Association between First-Generation Antihistamine Use in Children and Cardiac Arrhythmia and Ischemic Heart Disease: A Case-Crossover Study
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Association between Cardiovascular Events and First-Generation H1-Antihistamine
3. Discussion
4. Methods
4.1. Data Source and Ethical Consideration
4.2. Participants
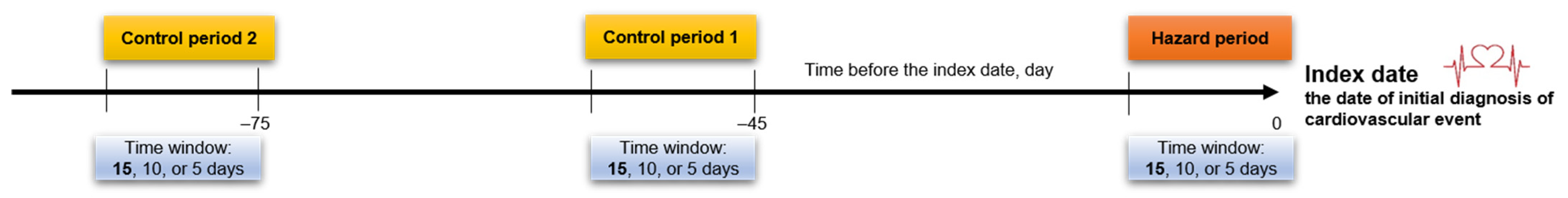
4.3. Case Cross-Over Study Design
4.4. Cardiovascular Events
4.5. First-Generation H1-Antihistamine Use
4.6. Covariates
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fitzsimons, R.; van der Poel, L.-A.; Thornhill, W.; du Toit, G.; Shah, N.; Brough, H.A. Antihistamine use in children. Arch. Dis. Child. Educ. Pract. 2015, 100, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Parisi, G.; Leonardi, S.; Ciprandi, G.; Corsico, A.; Licari, A.; Del Giudice, M.M.; Peroni, D.; Salpietro, C.; Marseglia, G. Antihistamines in children and adolescents: A practical update. Allergol. Immunopathol. 2020, 48, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Yum, H.Y.; Ha, E.K.; Shin, Y.H.; Han, M.Y. Prevalence, comorbidities, diagnosis, and treatment of nonallergic rhinitis: Real-world comparison with allergic rhinitis. Clin. Exp. Pediatr. 2021, 64, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Church, M.; Maurer, M.; Simons, F.; Bindslev-Jensen, C.; Van Cauwenberge, P.; Bousquet, J.; Holgate, S.; Zuberbier, T. Risk of first-generation H1-antihistamines: A GA2LEN position paper. Allergy 2010, 65, 459–466. [Google Scholar] [CrossRef]
- Fein, M.N.; Fischer, D.A.; O’Keefe, A.W.; Sussman, G.L. CSACI position statement: Newer generation H1-antihistamines are safer than first-generation H1-antihistamines and should be the first-line antihistamines for the treatment of allergic rhinitis and urticaria. Allergy Asthma Clin. Immunol. 2019, 15, 1–6. [Google Scholar] [CrossRef]
- Anagnostou, K.; Swan, K.E.; Brough, H. The use of antihistamines in children. Paediatr. Child Health 2016, 26, 310–313. [Google Scholar] [CrossRef]
- Ali, Z.; Ismail, M.; Khan, F.; Sajid, H. Association of H1-antihistamines with torsade de pointes: A pharmacovigilance study of the food and drug administration adverse event reporting system. Expert Opin. Drug Saf. 2021, 20, 101–108. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. PRAC Recommends New Measures to Minimise Known Heart Risks of Hydroxyzine-Containing Medicines. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2015/02/WC500182462.pdf (accessed on 28 April 2022).
- Kim, J.H.; Lee, J.E.; Shim, S.M.; Ha, E.K.; Yon, D.K.; Kim, O.H.; Baek, J.H.; Koh, H.Y.; Chae, K.Y.; Lee, S.W.; et al. Cohort profile: National Investigation of Birth Cohort in Korea study 2008 (NICKs-2008). Clin. Exp. Pediatr. 2021, 64, 480–488. [Google Scholar] [CrossRef]
- Vigne, J.; Alexandre, J.; Fobe, F.; Milliez, P.; Loilier, M.; Fedrizzi, S.; Coquerel, A. QT prolongation induced by hydroxyzine: A pharmacovigilance case report. Eur. J. Clin. Pharmacol. 2015, 71, 379–381. [Google Scholar] [CrossRef]
- Magera, B.E.; Betlach, C.J.; Sweatt, A.P.; Derrick Jr, C.W. Hydroxyzine intoxication in a 13-month-old child. Pediatrics 1981, 67, 280–283. [Google Scholar] [CrossRef]
- Acosta-Materán, C.; Díaz-Oliva, E.; Fernández-Rodríguez, D.; Hernández-Afonso, J. QT interval prolongation and torsade de pointes: Synergistic effect of flecainide and H1 receptor antagonists. J. Pharmacol. Pharmacother. 2016, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Schlit, A.F.; Delaunois, A.; Colomar, A.; Claudio, B.; Cariolato, L.; Boev, R.; Valentin, J.P.; Peters, C.; Sloan, V.S.; Bentz, J.W. Risk of QT prolongation and torsade de pointes associated with exposure to hydroxyzine: Re-evaluation of an established drug. Pharmacol. Res. Perspect. 2017, 5, e00309. [Google Scholar] [CrossRef] [PubMed]
- Poluzzi, E.; Diemberger, I.; De Ridder, M.; Koci, A.; Clo, M.; Oteri, A.; Pecchioli, S.; Bezemer, I.; Schink, T.; Pilgaard Ulrichsen, S. Use of antihistamines and risk of ventricular tachyarrhythmia: A nested case-control study in five European countries from the ARITMO project. Eur. J. Clin. Pharmacol. 2017, 73, 1499–1510. [Google Scholar] [CrossRef]
- José de Abajo, F.; Rodríguez, L.A.G. Risk of ventricular arrhythmias associated with nonsedating antihistamine drugs. Br. J. Clin. Pharmacol. 1999, 47, 307–313. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, M.L.; Van Puijenbroek, E.P.; Egberts, A.; Hoes, A.W.; Leufkens, H.G. Non-sedating antihistamine drugs and cardiac arrhythmias–biased risk estimates from spontaneous reporting systems? Br. J. Clin. Pharmacol. 2002, 53, 370–374. [Google Scholar] [CrossRef]
- Hulhoven, R.; Rosillon, D.; Letiexhe, M.; Meeus, M.-A.; Daoust, A.; Stockis, A. Levocetirizine does not prolong the QT/QTc interval in healthy subjects: Results from a thorough QT study. Eur. J. Clin. Pharmacol. 2007, 63, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Engwall, M.J.; Baublits, J.; Chandra, F.A.; Jones, Z.W.; Wahlstrom, J.; Chui, R.W.; Vargas, H.M. Evaluation of levocetirizine in beagle dog and cynomolgus monkey telemetry assays: Defining the no QTc effect profile by timepoint and concentration-QTc analysis. Clin. Transl. Sci. 2023, 16, 436–446. [Google Scholar] [CrossRef]
- Taglialatela, M.; Castaldo, P.; Pannaccione, A.; Giorgio, G.; Genovese, A.; Marone, G.; Annunziato, L. Cardiac ion channels and antihistamines: Possible mechanisms of cardiotoxicity. Clin. Exp. Allergy 1999, 29, 182–189. [Google Scholar] [CrossRef]
- Mladěnka, P.; Applová, L.; Patočka, J.; Costa, V.M.; Remiao, F.; Pourová, J.; Mladěnka, A.; Karlíčková, J.; Jahodář, L.; Vopršalová, M. Comprehensive review of cardiovascular toxicity of drugs and related agents. Med. Res. Rev. 2018, 38, 1332–1403. [Google Scholar] [CrossRef]
- Khalifa, M.; Drolet, B.; Daleau, P.; Lefez, C.; Gilbert, M.; Plante, S.; O’Hara, G.E.; Gleeton, O.; Hamelin, B.A.; Turgeon, J. Block of potassium currents in guinea pig ventricular myocytes and lengthening of cardiac repolarization in man by the histamine H1 receptor antagonist diphenhydramine. J. Pharmacol. Exp. Ther. 1999, 288, 858–865. [Google Scholar]
- Salata, J.J.; Jurkiewicz, N.K.; Wallace, A.A.; Stupienski III, R.F.; Guinosso, P.J., Jr.; Lynch, J.J., Jr. Cardiac electrophysiological actions of the histamine H1-receptor antagonists astemizole and terfenadine compared with chlorpheniramine and pyrilamine. Circ. Res. 1995, 76, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zheng, Q.; Farley, J.M. Antimuscarinic actions of antihistamines on the heart. J. Biomed. Sci. 2006, 13, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Saternos, H.C.; Almarghalani, D.A.; Gibson, H.M.; Meqdad, M.A.; Antypas, R.B.; Lingireddy, A.; AbouAlaiwi, W.A. Distribution and function of the muscarinic receptor subtypes in the cardiovascular system. Physiol. Genom. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.D. Muscarinic receptor agonists and antagonists: Effects on cardiovascular function. Muscarinic Recept. 2012, 208, 299–316. [Google Scholar]
- Yun, J.; Kim, S.Y.; Yoon, K.S.; Shin, H.; Jeong, H.-S.; Chung, H.; Kim, Y.-H.; Shin, J.; Cha, H.J.; Han, K.m. P21 (Cdc42/Rac)-activated kinase 1 (pak1) is associated with cardiotoxicity induced by antihistamines. Arch. Pharmacal. Res. 2016, 39, 1644–1652. [Google Scholar] [CrossRef]
- Tamariz, L.; Harkins, T.; Nair, V. A systematic review of validated methods for identifying ventricular arrhythmias using administrative and claims data. Pharmacoepidemiol. Drug Saf. 2012, 21, 148–153. [Google Scholar] [CrossRef]
- Niesner, K.; Murff, H.J.; Griffin, M.R.; Wasserman, B.; Greevy, R.; Grijalva, C.G.; Roumie, C.L. Validation of VA administrative data algorithms for identifying cardiovascular disease hospitalization. Epidemiology 2013, 24, 334–335. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Inagaki, N.; Nakayama, T.; Morichi, S.; Nishimata, S.; Yamanaka, G.; Kashiwagi, Y. Cardiac Complications Caused by Respiratory Syncytial Virus Infection: Questionnaire Survey and a Literature Review. Glob. Pediatr. Health 2021, 8. [Google Scholar] [CrossRef]
- Steininger, C.; Holzmann, H.; Zwiauer, K.F.; Popow-Kraupp, T. Influenza A virus infection and cardiac arrhythmia during the neonatal period. Scand. J. Infect. Dis. 2002, 34, 782–784. [Google Scholar] [CrossRef]
- Babapoor-Farrokhran, S.; Rasekhi, R.T.; Gill, D.; Babapoor, S.; Amanullah, A. Arrhythmia in COVID-19. SN Compr. Clin. Med. 2020, 2, 1430–1435. [Google Scholar] [CrossRef]
- Song, M.K.; Kwon, B. Arrhythmia and COVID-19 in children. Clin. Exp. Pediatr. 2023, 66, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Garan, H.; Wan, E.Y.; Scully, B.E.; Biviano, A.; Yarmohammadi, H. Cardiac arrhythmias in viral infections. J. Interv. Card. Electrophysiol. 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Maclure, M. The case-crossover design: A method for studying transient effects on the risk of acute events. Am. J. Epidemiol. 1991, 133, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Shahn, Z.; Hernán, M.A.; Robins, J.M. A formal causal interpretation of the case-crossover design. Biometrics 2023, 79, 1330–1343. [Google Scholar] [CrossRef]
- Schneeweiss, S.; Stürmer, T.; Maclure, M. Case–crossover and case–time–control designs as alternatives in pharmacoepidemiologic research. Pharmacoepidemiol. Drug Saf. 1997, 6, S51–S59. [Google Scholar] [CrossRef]

| Variables a | N (%) | |
|---|---|---|
| Main Cohort b (N = 1992) | Second Cohort b (N = 1387) | |
| Age | ||
| 6–24 months | 348 (17.5) | 214 (15.4) |
| 24 months–6 years | 1194 (59.9) | 861 (62.1) |
| ≥7 years | 450 (22.6) | 312 (22.5) |
| Sex | ||
| Male | 1010 (50.7) | 704 (50.8) |
| Female | 982 (49.3) | 683 (49.2) |
| Residential area c | ||
| Seoul | 499 (25.3) | 360 (26.2) |
| Metropolitan | 389 (19.8) | 269 (19.6) |
| City | 942 (47.8) | 651 (47.4) |
| Rural | 139 (7.1) | 92 (6.7) |
| Economic status d | ||
| Below median | 876 (46.0) | 601 (45.4) |
| Median or over | 1027 (54.0) | 723 (54.6) |
| Season e | ||
| Spring (March–May) | 492 (24.7) | 331 (23.9) |
| Summer (June–August) | 511 (25.7) | 342 (24.7) |
| Fall (September–November) | 486 (24.4) | 359 (25.9) |
| Winter (December–February) | 503 (25.3) | 355 (25.6) |
| Comorbidities | ||
| Kawasaki disease | 40 (2.0) | 28 (2.0) |
| Obesity | 119 (6.7) | 82 (5.9) |
| Sleep apnea | 2 (0.1) | 2 (0.1) |
| Hyperthyroidism | 3 (0.2) | 3 (0.2) |
| Diabetes mellitus | 1 (0.1) | 1 (0.1) |
| Concomitant diseases f | ||
| Acute nasopharyngitis | 270 (13.6) | 181 (13.0) |
| Acute tonsillitis | 284 (14.3) | 202 (14.6) |
| Acute pharyngitis | 283 (14.2) | 199 (14.3) |
| Acute upper respiratory infection | 243 (12.2) | 165 (11.9) |
| Acute sinusitis | 193 (9.7) | 136 (9.8) |
| Acute suppurative otitis media | 122 (6.1) | 83 (6.0) |
| Acute bronchitis | 1087 (54.6) | 765 (55.2) |
| Acute bronchiolitis | 160 (8.0) | 109 (7.9) |
| Gastroenteritis and colitis | 108 (5.4) | 85 (6.1) |
| Allergic rhinitis | 229 (11.5) | 166 (12.0) |
| Concomitant medication f | ||
| Second-generation antihistamine | 395 (19.8) | 301 (21.7) |
| Nasal decongestant | 959 (48.1) | 691 (49.8) |
| Systemic steroid | 402 (20.2) | 282 (20.3) |
| Time Window before Index Date | Total N | N (%) | Crude OR (95% CI) | Adjusted OR (95% CI) a | ||
|---|---|---|---|---|---|---|
| Exposed during the Hazard Period | Exposed during the Control Period 1 | Exposed during the Control Period 2 | ||||
| Main cohort | ||||||
| 0–15 days | 1992 | 1011 (50.8) | 796 (40.0) | 767 (38.5) | 1.203 (1.139–1.270) | 1.201 (1.132–1.273) |
| 0–10 days | 1731 | 870 (50.3) | 647 (37.4) | 626 (36.2) | 1.237 (1.167–1.312) | 1.249 (1.170–1.333) |
| 0–5 days | 1297 | 639 (49.3) | 452 (34.8) | 445 (34.3) | 1.254 (1.172–1.341) | 1.250 (1.157–1.351) |
| Second cohort | ||||||
| 0–15 days | 1387 | 694 (50.0) | 560 (40.4) | 541 (39.0) | 1.181 (1.107–1.260) | 1.189 (1.110–1.274) |
| 0–10 days | 1200 | 607 (50.5) | 450 (37.5) | 431 (35.9) | 1.248 (1.164–1.339) | 1.264 (1.171–1.365) |
| 0–5 days | 927 | 457 (49.3) | 319 (34.4) | 326 (35.2) | 1.238 (1.143–1.341) | 1.235 (1.129–1.352) |
| Variables a | Total N = 1992 | ||
|---|---|---|---|
| N (%) | Adjusted OR (95% CI) b | p Value | |
| Age | |||
| 6–24 months | 348 (17.5) | 1.283 (1.095–1.503) | Ref |
| 24 months–6 years | 1194 (59.9) | 1.199 (1.113–1.292) | 0.390 |
| ≥7 years | 450 (22.6) | 1.159 (1.028–1.305) | 0.244 |
| Sex | |||
| Male | 1010 (50.7) | 1.207 (1.111–1.312) | Ref |
| Female | 982 (49.3) | 1.194 (1.098–1.298) | 0.829 |
| Residential area c | |||
| Seoul | 499 (25.3) | 1.229 (1.095–1.380) | Ref |
| Metropolitan | 389 (19.8) | 1.111 (0.973–1.269) | 0.165 |
| City | 942 (47.8) | 1.172 (1.077–1.275) | 0.439 |
| Rural | 139 (7.1) | 1.746 (1.346–2.265) | 0.039 |
| Economic status d | |||
| <Median | 876 (46.0) | 1.273 (1.166–1.390) | Ref |
| ≥Median | 1027 (54.0) | 1.144 (1.057–1.239) | 0.04 |
| Season | |||
| Spring (March–May) | 492 (24.7) | 1.255 (1.114–1.413) | Ref |
| Summer (June–August) | 511 (25.7) | 0.990 (0.883–1.110) | <0.001 |
| Fall (September–November) | 486 (24.4) | 1.482 (1.313–1.674) | 0.045 |
| Winter (December–February) | 503 (25.3) | 1.145 (1.017–1.290) | 0.200 |
| Cardiovascular events e | |||
| Arrhythmia | 1902 (95.5) | 1.192 (1.122–1.265) | Ref |
| Ischemic heart disease | 90 (4.5) | 1.414 (1.063–1.882) | 0.245 |
| With specific birth history f | |||
| No | 1639 (82.3) | 1.193 (1.120–1.271) | Ref |
| Yes | 353 (17.7) | 1.252 (1.066–1.470) | 0.526 |
| With underlying cardiovascular disease g | |||
| No | 1658 (83.2) | 1.208 (1.132–1.288) | Ref |
| Yes | 334 (16.8) | 1.165 (1.007–1.347) | 0.584 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Cha, H.R.; Ha, E.K.; Kwak, J.H.; Kim, H.; Shin, J.; Jee, H.M.; Han, M.Y. Association between First-Generation Antihistamine Use in Children and Cardiac Arrhythmia and Ischemic Heart Disease: A Case-Crossover Study. Pharmaceuticals 2023, 16, 1073. https://doi.org/10.3390/ph16081073
Kim JH, Cha HR, Ha EK, Kwak JH, Kim H, Shin J, Jee HM, Han MY. Association between First-Generation Antihistamine Use in Children and Cardiac Arrhythmia and Ischemic Heart Disease: A Case-Crossover Study. Pharmaceuticals. 2023; 16(8):1073. https://doi.org/10.3390/ph16081073
Chicago/Turabian StyleKim, Ju Hee, Hye Ryeong Cha, Eun Kyo Ha, Ji Hee Kwak, Hakjun Kim, Jeewon Shin, Hye Mi Jee, and Man Yong Han. 2023. "Association between First-Generation Antihistamine Use in Children and Cardiac Arrhythmia and Ischemic Heart Disease: A Case-Crossover Study" Pharmaceuticals 16, no. 8: 1073. https://doi.org/10.3390/ph16081073
APA StyleKim, J. H., Cha, H. R., Ha, E. K., Kwak, J. H., Kim, H., Shin, J., Jee, H. M., & Han, M. Y. (2023). Association between First-Generation Antihistamine Use in Children and Cardiac Arrhythmia and Ischemic Heart Disease: A Case-Crossover Study. Pharmaceuticals, 16(8), 1073. https://doi.org/10.3390/ph16081073







