [Google Scholar] [CrossRef]
- Shioga, T.; Kondo, R.; Ogasawara, S.; Akiba, J.; Mizuochi, S.; Kusano, H.; Mihara, Y.; Tanigawa, M.; Kinjyo, Y.; Naito, Y.; et al. Usefulness of Tumor Tissue Biopsy for Predicting the Biological Behavior of Hepatocellular Carcinoma. Anticancer Res. 2020, 40, 4105–4113. [Google Scholar] [CrossRef]
- Wang, S.M.; Zhang, K.; Tan, S.Y.; Xin, J.Y.; Yuan, Q.Y.; Xu, H.H.; Xu, X.; Liang, Q.; Christiani, D.C.; Wang, M.L.; et al. Circular RNAs in body fluids as cancer biomarkers: The new frontier of liquid biopsies. Mol. Cancer 2021, 20, 13. [Google Scholar] [CrossRef]
- Unterrainer, M.; Eze, C.; Ilhan, H.; Marschner, S.; Roengvoraphoj, O.; Schmidt-Hegemann, N.S.; Walter, F.; Kunz, W.G.; Rosenschold, P.M.A.; Jeraj, R.; et al. Recent advances of PET imaging in clinical radiation oncology. Radiat. Oncol. 2020, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Ming, Y.; Wu, N.; Qian, T.; Li, X.; Wan, D.Q.; Li, C.; Li, Y.; Wu, Z.; Wang, X.; Liu, J.; et al. Progress and Future Trends in PET/CT and PET/MRI Molecular Imaging Approaches for Breast Cancer. Front. Oncol. 2020, 10, 1301. [Google Scholar] [CrossRef]
- Liu, C.H.; Abrams, N.D.; Carrick, D.M.; Chander, P.; Dwyer, J.; Hamlet, M.R.J.; Kindzelski, A.L.; PrabhuDas, M.; Tsai, S.A.; Vedamony, M.M.; et al. Imaging inflammation and its resolution in health and disease: Current status, clinical needs, challenges, and opportunities. FASEB J. 2019, 33, 13085–13097. [Google Scholar] [CrossRef] [PubMed]
- Gangadaran, P.; Ahn, B.C. Molecular Imaging: A Useful Tool for the Development of Natural Killer Cell-Based Immunotherapies. Front. Immunol. 2017, 8, 1090. [Google Scholar] [CrossRef]
- Kang, N.Y.; Soetedjo, A.A.P.; Amirruddin, N.S.; Chang, Y.T.; Eriksson, O.; Teo, A.K.K. Tools for Bioimaging Pancreatic beta Cells in Diabetes. Trends Mol. Med. 2019, 25, 708–722. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Joshi, F.R.; Evans, N.R.; Chowdhury, M.M.; Figg, N.L.; Shah, A.V.; Starks, L.T.; Martin-Garrido, A.; Manavaki, R.; Yu, E.; et al. Detection of Atherosclerotic Inflammation by 68Ga-DOTATATE PET Compared to [18F]FDG PET Imaging. J. Am. Coll. Cardiol. 2017, 69, 1774–1791. [Google Scholar] [CrossRef] [PubMed]
- Emami, H.; Singh, P.; MacNabb, M.; Vucic, E.; Lavender, Z.; Rudd, J.H.; Fayad, Z.A.; Lehrer-Graiwer, J.; Korsgren, M.; Figueroa, A.L.; et al. Splenic metabolic activity predicts risk of future cardiovascular events: Demonstration of a cardiosplenic axis in humans. JACC Cardiovasc. Imaging 2015, 8, 121–130. [Google Scholar] [CrossRef]
- Kwiecinski, J.; Dey, D.; Cadet, S.; Lee, S.E.; Otaki, Y.; Huynh, P.T.; Doris, M.K.; Eisenberg, E.; Yun, M.; Jansen, M.A.; et al. Peri-Coronary Adipose Tissue Density Is Associated With 18F-Sodium Fluoride Coronary Uptake in Stable Patients With High-Risk Plaques. JACC Cardiovasc. Imaging 2019, 12, 2000–2010. [Google Scholar] [CrossRef]
- Grobman, M.; Cohn, L.; Knapp, S.; Bryan, J.N.; Reinero, C. F-18-FDG-PET/CT as adjunctive diagnostic modalities in canine fever of unknown origin. Vet. Radiol. Ultrasoun. 2018, 59, 107–115. [Google Scholar] [CrossRef]
- Coughlin, J.M.; Wang, Y.; Minn, I.; Bienko, N.; Ambinder, E.B.; Xu, X.; Peters, M.E.; Dougherty, J.W.; Vranesic, M.; Koo, S.M.; et al. Imaging of Glial Cell Activation and White Matter Integrity in Brains of Active and Recently Retired National Football League Players. JAMA Neurol. 2017, 74, 67–74. [Google Scholar] [CrossRef]
- Cipriano, P.W.; Yoon, D.; Gandhi, H.; Holley, D.; Thakur, D.; Hargreaves, B.A.; Kennedy, D.J.; Smuck, M.W.; Cheng, I.; Biswal, S. F-18-FDG PET/MRI in Chronic Sciatica: Early Results Revealing Spinal and Nonspinal Abnormalities. J. Nucl. Med. 2018, 59, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Neumann, T.; Oelzner, P.; Freesmeyer, M.; Hansch, A.; Opfermann, T.; Hein, G.; Wolf, G. Images in cardiovascular medicine. Diagnosis of large-vessel vasculitis by [18F] fluorodeoxyglucose-positron emission tomography. Circulation 2009, 119, 338–339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hess, S.; Madsen, P.H.; Iversen, E.D.; Frifelt, J.J.; Hoilund-Carlsen, P.F.; Alavi, A. Efficacy of FDG PET/CT imaging for venous thromboembolic disorders: Preliminary results from a prospective, observational pilot study. Clin. Nucl. Med. 2015, 40, e23–e26. [Google Scholar] [CrossRef]
- Tawakol, A.; Ishai, A.; Takx, R.A.P.; Figueroa, A.L.; Ali, A.; Kaiser, Y.; Truong, Q.A.; Solomon, C.J.E.; Calcagno, C.; Mani, V.; et al. Relation between resting amygdalar activity and cardiovascular events: A longitudinal and cohort study. Lancet 2017, 389, 834–845. [Google Scholar] [CrossRef]
- Rondina, M.T.; Lam, U.T.; Pendleton, R.C.; Kraiss, L.W.; Wanner, N.; Zimmerman, G.A.; Hoffman, J.M.; Hanrahan, C.; Boucher, K.; Christian, P.E.; et al. (18)F-FDG PET in the evaluation of acuity of deep vein thrombosis. Clin. Nucl. Med. 2012, 37, 1139–1145. [Google Scholar] [CrossRef]
- Marchese, A.; George, S.R.; Kolakowski, L.F., Jr.; Lynch, K.R.; O’Dowd, B.F. Novel GPCRs and their endogenous ligands: Expanding the boundaries of physiology and pharmacology. Trends Pharmacol. Sci. 1999, 20, 370–375. [Google Scholar] [CrossRef]
- Haba, R.; Shintani, N.; Onaka, Y.; Kanoh, T.; Wang, H.; Takenaga, R.; Hayata, A.; Hirai, H.; Nagata, K.Y.; Nakamura, M.; et al. Central CRTH2, a second prostaglandin D2 receptor, mediates emotional impairment in the lipopolysaccharide and tumor-induced sickness behavior model. J. Neurosci. 2014, 34, 2514–2523. [Google Scholar] [CrossRef]
- Wang, L.; Yao, D.; Deepak, R.; Liu, H.; Xiao, Q.; Fan, H.; Gong, W.; Wei, Z.; Zhang, C. Structures of the Human PGD2 Receptor CRTH2 Reveal Novel Mechanisms for Ligand Recognition. Mol. Cell. 2018, 72, 48–59.e4. [Google Scholar] [CrossRef]
- Eriksson, O. GPR44 as a Target for Imaging Pancreatic Beta-Cell Mass. Curr. Diabetes Rep. 2019, 19, 49. [Google Scholar] [CrossRef]
- Domingo, C.; Palomares, O.; Sandham, D.A.; Erpenbeck, V.J.; Altman, P. The prostaglandin D2 receptor 2 pathway in asthma: A key player in airway inflammation. Respir. Res. 2018, 19, 189. [Google Scholar] [CrossRef]
- Pettipher, R.; Hansel, T.T.; Armer, R. Antagonism of the prostaglandin D2 receptors DP1 and CRTH2 as an approach to treat allergic diseases. Nat. Rev. Drug Discov. 2007, 6, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, O.; Johnstrom, P.; Cselenyi, Z.; Jahan, M.; Selvaraju, R.K.; Jensen-Waern, M.; Takano, A.; Sorhede Winzell, M.; Halldin, C.; Skrtic, S.; et al. In Vivo Visualization of beta-Cells by Targeting of GPR44. Diabetes 2018, 67, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Salimi, M.; Panse, I.; Mjosberg, J.M.; McKenzie, A.N.; Spits, H.; Klenerman, P.; Ogg, G. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J. Allergy Clin. Immunol. 2014, 133, 1184–1194. [Google Scholar] [CrossRef]
- Moraes-Ferreira, R.; Brandao-Rangel, M.A.R.; Gibson-Alves, T.G.; Silva-Reis, A.; Souza-Palmeira, V.H.; Aquino-Santos, H.C.; Frison, C.R.; Oliveira, L.V.F.; Albertini, R.; Vieira, R.P. Physical Training Reduces Chronic Airway Inflammation and Mediators of Remodeling in Asthma. Oxidative Med. Cell. Longev. 2022, 2022, 5037553. [Google Scholar] [CrossRef]
- Wang, J.J.; Mak, O.T. Induction of apoptosis in non-small cell lung carcinoma A549 cells by PGD2 metabolite, 15d-PGJ2. Cell Biol. Int. 2011, 35, 1089–1096. [Google Scholar] [CrossRef]
- Zhang, B.; Bie, Q.; Wu, P.; Zhang, J.; You, B.; Shi, H.; Qian, H.; Xu, W. PGD2/PTGDR2 Signaling Restricts the Self-Renewal and Tumorigenesis of Gastric Cancer. Stem. Cells 2018, 36, 990–1003. [Google Scholar] [CrossRef]
- Qian, F.; Arner, B.E.; Kelly, K.M.; Annageldiyev, C.; Sharma, A.; Claxton, D.F.; Paulson, R.F.; Prabhu, K.S. Interleukin-4 treatment reduces leukemia burden in acute myeloid leukemia. FASEB J. 2022, 36, e22328. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, M.; Li, Z.; Ma, R.; Tang, H.; Zhang, J.; Marcucci, G.; Yu, J. Human ILC1s target leukemia stem cells and control development of AML. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Dash, P.; Ghatak, S.; Topi, G.; Satapathy, S.R.; Ek, F.; Hellman, K.; Olsson, R.; Mehdawi, L.M.; Sjolander, A. High PGD2 receptor 2 levels are associated with poor prognosis in colorectal cancer patients and induce VEGF expression in colon cancer cells and migration in a zebrafish xenograft model. Br. J. Cancer 2022, 126, 586–597. [Google Scholar] [CrossRef]
- Ma, X.B.; Xu, Y.Y.; Zhu, M.X.; Wang, L. Prognostic Signatures Based on Thirteen Immune-Related Genes in Colorectal Cancer. Front. Oncol. 2020, 10, 591739. [Google Scholar] [CrossRef]
- Wang, P.P.; Fu, Y.; Chen, Y.Y.; Li, Q.; Hong, Y.; Liu, T.; Ding, Z.Y. Nomogram Personalizes and Visualizes the Overall Survival of Patients with Triple-Negative Breast Cancer Based on the Immune Genome. BioMed Res. Int. 2020, 2020, 4029062. [Google Scholar] [CrossRef]
- Di Lullo, G.; Marcatti, M.; Heltai, S.; Brunetto, E.; Tresoldi, C.; Bondanza, A.; Bonini, C.; Ponzoni, M.; Tonon, G.; Ciceri, F.; et al. Th22 cells increase in poor prognosis multiple myeloma and promote tumor cell growth and survival. Oncoimmunology 2015, 4, e1005460. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, X.Y.; Qiu, S.J.; Zhou, J.; Shi, Y.H.; Zhang, B.H.; Fan, J. Tumor stroma reaction-related gene signature predicts clinical outcome in human hepatocellular carcinoma. Cancer Sci. 2011, 102, 1522–1531. [Google Scholar] [CrossRef]
- Koyani, C.N.; Windischhofer, W.; Rossmann, C.; Jin, G.; Kickmaier, S.; Heinzel, F.R.; Groschner, K.; Alavian-Ghavanini, A.; Sattler, W.; Malle, E. 15-deoxy-Δ12,14-PGJ2 promotes inflammation and apoptosis in cardiomyocytes via the DP2/MAPK/TNFalpha axis. Int. J. Cardiol. 2014, 173, 472–480. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yue, L.; Haroun, S.; Parent, J.L.; de Brum-Fernandes, A.J. Prostaglandin D2 induces apoptosis of human osteoclasts through ERK1/2 and Akt signaling pathways. Bone 2014, 60, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Bie, Q.L.; Zhang, P.; Su, Z.L.; Zheng, D.; Ying, X.Y.; Wu, Y.M.; Yang, H.J.; Chen, D.Y.; Wang, S.J.; Xu, H.X. Polarization of ILC2s in Peripheral Blood Might Contribute to Immunosuppressive Microenvironment in Patients with Gastric Cancer. J. Immunol. Res. 2014, 2014, 923135. [Google Scholar] [CrossRef]
- Murata, T.; Lin, M.I.; Aritake, K.; Matsumoto, S.; Narumiya, S.; Ozaki, H.; Urade, Y.; Hori, M.; Sessa, W.C. Role of prostaglandin D2 receptor DP as a suppressor of tumor hyperpermeability and angiogenesis in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 20009–20014. [Google Scholar] [CrossRef]
- Hirai, H.; Tanaka, K.; Yoshie, O.; Ogawa, K.; Kenmotsu, K.; Takamori, Y.; Ichimasa, M.; Sugamura, K.; Nakamura, M.; Takano, S.; et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J. Exp. Med. 2001, 193, 255–262. [Google Scholar] [CrossRef]
- Monneret, G.; Gravel, S.; Diamond, M.; Rokach, J.; Powell, W.S. Prostaglandin D2 is a potent chemoattractant for human eosinophils that acts via a novel DP receptor. Blood 2001, 98, 1942–1948. [Google Scholar] [CrossRef]
- Kupczyk, M.; Kuna, P. Targeting the PGD2/CRTH2/DP1 Signaling Pathway in Asthma and Allergic Disease: Current Status and Future Perspectives. Drugs 2017, 77, 1281–1294. [Google Scholar] [CrossRef]
- Oyesola, O.O.; Shanahan, M.T.; Kanke, M.; Mooney, B.M.; Webb, L.M.; Smita, S.; Matheson, M.K.; Campioli, P.; Pham, D.; Fruh, S.P.; et al. PGD2 and CRTH2 counteract Type 2 cytokine-elicited intestinal epithelial responses during helminth infection. J. Exp. Med. 2021, 218, e20202178. [Google Scholar] [CrossRef]
- Luna-Gomes, T.; Magalhaes, K.G.; Mesquita-Santos, F.P.; Bakker-Abreu, I.; Samico, R.F.; Molinaro, R.; Calheiros, A.S.; Diaz, B.L.; Bozza, P.T.; Weller, P.F.; et al. Eosinophils as a Novel Cell Source of Prostaglandin D2: Autocrine Role in Allergic Inflammation. J. Immunol. 2011, 187, 6518–6526. [Google Scholar] [CrossRef]
- Rittchen, S.; Heinemann, A. Therapeutic Potential of Hematopoietic Prostaglandin D2 Synthase in Allergic Inflammation. Cells 2019, 8, 619. [Google Scholar] [CrossRef]
- Jahan, M.; Johnström, P.; Selvaraju, R.K.; Svedberg, M.; Winzell, M.S.; Bernström, J.; Kingston, L.; Schou, M.; Jia, Z.; Skrtic, S.; et al. The development of a GPR44 targeting radioligand [(11)C]AZ12204657 for in vivo assessment of beta cell mass. EJNMMI Res. 2018, 8, 113. [Google Scholar] [CrossRef]
- Gallant, M.; Beaulieu, C.; Berthelette, C.; Colucci, J.; Crackower, M.A.; Dalton, C.; Denis, D.; Ducharme, Y.; Friesen, R.W.; Guay, D.; et al. Discovery of MK-7246, a selective CRTH2 antagonist for the treatment of respiratory diseases. Bioorg. Med. Chem. Lett. 2011, 21, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, B.S.; Chi, D.Y. High efficiency synthesis of F-18 fluoromethyl ethers: An attractive alternative for C-11 methyl groups in positron emission tomography radiopharmaceuticals. Org. Lett. 2013, 15, 4346–4349. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Hao, G.; Guan, B.; Thapa, P.; Hao, J.; Hammers, H.; Sun, X. Theranostic Small-Molecule Prodrug Conjugates for Targeted Delivery and Controlled Release of Toll-like Receptor 7 Agonists. Int. J. Mol. Sci. 2022, 23, 7160. [Google Scholar] [CrossRef]
- Debnath, S.; Zhou, N.; McLaughlin, M.; Rice, S.; Pillai, A.K.; Hao, G.; Sun, X. PSMA-Targeting Imaging and Theranostic Agents-Current Status and Future Perspective. Int. J. Mol. Sci. 2022, 23, 1158. [Google Scholar] [CrossRef]
- Huang, L.A.; Huang, K.X.; Tu, J.; Kandeel, F.; Li, J. Ramatroban-Based Analogues Containing Fluorine Group as Potential 18F-Labeled Positron Emission Tomography (PET) G-Protein Coupled Receptor 44 (GPR44) Tracers. Molecules 2021, 26, 1433. [Google Scholar] [CrossRef]
- Hirai, H.; Tanaka, K.; Takano, S.; Ichimasa, M.; Nakamura, M.; Nagata, K. Cutting edge: Agonistic effect of indomethacin on a prostaglandin D2 receptor, CRTH2. J. Immunol. 2002, 168, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; Bell, F.M.; Akam, E.; Marshall, C.; Dainty, I.A.; Heinemann, A.; Dougall, I.G.; Bonnert, R.V.; Sargent, C.A. Biochemical and pharmacological characterization of AZD1981, an orally available selective DP2 antagonist in clinical development for asthma. Br. J. Pharmacol. 2013, 168, 1626–1638. [Google Scholar] [CrossRef] [PubMed]
- Torisu, K.; Kobayashi, K.; Iwahashi, M.; Nakai, Y.; Onoda, T.; Nagase, T.; Sugimoto, I.; Okada, Y.; Matsumoto, R.; Nanbu, F.; et al. Discovery of a new class of potent, selective, and orally active prostaglandin D2 receptor antagonists. Bioorg. Med. Chem. 2004, 12, 5361–5378. [Google Scholar] [CrossRef] [PubMed]
- Torisu, K.; Kobayashi, K.; Iwahashi, M.; Nakai, Y.; Onoda, T.; Nagase, T.; Sugimoto, I.; Okada, Y.; Matsumoto, R.; Nanbu, F.; et al. Discovery of orally active prostaglandin D2 receptor antagonists. Bioorg. Med. Chem. Lett. 2004, 14, 4891–4895. [Google Scholar] [CrossRef] [PubMed]
- Torisu, K.; Kobayashi, K.; Iwahashi, M.; Egashira, H.; Nakai, Y.; Okada, Y.; Nanbu, F.; Ohuchida, S.; Nakai, H.; Toda, M. Development of a prostaglandin D2 receptor antagonist: Discovery of a new chemical lead. Eur. J. Med. Chem. 2005, 40, 505–519. [Google Scholar] [CrossRef]
- Iwahashi, M.; Shimabukuro, A.; Onoda, T.; Matsunaga, Y.; Okada, Y.; Matsumoto, R.; Nambu, F.; Nakai, H.; Toda, M. Discovery of selective indole-based prostaglandin D₂ receptor antagonist. Bioorg. Med. Chem. 2011, 19, 4574–4588. [Google Scholar] [CrossRef]
- Armer, R.E.; Ashton, M.R.; Boyd, E.A.; Brennan, C.J.; Brookfield, F.A.; Gazi, L.; Gyles, S.L.; Hay, P.A.; Hunter, M.G.; Middlemiss, D.; et al. Indole-3-acetic acid antagonists of the prostaglandin D2 receptor CRTH2. J. Med. Chem. 2005, 48, 6174–6177. [Google Scholar] [CrossRef]
- Shen, T.Y.; Winter, C.A. Chemical and biological studies on indomethacin, sulindac and their analogs. Adv. Drug Res. 1977, 12, 90–245. [Google Scholar]
- Gervais, F.G.; Morello, J.P.; Beaulieu, C.; Sawyer, N.; Denis, D.; Greig, G.; Malebranche, A.D.; O’Neill, G.P. Identification of a potent and selective synthetic agonist at the CRTH2 receptor. Mol. Pharmacol. 2005, 67, 1834–1839. [Google Scholar] [CrossRef]
- Norman, P. DP2 receptor antagonists in development. Expert Opin. Investig. Drugs 2010, 19, 947–961. [Google Scholar] [CrossRef]
- Armer, R.E.; Pettipher, E.R.; Whittaker, M.; Wynne, G.M.; Vile, J.; Schroer, F. Compounds Having Crth2 Antagonist Activity. European Patent EP2250161B1, 19 January 2009. [Google Scholar]
- Pettipher, R.; Vinall, S.L.; Xue, L.; Speight, G.; Townsend, E.R.; Gazi, L.; Whelan, C.J.; Armer, R.E.; Payton, M.A.; Hunter, M.G. Pharmacologic profile of OC000459, a potent, selective, and orally active D prostanoid receptor 2 antagonist that inhibits mast cell-dependent activation of T helper 2 lymphocytes and eosinophils. J. Pharmacol. Exp. Ther. 2012, 340, 473–482. [Google Scholar] [CrossRef]
- Sokolowska, M.; Rovati, G.E.; Diamant, Z.; Untersmayr, E.; Schwarze, J.; Lukasik, Z.; Sava, F.; Angelina, A.; Palomares, O.; Akdis, C.A.; et al. Effects of non-steroidal anti-inflammatory drugs and other eicosanoid pathway modifiers on antiviral and allergic responses: EAACI task force on eicosanoids consensus report in times of COVID-19. Allergy 2022, 77, 2337–2354. [Google Scholar] [CrossRef] [PubMed]
- Currie, C.; Framroze, B.; Singh, D.; Lea, S.; Bjerknes, C.; Hermansen, E. Assessing the Anti-Inflammatory Effects of an Orally Dosed Enzymatically Liberated Fish Oil in a House Dust Model of Allergic Asthma. Biomedicines 2022, 10, 2574. [Google Scholar] [CrossRef]
- Vatrella, A.; Maglio, A.; Pelaia, C.; Ciampo, L.; Pelaia, G.; Vitale, C. Eosinophilic inflammation: An Appealing Target for Pharmacologic Treatments in Severe Asthma. Biomedicines 2022, 10, 2181. [Google Scholar] [CrossRef] [PubMed]
- Farne, H.; Glanville, N.; Johnson, N.; Kebadze, T.; Aniscenko, J.; Regis, E.; Zhu, J.; Trujillo-Torralbo, M.B.; Kon, O.M.; Mallia, P.; et al. Effect of CRTH2 antagonism on the response to experimental rhinovirus infection in asthma: A pilot randomised controlled trial. Thorax 2021, 77, 950–959. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, Y.; Chen, Y. The impact of CRTH2 antagonist OC 000459 on pulmonary function of asthma patients: A meta-analysis of randomized controlled trials. Postepy Dermatol. Alergol. 2021, 38, 566–571. [Google Scholar] [CrossRef]
- Bonafoux, D.; Abibi, A.; Bettencourt, B.; Burchat, A.; Ericsson, A.; Harris, C.M.; Kebede, T.; Morytko, M.; McPherson, M.; Wallace, G.; et al. Thienopyrrole acetic acids as antagonists of the CRTH2 receptor. Bioorganic Med. Chem. Lett. 2011, 21, 1861–1864. [Google Scholar] [CrossRef] [PubMed]
- Sykes, D.A.; Bradley, M.E.; Riddy, D.M.; Willard, E.; Reilly, J.; Miah, A.; Bauer, C.; Watson, S.J.; Sandham, D.A.; Dubois, G.; et al. Fevipiprant (QAW039), a Slowly Dissociating CRTh2 Antagonist with the Potential for Improved Clinical Efficacy. Mol. Pharmacol. 2016, 89, 593–605. [Google Scholar] [CrossRef]
- Barnes, N.; Pavord, I.; Chuchalin, A.; Bell, J.; Hunter, M.; Lewis, T.; Parker, D.; Payton, M.; Collins, L.P.; Pettipher, R.; et al. A randomized, double-blind, placebo-controlled study of the CRTH2 antagonist OC000459 in moderate persistent asthma. Clin. Exp. Allergy 2012, 42, 38–48. [Google Scholar] [CrossRef]
- Mangiavacchi, F.; Crociani, L.; Sancineto, L.; Marini, F.; Santi, C. Continuous Bioinspired Oxidation of Sulfides. Molecules 2020, 25, 2711. [Google Scholar] [CrossRef]
- Sturino, C.F.; O’Neill, G.; Lachance, N.; Boyd, M.; Berthelette, C.; Labelle, M.; Li, L.; Roy, B.; Scheigetz, J.; Tsou, N.; et al. Discovery of a potent and selective prostaglandin D2 receptor antagonist, [(3R)-4-(4-chloro-benzyl)-7-fluoro-5-(methylsulfonyl)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl]-acetic acid (MK-0524). J. Med. Chem. 2007, 50, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Aguiar, J.; Ardati, A.; Cao, B.; Gardner, C.J.; Gillespy, T.; Harris, K.; Lim, S.; Marcus, R.; Morize, I.; et al. Understanding DP receptor antagonism using a CoMSIA approach. Bioorg. Med. Chem. Lett. 2011, 21, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Cadden, P.; Hunter, M.; Pearce Collins, L.; Perkins, M.; Pettipher, R.; Townsend, E.; Vinall, S.; O’Connor, B. Inhibition of the asthmatic allergen challenge response by the CRTH2 antagonist OC000459. Eur. Respir. J. 2013, 41, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Radnai, B.; Sturm, E.M.; Stancic, A.; Jandl, K.; Labocha, S.; Ferreiros, N.; Grill, M.; Hasenoehrl, C.; Gorkiewicz, G.; Marsche, G.; et al. Eosinophils Contribute to Intestinal Inflammation via Chemoattractant Receptor-homologous Molecule Expressed on Th2 Cells, CRTH2, in Experimental Crohn’s Disease. J. Crohn’s Colitis 2016, 10, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Birkinshaw, T.N.; Teague, S.J.; Beech, C.; Bonnert, R.V.; Hill, S.; Patel, A.; Reakes, S.; Sanganee, H.; Dougall, I.G.; Phillips, T.T.; et al. Discovery of potent CRTh2 (DP2) receptor antagonists. Bioorg. Med. Chem. Lett. 2006, 16, 4287–4290. [Google Scholar] [CrossRef]
- Luker, T.; Bonnert, R.; Brough, S.; Cook, A.R.; Dickinson, M.R.; Dougall, I.; Logan, C.; Mohammed, R.T.; Paine, S.; Sanganee, H.J.; et al. Substituted indole-1-acetic acids as potent and selective CRTh2 antagonists-discovery of AZD1981. Bioorg. Med. Chem. Lett. 2011, 21, 6288–6292. [Google Scholar] [CrossRef]
- Pettipher, R.; Whittaker, M. Update on the development of antagonists of chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2). From lead optimization to clinical proof-of-concept in asthma and allergic rhinitis. J. Med. Chem. 2012, 55, 2915–2931. [Google Scholar] [CrossRef]
- Andrés, M.; Bravo, M.; Buil, M.A.; Calbet, M.; Castillo, M.; Castro, J.; Eichhorn, P.; Ferrer, M.; Lehner, M.D.; Moreno, I.; et al. 2-(1H-Pyrazol-1-yl)acetic acids as chemoattractant receptor-homologous molecule expressed on Th2 lymphocytes (CRTh2) antagonists. Eur. J. Med. Chem. 2014, 71, 168–184. [Google Scholar] [CrossRef]
- Bauer, P.H.; Ceng, J.B.; Gladue, R.P.; Li, B.; Neote, K.S.; Zhang, J. Methods for the Identification of Compounds Useful for the Treatment of Disease States Mediated by Prostaglandin D2. European Patent EP1170594, 9 January 2002. [Google Scholar]
- Kaila, N.; Huang, A.; Moretto, A.; Follows, B.; Janz, K.; Lowe, M.; Thomason, J.; Mansour, T.S.; Hubeau, C.; Page, K.; et al. Diazine indole acetic acids as potent, selective, and orally bioavailable antagonists of chemoattractant receptor homologous molecule expressed on Th2 cells (CRTH2) for the treatment of allergic inflammatory diseases. J. Med. Chem. 2012, 55, 5088–5109. [Google Scholar] [CrossRef]
- Babu, S.; Rupa, M.; Nagarajan, S.K.; Sohn, H.; Madhavan, T. Molecular Modeling Study on Diazine Indole Acetic Acid Derivatives for CRTH2 Inhibitory Activity. Comb. Chem. High Throughput Screen. 2016, 19, 444–460. [Google Scholar] [CrossRef]
- Andrés, M.; Bravo, M.; Buil, M.A.; Calbet, M.; Castro, J.; Domènech, T.; Eichhorn, P.; Ferrer, M.; Gómez, E.; Lehner, M.D.; et al. 2-(1H-Pyrazol-4-yl)acetic acids as CRTh2 antagonists. Bioorg. Med. Chem. Lett. 2013, 23, 3349–3353. [Google Scholar] [CrossRef]
- Buil, M.A.; Calbet, M.; Castillo, M.; Castro, J.; Esteve, C.; Ferrer, M.; Forns, P.; González, J.; López, S.; Roberts, R.S.; et al. Structure-activity relationships (SAR) and structure-kinetic relationships (SKR) of sulphone-based CRTh2 antagonists. Eur. J. Med. Chem. 2016, 113, 102–133. [Google Scholar] [CrossRef]
- Pothier, J.; Riederer, M.A.; Peter, O.; Leroy, X.; Valdenaire, A.; Gnerre, C.; Fretz, H. Novel 2-(2-(benzylthio)-1H-benzo[d]imidazol-1-yl)acetic acids: Discovery and hit-to-lead evolution of a selective CRTh2 receptor antagonist chemotype. Bioorg. Med. Chem. Lett. 2012, 22, 4660–4664. [Google Scholar] [CrossRef] [PubMed]
- Valdenaire, A.; Pothier, J.; Renneberg, D.; Riederer, M.A.; Peter, O.; Leroy, X.; Gnerre, C.; Fretz, H. Evolution of novel tricyclic CRTh2 receptor antagonists from a (E)-2-cyano-3-(1H-indol-3-yl)acrylamide scaffold. Bioorg. Med. Chem. Lett. 2013, 23, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Ly, T.W.; Bacon, K.B. Small-molecule CRTH2 antagonists for the treatment of allergic inflammation: An overview. Expert Opin. Investig. Drugs 2005, 14, 769–773. [Google Scholar] [CrossRef]
- Campos, K.R.; Journet, M.; Lee, S.; Grabowski, E.J.; Tillyer, R.D. Asymmetric synthesis of a prostaglandin D2 receptor antagonist. J. Org. Chem. 2005, 70, 268–274. [Google Scholar] [CrossRef]
- Chang, S.W.; Reddy, V.; Pereira, T.; Dean, B.J.; Xia, Y.Q.; Seto, C.; Franklin, R.B.; Karanam, B.V. The pharmacokinetics and disposition of MK-0524, a Prosglandin D2 Receptor 1 antagonist, in rats, dogs and monkeys. Xenobiotica 2007, 37, 514–533. [Google Scholar] [CrossRef]
- Dean, B.J.; Chang, S.; Silva Elipe, M.V.; Xia, Y.Q.; Braun, M.; Soli, E.; Zhao, Y.; Franklin, R.B.; Karanam, B. Metabolism of MK-0524, a prostaglandin D2 receptor 1 antagonist, in microsomes and hepatocytes from preclinical species and humans. Drug Metab. Dispos. 2007, 35, 283–292. [Google Scholar] [CrossRef]
- Karanam, B.; Madeira, M.; Bradley, S.; Wenning, L.; Desai, R.; Soli, E.; Schenk, D.; Jones, A.; Dean, B.; Doss, G.; et al. Absorption, metabolism, and excretion of [14C]MK-0524, a prostaglandin D2 receptor antagonist, in humans. Drug Metab. Dispos. 2007, 35, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, C.; Guay, D.; Wang, Z.; Leblanc, Y.; Roy, P.; Dufresne, C.; Zamboni, R.; Berthelette, C.; Day, S.; Tsou, N.; et al. Identification of prostaglandin D2 receptor antagonists based on a tetrahydropyridoindole scaffold. Bioorg. Med. Chem. Lett. 2008, 18, 2696–2700. [Google Scholar] [CrossRef]
- Ito, S.; Terasaka, T.; Zenkoh, T.; Matsuda, H.; Hayashida, H.; Nagata, H.; Imamura, Y.; Kobayashi, M.; Takeuchi, M.; Ohta, M. Discovery of novel and potent CRTH2 antagonists. Bioorg. Med. Chem. Lett. 2012, 22, 1194–1197. [Google Scholar] [CrossRef]
- Cheung, P.; Zhang, B.; Puuvuori, E.; Estrada, S.; Amin, M.A.; Ye, S.; Korsgren, O.; Odell, L.R.; Eriksson, J.; Eriksson, O. PET Imaging of GPR44 by Antagonist [11C]MK-7246 in Pigs. Biomedicines 2021, 9, 434. [Google Scholar] [CrossRef]
- Eriksson, J.; Roy, T.; Sawadjoon, S.; Bachmann, K.; Skold, C.; Larhed, M.; Weis, J.; Selvaraju, R.K.; Korsgren, O.; Eriksson, O.; et al. Synthesis and preclinical evaluation of the CRTH2 antagonist [11C]MK-7246 as a novel PET tracer and potential surrogate marker for pancreatic beta-cell mass. Nucl. Med. Biol. 2019, 71, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.; Amin, M.A.; Zhang, B.; Lechi, F.; Korsgren, O.; Eriksson, J.; Odell, L.R.; Eriksson, O. [(18)F]MK-7246 for Positron Emission Tomography Imaging of the Beta-Cell Surface Marker GPR44. Pharmaceutics 2023, 15, 499. [Google Scholar] [CrossRef]
- Kandeel, F.; Li, J.; Peng, J. Compositions and Methods for G-Protein-Coupled Receptor 44 Detection. U.S. Patent Application No. PCT/US2021/047091, 23 August 2021. [Google Scholar]
- van der Born, D.; Pees, A.; Poot, A.J.; Orru, R.V.A.; Windhorst, A.D.; Vugts, D.J. Fluorine-18 labelled building blocks for PET tracer synthesis. Chem. Soc. Rev. 2017, 46, 4709–4773. [Google Scholar] [CrossRef] [PubMed]
- Tredwell, M.; Gouverneur, V. 18F labeling of arenes. Angew. Chem. Int. Ed. Engl. 2012, 51, 11426–11437. [Google Scholar] [CrossRef]
- Jacobson, O.; Kiesewetter, D.O.; Chen, X. Fluorine-18 radiochemistry, labeling strategies and synthetic routes. Bioconjug. Chem. 2015, 26, 1–18. [Google Scholar] [CrossRef]
- Ajenjo, J.; Destro, G.; Cornelissen, B.; Gouverneur, V. Closing the gap between 19F and 18F chemistry. EJNMMI Radiopharm. Chem. 2021, 6, 33. [Google Scholar] [CrossRef]
- Neumann, C.N.; Hooker, J.M.; Ritter, T. Concerted nucleophilic aromatic substitution with 19F− and 18F−. Nature 2016, 534, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Coenen, H.H. Fluorine-18 labeling methods: Features and possibilities of basic reactions. In Ernst Schering Research Foundation Workshop; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Tewson, T.J.; Raichle, M.E.; Welch, M.J. Preliminary studies with [18F]haloperidol: A radioligand for in vivo studies of the dopamine receptors. Brain Res. 1980, 192, 291–295. [Google Scholar] [CrossRef]
- Pike, V.W.; Aigbirhio, F.I. Reactions of Cyclotron-Produced [18F]Fluoride with Diaryliodonium-Salts—A Novel Single-Step Route to No-Carrier-Added [18]Fluoroarenes. J. Chem. Soc. Chem. Comm. 1995, 21, 2215–2216. [Google Scholar] [CrossRef]
- Basuli, F.; Wu, H.T.; Griffiths, G.L. Syntheses of meta-[18F]fluorobenzaldehyde and meta-[18F]fluorobenzylbromide from phenyl(3-Formylphenyl) iodonium salt precursors. J. Label. Compd. Rad. 2011, 54, 224–228. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, C.P. Revisiting the Balz-Schiemann Reaction of Aryldiazonium Tetrafluoroborate in Different Solvents under Catalyst- and Additive-Free Conditions. ACS Omega 2021, 6, 21595–21603. [Google Scholar] [CrossRef]
- Preshlock, S.; Tredwell, M.; Gouverneur, V. 18F-Labeling of Arenes and Heteroarenes for Applications in Positron Emission Tomography. Chem. Rev. 2016, 116, 719–766. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cerny, R.L.; Uppaluri, S.; Kempinger, J.J.; Dimagno, S.G. Fluoride-Promoted Ligand Exchange in Diaryliodonium Salts. J. Fluor. Chem. 2010, 131, 1113–1121. [Google Scholar] [CrossRef][Green Version]
- Ichiishi, N.; Canty, A.J.; Yates, B.F.; Sanford, M.S. Cu-catalyzed fluorination of diaryliodonium salts with KF. Org. Lett. 2013, 15, 5134–5137. [Google Scholar] [CrossRef]
- Chen, W.; Huang, Z.; Tay, N.E.S.; Giglio, B.; Wang, M.; Wang, H.; Wu, Z.; Nicewicz, D.A.; Li, Z. Direct arene C-H fluorination with 18F− via organic photoredox catalysis. Science 2019, 364, 1170–1174. [Google Scholar] [CrossRef]
- Tay, N.E.S.; Chen, W.; Levens, A.; Pistritto, V.A.; Huang, Z.; Wu, Z.H.; Li, Z.B.; Nicewicz, D.A. 19F- and 18F-arene deoxyfluorination via organic photoredox-catalysed polarity-reversed nucleophilic aromatic substitution. Nat. Catal. 2020, 3, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, S.; Stenhagen, I.S.R.; O’Duill, M.; Wolstenhulme, J.; Kirjavainen, A.K.; Forsback, S.J.; Tredwell, M.; Sandford, G.; Moore, P.R.; Huiban, M.; et al. Catalytic Decarboxylative Fluorination for the Synthesis of Tri- and Difluoromethyl Arenes. Org. Lett. 2013, 15, 2648–2651. [Google Scholar] [CrossRef]
- Huiban, M.; Tredwell, M.; Mizuta, S.; Wan, Z.; Zhang, X.; Collier, T.L.; Gouverneur, V.; Passchier, J. A broadly applicable [18F]trifluoromethylation of aryl and heteroaryl iodides for PET imaging. Nat. Chem. 2013, 5, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, T.; Rafique, W.; Lien, V.T.; Riss, P.J. Cu(I)-mediated 18F-trifluoromethylation of arenes: Rapid synthesis of 18F-labeled trifluoromethyl arenes. Chem. Commun. 2014, 50, 6056–6059. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Brindisi, M. Organic Carbamates in Drug Design and Medicinal Chemistry. J. Med. Chem. 2015, 58, 2895–2940. [Google Scholar] [CrossRef] [PubMed]





| Compd. | Binding Ki (µM) | IC50 (µM) b | ||||||
|---|---|---|---|---|---|---|---|---|
| mEP1 | mEP2 | mEP3 | mEP4 | hIP | mDP | hDP | hDP | |
| 1 | >10 | 2.1 | 1.7 | >10 | 0.26 | 0.023 | 0.0053 | 0.00081 |
| 2 | 0.27 | 0.24 | 1.4 | 1.9 | 0.026 | 0.0024 | 0.046 | 0.15 |
| 3 | 1.2 | 0.73 | 2.0 | 1.6 | NT a | 0.019 | 0.20 | 0.37 |
| 4 | 0.20 | 0.71 | 2.8 | 3.5 | 0.29 | 0.0093 | 0.13 | 0.47 |
| 5 | >10 | 2.1 | 1.7 | >10 | NT a | 0.023 | 0.0053 | 0.00081 |
| 6 | >10 | 2.8 | >10 | >10 | NT a | 0.11 | NT a | NT a |
| Comp. | X | Y | Binding Ki (nM) | IC50 a (nM) | Binding Ki (nM) | |
|---|---|---|---|---|---|---|
| mDP | hIP | |||||
| 8 |  | H | 2-F | 3.8 | 2.5 | 110 |
| 9 | H | 3-F | 240 | NT b | 210 | |
| 10 | F | 2-Me | 2.7 | 3.2 | 130 | |
| 11 | F | 2-Cl | 5.2 | 1.6 | 200 | |
| 12 | F | 2-F | 3.8 | 0.7 | 320 | |
| 13 | F | 3-Me | 59 | 42 | 160 | |
| 14 | F | 3-OMe | NT b | 68 | 1500 | |
| 15 | F | 2,3-Me | NT b | 32 | 230 | |
| 16 | F | 2,5-Me | 14 | 2.8 | 93 | |
| Compound | Actual pIC50 | Predicted pIC50 | Residual |
|---|---|---|---|
| 36 * | 7.699 | 7.892 | −0.193 |
| 37 * | 7.638 | 7.776 | −0.138 |
| 38 | 7.046 | 6.902 | 0.144 |
| 39 | 7.119 | 6.990 | 0.129 |
| 40 * | 6.611 | 6.190 | 0.421 |
| 42 | 6.030 | 6.149 | −0.119 |
| 43 * | 7.959 | 8.317 | −0.358 |
| 45 | 8.824 | 8.971 | −0.147 |
| 46 | 8.046 | 8.083 | −0.037 |
| 47 | 8.523 | 8.453 | 0.070 |
| 48 * | 8.398 | 8.266 | 0.132 |
| 50 | 8.301 | 9.168 | −0.867 |
| 51 | 8.222 | 8.177 | 0.045 |
| 52 | 9.097 | 9.120 | −0.023 |
| 53 | 7.699 | 7.807 | −0.108 |
| 54 | 7.921 | 7.889 | 0.032 |
| 55 * | 7.796 | 8.134 | −0.338 |
| 56 | 8.155 | 8.109 | 0.046 |
| 58 | 7.553 | 7.663 | −0.110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, R.; Huang, K.X.; Huang, L.A.; Ji, M.; Zhao, H.; Li, K.; Gao, A.; Chen, J.; Li, Z.; Liu, T.; et al. Indole-Based and Cyclopentenylindole-Based Analogues Containing Fluorine Group as Potential 18F-Labeled Positron Emission Tomography (PET) G-Protein Coupled Receptor 44 (GPR44) Tracers. Pharmaceuticals 2023, 16, 1203. https://doi.org/10.3390/ph16091203
Yin R, Huang KX, Huang LA, Ji M, Zhao H, Li K, Gao A, Chen J, Li Z, Liu T, et al. Indole-Based and Cyclopentenylindole-Based Analogues Containing Fluorine Group as Potential 18F-Labeled Positron Emission Tomography (PET) G-Protein Coupled Receptor 44 (GPR44) Tracers. Pharmaceuticals. 2023; 16(9):1203. https://doi.org/10.3390/ph16091203
Chicago/Turabian StyleYin, Runkai, Kelly X. Huang, Lina A. Huang, Melinda Ji, Hanyi Zhao, Kathy Li, Anna Gao, Jiaqi Chen, Zhixuan Li, Tianxiong Liu, and et al. 2023. "Indole-Based and Cyclopentenylindole-Based Analogues Containing Fluorine Group as Potential 18F-Labeled Positron Emission Tomography (PET) G-Protein Coupled Receptor 44 (GPR44) Tracers" Pharmaceuticals 16, no. 9: 1203. https://doi.org/10.3390/ph16091203
APA StyleYin, R., Huang, K. X., Huang, L. A., Ji, M., Zhao, H., Li, K., Gao, A., Chen, J., Li, Z., Liu, T., Shively, J. E., Kandeel, F., & Li, J. (2023). Indole-Based and Cyclopentenylindole-Based Analogues Containing Fluorine Group as Potential 18F-Labeled Positron Emission Tomography (PET) G-Protein Coupled Receptor 44 (GPR44) Tracers. Pharmaceuticals, 16(9), 1203. https://doi.org/10.3390/ph16091203








