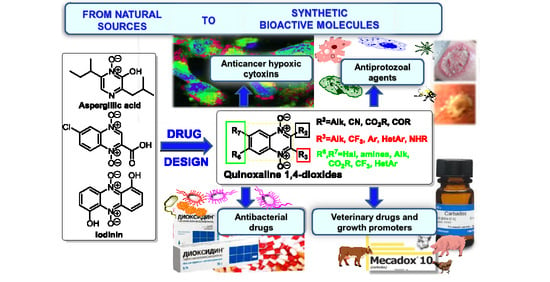
Quinoxaline 1,4-Dioxides: Advances in Chemistry and Chemotherapeutic Drug Development
Abstract
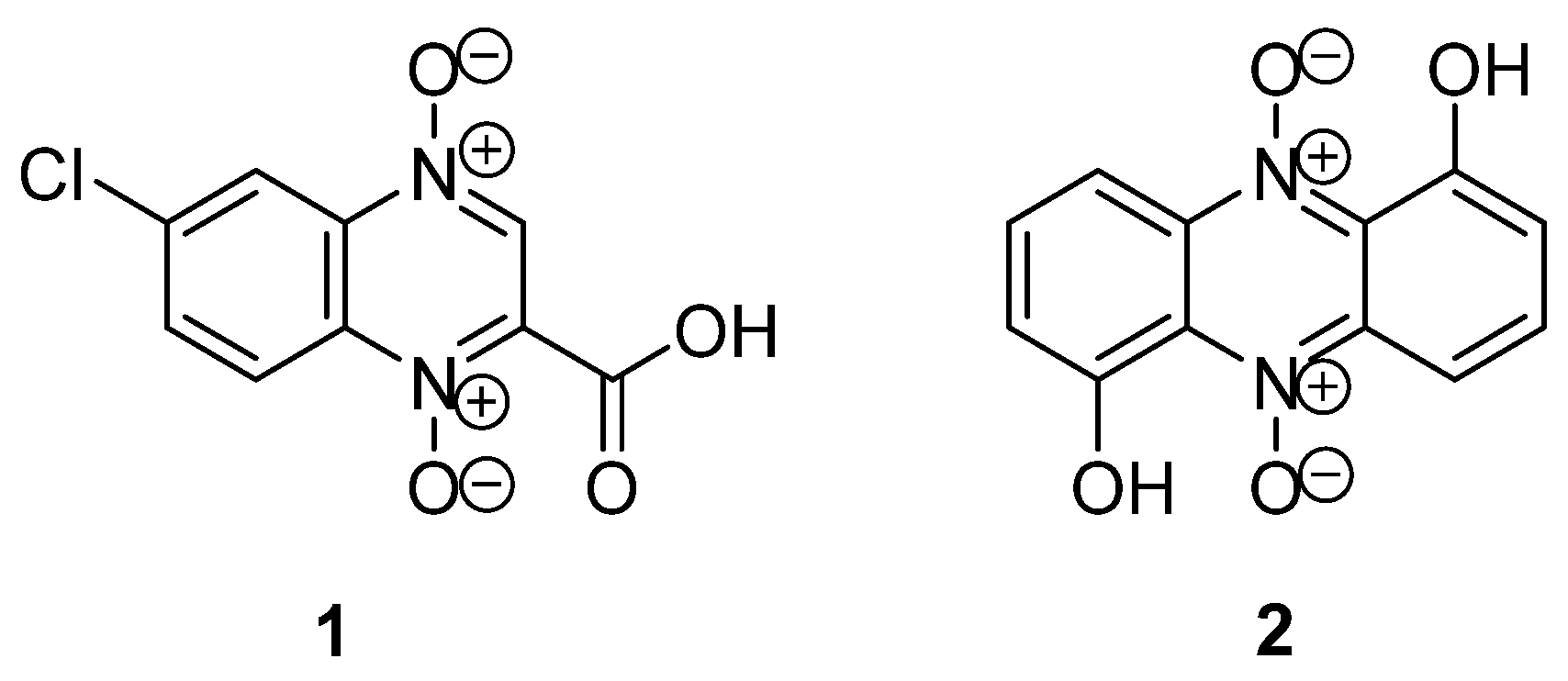
1. Introduction
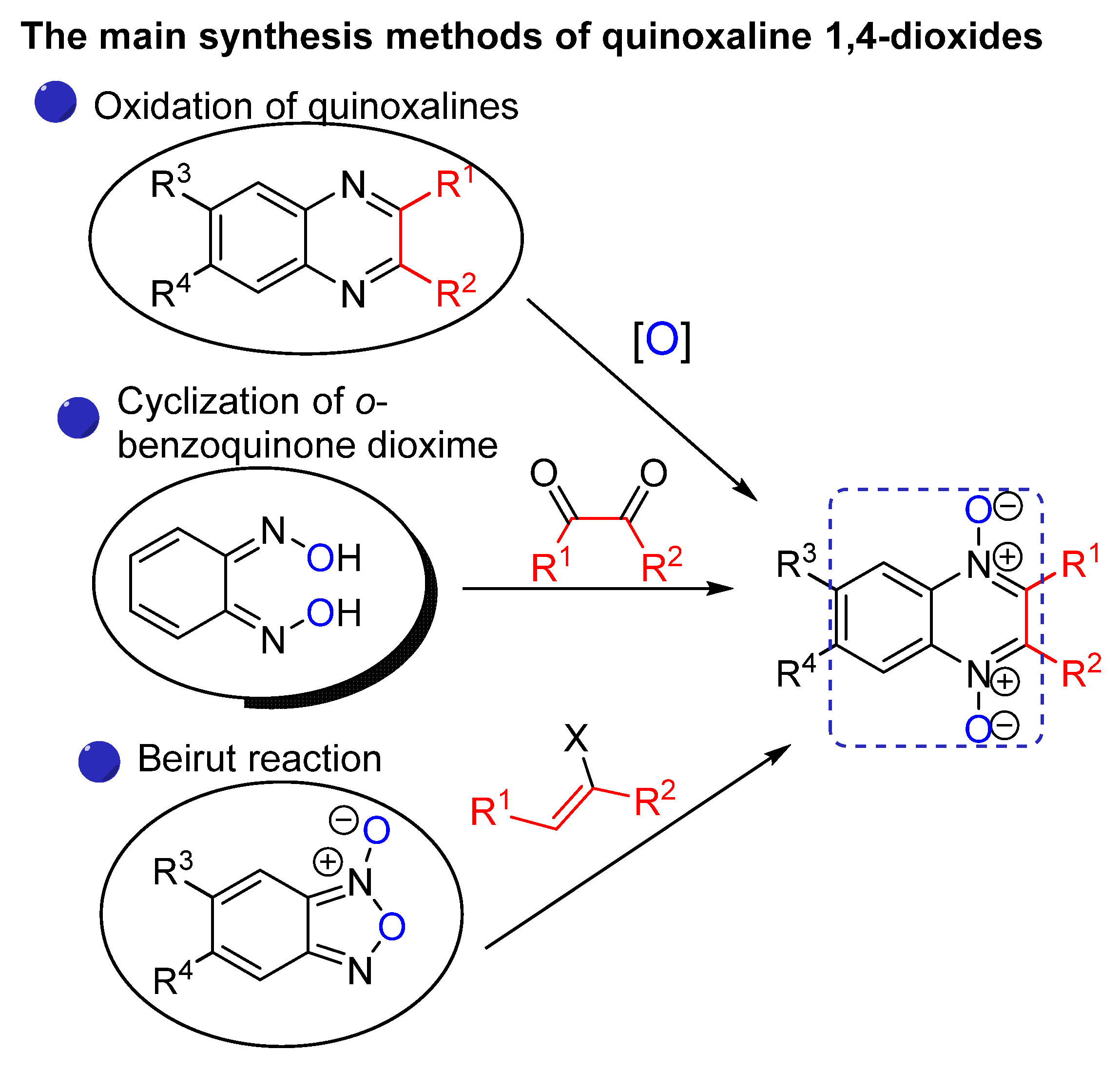
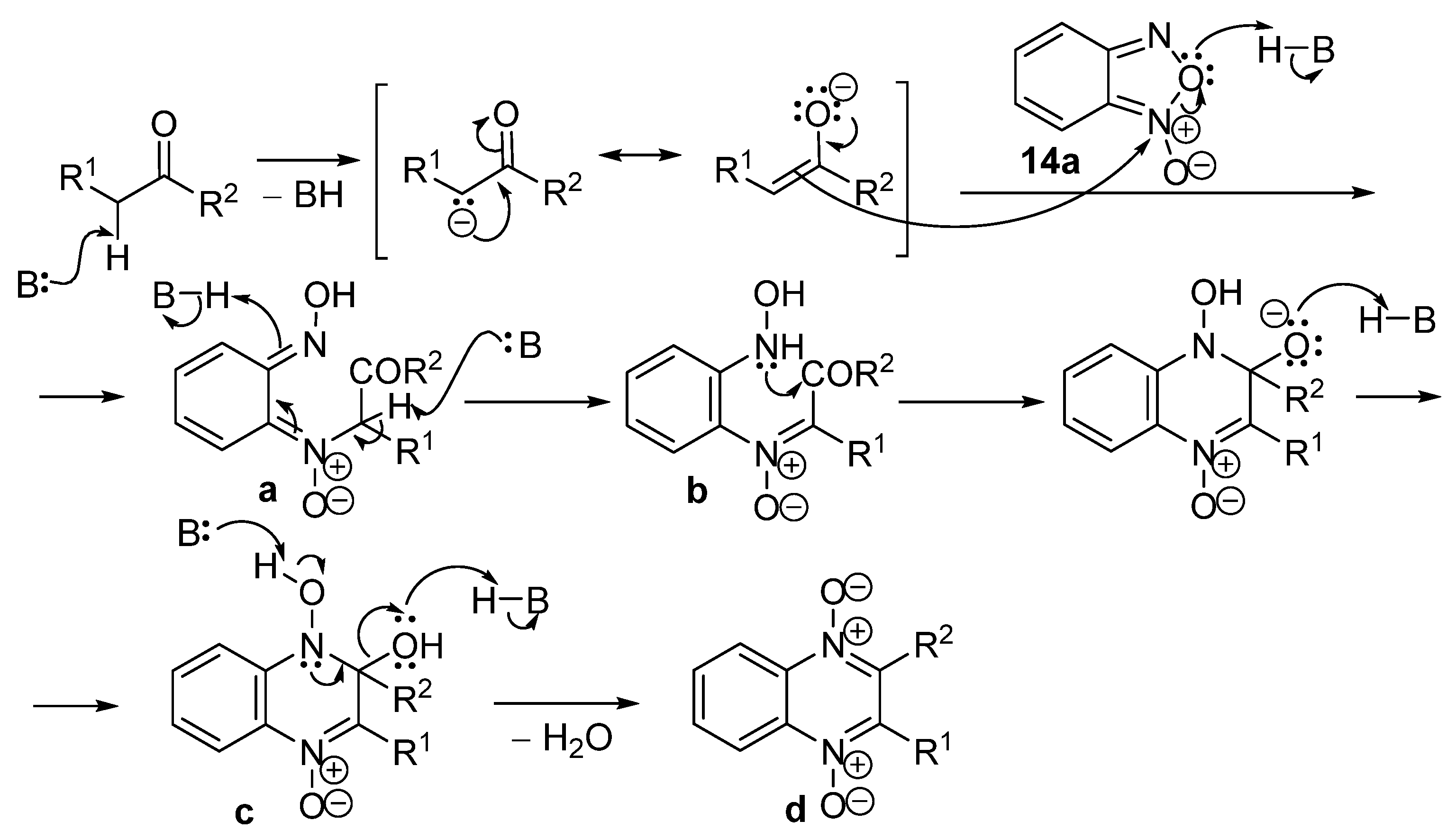
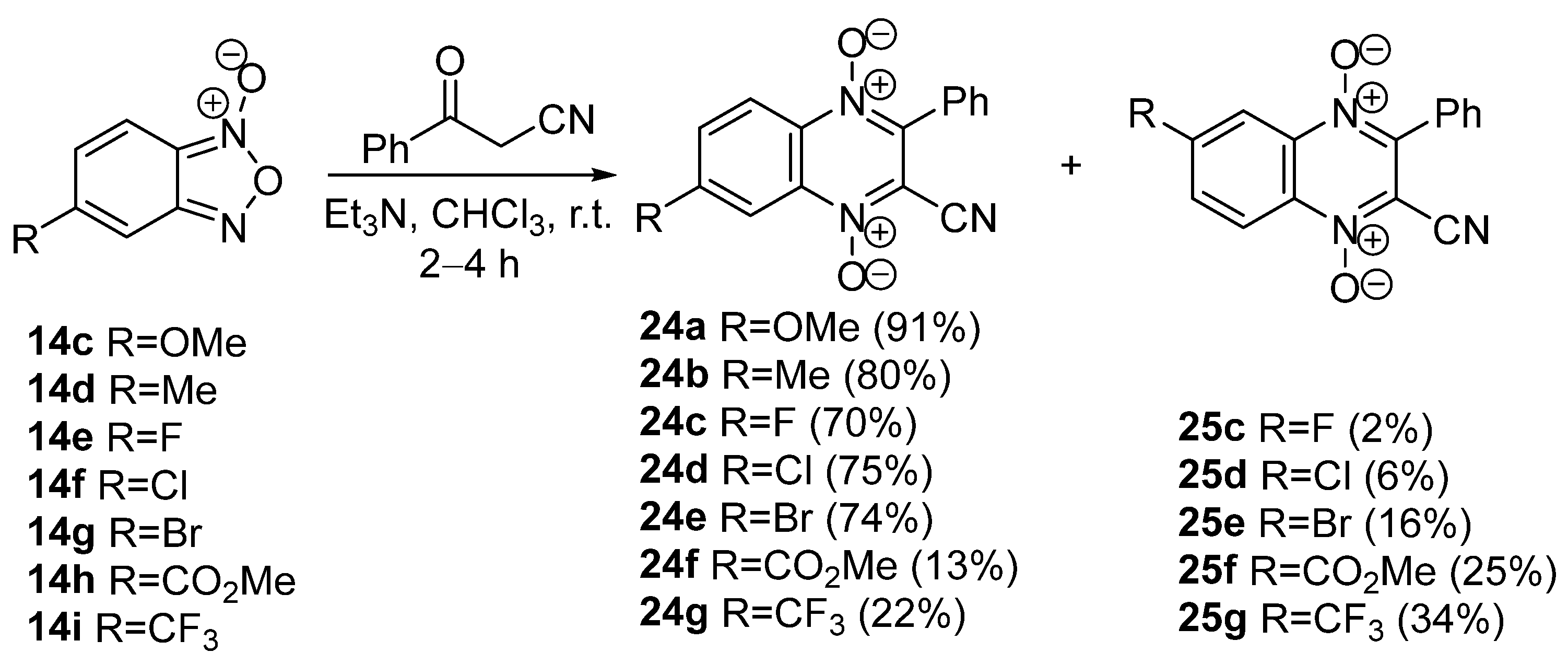
2. Methods of the Synthesis of Quinoxaline 1,4-Dioxides
3. Chemical Properties of Quinoxaline 1,4-Dioxides
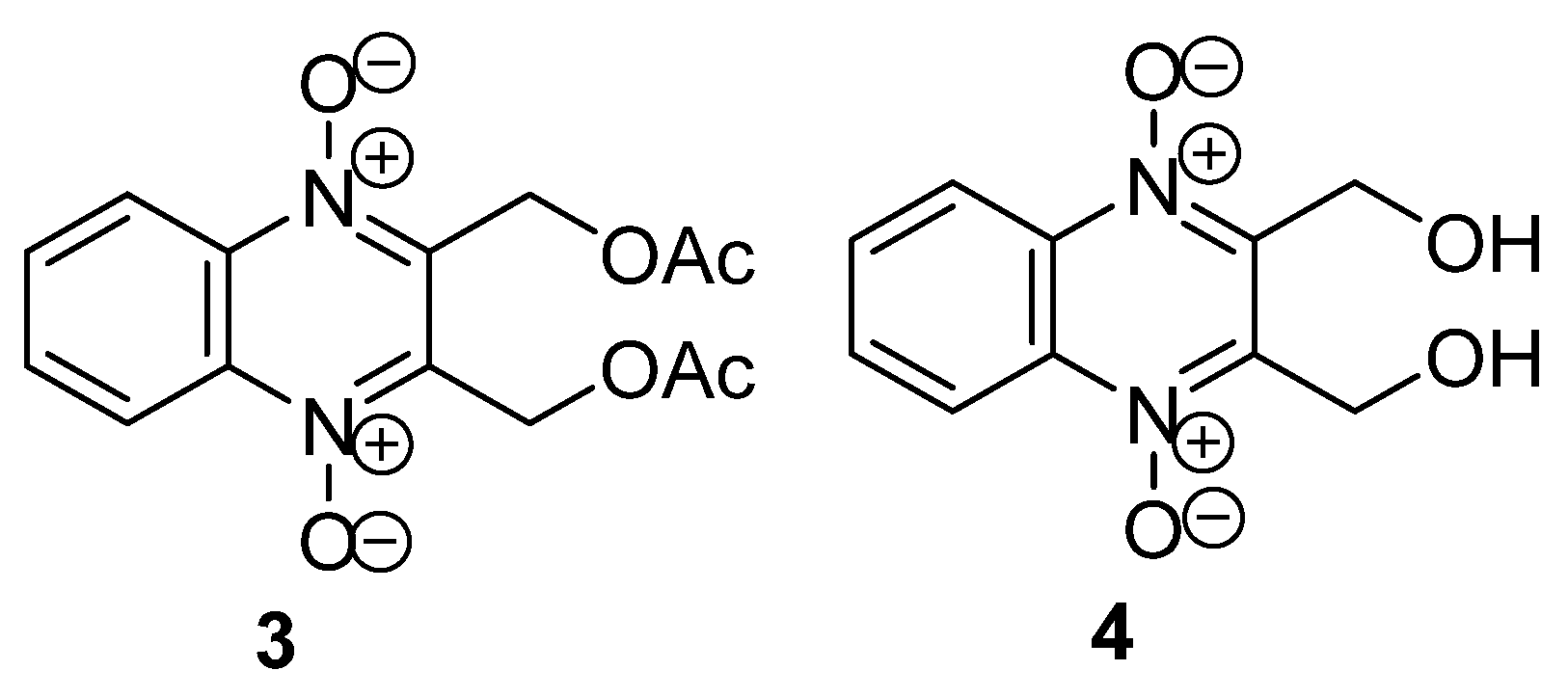
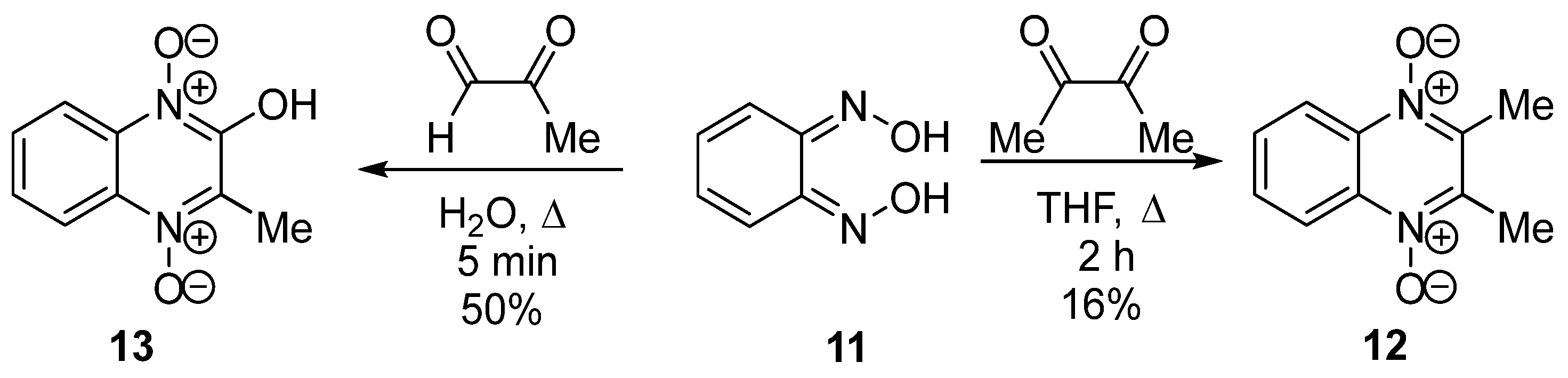
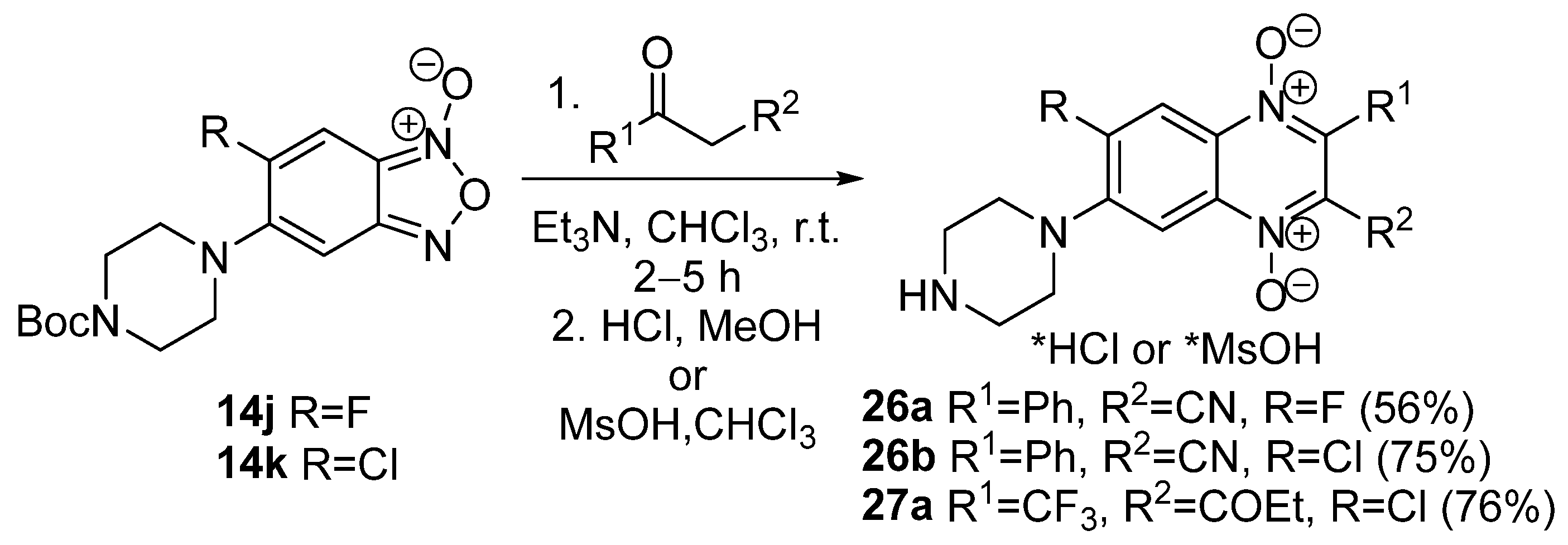
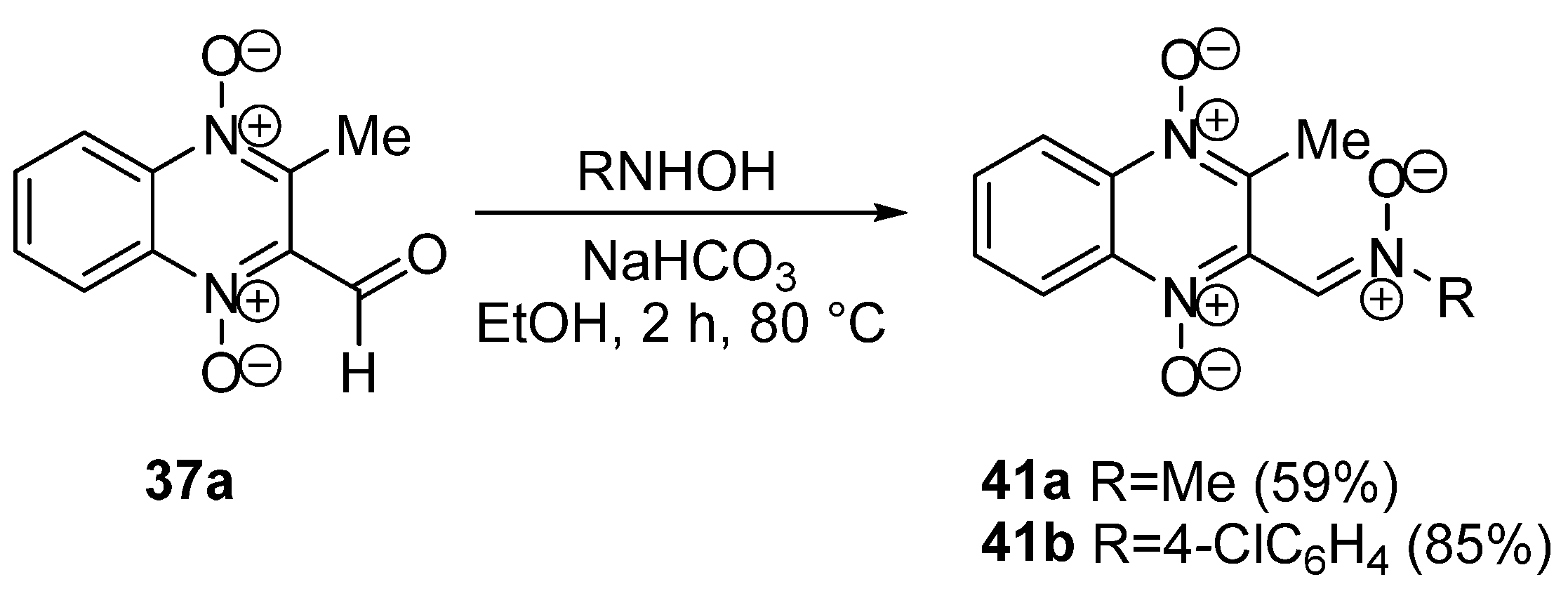
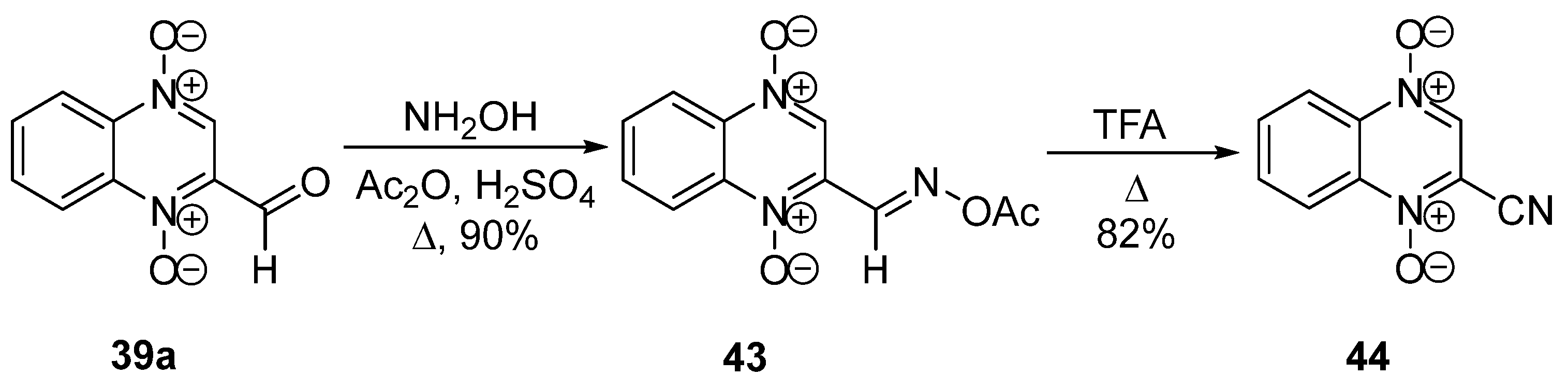
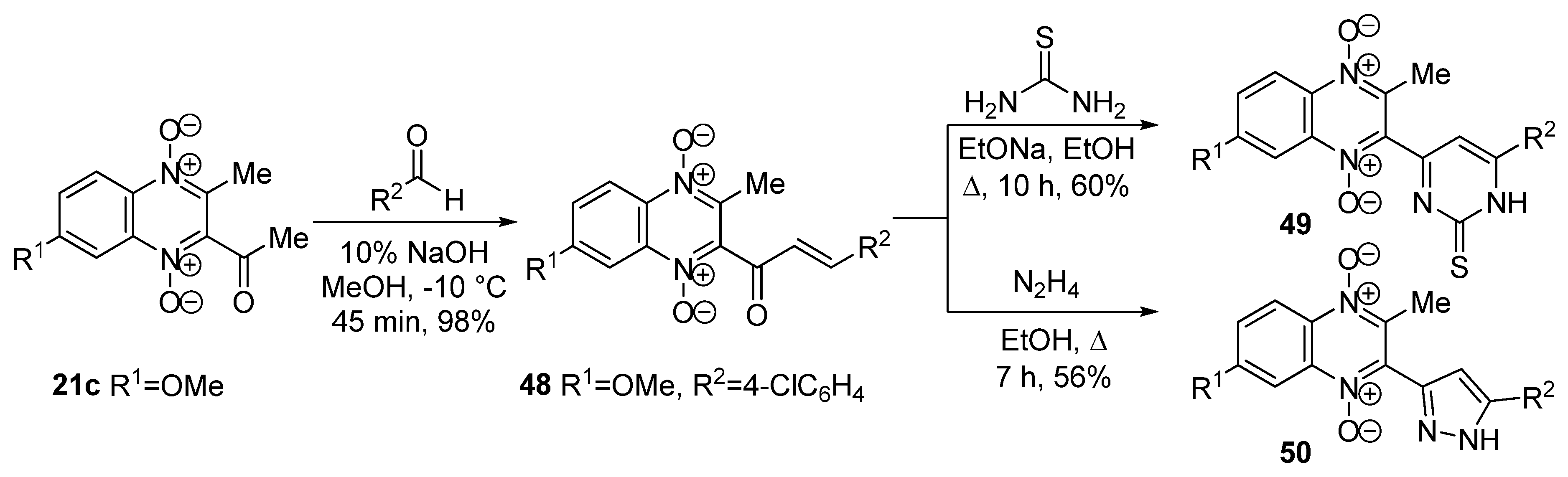
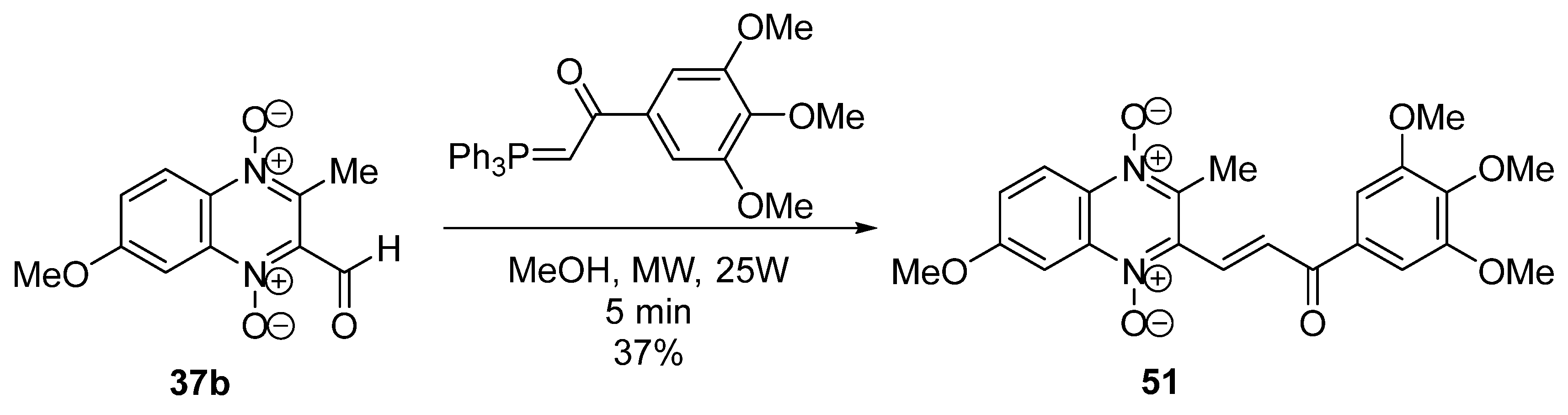
3.1. Transformations of Alkyl and Acyl Groups
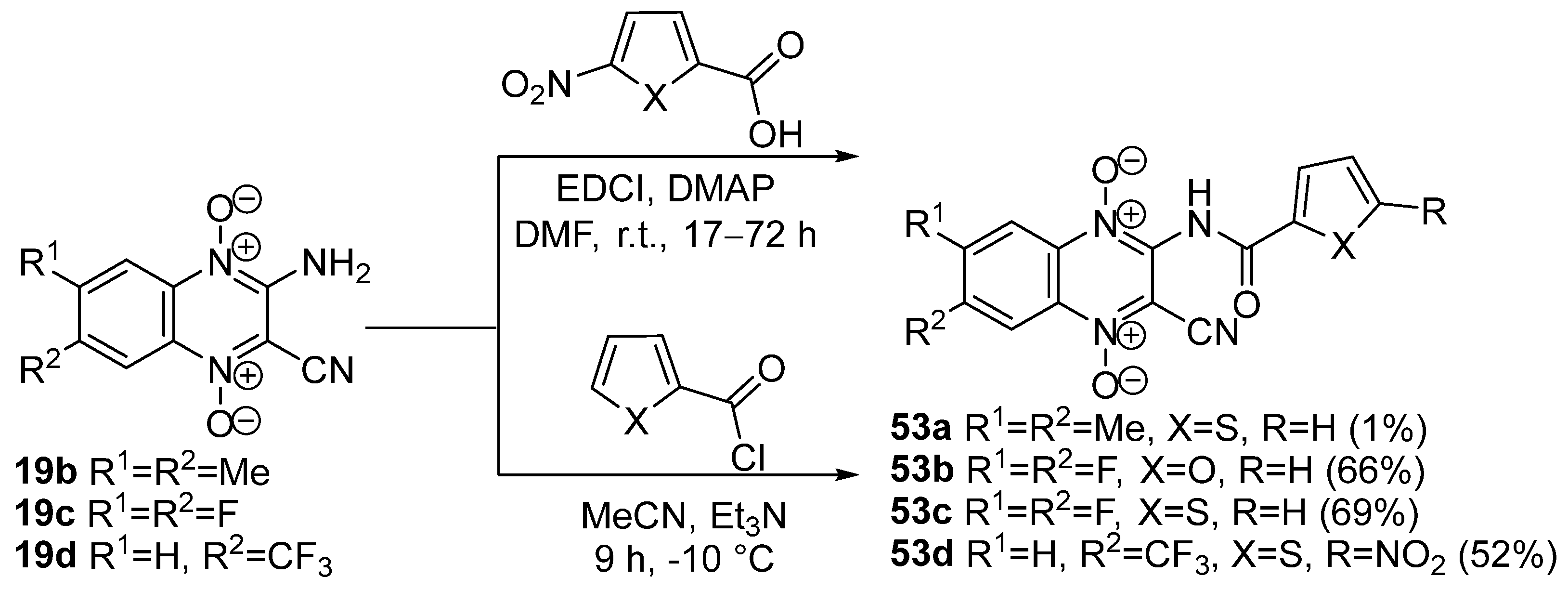
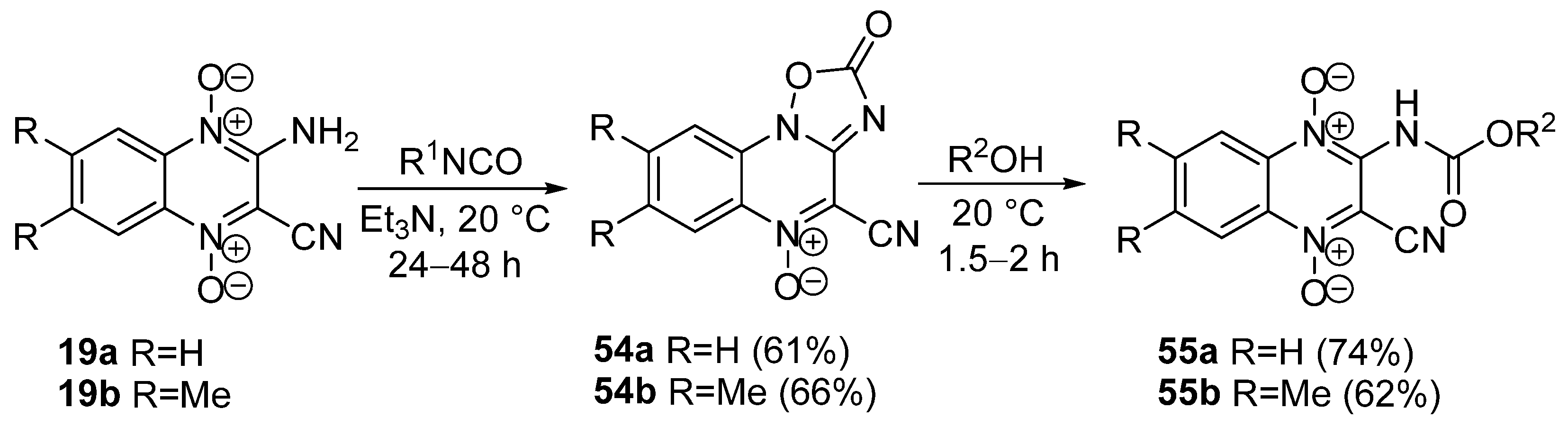
3.2. Reactions of Amino Derivatives of Quinoxaline 1,4-Dioxides
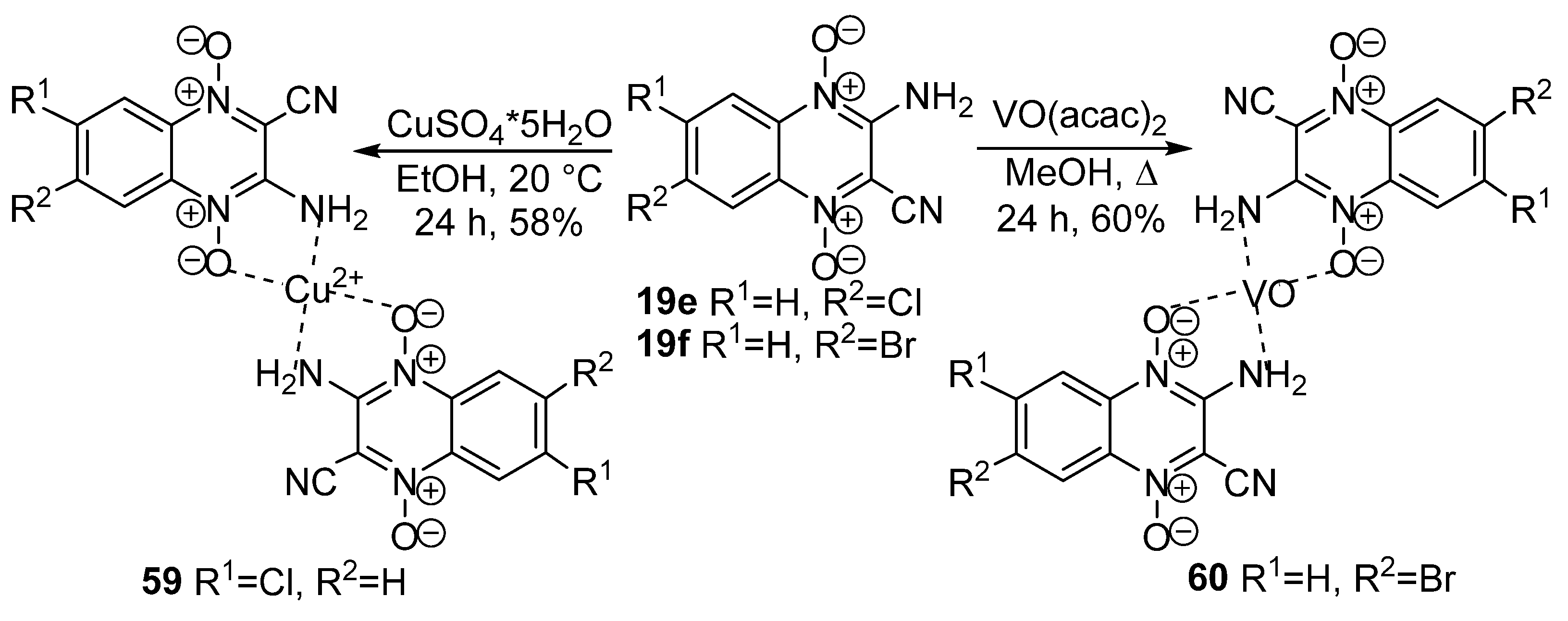
3.3. Metal-Containing Chelates of Quinoxaline 1,4-Dioxides
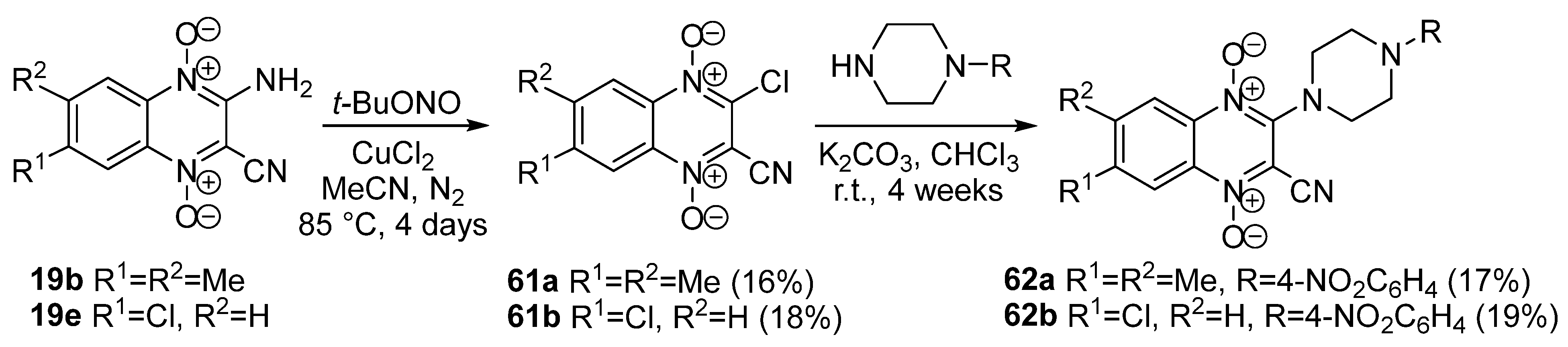
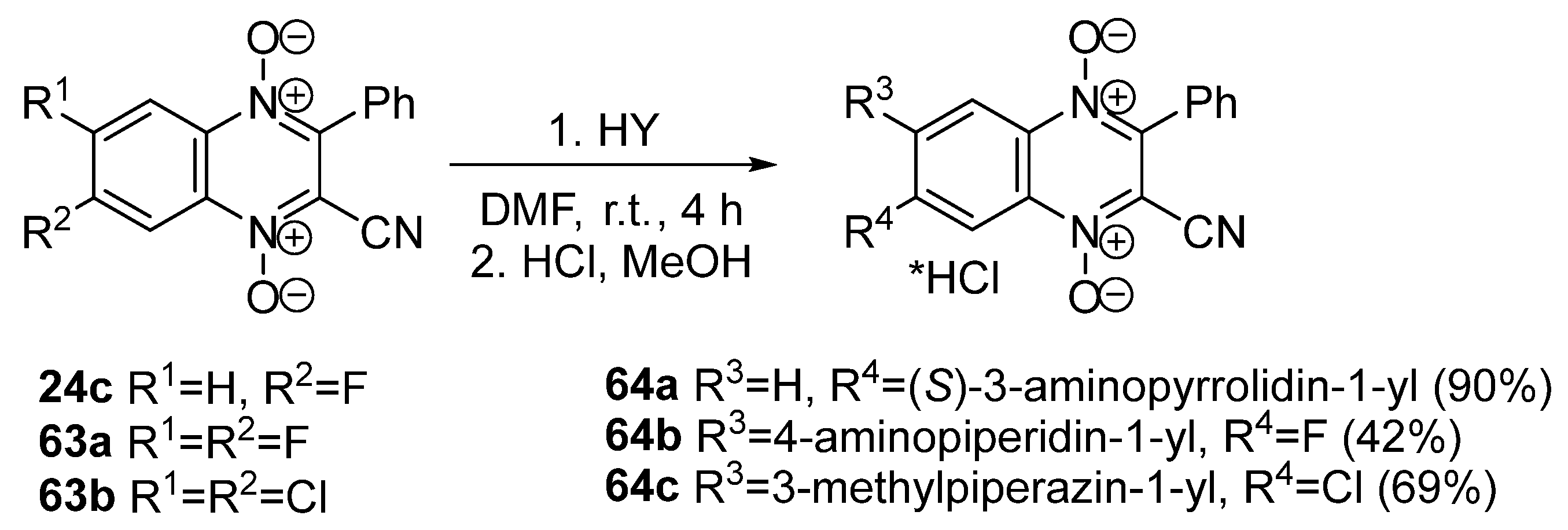
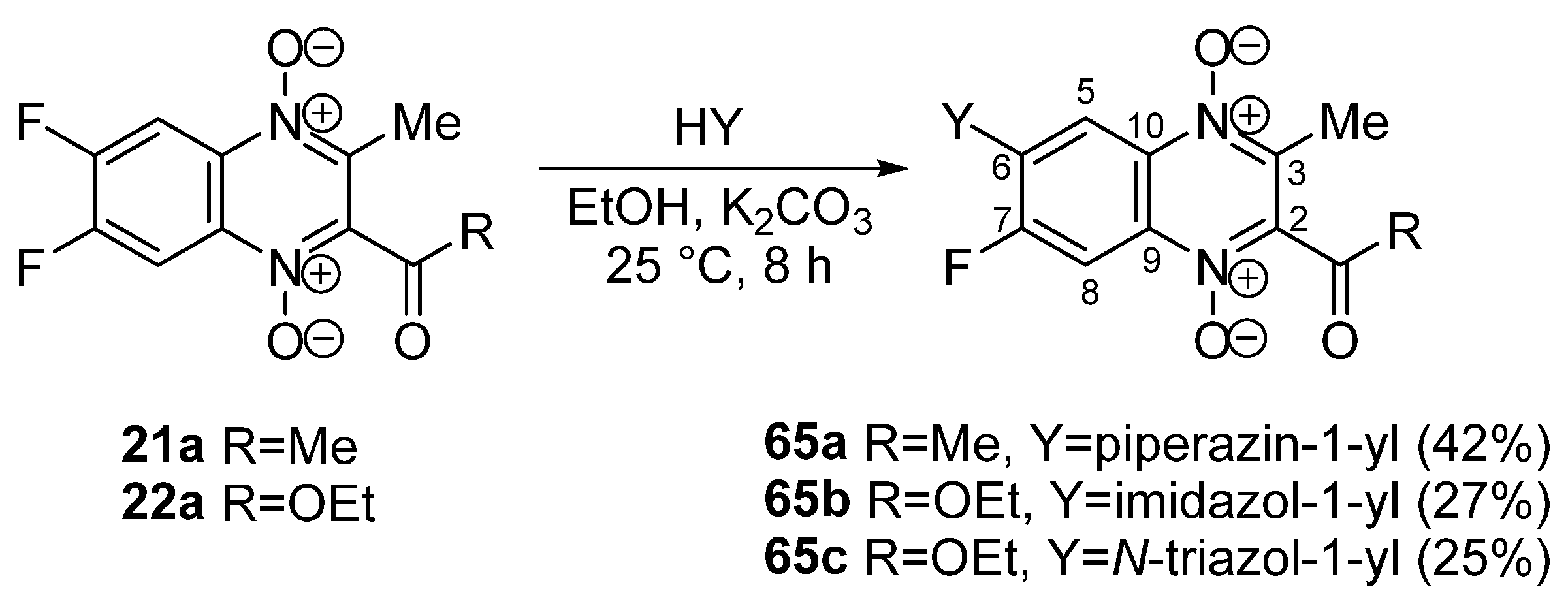
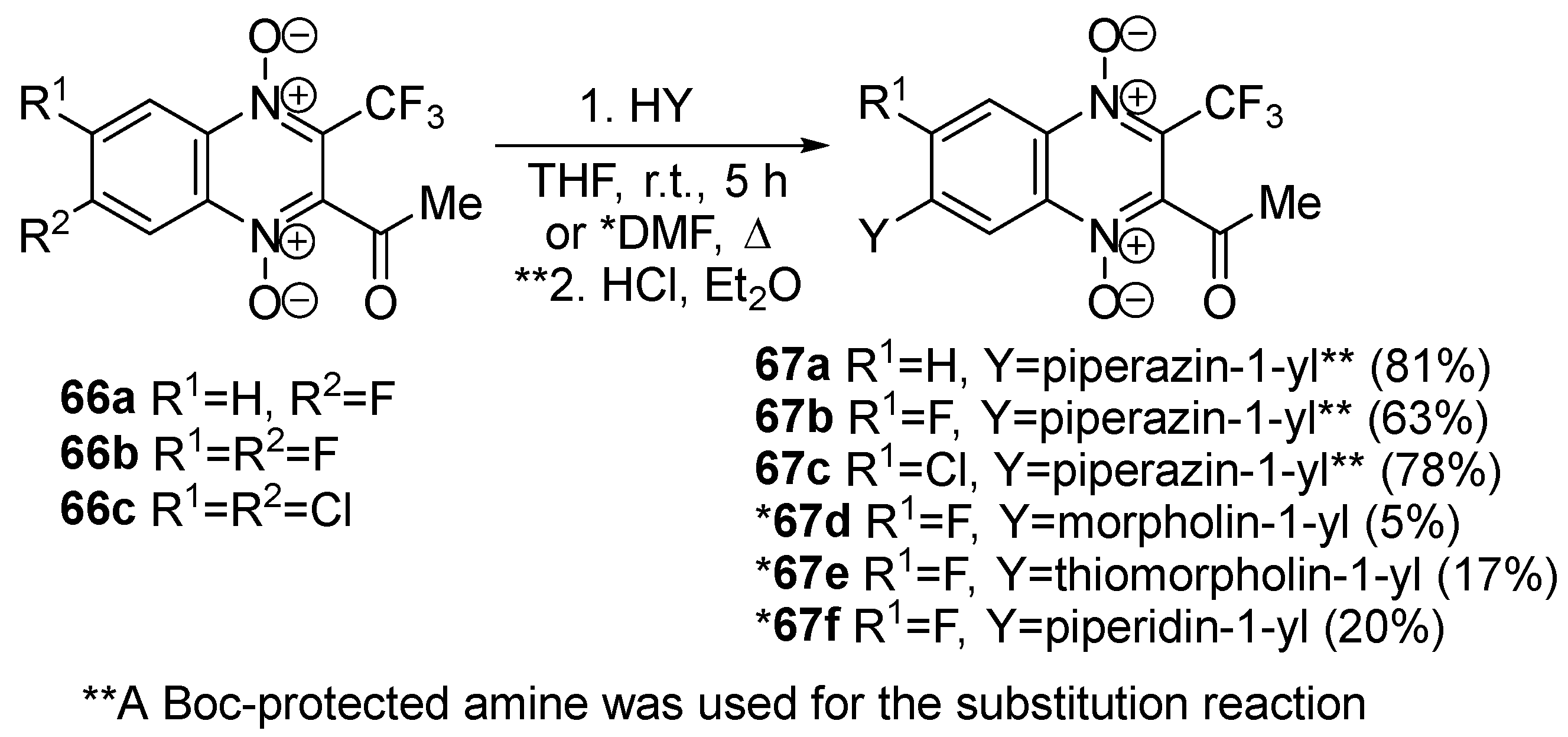
3.4. Nucleophilic Substitution of Halogen Atoms
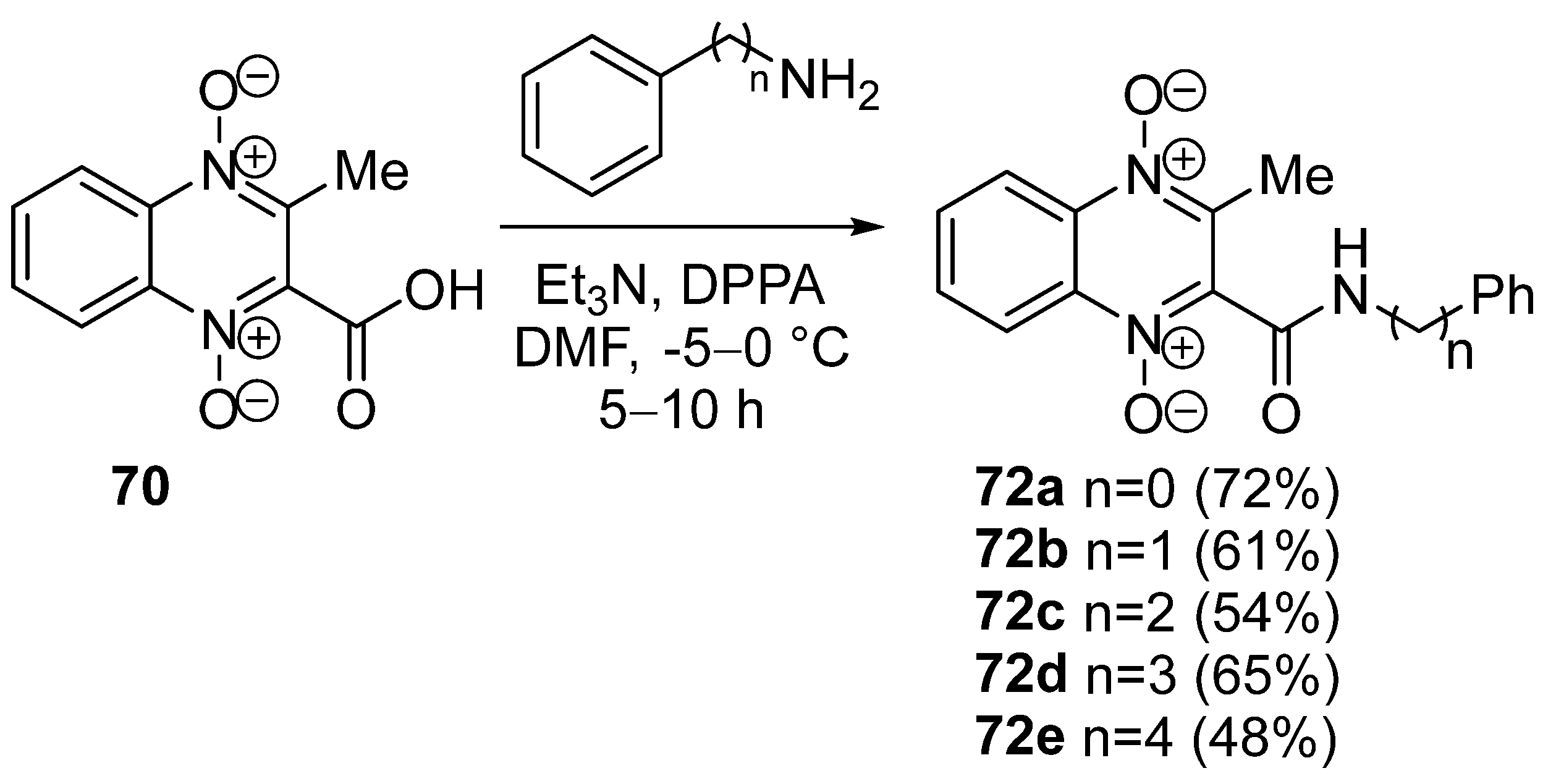
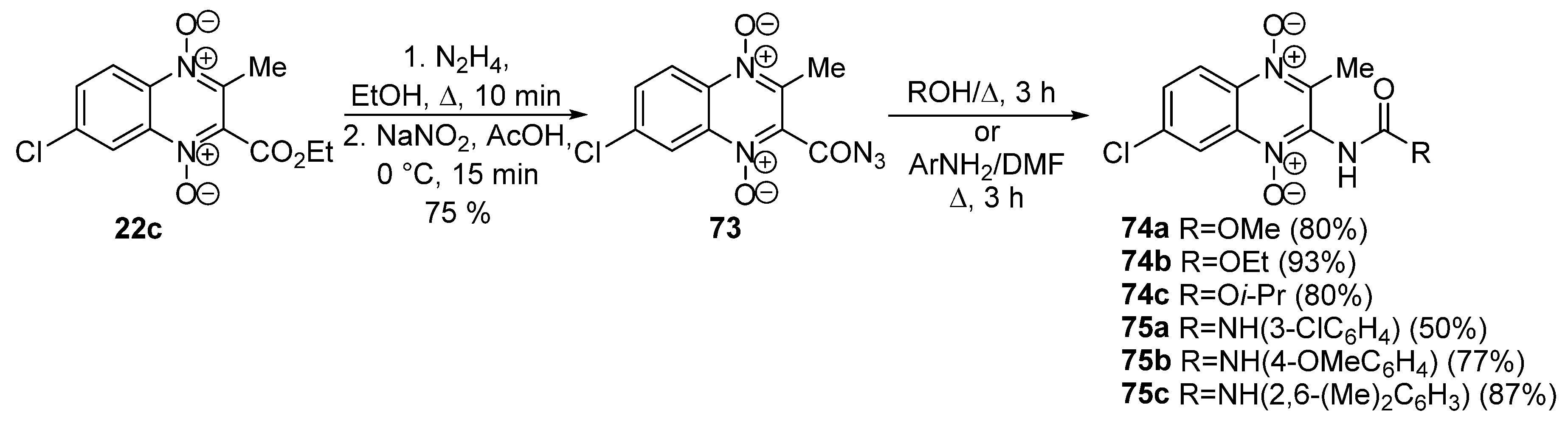
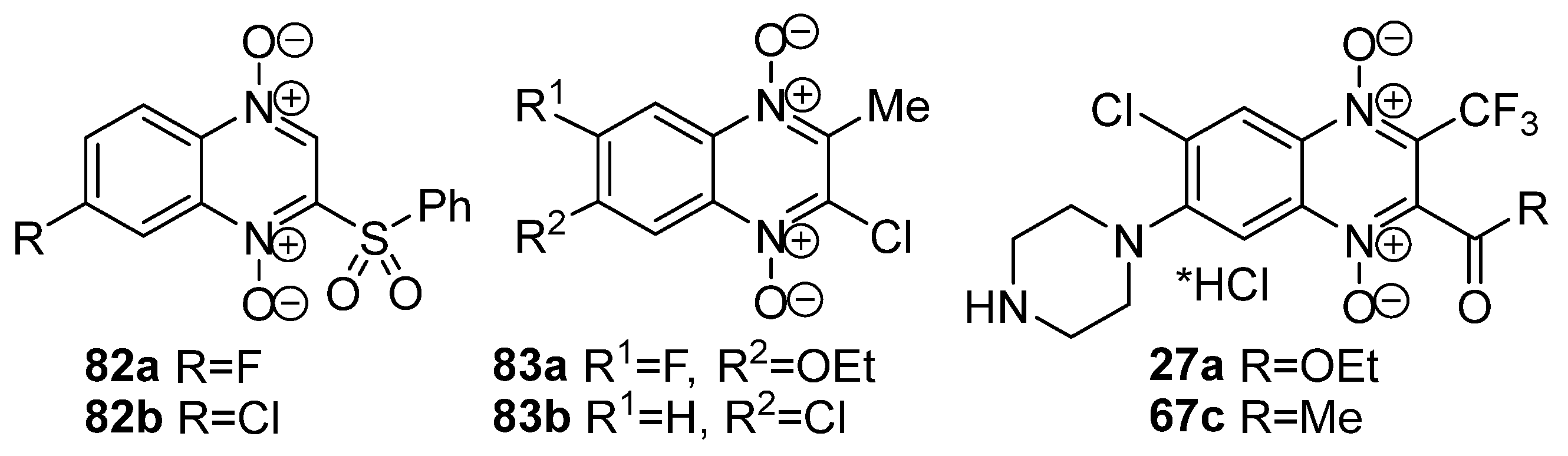
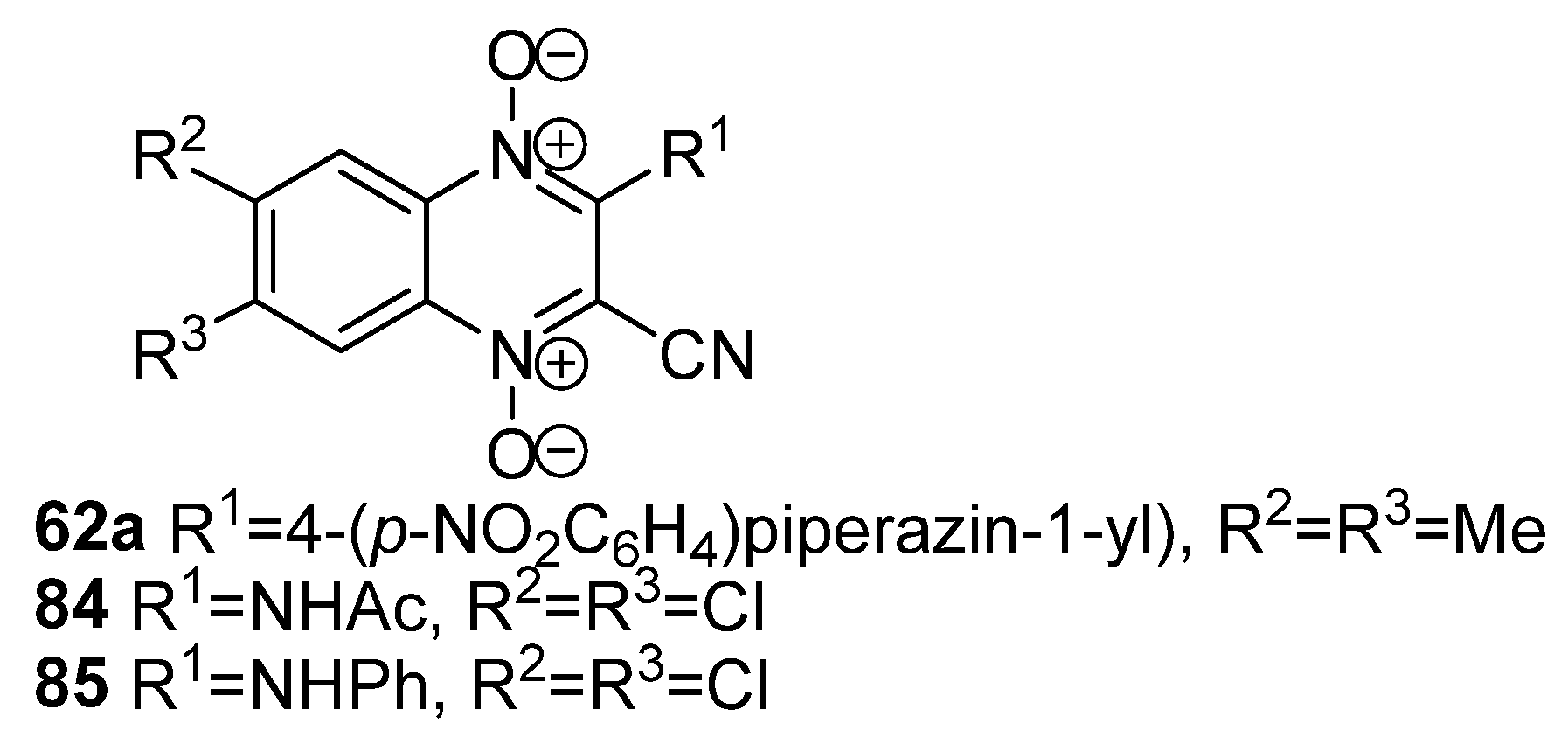
3.5. Transformations of Carboxylic Acids and Their Derivatives
4. Chemotherapeutic Properties of Quinoxaline 1,4-Dioxides
4.1. Antibacterial and Antifungal Activity of Quinoxaline 1,4-Dioxides
4.2. Antimycobacterial Activity of Quinoxaline 1,4-Dioxides
4.3. Antiparasitic Activity of Quinoxaline 1,4-Dioxides
4.4. Anticancer Properties of Quinoxaline 1,4-Dioxides
5. Targeting Signaling Pathways in Cells by Quinoxaline 1,4-Dioxide Derivatives
6. Agricultural Use of Quinoxaline 1,4-Dioxide Derivatives
7. Toxicological Properties of Quinoxaline 1,4-Dioxides
8. Conclusions
- -
- the introduction of electron-withdrawing substituents at positions 3 and 6/7 of the quinoxaline moiety promotes more readily reduction by bacterial or eukaryotic reductases, resulting in increased cytotoxicity and antimicrobial activity of such derivatives;
- -
- the presence of a carbonitrile moiety at position 2 of the pyrazine ring enhances antiproliferative activity, while ester or carboxamide fragments at this position improve the antibacterial properties of quinoxaline 1,4-dioxides;
- -
- overall, the presence of a halogen atom in the quinoxaline core enhances the biological activity of the compounds, with chlorine being the most significant;
- -
- the introduction of electron-donating groups at C3 or C7 carbon atoms of the heterocycle increases the hypoxic cytotoxicity of quinoxaline-2-carbonitrile 1,4-dioxide derivatives.
- -
- lipophilic substituents at positions 2, 6, or 7 improve the antituberculous potential of the derivatives;
- -
- acylamino groups at position 3 of the quinoxaline ring have a positive effect on suppressing the growth of protozoa (Leishmania spp. and Plasmodium spp);
- -
- the introduction of salt-forming fragments leads to water-soluble derivatives while retaining their biological potency.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.D.; Anilkumar, N.C.; Rangappa, S.; Shanmugam, M.K.; Mishra, S.; Chinnathambi, A.; Alharbi, S.A.; Bhattacharjee, A.; Sethi, G.; Kumar, A.P.; et al. Novel 1,3,4-oxadiazole induces anticancer activity by targeting NF-κB in hepatocellular carcinoma cells. Front. Oncol. 2018, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, C.; De Franco, M.; Carbone, D.; Bassani, D.; Pavan, M.; Cascioferro, S.; Parrino, B.; Cirrincione, G.; Dall’Acqua, S.; Moro, S.; et al. 1,2,4-Amino-triazine derivatives as pyruvate dehydrogenase kinase inhibitors: Synthesis and pharmacological evaluation. Eur. J. Med. Chem. 2023, 249, 115134. [Google Scholar] [CrossRef]
- Moniot, S.; Forgione, M.; Lucidi, A.; Hailu, G.S.; Nebbioso, A.; Carafa, V.; Baratta, F.; Altucci, L.; Giacché, N.; Passeri, D.; et al. Development of 1,2,4-oxadiazoles as potent and selective inhibitors of the human deacetylase sirtuin 2: Structure-activity relationship, X-ray crystal ctructure, and anticancer activity. Eur. J. Med. Chem. 2017, 60, 2344–2360. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.; De Franco, M.; Pecoraro, C.; Bassani, D.; Pavan, M.; Cascioferro, S.; Parrino, B.; Cirrincione, G.; Dall’Acqua, S.; Moro, S.; et al. Discovery of the 3-amino-1,2,4-triazine-based library as selective PDK1 inhibitors with therapeutic potential in highly aggressive pancreatic ductal adenocarcinoma. Int. J. Mol. Sci. 2023, 24, 3679. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.K.; Dokla, E.M.E.; Abouzid, K.A.M.; Minucci, S. Discovery of EMD37, a 1,2,4-oxadiazole derivative, as a novel endoplasmic reticulum stress inducer with potent anticancer activity. Biochem. Pharmacol. 2022, 206, 115316. [Google Scholar] [CrossRef]
- Patterson, L.H. Rationale for the use of aliphatic N-oxides of cytotoxic anthraquinones as prodrug DNA binding agents: A new class of bioreductive agent. Cancer Metastasis Rev. 1993, 12, 119–134. [Google Scholar] [CrossRef]
- Shen, X.; Gates, K.S. Enzyme-activated generation of reactive oxygen species from heterocyclic N-oxides under aerobic and anaerobic conditions and its relevance to hypoxia-selective prodrugs. Chem. Res. Toxicol. 2019, 32, 348–361. [Google Scholar] [CrossRef]
- Nicola, O.; O’Neill, A.J. Revisiting unexploited antibiotics in search of new antibacterial drug candidates: The case of MSD-819 (6-chloro-2-quinoxalinecarboxylic acid 1,4-dioxide). J. Antibiot. 2017, 70, 317–319. [Google Scholar] [CrossRef][Green Version]
- Turner, J.M.; Messenger, A.J. Occurrence, biochemistry and physiology of phenazine pigment production. Adv. Microb. Physiol. 1986, 27, 211–275. [Google Scholar] [CrossRef]
- Cimmino, A.; Evidente, A.; Mathieu, V.; Andolfi, A.; Lefranc, F.; Kornienko, A.; Kiss, R. Phenazines and cancer. Nat. Prod. Rep. 2012, 29, 487–501. [Google Scholar] [CrossRef]
- McIlwain, H. Bacterial inhibition by metabolite analogues. Part V. Reactions and antibacterial properties of p-diazine di-N-oxides. J. Chem. Soc. 1943, 88, 322–325. [Google Scholar] [CrossRef]
- Viktorsson, E.Ö.; Melling Grøthe, B.; Aesoy, R.; Sabir, M.; Snellingen, S.; Prandina, A.; Åstrand, O.A.H.; Bonge-Hansen, T.; Døskeland, S.O.; Herfindal, L.; et al. Total synthesis and antileukemic evaluations of the phenazine 5,10-dioxide natural products iodinin, myxin and their derivatives. Bioorg. Med. Chem. 2017, 25, 2285–2293. [Google Scholar] [CrossRef]
- Myhren, L.; Nygaard, G.; Gausdal, G.; Sletta, H.; Teigen, K.; Degnes, K.; Zahlsen, K.; Brunsvik, A.; Bruserud, Ø.; Døskeland, S.O.; et al. Iodinin (1,6-Dihydroxyphenazine 5,10-dioxide) from Streptosporangium sp. induces apoptosis selectively in myeloid leukemia cell lines and patient cells. Mar. Drugs 2013, 11, 332–349. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.S.; Pollak, J.; Bailey-Davis, L.; Hirsch, A.G.; Cosgrove, S.E.; Nau, C.; Kress, A.M.; Glass, T.A.; Bandeen-Roche, K. Antibiotic use and childhood body mass index trajectory. Int. J. Obes. 2016, 40, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Dvoryantseva, G.G.; Lindeman, S.V.; Aleksanyan, M.S.; Struehkov, Y.T.; Teten’ehuk, K.P.; Khabarova, L.S.; Elina, A.S. Connection between the structure and the antibacterial activity of the N-oxides of quinoxalines. Molecular structure of dioxidine and quinoxidine. Pharm. Chem. J. 1990, 24, 672–677. [Google Scholar] [CrossRef]
- Vicente, E.; Lima, L.M.; Bongard, E.; Charnaud, S.; Villar, R.; Solano, B.; Burguete, A.; Perez-Silanes, S.; Aldana, I.; Vivas, L.; et al. Synthesis and structure-activity relationship of 3-phenylquinoxaline 1,4-di-N-oxide derivatives as antimalarial agents. Eur. J. Med. Chem. 2008, 43, 1903–1910. [Google Scholar] [CrossRef]
- Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A. Synthesis and antimycobacterial activity of new quinoxaline-2-carboxamide 1,4-di-N-oxide derivatives. J. Med. Chem. 2003, 11, 2149–2156. [Google Scholar] [CrossRef]
- Edwards, M.L.; Bambury, R.E.; Ritter, H.W. 3-Substituted 2-formylquinoxaline 1,4-dioxides. J. Med. Chem. 1975, 18, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Kleim, J.P.; Bender, R.; Billhardt, U.M.; Meichsner, C.; Riess, G.; Rösner, M.; Winkler, I.; Paessens, A. Activity of a novel quinoxaline derivative against human immunodeficiency virus type 1 reverse transcriptase and viral replication. Antimicrob. Agents Chemother. 1993, 37, 1659–1664. [Google Scholar] [CrossRef]
- Monge, A.; Palop, J.A.; Cerain, A.L.; Senador, V.; Martinez-Crespo, F.J.; Sainz, Y.; Narro, S.; Garcia, E.; de Miguel, C.; Gonzalez, M.; et al. Greenhow hypoxia-selective agents derived from quinoxaline 1,4-di-N-oxides. J. Med. Chem. 1995, 38, 1786–1792. [Google Scholar] [CrossRef]
- Tucker, M.J. Carcinogenic action of quinoxaline 1,4-dioxide in rats. J. Natl. Cancer Inst. 1975, 55, 137–146. [Google Scholar] [CrossRef]
- Zaynoun, S.; Johnson, B.; Frain-Bell, W. The investigation of Quindoxin photosensitivity. Contact Derm. 1976, 2, 343–352. [Google Scholar] [CrossRef] [PubMed]
- De Vries, H.; Bojarski, J.; Donker, A.A.; Bakri, A.; Beyersbergen van Henegouwen, G.M. Photochemical reactions of Quindoxin, Olaquindox, Carbadox and Cyadox with protein, indicating photoallergic properties. Toxicology 1990, 63, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.; Pinheiro, C.; Fernandes, R.; Noronha, J.P.; Prudencio, C. Antimicrobial activity of quinoxaline 1,4-dioxide with 2-and 3-substituted derivatives. Microbiol. Res. 2014, 169, 287–293. [Google Scholar] [CrossRef]
- Carta, A.; Corona, P.; Loriga, M. Quinoxaline 1,4-dioxide: A versatile scaffold endowed with manifold activities. Curr. Med. Chem. 2005, 12, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.M.; do Amaral, D.N. Beirut reaction and its application in the synthesis of quinoxaline-N,N′-dioxides bioactive compounds. Rev. Virtual Quim. 2013, 5, 1075–1100. [Google Scholar] [CrossRef]
- Agrawal, N.; Bhardwaj, A. An appraisal on synthetic and pharmaceutical perspectives of quinoxaline 1,4-di-N-oxide scaffold. Chem. Biol. Drug Des. 2022, 100, 346–363. [Google Scholar] [CrossRef]
- Rivera, G. Quinoxaline 1,4-di-N-oxide derivatives: Are they unselective or selective inhibitors? Mini Rev. Med. Chem. 2022, 22, 15–25. [Google Scholar] [CrossRef]
- Dos Santos Fernandes, G.F.; Pavan, A.R.; dos Santos, J.L. Heterocyclic N-oxides—A promising class of agents against tuberculosis, malaria and neglected tropical diseases. Curr. Pharm. Des. 2018, 24, 1325–1340. [Google Scholar] [CrossRef]
- Hamama, W.S.; Waly, S.M.; Said, S.B.; Zoorob, H.H. Highlights on the chemistry of 2-amino-3-cyano-quinoxaline 1,4-dioxides and their derivatives. Synth. Commun. 2020, 50, 1737–1757. [Google Scholar] [CrossRef]
- Cheng, G.; Sa, W.; Cao, C.; Guo, L.; Hao, H.; Liu, Z.; Wang, X.; Yuan, Z. Quinoxaline 1,4-di-N-Oxides: Biological activities and mechanisms of actions. Front. Pharmacol. 2016, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- González, M.; Cerecetto, H.; Monge, A. Quinoxaline 1,4-dioxide and phenazine 5,10-dioxide. Bioact. Heterocycl. V 2007, 11, 179–211. [Google Scholar] [CrossRef]
- Vicente, E.; Villar, R.; Pérez-Silanes, S.; Aldana, I.; Goldman, R.C.; Monge, A. Quinoxaline 1,4-di-N-oxide and the potential for treating tuberculosis infectious disorders. Curr. Drug Targets 2011, 11, 196–204. [Google Scholar] [CrossRef]
- Keri, R.S.; Pandule, S.S.; Budagumpi, S.; Nagaraja, B.M. Quinoxaline and quinoxaline-1,4-di-N-oxides: An emerging class of antimycobacterials. Arch. Pharm. 2018, 351, e1700325. [Google Scholar] [CrossRef]
- Montana, M.; Montero, V.; Khoumeri, O.; Vanelle, P. Quinoxaline moiety: A potential scaffold against Mycobacterium tuberculosis. Molecules 2021, 26, 4742. [Google Scholar] [CrossRef]
- Carmeli, M.; Rozen, S. A new efficient route for the formation of quinoxaline N-oxides and N,N′-dioxides using HOF-CH3CN. J. Org. Chem. 2006, 71, 5761–5765. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, M.; Wang, W.; Wang, X.; Yuan, Y.; Li, J. Synthesis, crystal structure and calculation of oxides of 2-methylamino-3-methyl quinoxaline. J. Mol. Struct. 2020, 1222, 128826. [Google Scholar] [CrossRef]
- Abushanab, E. One-step synthesis of quinoxaline 1,4-dioxides and related compounds. J. Org. Chem. 1970, 35, 4279–4280. [Google Scholar] [CrossRef]
- Haddadin, M.J.; Issidorides, C.H. Enamines with isobenzofuroxan: A novel synthesis of quinoxaline-di-N-oxides. Tetrahedron Lett. 1965, 6, 3253–3256. [Google Scholar] [CrossRef]
- Jie, J.L. Name Reactions: A Collection of Detailed Reaction Mechanisms, 3rd ed.; Springer: New York, NY, USA, 2006; Volume 1, p. 504. [Google Scholar]
- El-Haj, M.J.A.; Dominy, B.W.; Johnston, J.D.; Haddadin, M.J.; Issidorides, C.H. New route to phenazine 5,10-dioxides and related compounds. J. Org. Chem. 1972, 37, 589–593. [Google Scholar] [CrossRef]
- Tanaka, A.A.; Usui, T. Studies on furan derivatives. Preparation of 2-substituted 3-(5-nitro-2-furyl) quinoxaline 1,4-dioxides and determination of their antibacterial activity. Chem. Pharm. Bull. 1981, 29, 110–115. [Google Scholar] [CrossRef]
- Li, J.; Ji, M.; Hua, W.; Hu, H. Synthesis of 2-substituted-quinoxaline 1,4-dioxides. Ind. J. Chem. B 2001, 40, 1230–1231. [Google Scholar] [CrossRef]
- McFarland, J.W. 2,3-Dihydroquinoxaline 1,4-dioxides as intermediates in the reaction between benzofurazan 1-oxide and enamines. J. Org. Chem. 1971, 36, 1842–1843. [Google Scholar] [CrossRef]
- Atfah, A.; Hill, J. Reaction of 2,1,3-benzoxadiazole 1-oxide with ethyl 2,4-dioxo-4-phenylbutyrate. A route to 2-benzoylquinoxaline, its 1,4-dioxide, and related compounds. J. Chem. Soc. Perkin Trans. 1 1989, 2, 221–224. [Google Scholar] [CrossRef]
- Monge, A.; Palop, J.A.; Urbasos, I.; Fernandez-Alvarez, E. New quinoxaline and pyrimido [4,5-b]quinoxaline derivatives. Potential antihypertensive and blood platelet antiaggregating agents J. Heterocycl. Chem. 1989, 26, 1623–1626. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, F.; Yao, Z.; Zhang, M.; Jiang, S. Synthesis of quinoxaline 1,4-di-N-oxide analogues and crystal structure of 2-carbomethoxy-3-hydroxyquinoxaline-di-N-oxide. Molecules 2011, 16, 6894–6901. [Google Scholar] [CrossRef]
- Anderson, R.F.; Yadav, P.; Shinde, S.S.; Hong, C.R.; Pullen, S.M.; Reynisson, J.; Wilso, W.R.; Hay, M.P. Radical chemistry and cytotoxicity of bioreductive 3-substituted quinoxaline di-N-oxides. Chem. Res. Toxicol. 2016, 29, 1310–1324. [Google Scholar] [CrossRef] [PubMed]
- Chupakhin, O.N.; Kotovskaya, S.K.; Perova, N.M.; Baskakova, Z.M.; Charushin, V.N. Synthesis of new fluorine-containing derivatives of quinoxaline 1,4-dioxides and condensed systems derived from them. Chem. Heterocycl. Compd. 1999, 35, 459–469. [Google Scholar] [CrossRef]
- Lima, L.M.; Zarranz, B.; Marin, A.; Solano, B.; Vicente, E.; Perez Silanes, S.; Aldana, I.; Monge, A. Comparative use of solvent-free KF-Al2O3 and K2CO3 in acetone in the synthesis of quinoxaline 1,4-dioxide derivatives designed as antimalarial drug candidates. J. Heterocycl. Chem. 2005, 42, 1381–1385. [Google Scholar] [CrossRef]
- Sun, T.; Zhao, W.-J.; Hao, A.-Y.; Sun, L.-Z. Effective promotion of Beirut reaction by β-cyclodextrin in water. Synth. Commun. 2011, 41, 3097–3105. [Google Scholar] [CrossRef]
- Bonilla-Ramírez, L.; Rios, A.; Quiliano, M.; Ramírez-Calderon, G.; Beltrán-Hortelano, I.; Franetich, J.F.; Corcuera, L.; Bordessoulles, M.; Vettorazzi, A.; López de Cerain, A.; et al. Novel antimalarial chloroquine- and primaquine-quinoxaline 1,4-di-N-oxide hybrids: Design, synthesis, Plasmodium life cycle stage profile, and preliminary toxicity studies. Eur. J. Med. Chem. 2018, 158, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Srinivasarao, S.; Nandikolla, A.; Suresh, A.; Ewa, A.-K.; Głogowska, A.; Ghosh, B.; Kumar, B.K.; Murugesan, S.; Pulya, S.; Aggrawal, H.; et al. Discovery of 1,2,3-triazole based quinoxaline-1,4-di-N-oxide derivatives as potential anti-tubercular agents. Bioorg. Chem. 2020, 100, 103955. [Google Scholar] [CrossRef]
- Gasco, A.M.; Ermondi, G.; Fruttero, R.; Gasco, A. Benzofurazanyl- and benzofuroxanyl-1,4-dihydropyridines: Synthesis, structure and calcium entry blocker activity. Eur. J. Med. Chem. 1996, 31, 3–10. [Google Scholar] [CrossRef]
- Haddadin, M.J.; Taha, M.U.; Jarrar, A.A.; Issidorides, C.H. Reaction of benzofurazan oxide with unsymmetrical 1,3-diketones; steric and polar effects. Tetrahedron 1976, 32, 719–724. [Google Scholar] [CrossRef]
- Haddadin, M.J.; Issidorides, C.H. The Beirut reaction. Heterocycles 1993, 3, 1503–1525. [Google Scholar] [CrossRef]
- Cerecetto, H.; Gonzalez, M.; Lavaggi, M.L.; Porcal, W. Preparation of phenazine N5,N10-dioxides. Effects of benzofuroxan substituents in the outcome of their expansion reaction with phenolates. J. Braz. Chem. Soc. 2005, 16, 1290–1296. [Google Scholar] [CrossRef]
- Ermondi, G.; Visentin, S.; Boschi, D.; Fruttero, R.; Gasco, A. Structural investigation of Ca2+ antagonists benzofurazanyl and benzofuraxanyl-1,4-dihydropiridines. J. Mol. Struct. 2000, 523, 149–154. [Google Scholar] [CrossRef]
- Buravchenko, G.I.; Scherbakov, A.M.; Korlukov, A.D.; Dorovatovskii, P.V.; Shchekotikhin, A.E. Revision of the regioselectivity of the Beirut reaction of monosubstituted benzofuroxans with benzoylacetonitrile. 6-Substituted quinoxaline-2-carbonitrile 1,4-dioxides: Structural characterization and estimation of anticancer activity and hypoxia selectivity. Curr. Org. Chem. 2020, 17, 29–39. [Google Scholar] [CrossRef]
- Buravchenko, G.I.; Scherbakov, A.M.; Dezhenkova, L.G.; Monzote, L.; Shchekotikhin, A.E. Synthesis of 7-amino-6-halogeno-3-phenylquinoxaline-2-carbonitrile 1,4-dioxides: A way forward for targeting hypoxia and drug resistance of cancer cells. RSC Adv. 2021, 11, 38782–38795. [Google Scholar] [CrossRef]
- Buravchenko, G.I.; Maslov, D.A.; Alam, M.S.; Grammatikova, N.E.; Frolova, S.G.; Vatlin, A.A.; Tian, X.; Ivanov, I.V.; Bekker, O.B.; Kryakvin, M.A.; et al. Synthesis and characterization of novel 2-acyl-3-trifluoromethylquinoxaline 1,4-dioxides as potential antimicrobial agents. Pharmaceuticals 2022, 15, 155. [Google Scholar] [CrossRef]
- Dirlam, J.P.; McFarland, J.W. Selective monodeoxygenation of certain quinoxaline 1,4-dioxides with trimethyl phosphite. J. Org. Chem. 1977, 42, 1360–1364. [Google Scholar] [CrossRef]
- Kluge, A.F.; Maddox, M.L.; Lewis, G.S. Formation of quinoxaline monoxides from reaction of benzofurazan oxide with enones and carbon-13 NMR correlations of quinoxaline N-oxides. J. Org. Chem. 1980, 45, 1909–1914. [Google Scholar] [CrossRef]
- Demirdji, S.H.; Haddadin, M.J.; Issidorides, C.H. Deoxygenation of quinoxaline and phenazine N-oxides by catalytic transfer reduction and by iodide in the presence of pyridine/sulfur trioxide complex. J. Heterocycl. Chem. 1983, 20, 1735–1737. [Google Scholar] [CrossRef]
- Novacek, L.; Nechvatal, M. Partial reduction of quinoxaline 1,4-dioxide derivatives with L-ascorbic acid. Collect. Czech. Chem. Commun. 1988, 53, 1302–1306. [Google Scholar] [CrossRef]
- Homaidan, F.R.; Issidorides, C.H. Deoxygenation of 2,3-disubstituted quinoxaline 1,4-dioxides. Heterocycles 1981, 16, 411–415. [Google Scholar] [CrossRef]
- Jarrar, A.A.; Fataftah, Z.A. Photolysis of some quinoxaline-1,4-di-oxides. Tetrahedron 1977, 33, 2127–2129. [Google Scholar] [CrossRef]
- Hirota, T.; Namba, T.; Sasaki, K. Polycyclic N-hetero compounds. XXII Reaction of pyridine N-oxides and pyrazine di-N-oxides with formamide. J. Heterocycl. Chem. 1987, 24, 949–953. [Google Scholar] [CrossRef]
- Koyama, T.; Nanba, T.; Hirota, T.; Ohmori, S.; Yamato, M. Polycyclic N-hetero compounds. XIII. Reactions of pyridine N-oxides with formamide. Chem. Pharm. Bull. 1977, 25, 964–967. [Google Scholar] [CrossRef]
- Elina, A.S.; Musatova, I.S.; Titkova, R.M.; Dubinskii, R.A.; Goizman, M.S. Redox transformations of 2-formylquinoxaline hydrate and diethylacetal N,N′-dioxides. Chem. Heterocycl. Compd. 1980, 16, 861–863. [Google Scholar] [CrossRef]
- Epstein, W.W.; Sweat, F.W. Dimethyl sulfoxide oxidations. Chem. Rev. 1967, 67, 247–260. [Google Scholar] [CrossRef]
- Elina, A.S.; Tsyrul’nikova, L.G.; Syrova, G.P. N-oxides of the quinoxaline series. XVI. Redox reactions in the N-oxides of α-hydroxymethyl derivatives of quinoxaline. Chem. Heterocycl. Compd. 1969, 5, 115–117. [Google Scholar] [CrossRef]
- Jarrar, A.A. Photochemistry of some quinoxaline 1,4-dioxides. J. Heterocycl. Chem. 1978, 15, 177–179. [Google Scholar] [CrossRef]
- Vega, A.O.; Gil, M.J.; Fernandez-Alvarez, E. Synthesis of 1-hydrazinopyridazino [4,5-b]quinoxaline and related compounds. J. Heterocycl. Chem. 1984, 21, 1271–1276. [Google Scholar] [CrossRef]
- Lima, L.M.; Vicente, E.; Solano, B.; Perez-Silanes, S.; Aldana, I.; Monge, A. Unexpected reduction of ethyl 3-phenylquinoxaline-2-carboxylate 1,4-di-N-oxide derivatives by amines. Molecules 2008, 13, 78–85. [Google Scholar] [CrossRef]
- Ahmed, Y.; Qureshi, M.I.; Habib, M.S.; Farooqi, M.A. Quinoxaline derivatives. XII. The reactions of quinoxaline 1,4-dioxides with acetic anhydride. Bull. Chem. Soc. Jpn. 1987, 60, 1145–1148. [Google Scholar] [CrossRef]
- Ahmed, Y.; Habib, M.S.; Qureshi, M.I.; Faroogi, M.A. Quinoxaline derivatives. XI. Reaction of quinoxaline 1,4-dioxide and some of its derivatives with acetyl chloride. J. Org. Chem. 1973, 38, 2176–2179. [Google Scholar] [CrossRef]
- Elina, A.S.; Tsyrul’nikova, L.G.; Musatova, I.S. N-oxides of the quinoxaline series XVIII. Reactions of di-N-oxides of α-methyl derivatives of quinoxaline and pyrazine with p-nitrosodimethylaniline. Pharm. Chem. J. 1967, 1, 241–244. [Google Scholar] [CrossRef]
- Ley, K.; Seng, F.; Eholzer, U.; Nast, R.; Schubart, R. New syntheses of quinoxaline and phenazine di-N-oxides. Angew. Chem. 1969, 8, 596–597. [Google Scholar] [CrossRef]
- Amin, K.M.; Ismail, M.M.F.; Noaman, E.; Soliman, D.H.; Ammar, Y.A. New quinoxaline 1,4-di-N-oxides. Part 1: Hypoxia-selective cytotoxins and anticancer agents derived from quinoxaline 1,4-di-N-oxides. Bioorg. Med. Chem. 2006, 14, 6917–6923. [Google Scholar] [CrossRef]
- Said, S.; El-Ablack, F.; Elbeheiry, H.M. Synthesis and characterization of newly fused 1,2-dihydropyrido [3,4-b], bridged oxadiazol-2-yl, 4-substituted-benzylidene hydrazide and arylidene 6-chloroquinoxaline 1,4-dioxides. J. Braz. Chem. Soc. 2018, 29, 2060–2071. [Google Scholar] [CrossRef]
- Quiliano, M.; Pabón, A.; Ramirez-Calderon, G.; Barea, C.; Deharo, E.; Galiano, S.; Aldana, I. New hydrazine and hydrazide quinoxaline 1,4-di-N-oxide derivatives: In silico ADMET, antiplasmodial and antileishmanial activity. Bioorg. Med. Chem. Lett. 2017, 27, 1820–1825. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Moreno, E.; Ancizu, S.; Barea, C.; Galiano, S.; Aldana, I.; Monge, A.; Pérez-Silanes, S. New 1,4-di-N-oxide-quinoxaline-2-ylmethylene isonicotinic acid hydrazide derivatives as anti-Mycobacterium tuberculosis agents. Bioorg. Med. Chem. Lett. 2011, 21, 3699–3703. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Qu, W.; Xie, S.; Huang, L.; Chen, D.; Tao, Y.; Liu, Z.; Pan, Y.; Yuan, Z. Design, synthesis, and biological evaluation of novel thiazolidinone-containing quinoxaline-1,4-di-N-oxides as antimycobacterial and antifungal agents. Front. Chem. 2020, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Miller, L.F.; Bambury, R.E.; Ritter, H.W. Nitrones. 7. alpha-Quinoxalinyl-N-substituted nitrone 1,4-dioxides. J. Med. Chem. 1977, 20, 557–560. [Google Scholar] [CrossRef]
- Elina, A.S. 1,4-Dioxide nitrone derivatives quinoxalines and their biological action. Khim. Farm. Zh. 1967, 1, 69–73. [Google Scholar]
- Michniak, B.B. Amines and amides as penetration enhancers. In Percutaneous Penetration Enhancers; Smith, E.W., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 79–82. ISBN 0-8493-2605-2. [Google Scholar]
- Pan, Y.; Li, P.; Xie, S.; Tao, Y.; Chen, D.; Dai, M.; Hao, H.; Huang, L.; Wang, Y.; Wang, L.; et al. Synthesis, 3D-QSAR analysis and biological evaluation of quinoxaline 1,4-di-N-oxide derivatives as antituberculosis agents. Bioorg. Med. Chem. Let. 2016, 26, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Pabón, A.; Galiano, S.; Burguete, A.; Pérez-Silanes, S.; Deharo, E.; Monge, A.; Aldana, I. Synthesis, biological evaluation and structure-activity relationships of new quinoxaline derivatives as anti-Plasmodium falciparum agents. Molecules 2014, 19, 2166–2180. [Google Scholar] [CrossRef] [PubMed]
- Burguete, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Ancizu, S.; Villar, R.; Solano, B.; Moreno, E.; Torres, E.; Pe´rez, S.; Aldana, I.; et al. Synthesis and biological evaluation of new quinoxaline derivatives as antioxidant and anti-inflammatory agents. Chem. Biol. Drug Des. 2011, 77, 255–267. [Google Scholar] [CrossRef]
- Burguete, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Villar, R.; Vicente, E.; Solano, B.; Ancizu, S.; Perez-Silanes, S.; Aldana, I.; Monge, A. Synthesis and anti-inflammatory/antioxidant activities of some new ring substituted 3-phenyl-1-(1,4-di-N-oxidequinoxalin-2-yl)-2-propen-1-one derivatives and of their 4,5-dihydro-(1H)-pyrazole an alogues. Bioorg. Med. Chem. Lett. 2007, 17, 6439–6443. [Google Scholar] [CrossRef]
- Ancizu, S.; Moreno, E.; Torres, E.; Burguete, A.; Pérez-Silanes, S.; Benítez, D.; Villar, R.; Solano, B.; Marín, A.; Aldana, I.; et al. Heterocyclic-2-carboxylic acid (3-cyano-1,4-di-N-oxidequinoxalin-2-yl)amide derivatives as hits for the development of neglected disease drugs. Molecules 2009, 14, 2256–2272. [Google Scholar] [CrossRef] [PubMed]
- Martínez Crespo, F.J.; Palop, J.A.; Sainz, Y.; Narro, S.; Senador, V.; Gonzalez, M.; Lopez De Cerain, A.; Monge, A.; Hamilton, E.; Barker, A.J. 4-Cyano-2-oxo-1,2,4-oxadiazolo [2,3-a]quinoxaline 5-N-oxides. New synthetic method and reaction with alcohols. Potential cytotoxic activity. J. Heterocycl. Chem. 1996, 33, 1671–1677. [Google Scholar] [CrossRef]
- Abdel-Sattar, S.; Elgazwy, H.; Soliman, D.H. Design, synthesis and evaluation of 1,3,2-diazaphosphorin [4,5-b]quinoxaline-5,10-di-N-oxide derivatives as novel VEGFR-2 and SRC Kinase inhibitors in the treatment of prostate cancer. JPS Conf. Proc. 2013, 4, 77–86. [Google Scholar] [CrossRef][Green Version]
- Urquiola, C.; Gambino, D.; Cabrera, M.; Lavaggi, M.L.; Cerecetto, H.; Gonza´lez, M.; Lo´pez de Cerain, A.; Monge, A.; Costa-Filho, A.J.; Torre, M.H. New copper-based complexes with quinoxaline N1,N4-dioxide derivatives, potential antitumoral agents. J. Inorg. Biochem. 2008, 102, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Stanila, A.; Marcu, A.; Rusu, D.; Rusu, M.; David, L. Spectroscopic studies of some copper(II) complexes with amino acids. J. Mol. Struct. 2007, 834–836, 364–368. [Google Scholar] [CrossRef]
- Marın-Hernandez, A.; Gracia-Morb, I.; Ruiz-Ramırez, L.; Moreno-Sanchez, R. Toxic effects of copper-based antineoplastic drugs (Casiopeinas 1) on mitochondrial functions. Biochem. Pharmacol. 2003, 65, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Kállay, C.; Várnagy, K.; Micera, G.; Sanna, D.; Sóvágó, I. Copper(II) complexes of oligopeptides containing aspartyl and glutamyl residues. Potentiometric and spectroscopic studies. J. Inorg. Biochem. 2005, 99, 1514–1525. [Google Scholar] [CrossRef]
- Matera-Witkiewicz, A.; Brasuń, J.; Świątek-Kozłowska, J.; Pratesi, A.; Ginanneschi, M.; Messori, L. Short-chain oligopeptides with copper(II) binding properties: The impact of specific structural modifications on the copper(II) coordination abilities. J. Inorg. Biochem. 2009, 103, 678–688. [Google Scholar] [CrossRef]
- Pahontu, E.; Fala, V.; Gulea, A.; Poirier, D.; Tapcov, V.; Rosu, T. Synthesis and characterization of some new Cu(II), Ni(II) and Zn(II) complexes with salicylidene thiosemicarbazones: Antibacterial, antifungal and in vitro antileukemia activity. Molecules 2013, 18, 8812–8836. [Google Scholar] [CrossRef]
- Lim, S.; Price, K.A.; Chong, S.-F.; Paterson, B.M.; Caragounis, A.; Barnham, K.J.; Crouch, P.J.; Peach, J.M.; Dilworth, J.R.; White, A.R.; et al. Copper and zinc bis(thiosemicarbazonato) complexes with a fluorescent tag: Synthesis, radiolabelling with copper-64, cell uptake and fluorescence studies. J. Biol. Inorg. Chem. 2009, 15, 225–235. [Google Scholar] [CrossRef]
- Karayannis, N.M.; Speca, A.N.; Chasan, D.E.; Pytlewski, L.L. Coordination complexes of the N-oxides of aromatic diimines and diazines. Coord. Chem. Rev. 1976, 20, 37–80. [Google Scholar] [CrossRef]
- Torre, M.H.; Gambino, D.; Araujo, J.; Cerecetto, H.; González, M.; Lavaggi, M.L.; Azqueta, A.; López de Cerain, A.; Monge, A.; Abra, U.; et al. Novel Cu(II) quinoxaline N1,N4-dioxide complexes as selective hypoxic cytotoxins. Eur. J. Med. Chem. 2005, 40, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Noblia, P.; Vieites, M.; Torre, M.H.; Costa-Filho, A.J.; Cerecetto, H.; Gonzalez, M.; Lavaggi, M.L.; Adachi, Y.; Sakurai, H.; Gambino, D. Novel vanadyl complexes with quinoxaline 1,4-dioxide derivatives as potent in vitro insulin-mimetic compounds. J. Inorg. Biochem. 2006, 100, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xia, Q.; Shangguan, S.; Liu, X.; Hu, Y.; Sheng, R. Synthesis and biological evaluation of 3-aryl-quinoxaline-2-carbonitrile 1,4-di-N-oxide derivatives as hypoxic selective anti-tumor agents. Molecules 2012, 17, 9683–9696. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Morancho, M.J.; Martinez-Crespo, F.J.; Sainz, Y.; Montoya, M.E.; Lopez de Cerain, A.; Monge, A. New quinoxalinecarbonitrile 1,4-di-N-oxide derivatives as hypoxic-cytotoxic agents. Eur. J. Med. Chem. 2000, 35, 21–30. [Google Scholar] [CrossRef]
- Aguirre, G.; Cerecetto, H.; Di Maio, R.; González, M.; Alfaro, M.E.M.; Jaso, A.; Zarranz, B.; Ortega, M.A.; Aldana, I.; Monge-Vega, A. Quinoxaline N,N′-dioxide derivatives and related compounds as growth inhibitors of Trypanosoma cruzi. Structure–activity relationships. Bioorg. Med. Chem. Lett. 2004, 14, 3835–3839. [Google Scholar] [CrossRef]
- Usta, J.A.; Haddadin, M.J.; Issidorides, C.H.; Jarrar, A.A. Preparation of some functionalized quinoxaline 1,4-dioxides. J. Heterocycl. Chem. 1981, 18, 655–658. [Google Scholar] [CrossRef]
- Vicente, E.; Villar, R.; Burguete, A.; Solano, B.; Ancizu, S.; Perez-Silanes, S.; Aldana, I.; Monge, A. Substitutions of fluorine atoms and phenoxy groups in the synthesis of quinoxaline 1,4-di-N-oxide derivatives. Molecules 2008, 13, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Monge, A.; Martinez-Crespo, F.J.; Lopez de Cerain, A.; Palop, J.A.; Narro, S.; Senador, V.; Marin, A.; Sainz, Y.; Gonzalez, M.; Hamilton, E.; et al. Hypoxia-selective agents derived from 2-quinoxalinecarbonitrile 1,4-di-N-oxides. J. Med. Chem. 1995, 38, 4488–4494. [Google Scholar] [CrossRef]
- Aldana, I.; Ortega, M.A.; Jaso, A.; Zarranz, B.; Oporto, P.; Gimenez, A.; Monge, A.; Deharo, E. Anti-malarial activity of some 7-chloro-2-quinoxalinecarbonitrile-1,4-di-N-oxide derivatives. Pharmazie 2003, 58, 68–69. [Google Scholar]
- Buravchenko, G.I.; Scherbakov, A.M.; Dezhenkova, L.G.; Bykov, E.E.; Solovieva, S.E.; Korlukov, A.A.; Sorokin, D.N.; Fidalgo, L.M.; Shchekotikhin, A.E. Discovery of derivatives of 6(7)-amino-3-phenylquinoxaline-2-carbonitrile 1,4-dioxides: Novel, hypoxia-selective HIF-1α inhibitors with strong antiestrogenic potency. Bioorg. Chem. 2020, 104, 104324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lu, Q.; Zhang, J.; Qu, W.; Xie, S.; Huang, L.; Yuan, Z.; Pan, Y. Discovery of novel nitrogenous heterocyclic-containing quinoxaline-1,4-di-N-oxides as potent activator of autophagy in M. tb-infected macrophages. Eur. J. Med. Chem. 2021, 223, 113657. [Google Scholar] [CrossRef] [PubMed]
- Santivañez-Veliz, M.; Pérez-Silanes, S.; Torres, E.; Moreno-Viguri, E. Design and synthesis of novel quinoxaline derivatives as potential candidates for treatment of multidrug-resistant and latent tuberculosis. Bioorg. Med. Chem. Lett. 2016, 26, 2188–2193. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Silanes, S.; Torres, E.; Arbillaga, L.; Varela, J.; Cerecetto, H.; González, M.; Azqueta, A.; Moreno-Viguri, E. Synthesis and biological evaluation of quinoxaline di-N-oxide derivatives with in vitro trypanocidal activity. Bioorg. Med. Chem. Lett. 2016, 26, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Silanes, S.; Devarapally, G.; Torres, E.; Moreno-Viguri, E.; Aldana, I.; Monge, A.; Crawford, P.W. Cyclic voltammetric study of some anti-chagas-active 1,4-dioxidoquinoxalin-2-ylketone derivatives. Helv. Chim. Acta 2013, 96, 217–227. [Google Scholar] [CrossRef]
- Stumm, G.; Niclas, H.-J. An improved and efficient synthesis of quinoxalinecarboxamide 1,4-dioxides from benzofuroxan and acetoacetamides in the presence of calcium salts. J. Prakt. Chem. 1989, 331, 736–744. [Google Scholar] [CrossRef]
- Sabri, S.S.; El-Abadelah, M.M.; Al-Bitar, B.A. Synthesis and spectroscopic studies on some new substituted 2-quinoxalinecarboxamides and their N-oxides. Heterocycles 1987, 26, 699–711. [Google Scholar] [CrossRef]
- Ismail, M.M.F.; Amin, K.M.; Noaman, E.; Soliman, D.H.; Ammar, Y.A. New quinoxaline 1,4-di-N-oxides: Anticancer and hypoxia-selective therapeautic agents. Eur. J. Med. Chem. 2010, 45, 2733–2738. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Fu, Z.Q.; Liu, F. Monooxygenase LaPhzX is involved in self-resistance mechanisms during the biosynthesis of N-oxide phenazine Myxin. J. Agric. Food Chem. 2021, 69, 13524–13532. [Google Scholar] [CrossRef]
- Dutcher, J.D.; Wintersteiner, O. The Structure of aspergillic acid. J. Biol. Chem. 1944, 155, 359–360. [Google Scholar] [CrossRef]
- Watanabe, K.; Hotta, K.; Praseuth, A.; Koketsu, K.; Migita, A.; Boddy, C.N.; Wang, C.C.C.; Oguri, H.; Oikawa, H. Total biosynthesis of antitumor nonribosomal peptides in Escherichia coli. Nat. Chem. Biol. 2006, 2, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Clemo, G.R.; McIlwain, H. The Phenazine Series. Part VII. The Pigment of Chromobacterium iodinum; The phenazine di-N-oxides. J. Chem. Soc. 1938, 100, 479–483. [Google Scholar] [CrossRef]
- Suter, W.; Rosselet, A.; Knusel, F. Mode of action of quindoxin and substituted quinoxaline-di-N-oxides on Escherichia coli. Antimicrob. Agents Chemother. 1978, 13, 770–783. [Google Scholar] [CrossRef]
- Fadeeva, N.I.; Padeĭskaia, E.N.; Degtiareva, I.N.; Pershin, G.N. Effect of quinolaline di-N-oxide derivatives on the DNAse and plasmocoagulase of Staphylococcus aureus. Farmakol. Toksikol. 1978, 41, 613–617. [Google Scholar] [PubMed]
- Huang, X.-J.; Zhang, H.-H.; Wang, X.; Huang, L.-L.; Zhang, L.-Y.; Yan, C.-X.; Liu, Y.; Yuan, Z.-H. ROS mediated cytotoxicity of porcine adrenocortical cells induced by QdNOs derivatives in vitro. Chem. Biol. Interact. 2010, 185, 227–234. [Google Scholar] [CrossRef]
- Xu, F.; Cheng, G.; Hao, H.; Wang, Y.; Wang, X.; Chen, D.; Peng, D.; Liu, Z.; Yuan, Z.; Dai, M. Mechanisms of antibacterial action of quinoxaline 1,4-di-N-oxides against Clostridium perfringens and Brachyspira hyodysenteriae. Front. Microbiol. 2016, 7, 1948–1959. [Google Scholar] [CrossRef] [PubMed]
- Azqueta, A.; Pachon, G.; Cascante, M.; Creppy, E.E.; de Cerain, A.L. DNA damage induced by a quinoxaline 1, 4-di-N-oxide derivative (hypoxic selective agent) in Caco-2 cells evaluated by the comet assay. Mutagenesis 2005, 20, 165–171. [Google Scholar] [CrossRef]
- Popov, D.A.; Anuchina, N.M.; Terentyev, A.A.; Kostyuk, G.V.; Blatun, L.A.; Rusanova, E.V.; Aleksandrova, I.A.; Pkhakadze, T.Y.; Bogomolova, N.S.; Terekhova, L.P. Dioxidin: Antimicrobial activity and prospects of its clinical use at present. Antibiot. Chemother. 2013, 58, 37–42. (In Russian) [Google Scholar]
- Shabatina, T.I.; Morosov, Y.N.; Soloviev, A.V.; Shabatin, A.V.; Vernaya, O.I.; Melnikov, M.Y. Cryochemical production of drug nanoforms: Particle size and crystal phase control of the antibacterial medication 2,3-quinoxalinedimethanol-1,4-dioxide (Dioxidine). Nanomaterials 2021, 11, 1588. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, M.; Roshchina, E.; Loseva, C.; Gurov, A. Assessment of the toxicological profile of the new dioxidine dosage form (solution for topical and external use, 0.025%). Antibiot. Chemother. 2022, 67, 24–32. [Google Scholar] [CrossRef]
- Usai, D.; Sanna, P.; Sechi, L.A.; Carta, A.; Paglietti, G.; Zanettu, S. In vitro activity of quinoxaline 1, 4-dioxides derivatives against enterococci clinical strains. Ig. Mod. 2004, 121, 289–300. [Google Scholar]
- Carta, A.; Paglietti, G.; Nikookar, M.E.R.; Sanna, P.; Sechi, L.; Zanetti, S. Novel substituted quinoxaline 1,4-dioxides with in vitro antimycobacterial and anticandida activity. Eur. J. Med. Chem. 2002, 37, 355–366. [Google Scholar] [CrossRef]
- Iland, C.N. Effect of antibacterial analogues of vitamin K on M. tuberculosis. Nature 1948, 161, 1010. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Ancizu, S.; Pérez-Silanes, S.; Torres, E.; Aldana, I.; Monge, A. Synthesis and antimycobacterial activity of new quinoxaline-2-carboxamide 1,4-di-N-oxide derivatives. Eur. J. Med. Chem. 2010, 45, 4418–4426. [Google Scholar] [CrossRef]
- Sainz, Y.; Montoya, M.E.; Martinez-Crespo, F.J.; Ortega, M.A.; Lopez de Cerain, A.; Monge, A. New quinoxaline 1,4-di-N-oxides for treatment of tuberculosis. Arzneim. Forsch. Drug Res. 1999, 49, 55–59. [Google Scholar] [CrossRef]
- Ortega, M.A.; Sainz, Y.; Montoya, M.E.; Lopez De Cerain, A.; Monge, A. Synthesis and antituberculosis activity of new 2-quinoxalinecarbonitrile 1,4-di-N-oxides. Pharmazie 1999, 54, 24–25. [Google Scholar] [PubMed]
- Montoya, M.E.; Sainz, Y.; Ortega, M.A.; Lopez De Cerain, A.; Monge, A. Synthesis and antituberculosis activity of some new 2-quinoxalinecarbonitriles. Farmaco 1998, 53, 570–573. [Google Scholar] [CrossRef]
- Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Synthesis of new quinoxaline-2-carboxylate 1,4-dioxide derivatives as anti-Mycobacterium tuberculosis agents. J. Med. Chem. 2005, 48, 2019–2025. [Google Scholar] [CrossRef]
- Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Synthesis of new 2-acetyl and 2-benzoyl quinoxaline 1,4-di-N-oxide derivatives as anti-Mycobacterium tuberculosis agents. Eur. J. Med. Chem. 2003, 38, 791–800. [Google Scholar] [CrossRef]
- Carta, A.; Loriga, M.; Paglietti, G.; Mattana, A.; Fiori, P.L.; Mollicotti, P.; Sechi, L.; Zanettic, S. Synthesis, antimycobacterial, antitrichomonas and anticandida in vitro activities of 2-substituted-6,7-difluoro-3-methylquinoxaline 1,4-dioxides. Eur. J. Med. Chem. 2004, 39, 195–203. [Google Scholar] [CrossRef]
- Zanetti, S.; Sechi, L.A.; Molicotti, P.; Cannas, S.; Bua, A.; Deriu, A.; Carta, A.; Paglietti, G. In vitro activity of new quinoxaline 1,4-dioxide derivatives against strains of Mycobacterium tuberculosis and other mycobacteria. Int. J. Antimicrob. Agents 2005, 25, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Noreen, N.; Ullah, A.; Salman, S.M.; Mabkhot, Y.; Alsayari, A.; Badshah, S.L. New insights into the spread of resistance to artemisinin and its analogues. J. Glob. Antimicrob. Resist. 2021, 27, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A.; Maurel, S.; Deharo, E.; Jullian, V.; Sauvain, M. Synthesis and antimalarial activity of new 3-arylquinoxaline-2-carbonitrile derivatives. Arzneim. Forsch./Drug Res. 2004, 55, 754–761. [Google Scholar] [CrossRef]
- Zarranz, B.; Jaso, A.; Lima, L.M.; Aldana, I.; Monge, A.; Maurel, S.; Sauvain, M. Antiplasmodial activity of 3-trifluoromethyl-2-carbonylquinoxaline di-N-oxide derivatives. Rev. Bras. Cienc. Farm. 2006, 42, 357–361. [Google Scholar] [CrossRef]
- Brizuela, M.; Huang, H.M.; Smith, C.; Burgio, G.; Foote, S.J.; McMorran, B.J. Treatment of erythrocytes with 2-cys peroxiredoxin inhibitor, Conoidin A, prevents the growth of Plasmodium falciparum and enhances parasite sensitivity to chloroquine oxides. PLoS ONE 2014, 9, e92411. [Google Scholar] [CrossRef]
- Barea, C.; Pabon, A.; Galiano, S.; Perez-Silanes, S.; Gonzalez, G.; Deyssard, C.; Monge, A.; Deharo, E.; Aldana, I. Antiplasmodial and leishmanicidal activities of 2-cyano-3-(4-phenylpiperazine-1-carboxamido)quinoxaline 1,4-dioxide derivatives. Molecules 2012, 17, 9451–9461. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Rocha, J.C.; Sanchez-Torres, L.; Nogueda-Torres, B.; Segura-Cabrera, A.; Garcia-Perez, C.A.; Bocanegra-Garcia, V.; Palos, I.; Monge, A.; Rivera, G. Anti-Trypanosoma cruzi and anti-leishmanial activity by quinoxaline-7-carboxylate 1,4-di-N-oxide derivatives. Parasitol. Res. 2014, 113, 2027–2035. [Google Scholar] [CrossRef]
- Chacón-Vargas, K.F.; Andrade-Ochoa, S.; Nogueda-Torres, B.; Juárez-Ramírez, D.C.; Lara-Ramírez, E.E.; Mondragón-Flores, R.; Monge, A.; Rivera, G.; Sánchez-Torres, L.E. Isopropyl quinoxaline-7-carboxylate 1,4-di-N-oxide derivatives induce regulated necrosis like cell death on Leishmania (Leishmania) mexicana. Parasitol. Res. 2017, 117, 45–58. [Google Scholar] [CrossRef]
- Duque-Montano, B.E.; Gomez-Caro, L.C.; Sanchez-Sanchez, M.; Monge, A.; Hernandez-Baltazar, E.; Rivera, G.; Torres-Angeles, O. Synthesis and in vitro evaluation of new ethyl and methyl quinoxaline-7-carboxylate 1,4-di-N-oxide against Entamoeba histolytica. Bioorg. Med. Chem. 2013, 21, 4550–4558. [Google Scholar] [CrossRef]
- Jones, W.R.; Landquist, J.K.; Stewart, G.T. Synthetic amoebicides. II. The anti-amoebic action of quinoxaline-1,4-dioxide and some derivatives. Br. J. Pharmacol. Chemother. 1953, 8, 286–289. [Google Scholar] [CrossRef]
- Glazer, E.A.; Chappel, L.R. Pyridoquinoxaline N-oxides. A new class of antitrichomonal agents. J. Med. Chem. 1982, 25, 868–870. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Moreno-Viguri, E.; Galiano, S.; Devarapally, G.; Crawford, P.W.; Azqueta, A.; Arbillaga, L.; Varela, J.; Birriel, E.; Di Maio, R.; et al. Novel quinoxaline 1,4-di-N-oxide derivatives as new potential antichagasic agents. Eur. J. Med. Chem. 2013, 66, 324–334. [Google Scholar] [CrossRef]
- Gerpe, A.; Boiani, L.; Hernandez, P.; Sortino, M.; Zacchino, S.; Gonzalez, M.; Cerecetto, H. Naftifine-analogues as anti-Trypanosoma cruzi agents. Eur. J. Med. Chem. 2010, 45, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Xia, Q.; Cella, M.; Nenninger, A.; Mruzik, M.; Brillos-Monia, K.; Hu, Y.Z.; Sheng, R.; Ragain, C.M.; Crawford, P. Voltammetric study of some 3-aryl-quinoxaline-2-carbonitrile 1,4-di-N-oxide derivatives with anti-tumor activities. Molecules 2017, 22, 1442. [Google Scholar] [CrossRef] [PubMed]
- Das, U.; Pati, H.N.; Panda, A.K.; De Clercq, E.; Balzarini, J.; Molnar, J.; Barath, Z.; Ocsovszki, I.; Kawase, M.; Zhou, L.; et al. 2-(3-Aryl-2-propenoyl)-3-methylquinoxaline-1,4-dioxides: A novel cluster of tumor-specific cytotoxins which reverse multidrug resistance. Bioorg. Med. Chem. 2009, 17, 3909–3915. [Google Scholar] [CrossRef]
- Ganley, B.; Chowdhury, G.; Bhansali, J.; Daniels, J.S.; Gates, K.S. Redox-activated, hypoxia-selective DNA cleavage by quinoxaline 1,4-di-N-oxide. Bioorg. Med. Chem. 2001, 9, 2395–2401. [Google Scholar] [CrossRef]
- Urquiola, C.; Vieites, M.; Torre, M.H.; Cabrera, M.; Lavaggi, M.L.; Cerecetto, H.; González, M.; López de Cerain, A.; Monge, A.; Smircich, P.; et al. Cytotoxic palladium complexes of bioreductive quinoxaline N1,N4-dioxide prodrugs. Bioorg. Med. Chem. 2009, 17, 1623–1629. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.U.; Haddadin, M.J.; Rahhal, D.N.; Younes, I.H. Quinoxaline 1,4-dioxides as anticancer and hypoxia-selective drugs. Oncol. Rep. 2001, 8, 679–684. [Google Scholar] [CrossRef]
- Diab-Assef, M.; Haddadin, M.J.; Yared, P.; Assaad, C.; Gali-Muhtasib, H.U. Quinoxaline 1,4-dioxides: Hypoxia-selective therapeutic agents. Mol. Carcinog. 2002, 33, 198–205. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.; Sidani, M.; Geara, F.; Mona, A.-D.; Al-Hmaira, J.; Haddadin, M.J.; Zaatari, G. Quinoxaline 1,4-dioxides are novel angiogenesis inhibitors that potentiate antitumor effects of ionizing radiation. Int. J. Oncol. 2004, 2, 1121–1131. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.U.; Diab-Assaf, M.K.; Haddadin, M.J. Quinoxaline 1,4-dioxides induce G2/M cell cycle arrest and apoptosis in human colon cancer cells. Cancer Chemother. Pharmacol. 2005, 55, 369–378. [Google Scholar] [CrossRef]
- Weng, Q.; Wang, D.; Guo, P.; Fang, L.; Hu, Y.; He, Q.; Yang, B. Q39, a novel synthetic quinoxaline 1,4-di-N-oxide compound with anti-cancer activity in hypoxia. Eur. J. Pharmacol. 2008, 581, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, A.M.; Borunov, A.M.; Buravchenko, G.I.; Andreeva, O.E.; Kudryavtsev, I.A.; Dezhenkova, L.G.; Shchekotikhin, A.E. Novel quinoxaline-2-carbonitrile-1,4-dioxide derivatives suppress HIF1α activity and circumvent MDR in cancer cells. Cancer Investig. 2018, 36, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Glaser, R.; Gates, K.S. On the reaction mechanism of tirapazamine reduction chemitry: Unimolecular N-OH homolysis, stepwise dehydration, or triazenering-opening. Chem. Res. Toxicol. 2012, 25, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Huang, L.; Pan, Y.; Li, J.; Chen, D.; Cheng, G.; Hao, H.; Tao, Y.; Liu, Z.; et al. Deoxidation rates play a critical role in DNA damage mediated by important synthetic drugs, quinoxaline 1,4-dioxides. Chem. Res. Toxicol. 2015, 28, 470–481. [Google Scholar] [CrossRef]
- Zeng, Y.; Ma, J.; Zhan, Y.; Xu, X.; Zeng, Q.; Liang, J.; Chen, X. Hypoxia-activated prodrugs and redox-responsive nanocarriers. Int. J. Nanomed. 2018, 13, 6551–6574. [Google Scholar] [CrossRef]
- Sibiya, M.A.; Raphoko, L.; Mangokoana, D.; Makola, R.; Nxumalo, W.; Matsebatlela, T.M. Induction of cell death in human A549 cells using 3-(quinoxaline-3-yl)prop-2-ynyl methanosulphonate and 3-(quinoxaline-3-yl)prop-2-yn-1-ol. Molecules 2019, 24, 407–423. [Google Scholar] [CrossRef]
- Zamudio-Vazquez, R.; Ivanova, S.; Moreno, M.; Hernandez-Alvarez, M.I.; Giralt, E.; Bidon-Chanal, A.; Zorzano, A.; Albericio, F.; Tulla-Puche, J. A new quinoxaline-containing peptide induces apoptosis in cancer cells by autophagy modulation. Chem. Sci. 2015, 6, 4537–4549. [Google Scholar] [CrossRef]
- El-Khatib, M.; Geara, F.; Haddadin, M.J.; Gali-Muhtasib, H. Cell death by the quinoxaline dioxide DCQ in human colon cancer cells is enhanced under hypoxia and is independent of p53 and p21. Radiat. Oncol. 2010, 5, 107. [Google Scholar] [CrossRef]
- Li, D.; Dai, C.; Yang, X.; Li, B.; Xiao, X.; Tang, S. GADD45a regulates olaquindox-induced DNA damage and S-phase arrest in human hepatoma G2 cells via JNK/p38 pathways. Molecules 2017, 22, 124. [Google Scholar] [CrossRef]
- Chacón-Vargas, K.; Nogueda-Torres, B.; Sánchez-Torres, L.; Suarez-Contreras, E.; Villalobos-Rocha, J.; Torres-Martinez, Y.; Lara-Ramirez, E.E.; Fiorani, G.; Krauth-Siegel, R.L.; Bolognesi, M.L.; et al. Trypanocidal activity of quinoxaline 1,4-di-N-oxide derivatives as Trypanothione reductase inhibitors. Molecules 2017, 22, 220. [Google Scholar] [CrossRef] [PubMed]
- Soto-Sánchez, J.; Caro-Gómez, L.A.; Paz-González, A.D.; Marchat, L.A.; Rivera, G.; Moo-Puc, R.; Arias, D.G.; Ramírez-Moreno, E. Biological activity of esters of quinoxaline-7-carboxylate 1,4-di-N-oxide against E. histolytica and their analysis as potential thioredoxin reductase inhibitors. Parasitol. Res. 2020, 119, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Norris, L.C. The significant advances of the past fifty years in poultry nutrition. Poult. Sci. 1958, 37, 256–274. [Google Scholar] [CrossRef]
- Cheng, G.; Li, B.; Wang, C.; Zhang, H.; Liang, G.; Weng, Z.; Hao, H.; Wang, X.; Liu, Z.; Dai, M.; et al. Systematic and molecular basis of the antibacterial action of quinoxaline 1,4-di-N-oxides against Escherichia coli. PLoS ONE 2015, 10, e0136450. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.-J.; Zheng, Q.-F.; Huang, X.-J.; Wang, X.; Ihsan, A. Potential benefits of quinoxaline 1,4-dioxides in aldosterone dysmetabolism disease—A medical hypothesis. J. Anim. Sci. 2011, 1, 121–127. [Google Scholar] [CrossRef][Green Version]
- Butaye, P.; Devriese, L.A.; Haesebrouck, F. Antimicrobial growth promoters used in animal feed: Effects of less well known antibiotics on Gram-positive bacteria. Clin. Microbiol. Rev. 2003, 16, 175–188. [Google Scholar] [CrossRef]
- Yen, J.T.; Nienaber, J.A.; Pond, W.G.; Varel, V.H. Effect of carbadox on growth, fasting metabolism, thyroid function and gastrointestinal tract in young pigs. J. Nutr. 1985, 115, 970–979. [Google Scholar] [CrossRef]
- Yen, J.T.; Pond, W.G. Effects of carbadox, copper, or Yucca shidigera extract on growth performance and visceral weight of young pigs. J. Anim. Sci. 1993, 71, 2140–2146. [Google Scholar] [CrossRef]
- Constable, P.; Hinchcliff, K.W.; Done, S.; Gruenberg, W. Practical Antimicrobial Therapeutics, 11th ed.; Saunders Ltd.: Nottingham, UK, 2017; pp. 153–174. [Google Scholar] [CrossRef]
- Yang, B.; Huang, L.; Wang, Y.; Liu, Y.; Tao, Y.; Chen, D.; Liu, Z.; Fang, K.; Chen, Y.; Yuan, Z. Residue depletion and tissue-plasma correlation of methyl-3-quinoxaline-2-carboxylic acid after dietary administration of Olaquindox in pigs. J. Agric. Food Chem. 2010, 58, 937–942. [Google Scholar] [CrossRef]
- Čihák, R.; Vontorková, M. Cytogenetic effects of quinoxaline-1,4-dioxide-type growth-promoting agents. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1983, 117, 311–316. [Google Scholar] [CrossRef]
- Huang, L.; Yin, F.; Pan, Y.; Chen, D.; Li, J.; Wan, D.; Liu, Z.; Yuan, Z. Metabolism, distribution, and elimination of mequindox in pigs, chickens, and rats. J. Agric. Food Chem. 2015, 63, 9839–9849. [Google Scholar] [CrossRef]
- Li, J.S.; Zhao, R.C.; Lu, R.H. Quincetone growth promoter in fat chicklings. Ind. Vet. J. 2005, 82, 1149–1151. [Google Scholar]
- Hao, H.; Guo, W.; Iqbal, Z.; Cheng, G.; Wang, X.; Dai, M.; Huang, L.; Wang, Y.; Peng, D.; Liu, Z.; et al. Impact of cyadox on human colonic microflora in chemostat models. Regul. Toxicol. Pharmacol. 2013, 67, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Cerecetto, H.; Dias, E.; Di Maio, R.; Gonzalez, M.; Pacce, S.; Saenz, P.; Seoane, G.; Suescun, L.; Mombru, A.; Fernandez, G.; et al. Synthesis and herbicidal activity of N-oxide derivatives. J. Agric. Food Chem. 2000, 48, 2995–3002. [Google Scholar] [CrossRef] [PubMed]
- Negishi, T.; Tanaka, K.; Hayatsu, H. Mutagenicity of carbadox and several quinoxaline 1,4-dioxide derivatives. Chem. Pharm. Bull. 1980, 28, 1347–1349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cihak, R.; Srb, V. Cytogenetic effects of quinoxaline-1,4-dioxide-type growth-promoting agents: I. Micronucleus test in rats. Mutat. Res. Genet. Toxicol. 1983, 116, 129–135. [Google Scholar] [CrossRef]
- Yoshimura, H. Teratogenic assessment of carbadox in rats. Toxicol. Lett. 2002, 129, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Fonshteĭn, L.M.; Revazova, I.A.; Zolotareva, G.N.; Abilev, S.K.; Akin’shina, L.N. Mutagenic activity of dioxydine. Genetika 1978, 14, 900–908. [Google Scholar]
- Elina, A.S.; Musatova, I.S.; Padeiskaya, E.N.; Shvarts, Y.G. Esters of 2,3-bis(hydroxymethyl)quinoxaline 1,4-di-N-oxide and their biological action. Pharm. Chem. J. 1977, 11, 781–785. [Google Scholar] [CrossRef]
- Fuchs, T.; Gates, K.S.; Hwang, J.T.; Greenberg, M.M. Photosensitization of guanine-specific DNA damage by a cyano-substituted quinoxaline di-N-oxide. Chem. Res. Toxicol. 1999, 12, 1190–1194. [Google Scholar] [CrossRef]

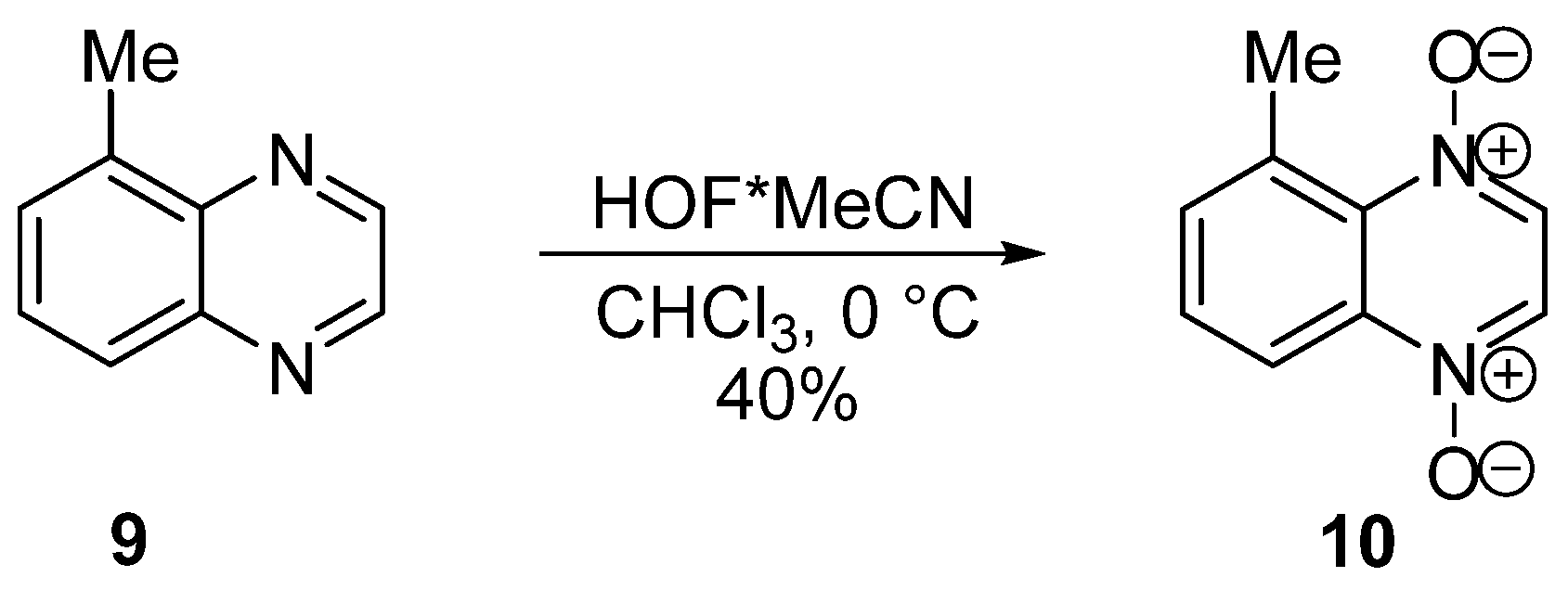






















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buravchenko, G.I.; Shchekotikhin, A.E. Quinoxaline 1,4-Dioxides: Advances in Chemistry and Chemotherapeutic Drug Development. Pharmaceuticals 2023, 16, 1174. https://doi.org/10.3390/ph16081174
Buravchenko GI, Shchekotikhin AE. Quinoxaline 1,4-Dioxides: Advances in Chemistry and Chemotherapeutic Drug Development. Pharmaceuticals. 2023; 16(8):1174. https://doi.org/10.3390/ph16081174
Chicago/Turabian StyleBuravchenko, Galina I., and Andrey E. Shchekotikhin. 2023. "Quinoxaline 1,4-Dioxides: Advances in Chemistry and Chemotherapeutic Drug Development" Pharmaceuticals 16, no. 8: 1174. https://doi.org/10.3390/ph16081174
APA StyleBuravchenko, G. I., & Shchekotikhin, A. E. (2023). Quinoxaline 1,4-Dioxides: Advances in Chemistry and Chemotherapeutic Drug Development. Pharmaceuticals, 16(8), 1174. https://doi.org/10.3390/ph16081174