Reactive Palladium–Ligand Complexes for 11C-Carbonylation at Ambient Pressure: A Breakthrough in Carbon-11 Chemistry
Abstract
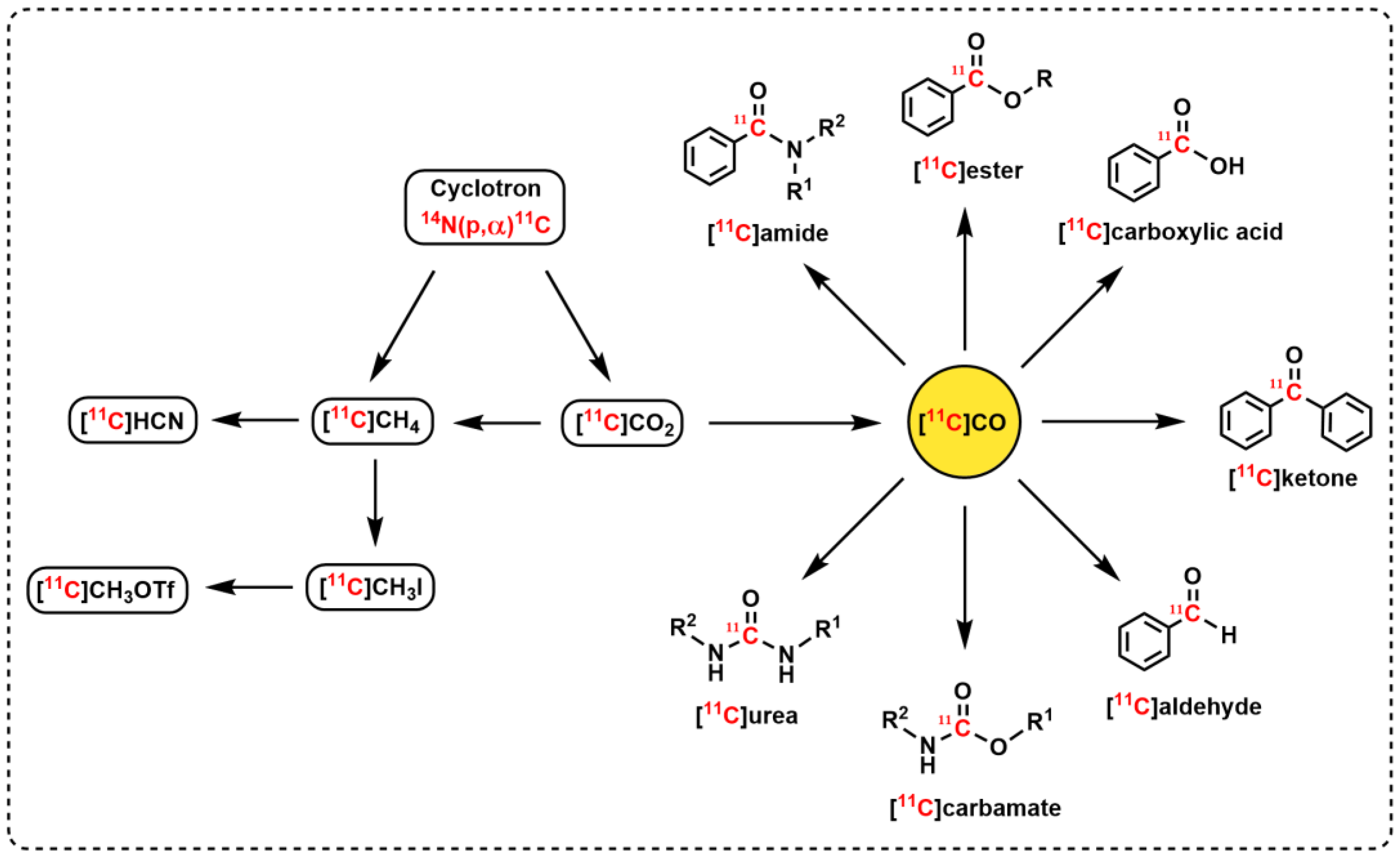
1. Introduction
2. 11C-Carbonylation
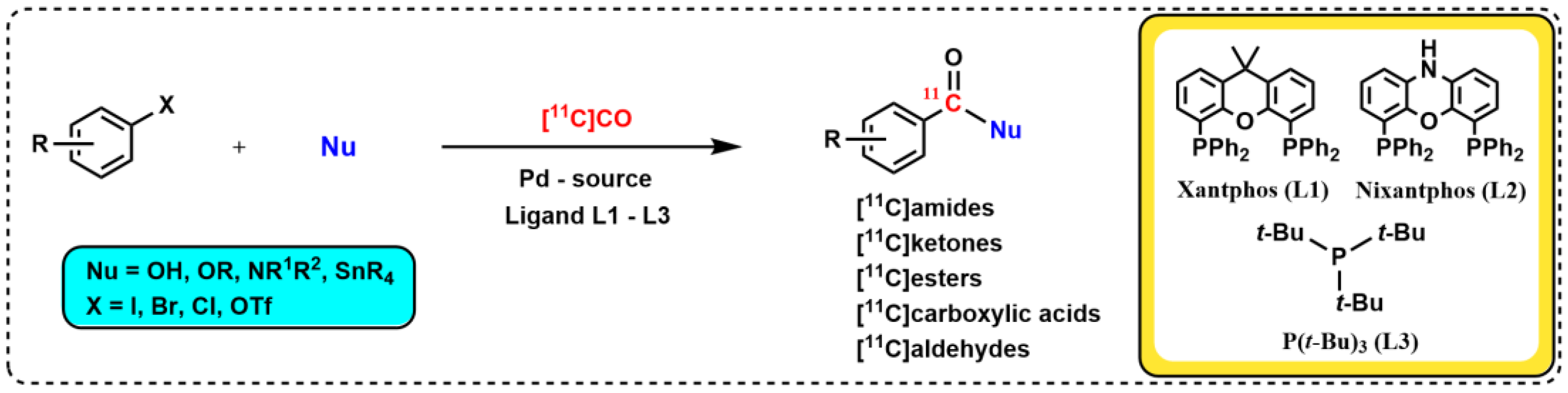
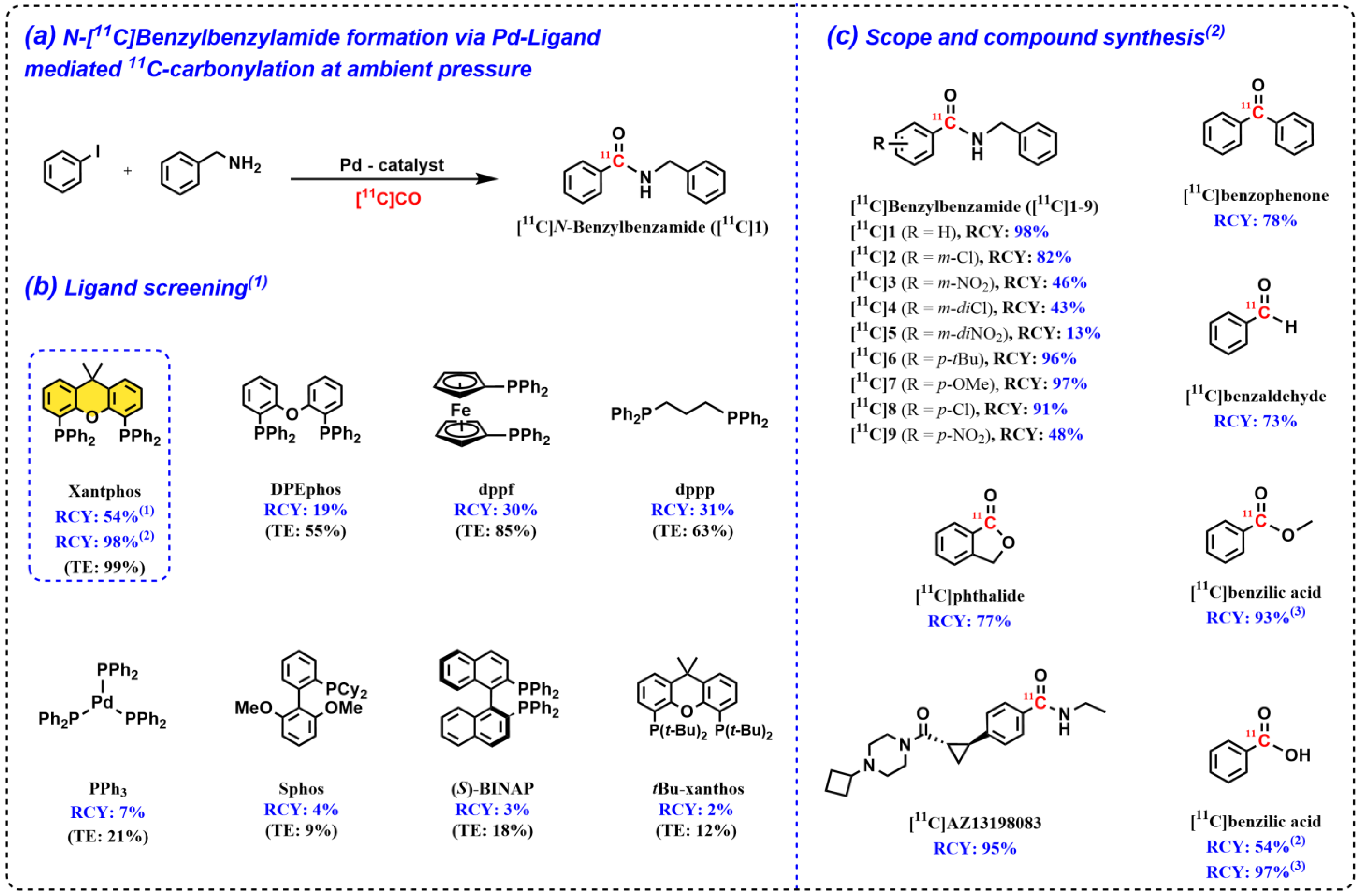
2.1. Discovery and Development of the Pd–Xantphos Protocol for 11C-Carbonylation Radiochemistry at Ambient Pressure
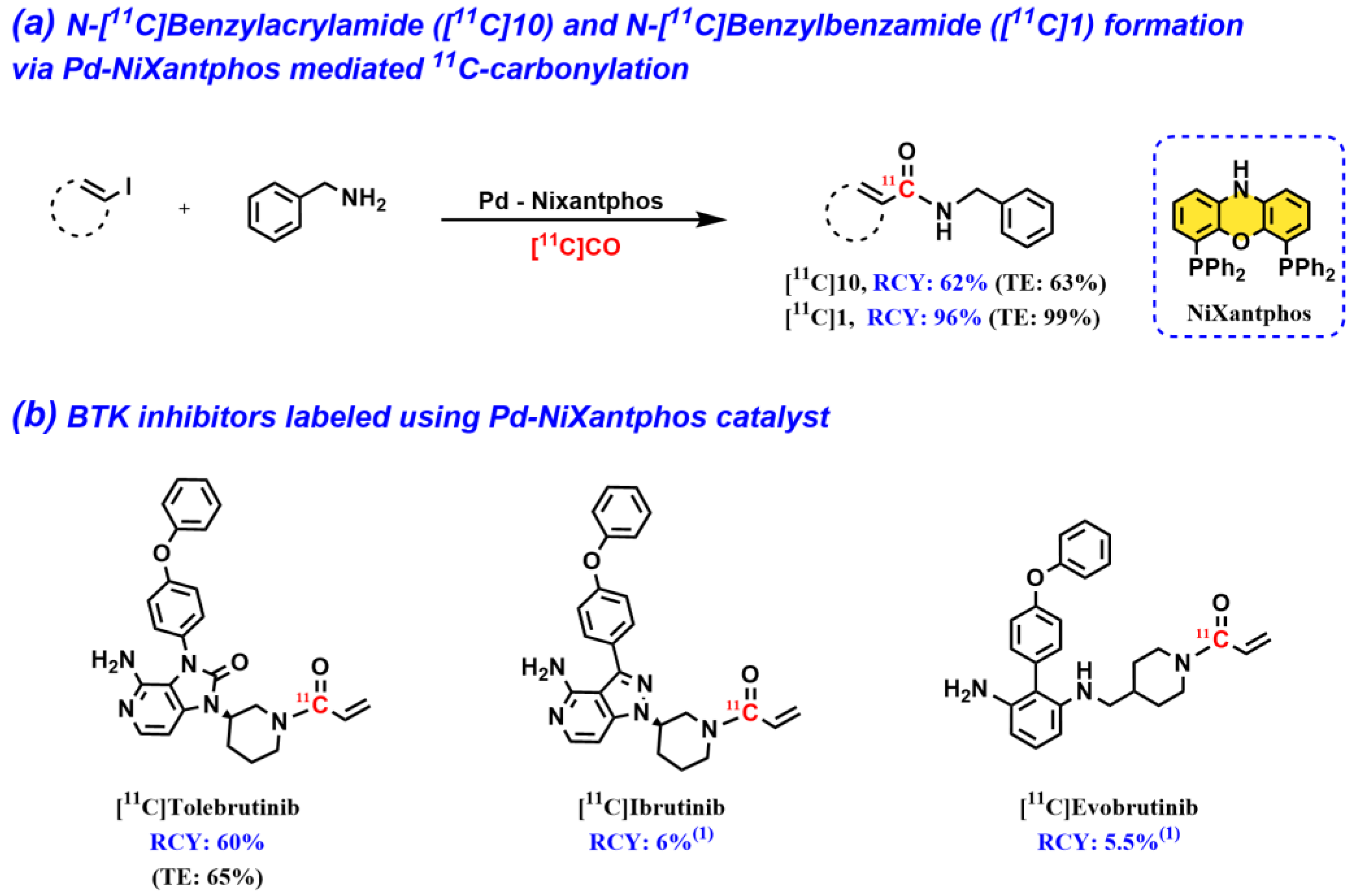
2.2. Pd–NiXantphos: An Alternative Pd-Ligand Complex for 11C-Carbonylation
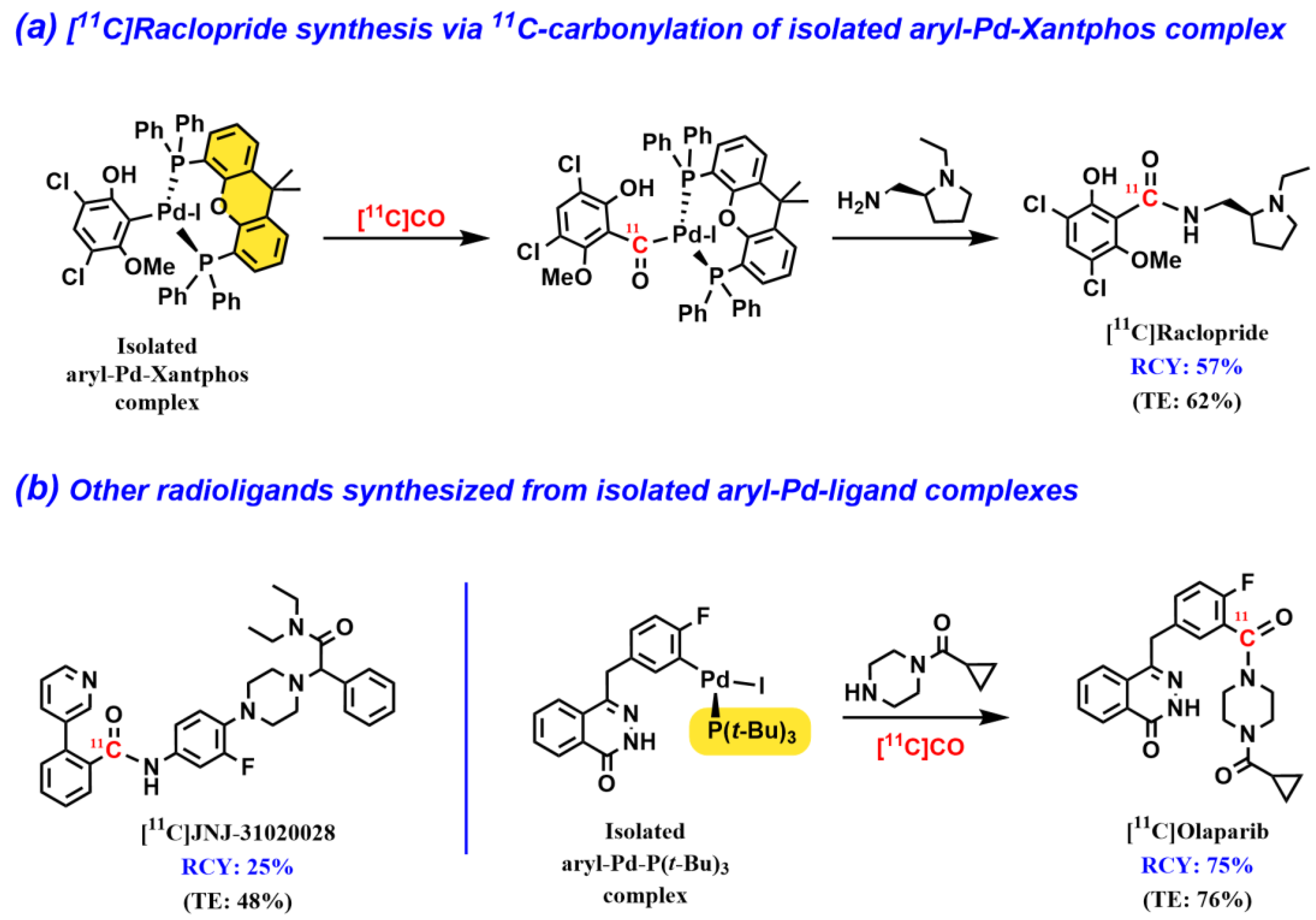
2.3. Isolated Aryl–Pd–Ligand Complexes for 11C-Carbonylation Radiochemistry
2.4. “In-loop” 11C-Carbonylation Reaction
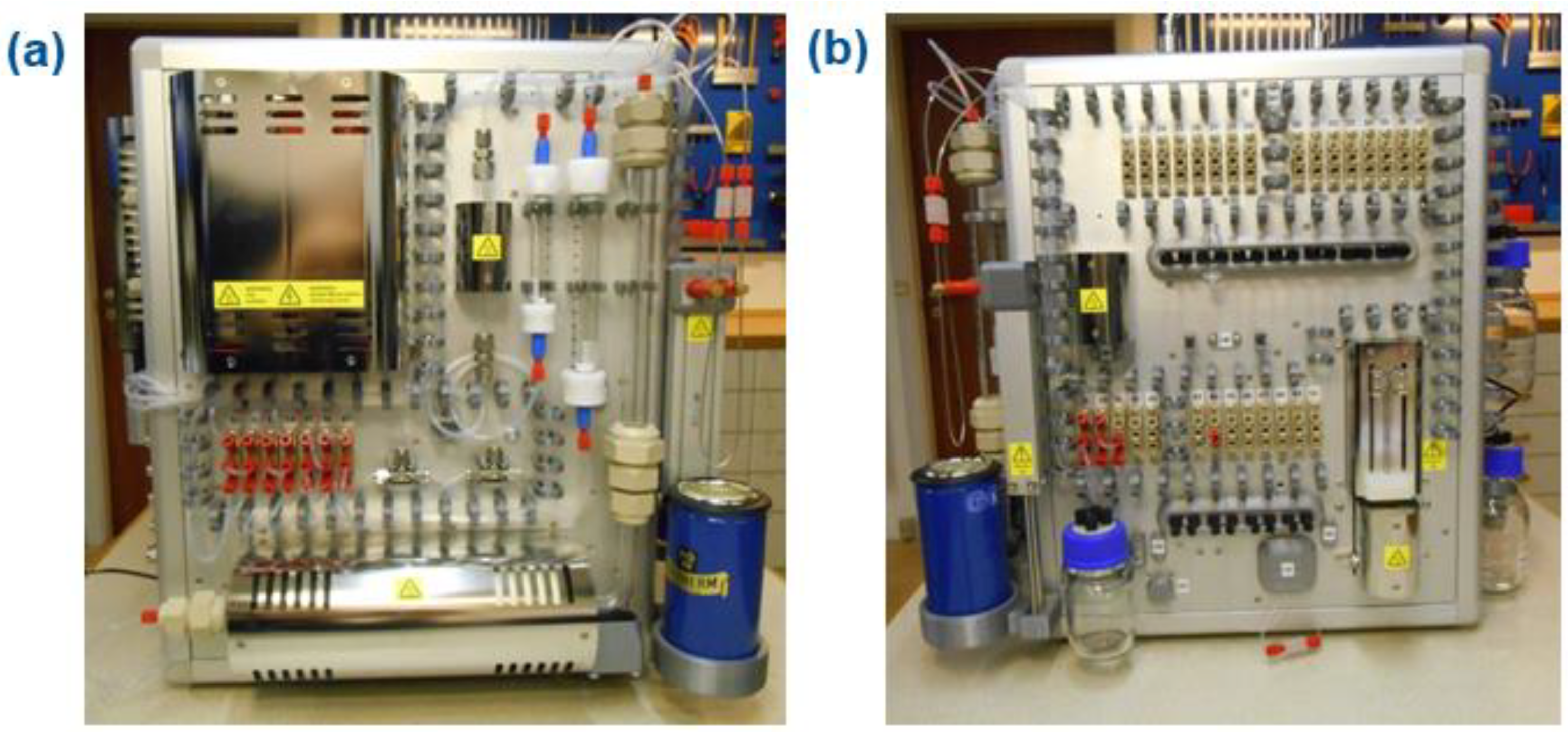
2.5. TracerMakerTM: A Fully Automated and GMP-Compliant Synthesis of [11C]CO-Labeled Radiopharmaceuticals
3. Pd–Xantphos-Based Radiochemistry for the Synthesis of Bioactive Molecules and Their Applications in PET Imaging
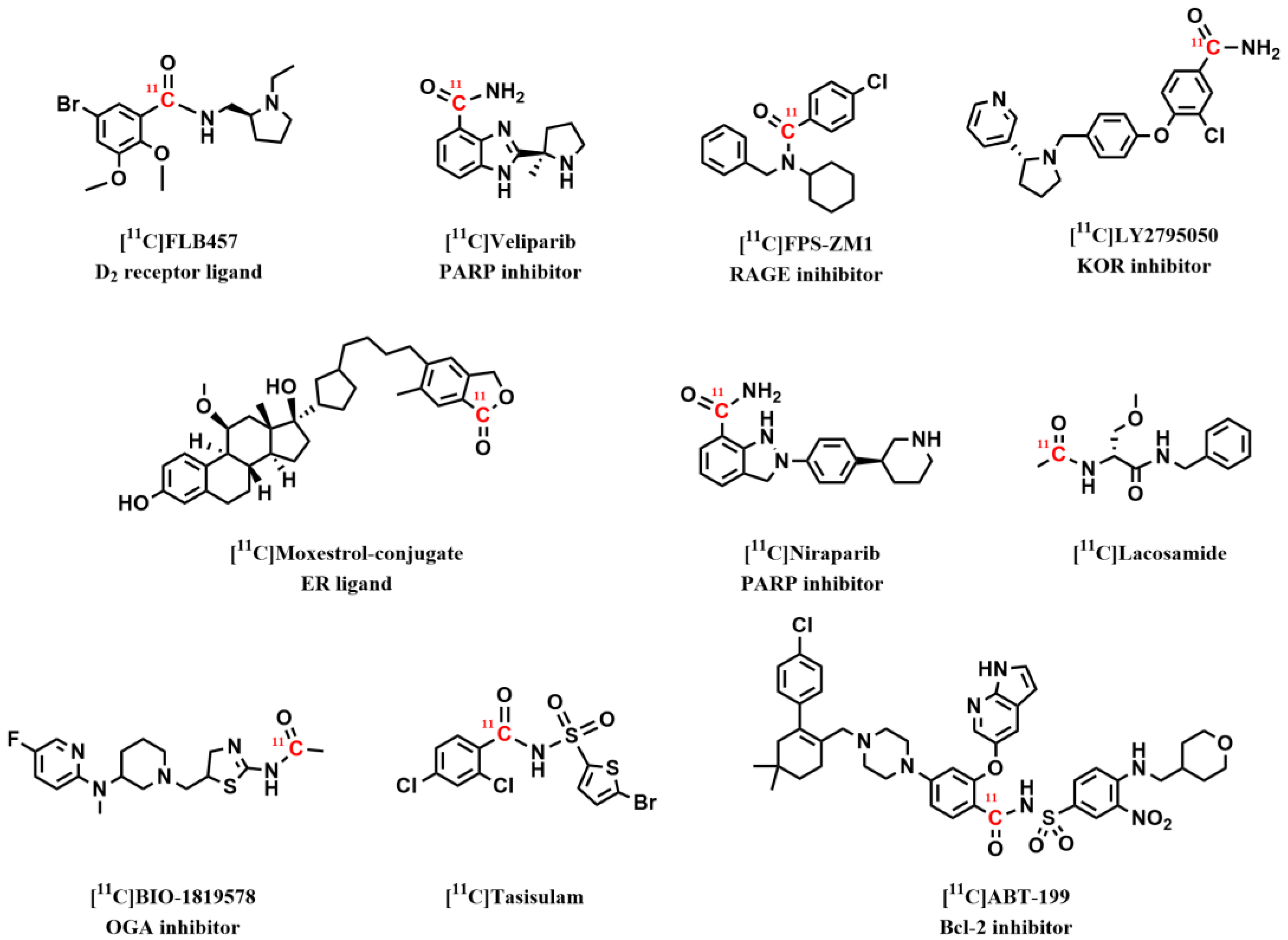
3.1. Radiopharmaceuticals for PET Imaging in the Central Nervous System
3.2. Radiopharmaceuticals for PET Imaging in Oncology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ametamey, S.M.; Honer, H.; Schubiger, P.A. Molecular imaging with PET. Chem. Rev. 2008, 108, 1501–1516. [Google Scholar] [CrossRef] [PubMed]
- Halldin, C.; Gulyas, B.; Langer, O.; Farde, L. Brain radioligands—State of the art and new trends. J. Nucl. Med. 2001, 45, 139–152. [Google Scholar]
- Rohren, E.M.; Turkington, T.G.; Coleman, R.E. Clinical Applications of PET in Oncology. Radiology 2004, 231, 305–332. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, M.; Ziegler, S.; Nekolla, S.G. PET/CT: Challenge for Nuclear Cardiology. J. Nucl. Med. 2005, 46, 1664–1678. [Google Scholar]
- Nerella, S.G.; Singh, P.; Sanam, T.; Digwal, C.S. PET Molecular Imaging in Drug Development: The Imaging and Chemistry Perspective. Front. Med. 2022, 9, 12270. [Google Scholar] [CrossRef]
- Miller, P.W.; Long, N.J.; Vilar, R.; Gee, A.D. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew. Chem. Int. Ed. 2008, 47, 8998–9033. [Google Scholar] [CrossRef]
- Goud, N.S.; Bhattacharya, A.; Joshi, R.K.; Nagaraj, C.; Bharath, R.D.; Kumar, P. Carbon-11: Radiochemistry and Target-Based PET Molecular Imaging Applications in Oncology, Cardiology, and Neurology. J. Med. Chem. 2021, 64, 1223–1259. [Google Scholar] [CrossRef]
- Antoni, G. Development of carbon-11 labelled PET tracers—Radiochemical and technological challenges in a historic perspective. J. Label. Compd. Radiopharm. 2015, 58, 65–72. [Google Scholar] [CrossRef]
- Shegani, A.; Kealey, S.; Luzi, F.; Basagni, F.; do Mar Machado, J.; Ekici, S.D.; Ferocino, A.; Gee, A.D.; Bongarzone, S. Radiosynthesis, Preclinical, and Clinical Positron Emission Tomography Studies of Carbon-11 Labeled Endogenous and Natural Exogenous Compounds. Chem. Rev. 2023, 123, 105–229. [Google Scholar] [CrossRef]
- Scott, P. Methods for the incorporation of carbon-11 to generate radiopharmaceuticals for PET imaging. Angew. Chem. Int. Ed. 2009, 48, 6001–6004. [Google Scholar] [CrossRef]
- Toddei, C.; Gee, A.D. Recent progress in [11C]carbon dioxide ([11C]CO2) and [11C]carbon monoxide ([11C]CO) chemistry. J. Label. Compd. Radiopharm. 2017, 61, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Kealey, S.; Gee, A.D.; Miller, P.W. Transition metal mediated [11C]carbonylation reactions: Recent advances and applications. J. Label. Compd. Radiopharm. 2014, 57, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Rahman, O. [11C]Carbon monoxide in labeling chemistry and positron emission tomography tracer development: Scope and limitations. J. Label. Compd. Radiopharm. 2015, 58, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Taddei, C.; Pike, V.W. [11C]Carbon monoxide: Advances in production and application to PET radiotracer development over the past 15 years. EJNMMI Radiopharm. Chem. 2019, 4, 25–56. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Antoni, G.; Långström, B.; Itsenko, O. The development of 11C-carbonylation chemistry: A systematic view. Nucl. Med. Biol. 2021, 92, 115–137. [Google Scholar] [CrossRef] [PubMed]
- Dahl, K.; Schou, M.; Amini, N.; Halldin, C. Palladium-mediated [11C]carbonylation at atmospheric pressure: A general method using xantphos as supporting ligand. Eur. J. Org. Chem. 2013, 2013, 1228–1231. [Google Scholar] [CrossRef]
- Brennführer, A.; Neumann, H.; Beller, M. Palladium-catalyzed carbonylation reactions of aryl halides and related compounds. Angew. Chem. Int. Ed. 2009, 48, 4114–4133. [Google Scholar] [CrossRef]
- Kihlberg, T.; Långström, B. Biologically Active 11C-Labeled Amides Using Palladium-Mediated Reactions with Aryl Halides and [11C]Carbon Monoxide. J. Org. Chem. 1999, 64, 9201–9205. [Google Scholar] [CrossRef]
- Långström, B.; Itsenko, O.; Rahman, O. [11C]carbon monoxide, a versatile and useful precursor in labelling chemistry for PET-ligand development. J. Label. Compd. Radiopharm. 2007, 50, 794–810. [Google Scholar] [CrossRef]
- Audrain, H.; Martarello, L.; Gee, A.; Bender, D. Utilization of [11C]-labelled boron carbonyl complexes in palladium carbonylation reaction. Chem. Comm. 2004, 10, 558–559. [Google Scholar] [CrossRef]
- Kealey, S.; Miller, P.W.; Long, N.; Plisson, C.; Martarello, L.; Gee, A.D. Copper(I) scorpionate complexes and their application in palladium-mediated [11C]carbonylation reactions. Chem. Commun. 2009, 3696–3698. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; van den Hoek, J.; Windhorst, A.D. Transition metal mediated synthesis using [11C]CO at low pressure—A simplified method for 11C-carbonylation. J. Label. Compd. Radiopharm. 2012, 55, 223–228. [Google Scholar] [CrossRef]
- Takashima-Hirano, M.; Ishii, H.; Suzuki, M. Synthesis of [11C]Am80 via Novel Pd(0)-Mediated Rapid [11C]Carbonylation Using Arylboronate and [11C]Carbon Monoxide. ACS Med. Chem. Lett. 2012, 3, 804–807. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, P.W.N.M.; Kamer, P.C.J. Featuring Xantphos. Catal. Sci. Technol. 2018, 8, 26–113. [Google Scholar] [CrossRef]
- van Leeuwen, P.; Kamer, P.; Reek, J.; Dierkes, P. Ligand bite angle effects in metal-catalyzed c-c bond formation. Chem. Rev. 2000, 100, 2741–2769. [Google Scholar] [CrossRef]
- Martinelli, J.R.; Freckmann, D.M.M.; Buchwald, S.L. Convenient Method for the Preparation of Weinreb Amides via Pd-Catalyzed Aminocarbonylation of Aryl Bromides at Atmospheric Pressure. Org. Lett. 2006, 8, 4843–4846. [Google Scholar] [CrossRef]
- Martinelli, J.R.; Watson, D.A.; Freckmann, D.M.M.; Barder, T.E.; Buchwald, S.L. Palladium-Catalyzed Carbonylation Reactions of Aryl Bromides at Atmospheric Pressure: A General System Based on Xantphos. J. Org. Chem. 2008, 73, 7102–7107. [Google Scholar] [CrossRef]
- Miller, P.W.; Jennings, L.E.; deMello, A.J.; Gee, A.D.; Long, N.J.; Vilar, R. A Microfluidic Approach to the Rapid Screening of Palladium-Catalysed Aminocarbonylation Reactions. Adv. Synth. Catal. 2009, 351, 3260–3268. [Google Scholar] [CrossRef]
- Miller, P.W.; Audrain, H.; Bender, D.; deMello, A.J.; Gee, A.D.; Long, N.J.; Vilar, R. Rapid Carbon-11 Radiolabelling for PET Using Microfluidics. Chem. Eur. J. 2011, 17, 460–463. [Google Scholar] [CrossRef]
- Dahl, K.; Schou, M.; Rahman, O.; Halldin, C. Improved Yields for the Palladium-Mediated 11C-Carbonylation Reaction Using Microwave Technology. Eur. J. Org. Chem. 2013, 2014, 304–310. [Google Scholar]
- Dahl, K.; Turner, T.; Vasdev, N. Radiosynthesis of a Bruton’s tyrosine kinase inhibitor, [11C]Tolebrutinib, via palladium-NiXantphos-mediated carbonylation. J. Label. Compd. Radiopharm. 2020, 63, 482–487. [Google Scholar] [CrossRef]
- Lindberg, A.; Boyle, A.J.; Tong, J.; Harkness, M.B.; Garcia, A.; Tran, T.; Zhai, D.; Liu, F.; Donnelly, D.J.; Vasdev, N. 11C-Radiosynthesis of [11C]Ibrutinib via Pd-Mediated [11C]CO Carbonylation: Preliminary PET Imaging in Experimental Autoimmune Encephalomyelitis Mice. Front. Nucl. Med. 2021, 1, 772289. [Google Scholar] [CrossRef]
- Boyle, A.J.; Lindberg, A.; Tong, J.; Vasdev, N. Radiosynthesis of [11C]Evobrutinib via Palladium-NiXantphos-Mediated 11C-Carbonylation and Preliminary Imaging in Mice. J. Label. Compd. Radiopharm. 2023; Submitted. [Google Scholar]
- Eriksson, J.; Åberg, O.; Långström, B. Synthesis of [11C]/[13C]Acrylamides by Palladium-Mediated Carbonylation. Eur. J. Org. Chem. 2007, 2007, 455–461. [Google Scholar] [CrossRef]
- Åberg, O.; Långström, B. Combinatorial synthesis of labelled drugs and PET tracers: Synthesis of a focused library of 11C-carbonyl-labelled acrylamides as potential biomarkers of EGFR expression. J. Label. Compd. Radiopharm. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- van der Wildt, B.; Wilhelmus, M.M.M.; Bijkerk, J.; Haveman, L.Y.F.; Kooijman, E.J.M.; Schuit, R.C.; Bol, J.G.J.M.; Jongenelen, C.A.M.; Lammertsma, A.A.; Drukarch, B.; et al. Development of carbon-11 labeled acryl amides for selective PET imaging of active tissue transglutaminase. Nucl. Med. Biol. 2016, 43, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Mossine, A.V.; Brooks, A.F.; Jackson, I.M.; Quesada, C.A.; Sherman, P.; Cole, E.L.; Donnelly, D.J.; Scott, P.J.H.; Shao, X. Synthesis of Diverse 11C-Labeled PET Radiotracers via Direct Incorporation of [11C]CO2. Bioconjug. Chem. 2016, 27, 1382–1389. [Google Scholar] [CrossRef]
- Andersen, T.; Friis, S.; Audrain, H.; Nordeman, P.; Antoni, G.; Skrydstrup, T. Efficient 11C-carbonylation of isolated aryl palladium complexes for PET: Application to challenging radiopharmaceutical synthesis. J. Am. Chem. Soc. 2015, 137, 1548–1555. [Google Scholar] [CrossRef]
- Andersen, T.L.; Nordeman, P.; Christoffersen, H.F.; Audrain, H.; Antoni, G.; Skrydstrup, T. Application of Methyl Bisphosphine-Ligated Palladium Complexes for Low Pressure N-11C-Acetylation of Peptides. Angew. Chem. Int. Ed. 2017, 56, 4549–4553. [Google Scholar] [CrossRef]
- Ferrat, M.; Dahl, K.; Halldin, C.; Schou, M. “In-loop” carbonylation—A simplified method for carbon-11 labelling of drugs and radioligands. J. Label. Compd. Radiopharm. 2020, 63, 100–107. [Google Scholar] [CrossRef]
- Donnelly, D.J.; Preshlock, S.; Kaur, T.; Tran, T.; Wilson, T.C.; Mhanna, K.; Henderson, B.D.; Batalla, D.; Scott, P.J.H.; Shao, X. Synthesis of Radiopharmaceuticals via “In-Loop” 11C-Carbonylation as Exemplified by the Radiolabeling of Inhibitors of Bruton’s Tyrosine Kinase. Front. Nucl. Med. 2022, 1, 820235. [Google Scholar] [CrossRef]
- Ferrat, M.; El Khoury, Y.; Larsen, P.; Dahl, K.; Halldin, C.; Schou, M. Development of a fully automated low-pressure [11C]CO carbonylation apparatus. J. Label. Compd. Radiopharm. 2020, 63, 517–522. [Google Scholar]
- Duffy, I.R.; Vasdev, N.; Dahl, K. Copper(I)-Mediated 11C-Carboxylation of (Hetero)arylstannanes. ACS Omega 2020, 5, 8242–8825. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswami, V.; Tong, J.; Schifani, C.; Bloomfield, P.M.; Dahl, K.; Vasdev, N. Preclinical Evaluation of TSPO and MAO-B PET Radiotracers in an LPS Model of Neuroinflammation. PET Clin. 2021, 2, 233–247. [Google Scholar] [CrossRef]
- Dahl, K.; Nakao, R.; Amini, N.; Moein, M.M.; Finnema, S.; Malmquist, J.; Varnäs, K.; Schou, M. Development of [Carbonyl-11C]AZ13198083, a Novel Histamine Type-3 Receptor Radioligand with Favorable Kinetics. ACS Chem. Neurosci. 2018, 9, 906–911. [Google Scholar] [CrossRef]
- Rahman, O.; Takano, A.; Amini, N.; Dahl, K.; Kanegawa, N.; Långström, B.; Farde, L.; Halldin, C. Synthesis of ([11C]carbonyl)raclopride and a comparison with ([11C]methyl)raclopride in a monkey PET study. Nucl. Med. Biol. 2015, 42, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Luzi, F.; Savickas, V.; Taddei, C.; Hader, S.; Singh, N.; Gee, A.D.; Bongarzone, S. Radiolabelling of [11C]FPS-ZM1, a RAGE-targeting PET radiotracer, using a [11C]CO2-to-[11C]CO chemical conversion. Future Med. Chem. 2019, 12, 511–521. [Google Scholar] [CrossRef]
- Taddei, C.; Bongarzone, S.; Dheere, A.K.H.; Gee, A.D. [11C]CO2 to [11C]CO conversion mediated by [11C]silanes: A novel route for [11C]carbonylation reactions. Chem. Commun. 2015, 51, 11795–11797. [Google Scholar] [CrossRef]
- Kaur, T.; Scott, P.J.H.; Shao, X. Radiosynthesis of Kappa Opioid Receptor Radioligand [11C]LY2795050, via In-loop [11C]Carbonylation Chemistry. In Proceedings of the Society of Nuclear Medicine & Molecular Imaging, Virtual Event, 11–16 June 2021. [Google Scholar]
- Nag, S.; Arakawa, R.; Datta, P.; Forsberg, A.; Moein, M.M.; Bolin, M.; Lin, T.; Genung, N.; Heiring, H.; Guckian, K.; et al. Development of a novel 11CO labelled PET radioligand 11C-BIO-1819578 for the detection of O-GlcNAcase (OGA) enzyme in the living brain. In Proceedings of the European Association of Nuclear Medicine, Virtual Event, 15–19 October 2022. [Google Scholar]
- Zmuda, F.; Blair, A.; Liuzzi, M.C.; Malviya, G.; Chalmers, A.J.; Lewis, D.; Sutherland, A.; Pimlott, S.L. An 18F-Labeled Poly(ADP-ribose) Polymerase Positron Emission Tomography Imaging Agent. J. Med. Chem. 2018, 61, 4103–4114. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.C.; Xavier, M.-A.; Knight, J.; Verhoog, S.; Torres, J.B.; Mosley, M.; Hopkins, M.M.; Wallington, S.; Allen, P.D.; Kersemans, V.; et al. PET Imaging of PARP Expression Using 18F-Olaparib. J. Nucl. Med. 2019, 60, 504–510. [Google Scholar] [CrossRef]
- Ferrat, M.; Dahl, K.; Schou, M. One-Pot Synthesis of 11C-Labelled Primary Benzamides via Intermediate [11C]Aroyl Dimethylaminopyridinium Salts. Chem. Eur. J. 2021, 21, 8689–8693. [Google Scholar] [CrossRef]
- van der Wildt, B.; Shen, B.; Chin, F.T. Efficient synthesis of carbon-11 labelled acylsulfonamides using [11C]CO carbonylation chemistry. Chem. Commun. 2019, 55, 3124–3127. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.R.; Ilaria, R.L., Jr.; Sovak, M.A.; Williams, C.C.; Haura, E.B.; Cleverly, A.L.; Sykes, A.K.; Wagner, M.M.; de Alwis, D.P.; Slapak, C.A.; et al. A phase I study of tasisulam sodium (LY573636 sodium), a novel anticancer compound in patients with refractory solid tumors. Cancer Chemother. Pharmacol. 2011, 68, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Cornilleau, T.; Audrain, H.; Guillemet, A.; Hermange, P.; Fouquet, E. General Last-Step Labeling of Biomolecule-Based Substrates by [12C], [13C], and [11C]Carbon Monoxide. Org. Lett. 2015, 17, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Cornilleau, T.; Simonsen, M.; Vang, M.; Taib-Maamar, N.; Dessolin, J.; Audrain, H.; Hermange, P.; Fouquet, E. Last-Step Pd-Mediated [11C]CO Labeling of a Moxestrol-Conjugated o-Iodobenzyl Alcohol: From Model Experiments to in Vivo Positron Emission Tomography Studies. Bioconjug. Chem. 2017, 28, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahl, K.; Lindberg, A.; Vasdev, N.; Schou, M. Reactive Palladium–Ligand Complexes for 11C-Carbonylation at Ambient Pressure: A Breakthrough in Carbon-11 Chemistry. Pharmaceuticals 2023, 16, 955. https://doi.org/10.3390/ph16070955
Dahl K, Lindberg A, Vasdev N, Schou M. Reactive Palladium–Ligand Complexes for 11C-Carbonylation at Ambient Pressure: A Breakthrough in Carbon-11 Chemistry. Pharmaceuticals. 2023; 16(7):955. https://doi.org/10.3390/ph16070955
Chicago/Turabian StyleDahl, Kenneth, Anton Lindberg, Neil Vasdev, and Magnus Schou. 2023. "Reactive Palladium–Ligand Complexes for 11C-Carbonylation at Ambient Pressure: A Breakthrough in Carbon-11 Chemistry" Pharmaceuticals 16, no. 7: 955. https://doi.org/10.3390/ph16070955
APA StyleDahl, K., Lindberg, A., Vasdev, N., & Schou, M. (2023). Reactive Palladium–Ligand Complexes for 11C-Carbonylation at Ambient Pressure: A Breakthrough in Carbon-11 Chemistry. Pharmaceuticals, 16(7), 955. https://doi.org/10.3390/ph16070955






