Pharmacological Activities of Schiff Bases and Their Derivatives with Low and High Molecular Phosphonates
Abstract
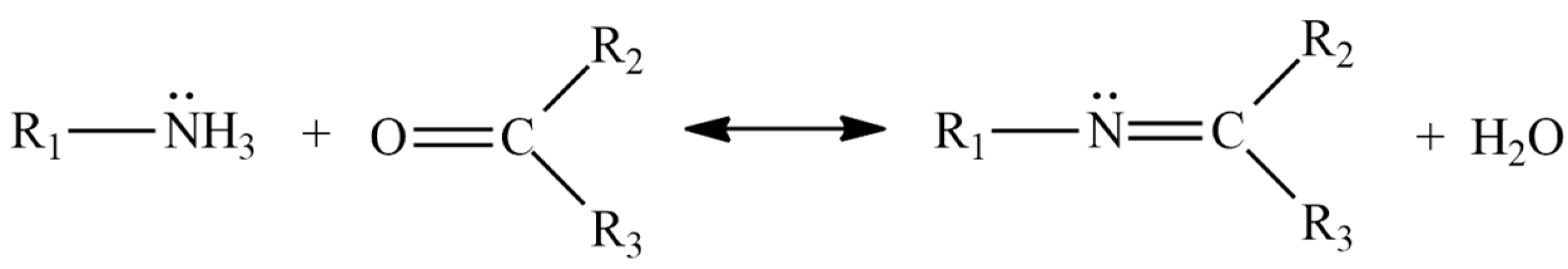
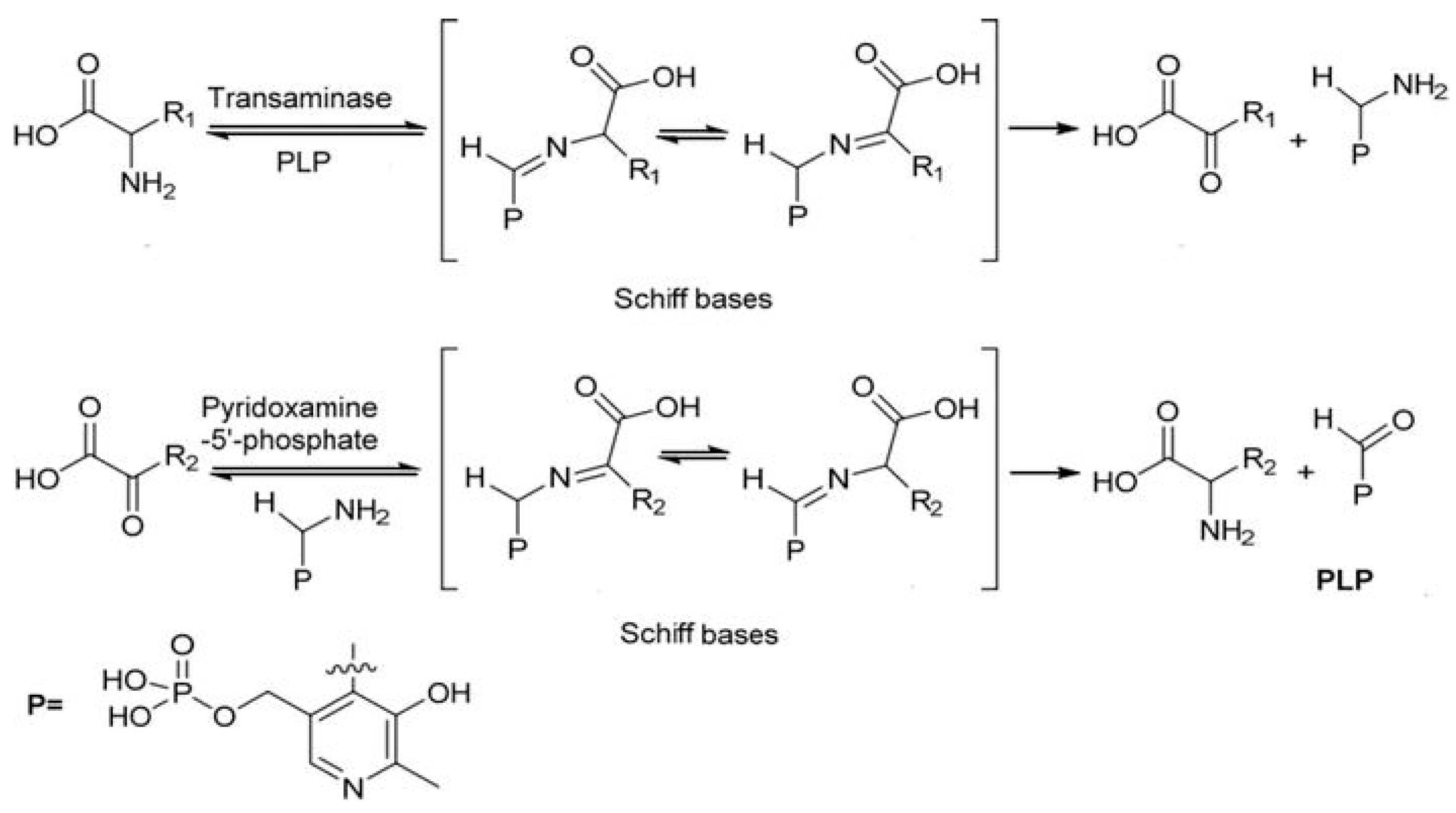
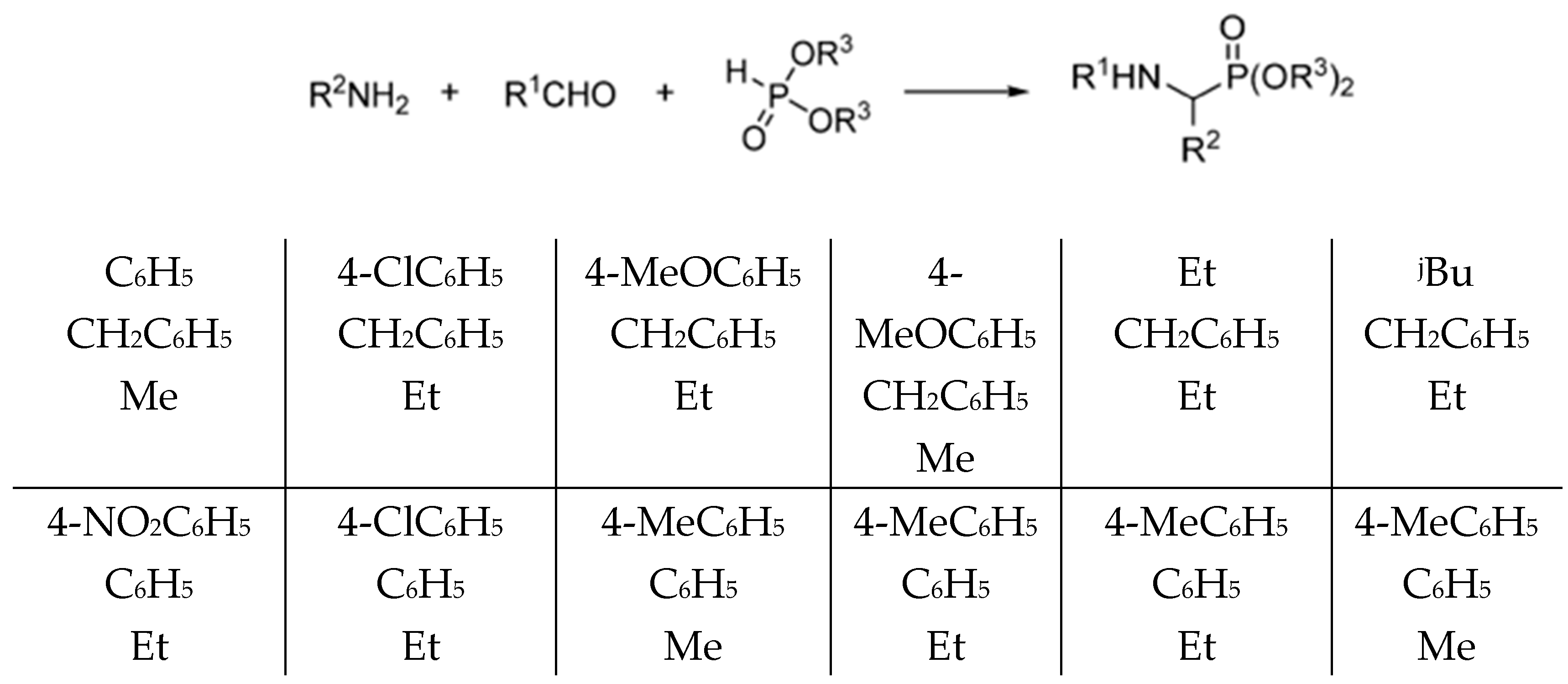
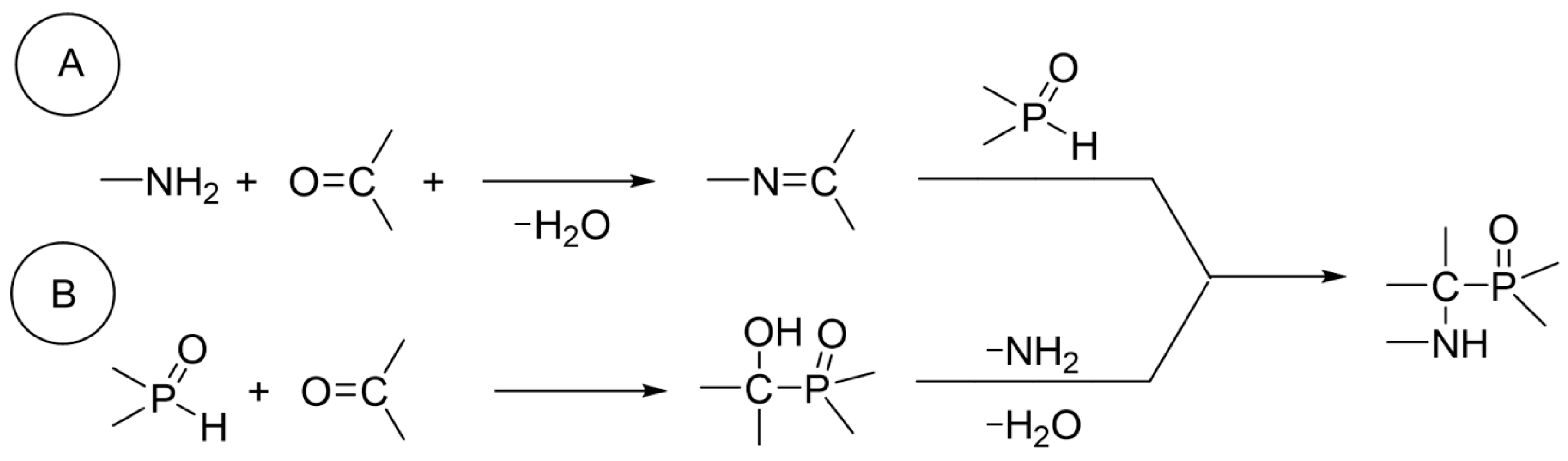
1. Introduction
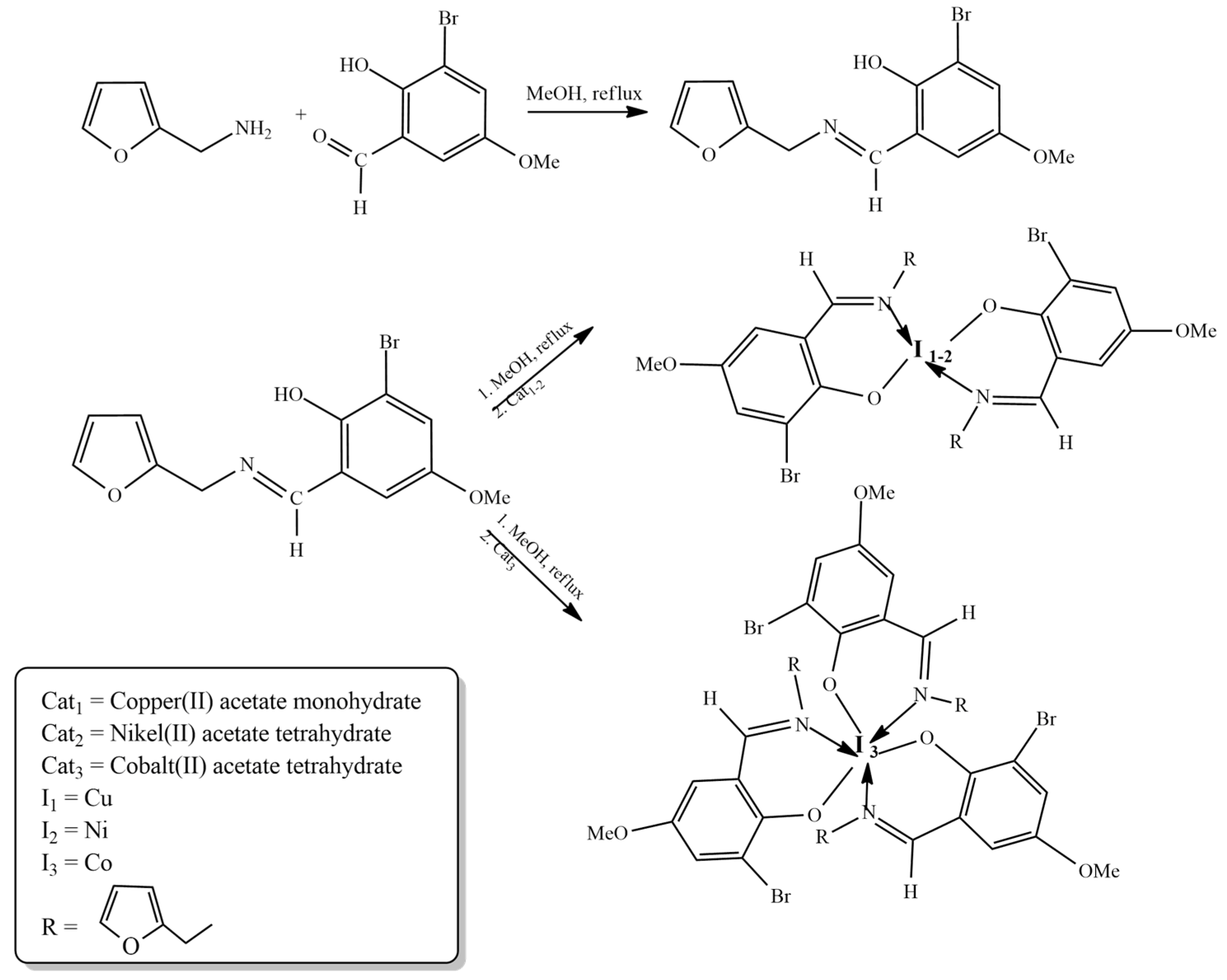
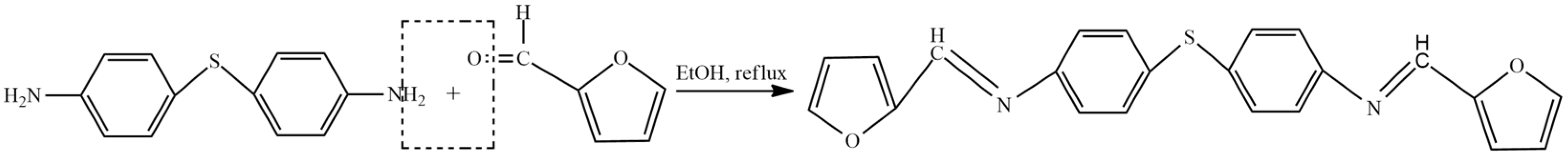
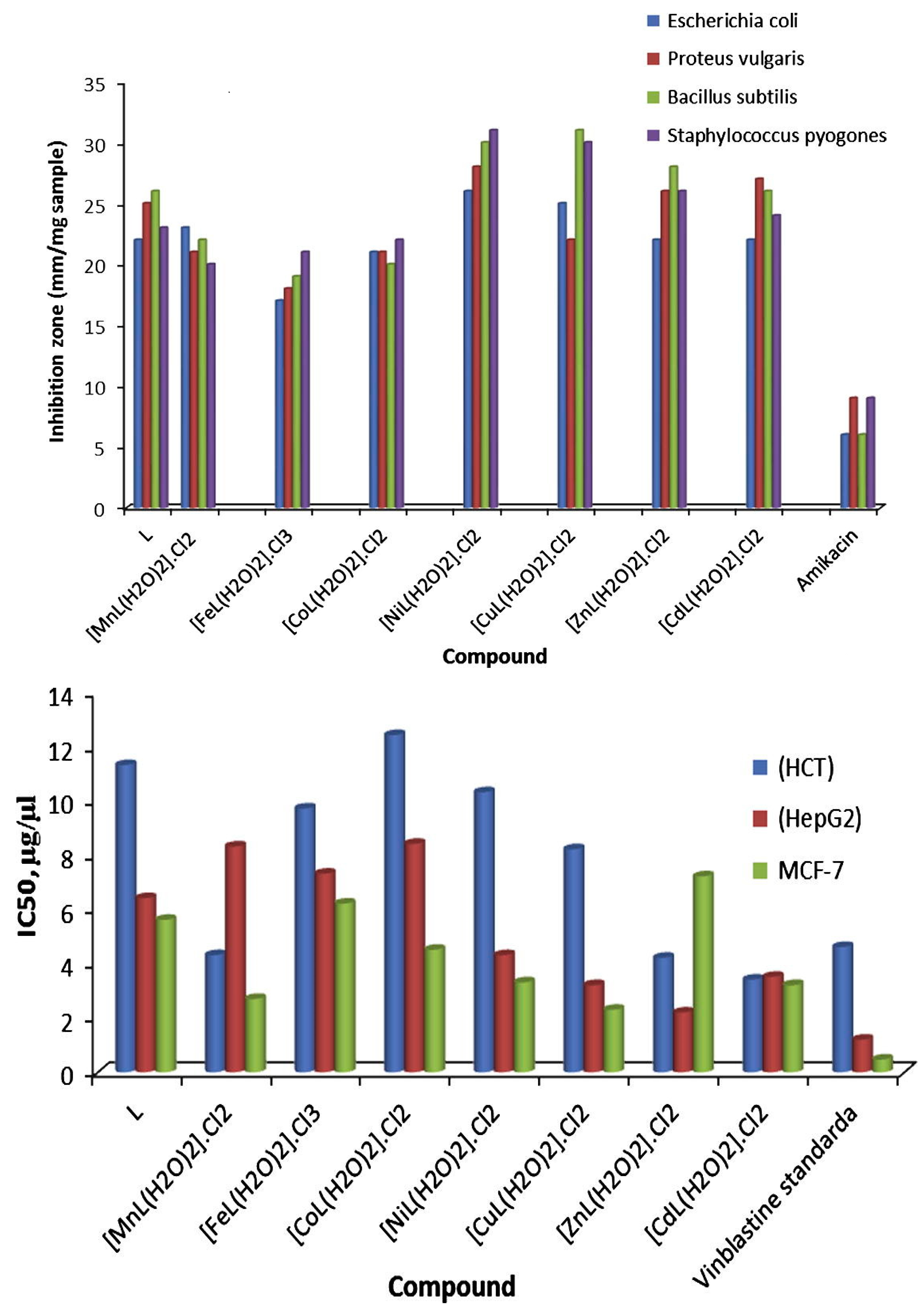
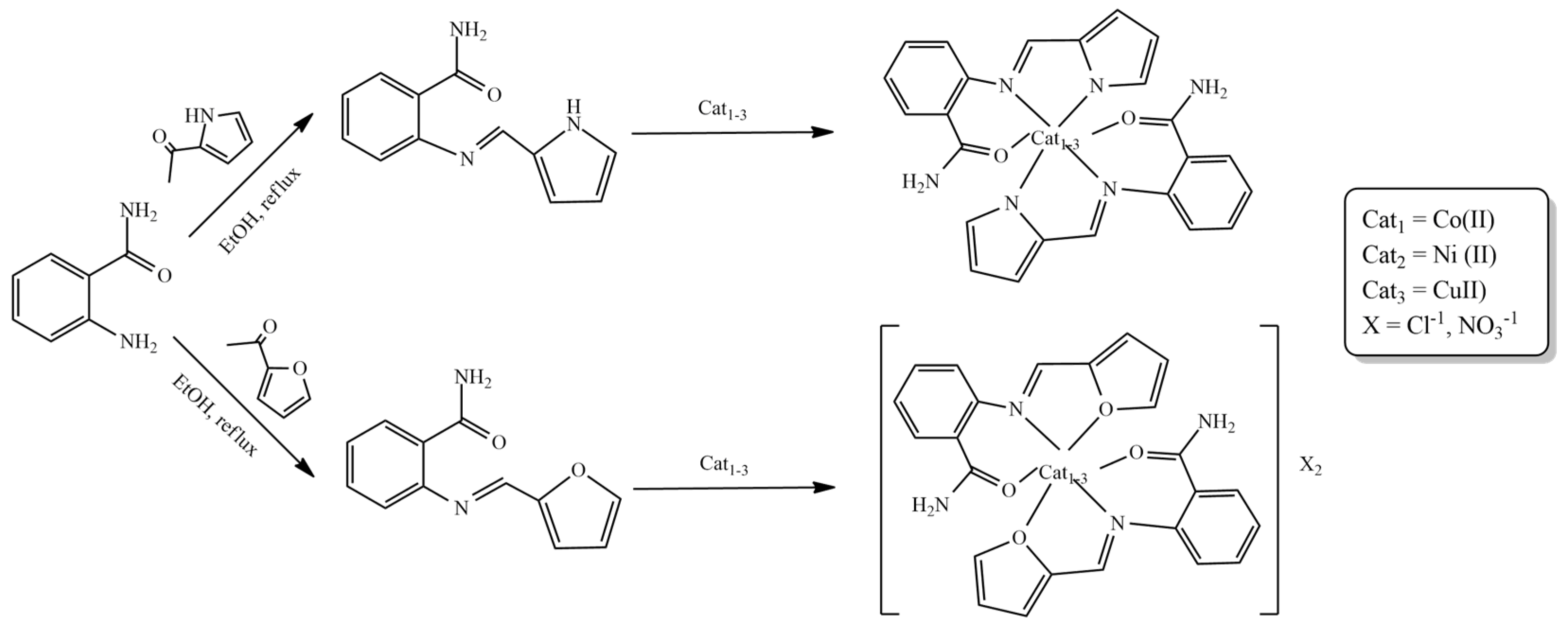
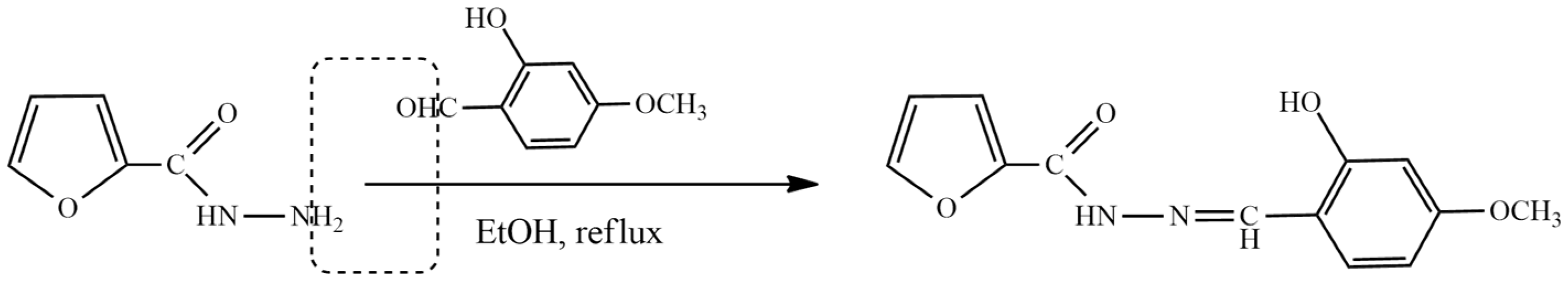
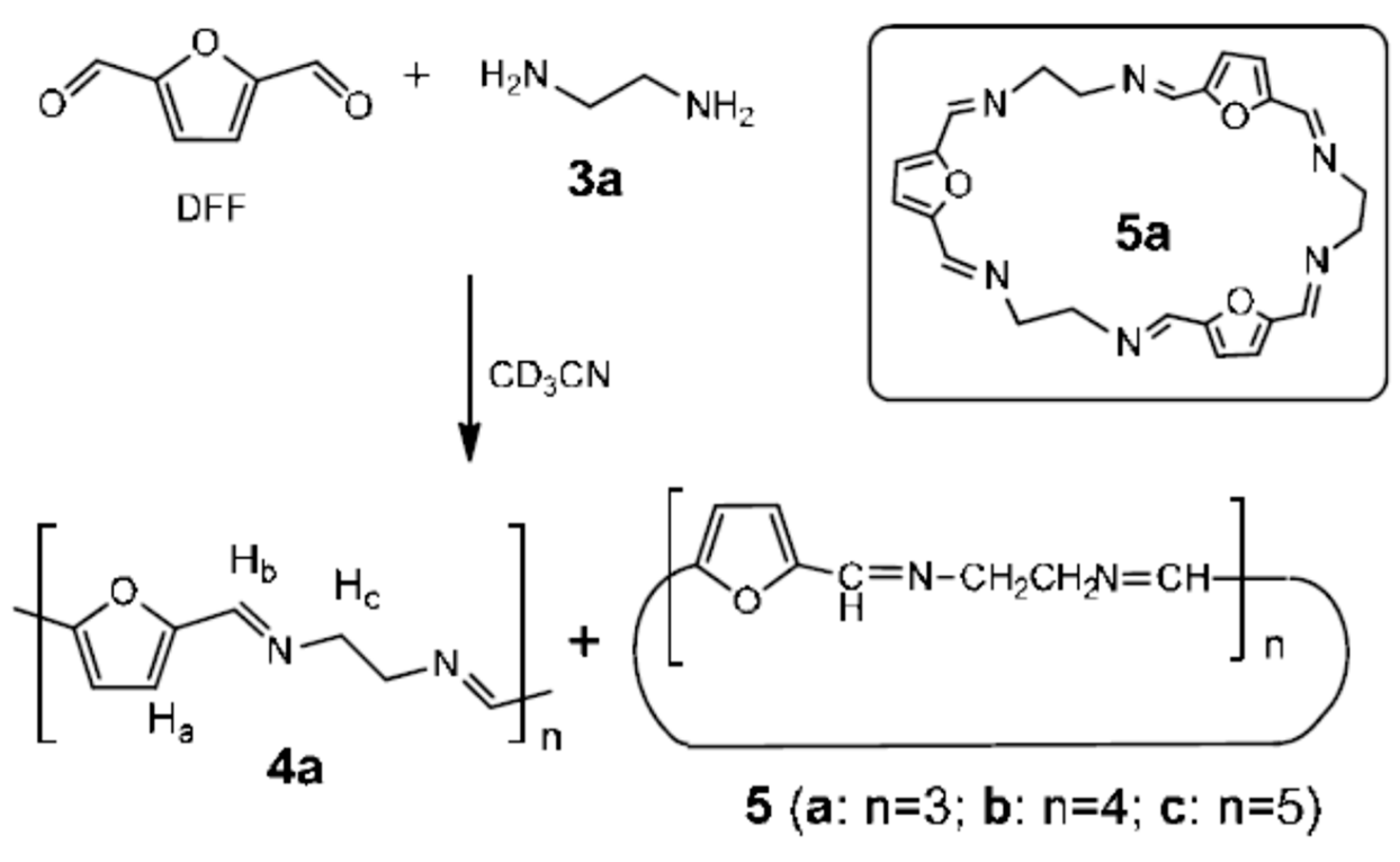
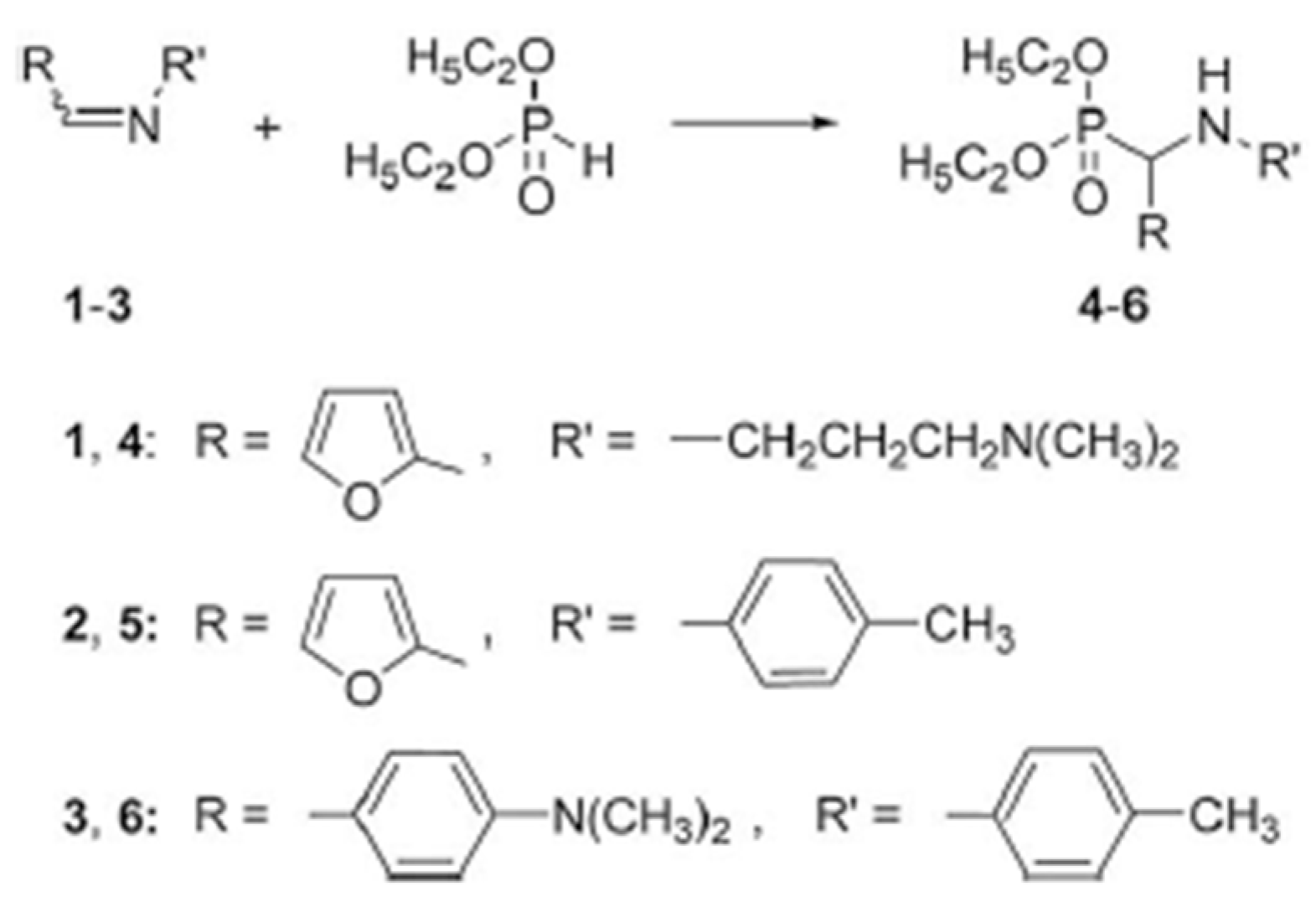
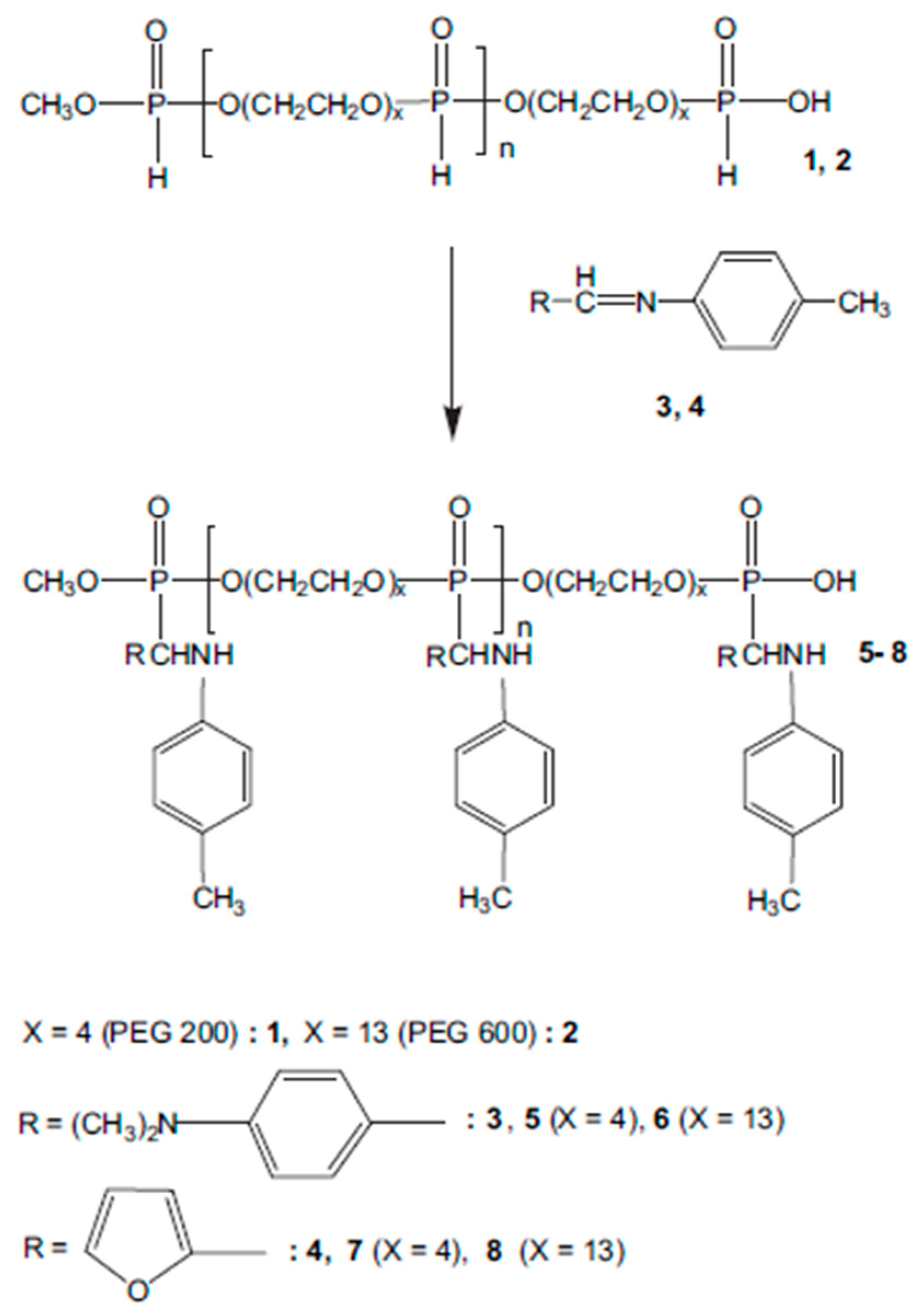
2. Furan-Containing Schiff Bases
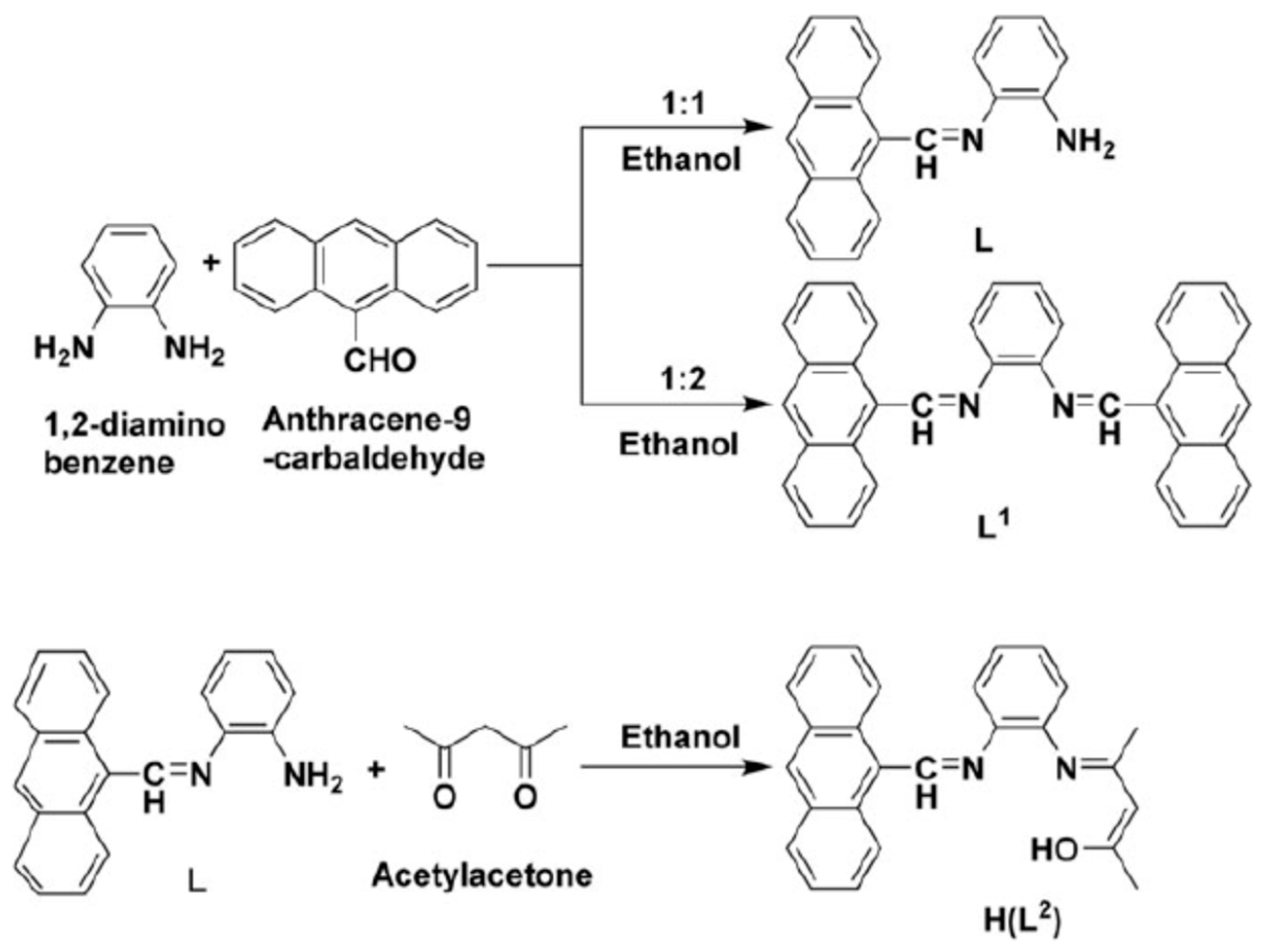
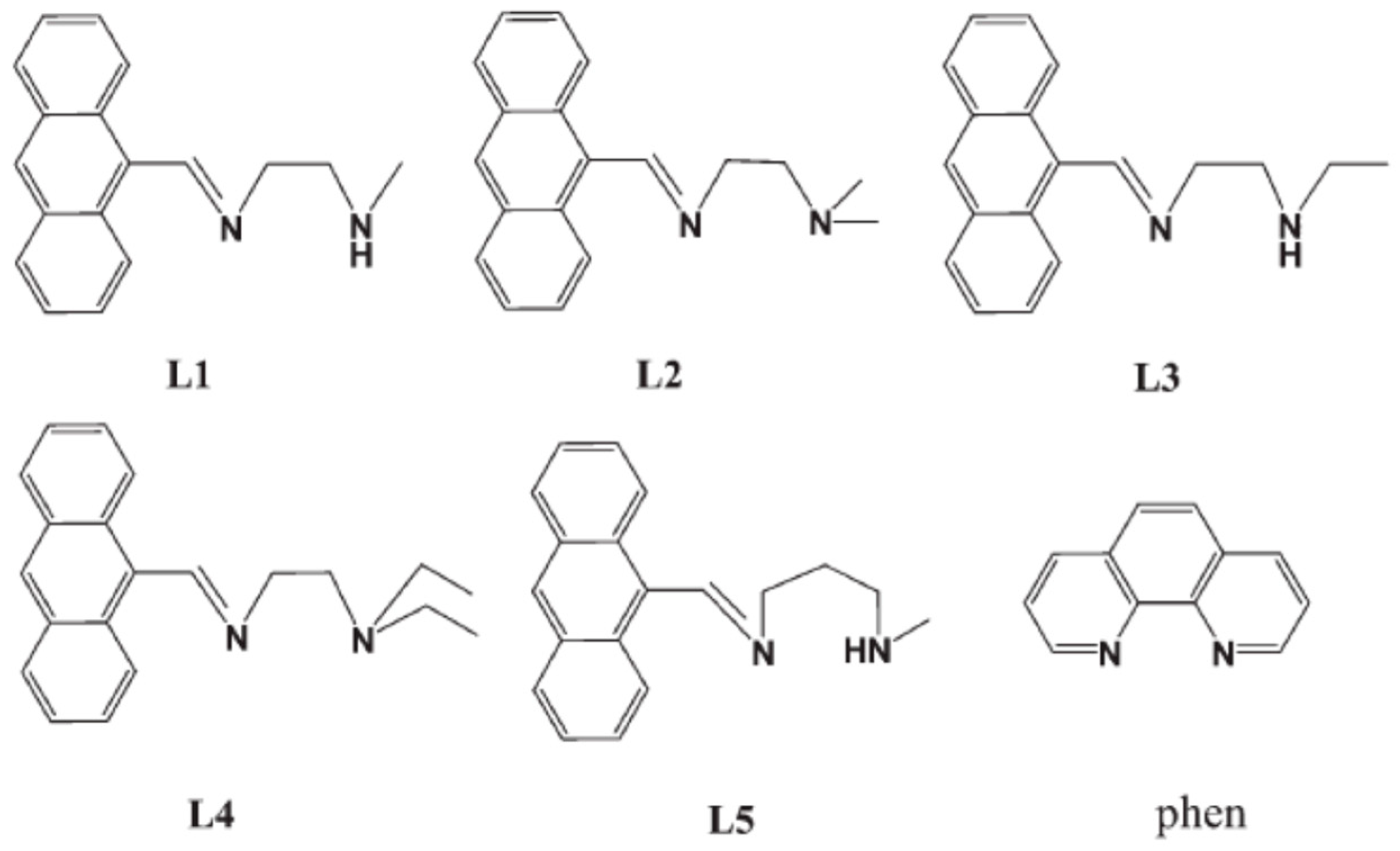
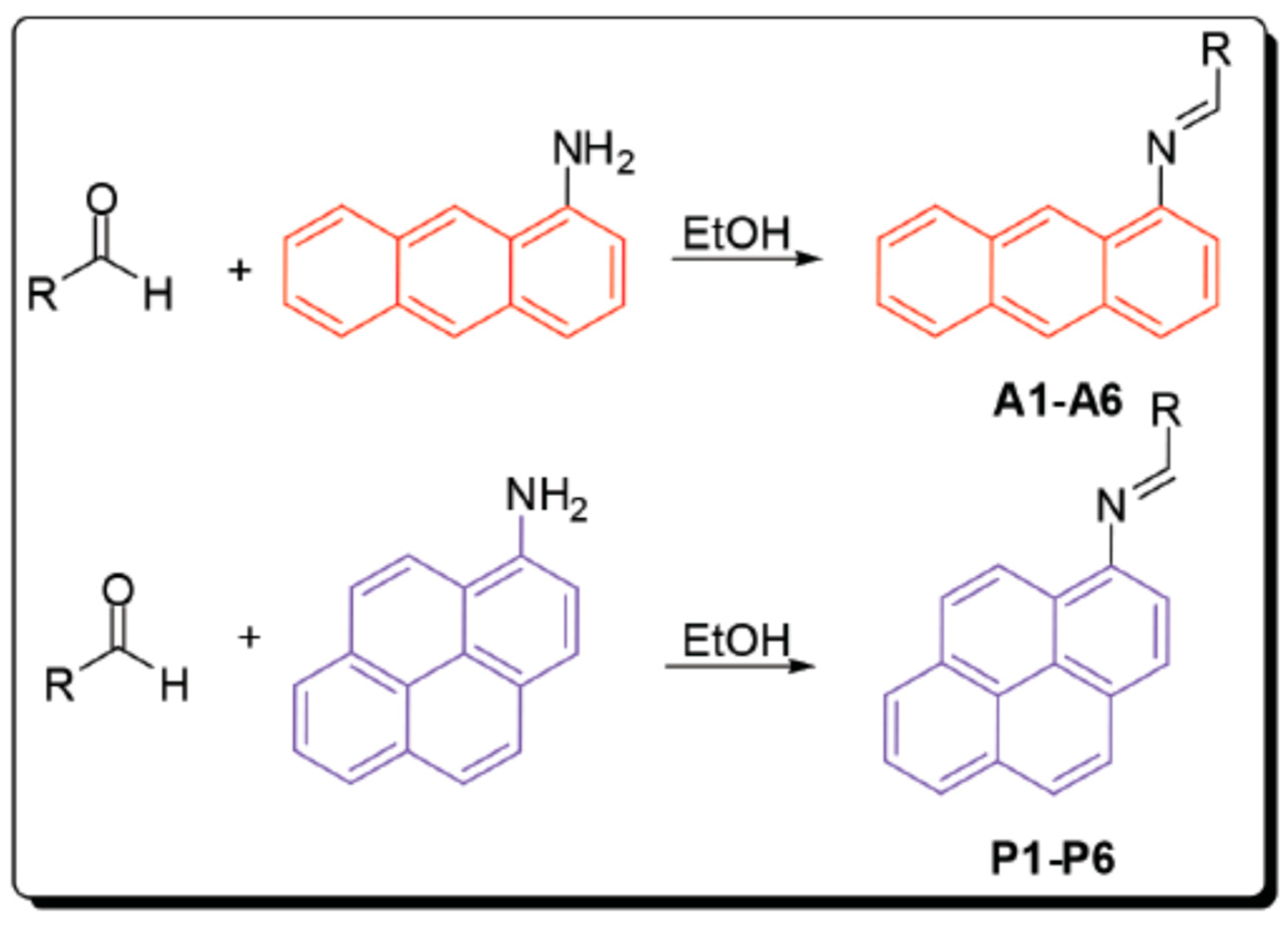
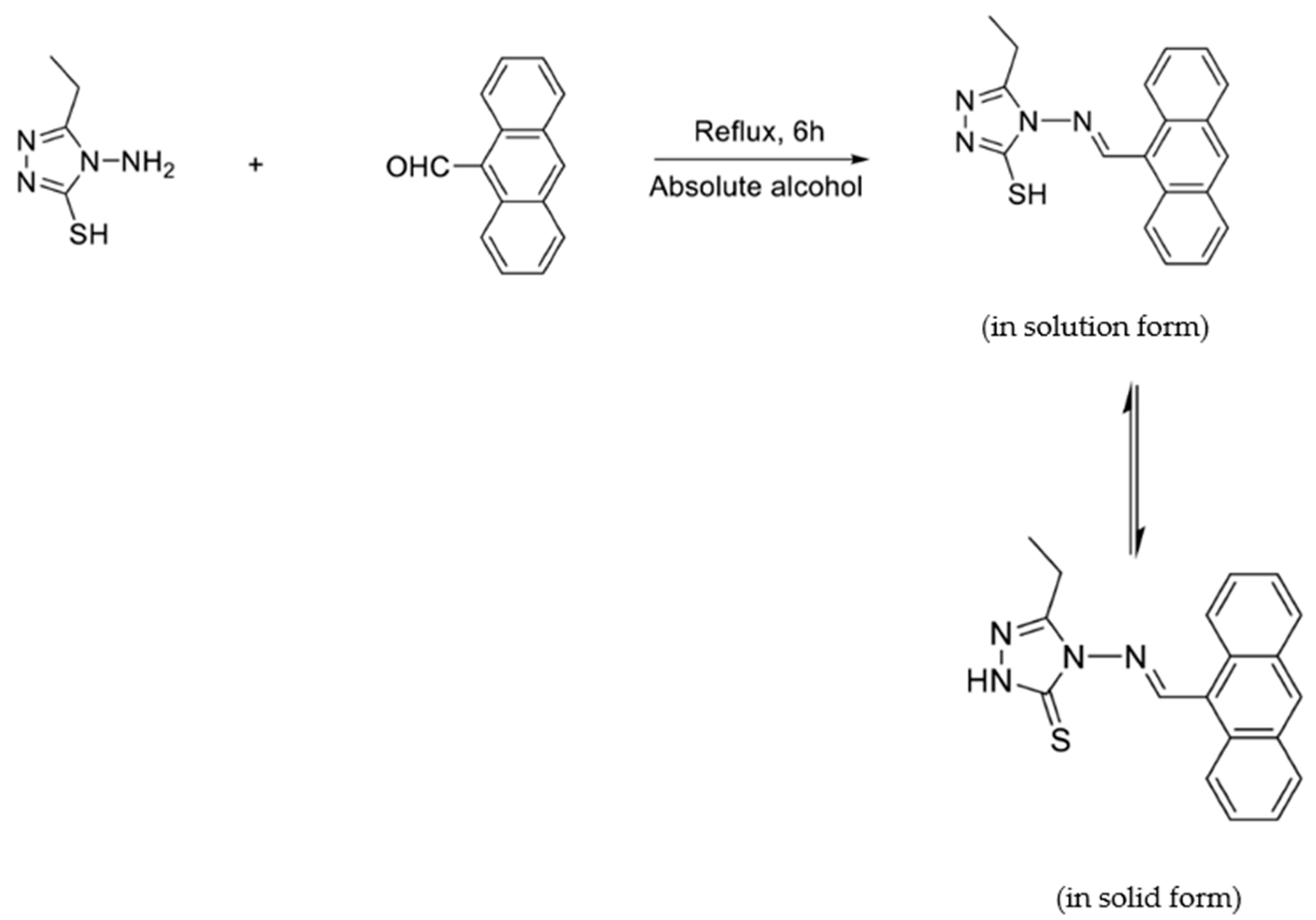
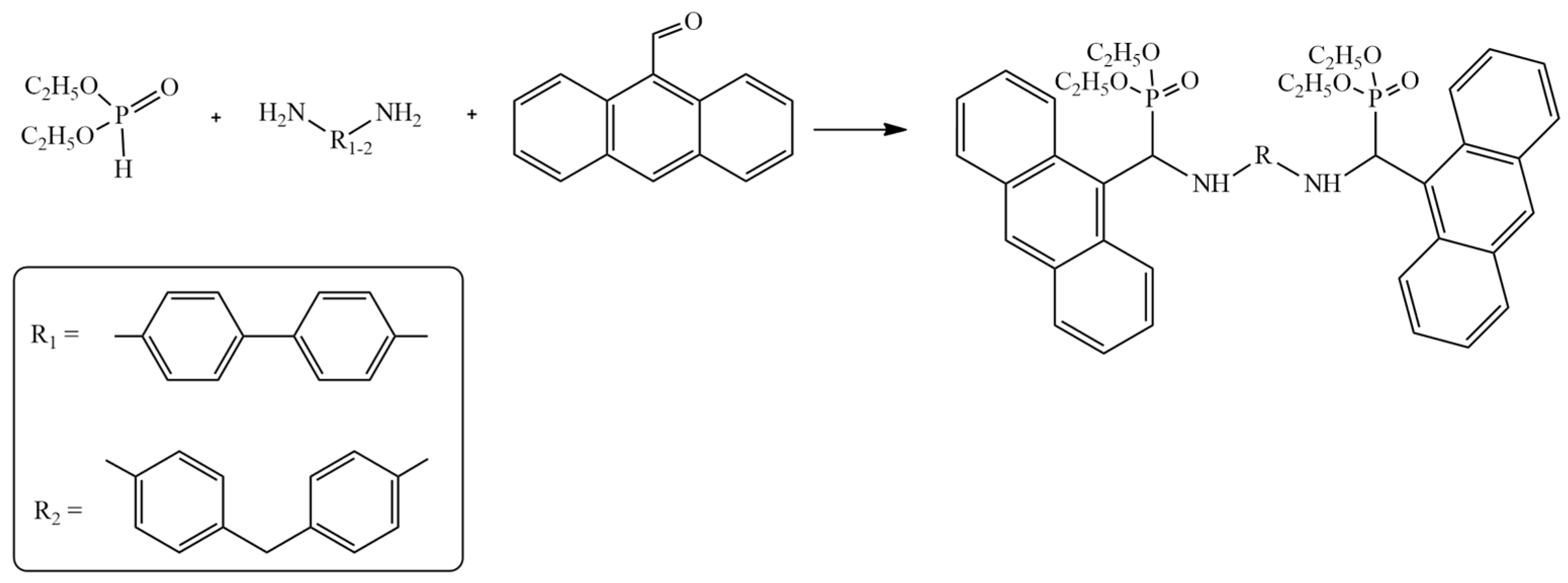
3. Anthracene-Containing Schiff Bases
3.1. In Vitro Antitumor Activity
3.2. In Vitro Safety Testing
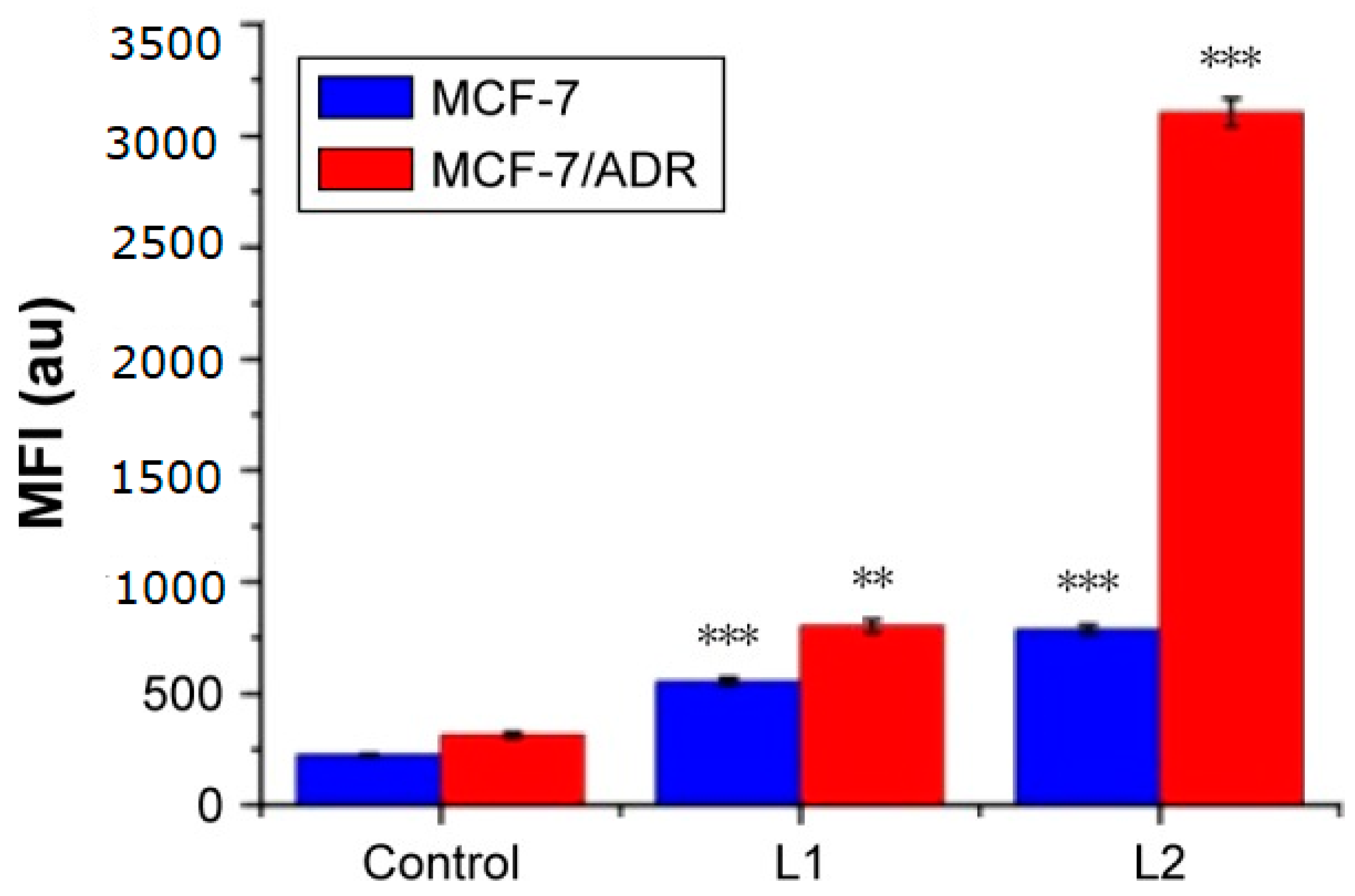
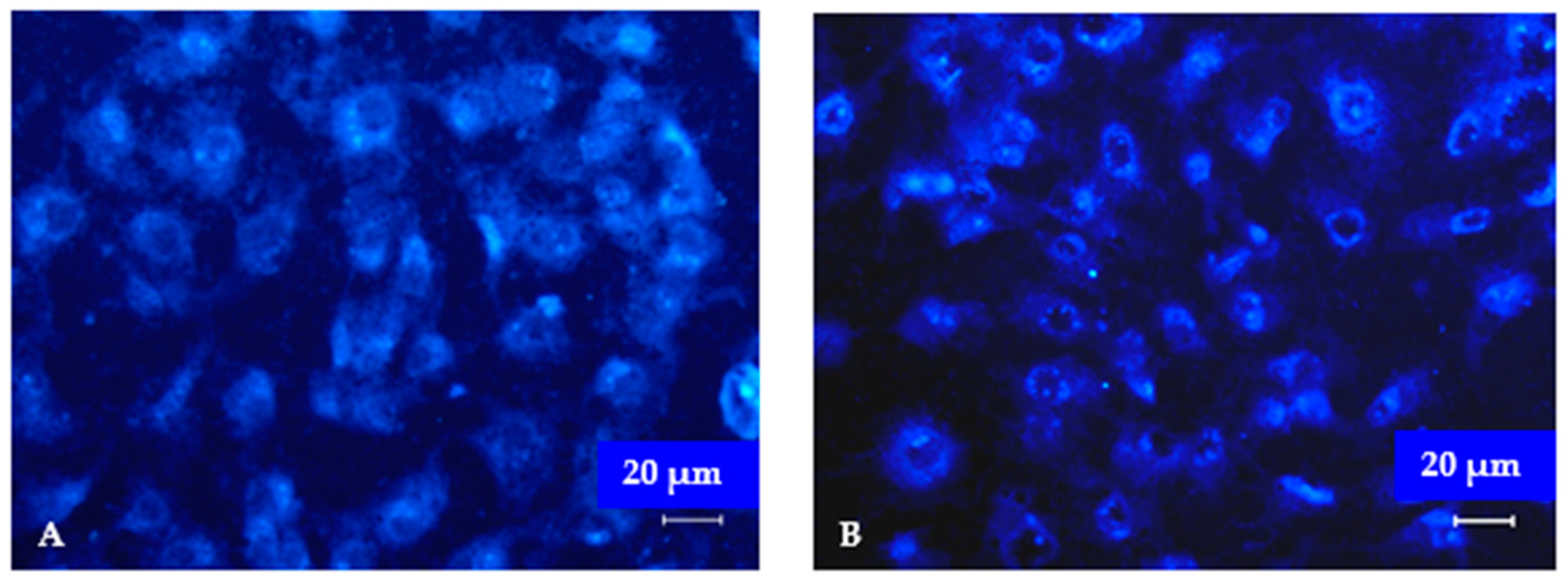
3.3. Fluorescent Studies
3.4. In Vitro Antitumor Activity of Bis-Aminophosphonates
3.5. In Vitro Safety Testing of Bis-Aminophosphonates
3.6. In Vivo Safety Testing of Bis-Aminophosphonates
3.7. Fluorescent Studies of Bis-Aminophosphonates
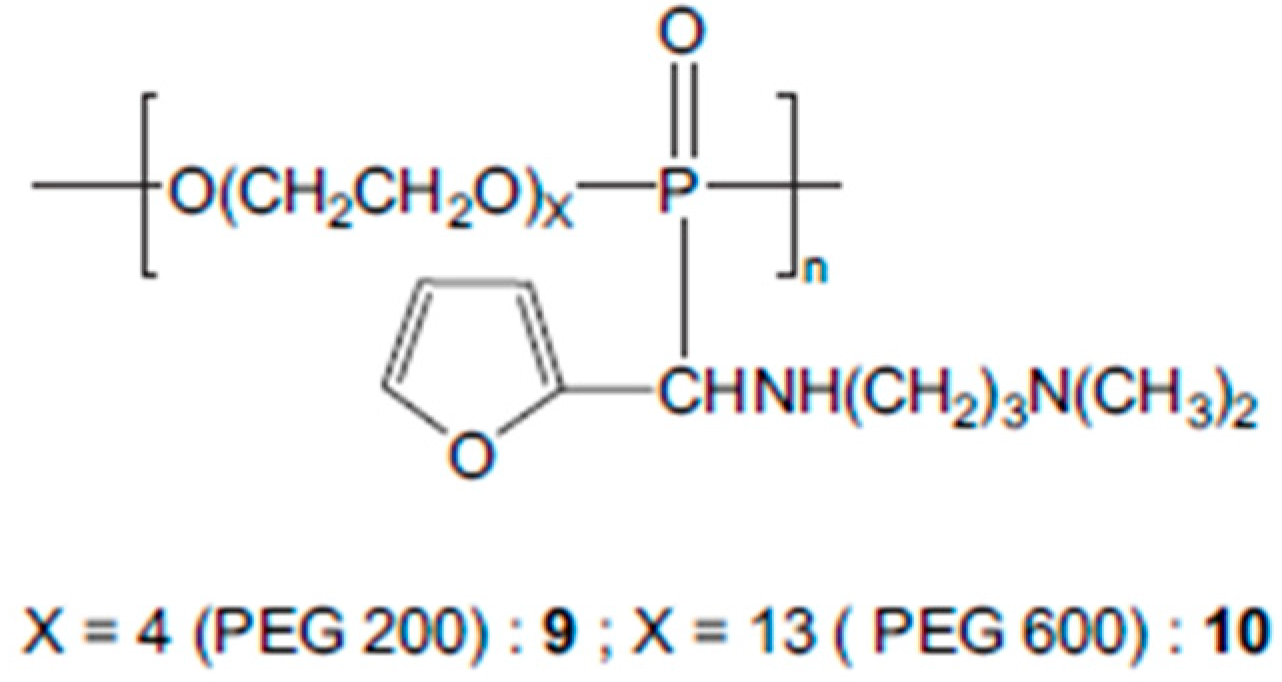
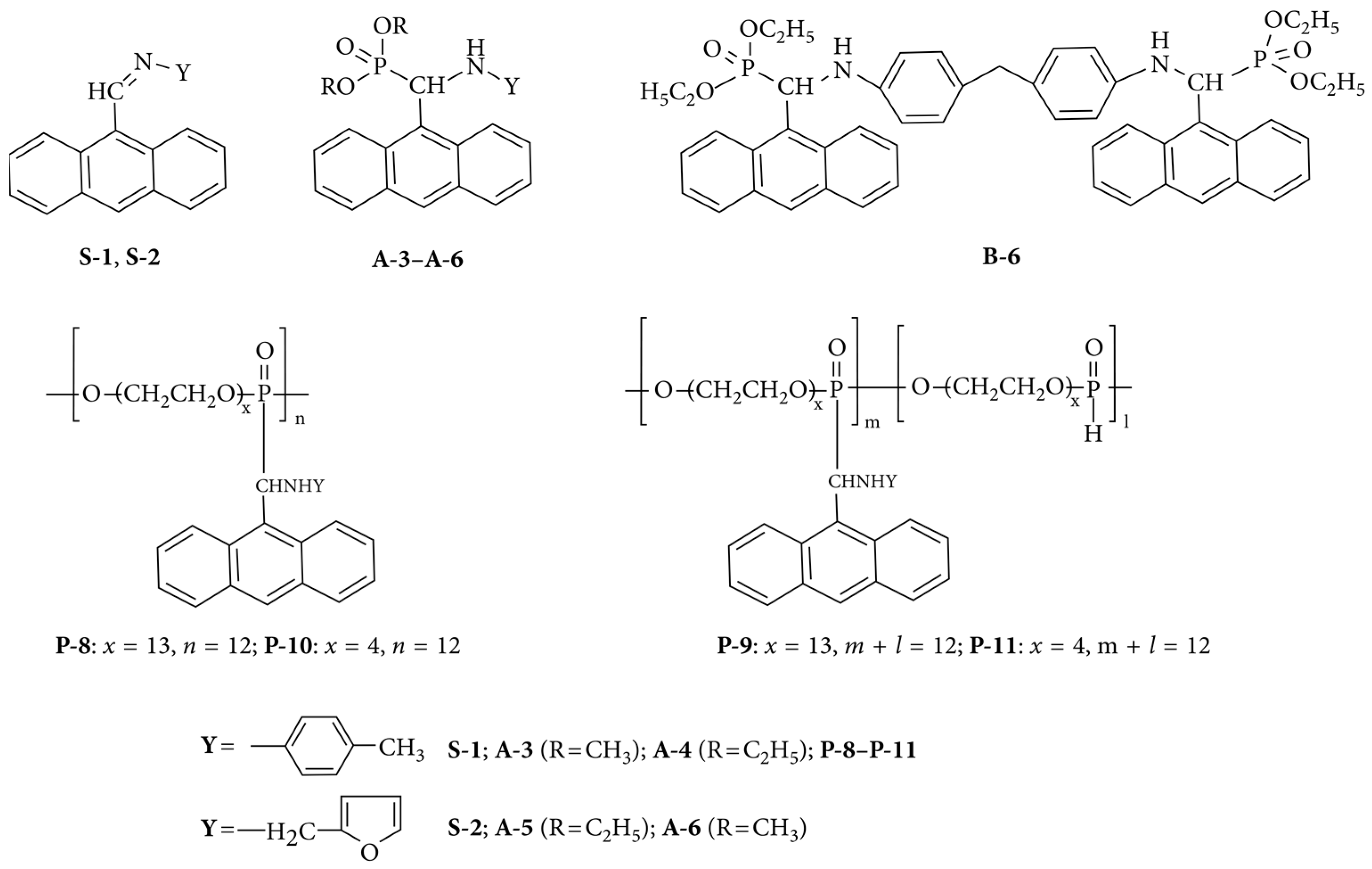
3.8. Polyaminophosphonates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sangle, S.L. Introduction to Schiff Base. In Schiff Base in Organic, Inorganic and Physical Chemistry; IntechOpen: London, UK, 2023; pp. 1–13. [Google Scholar] [CrossRef]
- Subasi, N.T. Overview of Schiff Bases. In Schiff Base in Organic, Inorganic and Physical Chemistry; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Aziz, A.A.A.; Salem, A.N.M.; Sayed, M.A.; Aboaly, M.M. Synthesis, structural characterization, thermal studies, catalytic efficiency and antimicrobial activity of some M(II) complexes with ONO tridentate Schiff base N-salicylidene-o-aminophenol (saphH2). J. Mol. Struct. 2012, 1010, 130–131. [Google Scholar] [CrossRef]
- Sinha, D.; Tiwari, A.K.; Singh, S.; Shukla, G.; Mishra, P.; Chandra, H.; Mishra, A.K. Synthesis, characterization and biological activity of Schiff base analogues of indole-3-carboxaldehyde. Eur. J. Med. Chem. 2008, 43, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Ronad, P.M.; Noolvi, M.N.; Sapkal, S.; Dharbhamulla, S.; Maddi, V.S. Synthesis and antimicrobial activity of 7-(2-substituted phenylthiazolidinyl)-benzopyran-2-one derivatives. Eur. J. Med. Chem. 2010, 45, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Krammer, B.; Abdel-Kader, N.; Verwanger, T.; El-Ansary, A. Antibacterial effect of some benzopyrone derivatives. Eur. J. Med. Chem. 2010, 45, 372–373. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, M.S.; Prasad, D.J.; Poojary, B.; Bhat, K.S.; Holla, B.S.; Kumari, N.S. Synthesis and biological activity of Schiff and Mannich bases bearing 2,4-dichloro-5-fluorophenyl moiety. Bioorg. Med. Chem. 2006, 14, 7482–7489. [Google Scholar] [CrossRef]
- Saravanan, G.; Pannerselvam, P.; Prakash, C.R. Synthesis and anti-microbial screening of novel Schiff bases of 3-amino-2-methyl quinazolin 4-(3H)-one. J. Adv. Pharm. Technol. Res. 2010, 1, 320–325. [Google Scholar] [CrossRef]
- De Souza, A.O.; Galetti, F.C.S.; Silva, C.L.; Bicalho, B.; Parma, M.M.; Fonseca, S.F.; Marsaioli, A.J.; Trindade, A.C.L.B.; Gil, R.P.F.; Franciglauber, S.; et al. Antimycobacterial and cytotoxicity activity of synthetic and natural compounds. Quim. Nova 2007, 30, 1563–1566. [Google Scholar] [CrossRef]
- Gümüş, A.; Okumuş, V.; Gümüş, S. Synthesis, biological evaluation of antioxidant-antibacterial activities and computational studies of novel anthracene- and pyrene-based Schiff base derivatives. Turk. J. Chem. 2020, 44, 1200–1215. [Google Scholar] [CrossRef]
- Young, D.W. Heterocyclic Chemistry, 1st ed.; Longman Group Ltd.: London, UK, 1975. [Google Scholar]
- Kumar, K.S.; Ganguly, S.; Veerasamy, R.; De Clercq, E. Synthesis, antiviral activity and cytotoxicity evaluation of Schiff bases of some 2-phenyl quinazoline-4 (3) H-ones. Eur. J. Med. Chem. 2010, 45, 5474–5479. [Google Scholar] [CrossRef]
- Güngör, O.; Gürkan, P. Synthesis and characterization of higher amino acid Schiff bases, as monosodium salts and neutral forms. Investigation of the intramolecular hydrogen bonding in all Schiff bases, antibacterial and antifungal activities of neutral forms. J. Mol. Struct. 2014, 1074, 62–70. [Google Scholar] [CrossRef]
- Shanty, A.A.; Philip, J.E.; Sneha, E.J.; Kurup, M.R.P.; Balachandran, S.; Mohanan, P.V. Synthesis, characterization and biological studies of Schiff bases derived from heterocyclic moiety. Bioorg. Chem. 2017, 70, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D.; Chaviara, A.T. Evaluation of anti-inflammatory and antioxidant activities of copper (II) Schiff mono-base and copper (II) Schiff base coordination compounds of dien with heterocyclic aldehydes and 2-amino-5-methyl-thiazole. J. Enzyme Inhib. Med. Chem. 2008, 23, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.; El-Wakiel, N.; El-Ghamry, H. Synthesis, spectral, antitumor and antimicrobial studies on Cu (II) complexes of purine and triazole Schiff base. J. Mol. Struct. 2013, 1049, 326–335. [Google Scholar] [CrossRef]
- El-wakiel, N.; El-Keiy, M.; Gaber, M. Synthesis, spectral, antitumor, antioxidant and antimicrobial studies on Cu (II), Ni (II) and Co (II) complexes of 4-[(1HBenzoimidazol-2-ylimino)-methyl]-benzene-1, 3-diol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 147, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Bensaber, S.M.; Allafe, H.; Ermeli, N.B.; Mohamed, S.B.; Zetrini, A.A.; Alsabri, S.G.; Erhuma, M.; Hermann, A.; Jaeda, M.I.; Gbaj, A.M. Chemical synthesis, molecular modelling, and evaluation of anticancer activity of some pyrazol-3-one Schiff base derivatives. Med. Chem. Res. 2014, 23, 5120–5134. [Google Scholar] [CrossRef]
- Da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.; de Fatima, A. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Redshaw, C. Use of metal catalysts bearing Schiff Base macrocycles for the ring opening polymerization (ROP) of cyclic esters. Catalysts 2017, 7, 165. [Google Scholar] [CrossRef]
- Roberts, D.W.; Schultz, T.W.; Api, A.M. Skin sensitization QMM for HRIPT NOEL data: Aldehyde Schiff-Base domain. Chem. Res. Toxicol. 2017, 30, 1309. [Google Scholar] [CrossRef]
- DiRisio, R.J.; Armstrong, J.E.; Frank, M.A.; Lake, W.R.; McNamara, W.R. Cobalt Schiff-base complexes for electrocatalytic hydrogen generation. Dalton Trans. 2017, 46, 10418–10425. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Kumar, A.; Upadhyay, S.; Mishra, P.C. Synthesis, characterization, structural optimization using density functional theory and superoxide ion scavenging activity of some Schiff bases. J. Mol. Struct. 2008, 873, 5–16. [Google Scholar] [CrossRef]
- Vigato, P.A.; Tamburini, S. The challenge of cyclic and acyclic Schiff bases and related derivatives. Coord. Chem. Rev. 2004, 248, 1717–2128. [Google Scholar]
- Zhang, J.; Xu, L.; Wong, W.Y. Energy materials based on metal Schiff base complexes. Coord. Chem. Rev. 2018, 355, 180–198. [Google Scholar]
- Yeap, G.Y.; Ha, S.T.; Ishizawa, N.; Suda, K.; Boey, P.L.; Mahmood, W.A.K. Synthesis, crystal structure and spectroscopic study of para substituted 2-hydroxy-3-methoxybenzalideneanilines. J. Mol. Struct. 2003, 658, 87–99. [Google Scholar] [CrossRef]
- Jesmin, M.; Ali, M.M.; Salahuddin, M.S.; Habib, M.R.; Khanam, J.A. Antimicrobial activity of some schiff bases derived from benzoin, salicylaldehyde, aminophenol and 2,4 dinitrophenyl hydrazine. Mycobiology 2008, 36, 70–73. [Google Scholar] [CrossRef]
- Bálint, E.; Tripolszky, A.; Tajti, A. Synthesis of α-aminophosphonates by the Kabachnik–Fields reaction and by the Pudovik reaction. In Organophosphorus Chemistry; De Gruyter: Berlin, Germany, 2018; pp. 108–147. [Google Scholar]
- Aissa, R.; Guezane-Lakoud, A.; Gali, L.; Toffano, M.; Ignaczak, A.; Adamiak, M.; Merabet-Khelassi, M.; Guillot, R.; Aribi-Zouioueche, L. New promising generation of phosphates α-aminophosphonates: Design, synthesis, In-Vitro biological evaluation and computational study. J. Mol. Struct. 2022, 1247, 131336. [Google Scholar] [CrossRef]
- Varga, P.R.; Keglevich, G. Synthesis of α-Aminophosphonates and Related Derivatives; The Last Decade of the Kabachnik–Fields Reaction. Molecules 2021, 26, 2511. [Google Scholar] [CrossRef] [PubMed]
- Lejczak, P.K. Aminophosphonic acids of potential medical importance. Curr. Med. Chem. Anti-Cancer Agents 2001, 1, 301–312. [Google Scholar]
- Danila, D.C.; Wang, X.; Hubble, H.; Antipin, I.S.; Pinkhassik, E. Increasing permeability of phospholipid bilayer membranes to alanine with synthetic α-aminophosphonate carriers. Bioorg. Med. Chem. Lett. 2008, 18, 2320–2323. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, K.; Song, B.; Xu, G.; Yang, S.; Xue, W.; Hu, D.; Lu, P.; Ouyang, G.; Jin, L.; et al. Synthesis and antiviral bioactivities of alpha-aminophosphonates containing alkoxyethyl moieties. Molecules 2006, 11, 666–676. [Google Scholar] [CrossRef]
- Pudovik, A.N.; Konovalova, I.V. Addition reactions of esters of phosphorus(III) acids with unsaturated systems. Synthesis 1979, 1979, 81–96. [Google Scholar] [CrossRef]
- Stiernet, P.; Debuigne, A. Imine-based multicomponent polymerization: Concepts, structural diversity and applications. Prog. Polym. Sci. 2022, 128, 101528. [Google Scholar] [CrossRef]
- Fields, E.K. The Synthesis of Esters of Substituted Amino Phosphonic Acids1a. J. Am. Chem. Soc. 1952, 74, 1528. [Google Scholar] [CrossRef]
- Cherkasov, R.A.; Galkin, V.I. The Kabachnik ± Fields reaction: Synthetic potential and the problem of the mechanism. Russ. Chem. Rev. 1998, 67, 857–882. [Google Scholar] [CrossRef]
- Kabachnik, M.I.; Medved, T.Y. New synthesis of aminophosphonic acids. Dokl. Akad. Nauk. SSSR 1952, 83, 689–692. [Google Scholar]
- Kafarski, P.B.; Lejczak, B. Biological Activity of Aminophosphonic Acids. Phosphorus Sulfur Silicon Relat. Elem. 1991, 63, 193. [Google Scholar] [CrossRef]
- Horiguchi, M.; Kandatsu, M. Isolation of 2-Aminoethane Phosphonic Acid from Rumen Protozoa. Nature 1959, 184, 901. [Google Scholar] [CrossRef]
- Gancarz, R.; Gansarz, I. Failure of aminophosphonate synthesis due to facile hydroxyphosphonate—Phosphate rearrangement. Tetrahedron Lett. 1993, 34, 145–148. [Google Scholar] [CrossRef]
- Savignac, P.; Iorga, B. Modern Phosphonate Chemistry; CRC Press LLC: Boca Raton, FL, USA, 2003; pp. 259–260, 432–433. [Google Scholar]
- Enders, D.; Saint-Dizier, A.; Lannou, M.-I.; Lenzen, A. The PhosphaMichael addition in organic synthesis. Eur. J. Org. Chem. 2006, 2006, 29–49. [Google Scholar] [CrossRef]
- Rulev, A.Y. Recent advances in Michael addition of H-phosphonates. RSC Adv. 2014, 4, 26002–26012. [Google Scholar] [CrossRef]
- Salina, A.V.; Il’ina, A.V.; Shamsutdinova, F.G.; Fatkhutdinova, A.R.; Islamova, D.R.; Kataeva, O.N.; Galkina, V.I. The Pudovik Reaction Catalyzed by Tertiary Phosphines. Curr. Org. Synth. 2016, 13, 132–141. [Google Scholar] [CrossRef]
- Zhu, X.-F.; Zhang, J.; Sun, S.; Guo, Y.C.; Cao, S.X.; Zhao, Y.F. Synthesis and structure-activity relationships study of α-aminophosphonate derivatives containing a quinoline moiety. Chin. Chem. Lett. 2017, 28, 1514–1518. [Google Scholar] [CrossRef]
- Abdelwahed, R.E.; Radhi, A.H.; Awad, H.M.; Gokha, A.A.E.; Goda, A.E.S.; El Sayed, I.E.T. Synthesis and Anti-Proliferative Activity of New α-Amino Phosphonate Derivatives Bearing Heterocyclic Moiety. Pharm. Chem. J. 2021, 55, 231–239. [Google Scholar] [CrossRef]
- Elsherbiny, D.A.; Abdelgawad, A.M.; Shaheen, T.I.; Abdelwahed, N.A.M.; Jockenhoevel, S.; Ghazanfari, S. Thermoresponsive nanofibers loaded with antimicrobial α-aminophosphonate-o/w emulsion supported by cellulose nanocrystals for smart wound care patches. Int. J. Biol. Macromol. 2023, 233, 123655. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, K.; Daravath, S.; Ramesh, G.; Lakshmi, P.V.A.; Shivaraj. Investigation of DNA binding and bioactivities of furan cored Schiff base Cu (II), Ni (II), and Co (III) complexes: Synthesis, characterization and spectroscopic properties. Appl. Organomet. Chem. 2021, 35, 1–20. [Google Scholar] [CrossRef]
- Mesbah, M.; Douadi, T.; Sahli, F.; Issaadi, S.; Boukazoula, S.; Chafaa, S. Synthesis, characterization, spectroscopic studies and antimicrobial activity of three new Schiff bases derived from Heterocyclic moiety. J. Mol. Struct. 2018, 1151, 41–48. [Google Scholar] [CrossRef]
- Mohamed, G.G.; Zayed, E.M.; Hindy, A.M.; Ahmed, M.M. Coordination behavior of new bis Schiff base ligand derived from 2-furan carboxaldehyde and propane-1,3-diamine. Spectroscopic, thermal, anticancer and antibacterial activity studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 76–84. [Google Scholar] [CrossRef]
- Tyagi, P.; Chandra, S.; Saraswat, B.S.; Sharma, D. Design, spectral characterization, DFT and biological studies of transition metal complexes of Schiff base derived from 2-aminobenzamide, pyrrole and furan aldehyde. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 143, 1–11. [Google Scholar] [CrossRef]
- Manivel, S.; Gangadharappa, B.S.; Elangovan, N.; Thomas, R.; Abu, A.; Ola, A.; Saleh, D.I. Schiff base (Z)-4-((furan-2-ylmethylene)amino) benzenesulfonamide: Synthesis, solvent interactions through hydrogen bond, structural and spectral properties, quantum chemical modeling and biological studies. J. Mol. Liq. 2022, 350, 118531. [Google Scholar] [CrossRef]
- Peng, H.N.; Liu, Y.-Q.; Huang, J.-Q.; Huang, S.-S.; Cai, X.-P.; Xu, S.-J.; Huang, A.; Zeng, Q.; Xu, M. A simple fluorescent probe for selective detection of Al3+ based on furan Schiff base and its crystal structure. J. Mol. Struct. 2021, 1229, 129866. [Google Scholar] [CrossRef]
- Bai, L.; Xu, Y.; Li, L.; Tao, F.; Wang, S.; Wang, L.; Li, G. An efficient water-soluble fluorescent chemosensor based on furan Schiff base functionalized PEG for the sensitive detection of Al 3+ in pure aqueous solution. New J. Chem. 2020, 44, 11148–11154. [Google Scholar] [CrossRef]
- Xiang, T.; Liu, X.; Yi, P.; Guo, M.; Chen, Y.; Wesdemiotis, C.; Xu, J.; Pang, Y. Schiff base polymers derived from 2,5-diformylfuran. Polym. Int. 2013, 62, 1517–1523. [Google Scholar] [CrossRef]
- Alizadeh, M.; Jalal, M.; Hamed, K.; Saber, A.; Kheirouri, S.; Pourteymour Fard Tabrizi, F.; Kamari, N. Recent Updates on Anti-Inflammatory and Antimicrobial Effects of Furan Natural Derivatives. J. Inflamm. Res. 2020, 13, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Lewkowski, J.; Morawska, M.; Kowalczyk, A. Antibacterial action of (5-nitrofurfuryl)-derived aminophosphonates and their parent imines. Chem. Pap. 2019, 73, 365–374. [Google Scholar] [CrossRef]
- Lewkowski, J.; Rzeszotarska, E.; Matusiak, A.; Morawska, M.; Gajek, G.; Nowak, K.; Kontek, R. Cytotoxic Action of N-aryl, Furan-derived Aminophosphonates against HT29 and HCT116 Cancer Cell Lines. Anti-Cancer Agents Med. 2019, 19, 453–462. [Google Scholar] [CrossRef]
- Patnala, H.; Abbo, H.S.; Potla, K.M.; Titinchi, S.J.J.; Chinnam, S. Polyethylene glycol (PEG-400): An efficient one-pot green synthesis and anti-viral activity of novel α-diaminophosphonates. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 1035–1039. [Google Scholar] [CrossRef]
- Uparkar, J.; Dhavan, P.P.; Jadhav, B.L.; Pawar, S.D. Design, synthesis and biological evaluation of furan based α-aminophosphonate derivatives as anti-Alzheimer agent. J. Iran. Chem. Soc. 2022, 19, 3103–3116. [Google Scholar] [CrossRef]
- Kraicheva, I.; Bogomilova, A.; Tsacheva, I.; Momekov, G.; Troev, K. Synthesis, NMR characterization and in vitro antitumor evaluation of new aminophosphonic acid diesters. Eur. J. Med. Chem. 2009, 44, 3363–3367. [Google Scholar] [CrossRef]
- Sajjadi, S.E.; Ghanadian, M.; Haghighi, M.; Mouhebat, L. Cytotoxic effect of Cousiniaver bascifolia bunge against OVCAR-3 and HT-29 cancer cells. J. HerbMed Pharmacol. 2015, 4, 15–22. [Google Scholar]
- Kril, A.; Iliev, I.; Topashka-Ancheva, M.; Gerasimova, T.; Kraicheva, I.; Tsacheva, I.; Vodenicharova, E.; Ivanov, I.; Troev, K. In vitro Antitumor Activity and Safety Testing of an Aminophosphonate Bearing a Furan Ring. Biotechnol. Biotechnol. Equip. 2011, 25, 2663–2667. [Google Scholar] [CrossRef]
- Shanmugaraju, S.; Jadhav, H.; Karthik, R.; Mukherjee, P.S. Electron rich supramolecular polymers as fluorescent sensors for nitroaromatics. RSC Adv. 2013, 3, 4940. [Google Scholar] [CrossRef]
- Suguna, S.; Nandhakumar, R.; Prabhu, J. Anthracene benzene conjugate (ABC): An asymmetric Schiff base for the selective detection of Ag+ ion using fluorimetry and its applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 288, 122196. [Google Scholar] [CrossRef]
- Sek, D.; Siwy, M.; Grucela, M.; Małecki, G.; Nowak, E.M.; Lewinska, G.; Santera, J.; Laba, K.; Lapkowski, M.; Kotowicz, S.; et al. New anthracene-based Schiff bases: Theoretical and experimental investigations of photophysical and electrochemical properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 175, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Gubendran, A.; Kumar, G.G.V.; Kesavan, M.P.; Rajagopal, G.; Athappan, P.; Rajesh, J. New anthracene based Schiff base ligands appended Cu(II) complexes: Theoretical study, DNA binding and cleavage activities. Appl. Organomet. Chem. 2018, 32, 4128. [Google Scholar] [CrossRef]
- Jaividhya, P.; Ganeshpandian, M.; Dhivya, R.; Akbarsha, M.A.; Palaniandavar, M. Fluorescent mixed ligand copper(II) complexes of anthracene-appended Schiff bases: Studies on DNA binding, nuclease activity and cytotoxicity. Dalton Trans. 2015, 44, 11997–12010. [Google Scholar] [CrossRef] [PubMed]
- Kumari, B.; Singh, K.; Sharma, A. Synthesis, crystal structure and molecular docking studies of novel Schiff base ligand 9-(((3-ethyl-5-mercapto/thio-4H-1,2,4-triazole-4-yl)imino)methyl)-anthracene and its complexes with Ni(II), Cu(II), Zn(II) and Cd(II): Comparative spectral, thermo-kinetics, radical scavenging and antimicrobial studies. Chem. Data Collect. 2022, 38, 100833. [Google Scholar]
- Prakash, A.; Shamim, A.S. Synthesis and characterisation of Schiff base complexes with Ti (III), Cr (III) and Ni (II). Orient. J. Chem. 2009, 25, 1035–1040. [Google Scholar]
- Bai, J.; Wang, R.-H.; Qiao, Y.; Wang, A.; Fang, C.-J. Schiff base derived from thiosemicarbazone and anthracene showed high potential in overcoming multidrug resistance in vitro with low drug resistance index. Drug Des. Dev. Ther. 2017, 11, 2227–2237. [Google Scholar] [CrossRef]
- Turibius, S.; Muthaiah, S.; Venkatesan, S.; Ching-Chang, L.; Fu-Hsiang, K.; Wen, K.S.; Lin, M. Novel anthracene and pyridine comprising schiff base probe for selective “OFF-ON” fluorescent determination of Cu2+ ions towards live cell application. New J. Chem. 2016, 40, 1–26. [Google Scholar]
- Saifi, A.; Negi, C.; Kumar, K. Visible light responsive soft actuator based on functional anthracene dye. Eur. Polym. J. 2022, 171, 111176. [Google Scholar] [CrossRef]
- Densil, S.; Chang, C.-H.; Chen, C.-L.; Mathavan, A.; Ramdass, A.; Sathish, V.; Thanasekaran, P.; Li, W.-S.; Rajagopal, S. Aggregation-induced emission enhancement of anthracene-derived Schiff base compounds and their application as a sensor for bovine serum albumin and optical cell imaging. Luminescence 2018, 33, 780–789. [Google Scholar] [CrossRef]
- Kraicheva, I.; Tsacheva, I.; Nikolova, R.; Topashka-Ancheva, M.; Stoineva, I.; Shivachev, B. Microwave assisted synthesis and X-ray structure of a novel anthracene-derived aminophosphonate. Enantioseparation of two α-aminophosphonates and genotoxicity in vivo. Phosphorus Sulfur Silicon 2017, 192, 403–409. [Google Scholar] [CrossRef]
- Kraicheva, I.; Tsacheva, I.; Vodenicharova, E.; Tashev, E.; Tosheva, T.; Kril, A.; Topashka-Ancheva, M.; Iliev, I.; Gerasimova, T.; Troev, K. Synthesis, antiproliferative activity and genotoxicity of novel anthracene-containing aminophosphonates and a new anthracene-derived Schiff base. Bioorg. Med. Chem. 2012, 20, 117–124. [Google Scholar] [CrossRef]
- Kraicheva, I.; Vodenicharova, E.; Shivachev, B.; Nikolova, R.; Kril, A.; Topashka-Ancheva, M.; Iliev, I.; Georgieva, A.; Gerasimova, T.; Tosheva, T.; et al. Anthracene-Delivered Bis-Aminiphosphonates: Crystal Structure, In vitro Antitumor Activity, and Genotoxicity in vivo. Phosphorus Sulfur Silicon 2013, 188, 1535–1547. [Google Scholar] [CrossRef]
- Elvira, C.; Gallardo, A.; San Roman, J.; Cifuentes, A. Covalent Polymer-Drug Congugates. Molecules 2005, 10, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Hershfield, M.S. Adenosine deaminase deficiency: Clinical expression, molecular basis, and therapy. Semin. Hematol. 1998, 35, 291–298. [Google Scholar]
- Maeda, H. SMANCS and polymer-conjugated macromolecular drugs: Advantages in cancer chemotherapy. Adv. Drug Deliv. Rev. 1991, 6, 181–202. [Google Scholar] [CrossRef]
- Lowman, A.M.; Peppas, N.A. Solute transport analysis in pH-responsive, complexing hydrogels of poly(methacrylic acid-g-ethylene glycol). J. Biomater. Sci. Polym. Ed. 2012, 10, 999–1090. [Google Scholar] [CrossRef]
- Vicent, M.J. Polymer-drug conjugates as modulators of cellular apoptosis. Am. Assoc. Pharm. Sci. J. 2007, 9, E200–E207. [Google Scholar] [CrossRef]
- Luten, J.; van Nostrum, C.F.; De Smedt, S.C.; Hennink, W.E. Biodegradable polymers as non-viral carriers for plasmid DNA delivery. J. Control. Release 2008, 126, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, J.; Mao, H.-Q.; Leong, K.W. Polyphosphoesters in drug and gene delivery. Adv. Drug Deliv. Rev. 2003, 55, 483–499. [Google Scholar] [CrossRef]
- Dahiyat, B.I.; Richards, M.; Leong, K.W. Controlled release from poly(phosphoester) matrices. J. Control. Release 1995, 33, 13–21. [Google Scholar] [CrossRef]
- Brosse, J.-C.; Derouet, D.; Fontaine, L.; Chairatanathavorn, S. Fixation of pharmacologically active amines on polyphosphonates, 2. Application to benzocaïne and phenethylamine. Die Makromol. Chem. 1989, 190, 2339–2345. [Google Scholar] [CrossRef]
- Huang, S.-W.; Zhuo, R.-X. Recent Advances in Polyphosphoester and Polyphosphoramidate-Based Biomaterials. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 340–348. [Google Scholar] [CrossRef]
- Kraicheva, I.; Tsacheva, I.; Troev, K. Poly(oxyethylene aminophosphonate)s—Novel promising biologically active polymers and drug carriers. Design and NMR characterization. Bulg. Chem. Commun. 2008, 40, 54–58. [Google Scholar]
- Kraicheva, I.; Bogomilova, A.; Tsacheva, I.; Momekov, G.; Momekova, D.; Troev, K. Synthesis, NMR characterization and in vitro cytotoxicity evaluation of new poly(oxyethylene aminophosphonate)s. Eur. J. Med. Chem. 2010, 45, 6039–6044. [Google Scholar] [CrossRef]
- Martínez, R.; Chacón-García, L. The Search of DNA-Intercalators as Antitumoral Drugs: What it Worked and What did not Work. Curr. Med. Chem. 2005, 12, 127–151. [Google Scholar] [CrossRef]
- Bowden, G.T.; García, D.; Peng, Y.-M.; Alberts, D.S. Molecular pharmacology of the anthracycline drug 9,10-anthracenedi-carboxaldehyde bis[(4,5 dihydro-1H-imidazol-2-yl) hydrazone] dihydrochloride (CL 216,942). Cancer Res. 1982, 42, 2660–2665. [Google Scholar] [PubMed]
- Prinz, H.; Schmidt, P.; Böhm, K.J.; Baasner, S.; Müller, K.; Gerlach, M.; Günther, E.G.; Unger, E. Phenylimino-10H-anthracen-9-ones as novel antimicrotubule agents—Synthesis, antiproliferative activity and inhibition of tubulin polymerization. Bioorg. Med. Chem. 2011, 19, 4183–4191. [Google Scholar] [CrossRef]
- Herrmann, U.; Tummler, B.; Maass, G.; Mew, P.K.T.; Vögtle, F. Anthracenyl crown ethers and cryptands as fluorescent probes for solid-phase transitions of phosphatidylcholines: Syntheses and phospholipid membrane studies. Biochemistry 1984, 23, 4059–4067. [Google Scholar] [CrossRef]
- Kraicheva, I.; Vodenicharova, E.; Shenkov, S.; Tashev, E.; Tosheva, T.; Tsacheva, I.; Kril, A.; Topashka-Ancheva, M.; Georgieva, A.; Iliev, I.; et al. Synthesis, characterization, antitumor activity and safety testing of novel polyphosphoesters bearing anthracene-derived aminophosphonate units. Bioorg. Med. Chem. 2014, 22, 874–882. [Google Scholar] [CrossRef]
- Kraicheva, I.; Momekov, G.; Mihaylova, R.; Topashka-Ancheva, M.; Tsacheva, I.; Stoineva, I.; Vodenicharova, E.; Nedialkov, P. Synthesis of Two Novel Homologous Polyphosphoesters Containing Aminophosphonate Units and Cytotoxicity of Some Low-Molecular and Polymeric Aminophosphonate Derivatives. Adv. Mater. Sci. Eng. 2018, 2018, 9565401. [Google Scholar] [CrossRef]


| Cell Line | IC50 Value (µM) a | |||||
|---|---|---|---|---|---|---|
| Cisplatin | 2 | 3 | 4 | 5 | 6 | |
| LAMA-84 b | 18.2 ± 1.7 | 39.9 ± 2.1 | 251.9 ± 7.3 | >400.0 | 71.2 ± 2.4 | 119.4 ± 6.3 |
| K-562 b | 25.7 ± 2.1 | 29.9 ± 1.9 | 212.9 ± 12.1 | 352.9 ± 11.7 | 22.9 ± 0.9 | 42.4 ± 3.0 |
| HL-60 b | 7.8 ± 1.1 | >400.0 | >400.0 | 163.4 ± 5.3 | 74.8 ± 2.7 | >400.0 |
| HL-60/DOX c,d | 14.5 ± 1.4 | 68.6 ± 4.0 | 226.1 ± 5.9 | 190.0 ± 4.7 | 115.2 ± 7.1 | 107.2 ± 4.1 |
| Cell Lines | IC50 Value (µM) a | |||||
|---|---|---|---|---|---|---|
| Doxorubicin | 1 | 2 | 3 | 4 | 5 | |
| MCF-7 | <125 | 1590 ± 60.1 | 912.3 ± 49.1 | 1111.1 ± 59.3 | >4600 | 236.4 ± 7.1 |
| MDA-MB-231 | <125 | 424 ± 24.7 | 1578.9 ± 498.2 | 2172.8 ± 56.8 | >4600 | 165.5 ± 2.4 |
| HBL-100 | 257.6 ± 20.23 | 1095 ± 95.4 | 1614 ± 63.2 | 49.4 ± 14.8 | 1685.9 ± 53.11 | 165.5 ± 2.4 |
| HepG2 | <125 | 636 ± 38.9 | 421 ± 17.5 | 2049 ± 61.7 | >4600 | 260 ± 4.7 |
| HT-29 | 1067.1 ± 23.9 | 282.7 ± 10.6 | 701.8 ± 42.1 | >4600 | >4600 | 260 ± 2.4 |
| 647-V | <125 | 459.4 ± 14.1 | 280.7 ± 7 | 1481.5 ± 51.9 | >4600 | 165.5 ± 2.4 |
| 138.6HeLa | <125 | 600.7 ± 14.1 | 1228 ± 0.03 | >4600 | 4480.4 ± 138.6 | 283.7 ± 2.4 |
| Cell Lines | IC50 Value (µM) a | ||
|---|---|---|---|
| Doxorubicin | 3 | 4 | |
| MCF-7 | <125.1 | 865.8 ± 46.8 | 757.6 ± 13.3 |
| MDA-MB-231 | <125.1 | >1200 | >1176 |
| HBL-100 | 257.8 ± 20.2 | >1200 | 1129.4 ± 28.5 |
| HepG2 | <125.1 | >1200 | >1176 |
| HT-29 | 1067.1 ± 23.9 | 539.1 ± 5.2 | 512.9 ± 5.2 |
| 647-V | <125.1 | >1200 | >1176 |
| HeLa | <125.1 | 1103.1 ± 6.8 | 1034.1 ± 6.2 |
| Bis- Aminophosphonates and Doses | Time after Treatment | Number of Metaphases Scored | Type of Chromosome Aberrations | Mitotic Index (‰) (X ± m) | Percentage of Cells with Aberrations (X ± m) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Breaks | Fragments | Rearrangements | |||||||
| c/c | t/t | c/t | |||||||
| (3) 10 mg/kg | 24 h | 400 | 0 | 3 | 17 | 0 | 0 | 12.07 ± 0.99 | 5.00 ± 0.84 |
| 48 h | 450 | 4 | 5 | 11 | 0 | 0 | 11.18 ± 0.68 | 4.37 ± 0.59 | |
| (3) 100 mg/kg | 24 h | 400 | 10 | 13 | 13 | 3 | 0 | 8.20 ± 0.72 | 9.75 ± 0.88 |
| 48 h | 400 | 9 | 7 | 8 | 1 | 0 | 7.27 ± 0.83 | 6.25 ± 0.70 | |
| (4) 10 mg/kg | 24 h | 400 | 5 | 5 | 10 | 2 | 0 | 8.39 ± 0.35 | 5.50 ± 0.33 |
| 48 h | 400 | 3 | 8 | 6 | 1 | 0 | 8.93 ± 0.51 | 4.50 ± 0.33 | |
| (4) 100 mg/kg | 24 h | 400 | 21 | 8 | 17 | 0 | 0 | 10.08 ± 0.73 | 11.50 ± 0.50 |
| 48 h | 400 | 8 | 8 | 13 | 0 | 0 | 7.90 ± 0.34 | 7.25 ± 0.53 | |
| Mit. C 3.5 mg/kg | 24 h | 200 | 17 | 30 | 7 | 1 | 0 | 5.49 ± 0.19 | 30.50 ± 2.36 |
| 48 h | 400 | 17 | 24 | 20 | 0 | 0 | 7.29 ± 0.34 | 15.80 ± 0.81 | |
| DMSO | 24 h | 500 | 1 | 1 | 4 | 0 | 0 | 15.14 ± 0.46 | 1.40 ± 0.30 |
| 48 h | 500 | 2 | 0 | 2 | 0 | 0 | 12.47 ± 1.07 | 0.80 ± 0.32 | |
| Control 0.9% NaCl | 24 h | 700 | 4 | 0 | 4 | 0 | 0 | 20.06 ± 1.38 | 1.14 ± 0.34 |
| 48 h | 500 | 0 | 0 | 3 | 0 | 0 | 16.88 ± 0.56 | 0.60 ± 0.30 | |
| Compounds | IC50 (µmol/L) | |||
|---|---|---|---|---|
| K-562 a | LAMA-84 a | HL-60/Dox a, * | HL-60 b | |
| 5 | >400 | >400.0 | >400.0 | 105.9 |
| 6 | 14.9 | 17.2 | 27.2 | 19.2 |
| 7 | 15.2 | 14.0 | 20.4 | 19.9 |
| 8 | 13.7 | 12.3 | 14.4 | 14.2 |
| 9 | 62.3 | 88.8 | 159.1 | 157.5 |
| 10 | 18.3 | 41.9 | 16.2 | 66.2 |
| Cisplatin | 25.7 | 18.2 | 14.5 | 7.8 |
| Compounds | IC50 (µM) | |||
|---|---|---|---|---|
| K-562 (CML) | SKW-3 (CLL) | REH (ALL) | HL-60 (APML) | |
| S-1 | 80.8 ± 11.4 | 57.2 ± 4.6 | 19.8 ± 2.7 | 47.7 ± 6.9 |
| S-2 | 89.7 ± 9.1 | 34.3 ± 7.1 | 18.3 ± 3.2 | 37.3 ± 4.6 |
| A-3 | >200 | 37.0 ± 5.6 | 30.5 ± 7.8 | 35.6 ± 6.1 |
| A-4 | 18.7 ± 4.9 | 8.4 ± 1.5 | 14.1 ± 1.9 | 21.2 ± 4.4 |
| A-5 | 139.1 ± 8.1 | 76.3 ± 7.9 | 114 ± 14.7 | 76.8 ± 12.9 |
| A-6 | 47.3 ± 6.5 | 51.4 ± 7.3 | 42.8 ± 6.9 | 64.1 ± 9.8 |
| A-5a | 58.6 ± 2.0 | 10.5 ± 2.5 | 21.4 ± 0.7 | 30.2 ± 2.1 |
| A-5b | 79.7 ± 3.3 | 20.5 ± 2.0 | 17.6 ± 0.8 | 30.3 ± 2.1 |
| B6 | >200 | 187.2 ± 14.4 | 165.9 ± 21.5 | 139.1 ± 11.3 |
| P-8 | 35.6 ± 7.3 | 3.1 ± 1.2 | 7.9 ± 1.4 | 2.1 ± 0.6 |
| P-9 | 24.8 ± 1.1 | 5.4 ± 1.2 | 16.8 ± 1.2 | 5.5 ± 1.2 |
| P-10 | 163.0 ± 22.3 | 38.5 ± 4.3 | 98.7 ± 8.8 | 49.4 ± 7.3 |
| P-11 | >200 | 61.1 ± 9.2 | 113.4 ± 10.5 | 116.9 ± 10.4 |
| P-12 | 26.9 ± 5.9 | 29.5 ± 5.2 | 33.8 ± 6.4 | 40.1 ± 7.6 |
| Cisplatin | 18.6 ± 3.9 | 11.2 ± 0.8 | 17.2 ± 2.1 | 8.1 ± 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsacheva, I.; Todorova, Z.; Momekova, D.; Momekov, G.; Koseva, N. Pharmacological Activities of Schiff Bases and Their Derivatives with Low and High Molecular Phosphonates. Pharmaceuticals 2023, 16, 938. https://doi.org/10.3390/ph16070938
Tsacheva I, Todorova Z, Momekova D, Momekov G, Koseva N. Pharmacological Activities of Schiff Bases and Their Derivatives with Low and High Molecular Phosphonates. Pharmaceuticals. 2023; 16(7):938. https://doi.org/10.3390/ph16070938
Chicago/Turabian StyleTsacheva, Ivelina, Zornica Todorova, Denitsa Momekova, Georgi Momekov, and Neli Koseva. 2023. "Pharmacological Activities of Schiff Bases and Their Derivatives with Low and High Molecular Phosphonates" Pharmaceuticals 16, no. 7: 938. https://doi.org/10.3390/ph16070938
APA StyleTsacheva, I., Todorova, Z., Momekova, D., Momekov, G., & Koseva, N. (2023). Pharmacological Activities of Schiff Bases and Their Derivatives with Low and High Molecular Phosphonates. Pharmaceuticals, 16(7), 938. https://doi.org/10.3390/ph16070938






