ED Formula, a Complex of Ecklonia cava and Chrysanthemum indicum, Ameliorates Airway Inflammation in Lipopolysaccharide-Stimulated RAW Macrophages and Ovalbumin-Induced Asthma Mouse Model
Abstract
:1. Introduction
2. Results
2.1. Dieckol and Luteolin Content in ED
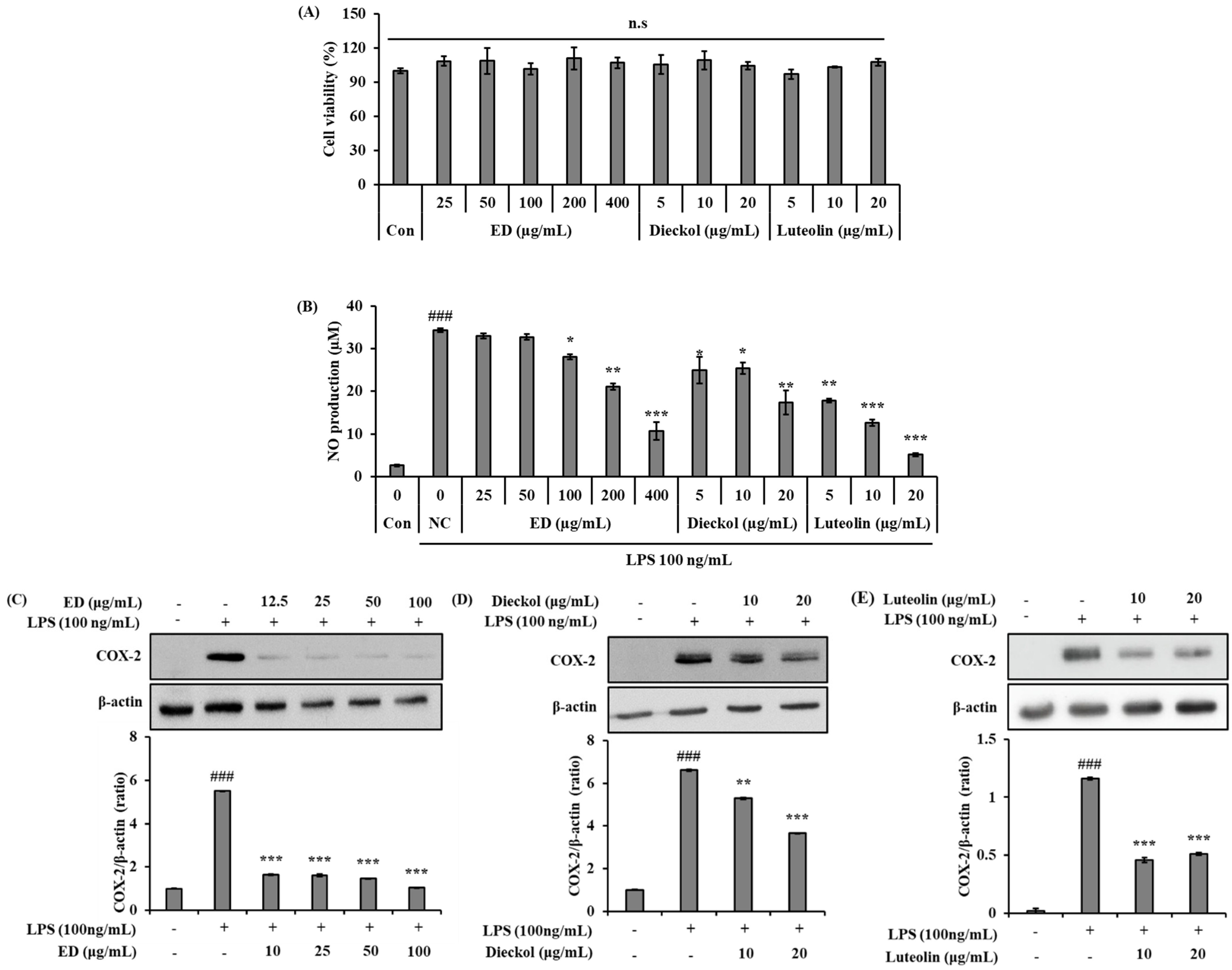
2.2. ED and Its Active Compounds Suppress LPS-Induced Inflammation in RAW 264.7 Cells
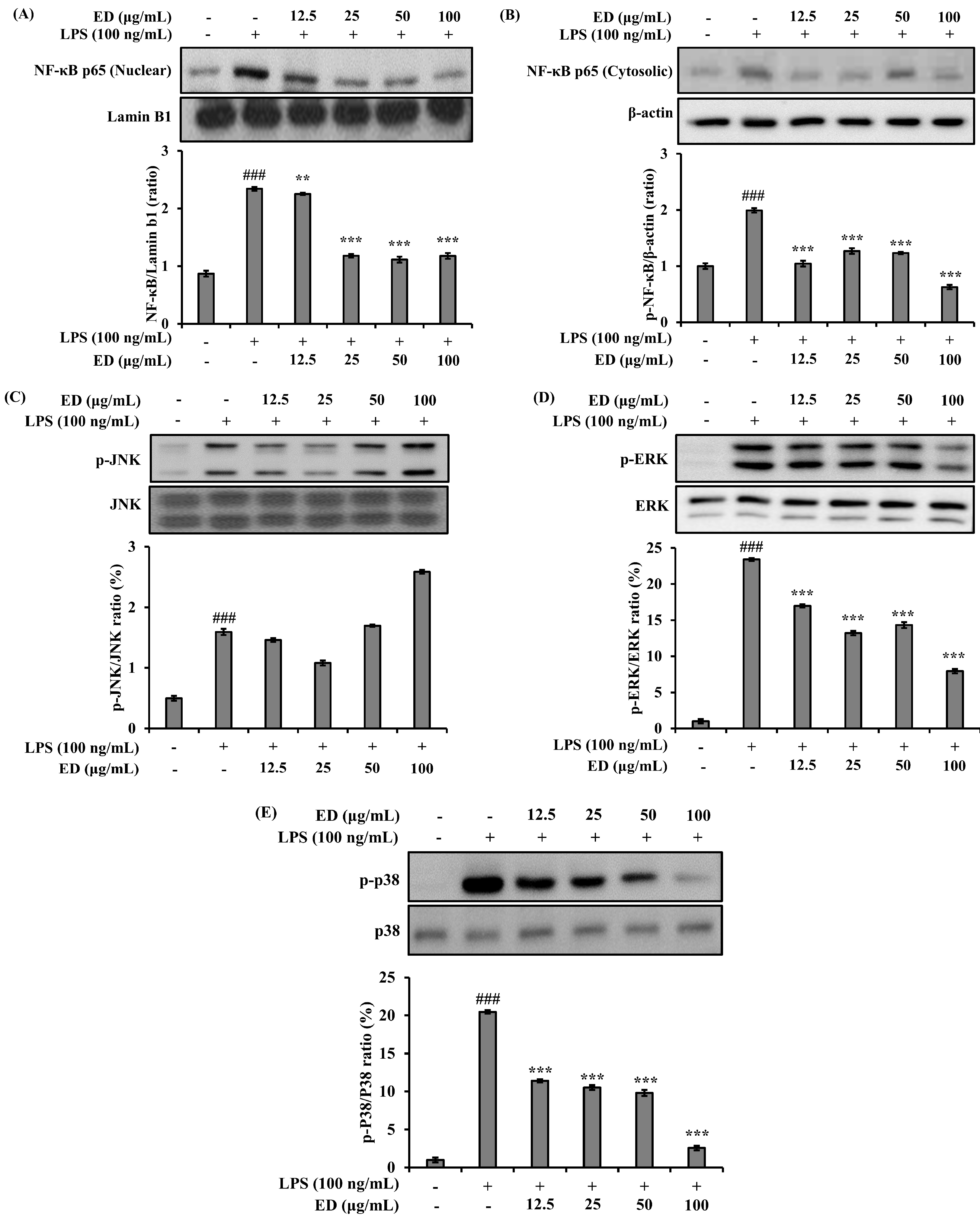
2.3. ED Inhibits LPS-Induced Nuclear Factor-kappa B (NF-κB) and Mitogen-Activated Protein Kinase (MAPK) Signaling Activation
2.4. ED Ameliorates Airway Inflammation in OVA-Induced Asthmatic Mice
2.4.1. Treatment with ED Reduced the Number of Total Cells in BALF
2.4.2. Effects of ED on Lung Histological Changes
2.4.3. Effects of ED on Inflammatory Cytokine Interleukin (IL)-6 in Serum
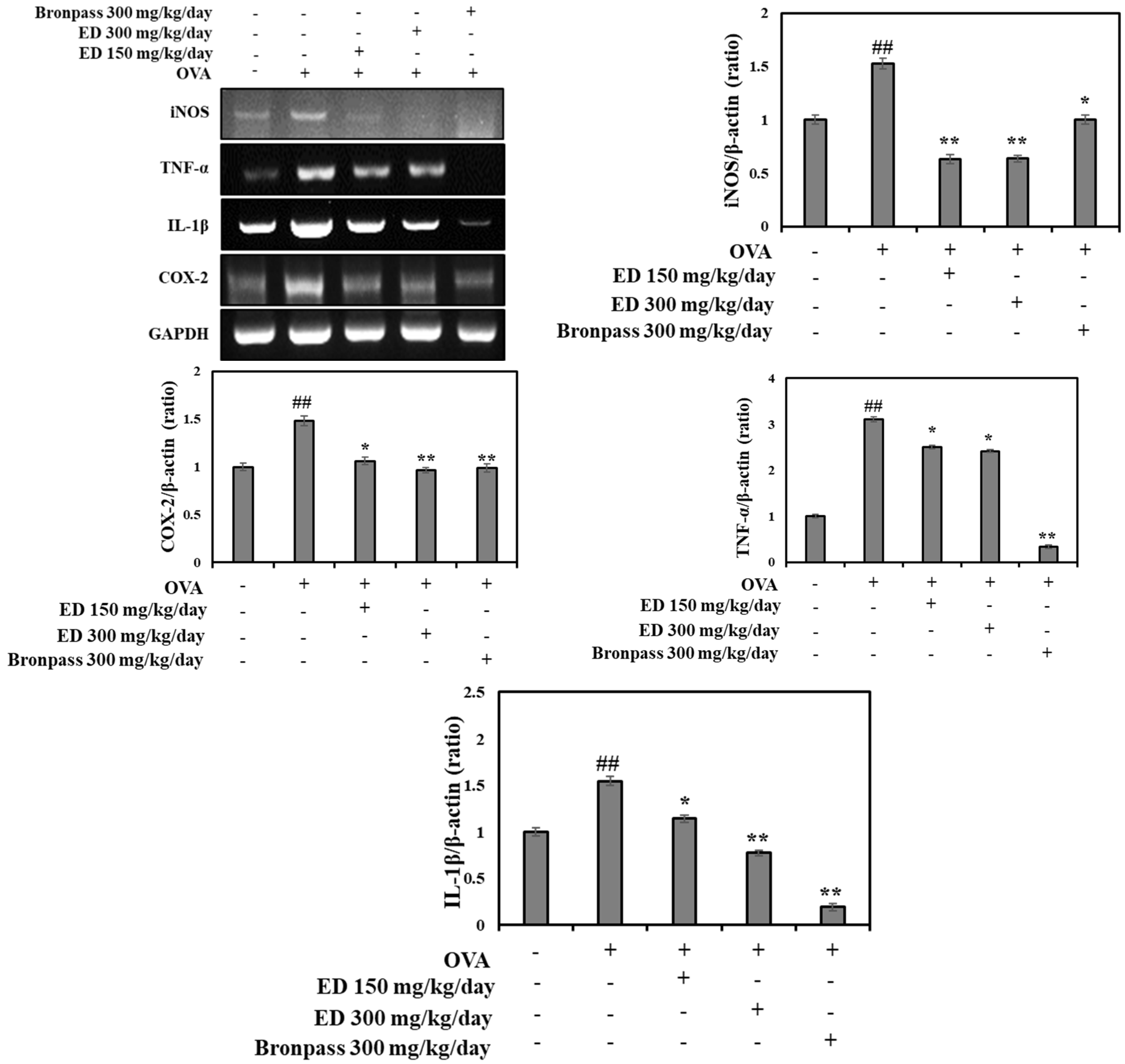
2.4.4. Effects of ED on the mRNA Expression Levels of Inflammatory Mediators in the Lung Tissue
3. Discussion
4. Materials and Methods
4.1. Preparation of ED
4.2. Quantification of Active Compounds of ED
4.2.1. Standards, Reagents, and Materials
4.2.2. HPLC Analysis
4.3. In Vitro Anti-Inflammatory Activity of ED in LPS-Induced RAW 264.7 Macrophages
4.3.1. Cell Culture
4.3.2. Cell Viability Assay
4.3.3. Measurement of NO Production
4.3.4. Western Blot Analysis
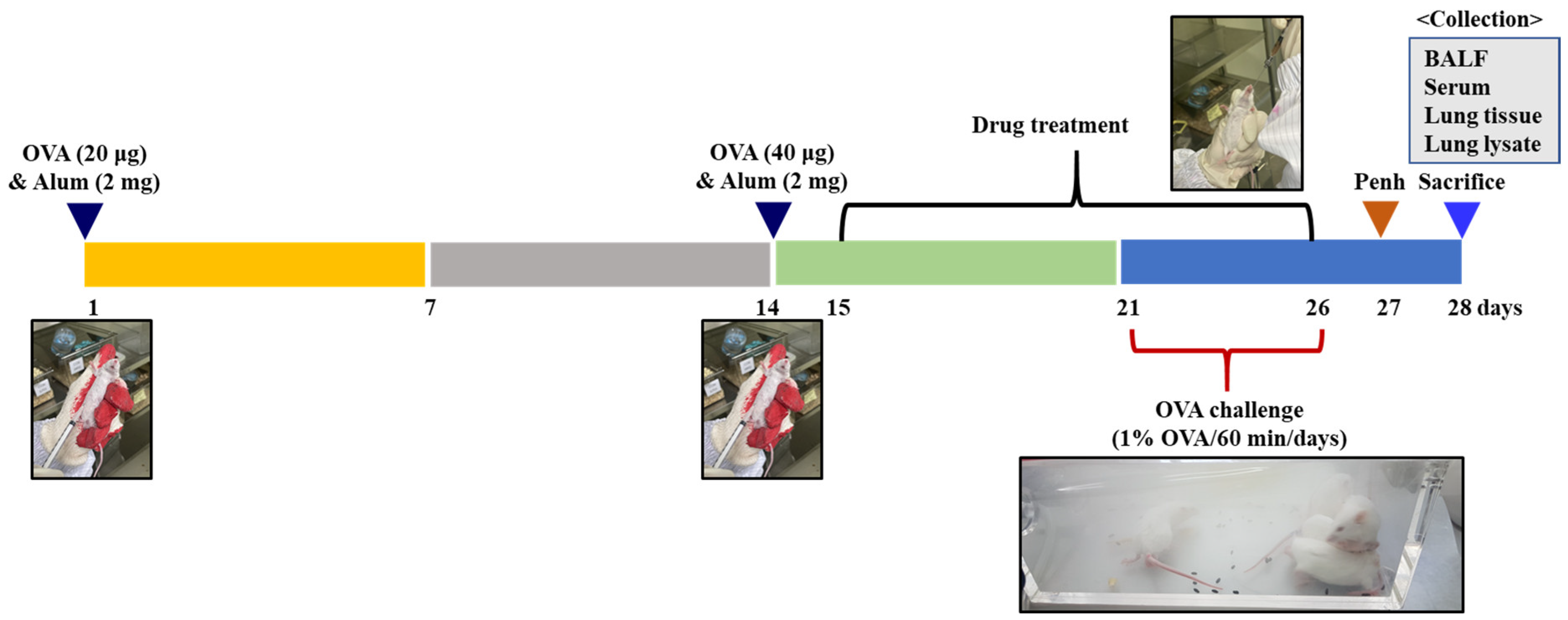
4.4. Evaluation of Anti-Airway Inflammation Activity of ED in OVA-induced Asthma Mouse Model
4.4.1. Experimental Animals
4.4.2. Animal Sensitization and Treatment
4.4.3. Measurement of Inflammatory Cell Counts in BALF
4.4.4. Determination of IL-6 in Serum
4.4.5. Histopathological Evaluation of Lungs
4.4.6. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Asthma Facts—CDC’s National Asthma Control Program Grantees; Public Presentation; Department of Health and Human Services: Atlanta, GA, USA, 2013. [Google Scholar]
- Baïz, N.; Annesi-Maesano, I. Is the asthma epidemic still ascending? Clin. Chest Med. 2012, 33, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, K.; Doyle-Waters, M.M.; Marra, C.; Lynd, L.; Alasaly, K.; Swiston, J.; FitzGerald, J.M. Economic burden of asthma: A systematic review. BMC Pulm. Med. 2009, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, Z.; Feng, J.; Tang, Y.; Qin, L.; Hu, X.; Zhang, Y.; He, R. Glucocorticoids modulate Th1 and Th2 responses in asthmatic mouse models by inhibition of Notch1 signaling. Int. Arch. Allergy Immunol. 2018, 175, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, T.; Obase, Y.; Kishikawa, R.; Mukae, H.; Iwanaga, T. Assessment of anti-inflammatory effect from addition of a long-acting beta-2 agonist to inhaled corticosteroid. Allergy Asthma Proc. 2016, 37, 293–387. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Kim, J.H.; Choi, J.H.; Lim, D.H. Preventive and therapeutic effects of vitamin D in a mouse model of allergic asthma. Asian Pac. J. Allergy Immunol. 2019, 37, 130–137. [Google Scholar] [PubMed]
- Kandhare, A.D.; Liu, Z.; Mukherjee, A.A.; Bodhankar, S.L. Therapeutic potential of morin in ovalbumin-induced allergic asthma via modulation of SUMF2/IL-13 and BLT2/NF-κB signaling pathway. Curr. Mol. Pharmacol. 2019, 12, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Patel, V. Inhibitory effects of catechin isolated from Acacia catechu on ovalbumin induced allergic asthma model. Nutr. Food Sci. 2019, 49, 18–31. [Google Scholar] [CrossRef]
- Chowdhury, M.T.H.; Bangoura, I.; Kang, J.Y.; Park, N.G.; Ahn, D.H.; Hong, Y.K. Distribution of phlorotannins in the brown alga Ecklonia cava and comparison of pretreatments for extraction. Fish. Aquat. Sci. 2011, 14, 198–204. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Z.J.; Ryu, B.; Lee, S.H.; Kim, M.M.; Kim, S.K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.K.; Jung, W.K.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. Vitr. 2009, 23, 1123–1130. [Google Scholar] [CrossRef]
- Yoon, O.H.; Cho, J.S. Optimization of extraction conditions for hot water extracts from Chrysanthemum indicum L. by response surface methodology. Korean J. Food Cook. Sci. 2007, 23, 1–8. [Google Scholar]
- Ryu, S.Y.; Choi, S.U.; Lee, C.O.; Lee, S.H.; Ahn, J.W.; Zee, O.P. Antitumor activity of some phenolic components in plants. Arch. Pharm. Res. 1994, 17, 42–44. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Müller-Newen, G.; Schaper, F.; Graeve, L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 1994, 334, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Cheon, M.S.; Yoon, T.S.; Lee, D.Y.; Choi, G.Y.; Moon, B.C.; Lee, A.Y.; Choo, B.K.; Kim, H.K. Chrysanthemum indicum Linné extract inhibits the inflammatory response by suppressing NF-kappaB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophages. J. Ethnopharmacol. 2009, 122, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Park, C.H.; Kwon, S.O.; Lee, S.G. Antioxidant and anti-inflammatory activities of Chrysanthemum indicum Linne extracts at different ethanol ratios. Korean J. Food Sci. Technol. 2021, 53, 416–422. [Google Scholar]
- Lee, S.G.; Kwon, S.O. Effects of Inhibiting Glycoprotein MUC5AC by Seaweed Ecklonia cava Extract in human Airway Epithelial Cells. Biomed. Sci. Lett. 2021, 27, 334–339. [Google Scholar] [CrossRef]
- Lee, S.G.; Kwon, S.O. Ecklonia cava-Hizikia fusiformis complex extract alleviates inflammation in human lung epithelia. J. Plant Biotechnol. 2022, 49, 90–98. [Google Scholar] [CrossRef]
- Endo, Y.; Hirahara, K.; Yagi, R.; Tumes, D.J.; Nakayama, T. Pathogenic memory type Th2 cells in allergic inflammation. Trends Immunol. 2014, 35, 69–78. [Google Scholar] [CrossRef]
- Wise, J. Corticosteroids for asthma may suppress growth in children in first year of treatment, researchers say. BMJ 2014, 349, g4623. [Google Scholar] [CrossRef]
- Ciriaco, M.; Ventrice, P.; Russo, G.; Scicchitano, M.; Mazzitello, G.; Scicchitano, F.; Russo, E. Corticosteroid-related central nervous system side effects. J. Pharmacol. Pharmacother. 2013, 4, 94–98. [Google Scholar] [CrossRef]
- Shin, H.C.; Hwang, H.J.; Kang, K.J.; Lee, B.H. An antioxidative and antiinflammatory agent for potential treatment of osteoarthritis from Ecklonia cava. Arch. Pharm. Res. 2006, 29, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.G.; Kang, O.H.; Brice, O.O.; Lee, Y.S.; Chae, H.S.; Oh, Y.C.; Sohn, D.H.; Park, H.; Choi, H.G.; Kim, S.G.; et al. Antibacterial activity of Ecklonia cava against methicillin-resistant Staphylococcus aureus and Salmonella spp. Foodborne Pathog. Dis. 2010, 7, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Seok, J.K.; Boo, Y.C. Ecklonia cava extract and dieckol attenuate cellular lipid peroxidation in keratinocytes exposed to PM10. Evid. Based Complement. Altern. Med. 2018, 2018, 8248323. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Li, J.; You, T.; Hu, C. Anti-inflammatory and immunomodulatory activities of the extracts from the inflorescence of Chrysanthemum indicum Linné. J. Ethnopharmacol. 2005, 101, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Choi, G.Y.; Yoon, T.S.; Cheon, M.S.; Choo, B.K.; Kim, H.K. Anti-inflammatory activity of Chrysanthemum indicum extract in acute and chronic cutaneous inflammation. J. Ethnopharmacol. 2009, 123, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Morikawa, T.; Murakami, T.; Toguchida, I.; Harima, S.; Matsuda, H. Medicinal flowers. I. Aldose reductase inhibitors and three new eudesmane-type sesquiterpenes, kikkanols A, B, and C, from the flowers of Chrysanthemum indicum L. Chem. Pharm. Bull. 1999, 47, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, L.; Jin, Y. The anti-inflammatory effects of total flavonoids of Chrysanthemum indicum and its partial mechanism. Anhui Yike Daxue Xuebao 2005, 40, 405–408. [Google Scholar]
- Wynn, T.A.; Barron, L. Macrophages: Master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef]
- Blatteis, C.M.; Li, S.; Li, Z.; Feleder, C.; Perlik, V. Cytokines, PGE2 and endotoxic fever: Are-assessment. Prostaglandins Other Lipid Mediat. 2005, 76, 1–18. [Google Scholar] [CrossRef]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Kumagai, Y.; Sobajima, J.; Higashi, M.; Ishiguro, T.; Fukuchi, M.; Ishibashi, K.; Mochiki, E.; Yakabi, K.; Kawano, T.; Tamaru, J.; et al. Coexpression of COX-2 and iNOS in angiogenesis of superficial esophageal squamous cell carcinoma. Int. Surg. 2015, 100, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Fu, J.; Zhao, Z.; Kong, X.; Huang, H.; Luo, L.; Yin, Z. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264. 7 cells through suppressing NF-κB and JNK/AP-1 activation. Int. Immunopharmacol. 2009, 9, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.M.; Zhang, W.Y.; Li, R.J.; Guo, C.; Wei, S.S.; Tian, X.M.; Luo, J.; Kong, L.Y. Anti-inflammatory activity of Khayandirobilide A from Khaya senegalensis via NF-kappaB, AP-1 and p38 MAPK/Nrf2/HO-1 signaling pathways in lipopolysaccharide-stimulated RAW 264.7 and BV-2 cells. Phytomedicine 2018, 42, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Enke, C.G.; Nagels, L.J. Undetected components in natural mixtures: How many? What concentrations? Do they account for chemical noise? What is needed to detect them. Anal. Chem. 2011, 83, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Merzenich, G.; Panek, D.; Zeitler, H.; Vetter, H.; Wagner, H. Drug development from natural products: Exploiting synergistic effects. Indian J. Exp. Biol. 2010, 48, 208–219. [Google Scholar] [PubMed]
- Han, J.M.; Jin, Y.Y.; Kim, H.Y.; Park, K.H.; Lee, W.S.; Jeong, T.S. Lavandulyl flavonoids from Sophora flavescens suppress lipopolysaccharide-induced activation of nuclear factor-κB and mitogen-activated protein kinases in RAW264.7 cells. Biol. Pharm. Bull. 2010, 33, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.S.; Shin, P.G.; Lee, J.H.; Kim, G.D. The regulatory effect of veratric acid on NO production in LPS-stimulated RAW264. 7 macrophage cells. Cell Immunol. 2012, 280, 164–170. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, W.; Zhang, Z.; Qian, M.; Du, B. Nitidine chloride inhibits LPS-induced inflammatory cytokines production via MAPK and NF-kappaB pathway in RAW 264.7 cells. J. Ethnopharmacol. 2012, 144, 145–150. [Google Scholar] [CrossRef]
- Lee, J.; Tae, N.; Lee, J.J.; Kim, T.; Lee, J.H. Eupatolide inhibits lipopolysaccharide-induced COX-2 and iNOS expression in RAW264.7 cells by inducing proteasomal degradation of TRAF6. Eur. J. Pharmacol. 2010, 636, 173–180. [Google Scholar] [CrossRef]
- Padrid, P.; Snook, S.; Finucane, T.; Shiue, P.; Cozzi, P.; Solway, J.; Leff, A.R. Persistent airway hyperresponsiveness and histologic alterations after chronic antigen challenge in cats. Am. J. Respir. Crit. Care Med. 1995, 151, 184–193. [Google Scholar] [CrossRef]
- Tiwari, M.; Dwivedi, U.N.; Kakkar, P. Tinospora cordifolia extract modulates COX-2 iNOS, ICAM-1, porinflammatory cytokines and redox status in murine model of asthma. J. Ethnopharmacol. 2014, 153, 326–337. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, D.Y.; Jung, W.K.; Kim, J.H.; Choi, I.; Park, S.G.; Seo, S.K.; Lee, S.W.; Lee, C.M.; Yea, S.S. Effects of Ecklonia cava ethanolic extracts on airway hyperresponsiveness and inflammation in a murine asthma model: Role of suppressor of cytokine signaling. Biomed. Pharmacother. 2008, 62, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Yang, E.J.; Yun, C.Y.; Kim, D.H.; Kim, I.S. Suppressive effect of Petasites japanicus extract on ovalbumin—Induced airway inflammation in an asthmatic mouse model. J. Ethnopharmacol. 2011, 133, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Kohno, N.; Fujino, S.; Hamada, H.; Inoue, Y.; Fujioka, S.; Ishida, S.; Hiwada, K. Circulating interleukin-6 levels in patients with bronchial asthma. Am. J. Respir. Crit. Care Med. 1995, 151, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Neveu, W.A.; Allard, J.L.; Raymond, D.M.; Bourassa, L.M.; Burns, S.M.; Bunn, J.Y.; Irvin, C.G.; Kaminsky, D.A.; Rincon, M. Elevation of IL-6 in the allergic asthmatic airway is independent of inflammation but associates with loss of central airway function. Respir. Res. 2010, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Virchow, J.C.J.; Kroegel, C.; Walker, C.; Matthys, H. Inflammatory determinants of asthma severity: Mediator and cellular changes in bronchoalveolar lavage fluid of patients with severe asthma. J. Allergy Clin. Immunol. 1996, 96, 33–40. [Google Scholar] [CrossRef]
- Peters, M.C.; McGrath, K.W.; Hawkins, G.A.; Hastie, A.T.; Levy, B.D.; Israel, E.; Phillips, B.R.; Mauger, D.T.; Comhair, S.A.; Erzurum, S.C.; et al. National Heart, Lung, and Blood Institute Severe Asthma Research Program. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: A cross-sectional analysis of two cohorts. Lancet Respir. Med. 2016, 4, 574–584. [Google Scholar] [CrossRef] [PubMed]
- McGregor, M.C.; Krings, J.G.; Nair, P.; Castro, M. Castro. Role of biologics in asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 433–445. [Google Scholar] [CrossRef]
- Myou, S.; Leff, A.R.; Myo, S.; Boetticher, E.; Tong, J.; Meliton, A.Y.; Liu, J.; Munoz, N.M.; Zhu, X. Blockade of inflammation and airway hyperresponsiveness in immunesensitized mice by dominant-negative phosphoinositide-3-kinase-TAT. J. Exp. Med. 2003, 198, 1573–1582. [Google Scholar] [CrossRef]
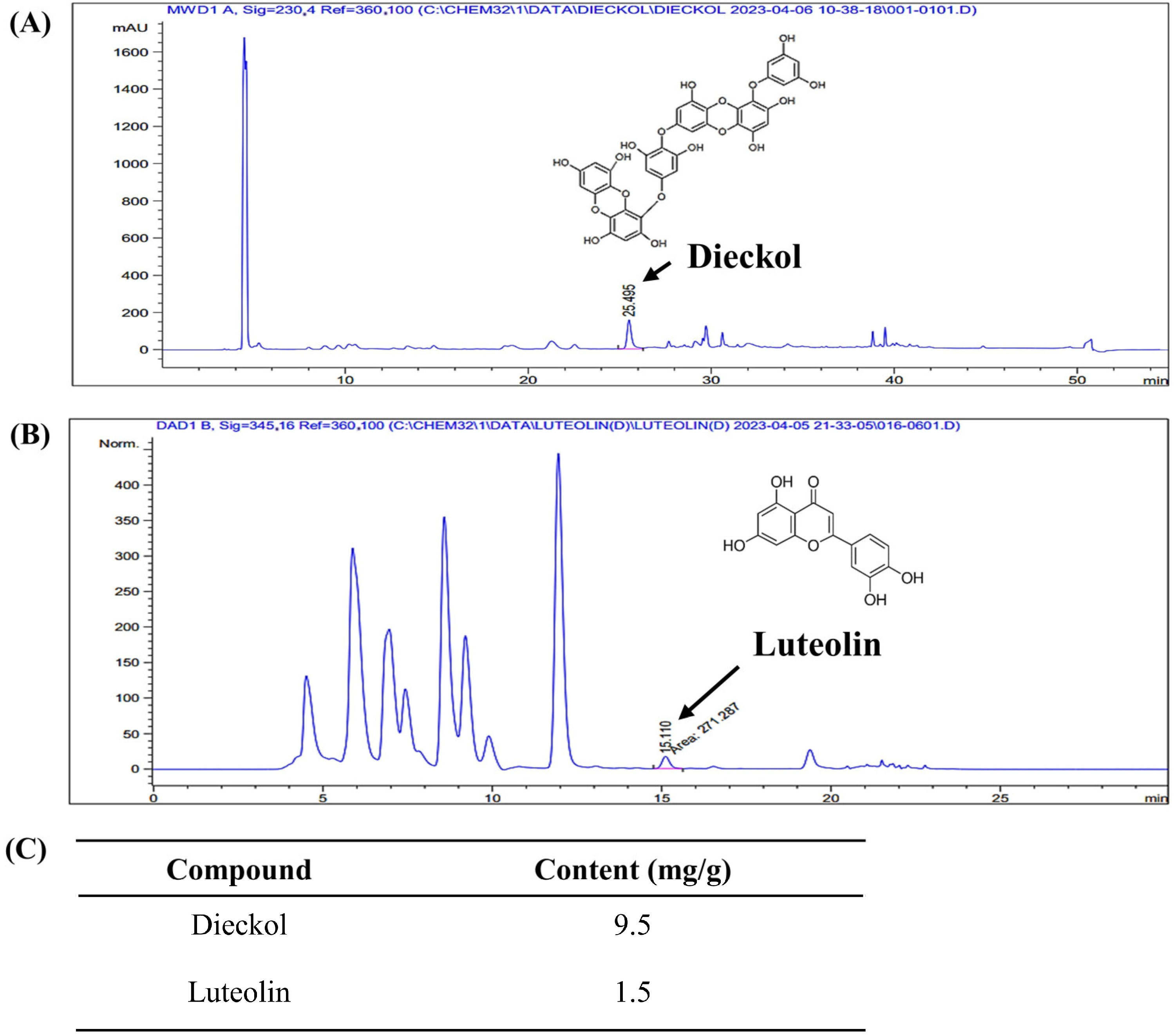



| Content | Dieckol | Luteolin |
|---|---|---|
| Injection volume | 5 μL | 10 μL |
| Column temperature | 25 °C | 30 °C |
| Mobile phase | A: 0.01% acetic acid in DW (90%) + MeOH (10%) | A: 0.1% trifluoroacetic acid in DW |
| B: Methanol | B: Methanol | |
| Flow rate | 0.7 mL/min | 0.8 mL/min |
| Detection wavelength | 230 nm | 345 nm |
| Purity of the standards | 95.6% | 99% |
| Gene | Size (bp) | Sequence |
|---|---|---|
| iNOS | 369 | Sense 5′-CTTGCAAGTCCAAGTCTTGC-3′ Antisense 5′-GTATGTGTCTGCAGATGTGCTG-3′ |
| TNF-α | 390 | Sense 5’-TTCGAGTGACAAGCCTGTAGC-3′ Antisense 5’-AGATTGACCTCAGCGCTGAGT-3′ |
| IL-1β | 385 | Sense 5’-CATATGAGCTGAAAGCTCTCCA-3′ Antisense 5′-GACACAGATTCCATGGTGAAGTC-3′ |
| COX-2 | 414 | Sense 5′-ACATCCCTGAGAACCTGCAGT-3′ Antisense 5′-CCAGGAGGATGGAGTTGTTGT-3′ |
| GAPDH | 378 | Sense 5’-CCAGTATGACTCCACTCACG-3′ Antisense 5’-CCTTCCACAATGCCAAGTT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.; Park, C.-H.; Kwon, S.-O.; Lee, S.-G. ED Formula, a Complex of Ecklonia cava and Chrysanthemum indicum, Ameliorates Airway Inflammation in Lipopolysaccharide-Stimulated RAW Macrophages and Ovalbumin-Induced Asthma Mouse Model. Pharmaceuticals 2023, 16, 1185. https://doi.org/10.3390/ph16081185
Kang H, Park C-H, Kwon S-O, Lee S-G. ED Formula, a Complex of Ecklonia cava and Chrysanthemum indicum, Ameliorates Airway Inflammation in Lipopolysaccharide-Stimulated RAW Macrophages and Ovalbumin-Induced Asthma Mouse Model. Pharmaceuticals. 2023; 16(8):1185. https://doi.org/10.3390/ph16081185
Chicago/Turabian StyleKang, Hyun, Chan-Hwi Park, Sang-Oh Kwon, and Sung-Gyu Lee. 2023. "ED Formula, a Complex of Ecklonia cava and Chrysanthemum indicum, Ameliorates Airway Inflammation in Lipopolysaccharide-Stimulated RAW Macrophages and Ovalbumin-Induced Asthma Mouse Model" Pharmaceuticals 16, no. 8: 1185. https://doi.org/10.3390/ph16081185
APA StyleKang, H., Park, C.-H., Kwon, S.-O., & Lee, S.-G. (2023). ED Formula, a Complex of Ecklonia cava and Chrysanthemum indicum, Ameliorates Airway Inflammation in Lipopolysaccharide-Stimulated RAW Macrophages and Ovalbumin-Induced Asthma Mouse Model. Pharmaceuticals, 16(8), 1185. https://doi.org/10.3390/ph16081185






