Depressive and Other Adverse CNS Effects of Fluoroquinolones
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligible Studies
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction
2.5. Data Quality
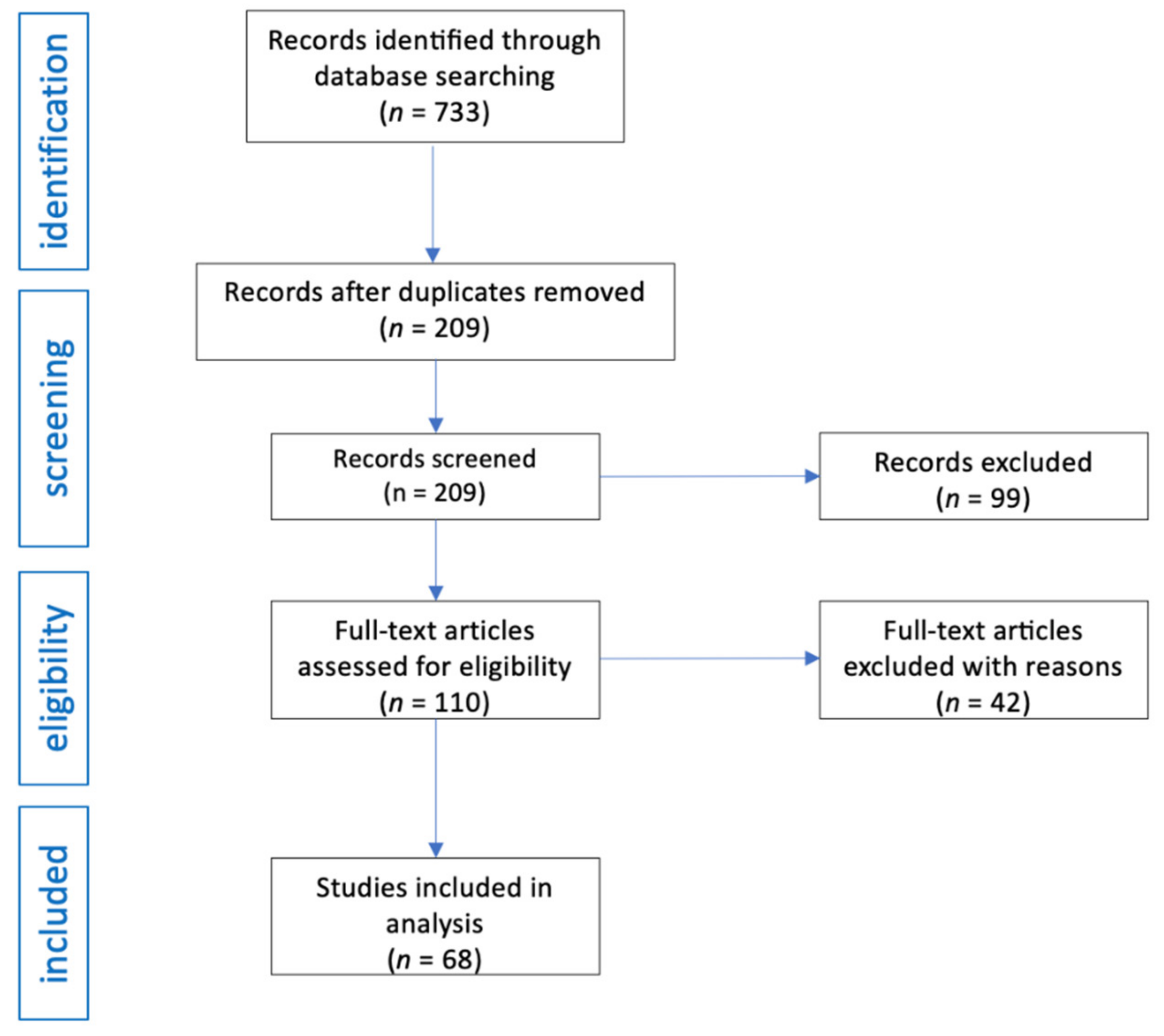
3. Results
3.1. Fluoroquinolone
3.2. Age and Sex
3.3. Indications for Fluoroquinolone Therapy
3.4. Route of Administration and Dose
3.5. Time of the Onset, Management, and Resolution of Psychiatric Symptoms
3.6. Concomitant Medication
3.7. Comorbidities and Substance Abuse
3.8. CNS Adverse Drug Reactions from Fluoroquinolones
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahoney, M.V.; Swords, K. Fluoroquinolones: Friends or Foes? Clin. Infect. Dis. 2021, 73, 857–858. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. MedChemComm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Hicks, L.A.; Bartoces, M.G.; Roberts, R.M.; Suda, K.J.; Hunkler, R.J.; Taylor, T.H., Jr.; Schrag, S.J. US Outpatient Antibiotic Prescribing Variation According to Geography, Patient Population, and Provider Specialty in 2011. Clin. Infect. Dis. 2015, 60, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- FDA in Brief: FDA Warns That Fluoroquino-Lone Antibiotics Can Cause Aortic Aneurysm in Certain Patients. Available online: https://www.fda.gov/news-events/fda-brief/fda-brief-fda-warns-fluoroquinolone-antibiotics-can-cause-aortic-aneurysm-certain-patients (accessed on 30 January 2021).
- Wang, J.; Gagne, J.J.; Kattinakere-Sreedhara, S.; Fischer, M.; Bykov, K. Association between initiation of fluoroquinolones and hospital admission or emergency department visit for suicidality: Population based cohort study. BMJ 2022, 379, e069931. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. FDA Drug Safety Communication: FDA Updates Warnings for Oral and Injectable Fluoroquinolone Antibiotics Due to Disabling Side Effects. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-up-dates-warnings-oral-and-injectable-fluoroquinolone-antibiotics (accessed on 4 November 2022).
- Tomé, A.M.; Filipe, A. Quinolones: Review of psychiatric and neurological adverse reactions. Drug Saf. 2011, 34, 465–488. [Google Scholar] [CrossRef]
- Zareifopoulos, N.; Panayiotakopoulos, G. Neuropsychiatric Effects of Antimicrobial Agents. Clin. Drug Investig. 2017, 37, 423–437. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Outpatient Antibiotic Prescriptions—United States, Annual Report 2016. Available online: https://www.cdc.gov/antibiotic-use/community/programs-measurement/state-local-activities/outpatient-antibiotic-prescriptions-US-2016.html (accessed on 4 November 2022).
- Buehrle, D.J.; Wagener, M.M.; Clancy, C.J. Outpatient Fluoroquinolone Prescription Fills in the United States, 2014 to 2020: Assessing the Impact of Food and Drug Administration Safety Warnings. Antimicrob. Agents Chemother. 2021, 65, e0015121. [Google Scholar] [CrossRef]
- World Health Organization. Model List of Essential Medicines—22nd List, 2021; WHO/MHP/HPS/EML/2021.02, Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Palma-Alvarez, R.F.; Duque-Yemail, J.; Ros-Cucurull, E.; Robles-Martínez, M.; Perea-Ortueta, M.; Grau-López, L.; Ramos-Quiroga, J. Quinolone-induced psychosis: An updated review. Actas Esp Psiquiatr. 2020, 48, 126–137. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Text Review; American Psychiatric Publishing, Inc.: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Odeh, M.; Kogan, Y.; Paz, A.; Elias, N. Delirium induced by levofloxacin. J. Clin. Neurosci. 2019, 66, 262–264. [Google Scholar] [CrossRef]
- Veličković-Radovanović, R.; Catić-Đorđević, A.; Dinić, K.; Radivojević, J.; Žikić, O.; Cvetković, T.; Mitić, B. Metronidazole- and levofloxacin-induced psychotic disorders in chronic kidney patient. Eur. J. Hosp. Pharm. 2019, 26, 347–349. [Google Scholar] [CrossRef]
- Muradian, M.; Khan, S. Levofloxacin-induced Psychosis in a Young Healthy Patient. Cureus 2019, 11, e6217. [Google Scholar] [CrossRef]
- Maharani, B.; Jafrin, A.L.; Bai, K.V.; Sivagnanam, G. Levofloxacin-induced tactile hallucination and acute anxiety reaction. Indian J. Pharmacol. 2019, 51, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.; Min, S.H.; Kim, J.-K.; Kang, K.W. Association between the Levofloxacin Plasma Concentration and Neurological Adverse Events in an Elderly Patient. J. Clin. Neurol. 2019, 15, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Annadatha, A.; Hepat, S.; Hulkoti, V.; Kumar, S. Levofloxacin induced psychosis in elderly: Case report. Ann. Geriatr. Educ. Med. Sci. 2019, 6, 33–34. [Google Scholar] [CrossRef]
- Steuber, H.; Williams, D.; Rech, M.A. Leave the levofloxacin? A case report of levofloxacin-induced psychosis. Am. J. Emerg. Med. 2018, 36, 1528.e1–1528.e2. [Google Scholar] [CrossRef]
- Kogan, Y.; Elias, N.; Paz, A.; Odeh, M. Acute Delirium Associated with Levofloxacin. J. Clin. Med. Res. 2018, 10, 725–727. [Google Scholar] [CrossRef]
- Takser, L.; Grad, R. Acute psychotic symptoms following a single dose of levofloxacin. Clin. Case Rep. 2017, 5, 2136–2137. [Google Scholar] [CrossRef]
- Ahan, E.; Setçelik, S. Levofloxacine Induced Acute Psychosis: Case Report. Turk. Klin. J. Case Rep. 2017, 25, 72–75. [Google Scholar]
- Khan, A.; Khan, E. F-’loco’-quinolones! Levofloxacin Induced Delirium with Psychotic Features. J. Am. Geriatr. Soc. 2017, 65, S194. [Google Scholar]
- Husain, N.S.F.D.; Goldman, P.M.; Goldrath, K.B.; Malas, N. Levofloxacin-Associated Neuroexcitation. J. Clin. Psychopharmacol. 2016, 36, 737–739. [Google Scholar] [CrossRef]
- Ghoshal, A.; Damani, A.; Salins, N.; Deodhar, J.; Muckaden, M. Management of levofloxacin induced anaphylaxis and acute delirium in a palliative care setting. Indian J. Palliat. Care 2015, 21, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Chowdhry, V.; Padhi, M.; Mohanty, B.B.; Mohapatra, S. Fluoroquinolones: An under-recognized cause for delirium. J. Anaesthesiol. Clin. Pharmacol. 2015, 31, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Chidozie, A.; Bikash, B.; Rawshan Ali, B.; Vikram, O.; Joseph, Q.; Marie, S.F.; Danilo, E. Levofloxacin-Induced Acute Psychosis. A Case Report. Chest 2015, 148, 209A. [Google Scholar] [CrossRef]
- Singh, D.; Kapoor, A.; Singhal, M.K.; Singh, V.; Kumar, H.S. Levofloxacin induced psychosis: A rare case report. Int. J. Basic Clin. Pharmacol. 2014, 3, 726–728. [Google Scholar]
- Raj, V.; Murthy, T. Levofloxacin induced delirium with psychotic features in a young patient. Med. J. Armed Forces India 2013, 69, 404–405. [Google Scholar] [CrossRef]
- Lertxundi, U.; Palacios, R.H.; Gutierrez, F.C.; Domingo-Echaburu, S.; García, M.G.; Gomez, C.A. Levofloxacin-induced delirium in a patient suffering from schizoaffective disorder and multiple sclerosis. Curr. Drug Saf. 2013, 8, 199–200. [Google Scholar]
- Pires, A.; Mariz, J.; Esperança, S.; Rua, A. Levofloxacin-Induced Acute Psychosis in an Elderly Man. Ann. Long-Term Care 2011, 19, 37–39. [Google Scholar]
- Kocyigit, I.; Dortdudak, S.; Sipahioglu, M.; Unal, A.; Yucel, H.E.; Tokgoz, B.; Eroglu, E.; Oymak, O.; Utas, C. Levofloxacin-Induced Delirium: Is It a Dangerous Drug in Patients with Renal Dysfunction? Ren. Fail. 2012, 34, 634–636. [Google Scholar] [CrossRef]
- Kandasamy, A.; Srinath, D. Levofloxacin-induced acute anxiety and insomnia. J. Neurosci. Rural. Pract. 2012, 3, 212–214. [Google Scholar] [CrossRef]
- LaSalvia, E.A.; Domek, G.J.; Gitlin, D.F. Fluoroquinolone-induced suicidal ideation. Gen. Hosp. Psychiatry 2010, 32, 108–110. [Google Scholar] [CrossRef]
- Slobodin, G.; Elias, N.; Zaygraikin, N.; Sheikh-Ahmad, M.; Sabetay, S.; Weller, B.; Odeh, M. Levofloxacin-induced delirium. Neurol. Sci. 2009, 30, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, N.; Raghavendra, N.; Venkatarathnamma, P. Levofloxacin-induced acute psychosis. Indian J. Psychiatry 2008, 50, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Kiangkitiwan, B.; Doppalapudi, A.; Fonder, M.; Solberg, K.; Bohner, B. Levofloxacin-induced delirium with psychotic features. Gen. Hosp. Psychiatry 2008, 30, 381–383. [Google Scholar] [CrossRef]
- Hakko, E.; Mete, B.; Ozaras, R.; Tabak, F.; Ozturk, R.; Mert, A. Levofloxacin-induced delirium. Clin. Neurol. Neurosurg. 2005, 107, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Yoshida, A.; Masuda, Y.; Fukayama, M.; Kita, Y.; Inamatsu, T. Levofloxacin-induced neurological adverse effects such as convulsion, involuntary movement (tremor, myoclonus and chorea like), visual hallucination in two elderly patients. Jpn. J. Geriatr. 1999, 36, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Jiang, B.; Tan, S.; Li, F. A Case of Ciprofloxacin-induced Acute Psychosis After Aortic Dissection Surgery. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Nazef, C.; Arif, A. Ciprofloxacin-Induced Acute Psychosis: A Case Report. Am. J. Med. Case Rep. 2019, 7, 143–144. [Google Scholar] [CrossRef]
- Weir, R.A. A Case Report of Ciprofloxacin-Induced Psychosis with Pseudobulbar Affect. J. Neuro-Psychiatry Clin. Neurosci. 2018, 30, E35. [Google Scholar]
- Rossi, G.; Mazoki, K. Acute Psychosis after Treatment of Epididymitis with Ciprofloxacin. Cureus 2018, 10, e2605. [Google Scholar] [CrossRef]
- Ransing, R.S.; Sarkar, D. Ciprofloxacin Induced Antibiomania. J. Clin. Diagn. Res. 2016, 10, VL01. [Google Scholar] [CrossRef]
- Dikici, S. A Case of Ciprofloxacin Induced Delirium. Konuralp Tip Derg. 2015, 7, 172–173. [Google Scholar]
- Grimm, O.; Alm, B.; Für Seelische, Z. A Case of Ciprofloxacin-Induced Acute Polymorphic Psychosis with a Distinct Deficit in Executive Functions. Psychosomatics 2007, 48, 269. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.; Kamat, R.; Padmanabh, R.V. Ciprofloxacin induced nightmares in an adult patient. Indian J. Psychiatry 2008, 50, 305–306. [Google Scholar] [CrossRef]
- Jayathissa, S.; Woolley, M.; Ganasegaram, M.; Holden, J.; Cu, E. Myoclonus and delirium associated with ciprofloxacin. Age Ageing 2010, 39, 762. [Google Scholar] [CrossRef]
- Ahmed, A.I.; van der Heijden, F.M.; Van den Berkmortel, H.; Kramers, K. A man who wanted to commit suicide by hanging himself: An adverse effect of ciprofloxacin. Gen. Hosp. Psychiatry 2011, 33, 82.e5–82.e7. [Google Scholar] [CrossRef] [PubMed]
- Denysenko, L.; Nicolson, S.E. Cefoxitin and Ciprofloxacin Neurotoxicity and Catatonia in a Patient on Hemodialysis. Psychosomatics 2011, 52, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Labay-Kamara, U.; Manning, S.; McMahon, T. Fluoroquinolone–Induced Suicidal Ideation and Suicidality. Psychosomatics 2012, 53, 97–98. [Google Scholar] [CrossRef]
- Ben-Chetrit, E.; Rothstein, N.; Munter, G. Ciprofloxacin-Induced Psychosis. Antimicrob. Agents Chemother. 2013, 57, 4079. [Google Scholar] [CrossRef][Green Version]
- Ranjan, A.; Praharaj, S.K. Ciprofloxacin-Induced Psychosis. J. Neuropsychiatry 2014, 26, E36–E37. [Google Scholar] [CrossRef]
- Steinert, T.; Studemund, H. Acute Delusional Parasitosis under Treatment with Ciprofloxacin. Pharmacopsychiatry 2006, 39, 159–160. [Google Scholar] [CrossRef]
- Bhalerao, S.; Talsky, A.; Hansen, K.; Kingstone, E.; Schroeder, B.; Karim, Z.; Fung, I. Ciprofloxacin-Induced Manic Episode. Psychosomatics 2006, 47, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Norra, C.; Skobel, E.; Breuer, C.; Haase, G.; Hanrath, P.; Hoff, P. Ciprofloxacin-induced acute psychosis in a patient with multidrug-resistant tuberculosis. Eur. Psychiatry 2003, 18, 262–263. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Chen, S.; O’Sullivan, S. Acute psychosis following the use of topical ciprofloxacin. Arch. Ophthalmol. 2002, 120, 665–666. [Google Scholar] [PubMed]
- Al-Ghamdi, S.M. Reversible Encephalopathy and Delirium in Patients with Chronic Renal Failure who had Received Ciprofloxacin. Saudi J. Kidney Dis. Transplant. 2002, 13, 163–170. [Google Scholar]
- Grassi, L.; Biancosino, B.; Pavanati, M.; Agostini, M.; Manfredini, R. Agostini Depression or hypoactive delirium? A report of ciprofloxacin-induced mental disorder in a patient with chronic obstructive pulmonary disease. Psychother. Psychosom. 2001, 70, 58–59. [Google Scholar] [CrossRef]
- James, E.; Demian, A.Z. Acute psychosis in a trauma patient due to ciprofloxacin. Postgrad. Med. J. 1998, 74, 189–190. [Google Scholar] [CrossRef][Green Version]
- Jay, G.T.; Fitzgerald, J.M. Ciprofloxacin-Induced Delirium. Ann. Pharmacother. 1997, 31, 252. [Google Scholar] [CrossRef]
- Mulhall, J.P.; Bergmann, L.S. Ciprofloxacin-induced acute psychosis. Urology 1995, 46, 102–103. [Google Scholar] [CrossRef]
- Farrington, J.; Stoudemire, A.; Tierney, J. The role of ciprofloxacin in a patient with delirium due to multiple etiologies. Gen. Hosp. Psychiatry 1995, 17, 47–53. [Google Scholar] [CrossRef]
- Reeves, R.R. Ciprofloxacin-Induced Psychosis. Ann. Pharmacother. 1992, 26, 930–931. [Google Scholar] [CrossRef]
- McCue, J.D.; Zandt, J.R. Acute psychoses associated with the use of ciprofloxacin and trime-thoprim-sulfamethoxazole. Am. J. Med. 1991, 90, 528–529. [Google Scholar] [CrossRef] [PubMed]
- Khadka, S.; Singh, P.; Khadka, M.; Chakrabarti, K.; Pandit, S.; Dhonju, G.; Gautam, S. A Case Report on Fluoro-Quinolones (Ofloxacin) Induced Psychosis at Nepal Medical College Teaching Hospital. J. Psychiatr. Assoc. Nepal 2019, 8, 66–67. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Sharan, R.; Praharaj, S.K. High Dose Ofloxacin-induced Bimodal Hallucinations in a 4 Years Old Child. Clin. Psychopharmacol. Neurosci. 2017, 15, 416–417. [Google Scholar] [CrossRef][Green Version]
- Chauhan, U.; Shanbag, P.; Kashid, P. Ofloxacin-induced hallucinations. Indian J. Pharmacol. 2013, 45, 189–190. [Google Scholar] [CrossRef]
- Koul, S.; Bhan-Kotwal, S.; Jenkins, H.; Carmaciu, C. Organic psychosis induced by ofloxacin and metronidazole. Br. J. Hosp. Med. 2009, 70, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Guven, E.O.; Balbay, D.; Kilciler, M. Unexpected, severe central nerve system toxicity of ofloxacine: Report of two cases. Int. Urol. Nephrol. 2007, 39, 647–649. [Google Scholar] [CrossRef]
- Hall, C.; Keegan, H.; Rogstad, K. Psychiatric side effects of ofloxacin used in the treatment of pelvic inflammatory disease. Int. J. STD AIDS 2003, 14, 636–637. [Google Scholar] [CrossRef]
- Uz, B. Moxifloxacin-Induced Visual Hallucinations, Alterations in Mood and Behavior, and Hyperglycemia. J. Pharm. Pract. 2020, 33, 368–371. [Google Scholar] [CrossRef]
- Higdon, E.; Twilla, J.D.; Sands, C. Moxifloxacin-Induced Visual Hallucinations: A Case Report and Review of the Literature. J. Pharm. Pract. 2017, 30, 375–377. [Google Scholar] [CrossRef]
- Mazhar, F.; Akram, S.; Haider, N. Moxifloxacin-induced acute psychosis: A case report with literature review. J. Res. Pharm. Pract. 2016, 5, 294–296. [Google Scholar] [CrossRef]
- Karagoz, E.; Ulcay, A.; Budakli, A.; Tutuncu, R. Moxifloxacin Induced Acute Delirium with Visual Hallucinations. Med. Sci. Int. Med. J. 2015, 4, 2694–2699. [Google Scholar] [CrossRef]
- Pepele, M.S.; Ertan, C.; Yucel, N. Moxifloxacin Hydrochloride Related Visual Hallucinations: A Case Presentation. Turk. J. Emerg. Med. 2013, 13, 141–143. [Google Scholar] [CrossRef]
- Tasleem, H.; Viswanathan, R. Moxifloxacin-Induced Delirium with Hallucinations. Psychosomatics 2011, 52, 472–474. [Google Scholar] [CrossRef]
- Sharma, D.; Aggarwal, A.; Sharma, R.; Kumar, R. A probable association of acute dystonia with gemifloxacin administration. Indian J. Med. Sci. 2009, 63, 557–560. [Google Scholar] [CrossRef][Green Version]
- Barrett, M.J.; Login, I.S. Gemifloxacin-Associated Neurotoxicity Presenting as Encephalopathy. Ann. Pharmacother. 2009, 43, 782–784. [Google Scholar] [CrossRef]
- Stroud, S.G.; Kandemir, U. Acute Delirium Induced by Ciprofloxacin in a Patient with Chronic Kidney Disease: A Case Report. JBJS Case Connect. 2020, 10, e0603. [Google Scholar] [CrossRef]
- Bangert, M.K.; Hasbun, R. Neurological and Psychiatric Adverse Effects of Antimicrobials. CNS Drugs 2019, 33, 727–753. [Google Scholar] [CrossRef]
- Sellick, J.; Mergenhagen, K.; Morris, L.; Feuz, L.; Horey, A.; Risbood, V.; Wojciechowski, A.; Ruh, C.; Bednarczyk, E.; Conway, E.; et al. Fluoroquinolone-Related Neuropsychiatric Events in Hospitalized Veterans. Psychosomatics 2018, 59, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Marinella, M.A. Myoclonus and generalized seizures associated with gatifloxacin treatment. Arch. Intern. Med. 2001, 161, 2261–2262. [Google Scholar] [CrossRef]
- Heyd, A.; Haverstock, D. Retrospective analysis of the safety profile of oral and intravenous ciprofloxacin in a geriatric population. Clin. Ther. 2000, 22, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Shimada, J.; Hori, S. Adverse effects of fluoroquinolones. Prog. Drug Res. 1992, 38, 133–143. [Google Scholar] [PubMed]
- Furuhama, K.; Akahane, K.; Tawara, K.; Takayama, S. Interaction of the new quinolone antibacterial agent levofloxacin with fenbufen in mice. Arzneimittelforschung 1992, 43, 406–408. [Google Scholar] [PubMed]
- Desai, C. Meyler’s side effects of drugs: The international encyclopedia of adverse drug reactions and interactions. Indian J. Pharmacol. 2016, 48, 224. [Google Scholar]
- Mehlhorn, A.J.; Brown, D. Safety Concerns with Fluoroquinolones. Ann. Pharmacother. 2007, 41, 1859–1866. [Google Scholar] [CrossRef]
- Zaudig, M.; von Bose, M.; Weber, M.M.; Bremer, D.; Zieglgänsberger, W. Psychotoxic Effects of Ofloxacin. Pharmacopsychiatry 1989, 22, 11–15. [Google Scholar] [CrossRef]
- Tango, R.C. Psychiatric side effects of medications prescribed in internal medicine. Dialog. Clin. Neurosci. 2003, 5, 155–165. [Google Scholar] [CrossRef]
- Kuriyama, A.; Jackson, J.L.; Doi, A.; Kamiya, T. Metronidazole-Induced Central Nervous System Toxicity: A systematic review. Clin. Neuropharmacol. 2011, 34, 241–247. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
| Fluoroquinolone | Number of Cases | Reference |
|---|---|---|
| Levofloxacin | 30 | [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40] |
| Ciprofloxacin | 27 | [41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66] |
| Ofloxacin | 8 | [67,68,69,70,71,72] |
| Moxifloxacin | 6 | [73,74,75,76,77,78] |
| Gemifloxacin | 2 | [79,80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzbiński, P.; Hubska, J.; Henzler, M.; Kucharski, B.; Bieś, R.; Krzystanek, M. Depressive and Other Adverse CNS Effects of Fluoroquinolones. Pharmaceuticals 2023, 16, 1105. https://doi.org/10.3390/ph16081105
Wierzbiński P, Hubska J, Henzler M, Kucharski B, Bieś R, Krzystanek M. Depressive and Other Adverse CNS Effects of Fluoroquinolones. Pharmaceuticals. 2023; 16(8):1105. https://doi.org/10.3390/ph16081105
Chicago/Turabian StyleWierzbiński, Piotr, Joanna Hubska, Michał Henzler, Bartłomiej Kucharski, Rafał Bieś, and Marek Krzystanek. 2023. "Depressive and Other Adverse CNS Effects of Fluoroquinolones" Pharmaceuticals 16, no. 8: 1105. https://doi.org/10.3390/ph16081105
APA StyleWierzbiński, P., Hubska, J., Henzler, M., Kucharski, B., Bieś, R., & Krzystanek, M. (2023). Depressive and Other Adverse CNS Effects of Fluoroquinolones. Pharmaceuticals, 16(8), 1105. https://doi.org/10.3390/ph16081105








