1. Introduction
An acanthopanax senticosus injection is a single preparation processed from acanthopanax senticosus through “water extraction and alcohol precipitation”, which is widely used in the treatment of cerebrovascular diseases, coronary heart disease, angina pectoris, acute cerebral infarction, and other diseases [
1]. However, with the continuous use of the acanthopanax senticosus injection in clinical practice, the occurrence of adverse reactions (ADR) also increases; the common adverse reactions include systemic damage, skin damage, etc. [
2]. One of the reasons is that the ineffective components remaining in acanthopanax senticosus injections, such as protein, condensed tannin, and pyrogen, are not completely removed, which leads to the generation of adverse reactions. Methods for removing macromolecules from traditional Chinese medicine injections generally have the disadvantages of high energy consumption, low efficiency, and high pollution, while the ultrafiltration technology is favored for its advantages of high separation efficiency and strong continuity.
The separation efficiency of ultrafiltration technology depends on the membrane materials. Common membrane materials include organic polymer and inorganic materials, among which the former is widely used. Organic polymer membrane materials mainly include polysulfone [
3,
4,
5], cellulose [
6,
7], polyamide [
8,
9], fluoropolymers, etc [
10,
11,
12]. Phenolphthalide polyethersulfone (PES-C) is a kind of polysulfone, its structure determines its good hydrolysis resistance, mechanical property, and thermodynamic stability, and it is widely used in the preparation of fuel cell membranes [
13], ultrafiltration/nanofiltration membranes [
14,
15] and nanofiber composite membranes [
16]. However, due to the inherent hydrophobic property of PES-C polymer, its service life is seriously affected. Therefore, the hydrophilic modification of the PES-C membrane is imperative. There are many modification methods for membrane materials, among which blending modification is the most widely used.
In recent years, natural products and their derivatives have gradually attracted researcher attention because of their decontamination and purification functions. Studies have shown that emodin isolated from the root of polygonum cuspidatum has antibacterial, anti-inflammatory, and other biological activities [
17,
18,
19]. Emodin has an obvious inhibitory effect on gram-positive bacteria such as Staphylococcus aureus. At the same time, the structure of emodin contains a certain number of hydroxyl groups, which lays a theoretical foundation for improving the hydrophilic and anti-pollution properties of the membrane, and it is expected to have a good application prospect in the membrane field.
In this paper, with emodin as an additive, a PES-C/emodin ultrafiltration membrane with excellent comprehensive performance was obtained. It was used to purify the acanthopanax senticosus injection to reduce the risk of adverse reactions.
2. Results and Discussions
According to the preliminary experimental results [
20], the best comprehensive performance of the prepared PES-C/emodin ultrafiltration membrane was as follows: filtration performance showed a water flux of 387.41 L∙m
−2∙h
−1, and a rejection rate of 99.83%; Hydrophilic had a water content of 6.78%, and a contact angle of 65.71°; Anti-pollution performance had a flux recovery rate of 57.05%, and a BSA adsorption rate of 1.44% and the antibacterial diameter of 2.30 cm on S. aureus. Moreover, compared with the PES-C ultrafiltration membrane, the optimal PES-C/emoin ultrafiltration membrane significantly reduced bacterial adhesion.
Preparation and appearance color detection results of the purified acanthopanax senticosus injection, acanthopanax senticosus injection, and acanthopanax senticosus injection containing macromolecules are shown in
Figure 1. From
Figure 1, compared with the original liquid of acanthopanax senticosus injection, the color of the purified acanthopanax senticosus injection was slightly lighter, and its clarity was improved. For the acanthopanax senticosus injection containing macromolecules, although there was no precipitation, its color was significantly dark, indicating that treatment of PES-C/emoin ultrafiltration membrane can effectively improve the clarity of an acanthopanax senticosus injection.
The area under the absorption curve of injection represents the turbidity degree of injection.
Figure 2,
Figure 3 and
Figure 4 shows the thermal stability test results of the original liquid of the acanthopanax senticosus injection, purified acanthopanax senticosus injection, and acanthopanax senticosus injection containing macromolecules at 0, 15, and 30 days. From
Figure 2,
Figure 3 and
Figure 4, compared with the original liquid of the acanthopanax senticosus injection on the first day, the area under the absorption curve for purified acanthopanax senticosus injection decreased, while that of an acanthopanax senticosus injection containing macromolecules increased. As the experiment progressed, the showed rules of the test samples on the 15th and 30th days became increasingly obvious. Especially on the 30th day, the areas under the absorption curves of the original liquid of the acanthopanax senticosus injection, purified acanthopanax senticosus injection, and acanthopanax senticosus injection containing macromolecules were 157.05 OD·nm, 141.49 OD·nm, and 174.37 OD·nm, respectively (
Figure 5). The purified acanthopanax senticosus injection had higher clarity and stability than the original solution of the acanthopanax senticosus injection, while the acanthopanax senticosus injection containing macromolecules had higher turbidity and poor stability, which were consistent with the results in
Figure 1.
Fingerprints of the original solution of the acanthopanax senticosus injection and purified acanthopanax senticosus injection are shown in
Figure 6. By comparison with
Figure 6A,B, it can be seen that the fingerprint of the original solution of the acanthopanax senticosus injection was highly similar to the purified acanthopanax senticosus injection; they both had 14 obvious peaks. The peak area of 14 chromatographic peaks was integrated, and the relative peak area of a single peak and total peak for the purified acanthopanax senticosus injection was calculated with the corresponding peak of the original solution of the acanthopanax senticosus injection as reference; the results are shown in
Table 1 and
Table 2. From
Table 1 and
Table 2, the relative peak area of each single peak was not less than 0.95, and the relative total peak area was greater than 0.96. As a result, the fingerprint of the acanthopanax senticosus injection purified by PES-C/emoin ultrafiltration membrane was not significantly changed, which showed its active component content did not change.
Photos of the interaction between the original solution of acanthopanax senticosus injection, purified acanthopanax senticosus injection, acanthopanax senticosus injection containing macromolecules (all the injections were diluted to 10% with the medium solution), and the blank group with RBL-2H3 cells are shown in
Figure 7. Compared with the original solution of the acanthopanax senticosus injection, the toxicity of the acanthopanax senticosus injection containing macromolecules on RBL-2H3 cells was enhanced; however, the toxicity of the purified acanthopanax senticosus injection weakened. Specifically, IC50 values of the acanthopanax senticosus injection, purified acanthopanax senticosus injection, and the acanthopanax senticosusa injection containing macromolecules by CCK8 method are shown in
Table 3,
Table 4 and
Table 5. From
Table 3,
Table 4 and
Table 5, the ability of cell proliferation decreased with the increase of drug concentration. According to the statistical analysis of SPSS26.0 software, the IC50 of the original solution of the acanthopanax senticosus injection was 11.18%, the IC50 of the purified acanthopanax senticosus injection and the acanthopanax senticosus injection containing macromolecules was 14.41% and 5.02%, respectively.
The standard curve of histamine standard is shown in
Figure 8. From
Figure 8, the standard curve equation of histamine standard is y = 0.0673x + 0.3396, R
2 = 0.9988, and the concentration of histamine standard is in the range of 2.5–40 μg∙L
−1. There was a good linear relationship between the histamine concentration and OD value.
According to the standard curve of histamine, the calculated results of histamine concentration in the test samples are shown in
Table 6. From
Table 6, compared with the original solution of the acanthopanax senticosus injection group, the release amount of histamine from the acanthopanax senticosus injection containing macromolecules significantly increased (
p < 0.05) when the interaction time with the drug was 10 min and 50 min, and that of the purified acanthopanax senticosus injection was slightly decreased (
p < 0.05). Compared with the blank group and the original solution of the acanthopanax senticosus injection group, the histamine release amount in the C48/80 group significantly increased (
p < 0.01). The results suggested that the acanthopanax senticosus injection containing macromolecules was prone to allergic reactions, while purified acanthopanax senticosus injection was safer.
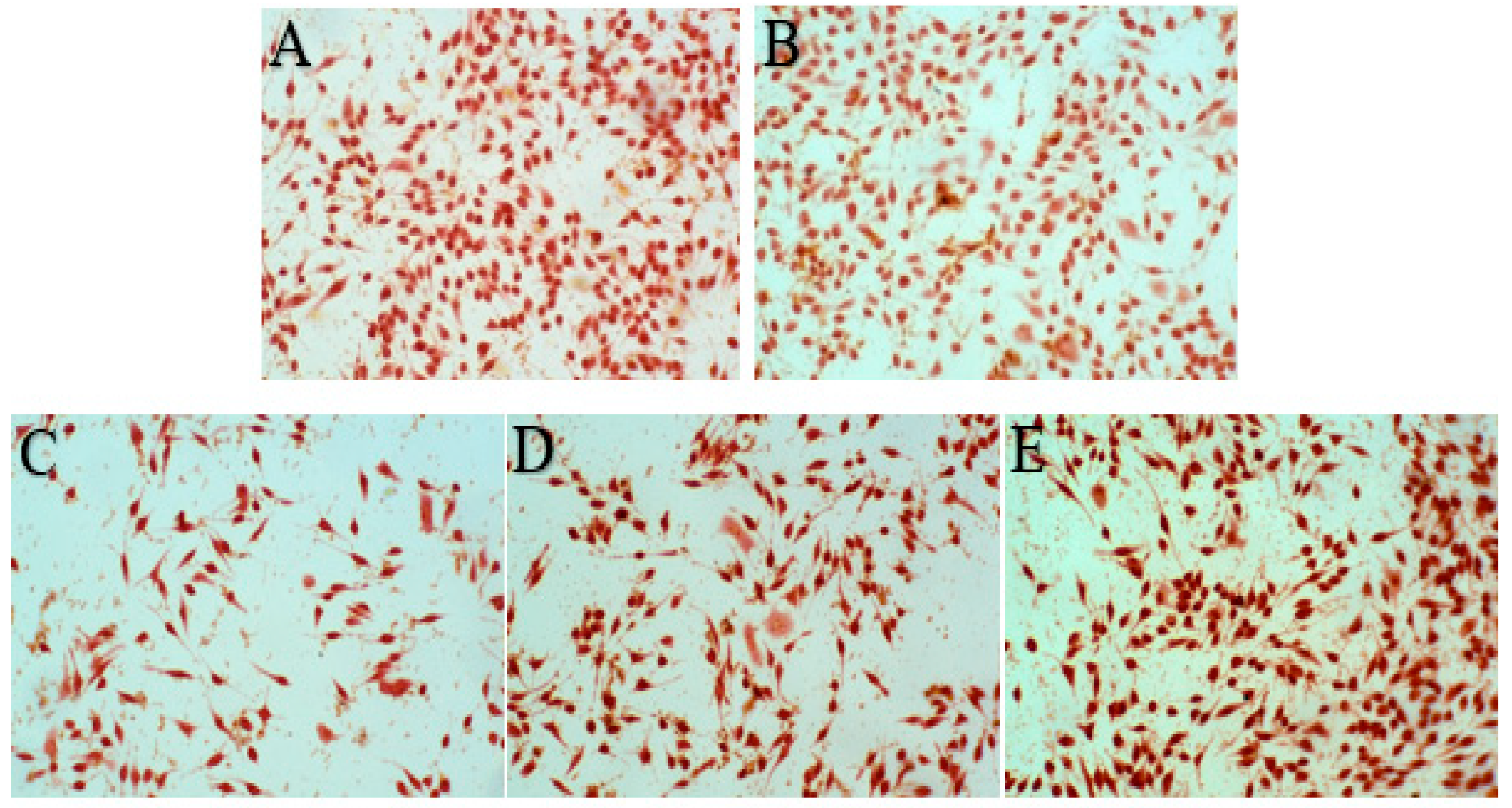
The morphology of RBL-2H3 cells treated with samples is shown in
Figure 9. Normal RBL-2H3 cells showed long spindle or pleomorphis with full cytoplasm and complete cell membrane, while the volume of degranulated cells increased, and even the cell membrane burst and the granular materials in the cells released. The results of the cell degranulation rate are shown in
Table 7. According to
Table 7, compared with the original solution of the acanthopanax senticosus injection group, the cell degranulation rate of purified acanthopanax senticosus injection was slightly lower (
p < 0.05), the cell degranulation rate of the acanthopanax senticosus injection containing macromolecules increased significantly (
p < 0.05). As a result, an acanthopanax senticosus injection containing macromolecules easily stimulated RBL-2H3 cells and triggered allergic reactions, while a purified acanthopanax senticosus injection significantly reduced the risk of allergic reactions, which was consistent with the histamine release result in
Table 6. The reason was that the acanthopanax senticosus injection containing macromolecules could easily stimulate RBL-2H3 cells to degranulate; thus, histamine, bradykinin, and other allergenic substances were released.
Routine blood test results of the blank group, acanthopanax senticosus injection, and acanthopanax senticosus injection containing macromolecules are shown in
Table 8. Five days after the mice were given the drug, compared with the original solution of the acanthopanax senticosus injection, the number of white blood cells, hemoglobin, and platelets in the acanthopanax senticosus injection containing macromolecules increased (
p < 0.05), because the macromolecular substances activated the relevant inflammatory system. In clinical practice, the increase in the number of white blood cells and platelets often means the chronic infection, tissue damage, and inflammation caused by bacteria and viruses. Compared with the blank group, the number of white blood cells, hemoglobin, and platelets in the acanthopanax senticosus injection group decreased. This was directly related to the effect of the acanthopanax senticosus injection on reinforcing the liver and kidneys. In addition, other detection indexes of the acanthopanax senticosus injection, acanthopanax senticosus injection containing macromolecules, and the blank group showed little change (
p > 0.05).
Table 9 shows the detection results of organ coefficients in the blank group (normal saline), the original solution of the acanthopanax senticosus group, and the acanthopanax senticosus injection containing macromolecules. From
Table 9, compared with the original solution of the acanthopanax senticosus injection, the liver and kidney indexes of the acanthopanax senticosus injection containing macromolecules had no significant difference (
p > 0.05). However, they increased to a certain extent, indicating that the acanthopanax senticosus injection containing macromolecules had a certain liver and kidney toxicity in mice. In addition, compared with the blank group, the changes in liver and kidney indexes for the original solution acanthopanax senticosus injection group were insignificant (
p > 0.05).
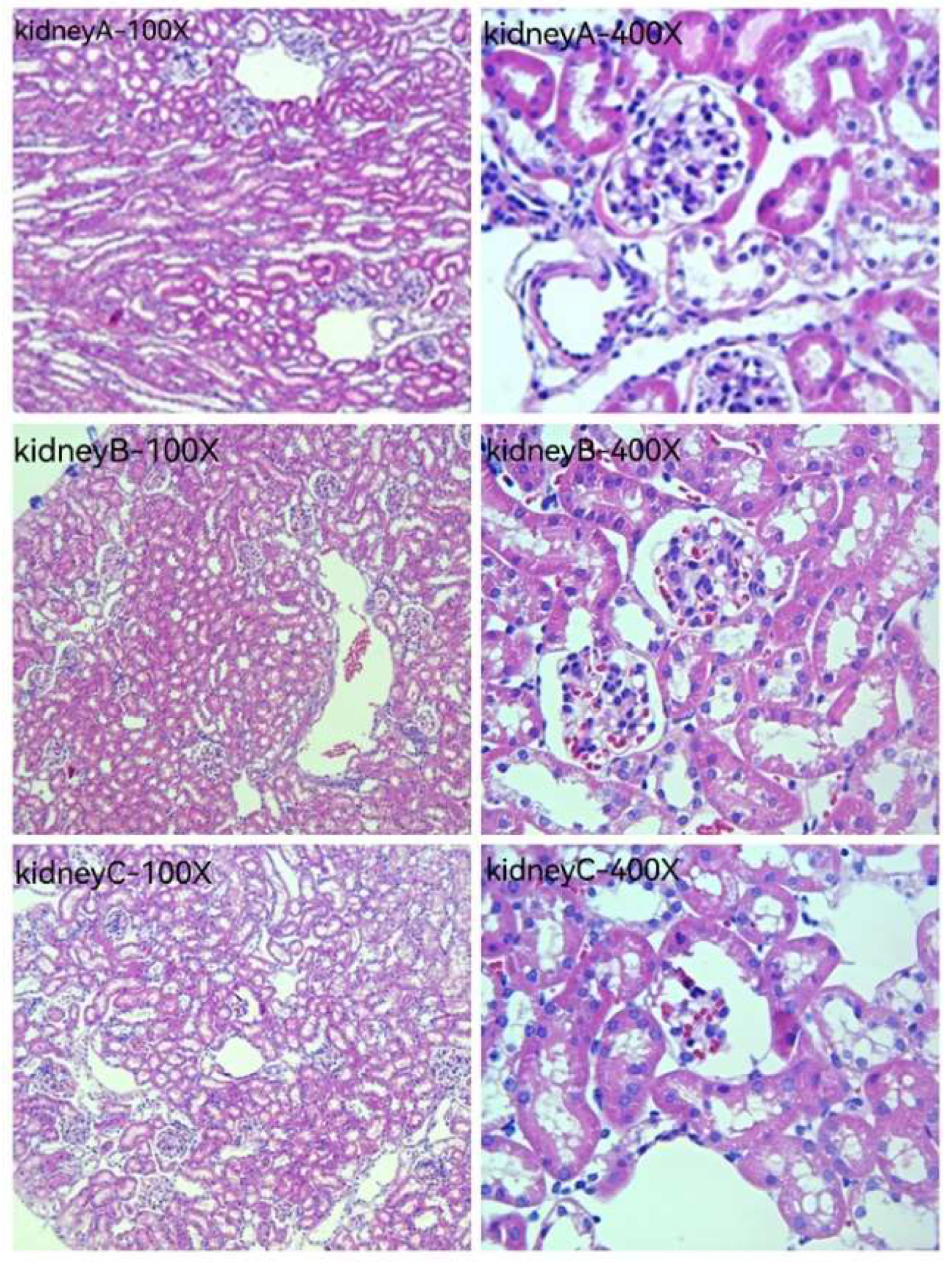
Figure 10 and
Figure 11 are HE staining photos of the liver and kidney for the blank group (normal saline), the original solution of the acanthopanax senticosus group, and the acanthopanax senticosus injection containing macromolecules. After administration of the original solution of the acanthopanax senticosus group and the acanthopanax senticosus injection containing macromolecules, the microscopic pathological changes of the liver and kidneys were observed in each group. The pathological changes of the acanthopanax senticosus injection containing macromolecules were more serious. In
Figure 10, liver A (blank group): the liver tissue section showed a completely normal hepatic lobule structure with radial central vein, there were no obvious changes in hepatic cord and hepatic sinusoid boundary and no cell infiltration around the central vein, and the nuclear position was clear. Liver B (original solution group of acanthopanax injection): hepatic lobules were normal in shape, hepatic cords were neatly arranged, and some nuclei were clearly located, while a small number of cells were degenerated, and inflammatory cells in the central venous area were lightly infiltrated. Liver C (acanthopanax injection group rich in macromolecules): the morphology of hepatic lobules changed with the occasional false lobules, hepatic cords were disorganized, the boundary of hepatic cords and hepatic sinus was unclear, nuclei were necrotic and fused into pieces, and a large number of inflammatory cells were infiltrated in portal duct area and central venous area. Kidney A (blank group): renal tissue section showed no hyperplasia of glomerulus distributed in the cortex, the intact structure of renal tubular epithelial cells, and normal morphology of renal interstitial cells. Kidney B (original solution group of acanthopanax injection): edema for the cortical glomerular epithelial cells was slightly absorbed, the renal tubular cells had slight congestion, and the infiltrated inflammatory cells and congestion were mild. Renal C (acanthopanax injection group rich in macromolecules): the glomerular epithelial cells distributed in the cortex had mild edema, the renal tubules had congestion, and there were infiltrated inflammatory cells and partial congestion in the interstitial cells.