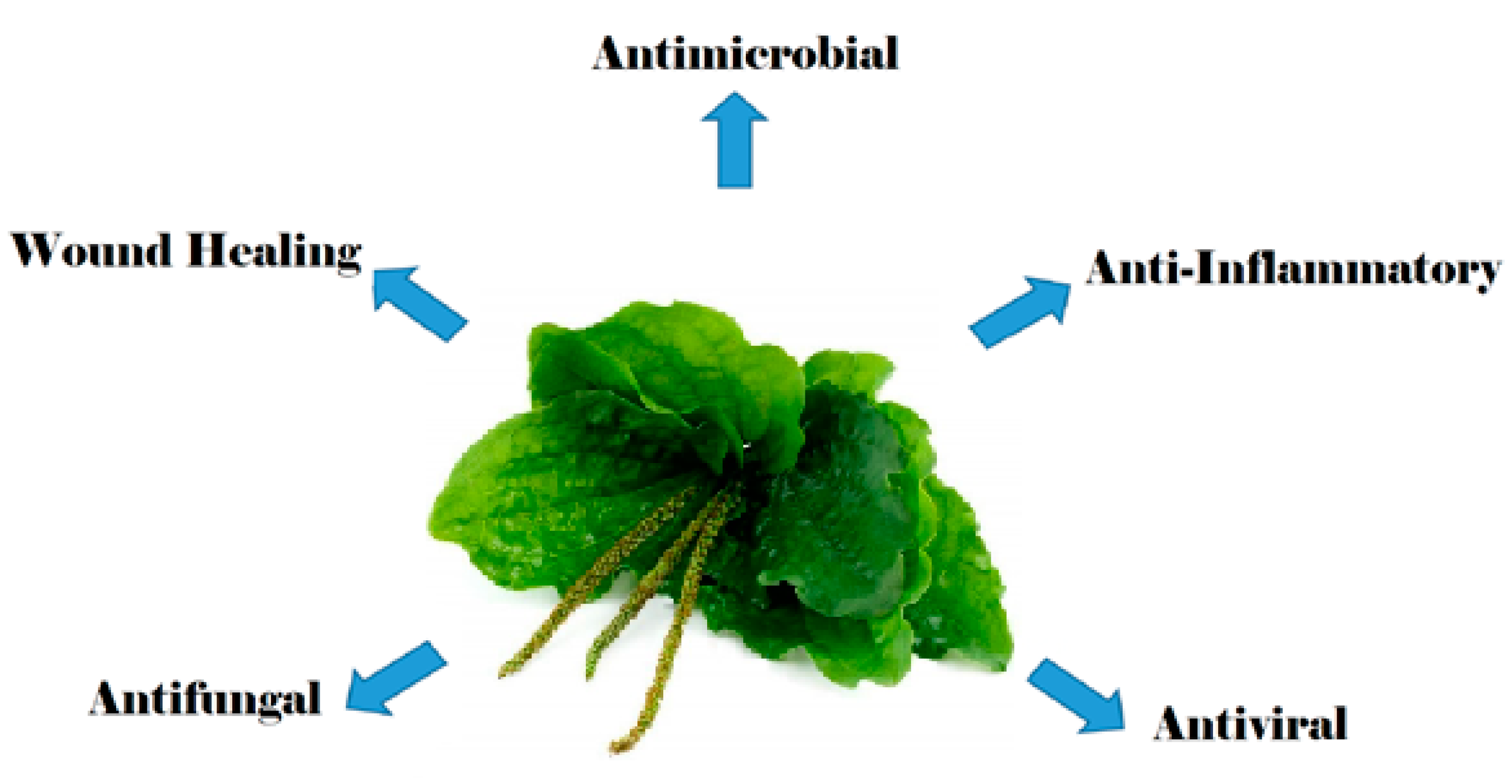
Antimicrobial and Other Biomedical Properties of Extracts from Plantago major, Plantaginaceae
Abstract
1. Introduction
2. Methods
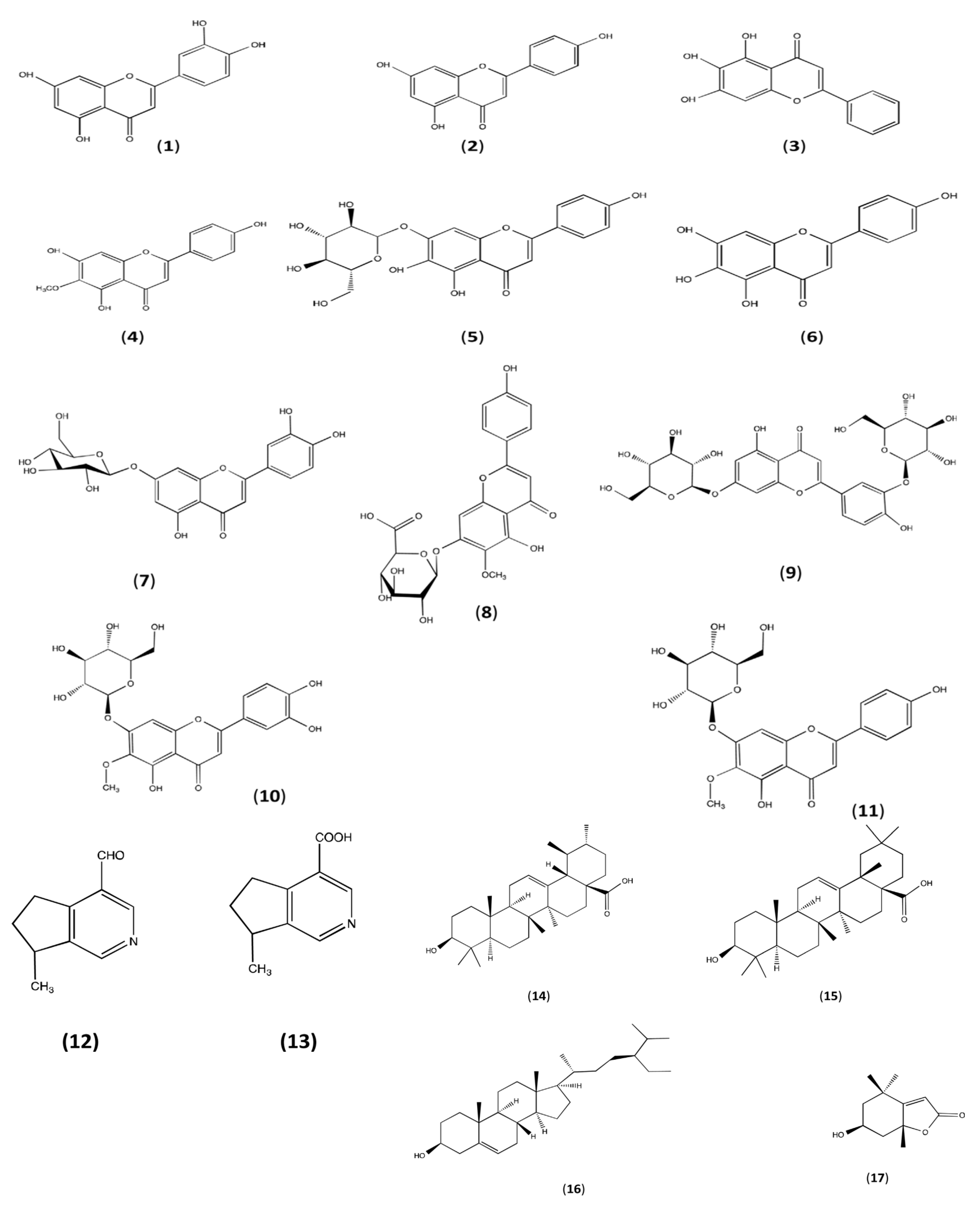
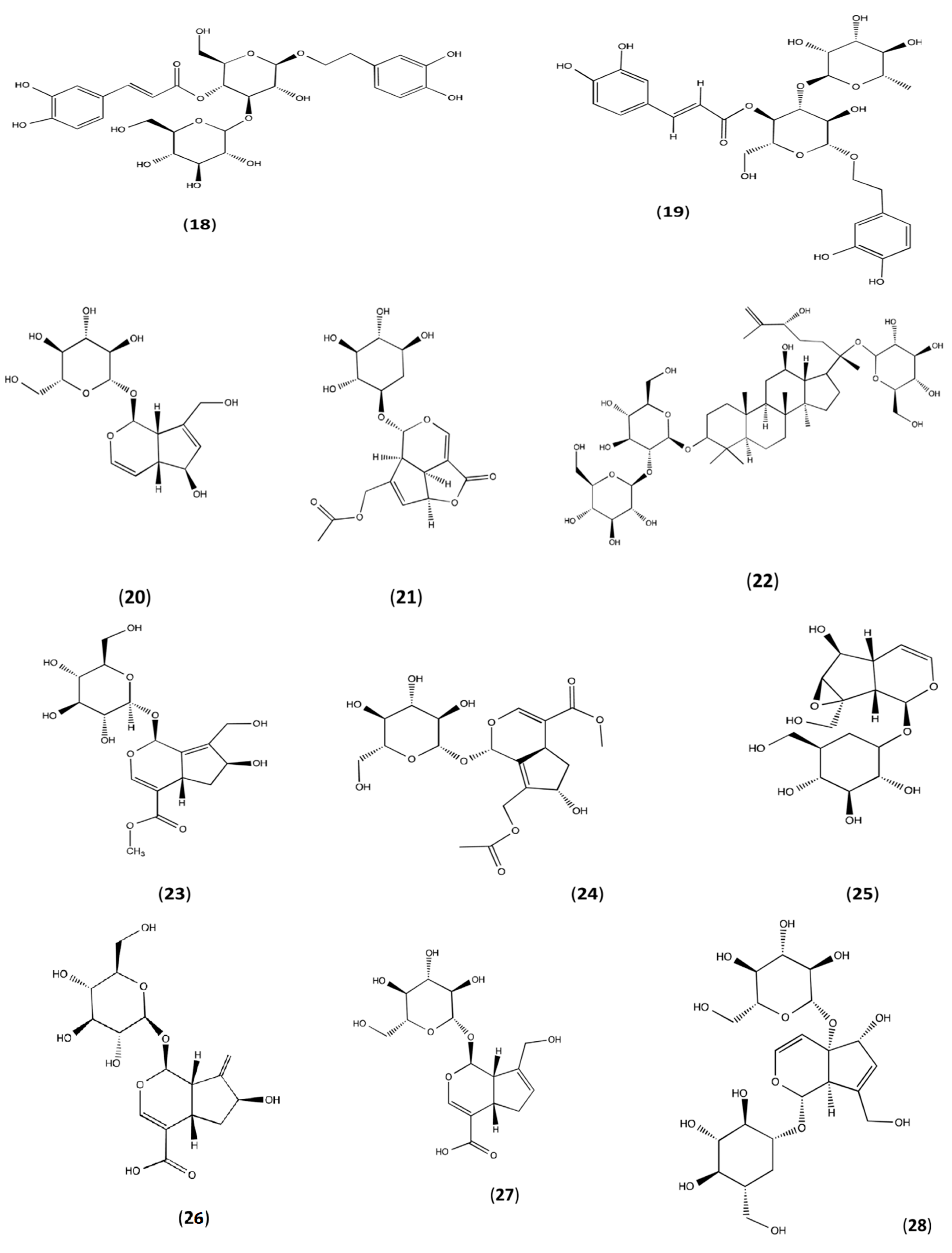
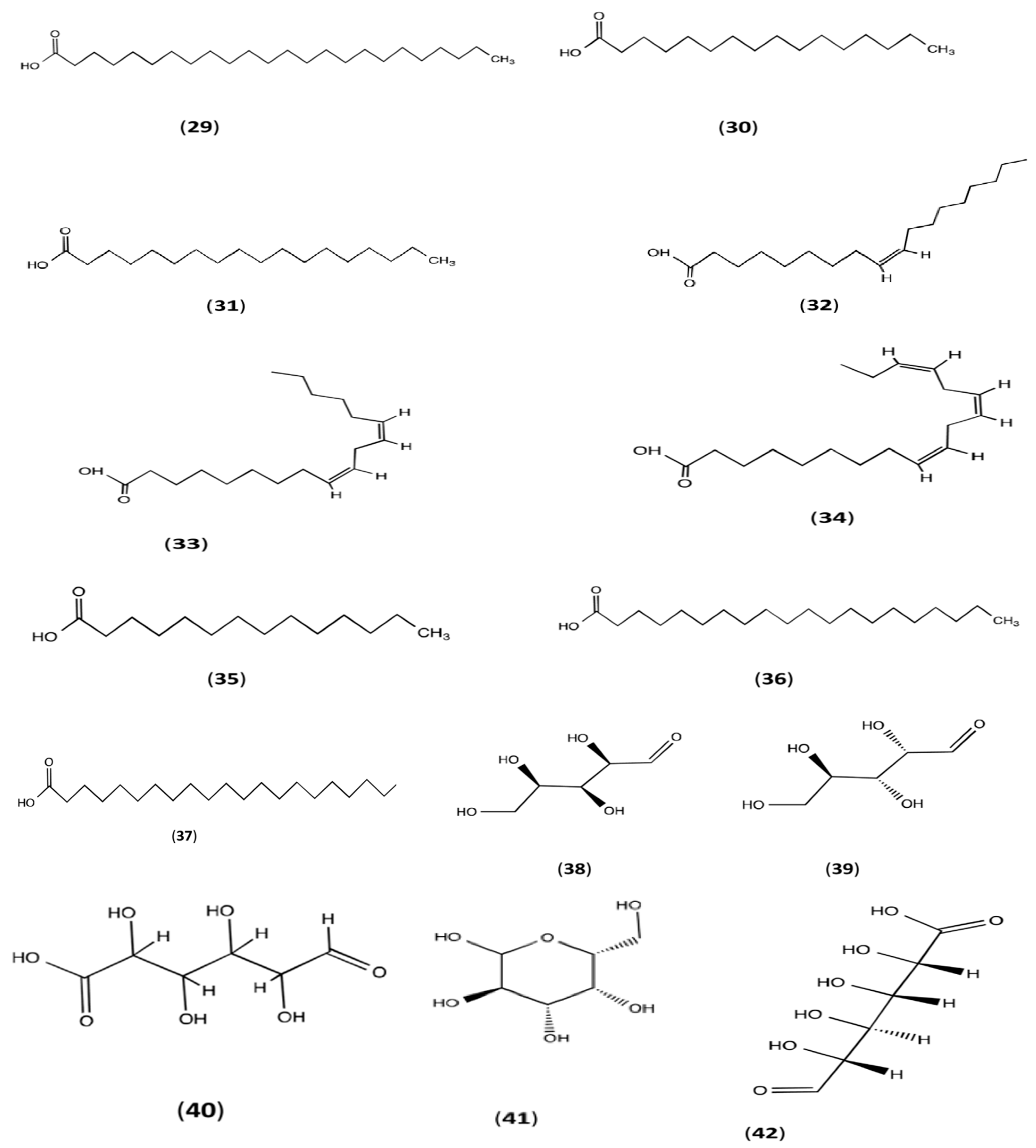
3. Phytoconstitutions
4. Antimicrobial Effects
5. Antiviral and Antifungal Effects
6. Anti-Inflammatory Activities
7. Wound-Healing Effects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kong, J.M.; Goh, N.K.; Chia, L.S.; Chia, T.F. Recent advances in traditional plant drugs and orchids. Acta Pharmacol. Sin. 2003, 24, 7–21. [Google Scholar] [PubMed]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed Pharmacol. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Samuelsen, A.B. The traditional uses chemical constituents biological activities of Plantago major, L. A review. J. Ethnopharmacol. 2000, 71, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Najafian, Y.; Hamedi, S.S.; Farshchi, M.K.; Feyzabadi, Z. Plantago major in Traditional Persian Medicine and modern phytotherapy: A narrative review. Electron. Physician 2018, 10, 6390. [Google Scholar] [CrossRef] [PubMed]
- Haddadian, K.; Haddadian, K.; Zahmatkash, M. A Review of Plantago Plant; NISCAIR-CSIR: Delhi, India, 2014. [Google Scholar]
- Sagar, G.R.; Harper, J.L. Plantago major L., P. Media L. and P. Lanceolata L. J. Ecol. 1964, 52, 189–221. [Google Scholar] [CrossRef]
- Iwanycki Ahlstrand, N.; Gopalakrishnan, S.; Vieira, F.G.; Bieker, V.C.; Meudt, H.M.; Dunbar-Co, S.; Rothfels, C.J.; Martinez-Swatson, K.A.; Maldonado, C.; Hassemer, G.; et al. Travel Tales of a Worldwide Weed: Genomic Signatures of Plantago major L. Reveal Distinct Genotypic Groups With Links to Colonial Trade Routes. Front. Plant Sci. 2022, 13, 838166. [Google Scholar] [CrossRef]
- Jamilah, J.; Sharifa, A.; Sharifah, N.R.S.A. GC-MS analysis of various extracts from leaf of Plantago major used as traditional medicine. World Appl. Sci. J. 2012, 17, 67–70. [Google Scholar]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.G.; Nakamura, C.V.; Dias Filho, B.P. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Memórias Do Inst. Oswaldo Cruz 2002, 97, 1027–1031. [Google Scholar] [CrossRef]
- Nazarizadeh, A.; Mikaili, P.; Moloudizargari, M.; Aghajanshakeri, S.; Javaherypour, S. Therapeutic uses and pharmacological properties of Plantago major L. and its active constituents. J. Basic Appl. Sci. Res. 2013, 3, 212–221. [Google Scholar]
- Akram, M.; Hamid, A.; Khalil, A.; Ghaffar, A.; Tayyaba, N.; Saeed, A.; Ali, M.; Naveed, A. Review on medicinal uses, pharmacological, phytochemistry and immunomodulatory activity of plants. Int. J. Immunopathol. Pharmacol. 2014, 27, 313–319. [Google Scholar] [CrossRef]
- Albahri, G.; Badran, A.; Hijazi, A.; Daou, A.; Baydoun, E.; Nasser, M.; Merah, O. The therapeutic wound healing bioactivities of various medicinal plants. Life 2023, 13, 317. [Google Scholar] [CrossRef]
- Subramani, R.; Narayanasamy, M.; Feussner, K.D. Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. 3 Biotech. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Sreelekshmi, R.; Latha, P.G.; Arafat, M.M.; Shyamal, S.; Shine, V.J.; Anuja, G.I.; Suja, S.R.; Rajasekharan, S. Anti-Inflammatory, Analgesic and Anti-Lipid Peroxidation Studies on Stem Bark of Ficus Religiosa Linn; CSIR: Delhi, India, 2007. [Google Scholar]
- Yatoo, M.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Tufani, N.A.; Chakraborty, S.; Tiwari, R.; Dhama, K.; Iqbal, H. Anti-inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders-a review. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti-and pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Nobahar, A.; Carlier, J.D.; Miguel, M.G.; Costa, M.C. A review of plant metabolites with metal interaction capacity: A green approach for industrial applications. BioMetals 2021, 34, 761–793. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A Comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, M.M.; Mohammed, M. Prospect of antifeedant secondary metabolites as post harvest material. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 8701–8708. [Google Scholar]
- Turgumbayeva, A.; Zhakipbekov, K.; Shimirova, Z.; Akhelova, S.; Amirkhanova, A.; Koilybayeva, M.; Seitimova, G.; Abdambayev, D. Study of phytochemical compounds of Plantago major leaves grown in Kazakhstan. Pharmacia 2022, 69, 1019–1026. [Google Scholar] [CrossRef]
- Adom, M.B.; Taher, M.; Mutalabisin, M.F.; Amri, M.S.; Kudos, M.B.A.; Sulaiman, M.W.A.W.; Sengupta, P.; Susanti, D. Chemical constituents and medical benefits of Plantago major. Biomed. Pharmacother. 2017, 96, 348–360. [Google Scholar] [CrossRef]
- Kawashty, S.A.; Abdalla, M.F.; Saleh, N.A.M. Flavonoids of Plantago species in Egypt. Biochem. Syst. Ecol. 1994, 22, 729–733. [Google Scholar] [CrossRef]
- Nishibe, S.; Tamayama, Y.; Sasahara, M.; Andary, C. A phenylethanoid glycoside from Plantago asiatica. Phytochemistry 1995, 38, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Zheng, R.L.; Jia, Z.J.; Ju, Y. Flavonoids as superoxide scavengers and antioxidants. Free Radic. Biol. Med. 1990, 9, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.J.; Ferrandiz, M.L.; Cejudo, M.; Terencio, M.C.; Gil, B.; Bustos, G.; Ubeda, A.; Gunasegaran, R.; Alcaraz, M.J. Influence of a series of natural flavonoids on free radical generating systems and oxidative stress. Xenobiotica 1994, 24, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Skari, K.P.; Malterud, K.E.; Haugli, T. Peroxidation from Plantago major, a medicinal plant. Nat. Antioxid. Anticarcinog. Nutr. Health Dis. 1999, 240, 200. [Google Scholar]
- Beara, I.N.; Lesjak, M.M.; Jovin, E.Đ.; Balog, K.J.; Anackov, G.T.; Orcic, D.Z.; Mimica-Dukic, N.M. Plantain (Plantago L.) species as novel sources of flavonoid antioxidants. J. Agric. Food Chem. 2009, 57, 9268–9273. [Google Scholar] [CrossRef] [PubMed]
- Mello, J.C.; Gonzalez, M.V.; Moraes, V.W.; Prieto, T.; Nascimento, O.R.; Rodrigues, T. Protective effect of Plantago major extract against t-BOOH-induced mitochondrial oxidative damage and cytotoxicity. Molecules 2015, 20, 17747–17759. [Google Scholar] [CrossRef]
- Schneider, G. Arzneidrogen: Ein Kompendium Für Pharmazeuten, Biologen UND Chemiker; BI-Wissenschaftsverlag: Mannheim, Germany, 1990. [Google Scholar]
- Haschke-Hofmeister, E. Contents from Plantago major. Planta Med. 1969, 17, 139–145. [Google Scholar]
- Hiltibran, R.C.; Wadkins, C.L.; Nicholas, H.J. The Distribution of Triterpenes in Rugel’s Plantain1. J. Am. Chem. Soc. 1953, 75, 5125–5126. [Google Scholar] [CrossRef]
- Ringbom, T.; Segura, L.; Noreen, Y.; Perera, P.; Bohlin, L. Ursolic acid from Plantago major, a selective inhibitor of cyclooxygenase-2 catalyzed prostaglandin biosynthesis. J. Nat. Prod. 1998, 61, 1212–1215. [Google Scholar] [CrossRef]
- Bakker, M.I.; Baas, W.J.; Sijm, D.T.; Kollöffel, C. Leaf wax of Lactuca sativa and Plantago major. Phytochemistry 1998, 47, 1489–1493. [Google Scholar] [CrossRef]
- Noro, Y. Pharmacognostical studies of plantaginis herba (VII) on the phenylethanoid contents of Plantago spp. Shoyakugaku Zasshi 1991, 45, 24–28. [Google Scholar]
- Long, C.; Moulis, C.; Stanislas, E.; Fouraste, I. L’aucuboside et le catalpol dans les feuilles de Plantago lanceolata L., Plantago major L. et Plantago media L. J. De Pharm. De Belg. 1995, 50, 484–488. [Google Scholar]
- Bianco, A.; Guiso, M.; Passacantilli, P.; Francesconi, A. Iridoid and phenypropanoid glycosides from new sources. J. Nat. Prod. 1984, 47, 901–902. [Google Scholar] [CrossRef]
- Handjieva, N.; Spassov, S.; Bodurova, G.; Saadi, H.; Popov, S.; Pureb, O.; Zamjansan, J. Majoroside, an iridoid glucoside from Plantago major. Phytochemistry 1991, 30, 1317–1318. [Google Scholar] [CrossRef]
- Taskova, R.; Handjieva, N.; Evstatieva, L.; Popov, S. Iridoid glucosides from Plantago cornuti, Plantago major and Veronica cymbalaria. Phytochemistry 1999, 52, 1443–1445. [Google Scholar] [CrossRef]
- Murai, M.; Tamayama, Y.; Nishibe, S. Phenylethanoids in the herb of Plantago lanceolata and inhibitory effect on arachidonic acid-induced mouse ear edema1. Planta Medica 1995, 61, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.F.; Hammouda, F.M.; Rizk, A.M.; Wassel, G.M. Phytochemical studies of Egyptian Plantago species. Planta Medica 1968, 16, 404–410. [Google Scholar] [CrossRef]
- Swiatek, L.; Kurowska, A.; Gora, J. Chemical-composition of some plantago species seed oil. Herba Pol. 1981, 26, 213–217. [Google Scholar]
- Ahmad, M.S.; Ahmad, M.U.; Osman, S.M. A new hydroxyolefinic acid from Plantago major seed oil. Phytochemistry 1980, 19, 2137–2139. [Google Scholar] [CrossRef]
- Guil, J.L.; Rodríguez-Garcí, I.; Torija, E. Nutritional and toxic factors in selected wild edible plants. Plant Foods Hum. Nutr. 1997, 51, 99–107. [Google Scholar] [CrossRef]
- Ahmed, Z.F.; Rizk, A.M.; Hammouda, F.M. Phytochemical studies of egyptian Plantago species (Glucides). J. Pharm. Sci. 1965, 54, 1060–1062. [Google Scholar] [CrossRef]
- Gorin, A.G. Polysaccharides from Plantago major leaves. I. Analysis of monosaccharide composition of polysaccharide complex. Chem. Abstr. 1966, 64, 8277. [Google Scholar]
- Samuelsen, A.; Lund, I.; Djahromi, J.; Paulsen, B.; Wold, J.; Knutsen, S. Structural features & anti-complementary activity of some heteroxylan polysaccharide fractions from the seeds of Plantago major L. Carbohydr. Polym. 1999, 38, 133–143. [Google Scholar]
- Samuelsen, A.B.; Paulsen, B.S.; Wold, J.K.; Otsuka, H.; Yamada, H.; Espevik, T. Isolation & partial characterization of biologically active polysaccharides from Plantago major L. Phytother. Res. 1995, 9, 211–218. [Google Scholar]
- Samuelsen, A.B.; Paulsen, B.S.; Wold, J.K.; Otsuka, H.; Kiyohara, H.; Yamada, H.; Knutsen, S.H. Characterization of a biologically active pectin from Plantago major L. Carbohydr. Polym. 1996, 30, 37–44. [Google Scholar] [CrossRef]
- Samuelsen, A.B.; Paulsen, B.S.; Wold, J.K.; Knutsen, S.H.; Yamada, H. Characterization of a biologically active arabinogalactan from the leaves of Plantago major L. Carbohydr. Polym. 1998, 35, 145–153. [Google Scholar] [CrossRef]
- Ravn, H.; Brimer, L. Structure and antibacterial activity of plantamajoside, a caffeic acid sugar ester from Plantago major subs major. Phytochemistry 1988, 27, 3433–3437. [Google Scholar] [CrossRef]
- Mammani, I.M.A. Antibacterial activity of ethanolic extracts of Plantago major leaves against Pseudomonas aeruginosa from burn infections. J. Infect. Dev. Ctries. 2023, 17, 276–280. [Google Scholar]
- Abbasi, A.; Maddah, S.M.; Ali, D.S.; Kamalinejad, M. Antibacterial Activity of Herbal Plantago major and Plantago lanceolata extracts on Pseudomonas aeruginosa with emphasis on Exotoxin A gene expression and Bioinformatics approach. 2022. [Google Scholar] [CrossRef]
- Stanisavljević, I.T.; Stojičević, S.S.; Veličković, D.T.; Lazić, M.L.; Veljković, V.B. Screening the antioxidant and antimicrobial properties of the extracts from plantain (Plantago major L.) leaves. Sep. Sci. Technol. 2008, 43, 3652–3662. [Google Scholar] [CrossRef]
- Akkuş, G.; Hiz-Çiçekliyurt, M.M. Antimicrobial Efficacy of Four Different Extracts of Plantago major: An In vitro Study. Asian J. Immunol. 2021, 5, 22–26. [Google Scholar]
- Astuti, A.D.; Etikawati, N.; Pangastuti, A. Antibacterial activity of Plantago major leaves against Streptococcus pyogenes ATCC 19615 as a cause of tonsilitis. Asian J. Trop. Biotechnol. 2020, 17, 14–21. [Google Scholar]
- Soliman, M.A.; Galal, T.M.; Naeim, M.A.; Khalafallah, A.A. Seasonal variation in the secondary metabolites and antimicrobial activity of Plantago major L. from Egyptian heterogenic habitats. Egypt. J. Bot. 2022, 62, 255–273. [Google Scholar] [CrossRef]
- Wijesundara, N.M.; Rupasinghe, H.V. Herbal tea for the management of pharyngitis: Inhibition of streptococcus pyogenes growth and biofilm formation by herbal infusions. Biomedicines 2019, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Ghezavat, K.; Sani, A.M.; Yavarmanesh, M. Assessment of the Plantago major extract for antimicrobial activities. BioTechnology 2014, 10, 65–66. [Google Scholar]
- Mirkalantari, S.; Fateh, K. The Effect of Plant Extracts of Plantago major and Laurus nobilis on the Antimicrobial Properties of some of Gram-positive and Gram-negative Bacteria. Complement. Med. J. 2018, 8, 3433–3443. [Google Scholar]
- Soliman Mozafar, S.; Majidi, E.; Bakhtiari, R.; Norouzi, M.; Kameli, S. Antimicrobial Effects of Ethanol and Acetone Extracts of Plantago major Seed on Streptococcus mutans. J. Res. Dent. Maxillofac. Sci. 2022, 8, 88–94. [Google Scholar]
- Safari, S.; Zaremahmoudabadi, R.; Arefnejad, M.; Hamedi, S.; Mehrabkhani, M. Antimicrobial Effect of Hydroalcoholic Extract of Plantago major Leaves with and without Zinc Oxide Nanoparticles on Streptococcus Mutans: An in vitro Study. J. Mashhad Dent. 2021, 45, 54–62. [Google Scholar]
- Kaya, Ö. Investigation of Antioxidant and Antimicrobial Effects of Plantago major Leaves. Master’s Thesis, Middle East Technical University, Ankara, Turkey, 2011. Available online: http://etd.lib.metu.edu.tr/upload/12613757/index.pdf (accessed on 9 September 2011).
- Teles, D.G.; Costa, M.M. Study of the joint antimicrobial action of aqueous extracts of broadleaf plantain (Plantago major L., Plantaginaceae) and Pomegranate (Punica granatum L., Punicaceae) and their interference in the in vitro activity of amoxicillin. Rev. Bras. De Plantas Med. 2014, 16, 323–328. [Google Scholar] [CrossRef]
- Chookalaii, H.; Riahi, H.; Shariatmadari, Z.; Mazarei, Z.; Seyed Hashtroudi, M. Enhancement of total flavonoid and phenolic contents in Plantago major L. with plant growth promoting cyanobacteria. J. Agric. Sci. Technol. 2020, 22, 505–518. [Google Scholar]
- Sahakyan, N.Z.; Ginovyan, M.M.; Petrosyan, M.T.; Trchounian, A.H. Antibacterial and anti-phage activity of $ plantago $$ major $ l. Raw material. Proc. YSU B Chem. Biol. Sci. 2019, 53, 59–64. [Google Scholar]
- Metiner, K.; Ozkan, O.; Ak, S. Antibacterial effects of ethanol and acetone extract of Plantago major L. on gram positive and gram negative bacteria. Kafkas Univ. Vet. Fak. Derg. 2012, 18, 503–505. [Google Scholar]
- Pensantes-Sangay, S.J.; Calla-Poma, R.D.; Requena-Mendizabal, M.F.; Alvino-Vales, M.I.; Millones-Gómez, P.A. Chemical composition and antibacterial effect of Plantago major Extract on periodontal pathogens. Pesqui. Bras. Em Odontopediatria E Clín. Integr. 2020, 20. [Google Scholar] [CrossRef]
- Abd Razik, B.M.; Hasan, H.A.; Murtadha, M.K. The study of antibacterial activity of Plantago major and Ceratonia siliqua. Iraqi Postgrad. Med. J. 2012, 11, 130–135. [Google Scholar]
- Sharma, H.; Yunus, G.Y.; Agrawal, R.; Kalra, M.; Verma, S.; Bhattar, S. Antifungal efficacy of three medicinal plants Glycyrrhiza glabra, Ficus religiosa, and Plantago major against oral Candida albicans: A comparative analysis. Indian J. Dent. Res. 2016, 27, 433. [Google Scholar] [CrossRef]
- Alvarado Villanueva, V.; Moromi Nakata, H. Medicinal Plants: Antibacterial Effect in Vitro de Plantago major L, Erythroxylum Novogranatense, Plowman Var Truxillense and Camellia Sinensis on Stomatologic Importance Bacteria. Odontología Sanmarquina 2010, 13, 21–25. [Google Scholar] [CrossRef][Green Version]
- Ursia, A.; Gulbaram, U.; Yulia, I.; Yevgeny, G.; Oksana, S.; Leonid, S. The study of the antimicrobial activity of CO2 extracts of Plantago major and Acorus calamus. World J. Pharm. Sci. 2015, 3, 826–829. [Google Scholar]
- Sukweenadhi, J.; Setiawan, K.I.; Avanti, C.; Kartini, K.; Rupa, E.J.; Yang, D.C. Scale-up of green synthesis and characterization of silver nanoparticles using ethanol extract of Plantago major L. leaf and its antibacterial potential. S. Afr. J. Chem. Eng. 2021, 38, 1–8. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227. [Google Scholar] [CrossRef]
- Arslan, E.; Aygan, A.; Kocabaş, Y.Z. Antimicrobial Activity of Plantago major Grown in Kahramanmaraş Against Bacteria Causing Hospital Infections. Ecology 2018. Available online: https://www.researchgate.net/publication/328064477_Antimicrobial_Activity_of_Plantago_major_Grown_in_Kahramanmaras_Against_Bacteria_Causing_Hospital_Infections (accessed on 19 June 2018).
- Chiang, L.C.; Chiang, W.; Chang, M.Y.; Ng, L.T.; Lin, C.C. Antiviral activity of Plantago major extracts and related compounds in vitro. Antivir. Res. 2002, 55, 53–62. [Google Scholar] [CrossRef]
- Chiang, L.C.; Chiang, W.; Chang, M.Y.; Lin, C.C. In vitro cytotoxic, antiviral and immunomodulatory effects of Plantago major and Plantago asiatica. Am. J. Chin. Med. 2003, 31, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Nikonorova, A.K.; Egorov, C.A.; Galkina, T.G.; Grishin, E.V.; Barakov, A.V. Antifungal activity of phenolic glicozide verbascoside from Plantago major seeds. Mikol. I Fitopatol. 2009, 43, 52–57. [Google Scholar]
- Edalatpanah, Y.; Rostampur, S.; Pouladi, I.; Rajaeenejad, S. Evaluation of Antifungal Effects of Prangos ferulace and Plantago major L Plants Against Fluconazole-resistant Candida albicans Species in Extracorporeal Conditions. Navid No 2020, 23, 44–52. [Google Scholar]
- Ferreira, C.; Oliveira, R. Protective antifungal activity of Plantago major extract against the phytopathogenic fungi Phytophthora cinnamomi, Diplodia corticola and Colletotrichum species. Proceedings 2020, 70, 94. [Google Scholar] [CrossRef]
- Della Loggia, R.; Sosa, S.; Cateni, F.; Zacchigna, M.; Tossi, A.; Tubaro, A. Topical anti-inflammatory activity of Plantago major L. leaves. Planta Medica 2006, 72, 323. [Google Scholar] [CrossRef]
- Zubair, M.; Widén, C.; Renvert, S.; Rumpunen, K. Water and ethanol extracts of Plantago major leaves show anti-inflammatory activity on oral epithelial cells. J. Tradit. Complement. Med. 2019, 9, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Farid, A.; Sheibani, M.; Shojaii, A.; Noori, M.; Motevalian, M. Evaluation of anti-inflammatory effects of leaf and seed extracts of Plantago major on acetic acid-induced ulcerative colitis in rats. J. Ethnopharmacol. 2022, 298, 115595. [Google Scholar] [CrossRef]
- Hussan, F.; Mansor, A.S.; Hassan, S.N.; Kamaruddin, T.N.E.; Tasnim, T.N.; Budin, S.B.; Othman, F. Anti-inflammatory property of Plantago major leaf extract reduces the inflammatory reaction in experimental acetaminophen-induced liver injury. Evid.-Based Complement. Altern. Med. 2015, 2015, 347861. [Google Scholar] [CrossRef]
- Motevalian, M.; Motahari, M.; Shiri, M. Study of the Anti-inflammatory Effect of Plantago major Seed Extract on Rat Paw Edema. 2007. Available online: https://sid.ir/paper/904687/en (accessed on 1 July 2007).
- Triastuti, A.; Indrati, O.; Hayati, F. Development of microemulsion containing Plantago major extracts: Formulation and evaluation of topical anti-inflammatory activities. In Proceedings of the MESMAP–5 Proceedings Book, Cappadocia, Turkey, 24–26 April 2019; p. 69. [Google Scholar]
- Turel, I.; Ozbek, H.; Erten, R.; Oner, A.C.; Cengiz, N.; Yilmaz, O. Hepatoprotective and anti-inflammatory activities of Plantago major L. Indian J. Pharmacol. 2009, 41, 120. [Google Scholar]
- Núñez Guillén, M.E.; da Silva Emim, J.A.; Souccar, C.; Lapa, A.J. Analgesic and anti-inflammatory activities of the aqueous extract of Plantago major L. Int. J. Pharmacogn. 1997, 35, 99–104. [Google Scholar] [CrossRef]
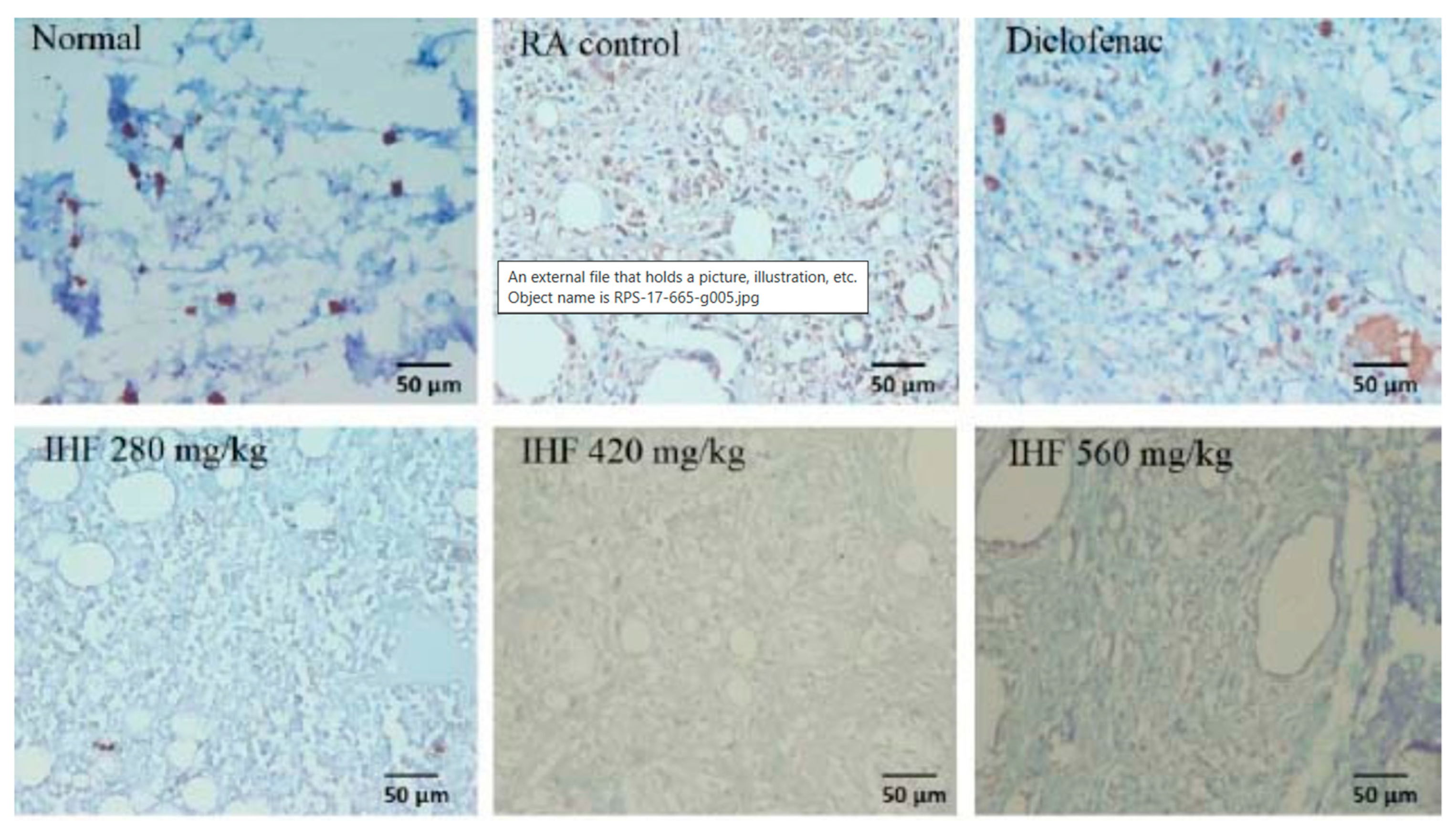
- Triastuti, A.; Pradana, D.A.; Setiawan, I.D.; Fakhrudin, N.; Himmi, S.K.; Widyarini, S.; Rohman, A. In vivo anti-inflammatory activities of Plantago major extract and fractions and analysis of their phytochemical components using a high-resolution mass spectrometry. Res. Pharm. Sci. 2022, 17, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Kartini, K.; Wati, N.; Gustav, R.; Wahyuni, R.; Anggada, Y.F.; Hidayani, R.; Raharjo, A.; Islamie, R.; Putra, S.E.D. Wound healing effects of Plantago major extract and its chemical compounds in hyperglycemic rats. Food Biosci. 2021, 41, 100937. [Google Scholar] [CrossRef]
- Ghanadian, M.; Soltani, R.; Homayouni, A.; Khorvash, F.; Jouabadi, S.M.; Abdollahzadeh, M. The effect of Plantago major hydroalcoholic extract on the healing of diabetic foot and pressure ulcers: A randomized open-label controlled clinical trial. Int. J. Low. Extrem. Wounds 2022. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Kherad, M.; Mehrabani, D.; Azarpira, N.; Panjehshahin, M.R.; Tanideh, N. Effect of Plantago major on burn wound healing in rat. J. Appl. Anim. Res. 2010, 37, 53–56. [Google Scholar] [CrossRef]
- Zubair, M.; Nybom, H.; Lindholm, C.; Brandner, J.M.; Rumpunen, K. Promotion of wound healing by Plantago major L. leaf extracts–ex-vivo experiments confirm experiences from traditional medicine. Nat. Prod. Res. 2016, 30, 622–624. [Google Scholar] [CrossRef]
- Thome, R.G.; Santos, H.B.D.; Santos, F.V.D.; Oliveira, R.J.D.S.; De Camargos, L.F.; Pereira, M.N.; Longatti, T.R.; Souto, C.M.; Franco, C.S.; De Oliveira Aquino Schüffner, R.; et al. Evaluation of healing wound and genotoxicity potentials from extracts hydroalcoholic of Plantago major and Siparuna guianensis. Exp. Biol. Med. 2012, 237, 1379–1386. [Google Scholar] [CrossRef]
- Anaya-Mancipe, J.M.; Queiroz, V.M.; Dos Santos, R.F.; Castro, R.N.; Cardoso, V.S.; Vermelho, A.B.; Dias, M.L.; Thiré, R.M. Electrospun Nanofibers Loaded with Plantago major L. Extract for Potential Use in Cutaneous Wound Healing. Pharmaceutics 2023, 15, 1047. [Google Scholar] [CrossRef]
- Zubair, M. Genetic Variation, Biochemical Contents and Wound Healing Activity of Plantago Major; Department of Plant Breeding and Biotechnology, Swedish University of Agricultural: Uppsala, Sweden, 2012; Volume 2012, Available online: https://res.slu.se/id/publ/79011 (accessed on 20 October 2012).
- Kartini, K.; Rambing, C.C.; Rahayu, I.B.W.S. Wound Healing Activity of Plantago major Extract on Hyperglicaemic Rat. 2017. Available online: http://repository.ubaya.ac.id/30558/ (accessed on 26 April 2017).
- Rad, N.M.; Shafie, F.; Chaghervand, M.M.; Kashfi, S.; Rashidipour, M.; Chehelcheraghi, F.; Mozaffarpur, S.A.; Rasoulian, B. The Wound Healing Effect of Plantago major Leaf Extract in a Rat Model: An Experimental Confirmation of a Traditional Belief in Persian Medicine. Herb. Med. J. 2018, 3, 26–30. [Google Scholar] [CrossRef]
- Bazafkan, M.H.; Hardani, A.; Zadeh, M.R.A.; Zargar, A.A.; Zadeh, M.O.; Hemmati, A.A.; Mansori, E.; Asadi-Samani, M.; Ghasemiboroon, M.; Kooti, V. Wound healing effect of an ointment made from a mixture of brassica oleracea var, Punica granatum, and Plantago major L Extracts in Rats. Jentashapir J. Health Res. 2014, 5, e21877. [Google Scholar] [CrossRef]
- Mahmood, M.M.; Mahdi, A.K. Experimental study of the effect of Plantago major leaves extract on contaminated excisional wound healing in rabbits. Iraqi J. Vet. Sci. 2022, 36, 31–39. [Google Scholar] [CrossRef]
- Cardoso, F.C.I.; Breder, J.C.; Apolinario, P.P.; Oliveia, H.C.; Saidel, M.G.B.; Dini, A.P.; Oliveira-Kumakura, A.R.; Lima, M.H.M. The Effect of Plantago major on Wound Healing in Preclinical Studies: A Systematic Review. Wound Manag. Prev. 2021, 67, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Tileuberdi, N.; Turgumbayeva, A.; Yeskaliyeva, B.; Sarsenova, L.; Issayeva, R. Extraction, isolation of bioactive compounds and therapeutic potential of rapeseed (Brassica napus L.). Molecules 2022, 27, 8824. [Google Scholar] [CrossRef] [PubMed]

| No. | Extraction | Part of Plant | Minimum Inhibitory Concentration (MIC) (mg/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bc * | Bs * | Sa * | Se * | Ec * | Kp * | Pa * | Pm * | Sen * | P. g * | F. n * | |||
| 1 | Aceton extract | Leaf, seed, root | 3.5 | 8. | 4.25 | 28.5 | 14.2 | 14.2 | 28.5 | 8.25 | 7.13 | ||
| 2 | Ethanol extract | Leaf, seed, root | 42.5 | R | R | R | 42.5 | R | R | R | R | - | 0.93 |
| 3 | AgNO3 | unknown | 6.85 | - | 5.7 | - | 9.5 | - | 6.8 | - | - | - | - |
| 4 | Diethyl ether extract | Leaf, seed | - | - | - | - | 5.83 | - | - | - | - | - | - |
| 5 | Water extract | Leaf, seed | - | - | - | - | - | - | 125 | - | - | - | - |
| 6 | Hydroalcoholic extract | Leaf, seed | - | - | 8.8 | - | - | - | - | - | - | 11.0 | |
| 7 | Ultrasonic Extract | Leaf | - | 10.7 | 10.7 | - | 11.7 | - | 11.3 | - | - | - | - |
| 8 | Classical Solvent Extract | Leaf | - | 11.0 | 11.3 | - | 10.9 | - | 11.2 | - | - | - | - |
| 9 | Ethyl acetate extract | Leaf | 33.5 | - | 16.7 | - | - | 67 | 33.5 | 67 | - | - | - |
| 10 | Aqueous extract | Leaf, seed | - | - | 57.2 | - | - | - | 114.5 | - | - | - | |
| 11 | Ag/AgCl nanoparticles | unknown | - | - | 0.8 | 1.6 | - | - | - | - | - | - | |
| 12 | Alcoholic extract | Leaf, seed | - | - | 0.04 | - | 0.03 | - | - | - | - | - | - |
| 13 | CO2 extract | unknown | - | 18 | 18 | - | 16 | - | - | - | - | - | - |
| 14 | Zinc oxide nanoparticles | Seed | - | - | 1.56 | - | - | - | - | - | - | - | - |
| No. | Pharmacological Activity | Extraction | Part of Plant | Concentration | Pre-Clinical Method | |
|---|---|---|---|---|---|---|
| mg/mL | % | |||||
| 1 | Antimicrobial | Aceton extract | Leaf, seed, root | 500 | - | in vivo |
| Ethanolic extract | Leaf, seed, root | 100, 75, 50, 25 | 10%, 75%, 100% | in vivo | ||
| AgNO3 | unknown | 20 | - | in vivo | ||
| Diethyl ether extract | Leaf, seed | 0.016–1.0 | - | in vivo | ||
| Hydroalcoholic extract | Leaf, seed | 25 | - | in vivo | ||
| Ultrasonic Extract | Leaf | 50 | - | in vivo | ||
| Metanolic Extract | Leaf, seed, root | 1000, 500, 250, 125 | - | in vivo | ||
| Ethyl acetate extract | Leaf | 0.078–5.0 | - | in vivo | ||
| Aqueous extract | Leaf, seed | 20, 25 | - | in vivo | ||
| Alcoholic extract | Leaf, seed | 25, 50, 75, 100, 1000 | 10%, 75%, 100% | in vivo | ||
| CO2-extract | unknown | 18 | - | in vivo | ||
| Zinc oxide nanoparticles | Seed | 1.56 | - | in vivo | ||
| 2 | Anti-Inflammatory | Ethanolic extract | Leaf | 0.1 | - | in vitro |
| Water extract | Leaf | 0.1 | - | in vitro | ||
| Metanolic Extract | Seed, leaf | 50 | - | in vitro | ||
| n-hexane | Seed, leaf | 280, 560 | - | in vitro | ||
| Chloroform | Seed, leaf | 280, 560 | - | in vitro | ||
| DCM | Seed, leaf | 280, 560 | - | in vitro | ||
| 3 | Wound Healing | Hydroalcoholic extract | Leaf, root | - | 50% | in vitro |
| Aqueous extract | Leaf | 1.0 | - | in vitro/ex vivo | ||
| Ethanol extract | Leaf, root | 1.0 | - | in vitro/ex vivo | ||
| Salve | Leaf | - | 10% | in vitro | ||
| 4 | Antiviral | Aqueous extract | Leaf | 14.2; 15.3; 87.3 | - | in vivo |
| 5 | Antifungal | Alcoholic extract | Seed, leaf | 7.5, 15, 30 | - | in vivo |
| Ethanol extract | Seed | 2000 | - | in vivo | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhakipbekov, K.; Turgumbayeva, A.; Issayeva, R.; Kipchakbayeva, A.; Kadyrbayeva, G.; Tleubayeva, M.; Akhayeva, T.; Tastambek, K.; Sainova, G.; Serikbayeva, E.; et al. Antimicrobial and Other Biomedical Properties of Extracts from Plantago major, Plantaginaceae. Pharmaceuticals 2023, 16, 1092. https://doi.org/10.3390/ph16081092
Zhakipbekov K, Turgumbayeva A, Issayeva R, Kipchakbayeva A, Kadyrbayeva G, Tleubayeva M, Akhayeva T, Tastambek K, Sainova G, Serikbayeva E, et al. Antimicrobial and Other Biomedical Properties of Extracts from Plantago major, Plantaginaceae. Pharmaceuticals. 2023; 16(8):1092. https://doi.org/10.3390/ph16081092
Chicago/Turabian StyleZhakipbekov, Kairat, Aknur Turgumbayeva, Raushan Issayeva, Aliya Kipchakbayeva, Gulnara Kadyrbayeva, Meruyert Tleubayeva, Tamila Akhayeva, Kuanysh Tastambek, Gaukhar Sainova, Elmira Serikbayeva, and et al. 2023. "Antimicrobial and Other Biomedical Properties of Extracts from Plantago major, Plantaginaceae" Pharmaceuticals 16, no. 8: 1092. https://doi.org/10.3390/ph16081092
APA StyleZhakipbekov, K., Turgumbayeva, A., Issayeva, R., Kipchakbayeva, A., Kadyrbayeva, G., Tleubayeva, M., Akhayeva, T., Tastambek, K., Sainova, G., Serikbayeva, E., Tolenova, K., Makhatova, B., Anarbayeva, R., Shimirova, Z., & Tileuberdi, Y. (2023). Antimicrobial and Other Biomedical Properties of Extracts from Plantago major, Plantaginaceae. Pharmaceuticals, 16(8), 1092. https://doi.org/10.3390/ph16081092







