Synthesis of 1,2,3-Triazole-Containing Methoxylated Cinnamides and Their Antileishmanial Activity against the Leishmania braziliensis Species
Abstract
1. Introduction
2. Results
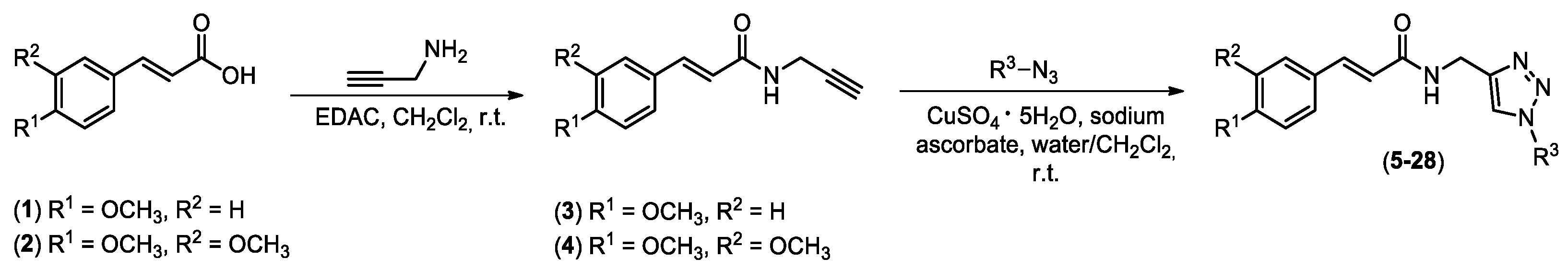
2.1. Synthesis of Chemical Derivatives
2.2. Antileishmanial Activity and Cytotoxicity on Mammalian Cells
2.3. Treatment of Infected Macrophages and Inhibition of Infection
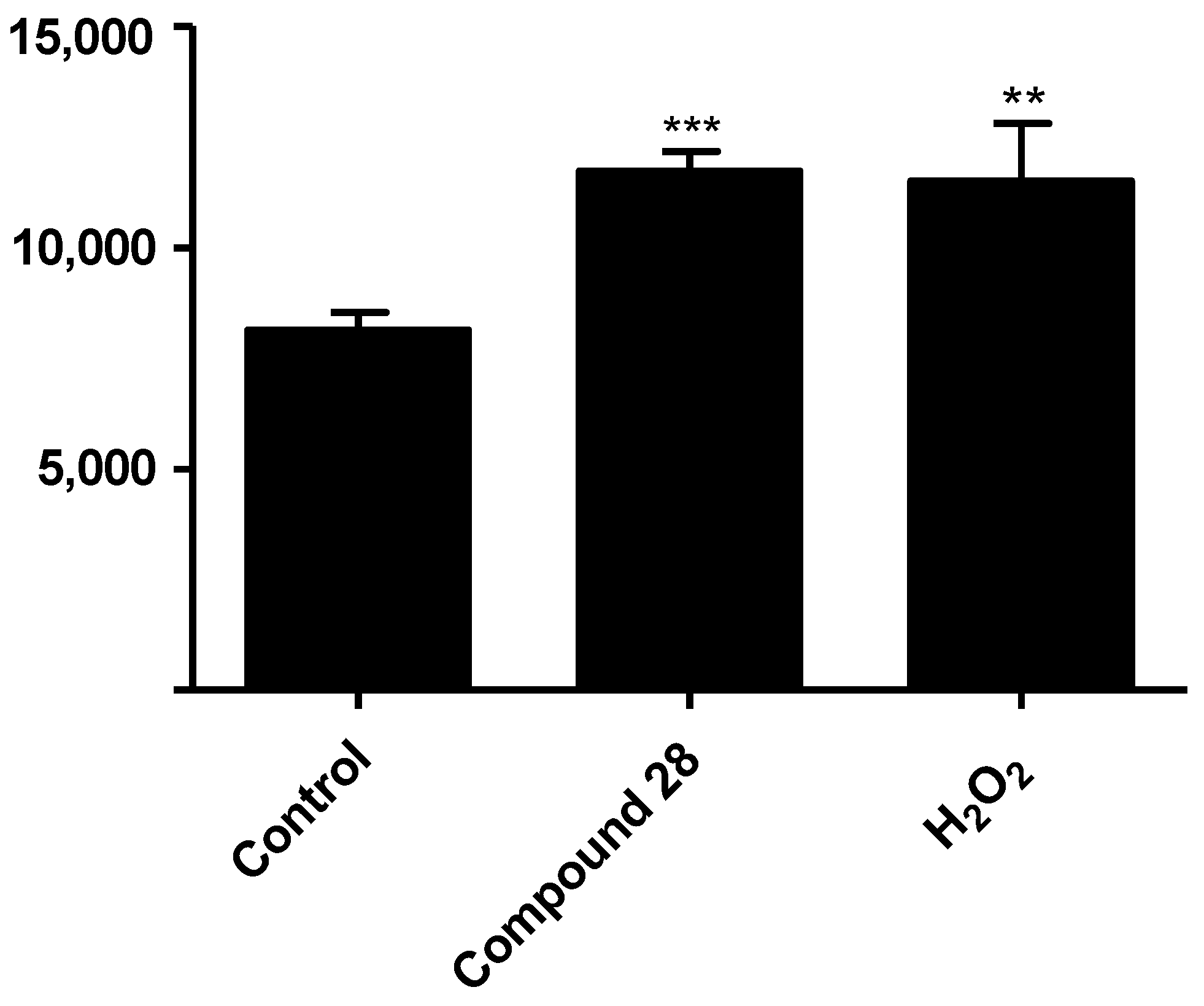
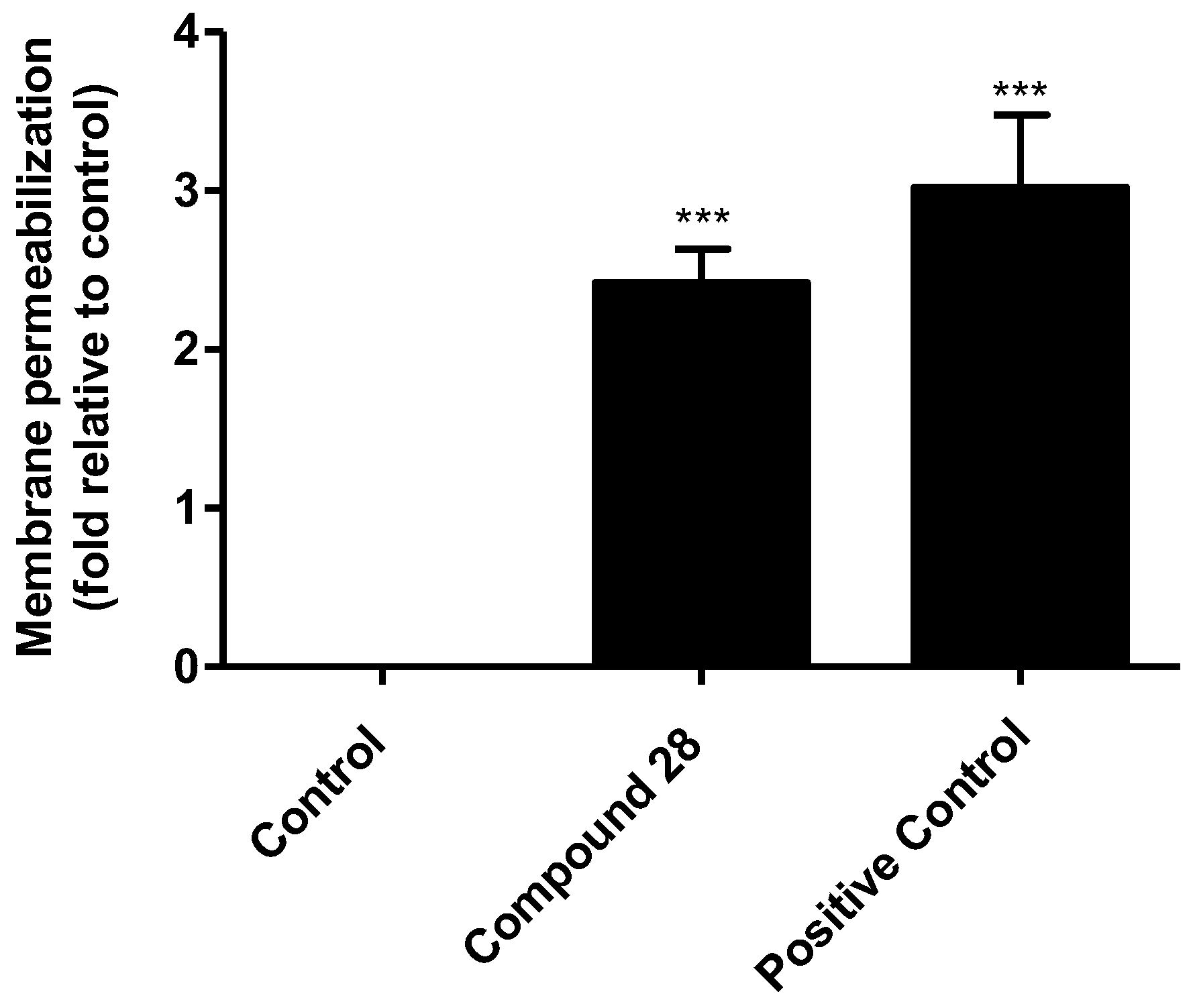
2.4. Preliminary Evaluation of the Mechanism of Action on Leishmania
2.5. Evaluation of Leishmania Targets by Compound 28
3. Discussion
4. Materials and Methods
4.1. Chemicals and General Information
4.2. Synthesis of Compounds 5–28 Exemplified through the Synthesis of N-((1-(4-methyl benzyl)-1H-1,2,3-triazol-4-yl)methyl) (4-methoxy) Cinnamide (5)
4.3. Parasite Culture, Antileishmanial Action, and Cytotoxicity in Mammalian Cells
4.4. Treatment of Infected Macrophages and Inhibition of Infection
4.5. ROS Levels in Leishmania-Infected Macrophages
4.6. Depolarization of Mitochondrial Membrane Potential (ΔΨm)
4.7. Evaluation of Reactive Oxygen Species (ROS) Production
4.8. Membrane Permeability Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Engels, D.; Zhou, X.-N. Neglected tropical diseases: An effective global response to local poverty-related disease priorities. Infect. Dis. Poverty 2020, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- WHO. Leishmaniasis, Fact Sheet No. 375; WHO: Geneva, Switzerland, 2015; Available online: http://www.who.int/mediacentre/factsheets/fs375/en/ (accessed on 13 August 2022).
- Serafim, T.D.; Coutinho-Abreu, I.V.; Dey, R.; Kissinger, R.; Valenzuela, J.G.; Oliveira, F.; Kamhawi, S. Leishmaniasis: The act of transmission. Trends Parasitol. 2021, 37, 976–987. [Google Scholar] [CrossRef]
- Kobets, T.; Grekov, I.; Lipoldová, M. Leishmaniasis: Prevention, parasite detection and treatment. Curr. Med. Chem. 2012, 19, 1443–1474. [Google Scholar] [CrossRef] [PubMed]
- Ashford, R.W. The leishmaniases as emerging and reemerging zoonoses. Int. J. Parasitol. 2000, 30, 1269–1281. [Google Scholar] [CrossRef]
- Uliana, S.R.B.; Trinconi, C.T.; Coelho, A.C. Chemotherapy of leishmaniasis: Present challenges. Parasitology 2018, 145, 464–480. [Google Scholar] [CrossRef]
- Frézard, F.; Demicheli, C.; Ribeiro, R.R. Pentavalent antimonials: New perspectives for old drugs. Molecules 2009, 14, 2317–2336. [Google Scholar] [CrossRef]
- Kumar, A.; Sen, P.; Roy, S. Use of antimony in the treatment of leishmaniasis: Current status and future directions. Mol. Biol. Int. 2011, 2011, 571242. [Google Scholar] [CrossRef]
- Lindoso, J.A.L.; Costa, J.M.L.; Goto, I.T.Q.H. Review of the current treatments for leishmaniasis. Res. Rep. Trop. Med. 2012, 3, 69–77. [Google Scholar] [CrossRef]
- de Macedo Silva, S.T.; Visbal, G.; Godinho, J.L.P.; Urbina, J.A.; de Souza, W.; Rodrigues, J.C.F. In vitro antileishmanial activity of ravuconazole, a triazole antifungal drug, as a potential treatment for leishmaniasis. J. Antimicrob. Chemother. 2018, 73, 2360–2373. [Google Scholar] [CrossRef]
- de Queiroz, V.T.; Botelho, B.D.O.; Guedes, N.A.; Cubides-Román, D.C.; Careta, F.D.P.; Freitas, J.C.C.; Cipriano, D.F.; Costa, A.V.; de Fátima, A.; Fernandes, S.A. Inclusion complexo f ketoconazole and p-sulfonic acid calix[6]arene improves antileishmanial activity and selectivity against Leishmania amazonensis and Leishmania infantum. Int. J. Pharm. 2023, 634, 122663. [Google Scholar] [CrossRef] [PubMed]
- Alrajhi, A.A.; Ibrahim, E.A.; de Vol, E.B.; Khairat, M.; Faris, R.M.; Maguire, J.H. Fluconazole for the treatment of cutaneous leishmaniasis cause by leishmania major. N. Engl. J. Med. 2002, 346, 891–895. [Google Scholar] [CrossRef] [PubMed]
- de Macedo-Silva, S.T.; Urbina, J.A.; de Souza, W.; Cola, J.; Rodrigues, F. In vitro activity of the antifungal azoles itraconazole and posaconazole against Leishmania amazonensis. PLoS ONE 2013, 8, e83427. [Google Scholar] [CrossRef] [PubMed]
- Gervazoni, L.F.O.; Barcellos, G.B.; Ferreira-Paes, T.; Almeida-Amaral, E.E. Use of natural products in leishmaniasis chemotherapy: An overview. Front. Chem. 2020, 8, 579891. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, J.A. The occurrence, metabolism and toxicity of cinnamic acid and related compounds. J. Appl. Toxicol. 1984, 4, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Perera-Córdova, W.H.; García, M.; Piñón, A.; Setzer, W.N. In-vitro and in-vivo activities of phenolic compounds against cutaneous leishmaniasis. Rec. Nat. Prod. 2016, 10, 269–276. [Google Scholar]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Costa, A.V.; de Oliveira, M.V.L.; Pinto, R.T.; Moreira, L.C.; Gomes, E.M.C.; de Assis Alves, T.; Pinheiro, P.F.; de Queiroz, V.T.; Vieira, L.F.A.; Teixeira, R.R.; et al. Synthesis of novel glycerol-derived 1,2,3-triazoles and evaluation of their fungicide, phytotoxic and cytotoxic activities. Molecules 2017, 22, 1666. [Google Scholar] [CrossRef]
- de Almeida Lima, G.D.; Rodrigues, M.P.; de Oliveira Mendes, T.A.; Moreira, G.A.; Siqueira, R.P.; da Silva, A.M.; Vaz, B.G.; Fietto, J.L.R.; Bresan, G.C.; Machado-Neves, M.; et al. Synthesis and antimetastic activity evaluation of cinnamic acid derivatives containing 1,2,3-triazolic portions. Toxicol. Vitr. 2018, 53, 1–9. [Google Scholar] [CrossRef]
- de Oliveira, A.S.; Gazolla, P.A.R.; Oliveira, A.F.C.S.; Pereira, W.L.; Viol, L.C.S.; Maia, A.F.S.; Santos, E.G.; Silva, I.E.P.; de Oliveira Mendes, T.A.; da Silva, A.M.; et al. Discovery of novel West Nile virus protease inhibitor based on isobenzofuranone and triazolic derivatives of eugenol and indan-1,3-dione scaffolds. PLoS ONE 2019, 14, e0223017. [Google Scholar] [CrossRef]
- do Vale, J.A.; Rodrigues, M.P.; Lima, A.M.A.; Santiago, S.S.; de Almeida Lima, G.D.; Lima, A.A.; de Oliveira, L.L.; Bressan, G.C.; Teixeira, R.R.; Machado-Neves, M. Synthesis of cinnamic acid ester derivatives with antiproliferative and antimetastic activities on murine melanoma cells. Biomed. Pharmacother. 2022, 148, 112689. [Google Scholar] [CrossRef]
- Santos, F.S.; do Vale, J.A.; Santos, L.S.; Gontijo, T.B.; Lima, G.D.A.; de Oliveira, L.L.; Machado-Neves, M.; Teixeira, R.R.; de Freitas, R.P. Synthesis of novel cinnamides and a bis cinnamate bearing 1,2,3-triazole functionalities with antiproliferative and antimetastic activities on melanoma cells. J. Braz. Chem. Soc. 2021, 32, 2174–2185. [Google Scholar] [CrossRef]
- Teixeira, R.R.; Gazolla, P.A.R.; da Silva, A.M.; Borsodi, M.P.G.; Bergmann, B.R.; Ferreira, R.S.; Vaz, B.G.; Vasconcelos, G.A.; Lima, W.P. Synthesis and antileishmanial activity of eugenol derivatives bearing 1,2,3-triazole functionalities. Eur. J. Med. Chem. 2018, 146, 274–286. [Google Scholar] [CrossRef]
- Teixeira, R.R.; da Silva, A.M.; Siqueira, R.P.; Gonçalves, V.H.S.; Pereira, H.S.; Ferreira, R.S.; Costa, A.V.; de Melo, E.B.; Paula, F.R.; Ferreira, M.M.C.; et al. Synthesis of nerol derivatives containing 1,2,3-triazole moiety and evaluation of their activities. J. Braz. Chem. Soc. 2019, 30, 541–561. [Google Scholar] [CrossRef]
- Evangelista, R.S.; Pereira, L.C.; de Souza, L.A.; Costa, A.V.; da Silva, D.A.; de Oliveira, F.M.; Vaz, B.G.; Bressan, G.C.; Fietto, J.L.R.; Teixeira, R.R. Synthesis and evaluation of the antileishmanial activity of novel eugenol analogs containing 1,2,3-triazole fragments against intracellular Leishmania braziliensis. J. Braz. Chem. Soc. 2023, in press. [CrossRef]
- Rodrigues, M.P.; Tomaz, D.C.; Ângelo de Souza, L.; Onofre, T.S.; de Menezes, W.A.; Almeida-Silva, J.; Suarez-Fontes, A.M.; de Almeida, M.R.; da Silva, A.M.; Bressan, G.C.; et al. Synthesis of cinnamic acid derivatives and antileishmanial activity against L. braziliensis. Eur. J. Med. Chem. 2019, 183, 11688. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef]
- Mandlik, V.; Patil, S.; Bopanna, R.; Basu, S.; Singh, S. Biological activity of coumarin derivatives as anti-leishmanial agents. PLoS ONE 2016, 11, e0164585. [Google Scholar] [CrossRef]
- Neises, B.; Steglich, W. Simple method for the esterification of carboxylic acids. Angew. Chem. Int. Ed. 1978, 17, 522–524. [Google Scholar] [CrossRef]
- Montalbetti, C.A.G.N.; Falque, V. Amide bond formation and peptide coupling. Tetrahedron 2005, 61, 10827–10852. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2598. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Petidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.S.; Chowdhury, S.; Koley, S. Advances of azide-alkyne cycloaddition-click chemistry over the recent decade. Tetrahedron 2016, 72, 5257–5283. [Google Scholar] [CrossRef]
- Borgati, T.F.; Alves, R.B.; Teixeira, R.R.; de Freitas, R.P.; Perdigão, T.G.; da Silva, S.F.; dos Santos, A.A.; Bastidas, A.J.O. Synthesis and phytotoxic activity of 1,2,3-triazole derivatives. J. Braz. Chem. Soc. 2013, 24, 953–961. [Google Scholar] [CrossRef]
- Sivakumar, K.; Xie, F.; Cash, M.; Long, S.; Barnhill, H.N.; Wang, Q. A fluorogenic 1,3-dipolar cycloaddition reaction of 3-azidocoumarinas and acetylenes. Org. Lett. 2004, 24, 4603–4606. [Google Scholar] [CrossRef]
- da Silva, E.R.; Come, J.A.A.S.; Brogi, S.; Calderone, V.; Chemi, G.; Campiani, G.; Oliveira, T.M.F.S.; Pham, T.-N.; Pudlo, M.; Girard, C.; et al. Cinnamides target Leishmania amazonensis arginase selectively. Molecules 2020, 25, 5271. [Google Scholar] [CrossRef]
- de Araújo-Vilges, K.M.; de Oliveira, S.V.; Couto, S.C.P.; Fokoue, H.H.; Romero, G.A.S.; Kato, M.J.; Romeiro, L.A.S.; Leite, J.R.S.A.; Kuckelhaus, S.A.S. Effect of piplartine and cinnamides on Leishmania amazonenses, Plasmodium falciparum and on peritoneal cells of Swiss mice. Pharm. Biol. 2017, 55, 1601–1607. [Google Scholar] [CrossRef]
- Nóbrega, F.R.; Silva, L.V.; Bezerra Filho, C.S.; Lima, T.C.; Castillo, Y.P.; Bezerra, D.P.; Lima, T.K.S.; de Sousa, D.P. Design, antileishmanial activity, and QSAR studies of a series of piplartine analogues. J. Chem. 2019, 2019, 4755756. [Google Scholar] [CrossRef]
- Chakravarty, J.; Sundar, S. Current and emerging medications for the treatment of leishmaniasis. Expert Opin. Pharmacother. 2019, 20, 1251–1265. [Google Scholar] [CrossRef]
- Sundar, S.; Singh, A.; Agrawal, N.; Chakravarty, J. Effectiveness of single-dose liposomal amphotericin B in visceral leishmaniasis in Bihar. Am. J. Trop. Med. Hyg. 2019, 101, 795–798. [Google Scholar] [CrossRef]
- Duran, G.; Duran, N.; Culha, G.; Ozcan, B.; Oztas, H.; Ozer, B. In vitro antileishmanial activity of Adana propolis samples on Leishmania tropica: A preliminary study. Parasitol. Res. 2008, 102, 1217–1225. [Google Scholar] [CrossRef]
- Holanda, V.N.; Silva, W.V.D.; Nascimento, P.H.D.; Silva, S.R.B.; Cabral Filho, P.E.; Assis, S.P.O.; Silva, C.A.D.; Oliveira, R.N.; Figueiredo, R.C.B.Q.; Lima, V.L.M. Antileishmanial activity of 4-phenyl-1-[2-phtalimido-2-yl)ethyl]-1H-1,2,3-triazole (PT4) derivative on Leishmania amazonensis and Leishmania braziliensis: In silico ADMET, in vitro activity, docking and molecular dynamic simulations. Bioorg. Chem. 2020, 105, 104437. [Google Scholar] [CrossRef]
- Koutsoni, O.; Barhoumi, M.; Guizani, I.; Dotsika, E. Leishmania eukaryotic initiation fator (LeIF) inhibits parasite growth in murine macrophages. PLoS ONE 2014, 9, e97319. [Google Scholar] [CrossRef]
- Fernandes, A.P.; Coelho, E.A.F.; Machado-Coelho, G.L.L.; Grimaldi, G., Jr.; Gazzinelli, R.T. Making an anti-amastigote vaccine for visceral leishmaniasis: Rational, update and perspectives. Curr. Opin. Microbiol. 2012, 15, 476–485. [Google Scholar] [CrossRef]
- Khouri, R.; Novais, F.; Santana, G.; de Oliveira, C.I.; dos Santos, M.A.V.; Barral, A.; Barral-Neto, M.; Weyenbergh, J.V. DETC induces Leishmania parasite killing in human in vitro and murine in vivo models: A promising therapeutic alternative in leishmaniasis. PLoS ONE 2010, 5, E14394. [Google Scholar] [CrossRef]
- Kian, D.; Lancheros, C.A.C.; Assolini, J.P.; Arakawa, N.S.; Veiga-Júnior, V.F.; Nakamura, C.V.; Pinge-Filho, P.; Conchon-Costa, I.; Pavanelli, W.R.; Yamada-Ogatta, S.F.; et al. Trypanocidal activity of copaiba oil and kaurenoic acid does not depend on macrophage killing machinery. Biomed. Pharmacother. 2018, 103, 1294–1301. [Google Scholar] [CrossRef]
- Holzmuller, P.; Bras-Gonçalves, R.; Lemsre, J.-L. Phenotypical characteristics, biochemical pathways, molecular targets and putative role of nitric oxide-mediated programmed cell death in Leishmania. Parasitology 2006, 132 (Suppl. 1), S19–S32. [Google Scholar] [CrossRef]
- Neris, D.M.; Ortolani, L.G.; de Castro, C.A.; de Oliveira Correia, R.; de Almeida Rodolpho, J.M.; Camillo, L.; Nogueira, C.T.; de Sousa, C.P.; de Freitas Anibal, F. In vitro modulator effect of total extract from the endophytic Paenibacillus polymyxa RNC-D in Leishmania (Leishmania) amazonensis and macrophages. Int. J. Microbiol. 2020, 2020, 8895308. [Google Scholar] [CrossRef]
- de Souza, W.; Attias, M.; Rodrigues, J.C.F. Particularities of mitochondrial structure in parasitic protists (Apicomplexa and Kinetoplastida). Int. J. Biochem. Cell Biol. 2009, 41, 2069–2080. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, L.M.; Gille, L. Mitochondria and trypanosomatids: Targets and drugs. Pharm. Res. 2011, 28, 2758–2770. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gálvez, S.-G.; Álvarez-Hernández, D.-A.; Gutiérrez-Kobeh, L.; Vázquez-López, R. Leishmania: Manipulation of signaling pathways to inhibit host cell apoptosis. Ther. Adv. Infect. Dis. 2021, 27, 20499361211014977. [Google Scholar] [CrossRef] [PubMed]
- Smirlis, D.; Duszenko, M.; Ruiz, A.J.; Scoulica, E.; Bastien, P.; Fasel, N.; Soteriadou, K. Targeting essential pathways in trypanosomatids gives insights into protozoan mechanisms of cell death. Parasites Vectors 2010, 17, 107. [Google Scholar] [CrossRef]
- Tomás, A.M.; Castro, H. Redox metabolisms in mitochondria of trypanosomatids. Antioxid. Redox Signal. 2013, 19, 696–707. [Google Scholar] [CrossRef]
- Turrens, J.F. Oxidative stress and antioxidant defenses: A target for the treatment of diseases caused by parasitic protozoa. Mol. Asp. Med. 2004, 25, 211–220. [Google Scholar] [CrossRef]
- de Souza, W.; Rodrigues, J.C.F. Sterol biosynthesis pathway as target for anti-trypanosomatid drugs. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 642502. [Google Scholar] [CrossRef]
- Raj, S.; Sasidharan, S.; Balaji, S.N.; Saudagar, P. An overview of biochemically characterized drug targets in metabolic pathway of Leishmania parasite. Parasitol. Res. 2020, 199, 2025–2037. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, V.; Tiwari, R.K.; Ravidas, V.; Pandey, K.; Kumar, A. Amphotericin B: A drug of choice for visceral leishmaniasis. Acta Trop. 2022, 235, 106661. [Google Scholar] [CrossRef]
- Darlo, T.P.C.; Balasegaram, M.; Beijnen, J.H.; de Vries, P.J. Miltefosine: A review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2576–2597. [Google Scholar] [CrossRef]
- Amaral, M.; de Sousa, F.S.; Silva, T.A.C.; Junior, A.J.G.; Taniwaki, N.N.; Johns, D.M.; Lago, J.H.G.; Anderson, E.A.; Tempone, A.G. A semi-synthetic neolignan derivative from dihydrodieugenol B selectively affects the bioenergetics system of Leishmania infantum and inhibits cell division. Sci. Rep. 2019, 9, 6114. [Google Scholar] [CrossRef]
- Glanzmann, N.; Antinarelli, L.M.R.; da Costa Nunes, I.K.; Pereira, J.M.G.; Coelho, E.A.F.; Coimbra, E.S.; da Silva, A.D. Synthesis and biological activity of novel 4-aminoquinoline/1,2,3-triazole hybrids against Leishmania amazonenesis. Biomed. Pharmacother. 2021, 141, 111857. [Google Scholar] [CrossRef]
- Morth, J.P.; Pedersen, B.P.; Buch-Pedersen, M.J.; Andersen, J.P.; Vilsen, B.; Palmgren, M.G.; Nissen, P. A structural overview of the plasma membrane Na+, K+-ATPase and H+-ATPase ions pumps. Nat. Rev. Mol. Cell Biol. 2011, 12, 60–70. [Google Scholar] [CrossRef]
- Coelho, E.A.; Tavares, C.A.; Carvalho, F.A.; Chaves, K.F.; Teixeira, K.N.; Rodrigues, R.C.; Charest, H.; Matlashewski, G.; Gazzinelli, R.T.; Fernandes, A.P. Immune responses induced by the Leishmania (Leishmania) donovani A2 antigen, but not by the LACK antigen, are protective against experimental Leishmania (Leishmania) amazonensis infection. Infect. Immun. 2003, 71, 3988–3994. [Google Scholar] [CrossRef]
- Valadares, D.G.; Duarte, M.C.; Oliveira, J.S.; Chávez-Fumagalli, M.A.; Martins, V.T.; Costa, L.E.; Leite, J.P.; Santoro, M.M.; Régis, W.C.; Tavares, C.A.; et al. Antileishmanial activity of the Agaricus blazei Murill in different Leishmania species. Parasitol. Int. 2011, 60, 357–363. [Google Scholar] [CrossRef]
- Tavares, G.S.V.; Mendonça, D.V.C.; Lage, D.P.; Antinarelli, L.M.R.; Soyer, T.G.; Senna, A.J.S.; Matos, G.F.; Dias, D.S.; Ribeiro, P.A.F.; Batista, J.P.T.; et al. In vitro and in vivo antileishmanial activity of a fluoroquinoline derivative against Leishmania infantum and Leishmania amazonensis species. Acta Trop. 2019, 191, 29–37. [Google Scholar] [CrossRef]
- Antinarelli, L.M.; Dias, R.M.; Souza, I.O.; Lima, W.P.; Gameiro, J.; da Silva, A.D.; Coimbra, E.S. 4-aminoquinoline derivatives as potential antileishmanial agentes. Chem. Biol. Drug Des. 2015, 86, 704–714. [Google Scholar] [CrossRef]


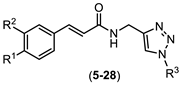 | ||||
|---|---|---|---|---|
| Compound | R1 | R2 | R3 | Yield (%) |
| 5 | OMe | H | 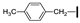 | 84 |
| 6 | OMe | H | 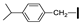 | 67 |
| 7 | OMe | H | 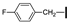 | 90 |
| 8 | OMe | H |  | 75 |
| 9 | OMe | H | 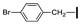 | 65 |
| 10 | OMe | H | 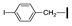 | 76 |
| 11 | OMe | H | 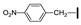 | 65 |
| 12 | OMe | H | 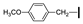 | 63 |
| 13 | OMe | H |  | 85 |
| 14 | OMe | H | 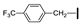 | 78 |
| 15 | OMe | H | 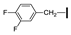 | 90 |
| 16 | OMe | OMe | 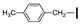 | 85 |
| 17 | OMe | OMe | 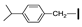 | 61 |
| 18 | OMe | OMe | 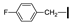 | 65 |
| 19 | OMe | OMe |  | 64 |
| 20 | OMe | OMe | 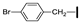 | 73 |
| 21 | OMe | OMe | 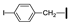 | 87 |
| 22 | OMe | OMe |  | 63 |
| 23 | OMe | OMe |  | 85 |
| 24 | OMe | OMe | 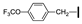 | 91 |
| 25 | OMe | OMe | 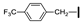 | 76 |
| 26 | OMe | OMe |  | 81 |
| 27 | OMe | H | 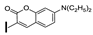 | 52 |
| 28 | OMe | OMe | 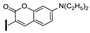 | 57 |
| Compound | IC50 (µg/mL) | CC50 (µg/mL) | |
|---|---|---|---|
| Promastigotes | Axenic Amastigotes | ||
| 5 | >600.0 | >600.0 | - |
| 6 | >600.0 | >600.0 | - |
| 7 | >600.0 | >600.0 | - |
| 8 | >600.0 | >600.0 | - |
| 9 | >600.0 | >600.0 | - |
| 10 | >600.0 | >600.0 | - |
| 11 | >600.0 | >600.0 | - |
| 12 | >600.0 | >600.0 | - |
| 13 | >600.0 | >600.0 | - |
| 14 | >600.0 | >600.0 | - |
| 15 | >600.0 | >600.0 | - |
| 16 | >600.0 | >600.0 | - |
| 17 | >600.0 | >600.0 | - |
| 18 | >600.0 | >600.0 | - |
| 19 | >600.0 | >600.0 | - |
| 20 | >600.0 | >600.0 | - |
| 21 | >600.0 | >600.0 | - |
| 22 | >600.0 | >600.0 | - |
| 23 | >600.0 | >600.0 | - |
| 24 | >600.0 | >600.0 | - |
| 25 | >600.0 | >600.0 | - |
| 26 | >600.0 | >600.0 | - |
| 27 | >600.0 | >600.0 | - |
| 28 | 105.7 ± 16.7 | 87.8 ± 9.7 | 1169.0 ± 113.2 |
| Amphotericin B | 0.974 ± 0.2 | 1.30 ± 0.4 | 8.90 ± 0.8 |
| Compounds | Concentration (µg/mL) | Percentage of Infected Macrophages after Treatment/Number of Amastigotes per Cell | Percentage of Infected Macrophages Using Pre-Incubated Parasites/Number of Amastigotes per Cell |
|---|---|---|---|
| 28 | 5.0 | 60.2/2.9 | 71.5/3.6 |
| 2.5 | 70.3/4.0 | 80.5/4.9 | |
| 1.0 | 79.5/6.7 | 84.5/5.8 | |
| 0 | 92.8/10.3 | 90.5/8.9 | |
| Amphotericin B | 5.0 | 19.8/1.8 | 51.4/5.3 |
| 2.5 | 27.6/3.5 | 67.6/6.5 | |
| 1.0 | 39.1/4.2 | 90.5/8.9 | |
| 0 | 92.8/10.3 | 90.5/8.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, F.S.d.; Freitas, R.P.d.; Freitas, C.S.d.; Mendonça, D.V.C.; Lage, D.P.; Tavares, G.d.S.V.; Machado, A.S.; Martins, V.T.; Costa, A.V.; Queiroz, V.T.d.; et al. Synthesis of 1,2,3-Triazole-Containing Methoxylated Cinnamides and Their Antileishmanial Activity against the Leishmania braziliensis Species. Pharmaceuticals 2023, 16, 1113. https://doi.org/10.3390/ph16081113
Santos FSd, Freitas RPd, Freitas CSd, Mendonça DVC, Lage DP, Tavares GdSV, Machado AS, Martins VT, Costa AV, Queiroz VTd, et al. Synthesis of 1,2,3-Triazole-Containing Methoxylated Cinnamides and Their Antileishmanial Activity against the Leishmania braziliensis Species. Pharmaceuticals. 2023; 16(8):1113. https://doi.org/10.3390/ph16081113
Chicago/Turabian StyleSantos, Fabíola Suelen dos, Rossimiriam Pereira de Freitas, Camila Simões de Freitas, Débora Vasconcelos Costa Mendonça, Daniela Pagliara Lage, Grasiele de Sousa Vieira Tavares, Amanda Sanchez Machado, Vivian Tamieti Martins, Adilson Vidal Costa, Vagner Tebaldi de Queiroz, and et al. 2023. "Synthesis of 1,2,3-Triazole-Containing Methoxylated Cinnamides and Their Antileishmanial Activity against the Leishmania braziliensis Species" Pharmaceuticals 16, no. 8: 1113. https://doi.org/10.3390/ph16081113
APA StyleSantos, F. S. d., Freitas, R. P. d., Freitas, C. S. d., Mendonça, D. V. C., Lage, D. P., Tavares, G. d. S. V., Machado, A. S., Martins, V. T., Costa, A. V., Queiroz, V. T. d., de Oliveira, M. B., Oliveira, F. M. d., Antinarelli, L. M. R., Coimbra, E. S., Pilau, E. J., da Silva, G. P., Coelho, E. A. F., & Teixeira, R. R. (2023). Synthesis of 1,2,3-Triazole-Containing Methoxylated Cinnamides and Their Antileishmanial Activity against the Leishmania braziliensis Species. Pharmaceuticals, 16(8), 1113. https://doi.org/10.3390/ph16081113







