Traditional Chinese Medicine for Topical Treatment of Skeletal Muscle Injury
Abstract
1. Introduction
- (1)
- Carthami Flos (Carthamus tinctorius L.). A Chinese herb traditionally used for cardiovascular disease and bone injury in China with pharmacological effects on improving blood circulation. Its proangiogenic effect has been proven in both in vitro and in vivo studies [19].
- (2)
- Dipsaci Radix (Dipsacus asperoides C.Y. Cheng T.M. Ai). A common herb in traumatology that promotes blood circulation to remove a hematoma and alleviate pain. Recent scientific studies illustrated that Akebia Saponin D, the most abundant constituent of Dipsaci Radix, exhibits anti-inflammatory effects [20,21].
- (3)
- Rhei Rhizoma (Rheum palmatum Linn). A widely and traditionally used Chinese herb for wound healing. One of its derivatives, emodin, has been reported to promote the repair of rat excisional wounds through a complex mechanism involving the stimulation of tissue regeneration and regulation of the Smads-mediated transforming growth factor-β1 signaling pathway [22].
2. Results
2.1. Effect of the Herbal Extract on Cell Viability of L6
2.2. Incapacitance Test
2.3. Morphological Change in Muscles after Contusion
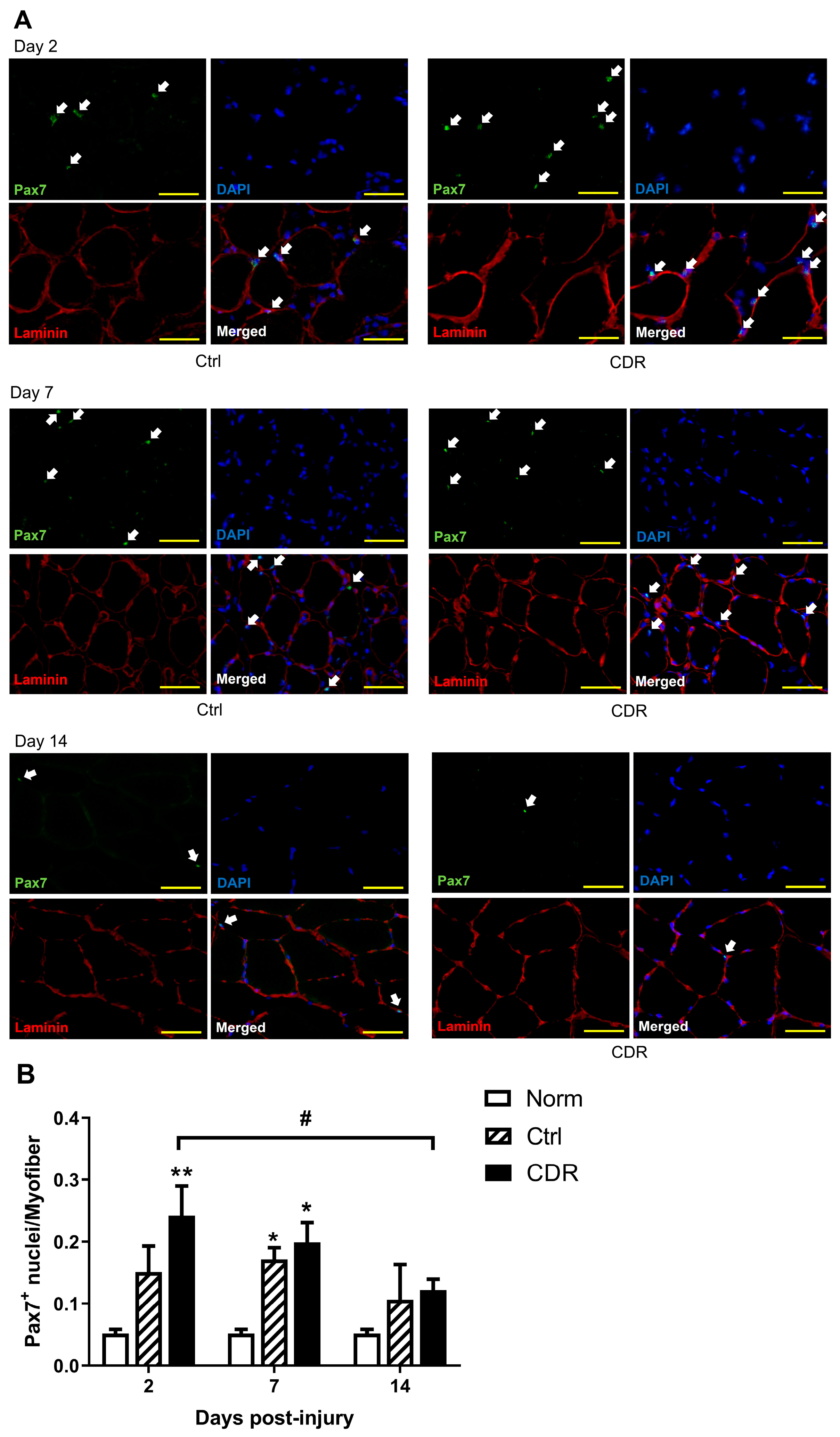
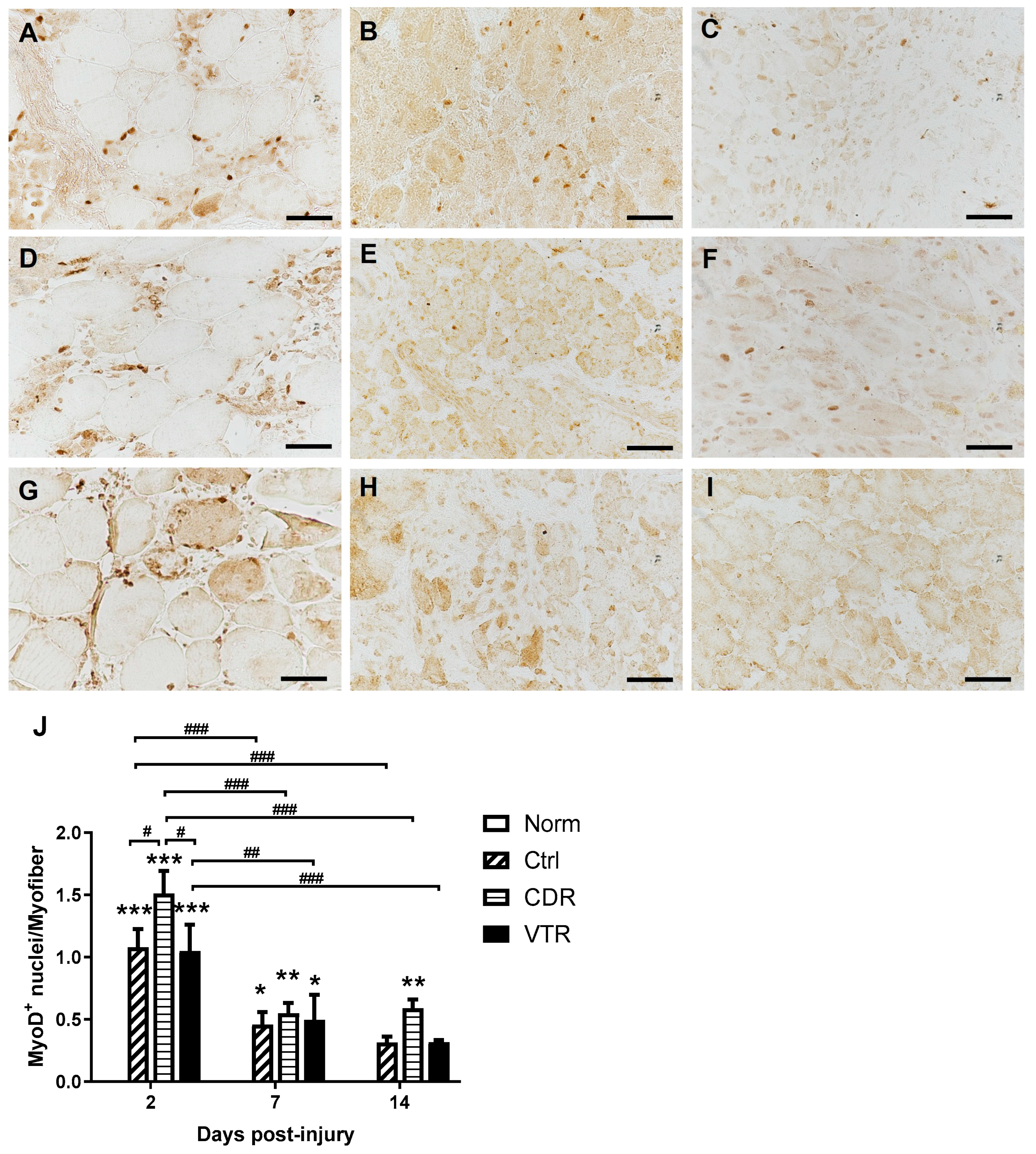
2.4. Immunostaining of Muscle
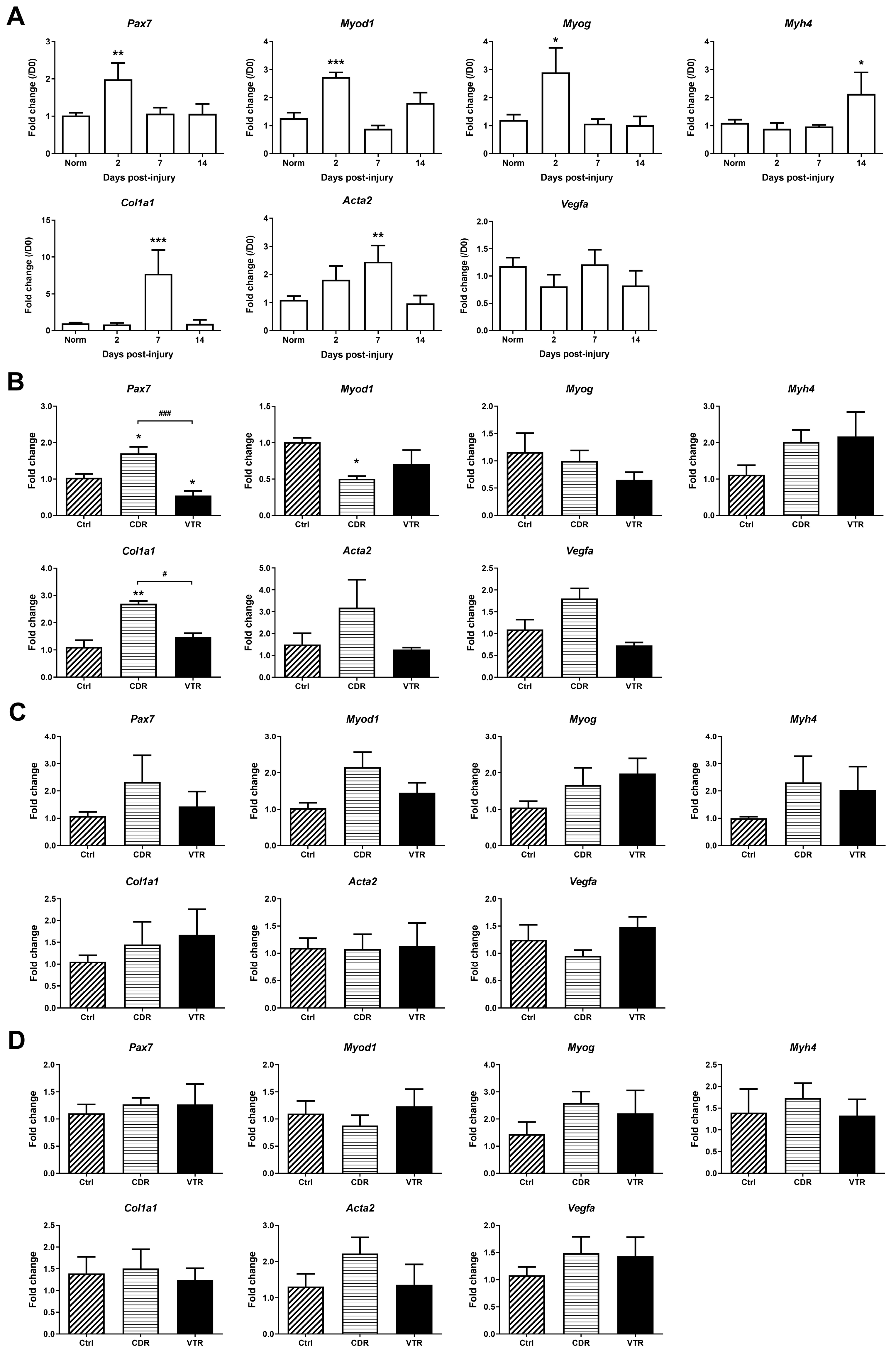
2.5. Gene Expression
3. Discussion
4. Materials and Methods
4.1. Herbal Materials and Authentication
4.2. In Vitro Study
4.3. Animal Model and In Vivo Study
4.4. Incapacitance Test
4.5. Histological Assessments
4.5.1. Immunofluorescence
4.5.2. Immunohistochemistry
4.5.3. Image Analysis
4.6. Gene Expression on Muscle Regeneration
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Bone and Joint Decade. Musculoskeletal Injuries. In The Burden of Musculoskeletal Diseases in the United States, 2nd ed.; American Academy of Orthopaedic Surgeon: Rosemont, IL, USA, 2010; pp. 129–180. [Google Scholar]
- Järvinen, M.J.; Lehto, M.U.K. The effects of early mobilisation and immobilisation on the healing process following muscle injuries. Sports Med. 1993, 15, 78–89. [Google Scholar] [CrossRef]
- Järvinen, T.A.H.; Järvinen, T.L.N.; Kääriäinen, M.; Kalimo, H.; Järvinen, M. Muscle injuries. Am. J. Sports Med. 2005, 33, 745–764. [Google Scholar] [CrossRef] [PubMed]
- Beiner, J.M.; Jokl, P. Muscle contusion injuries: Current treatment options. J. Am. Acad. Orthop. Surg. 2001, 9, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Stanos, S.P.; Galluzzi, K.E. Topical therapies in the management of chronic pain. Postgrad. Med. 2013, 125, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Almekinders, L.C. Anti-inflammatory treatment of muscular injuries in sport. An update of recent studies. Sports Med. 1999, 28, 383–388. [Google Scholar] [CrossRef]
- Jarvinen, M. Effect of some anti inflammatory agents on the healing of ruptured muscle: An experimental study in rats. J. Sports Traumatol. 1992, 14, 19–28. [Google Scholar]
- Vignaud, A.; Cebrian, J.; Martelly, I.; Caruelle, J.-P.; Ferry, A. Effect of anti-inflammatory and antioxidant drugs on the long-term repair of severely injured mouse skeletal muscle. Exp. Physiol. 2005, 90, 487–495. [Google Scholar] [CrossRef]
- Bondesen, B.A.; Mills, S.T.; Kegley, K.M.; Pavlath, G.K. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am. J. Physiol. Cell Physiol. 2004, 287, 475–483. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef]
- Xiao, W.; Liu, Y.; Chen, P. Macrophage depletion impairs skeletal muscle regeneration: The roles of pro-fibrotic factors, inflammation, and oxidative stress. Inflammation 2016, 39, 2016–2028. [Google Scholar] [CrossRef]
- Delos, D.; Maak, T.G.; Rodeo, S.A. Muscle injuries in athletes. Sports Health 2013, 5, 346–352. [Google Scholar] [CrossRef]
- Duchesne, E.; Dufresne, S.S.; Dumont, N.A. Impact of inflammation and anti-inflammatory modalities on skeletal muscle healing: From fundamental research to the clinic. Phys. Ther. 2017, 97, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Mirel, S.; Colobățiu, L.; Mirel, M.; Pop, S. Topical patches as treatments for the management of patient musculoskeletal and neuropathic pain. Balneo Res. J. 2017, 8, 21–25. [Google Scholar] [CrossRef]
- Ng, S. Topical Traditional Chinese Medicine: A report from Singapore. Arch. Dermatol. 1998, 134, 1395–1396. [Google Scholar] [CrossRef]
- Lee, T.Y.; Lam, T.H. Allergic contact dermatitis due to a Chinese orthopaedic solution Tieh Ta Yao Gin. Contact Ddermatitis 1993, 28, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Ho, M.S.; Ng, S.; Yosipovitch, G. Contact dermatitis: A common adverse reaction to topical traditional Chinese medicine. Int. J. Dermatol. 2010, 49, 1255–1260. [Google Scholar] [CrossRef]
- Yang, X.; Chen, A.; Ma, Y.; Gao, Y.; Gao, Z.; Fu, B.; Sun, F.; Qiao, J.; Li, Q.; Wan, S.; et al. Encyclopedic Reference of Traditional Chinese Medicine; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Zhou, X.; Siu, W.S.; Fung, C.H.; Cheng, L.; Wong, C.W.; Zhang, C.; Liu, C.L.; Kwok, H.F.; Lau, C.P.; Wat, E.; et al. Pro-angiogenic effects of Carthami Flos whole extract in human microvascular endothelial cells in vitro and in zebrafish in vivo. Phytomedicine 2014, 21, 1256–1263. [Google Scholar] [CrossRef]
- Yu, X.; Wang, L.; Du, Q.; Ma, L.; Chen, L.; You, R.; Liu, L.; Ling, J.J.; Yang, Z.L.; Ji, H. Akebia Saponin D attenuates amyloid β-induced cognitive deficits and inflammatory response in rats: Involvement of Akt/NF-κB pathway. Behav. Brain Res. 2012, 235, 200–209. [Google Scholar] [CrossRef]
- Gong, L.L.; Yang, S.; Liu, H.; Zhang, W.; Ren, L.L.; Han, F.F.; Lv, Y.L.; Wan, Z.R.; Liu, L.H. Anti-nociceptive and anti-inflammatory potentials of Akebia saponin D. Eur. J. Pharmacol. 2019, 845, 85–90. [Google Scholar] [CrossRef]
- Tang, T.; Yin, L.; Yang, J.; Shan, G. Emodin, an anthraquinone derivative from Rheum officinale Baill, enhances cutaneous wound healing in rats. Eur. Iournal Pharmacol. 2007, 567, 177–185. [Google Scholar] [CrossRef]
- Tse, L.F.; Cheng, H.S.; Tso, C.Y.; Hung, Y.W.; Hung, L.K.; Chen, J.Z.; Zhou, X.L.; Fung, C.H.; Pang, E.S.Y.; Cheng, K.F.; et al. Does topical agent help fracture healing? A pilot study using a herbal patch. Open J. Ther. Rehabil. 2015, 3, 35–39. [Google Scholar] [CrossRef]
- Tse, L.F.; Cheng, H.S.; Hung, L.K.; Pang, E.; Cheng, K.F.; Siu, W.S.; Chen, J.Z.; Zhou, X.L.; Fung, C.H.; Leung, P.C. A Pilot Study on the Effectiveness of a Novel Herbal Patch for the Treatment of Plantar Fasciitis. Int. J. Chin. Med. 2017, 1, 70–76. [Google Scholar]
- Siu, W.S.; Shiu, H.T.; Ko, C.H.; Shum, W.T.; Yu, H.N.; Lau, C.B.; Hung, L.K.; Leung, P.C. Integrative Approach to Facilitate Fracture Healing: Topical Chinese Herbal Paste with Oral Strontium Ranelate. Evid.-Based Complement. Altern. Med. 2017, 2017, 9795806. [Google Scholar] [CrossRef]
- Siu, W.S.; Shiu, H.T.; Shum, W.T.; Ko, C.H.; Lau, C.B.S.; Hung, L.K.; Leung, P.C. Chinese topical herbal medicine gives additive effect on pharmaceutical agent on fracture healing. J. Tradit. Chin. Med. 2019, 39, 853–860. [Google Scholar]
- Forcina, L.; Cosentino, M.; Musarò, A. Mechanisms regulating muscle regeneration: Insights into the interrelated and time-dependent phases of tissue healing. Cells 2020, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Musarò, A. The basis of muscle regeneration. Adv. Biol. 2014, 2014, 612471. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Qu, W.; Li, X.; Ma, S.; Wang, Z.; Liu, W.; Hou, S.; Fu, J. The effects of Xiangqing Anodyne Spray on treating acute soft-tissue injury mainly depend on suppressing activations of AKT and p38 pathways. Evid.-Based Complement. Altern. Med. 2016, 2016, 9213489. [Google Scholar] [CrossRef]
- Wang, S.; Qu, W.; Li, T.; Guo, K.; Liu, W.; Wang, Z.; Fu, J. Xiangqing Anodyne Spray (XQAS): A combination of ethanol extracts of Cynanchum paniculatum and Illicium henryi for treating soft-tissue injury. Int. J. Clin. Exp. Med. 2015, 8, 12716–12725. [Google Scholar]
- Duan, W.; Lu, J.; Xie, Y. Mechanisms of Topical analgesics in relieving pain in an animal model of muscular inflammation. Pain Med. 2013, 14, 1381–1387. [Google Scholar] [CrossRef]
- Xiao, B.; Li, Q.; Han, N.; Zhang, C.; Yin, J. Soft tissue contusion repairing effects of Hong Yao with different penetration enhancers. J. Ethnopharmacol. 2013, 148, 610–616. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, C.; Zhong, H.; Zhang, W.; Wang, D.; Wang, X.; Dong, F. In vivo effects of pain relieving plaster on closed soft tissue injury in rabbit ears. BMC Complement. Altern. Med. 2008, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Cai, Z.; Li, D.; Zhang, Y.; He, M.; Yang, Y.; Liu, D.; Xie, W.; Li, Y.; Xiao, W. Myogenic differentiation of stem cells for skeletal muscle regeneration. Stem Cells Int. 2021, 2021, 8884283. [Google Scholar] [CrossRef] [PubMed]
- Myburgh, K.H.; Kruger, M.J.; Smith, C. Accelerated skeletal muscle recovery after in vivo polyphenol administration. J. Nutr. Biochem. 2012, 23, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Jiang, S.; Zhang, M.; Wang, M.; Li, J.; Zhao, R.; Wang, L.; Li, S.; Liu, M.; Zhang, M.; et al. Detection of satellite cells during skeletal muscle wound healing in rats: Time-dependent expressions of Pax7 and MyoD in relation to wound age. Int. J. Leg. Med. 2016, 130, 163–172. [Google Scholar] [CrossRef]
- Hatade, T.; Takeuchi, K.; Fujita, N.; Arakawa, T.; Miki, A. Effect of heat stress soon after muscle injury on the expression of MyoD and myogenin during regeneration process. J. Musculoskelet. Neuronal Interact. 2014, 14, 325–333. [Google Scholar] [PubMed]
- Schmidt, M.; Schüler, S.C.; Hüttner, S.S.; von Eyss, B.; von Maltzahn, J. Adult stem cells at work: Regenerating skeletal muscle. Cell Mol. Life Sci. 2019, 76, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Srikuea, R.; Pholpramool, C.; Kitiyanant, Y.; Yimlamai, T. Satellite cell activity in muscle regeneration after contusion in rats. Clin. Exp. Pharmacol. Physiol. 2010, 37, 1078–1086. [Google Scholar] [CrossRef]
- Grounds, M.D.; Garret, K.L.; Lai, M.C.; Wright, W.E.; Beilharz, M.W. Identification of skeletal muscle precursor cells in vivo by use of MyoD1 and myogenin probes. Cell Tissue Res. 1992, 267, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal Muscle Extracellular Matrix—What do we know about its composition, regulation, and physiological roles? A narrative review. Front. Physiol. 2020, 11, 253. [Google Scholar] [CrossRef]
- Hurme, T.; Kalimo, H.; Sandberg, M.; Lehto, M.; Vuorio, E. Localization of type I and III collagen and fibronectin production in injured gastrocnemius muscle. Lab. Investig. 1991, 64, 76–84. [Google Scholar] [PubMed]
- Siu, W.S.; Shum, W.T.; Cheng, W.; Wong, C.W.; Shiu, H.T.; Ko, C.H.; Leung, P.C.; Lam, C.W.K.; Wong, C.K. Topical application of Chinese herbal medicine DAEP relieves the osteoarthritic knee pain in rats. Chin. Med. 2019, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Crisco, J.J.; Jokl, P.; Heinen, G.T.; Connell, M.D.; Panjabi, M.M. A muscle contusion injury model. Am. J. Sports Med. 1994, 22, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.C. Real-Time reverse transcription polymerase chain reaction to measure mRNA: Use, limitations, and presentation of results. Anat. Rec. 2012, 295, 1–3. [Google Scholar] [CrossRef] [PubMed]



| Gene | Sequence (5′ to 3′) | NCBI Accession No. |
|---|---|---|
| Acta2 | Forward: AACACGGCATCATCACCAACT Reverse: TTTCTCCCGGTTGGCCTTA | NM_031004.2 |
| Col1a1 | Forward: CCCAGCGGTGGTTATGACTT Reverse: GGGTTTGGGCTGATGTACCA | NM_053304.1 |
| Gapdh | Forward: CTCAGTTGCTGAGGAGTCCC Reverse: ATTCGAGAGAAGGGAGGGCT | NM_017008.4 |
| Myod1 | Forward: GGAGACATCCTCAAGCGATGC Reverse: AGCACCTGGTAAATCGGATTG | NM_176079.1 |
| Myog | Forward: GACCCTACAGGTGCCCACAA Reverse: ACATATCCTCCACCGTGATGCT | NM_017115.2 |
| Myh4 | Forward: CACACCAAAGTCATAAGCGAA Reverse: CCTTGATATACAGGACAGTGA | NM_019325.1 |
| Pax7 | Forward: GATTAGCCGAGTGCTCAGAATCAAG Reverse: GTCGGGTTCTGATTCCACGTC | NM_001191984.1 |
| Vegfa | Forward: TACCTCCACCATGCCAAGTG Reverse: TCTGCTCCCCTTCTGTCGTG | NM_031836.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siu, W.-S.; Ma, H.; Cheng, W.; Shum, W.-T.; Leung, P.-C. Traditional Chinese Medicine for Topical Treatment of Skeletal Muscle Injury. Pharmaceuticals 2023, 16, 1144. https://doi.org/10.3390/ph16081144
Siu W-S, Ma H, Cheng W, Shum W-T, Leung P-C. Traditional Chinese Medicine for Topical Treatment of Skeletal Muscle Injury. Pharmaceuticals. 2023; 16(8):1144. https://doi.org/10.3390/ph16081144
Chicago/Turabian StyleSiu, Wing-Sum, Hui Ma, Wen Cheng, Wai-Ting Shum, and Ping-Chung Leung. 2023. "Traditional Chinese Medicine for Topical Treatment of Skeletal Muscle Injury" Pharmaceuticals 16, no. 8: 1144. https://doi.org/10.3390/ph16081144
APA StyleSiu, W.-S., Ma, H., Cheng, W., Shum, W.-T., & Leung, P.-C. (2023). Traditional Chinese Medicine for Topical Treatment of Skeletal Muscle Injury. Pharmaceuticals, 16(8), 1144. https://doi.org/10.3390/ph16081144





