Natural Products Treat Colorectal Cancer by Regulating miRNA
Abstract
1. Introduction
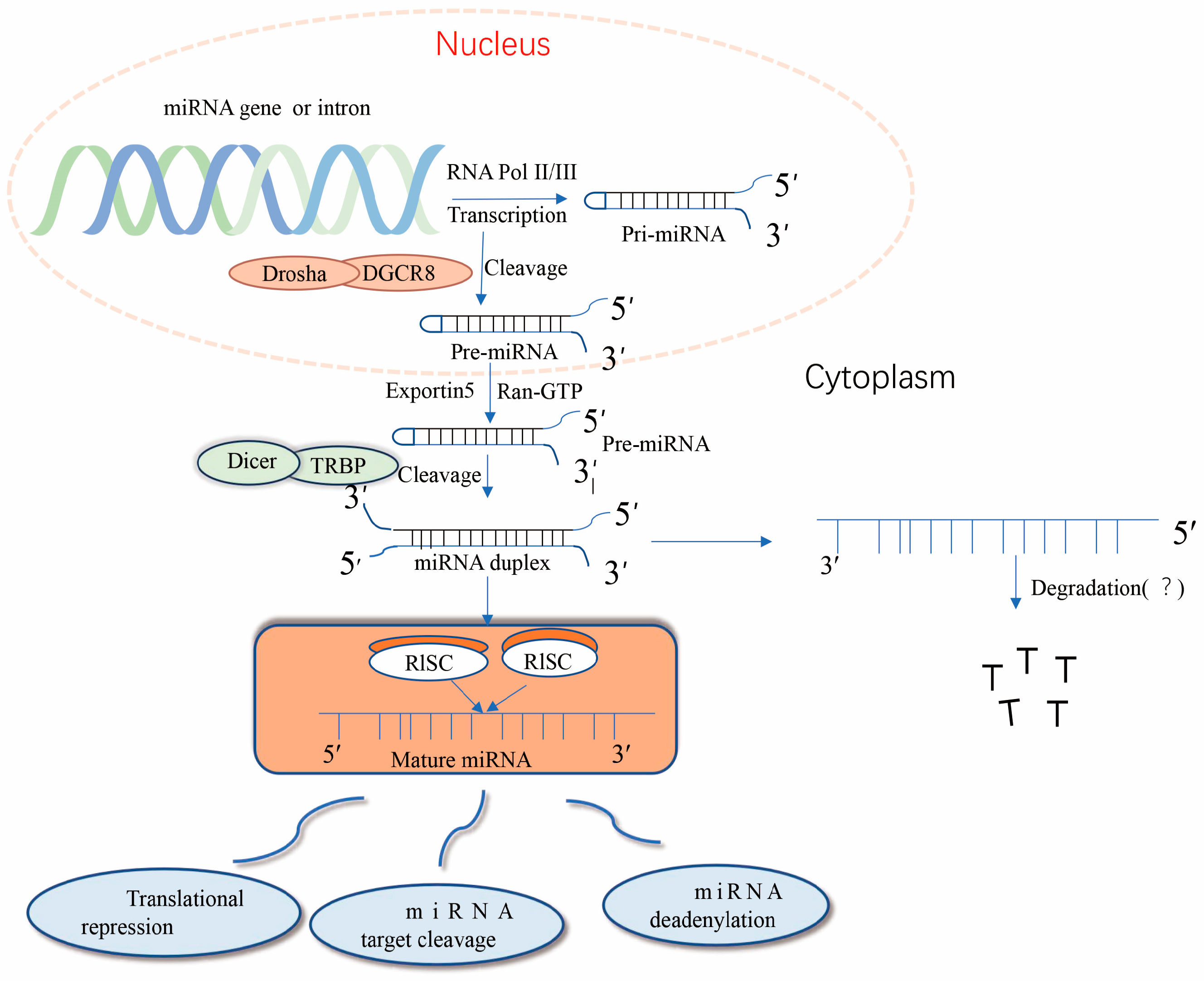
2. Biogenesis of miRNA Molecules and Their Mechanism of Action in Tumors
3. The Roles and Mechanisms of Natural Products in the Prevention and Treatment of Colon Cancer
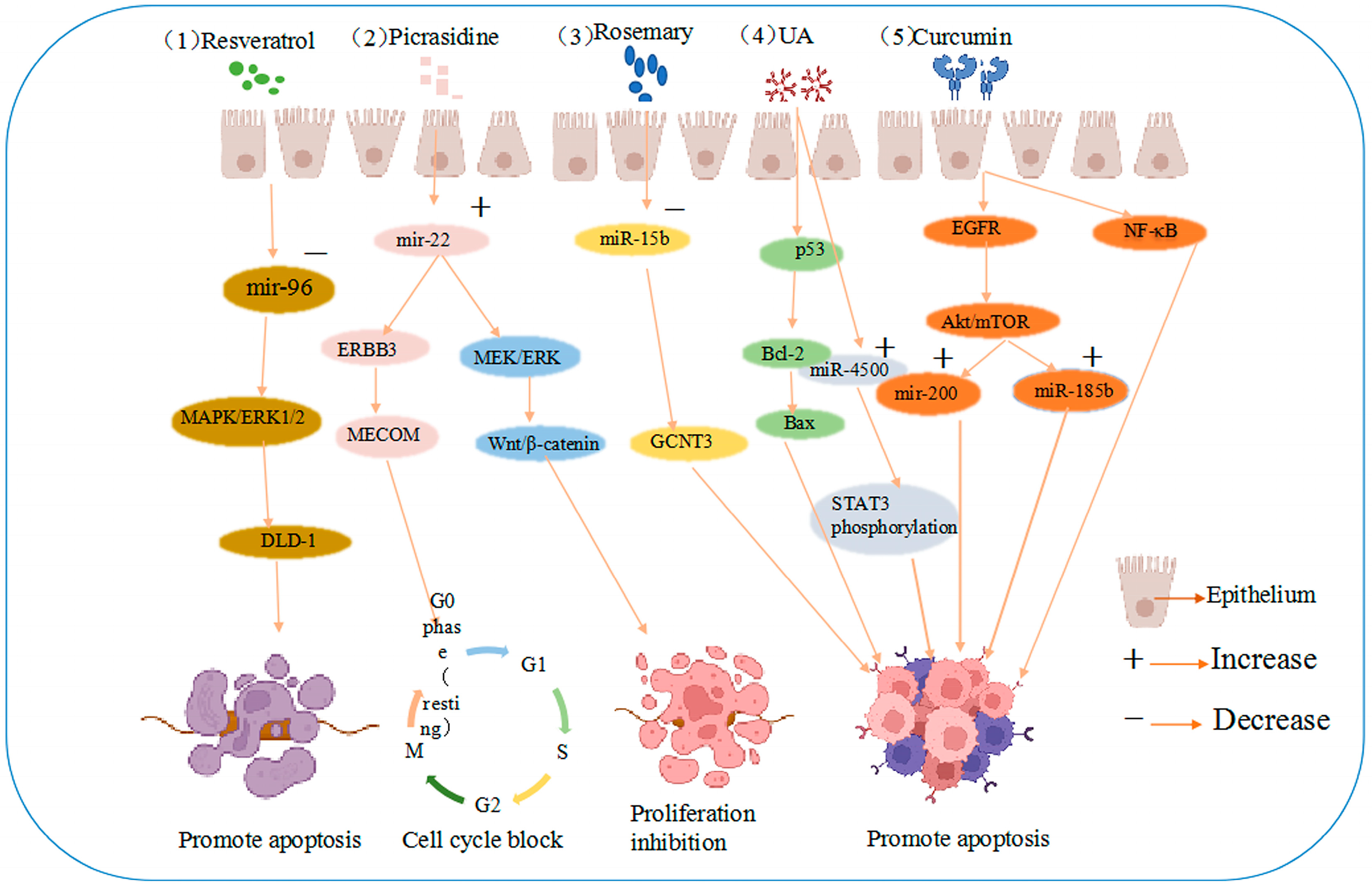
4. Natural Products Can Mediate miRNAs to Promote Apoptosis or Inhibit the Proliferation of CRC Cells
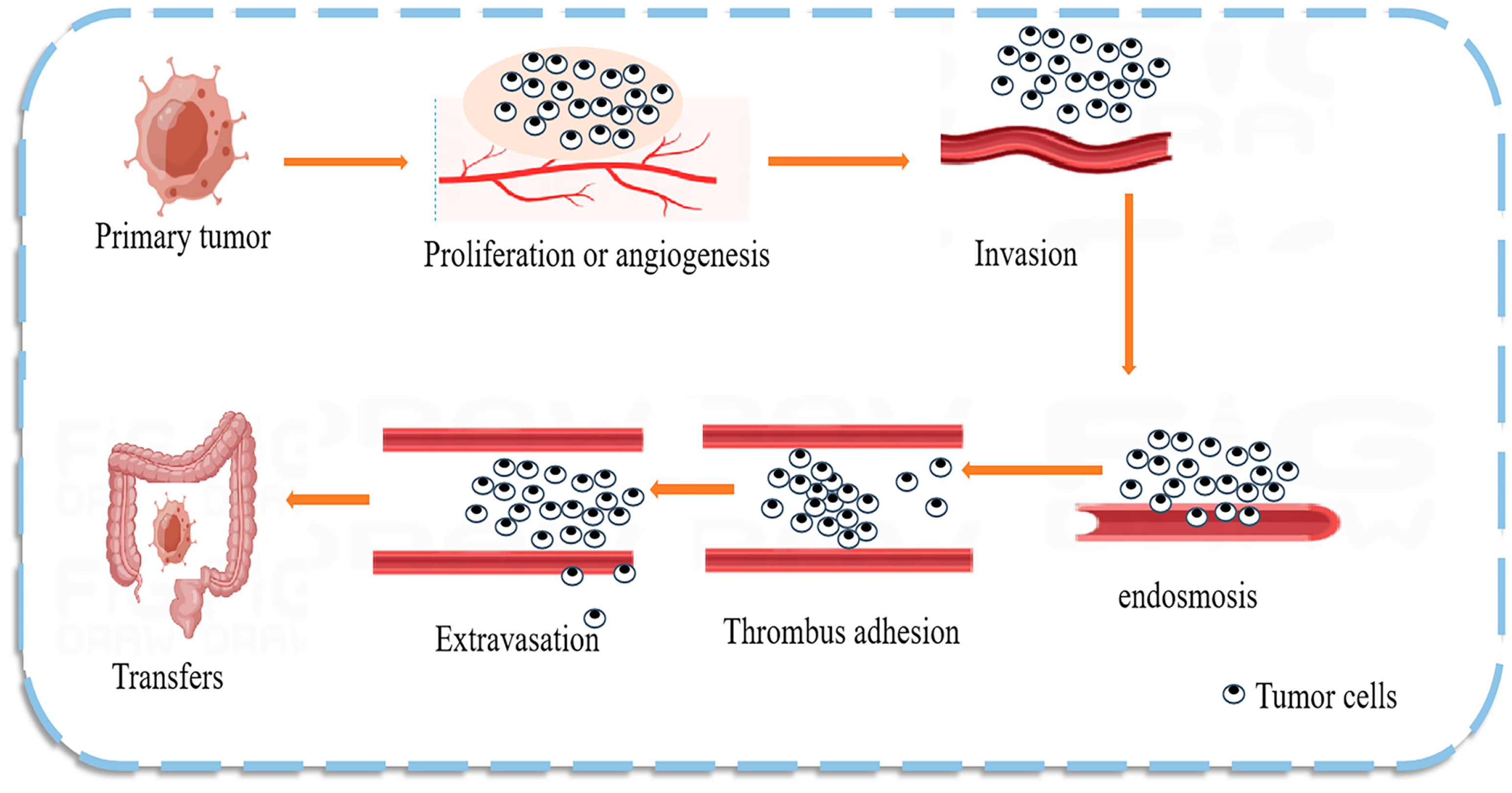
5. Novel Role of Natural Product-Targeted Regulatory miRNAs as Biomarkers in CRC
6. Natural Products Regulate miRNA Expression and Play Chemopreventive Roles in CRC
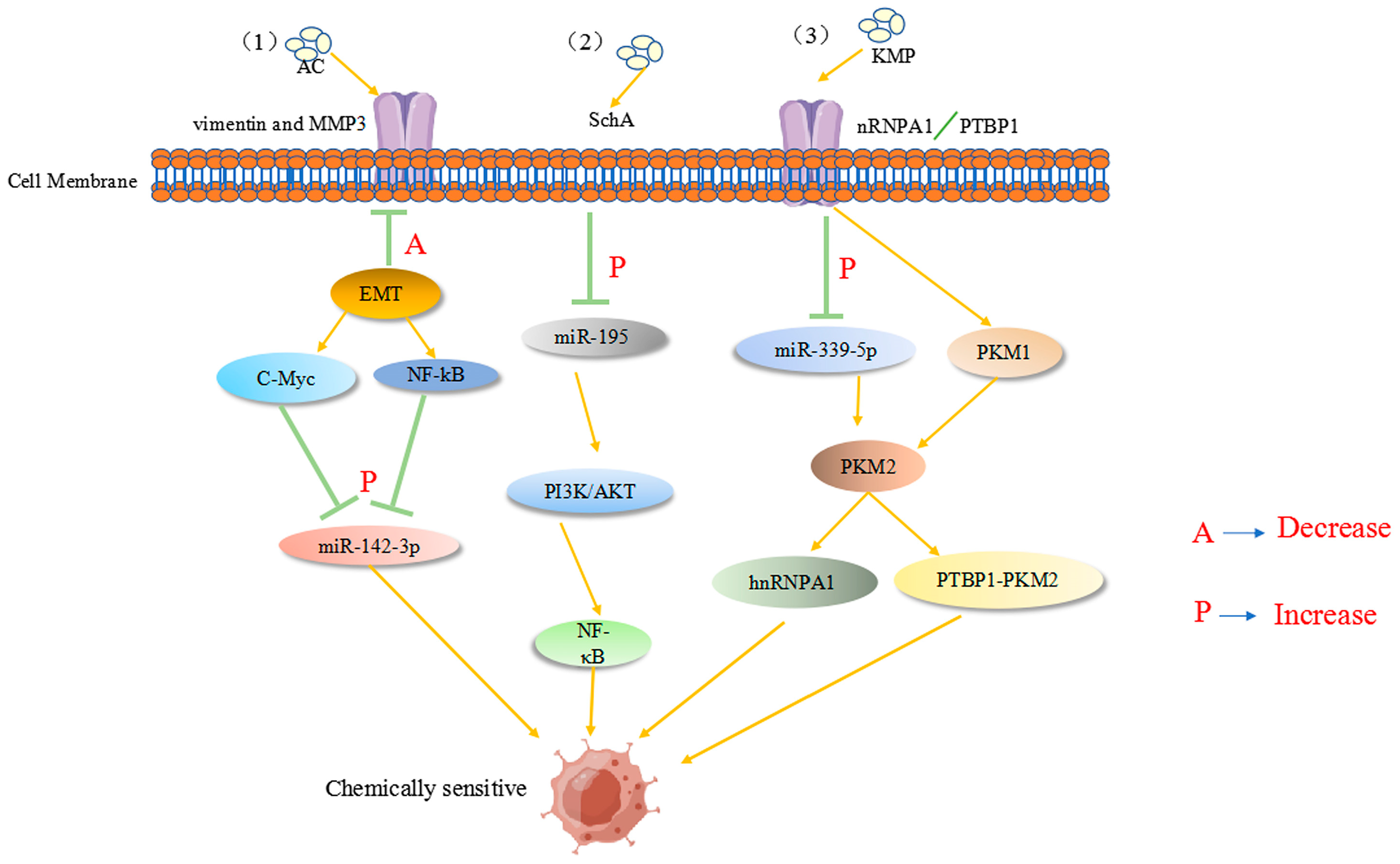
7. Natural Drugs Regulate ncRNA to Modulate Drug Resistance in Tumor Cells
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA. Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Fasano, C.; Grossi, V.; Forte, G.; Simone, C. Short Linear Motifs in Colorectal Cancer Interactome and Tumorigenesis. Cells 2022, 11, 3739. [Google Scholar] [CrossRef]
- Tang, X.; Ren, H.; Guo, M.; Qian, J.; Yang, Y.; Gu, C. Review on circular RNAs and new insights into their roles in cancer. Comput. Struct. Biotechnol. J. 2021, 19, 910–928. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Devadasan, V.; Raman, P.; Dasararaju, G. Anti-Cancer Compounds from Terrestrial and Marine Resources -In silico and Experimental Studies. Curr. Comput. Aided Drug Des. 2021, 17, 865–880. [Google Scholar] [CrossRef]
- Grigalunas, M.; Brakmann, S.; Waldmann, H. Chemical Evolution of Natural Product Structure. J. Am. Chem. Soc. 2022, 144, 3314–3329. [Google Scholar] [CrossRef]
- Vo, T.S. Natural products targeting FcεRI receptor for anti-allergic therapeutics. J. Food Biochem. 2020, 44, e13335. [Google Scholar] [CrossRef]
- Farroni, C.; Marasco, E.; Marcellini, V.; Giorda, E.; Valentini, D.; Petrini, S.; D’Oria, V.; Pezzullo, M.; Cascioli, S.; Scarsella, M.; et al. Dysregulated miR-155 and miR-125b Are Related to Impaired B-cell Responses in Down Syndrome. Front. Immunol. 2018, 9, 2683. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Ortiz, G.G.R.; Mohammadi, Y.; Nazari, A.; Ataeinaeini, M.; Kazemi, P.; Yasamineh, S.; Al-Naqeeb, B.Z.T.; Zaidan, H.K.; Gholizadeh, O. A state-of-the-art review on the MicroRNAs roles in hematopoietic stem cell aging and longevity. Cell Commun. Signal. 2023, 21, 85. [Google Scholar] [CrossRef]
- Nogami, M.; Miyamoto, K.; Hayakawa-Yano, Y.; Nakanishi, A.; Yano, M.; Okano, H. DGCR8-dependent efficient pri-miRNA processing of human pri-miR-9-2. J. Biol. Chem. 2021, 296, 100409. [Google Scholar] [CrossRef]
- Bayraktar, E.; Bayraktar, R.; Oztatlici, H.; Lopez-Berestein, G.; Amero, P.; Rodriguez-Aguayo, C. Targeting miRNAs and Other Non-Coding RNAs as a Therapeutic Approach: An Update. Noncoding RNA 2023, 9, 27. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.K.; Giri, R.; Kumar, D.; Sharma, R.; Valis, M.; Kuca, K.; Garg, N. The role of microRNA-21 in the onset and progression of cancer. Future Med. Chem. 2021, 13, 1885–1906. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Hussen, B.M.; Hidayat, H.J.; Salihi, A.; Sabir, D.K.; Taheri, M.; Ghafouri-Fard, S. MicroRNA: A signature for cancer progression. Biomed. Pharmacother. 2021, 138, 111528. [Google Scholar] [CrossRef]
- Szelągowski, A.; Kozakiewicz, M. A Glance at Biogenesis and Functionality of MicroRNAs and Their Role in the Neuropathogenesis of Parkinson’s Disease. Oxid. Med. Cell Longev. 2023, 2023, 7759053. [Google Scholar] [CrossRef]
- Hill, M.; Tran, N. miRNA interplay: Mechanisms and consequences in cancer. Dis. Model. Mech. 2021, 14, dmm047662. [Google Scholar] [CrossRef]
- Hill, M.; Tran, N. miRNA:miRNA Interactions: A Novel Mode of miRNA Regulation and Its Effect on Disease. Adv. Exp. Med. Biol. 2022, 1385, 241–257. [Google Scholar] [CrossRef]
- Taibi, A.; Lofft, Z.; Laytouni-Imbriaco, B.; Comelli, E.M. The role of intestinal microbiota and microRNAs in the anti-inflammatory effects of cranberry: From pre-clinical to clinical studies. Front. Nutr. 2023, 10, 1092342. [Google Scholar] [CrossRef]
- Desaulniers, D.; Vasseur, P.; Jacobs, A.; Aguila, M.C.; Ertych, N.; Jacobs, M.N. Integration of Epigenetic Mechanisms into Non-Genotoxic Carcinogenicity Hazard Assessment: Focus on DNA Methylation and Histone Modifications. Int. J. Mol. Sci. 2021, 22, 10969. [Google Scholar] [CrossRef]
- Saleh, H.A.; Yousef, M.H.; Abdelnaser, A. The Anti-Inflammatory Properties of Phytochemicals and Their Effects on Epigenetic Mechanisms Involved in TLR4/NF-κB-Mediated Inflammation. Front. Immunol. 2021, 12, 606069. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.J.; Qi, M.; Li, N.; Lei, Y.H.; Zhang, D.M.; Chen, J.X. Natural products and their derivatives: Promising modulators of tumor immunotherapy. J. Leukoc. Biol. 2020, 108, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jiang, Q. Twist1-mediated transcriptional activation of Claudin-4 promotes cervical cancer cell migration and invasion. Oncol. Lett. 2023, 26, 335. [Google Scholar] [CrossRef]
- Ma, Y.G.; Han, Y.Z.; Zhang, Z.S.; Yu, Y.; Xu, X.F.; Yuan, L. [MiR-451 regulates proliferation and migration of colorectal cells by targeting MIF]. Zhonghua Zhong Liu Za Zhi 2020, 42, 312–318. [Google Scholar] [CrossRef]
- Calixto, J.B. The role of natural products in modern drug discovery. An. Acad. Bras. Ciências 2019, 91 (Suppl. S3), e20190105. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.L.; Fu, W.J.; Liu, Z.H.; Lu, N.; Jia, X.Q.; Liu, Z.S. Research advance of natural products in tumor immunotherapy. Front. Immunol. 2022, 13, 972345. [Google Scholar] [CrossRef]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef]
- Cho, H.; Kim, J.E.; Hong, Y.S.; Kim, S.Y.; Kim, J.; Ryu, Y.M.; Kim, S.Y.; Kim, T.W. Comprehensive evaluation of the tumor immune microenvironment and its dynamic changes in patients with locally advanced rectal cancer treated with preoperative chemoradiotherapy: From the phase II ADORE study. Oncoimmunology 2022, 11, 2148374. [Google Scholar] [CrossRef]
- Chen, S.; Yang, J.; Wei, Y.; Wei, X. Epigenetic regulation of macrophages: From homeostasis maintenance to host defense. Cell Mol. Immunol. 2020, 17, 36–49. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Z.; Wu, T.; Zhao, Q.; Zhao, Q.; Cao, Y. LncGBP9/miR-34a axis drives macrophages toward a phenotype conducive for spinal cord injury repair via STAT1/STAT6 and SOCS3. J. Neuroinflamm. 2020, 17, 134. [Google Scholar] [CrossRef]
- Gazzillo, A.; Polidoro, M.A.; Soldani, C.; Franceschini, B.; Lleo, A.; Donadon, M. Relationship between Epithelial-to-Mesenchymal Transition and Tumor-Associated Macrophages in Colorectal Liver Metastases. Int. J. Mol. Sci. 2022, 23, 16197. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Sun, L.; Li, Q.; Guo, Y.; Yang, Q.; Yin, J.; Ran, Q.; Liu, L.; Zhao, Z.; Wang, Y.; Li, Y.; et al. Extract of Caulis Spatholobi, a novel platelet inhibitor, efficiently suppresses metastasis of colorectal cancer by targeting tumor cell-induced platelet aggregation. Biomed. Pharmacother. 2020, 123, 109718. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Tripathi, N.; Goel, B.; Jain, S.K. Anticancer Activity of Diosgenin and Its Semi-synthetic Derivatives: Role in Autophagy Mediated Cell Death and Induction of Apoptosis. Mini. Rev. Med. Chem. 2021, 21, 1646–1665. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.S.; Haynes, C.M. Folding the Mitochondrial UPR into the Integrated Stress Response. Trends Cell Biol. 2020, 30, 428–439. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Barakat, M.; Abu-Taha, M.I.; Mahmod, A.I. Targeting Drug Chemo-Resistance in Cancer Using Natural Products. Biomedicines 2021, 9, 1353. [Google Scholar] [CrossRef]
- Ding, F.; Yang, S. Epigallocatechin-3-gallate inhibits proliferation and triggers apoptosis in colon cancer via the hedgehog/phosphoinositide 3-kinase pathways. Can. J. Physiol. Pharmacol. 2021, 99, 910–920. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Y.; Cao, J. Matrine triggers colon cancer cell apoptosis and G0/G1 cell cycle arrest via mediation of microRNA-22. Phytother. Res. 2020, 34, 1619–1628. [Google Scholar] [CrossRef]
- Vedanayagam, J.; Chatila, W.K.; Aksoy, B.A.; Majumdar, S.; Skanderup, A.J.; Demir, E.; Schultz, N.; Sander, C.; Lai, E.C. Cancer-associated mutations in DICER1 RNase IIIa and IIIb domains exert similar effects on miRNA biogenesis. Nat. Commun. 2019, 10, 3682. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.P.; Sánchez-Martínez, R.; Vargas, T.; Herranz, J.; Martín-Hernández, R.; Mendiola, M.; Hardisson, D.; Reglero, G.; Feliu, J.; Redondo, A.; et al. The role of glycosyltransferase enzyme GCNT3 in colon and ovarian cancer prognosis and chemoresistance. Sci. Rep. 2018, 8, 8485. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Kwah, M.X.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef]
- Teodor, E.D.; Moroeanu, V.; Radu, G.L. Lignans from Medicinal Plants and their Anticancer Effect. Mini. Rev. Med. Chem. 2020, 20, 1083–1090. [Google Scholar] [CrossRef]
- Brockmueller, A.; Girisa, S.; Kunnumakkara, A.B.; Shakibaei, M. Resveratrol Modulates Chemosensitisation to 5-FU via β1-Integrin/HIF-1α Axis in CRC Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 4988. [Google Scholar] [CrossRef]
- Rahman, H.S. Preclinical Drug Discovery in Colorectal Cancer: A Focus on Natural Compounds. Curr. Drug Targets 2021, 22, 977–997. [Google Scholar] [CrossRef]
- Debnath, T.; Deb Nath, N.C.; Kim, E.K.; Lee, K.G. Role of phytochemicals in the modulation of miRNA expression in cancer. Food Funct. 2017, 8, 3432–3442. [Google Scholar] [CrossRef]
- Ebrahimpour, S.; Esmaeili, A.; Dehghanian, F.; Beheshti, S. Effects of quercetin-conjugated with superparamagnetic iron oxide nanoparticles on learning and memory improvement through targeting microRNAs/NF-κB pathway. Sci. Rep. 2020, 10, 15070. [Google Scholar] [CrossRef]
- Lan, C.Y.; Chen, S.Y.; Kuo, C.W.; Lu, C.C.; Yen, G.C. Quercetin facilitates cell death and chemosensitivity through RAGE/PI3K/AKT/mTOR axis in human pancreatic cancer cells. J. Food Drug Anal. 2019, 27, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.P.; Swetanshu; Singh, P.; Yadav, S.; Nigam, M.; Seidel, V.; Rodrigues, C.F. Role of the Dietary Phytochemical Curcumin in Targeting Cancer Cell Signalling Pathways. Plants 2023, 12, 1782. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Lertpiriyapong, K.; Steelman, L.S.; Abrams, S.L.; Yang, L.V.; Murata, R.M.; Rosalen, P.L.; Scalisi, A.; Neri, L.M.; Cocco, L.; et al. Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs. Aging 2017, 9, 1477–1536. [Google Scholar] [CrossRef]
- Lambring, C.; Varga, K.; Livingston, K.; Lorusso, N.; Dudhia, A.; Basha, R. Therapeutic Applications of Curcumin and Derivatives in Colorectal Cancer. Onco Ther. 2022, 9, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Abadi, A.J.; Mirzaei, S.; Mahabady, M.K.; Hashemi, F.; Zabolian, A.; Hashemi, F.; Raee, P.; Aghamiri, S.; Ashrafizadeh, M.; Aref, A.R.; et al. Curcumin and its derivatives in cancer therapy: Potentiating antitumor activity of cisplatin and reducing side effects. Phytother. Res. 2022, 36, 189–213. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Q.; Shi, X.; Zheng, Q.; Chen, L.; Sun, Y. Advances in plant-derived natural products for antitumor immunotherapy. Arch. Pharm. Res. 2021, 44, 987–1011. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Chen, L.; Guo, S.; Li, Y. Baicalin induced colon cancer cells apoptosis through miR-217/DKK1-mediated inhibition of Wnt signaling pathway. Mol. Biol. Rep. 2019, 46, 1693–1700. [Google Scholar] [CrossRef]
- Lu, L.; Cai, M.; Peng, M.; Wang, F.; Zhai, X. miR-491-5p functions as a tumor suppressor by targeting IGF2 in colorectal cancer. Cancer Manag. Res. 2019, 11, 1805–1816. [Google Scholar] [CrossRef]
- Panda, S.S.; Thangaraju, M.; Lokeshwar, B.L. Ursolic Acid Analogs as Potential Therapeutics for Cancer. Molecules 2022, 27, 8981. [Google Scholar] [CrossRef]
- Lin, W.; Ye, H. Anticancer activity of ursolic acid on human ovarian cancer cells via ROS and MMP mediated apoptosis, cell cycle arrest and downregulation of PI3K/AKT pathway. J. Buon 2020, 25, 750–756. [Google Scholar]
- Kim, K.; Shin, E.A.; Jung, J.H.; Park, J.E.; Kim, D.S.; Shim, B.S.; Kim, S.H. Ursolic Acid Induces Apoptosis in Colorectal Cancer Cells Partially via Upregulation of MicroRNA-4500 and Inhibition of JAK2/STAT3 Phosphorylation. Int. J. Mol. Sci. 2018, 20, 114. [Google Scholar] [CrossRef]
- Liu, Y.; Lang, T.; Jin, B.; Chen, F.; Zhang, Y.; Beuerman, R.W.; Zhou, L.; Zhang, Z. Luteolin inhibits colorectal cancer cell epithelial-to-mesenchymal transition by suppressing CREB1 expression revealed by comparative proteomics study. J. Proteom. 2017, 161, 1–10. [Google Scholar] [CrossRef]
- Yao, Y.; Rao, C.; Zheng, G.; Wang, S. Luteolin suppresses colorectal cancer cell metastasis via regulation of the miR-384/pleiotrophin axis. Oncol. Rep. 2019, 42, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Abdelnaby, H.B.; Abuhussein, A.A.; Fouad, A.M.; Alhashash, W.A.; Aldousari, A.S.; Abdelaleem, A.M.; Edelhamre, M.; Shahin, M.H.; Faisal, M. Histopathological and epidemiological findings of colonoscopy screening in a population with an average risk of colorectal cancer in Kuwait. Saudi J. Gastroenterol. 2021, 27, 158–165. [Google Scholar] [CrossRef]
- Shaukat, A.; Levin, T.R. Current and future colorectal cancer screening strategies. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 521–531. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, T.H.; Huang, Y.M.; Wei, P.L.; Lin, J.C. Involvement of microRNA in Solid Cancer: Role and Regulatory Mechanisms. Biomedicines 2021, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Cao, Y.H.; Chen, L.B.; Kang, R.; Huang, Z.X.; Lu, X.S. BMSC-derived exosomal lncRNA PTENP1 suppresses the malignant phenotypes of bladder cancer by upregulating SCARA5 expression. Cancer Biol. Ther. 2022, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Hu, X.; Du, Y.; Du, J. The role of miRNAs in colorectal cancer progression and chemoradiotherapy. Biomed. Pharmacother. 2021, 134, 111099. [Google Scholar] [CrossRef]
- Khalaf, K.; Hana, D.; Chou, J.T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, J.; Tong, Y.; Li, J.; Liu, B. miR-145-5p restrained cell growth, invasion, migration and tumorigenesis via modulating RHBDD1 in colorectal cancer via the EGFR-associated signaling pathway. Int. J. Biochem. Cell Biol. 2019, 117, 105641. [Google Scholar] [CrossRef]
- Li, Y.; Meng, L.; Li, B.; Li, Y.; Shen, T.; Zhao, B. The Exosome Journey: From Biogenesis to Regulation and Function in Cancers. J. Oncol. 2022, 2022, 9356807. [Google Scholar] [CrossRef]
- Aiello, P.; Sharghi, M.; Mansourkhani, S.M.; Ardekan, A.P.; Jouybari, L.; Daraei, N.; Peiro, K.; Mohamadian, S.; Rezaei, M.; Heidari, M.; et al. Medicinal Plants in the Prevention and Treatment of Colon Cancer. Oxid. Med. Cell Longev. 2019, 2019, 2075614. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, M.; Shan, X.; Zhou, X.; Wang, T.; Zhang, J.; Tao, J.; Cheng, W.; Chen, G.; Li, J.; et al. A panel of seven-miRNA signature in plasma as potential biomarker for colorectal cancer diagnosis. Gene 2019, 687, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Peng, W.W.; Wang, Y.; Zhong, L.; Zhang, X.; Zeng, L. β-catenin correlates with the progression of colon cancers and berberine inhibits the proliferation of colon cancer cells by regulating the β-catenin signaling pathway. Gene 2022, 818, 146207. [Google Scholar] [CrossRef] [PubMed]
- Anandappa, G.; Lampis, A.; Cunningham, D.; Khan, K.H.; Kouvelakis, K.; Vlachogiannis, G.; Hedayat, S.; Tunariu, N.; Rao, S.; Watkins, D.; et al. miR-31-3p Expression and Benefit from Anti-EGFR Inhibitors in Metastatic Colorectal Cancer Patients Enrolled in the Prospective Phase II PROSPECT-C Trial. Clin. Cancer Res. 2019, 25, 3830–3838. [Google Scholar] [CrossRef]
- Ganapathy, A.; Ezekiel, U. Phytochemical Modulation of MiRNAs in Colorectal Cancer. Medicines 2019, 6, 48. [Google Scholar] [CrossRef]
- Piao, Y.; Piao, M.; Ryu, K.H. Multiclass cancer classification using a feature subset-based ensemble from microRNA expression profiles. Comput. Biol. Med. 2017, 80, 39–44. [Google Scholar] [CrossRef]
- Wang, D.; Feng, M.; Ma, X.; Tao, K.; Wang, G. Transcription factor SP1-induced microRNA-146b-3p facilitates the progression and metastasis of colorectal cancer via regulating FAM107A. Life Sci. 2021, 277, 119398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, M.; Ding, Y.; Fan, Z.; Zhang, J.; Zhang, H.; Jiang, B.; Zhu, Y. Serum MicroRNA profile in patients with colon adenomas or cancer. BMC Med. Genom. 2017, 10, 23. [Google Scholar] [CrossRef]
- Zhao, Q.; Bi, Y.; Guo, J.; Liu, Y.; Zhong, J.; Liu, Y.; Pan, L.; Guo, Y.; Tan, Y.; Yu, X. Effect of pristimerin on apoptosis through activation of ROS/endoplasmic reticulum (ER) stress-mediated noxa in colorectal cancer. Phytomedicine 2021, 80, 153399. [Google Scholar] [CrossRef]
- Leone, A.; Longo, C.; Gerardi, C.; Trosko, J.E. Pro-Apoptotic Effect of Grape Seed Extract on MCF-7 Involves Transient Increase of Gap Junction Intercellular Communication and Cx43 Up-Regulation: A Mechanism of Chemoprevention. Int. J. Mol. Sci. 2019, 20, 3244. [Google Scholar] [CrossRef]
- Tian, Q.; Xu, Z.; Sun, X.; Deavila, J.; Du, M.; Zhu, M. Grape pomace inhibits colon carcinogenesis by suppressing cell proliferation and inducing epigenetic modifications. J. Nutr. Biochem. 2020, 84, 108443. [Google Scholar] [CrossRef]
- Hayakawa, S.; Ohishi, T.; Oishi, Y.; Isemura, M.; Miyoshi, N. Contribution of Non-Coding RNAs to Anticancer Effects of Dietary Polyphenols: Chlorogenic Acid, Curcumin, Epigallocatechin-3-Gallate, Genistein, Quercetin and Resveratrol. Antioxidants 2022, 11, 2352. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Bagherian, M.; Azami, N.; Bejandi, A.K.; Hushmandi, K.; Ang, H.L.; et al. Polychemotherapy with Curcumin and Doxorubicin via Biological Nanoplatforms: Enhancing Antitumor Activity. Pharmaceutics 2020, 12, 1084. [Google Scholar] [CrossRef] [PubMed]
- Tsoukas, M.A.; Ko, B.J.; Witte, T.R.; Dincer, F.; Hardman, W.E.; Mantzoros, C.S. Dietary walnut suppression of colorectal cancer in mice: Mediation by miRNA patterns and fatty acid incorporation. J. Nutr. Biochem. 2015, 26, 776–783. [Google Scholar] [CrossRef]
- Derry, M.M.; Raina, K.; Agarwal, R.; Agarwal, C. Characterization of azoxymethane-induced colon tumor metastasis to lung in a mouse model relevant to human sporadic colorectal cancer and evaluation of grape seed extract efficacy. Exp. Toxicol. Pathol. 2014, 66, 235–242. [Google Scholar] [CrossRef]
- Poudyal, D.; Cui, X.; Le, P.M.; Hofseth, A.B.; Windust, A.; Nagarkatti, M.; Nagarkatti, P.S.; Schetter, A.J.; Harris, C.C.; Hofseth, L.J. A key role of microRNA-29b for the suppression of colon cancer cell migration by American ginseng. PLoS ONE 2013, 8, e75034. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, X.; Chen, T.; Cheng, X.; Xiao, H.; Meng, X.; Jiang, Y. Inhibition and potential treatment of colorectal cancer by natural compounds via various signaling pathways. Front. Oncol. 2022, 12, 956793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hao, Y.; Yang, J.; Zhou, Y.; Li, J.; Yin, S.; Sun, C.; Ma, M.; Huang, Y.; Xi, J.J. Genome-wide functional screening of miR-23b as a pleiotropic modulator suppressing cancer metastasis. Nat. Commun. 2011, 2, 554. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhan, S.; Wang, Y.; Zhou, G.; Liang, H.; Chen, X.; Shen, H. Baicalin, the major component of traditional Chinese medicine Scutellaria baicalensis induces colon cancer cell apoptosis through inhibition of oncomiRNAs. Sci. Rep. 2018, 8, 14477. [Google Scholar] [CrossRef]
- Yeh, C.T.; Rao, Y.K.; Yao, C.J.; Yeh, C.F.; Li, C.H.; Chuang, S.E.; Luong, J.H.; Lai, G.M.; Tzeng, Y.M. Cytotoxic triterpenes from Antrodia camphorata and their mode of action in HT-29 human colon cancer cells. Cancer Lett. 2009, 285, 73–79. [Google Scholar] [CrossRef]
- Wang, S.; Mou, J.; Cui, L.; Wang, X.; Zhang, Z. Astragaloside IV inhibits cell proliferation of colorectal cancer cell lines through down-regulation of B7-H3. Biomed. Pharmacother. 2018, 102, 1037–1044. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, L.; Feng, J.; Lin, W.; Cai, Q.; Peng, J. Spica Prunellae extract suppresses the growth of human colon carcinoma cells by targeting multiple oncogenes via activating miR-34a. Oncol. Rep. 2017, 38, 1895–1901. [Google Scholar] [CrossRef]
- Hu, D.; Meng, R.Y.; Nguyen, T.V.; Chai, O.H.; Park, B.H.; Lee, J.S.; Kim, S.M. Inhibition of colorectal cancer tumorigenesis by ursolic acid and doxorubicin is mediated by targeting the Akt signaling pathway and activating the Hippo signaling pathway. Mol. Med. Rep. 2023, 27, 11. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, J.; Li, C.; Yuan, Y.; Fang, S.; Liu, W.; Qian, Y.; Ma, J.; Chang, L.; Chen, F.; et al. Exosome-mediated transfer of SNHG7 enhances docetaxel resistance in lung adenocarcinoma. Cancer Lett. 2022, 526, 142–154. [Google Scholar] [CrossRef]
- Vaghari-Tabari, M.; Majidinia, M.; Moein, S.; Qujeq, D.; Asemi, Z.; Alemi, F.; Mohamadzadeh, R.; Targhazeh, N.; Safa, A.; Yousefi, B. MicroRNAs and colorectal cancer chemoresistance: New solution for old problem. Life Sci. 2020, 259, 118255. [Google Scholar] [CrossRef]
- Quaglio, A.E.V.; Grillo, T.G.; De Oliveira, E.C.S.; Di Stasi, L.C.; Sassaki, L.Y. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J. Gastroenterol. 2022, 28, 4053–4060. [Google Scholar] [CrossRef]
- Yu, M.; Fang, Z.X.; Wang, W.W.; Zhang, Y.; Bu, Z.L.; Liu, M.; Xiao, X.H.; Zhang, Z.L.; Zhang, X.M.; Cao, Y.; et al. Wu-5, a novel USP10 inhibitor, enhances crenolanib-induced FLT3-ITD-positive AML cell death via inhibiting FLT3 and AMPK pathways. Acta Pharmacol. Sin. 2021, 42, 604–612. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Geng, L.; Yi, H.; Huo, W.; Talmon, G.; Kim, Y.C.; Wang, S.M.; Wang, J. Withdrawal: Transforming growth factor β mediates drug resistance by regulating the expression of pyruvate dehydrogenase kinase 4 in colorectal cancer. J. Biol. Chem. 2020, 295, 4368. [Google Scholar] [CrossRef]
- Al Bitar, S.; El-Sabban, M.; Doughan, S.; Abou-Kheir, W. Molecular mechanisms targeting drug-resistance and metastasis in colorectal cancer: Updates and beyond. World J. Gastroenterol. 2023, 29, 1395–1426. [Google Scholar] [CrossRef]
- Xiao, X.; Sticht, C.; Yin, L.; Liu, L.; Karakhanova, S.; Yin, Y.; Georgikou, C.; Gladkich, J.; Gross, W.; Gretz, N.; et al. Novel plant microRNAs from broccoletti sprouts do not show cross-kingdom regulation of pancreatic cancer. Oncotarget 2020, 11, 1203–1217. [Google Scholar] [CrossRef][Green Version]
- Medina-Lara, A.; Grigore, B.; Lewis, R.; Peters, J.; Price, S.; Landa, P.; Robinson, S.; Neal, R.; Hamilton, W.; Spencer, A.E. Cancer diagnostic tools to aid decision-making in primary care: Mixed-methods systematic reviews and cost-effectiveness analysis. Health Technol. Assess. 2020, 24, 1–332. [Google Scholar] [CrossRef]
- Shepshelovich, D.; Townsend, A.R.; Espin-Garcia, O.; Latifovic, L.; O’Callaghan, C.J.; Jonker, D.J.; Tu, D.; Chen, E.; Morgen, E.; Price, T.J.; et al. Fc-gamma receptor polymorphisms, cetuximab therapy, and overall survival in the CCTG CO.20 trial of metastatic colorectal cancer. Cancer Med. 2018, 7, 5478–5487. [Google Scholar] [CrossRef]
- Guo, C.; Liu, J.; Zhou, Q.; Song, J.; Zhang, Z.; Li, Z.; Wang, G.; Yuan, W.; Sun, Z. Exosomal Noncoding RNAs and Tumor Drug Resistance. Cancer Res. 2020, 80, 4307–4313. [Google Scholar] [CrossRef]
- Saad, E.D.; Buyse, M. Statistical Considerations for Trials in Adjuvant Treatment of Colorectal Cancer. Cancers 2020, 12, 3442. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, D.; Chu, X.; Wang, J. RETRACTED: Schizandrin A enhances chemosensitivity of colon carcinoma cells to 5-fluorouracil through up-regulation of miR-195. Biomed. Pharmacother. 2018, 99, 176–183. [Google Scholar] [CrossRef]
- Chen, J.F.; Tsai, Y.T.; Lai, Y.H.; Lin, C.C.; Chou, H.C.; Kuo, W.H.; Ko, M.L.; Wei, Y.S.; Wang, Y.S.; Lin, M.W.; et al. Proteomic analysis of Antrodia Cinnamomea-induced ER stress in liver cancer cells. J. Pharm. Biomed. Anal. 2020, 187, 113142. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Yuan, X.L.; Luo, Y.N.; Luo, M.N.; Zheng, Y. Effects of Culture Mechanism of Cinnamomum kanehirae and C. camphora on the Expression of Genes Related to Terpene Biosynthesis in Antrodia cinnamomea. Mycobiology 2022, 50, 121–131. [Google Scholar] [CrossRef]
- Huang, Y.J.; Yadav, V.K.; Srivastava, P.; Wu, A.T.; Huynh, T.T.; Wei, P.L.; Huang, C.F.; Huang, T.H. Antrodia cinnamomea Enhances Chemo-Sensitivity of 5-FU and Suppresses Colon Tumorigenesis and Cancer Stemness via Up-Regulation of Tumor Suppressor miR-142-3p. Biomolecules 2019, 9, 306. [Google Scholar] [CrossRef]
- Qin, T.; Zhu, W.; Kan, X.; Li, L.; Wu, D. Luteolin attenuates the chemoresistance of osteosarcoma through inhibiting the PTN/β-catenin/MDR1 signaling axis by upregulating miR-384. J. Bone Oncol. 2022, 34, 100429. [Google Scholar] [CrossRef]
- Han, H.; Li, Y.; Qin, W.; Wang, L.; Yin, H.; Su, B.; Yuan, X. miR-199b-3p contributes to acquired resistance to cetuximab in colorectal cancer by targeting CRIM1 via Wnt/β-catenin signaling. Cancer Cell Int. 2022, 22, 42. [Google Scholar] [CrossRef]
- Xia, D.; Li, W.; Tang, C.; Jiang, J. Astragaloside IV, as a potential anticancer agent. Front. Pharmacol. 2023, 14, 1065505. [Google Scholar] [CrossRef]
- Wu, H.; Du, J.; Li, C.; Li, H.; Guo, H.; Li, Z. Kaempferol Can Reverse the 5-Fu Resistance of Colorectal Cancer Cells by Inhibiting PKM2-Mediated Glycolysis. Int. J. Mol. Sci. 2022, 23, 3544. [Google Scholar] [CrossRef]
| Natural Products | ncRNA | Expression | Cell Models | Potential Clinical Values | Type of Bio-marker | Reference |
|---|---|---|---|---|---|---|
| Rosemary Extract | miR-15b | Decline | SW620, DLD-1 | Biomarkers | Diagnostics | [81] |
| Curcumin | miR-17-5p, miR-20a miR-27a, miR-21, miR-130a | Decline | RKO, SW480 HCT116 | Biomarkers | Diagnostics | [82] |
| Walnuts | miR-467c miR-1903, miR-3068 | Decline | HT-29 | Biomarkers | Prognosis | [83] |
| GSE | miR-19a, miR-20a, miR-103, miR-135b, miR-148a, miR-196a | Decline | HCT116, SW620 | Biomarkers | Prognosis | [84] |
| HAG | miR-29b | Raise | HCT116, DLD-1, LOVO | Biomarkers | Diagnostics | [85] |
| Rosmarinus officinalis L. | miR-15b | Raise | SW480 | Biomarkers | Diagnostics | [86] |
| Sulforaphane | miR-23b, miR-27b | Decline | NCM460, NCM356 | Biomarkers | Diagnostics | [87] |
| baicalein | miR-23a, miR-30a, miR-31c, miR-151, miR-205a, miR-29 | Raise Decline | HCT116, Panc-1, A549 | Biomarkers | Diagnostics | [88] |
| AC | miR-142-3p | Decline | HT-29, SW-480 | Biomarkers | Prognosis | [89] |
| AS-IV | miR-29c | Raise | SW620, HCT116 | Biomarkers | Diagnostics | [90] |
| Spica Prunellae | miR-34a | Decline | HCT-8 | Biomarkers | Prognosis | [91] |
| UA | miR-4500 | Raise | HCT-15, HCT-116, HT-29, Caco-2 | Biomarkers | Prognosis | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Chen, M.; Li, S.; Geng, Z.; Jin, Y.; Liu, D. Natural Products Treat Colorectal Cancer by Regulating miRNA. Pharmaceuticals 2023, 16, 1122. https://doi.org/10.3390/ph16081122
Guo S, Chen M, Li S, Geng Z, Jin Y, Liu D. Natural Products Treat Colorectal Cancer by Regulating miRNA. Pharmaceuticals. 2023; 16(8):1122. https://doi.org/10.3390/ph16081122
Chicago/Turabian StyleGuo, Shuoxi, Meiqi Chen, Shuangyang Li, Zijun Geng, Ye Jin, and Da Liu. 2023. "Natural Products Treat Colorectal Cancer by Regulating miRNA" Pharmaceuticals 16, no. 8: 1122. https://doi.org/10.3390/ph16081122
APA StyleGuo, S., Chen, M., Li, S., Geng, Z., Jin, Y., & Liu, D. (2023). Natural Products Treat Colorectal Cancer by Regulating miRNA. Pharmaceuticals, 16(8), 1122. https://doi.org/10.3390/ph16081122







