Efficacy of Ketamine with and without Lamotrigine in Treatment-Resistant Depression: A Preliminary Report
Abstract
:1. Introduction
2. Methods
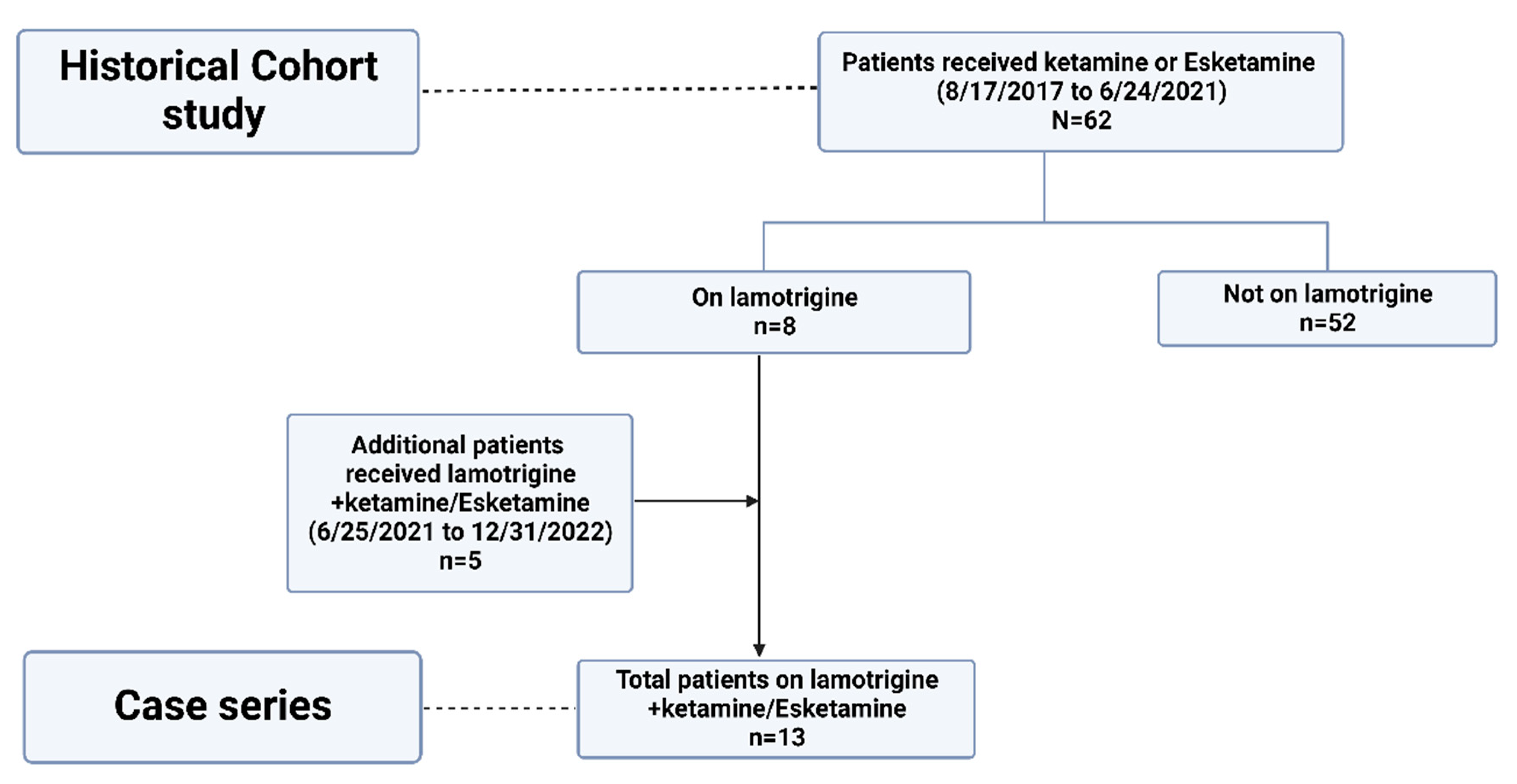
2.1. Historical Cohort Study
2.2. Case Series
2.3. Statistical Analysis
3. Results
3.1. Historical Cohort Study
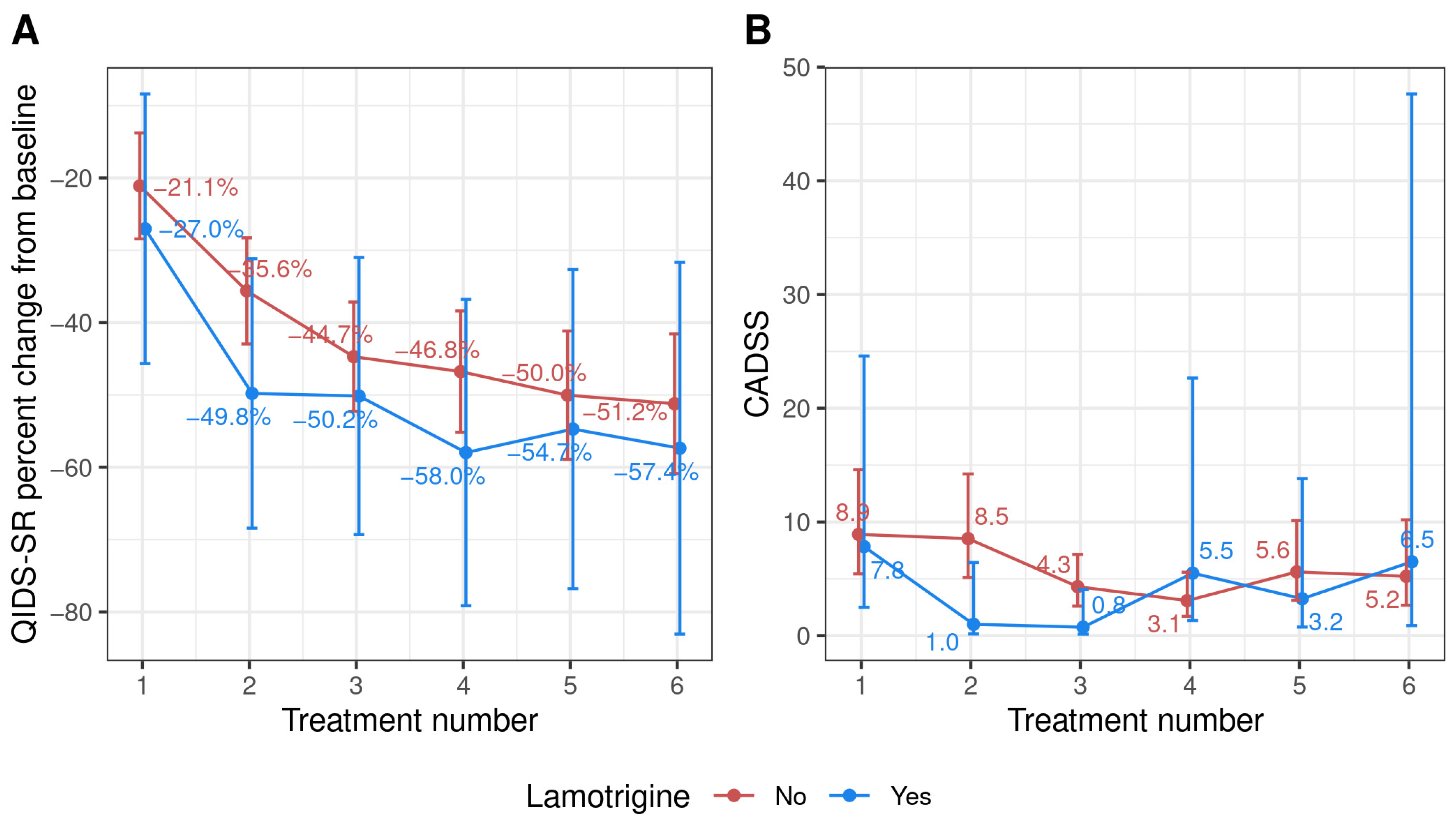
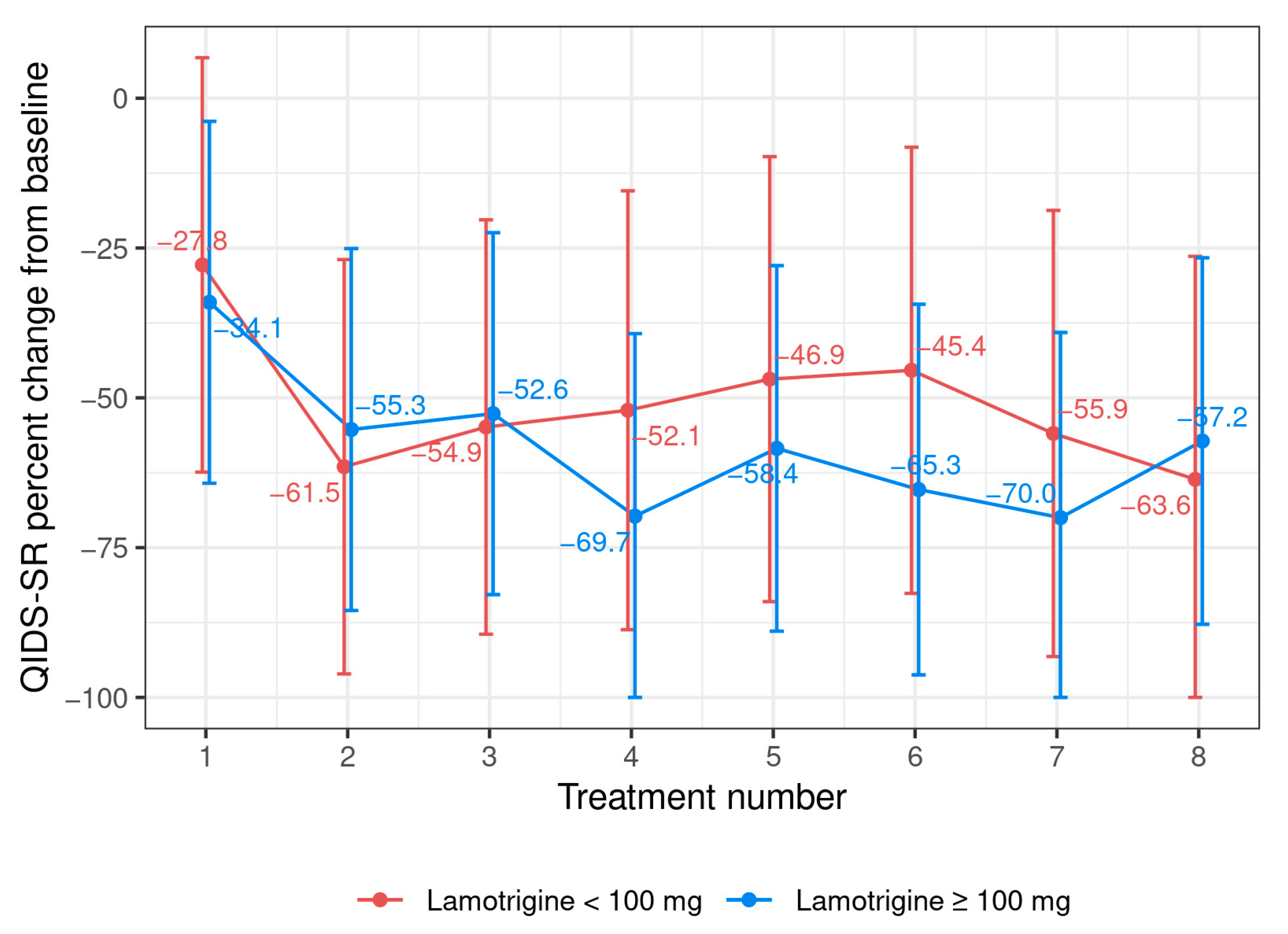
3.2. Case Series
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, B.; Vande Voort, J.L.; Frye, M.A.; Kung, S. Can ketamine be a safe option for treatment-resistant bipolar depression? Expert Opin. Drug Saf. 2022, 21, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Vande Voort, J.L.; Riva-Posse, P.; Pazdernik, V.M.; Frye, M.A.; Tye, S.J. Ketamine-Associated Change in Anhedonia and mTOR Expression in Treatment-Resistant Depression. Biol. Psychiatry 2023, 93, e65–e68. [Google Scholar] [CrossRef] [PubMed]
- Zarate, C.A.; Niciu, M.J. Ketamine for depression: Evidence, challenges and promise. World Psychiatry 2015, 14, 348–350. [Google Scholar] [CrossRef]
- Joseph, B.; Parsaik, A.K.; Ahmed, A.T.; Erwin, P.J.; Singh, B. A Systematic Review on the Efficacy of Intravenous Racemic Ketamine for Bipolar Depression. J. Clin. Psychopharmacol. 2021, 41, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Singh, B. Ketamine for Treatment-Resistant bipolar depression—A reality check! Bipolar Disord. 2023, 25, 247–248. [Google Scholar] [CrossRef]
- Large, C.H.; Webster, E.L.; Goff, D.C. The potential role of lamotrigine in schizophrenia. Psychopharmacology 2005, 181, 415–436. [Google Scholar] [CrossRef]
- Kumar, R.; Nunez, N.A.; Prokop, L.J.; Veldic, M.; Betcher, H.; Singh, B. Association of Optimal Lamotrigine Serum Levels and Therapeutic Efficacy in Mood Disorders: A Systematic Review. J. Clin. Psychopharmacol. 2021, 41, 681–686. [Google Scholar] [CrossRef]
- Wilkowska, A.; Wiglusz, M.S.; Jakuszkowiak-Wojten, K.; Cubala, W.J. Ketamine and Lamotrigine Combination in Psychopharmacology: Systematic Review. Cells 2022, 11, 645. [Google Scholar] [CrossRef]
- Anand, A.; Charney, D.S.; Oren, D.A.; Berman, R.M.; Hu, X.S.; Cappiello, A.; Krystal, J.H. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: Support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch. Gen. Psychiatry 2000, 57, 270–276. [Google Scholar] [CrossRef]
- Deakin, J.F.; Lees, J.; McKie, S.; Hallak, J.E.; Williams, S.R.; Dursun, S.M. Glutamate and the neural basis of the subjective effects of ketamine: A pharmaco-magnetic resonance imaging study. Arch. Gen. Psychiatry 2008, 65, 154–164. [Google Scholar] [CrossRef]
- Mathew, S.J.; Murrough, J.W.; aan het Rot, M.; Collins, K.A.; Reich, D.L.; Charney, D.S. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: A pilot randomized, placebo-controlled continuation trial. Int. J. Neuropsychopharmacol. 2010, 13, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, C.G.; Averill, C.L.; Salas, R.; Averill, L.A.; Baldwin, P.R.; Krystal, J.H.; Mathew, S.J.; Mathalon, D.H. Prefrontal Connectivity and Glutamate Transmission: Relevance to Depression Pathophysiology and Ketamine Treatment. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Kornhall, D.; Nielsen, E.W. Failure of ketamine anesthesia in a patient with lamotrigine overdose. Case Rep. Crit. Care 2014, 2014, 916360. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Rosenblat, J.D.; Nemeroff, C.B.; Sanacora, G.; Murrough, J.W.; Berk, M.; Brietzke, E.; Dodd, S.; Gorwood, P.; Ho, R.; et al. Synthesizing the Evidence for Ketamine and Esketamine in Treatment-Resistant Depression: An International Expert Opinion on the Available Evidence and Implementation. Am. J. Psychiatry 2021, 178, 383–399. [Google Scholar] [CrossRef]
- Singh, B.; Kung, S.; Pazdernik, V.; Schak, K.M.; Geske, J.; Schulte, P.J.; Frye, M.A.; Vande Voort, J.L. Comparative Effectiveness of Intravenous Ketamine and Intranasal Esketamine in Clinical Practice Among Patients with Treatment-Refractory Depression: An Observational Study. J. Clin. Psychiatry 2023, 84, 45331. [Google Scholar] [CrossRef]
- Singh, B.; Bobo, W.V.; Rasmussen, K.G.; Stoppel, C.J.; Rico, J.A., Jr.; Schak, K.M.; Biernacka, J.M.; Frye, M.A.; Vande Voort, J.L. The Association between Body Mass Index and Remission Rates in Patients with Treatment-Resistant Depression Who Received Intravenous Ketamine. J. Clin. Psychiatry 2019, 80, 19l12852. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.D.; Krystal, J.H.; Putnam, F.W.; Southwick, S.M.; Marmar, C.; Charney, D.S.; Mazure, C.M. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J. Trauma Stress 1998, 11, 125–136. [Google Scholar] [CrossRef]
- Frye, M.A.; Blier, P.; Tye, S.J. Concomitant benzodiazepine use attenuates ketamine response: Implications for large scale study design and clinical development. J. Clin. Psychopharmacol. 2015, 35, 334–336. [Google Scholar] [CrossRef]
- Rush, A.J.; Trivedi, M.H.; Ibrahim, H.M.; Carmody, T.J.; Arnow, B.; Klein, D.N.; Markowitz, J.C.; Ninan, P.T.; Kornstein, S.; Manber, R.; et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol. Psychiatry 2003, 54, 573–583. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: http://www.R-project.org/2016 (accessed on 5 May 2023).
- O’Brien, B.; Wilkinson, S.T.; Mathew, S.J. An Update on Community Ketamine Practices. Am. J. Psychiatry 2022, 179, 393–394. [Google Scholar] [CrossRef]
- Daly, E.J.; Trivedi, M.H.; Janik, A.; Li, H.; Zhang, Y.; Li, X.; Lane, R.; Lim, P.; Duca, A.R.; Hough, D.; et al. Efficacy of Esketamine Nasal Spray Plus Oral Antidepressant Treatment for Relapse Prevention in Patients with Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry 2019, 76, 893–903. [Google Scholar] [CrossRef]
- McMullen, E.P.; Lee, Y.; Lipsitz, O.; Lui, L.M.W.; Vinberg, M.; Ho, R.; Rodrigues, N.B.; Rosenblat, J.D.; Cao, B.; Gill, H.; et al. Strategies to Prolong Ketamine’s Efficacy in Adults with Treatment-Resistant Depression. Adv. Ther. 2021, 38, 2795–2820. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, S.; Takamiya, A.; Noda, Y.; Horita, N.; Wada, M.; Tsugawa, S.; Plitman, E.; Sano, Y.; Tarumi, R.; ElSalhy, M.; et al. Glutamatergic neurometabolite levels in major depressive disorder: A systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol. Psychiatry 2019, 24, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Price, R.B.; Shungu, D.C.; Mao, X.; Nestadt, P.; Kelly, C.; Collins, K.A.; Murrough, J.W.; Charney, D.S.; Mathew, S.J. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: Relationship to treatment resistance in major depressive disorder. Biol. Psychiatry 2009, 65, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Ballard, E.D.; Zarate, C.A., Jr. The role of dissociation in ketamine’s antidepressant effects. Nat. Commun. 2020, 11, 6431. [Google Scholar] [CrossRef] [PubMed]
- Haarsma, J.; Harmer, C.J.; Tamm, S. A continuum hypothesis of psychotomimetic rapid antidepressants. Brain Neurosci. Adv. 2021, 5, 23982128211007772. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Port, J.D.; Pazdernik, V.; Coombes, B.J.; Vande Voort, J.L.; Frye, M.A. Racemic ketamine treatment attenuates anterior cingulate cortex GABA deficits among remitters in treatment-resistant depression: A pilot study. Psychiatry Res. Neuroimaging 2022, 320, 111432. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Port, J.D.; Pazdernik, V.; Vande Voort, J.L.; Coombes, B.J.; Geske, J.R.; Lanza, I.R.; Morgan, R.J.; Frye, M.A. A preliminary study of the association of increased anterior cingulate gamma-aminobutyric acid with remission of depression after ketamine administration. Psychiatry Res. 2021, 301, 113953. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Georgiou, P.; Fischell, J.; Elmer, G.I.; Alkondon, M.; Yuan, P.; Pribut, H.J.; Singh, N.S.; et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016, 533, 481–486. [Google Scholar] [CrossRef]
- Williams, N.R.; Heifets, B.D.; Bentzley, B.S.; Blasey, C.; Sudheimer, K.D.; Hawkins, J.; Lyons, D.M.; Schatzberg, A.F. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol. Psychiatry 2019, 24, 1779–1786. [Google Scholar] [CrossRef]
- Stenovec, M. Ketamine Alters Functional Plasticity of Astroglia: An Implication for Antidepressant Effect. Life 2021, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Zanos, P.; Gould, T.D. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 2018, 23, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.W.; Marwood, L.; Oprea, E.; DeAngel, V.; Mather, S.; Valentini, B.; Zahn, R.; Young, A.H.; Cleare, A.J. Pharmacological Augmentation in Unipolar Depression: A Guide to the Guidelines. Int. J. Neuropsychopharmacol. 2020, 23, 587–625. [Google Scholar] [CrossRef] [PubMed]
- Lapidus, K.A.; Soleimani, L.; Murrough, J.W. Novel glutamatergic drugs for the treatment of mood disorders. Neuropsychiatr. Dis. Treat. 2013, 9, 1101–1112. [Google Scholar] [CrossRef]
- Barbee, J.G.; Jamhour, N.J. Lamotrigine as an augmentation agent in treatment-resistant depression. J. Clin. Psychiatry 2002, 63, 737–741. [Google Scholar] [CrossRef]
- Barbee, J.G.; Thompson, T.R.; Jamhour, N.J.; Stewart, J.W.; Conrad, E.J.; Reimherr, F.W.; Thompson, P.M.; Shelton, R.C. A double-blind placebo-controlled trial of lamotrigine as an antidepressant augmentation agent in treatment-refractory unipolar depression. J. Clin. Psychiatry 2011, 72, 1405–1412. [Google Scholar] [CrossRef]
- Coulter, D.A. Antiepileptic drug cellular mechanisms of action: Where does lamotrigine fit in? J. Child Neurol. 1997, 12 (Suppl. S1), S2–S9. [Google Scholar] [CrossRef]
- Doyle, O.M.; De Simoni, S.; Schwarz, A.J.; Brittain, C.; O’Daly, O.G.; Williams, S.C.; Mehta, M.A. Quantifying the attenuation of the ketamine pharmacological magnetic resonance imaging response in humans: A validation using antipsychotic and glutamatergic agents. J. Pharmacol. Exp. Ther. 2013, 345, 151–160. [Google Scholar] [CrossRef]
- Chan, L.F.; Eu, C.L.; Soh, S.Y.; Maniam, T.; Shahidii Kadir, Z.; Chong, B.T.W.; Loo, J.L.; Sharip, S.; Wong, V.C.W.; Loo, T.H.; et al. Is Ketamine the Future Clozapine for Depression? A Case Series and Literature Review on Maintenance Ketamine in Treatment-resistant Depression with Suicidal Behavior. J. Psychiatr. Pract. 2018, 24, 279–291. [Google Scholar] [CrossRef]
- Wilkowska, A.; Wlodarczyk, A.; Galuszko-Wegielnik, M.; Wiglusz, M.S.; Cubala, W.J. Intravenous Ketamine Infusions in Treatment-Resistant Bipolar Depression: An Open-Label Naturalistic Observational Study. Neuropsychiatr. Dis. Treat. 2021, 17, 2637–2646. [Google Scholar] [CrossRef]
- Wlodarczyk, A.; Cubala, W.J.; Galuszko-Wegielnik, M.; Szarmach, J. Central nervous system-related safety and tolerability of add-on ketamine to antidepressant medication in treatment-resistant depression: Focus on the unique safety profile of bipolar depression. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211011021. [Google Scholar] [CrossRef]
- Kuzniecky, R.; Ho, S.; Pan, J.; Martin, R.; Gilliam, F.; Faught, E.; Hetherington, H. Modulation of cerebral GABA by topiramate, lamotrigine, and gabapentin in healthy adults. Neurology 2002, 58, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Kantrowitz, J.T.; Dong, Z.; Milak, M.S.; Rashid, R.; Kegeles, L.S.; Javitt, D.C.; Lieberman, J.A.; John Mann, J. Ventromedial prefrontal cortex/anterior cingulate cortex Glx, glutamate, and GABA levels in medication-free major depressive disorder. Transl. Psychiatry 2021, 11, 419. [Google Scholar] [CrossRef] [PubMed]
- Vande Voort, J.L.; Morgan, R.J.; Kung, S.; Rasmussen, K.G.; Rico, J.; Palmer, B.A.; Schak, K.M.; Tye, S.J.; Ritter, M.J.; Frye, M.A.; et al. Continuation phase intravenous ketamine in adults with treatment-resistant depression. J. Affect. Disord. 2016, 206, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.G.; Lineberry, T.W.; Galardy, C.W.; Kung, S.; Lapid, M.I.; Palmer, B.A.; Ritter, M.J.; Schak, K.M.; Sola, C.L.; Hanson, A.J.; et al. Serial infusions of low-dose ketamine for major depression. J. Psychopharmacol. 2013, 27, 444–450. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | No (n = 54) | Yes (n = 8) | Total (n = 62) | p Value |
|---|---|---|---|---|
| Age | 0.97 (1) | |||
| - Mean (SD) | 47.1 (12.3) | 47.2 (12.1) | 47.1 (12.1) | |
| - Range | 19.9–68.4 | 29.4–63.1 | 19.9–68.4 | |
| Sex (Female) | 34 (63.0%) | 6 (75.0%) | 40 (64.5%) | 0.51 (2) |
| BMI | 0.93 (1) | |||
| - Mean (SD) | 29.5 (6.1) | 29.3 (6.1) | 29.5 (6.0) | |
| - Range | 20.8–50.9 | 22.1–39.6 | 20.8–50.9 | |
| Employed—Baseline | 0.50 (2) | |||
| - Unemployed | 2 (3.7%) | 1 (12.5%) | 3 (4.8%) | |
| - Employed | 30 (55.6%) | 3 (37.5%) | 33 (53.2%) | |
| - Disability due to depression | 12 (22.2%) | 3 (37.5%) | 15 (24.2%) | |
| - Homemaker/retired/student | 10 (18.5%) | 1 (12.5%) | 11 (17.7%) | |
| Diagnosis | 0.27 (2) | |||
| - MDD | 52 (96.3%) | 7 (87.5%) | 59 (95.2%) | |
| - Bipolar-II disorder | 1 (1.9%) | 1 (12.5%) | 2 (3.2%) | |
| - Bipolar-I disorder | 1 (1.9%) | 0 (0.0%) | 1 (1.6%) | |
| Duration of depressive episode (years) | 0.47 (1) | |||
| - Mean (SD) | 5.5 (6.5) | 3.9 (3.1) | 5.3 (6.1) | |
| - Range | 0.3–37.0 | 0.8–10.0 | 0.3–37.0 | |
| Comorbidities, n (%) | ||||
| PTSD | 5 (9.3%) | 1 (12.5%) | 6 (9.7%) | 0.77 (2) |
| GAD/anxiety disorders | 34 (63.0%) | 5 (62.5%) | 39 (62.9%) | 0.98 (2) |
| Fibromyalgia/chronic pain | 6 (11.1%) | 1 (12.5%) | 7 (11.3%) | 0.91 (2) |
| OCD | 2 (3.7%) | 0 (0.0%) | 2 (3.2%) | 0.58 (2) |
| Eating disorder | 3 (5.6%) | 0 (0.0%) | 3 (4.8%) | 0.49 (2) |
| Borderline personality disorder | 5 (9.3%) | 0 (0.0%) | 5 (8.1%) | 0.37 (2) |
| History of substance use disorder | 8 (14.8%) | 0 (0.0%) | 8 (12.9%) | 0.24 (2) |
| Intranasal Esketamine | 14 (25.9%) | 1 (12.5%) | 15 (24.2%) | 0.41 (2) |
| Baseline QIDS-SR | 0.47 (1) | |||
| - Mean (SD) | 17.5 (3.4) | 18.5 (3.9) | 17.7 (3.5) | |
| - Range | 9.0–24.0 | 11.0–24.0 | 9.0–24.0 | |
| QIDS-SR change from baseline to post-infusion #1 | 0.52 (1) | |||
| - Mean (SD) | −4.0 (4.6) | −5.1 (4.2) | −4.1 (4.6) | |
| - Range | −16.0–4.0 | −9.0–3.0 | −16.0–4.0 | |
| QIDS-SR percent change from baseline to post-infusion #1 | 0.57 (1) | |||
| - Mean (SD) | −23.3 (27.2) | −29.1 (26.7) | −24.1 (27.0) | |
| - Range | −81.2–26.7 | −63.6–18.8 | −81.2–26.7 | |
| Response | 31 (57.4%) | 5 (62.5%) | 36 (58.1%) | 0.79 (2) |
| Remission | 21 (38.9%) | 3 (37.5%) | 24 (38.7%) | 0.94 (2) |
| CADSS score, mean (SE) a | 6.08 (1.06) | 3.55 (2.65) | 5.73 (0.98) | 0.38 (3) |
| Change in the vitals | ||||
| Oxygen saturation, mean (SE) a | ||||
| Change at 40 min b | 0.05 (0.36) | 0.61 (0.88) | 0.13 (0.33) | 0.56 (3) |
| Change to endpoint c | −0.07 (0.37) | 0.63 (0.91) | 0.03 (0.34) | 0.48 (3) |
| Systolic BP, mean (SE) a | ||||
| Change at 40 min b | 5.41 (1.19) | 5.52 (2.93) | 5.41 (1.09) | 0.97 (3) |
| Change to endpoint c | 3.67 (1.22) | 5.63 (2.99) | 3.94 (1.12) | 0.55 (3) |
| Diastolic BP, mean (SE) a | ||||
| Change at 40 min b | 2.92 (0.73) | 2.19 (1.86) | 2.82 (0.67) | 0.72 (3) |
| Change to endpoint c | 2.16 (0.76) | 0.20 (1.95) | 1.90 (0.71) | 0.36 (3) |
| Heart rate, mean (SE) a | ||||
| Change at 40 min b | −0.41 (0.82) | 0.77 (2.01) | −0.24 (0.76) | 0.59 (3) |
| Change to endpoint c | −1.05 (0.86) | 0.46 (2.11) | −0.84 (0.80) | 0.51 (3) |
| Current # of psychotropics | 0.02 (1) | |||
| - Mean (SD) | 3.5 (1.4) | 4.8 (1.0) | 3.7 (1.4) | |
| - Range | 1.0–7.0 | 3.0–6.0 | 1.0–7.0 | |
| Individual psychotropic/class, n (%) | ||||
| SSRI | 15 (27.8%) | 1 (12.5%) | 16 (25.8%) | 0.36 (2) |
| SNRI | 18 (33.3%) | 3 (37.5%) | 21 (33.9%) | 0.82 (2) |
| TCA | 7 (13.0%) | 1 (12.5%) | 8 (12.9%) | 0.97 (2) |
| MAOIs | 2 (3.7%) | 0 (0.0%) | 2 (3.2%) | 0.58 (2) |
| Antipsychotics | 15 (27.8%) | 5 (62.5%) | 20 (32.3%) | 0.05 (2) |
| Mirtazapine | 5 (9.3%) | 0 (0.0%) | 5 (8.1%) | 0.37 (2) |
| Bupropion | 14 (25.9%) | 2 (25.0%) | 16 (25.8%) | 0.96 (2) |
| Stimulant | 12 (22.2%) | 2 (25.0%) | 14 (22.6%) | 0.86 (2) |
| Trazodone | 16 (29.6%) | 3 (37.5%) | 19 (30.6%) | 0.65 (2) |
| Gabapentin | 6 (11.1%) | 1 (12.5%) | 7 (11.3%) | 0.91 (2) |
| GABA-positive allosteric modulators | 27 (50.0%) | 5 (62.5%) | 32 (51.6%) | 0.51 (2) |
| Neuromodulation (tried in current episode) | ||||
| ECT | 16 (29.6%) | 3 (37.5%) | 19 (30.6%) | 0.65 (2) |
| TMS | 8 (14.8%) | 2 (25.0%) | 10 (16.1%) | 0.47 (2) |
| Patient | Age | Sex | BMI | Current Psychiatric Diagnosis | Concurrent Psychiatric Medications | Duration of Current Depressive Episode | Failed Interventions in Current Episode | IV Ketamine/ In Esketamine | Baseline QIDS-SR 16 Score | Response (Yes/No) | Remission (Yes/No) | Continued Ketamine/Esketamine after the Acute Phase |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 44 | F | 21.32 | MDD, GAD | Aripiprazole (2 mg/day), Venlafaxine-XR (375 mg/day), Lamotrigine (250 mg/day), Clonazepam (0.5 mg TID) | 5 years | Citalopram | IV ketamine | 19 | Yes | Yes | Yes |
| 2. | 61 | F | 33.27 | MDD, PTSD | Lurasidone (20 mg/day), Trazodone (75 mg/day), Lamotrigine (25 mg/day), Vilazodone (35 mg/day) | 10 years | Venlafaxine XR, Bupropion XL, Modafinil, Aripiprazole | IV, switched to IN esketamine after 3 treatments | 20 | Yes | No | Yes—Esketamine |
| 3. | 63 | M | 39.93 | BD-II, GAD | Bupropion (150 mg/day), Vortioxetine (10 mg/day), Lamotrigine (100 mg/day), Dextroamphetamine–Amphetamine (20 mg/day), Lorazepam (2 mg/day) | 5 years | Lithium, Venlafaxine, Quetiapine, Lurasidone, Brexpiprazole, Valproate, Carbamazepine, Olanzapine, Aripiprazole, Fluoxetine, Citalopram, Escitalopram, Sertraline, Nefazodone, Duloxetine, Vilazodone, Tranylcypromine, Phenelzine, Imipramine, ECT, TMS | IV ketamine | 15 | Yes | Yes | Yes |
| 4. | 54 | F | 27.98 | MDD, PTSD | Aripiprazole (5 mg/day), Duloxetine (120 mg/day), Lamotrigine (100 mg/day), Alprazolam (1.5 mg/day) | 5 years | Fluoxetine, Trazodone, Modafinil, Bupropion | IV ketamine | 18 | Yes | Yes | Yes |
| 5. | 36 | F | 27.05 | MDD, GAD | Nortriptyline (50 mg/day), Trazodone (50 mg/day), Lamotrigine (150 mg/day), Clonazepam (0.5 mg BID), Gabapentin (300 mg/day), Melatonin (10 mg/day) | 1 year | Bupropion, Duloxetine, ECT, Quetiapine | IV ketamine | 22 | Yes | No | No. Lost effectiveness after discontinuing nortriptyline (due to mild weight gain) |
| 6. | 38 | M | 33.3 | MDD, OCD, GAD, Personality disorder | Fluvoxamine (300 mg/day), Brexpiprazole (3 mg/day), Lamotrigine (25 mg/day) | 2 years | ECT, Dextroamphetamine–Amphetamine, Bupropion, Clomipramine, Lorazepam | IV ketamine | 16 | No | No | No. Lack of response |
| 7. | 29 | F | 29.57 | MDD, GAD, ADHD | Bupropion-XL (300 mg/day), Lamotrigine (125 mg BID), Quetiapine (25 mg/day), Dextroamphetamine-Amphetamine (25 mg/day) | 2 years | Sertraline, Melatonin | IV ketamine | 24 | Yes | No | Yes. Moved out of the state. |
| 8. | 48 | F | 22.11 | MDD, GAD | Iloperidone (3 mg/day), Vilazodone (10 mg/day), Lamotrigine (225 mg BID), Clonazepam (1 mg/day), Trazodone (100 mg/day), Buspirone (30 mg/day), clonidine 0.2 mg QHS | 0.8 years | TMS, IM ketamine | IV ketamine | 18 | No | No | No—Lack of response |
| 9. | 45 | F | 18.36 | MDD | Venlafaxine (31.25 mg/day), Sertraline (150 mg/day), Lamotrigine (25 mg/day) | 3 years | Aripiprazole, Quetiapine, Cariprazine, Lithium | IV ketamine | 15 | Yes | Yes | Yes |
| 10. | 23 | F | 33.4 | MDD with Anxious Distress | Vortioxetine (10 mg/day), Bupropion (150 mg/day), Lamotrigine (200 mg/day) | 1.25 years | Venlafaxine, Duloxetine, TMS, ECT, Quetiapine, Fluvoxamine | IN esketamine | 21 | No | No | No. Lack of response. Had side effects—nausea, vomiting, dissociations. Discontinued after 5 treatments |
| 11. | 43 | F | 30 | BD-II, GAD, Alcohol use disorder, in sustained remission | Cariprazine (3 mg/day), Duloxetine (80 mg/day), Lamotrigine (100 mg QAM, 200 mg QHS), Gabapentin (1200 mg TID) | 4.5 years | Lithium, Quetiapine, Lurasidone, Buspirone, Olanzapine, Dextroamphetamine-Amphetamine | IV ketamine | 23 | Yes | Yes | Yes |
| 12. | 43 | F | 25.23 | MDD, GAD, Borderline Personality Disorder | Fluoxetine (20 mg/day), Bupropion-XL (450 mg/day), Buspirone (60 mg/day), Lamotrigine (100 mg/day), Trazodone (100 mg/day), Hydroxyzine (25–50 mg TID) | 17 years | Venlafaxine, Duloxetine, Lisdexamfetamine, Risperidone | IV ketamine | 15 | No | No | No. Lack of response. Discontinued all the psychotropics after 2 treatments. |
| 13. | 46 | F | 33 | MDD, GAD | Clonazepam (2 mg/day), Pramipexole (0.5 mg/day), Lamotrigine (50 mg/day), Protriptyline (45 mg/day) | 10 years | Aripiprazole, Duloxetine, Venlafaxine, Bupropion, Buspirone, Trazodone, Vortioxetine, Oxcarbazepine, Selegiline, Lithium, ECT | IV, switched to IN esketamine after 3 treatments | 22 | Yes | Yes | No. Initial response with IV ketamine. Switched to esketamine but did not find prolonged benefit. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, B.; Nunez, N.A.; Kung, S.; Vande Voort, J.L.; Pazdernik, V.K.; Schak, K.M.; Boehm, S.M.; Carpenter, B.; Johnson, E.K.; Malyshev, G.; et al. Efficacy of Ketamine with and without Lamotrigine in Treatment-Resistant Depression: A Preliminary Report. Pharmaceuticals 2023, 16, 1164. https://doi.org/10.3390/ph16081164
Joseph B, Nunez NA, Kung S, Vande Voort JL, Pazdernik VK, Schak KM, Boehm SM, Carpenter B, Johnson EK, Malyshev G, et al. Efficacy of Ketamine with and without Lamotrigine in Treatment-Resistant Depression: A Preliminary Report. Pharmaceuticals. 2023; 16(8):1164. https://doi.org/10.3390/ph16081164
Chicago/Turabian StyleJoseph, Boney, Nicolas A. Nunez, Simon Kung, Jennifer L. Vande Voort, Vanessa K. Pazdernik, Kathryn M. Schak, Stacey M. Boehm, Brooke Carpenter, Emily K. Johnson, Grigoriy Malyshev, and et al. 2023. "Efficacy of Ketamine with and without Lamotrigine in Treatment-Resistant Depression: A Preliminary Report" Pharmaceuticals 16, no. 8: 1164. https://doi.org/10.3390/ph16081164
APA StyleJoseph, B., Nunez, N. A., Kung, S., Vande Voort, J. L., Pazdernik, V. K., Schak, K. M., Boehm, S. M., Carpenter, B., Johnson, E. K., Malyshev, G., Smits, N., Adewunmi, D. O., Brown, S. K., & Singh, B. (2023). Efficacy of Ketamine with and without Lamotrigine in Treatment-Resistant Depression: A Preliminary Report. Pharmaceuticals, 16(8), 1164. https://doi.org/10.3390/ph16081164









