The Antiproliferative Effect of Chloroform Fraction of Eleutherine bulbosa (Mill.) Urb. on 2D- and 3D-Human Lung Cancer Cells (A549) Model
Abstract
1. Introduction
2. Results and Discussion
2.1. 2D Culture Condition
2.1.1. Cytotoxic Activities of E. bulbosa Fractions and Cisplatin on A549 and MRC-5 Cells
2.1.2. Effect of E. bulbosa Chloroform Fraction on Clonogenic Potential and Survival of A549 Lung Cancer Cells
2.1.3. Effect of the E. bulbosa Chloroform Fraction on the Induction of Cell Death in A549 Cells
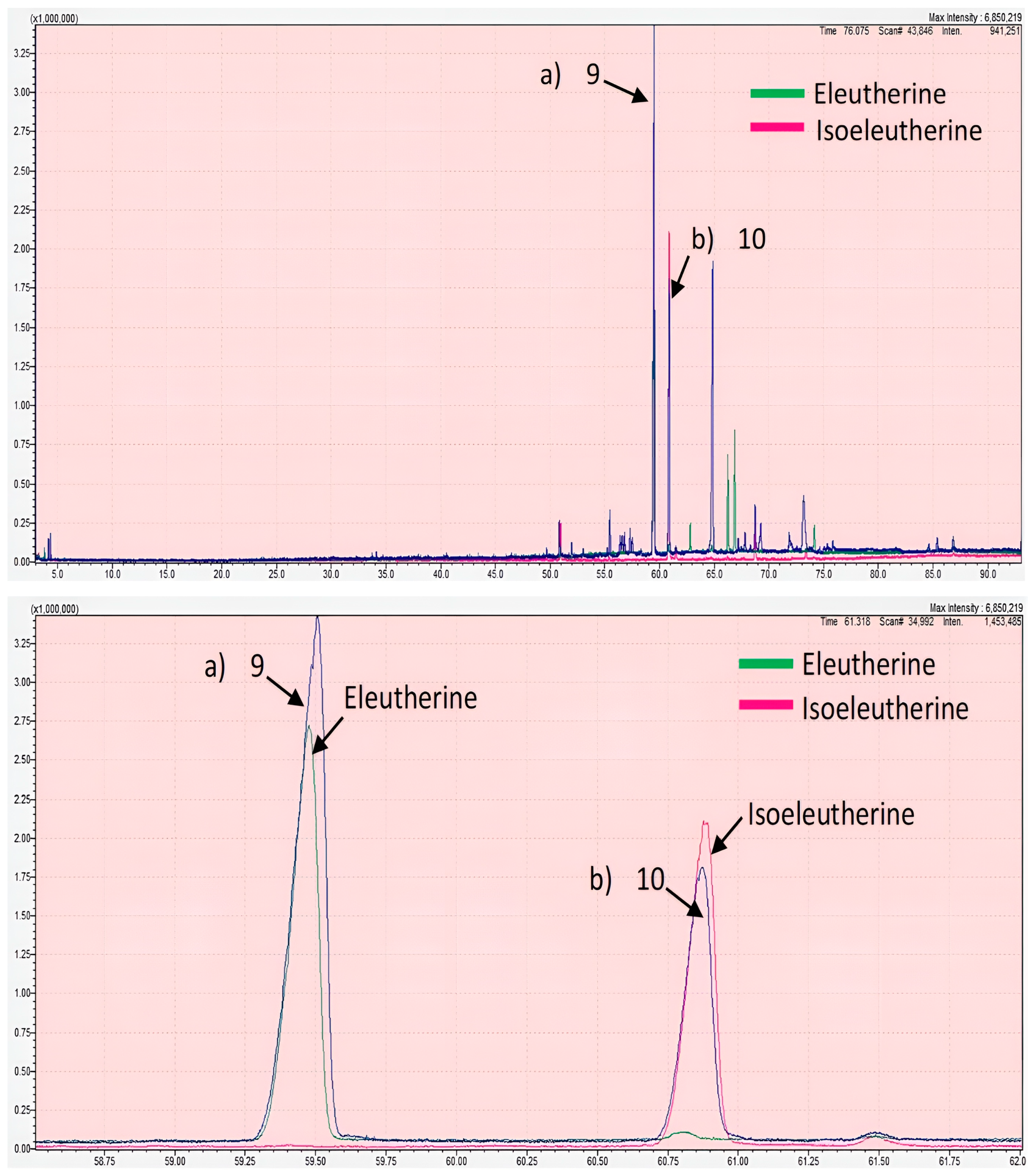
2.2. GC-MS Analysis
2.3. 3D Culture Condition
2.3.1. Cytotoxic Activity of the Chloroform Fraction of E. bulbosa on Lung Cancer Spheroids
2.3.2. Microscopy Analysis of Lung Cancer Spheroids
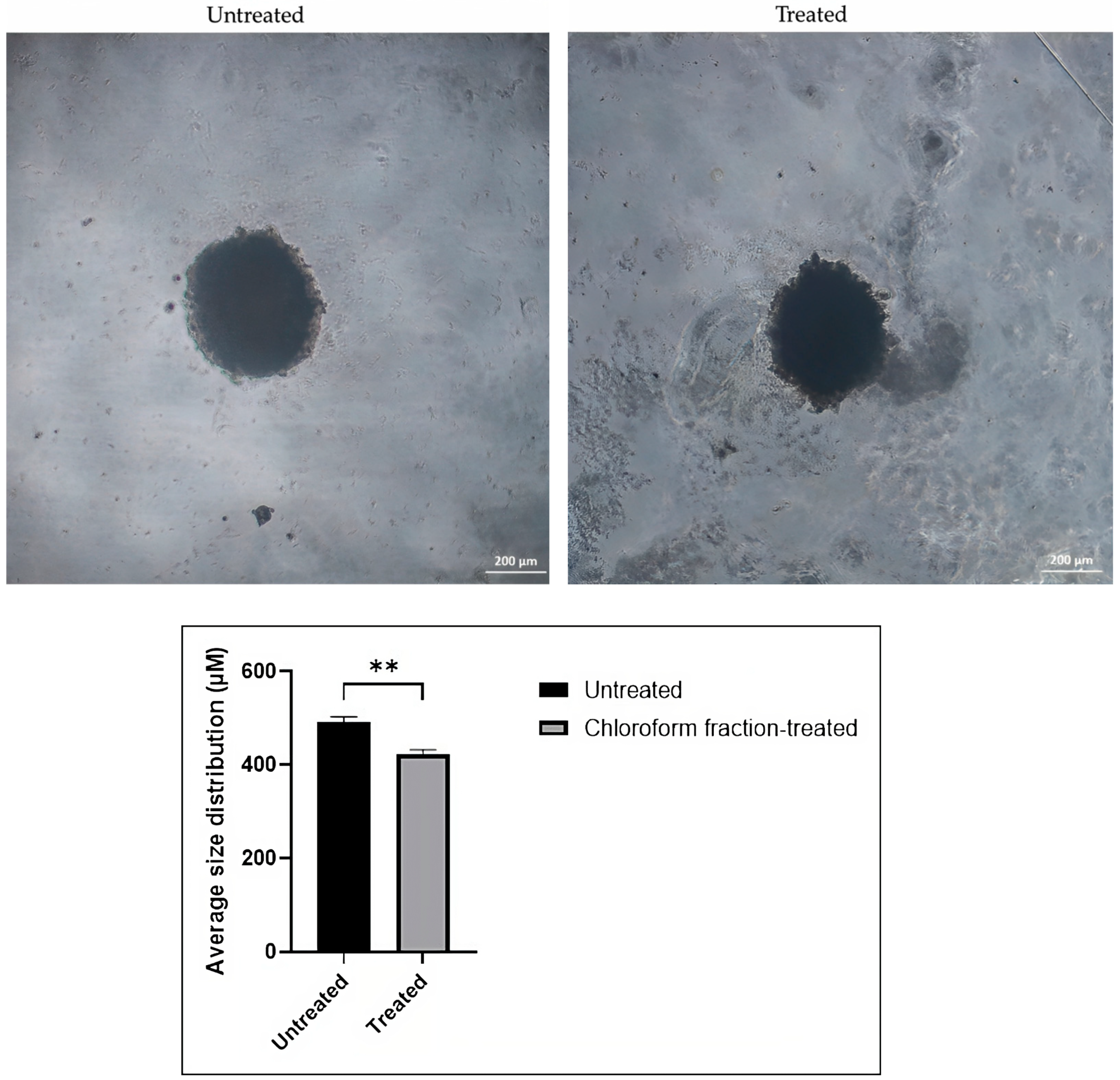
Effect of the Chloroform Fraction of E. bulbosa on the Size of the A549 Spheroid
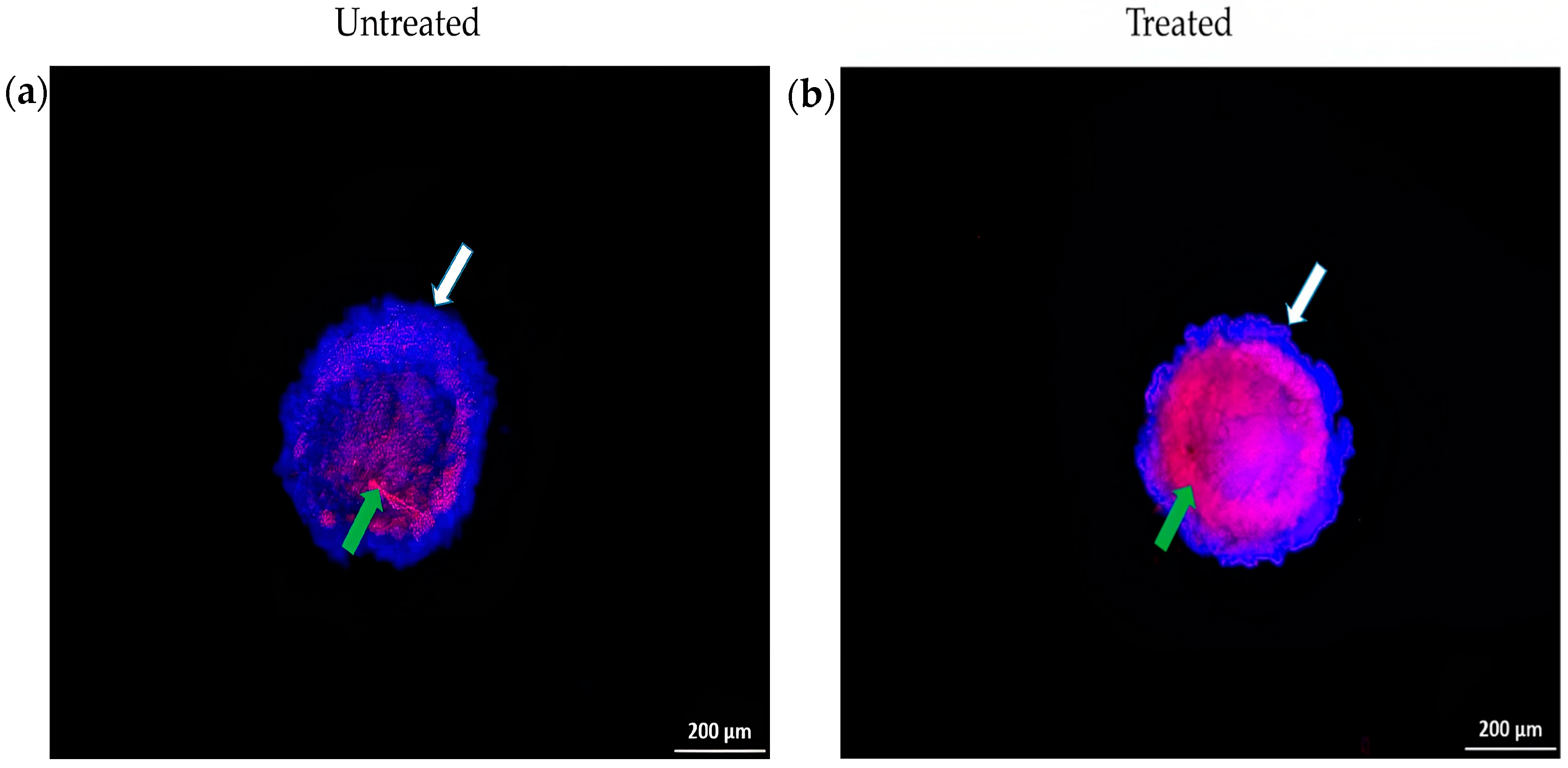
Cell Death Analysis of Spheroid Using Hoechst 33342/PI Staining
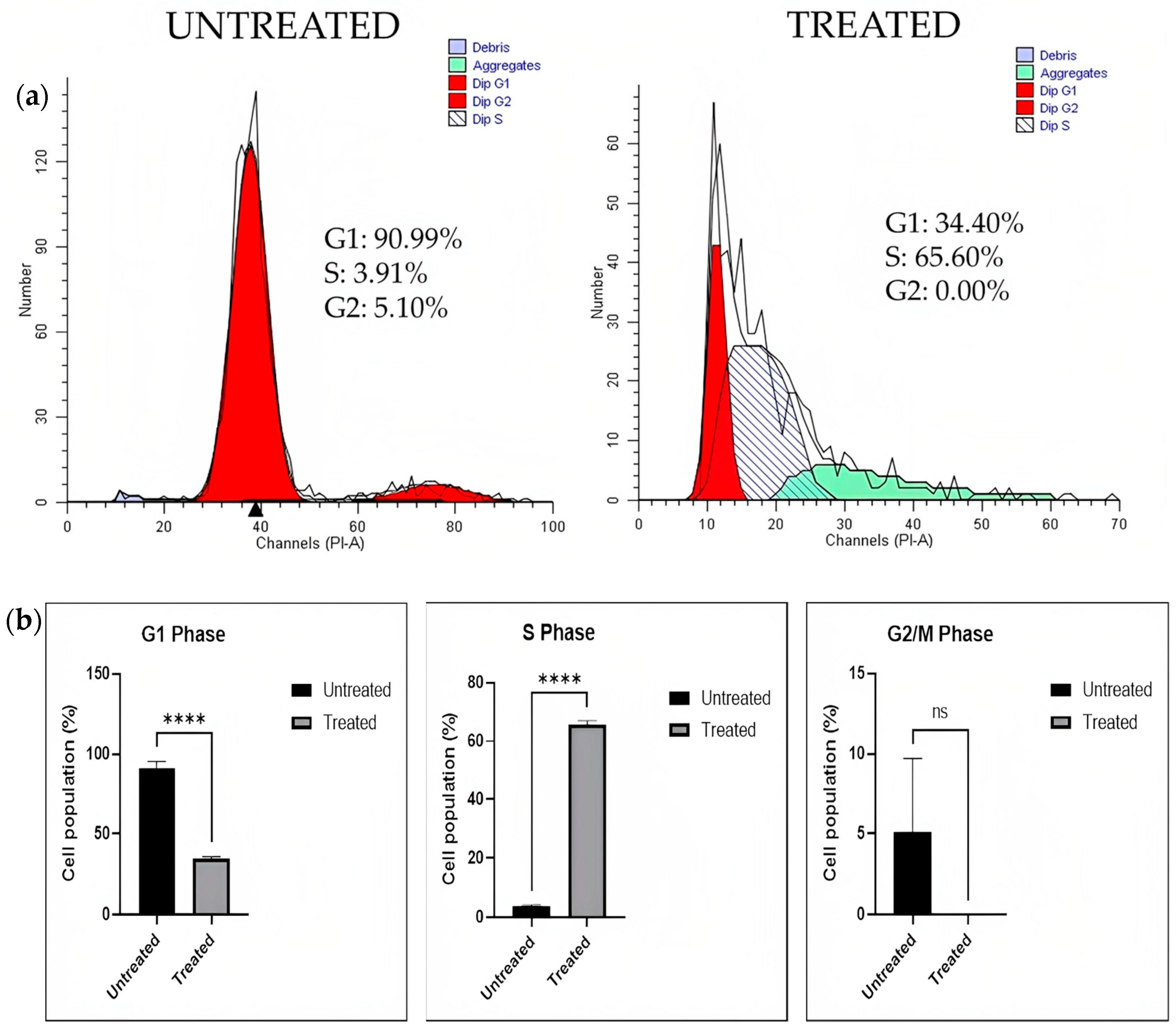
2.3.3. Flow Cytometry Analysis on Lung Cancer Spheroids
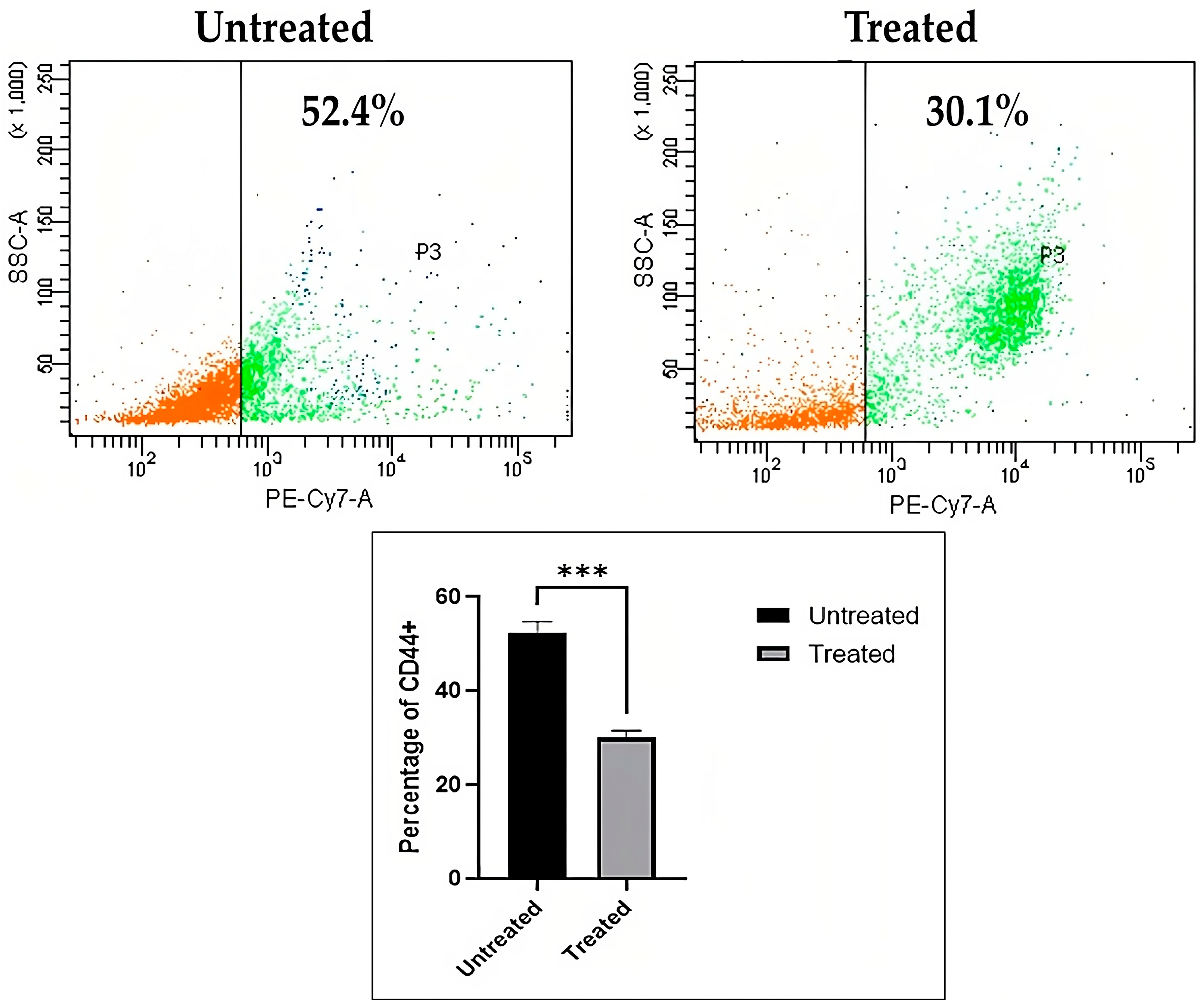
Immunophenotyping of CD44 Lung Cancer Stem Cells
Cell Cycle Analysis
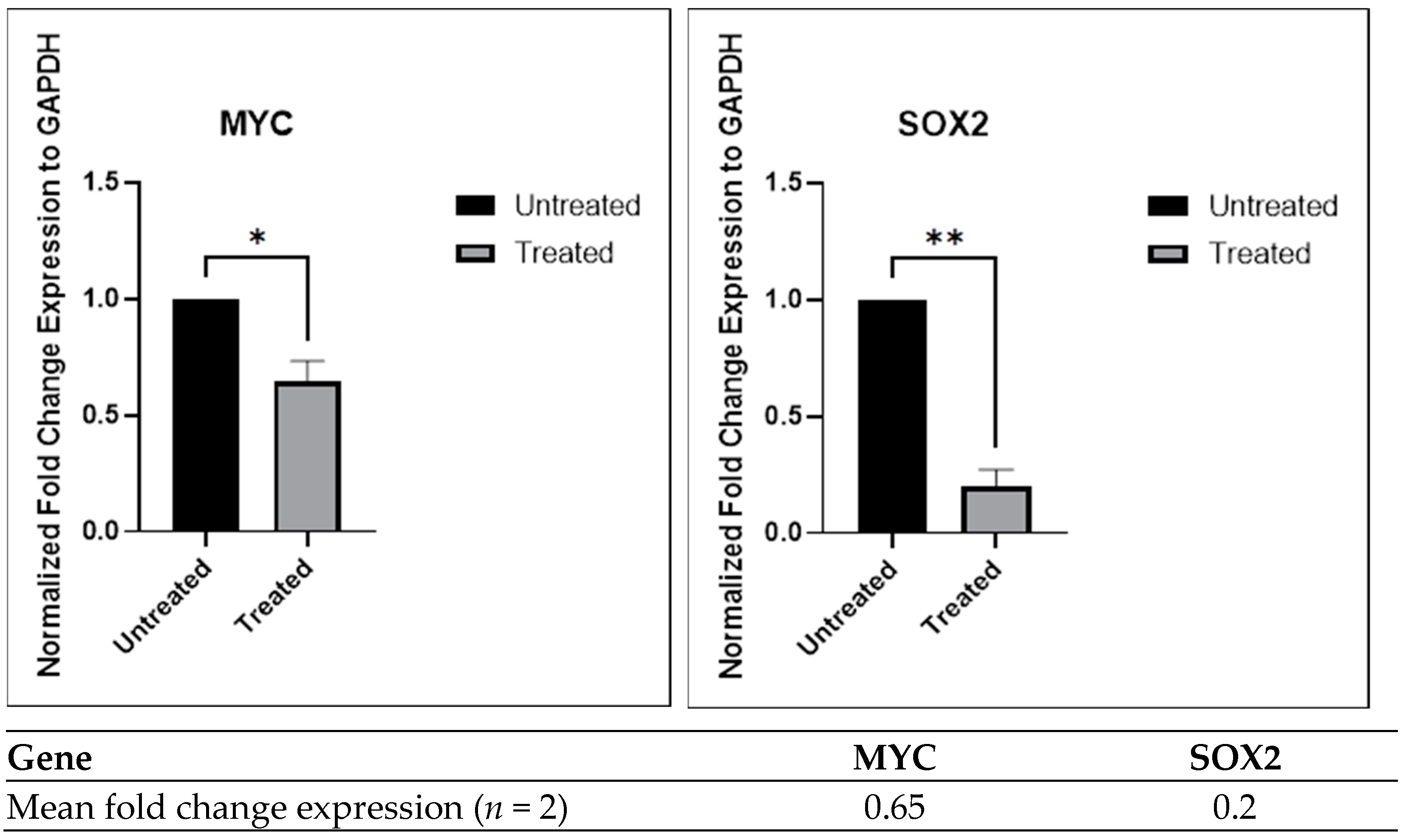
2.3.4. Inhibition of Gene Expression by the Chloroform Fraction of E. bulbosa in A549 Spheroids
3. Materials and Methods
3.1. Chemicals and Reagents
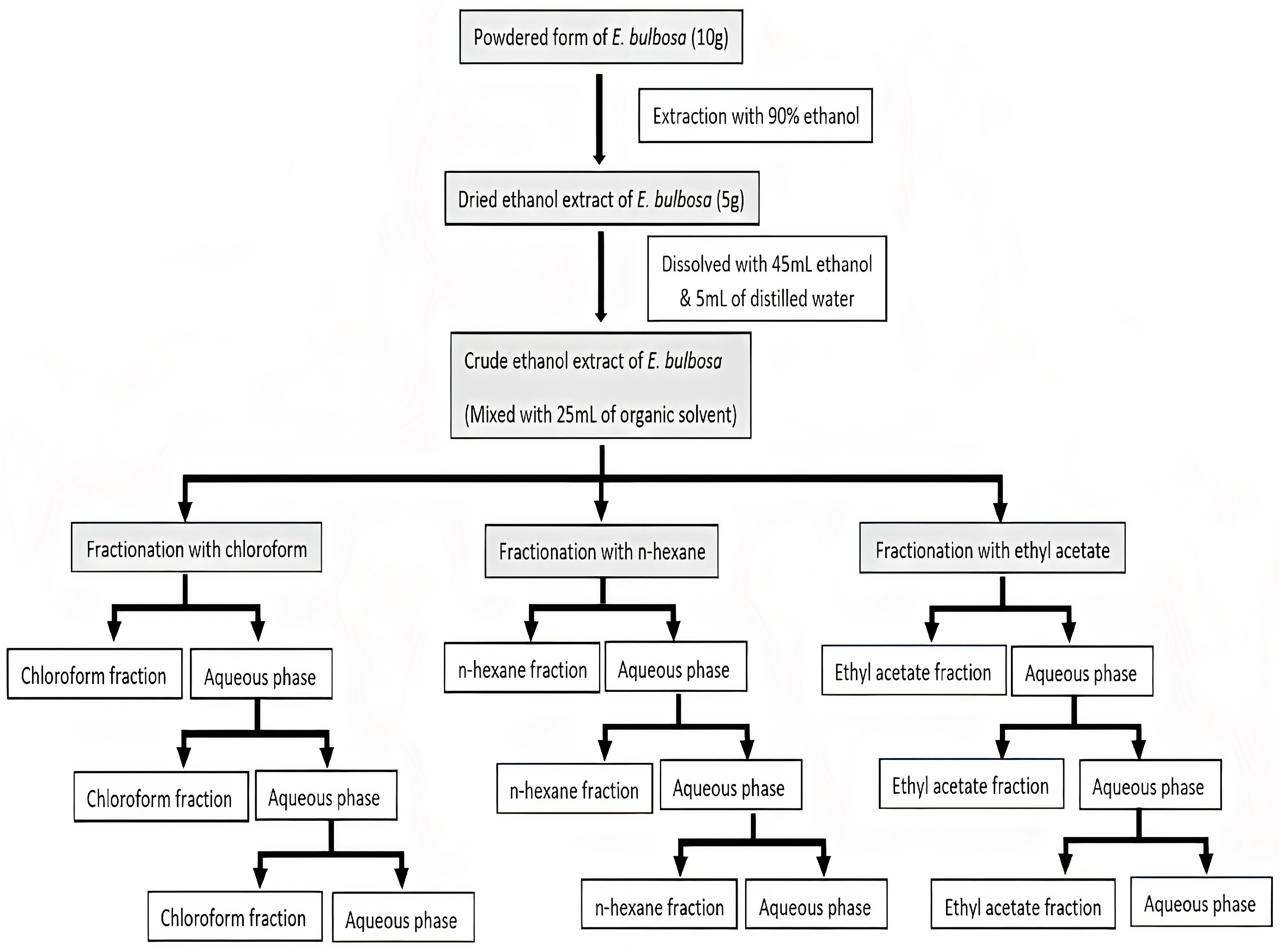
3.2. Raw Material and Sample Preparation
3.3. Ethanol Extraction and Liquid-Liquid Fractionation
3.4. 2D Culture Condition
3.4.1. Cell Lines and Culture
3.4.2. Cytotoxic Activity Assay
3.4.3. Clonogenic Survival Assay
3.4.4. Cell Death Analysis
3.5. GC-MS Analysis
3.6. 3D Culture Condition
3.6.1. Generation of Lung Cancer Spheroids
3.6.2. Cytotoxicity Activity on Lung Cancer Spheroids
3.6.3. Microscopy Analysis of Lung Cancer Spheroids
Spheroid Size Analysis
Cell Death Analysis of Spheroid Using Hoechst 33342/PI Staining
3.6.4. Flow Cytometry Analysis on Lung Cancer Spheroids
Immunophenotyping of CD44 Lung Cancer Stem Cells
Cell Cycle Analysis
3.6.5. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- National Strategic Plan for Cancer Control Programme. 2016–2020. Available online: https://www.iccp-portal.org/plans/national-strategic-plan-cancer-control-programme (accessed on 15 August 2022).
- Mustafa, M.; Azizi, A.J.; IIIzam, E.; Nazirah, A.; Sharifa, S.; Abbas, S. Lung cancer: Risk factors, management, and prognosis. IOSR J. Dent. Med. Sci. 2016, 15, 94–101. [Google Scholar] [CrossRef]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Wspolczesna Onkol. 2021, 25, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Lemjabbar-Alaoui, H.; Hassan, O.U.; Yang, Y.W.; Buchanan, P. Lung cancer: Biology and treatment options. Biochim. Biophys. Acta Rev. Cancer 2015, 1856, 189–210. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Wang, Z.-W.; Lu, T.-L.; Gomez, C.B.; Fang, H.-W.; Wei, Y.; Tseng, C.-L. The synergistic anticancer effect of dual drug- (cisplatin/epigallocatechin gallate) loaded gelatin nanoparticles for lung cancer treatment. J. Nanomater. 2020, 2020, 9181549. [Google Scholar] [CrossRef]
- Wen, T.; Song, L.; Hua, S. Perspectives and controversies regarding the use of natural products for the treatment of lung cancer. Cancer Med. 2021, 10, 2396–2422. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Boy HI, A.; Rutilla AJ, H.; Santos, K.A.; Ty AM, T.; Yu, A.I.; Mahboob, T.; Tangpoong, J.; Nissapatorn, V. Recommended medicinal plants as source of natural products: A review. Digit. Chin. Med. 2018, 1, 131–142. [Google Scholar] [CrossRef]
- Jin, H.; Park, S.B.; Yoon, J.H.; Lee, J.Y.; Kim, E.H.; Yoon, S.W. Traditional herbal medicine combined with firstline platinum-based chemotherapy for advanced non-small-cell lung cancer A PRISMA-compliant systematic review and meta-Analysis. Medicine 2021, 100, e27163. [Google Scholar] [CrossRef]
- Demir, S.; Turan, I.; Aliyazicioglu, R.; Yaman, S.O.; Aliyazicioglu, Y. Primula vulgaris extract induces cell cycle arrest and apoptosis in human cervix cancer cells. J. Pharm. Anal. 2018, 8, 307–311. [Google Scholar] [CrossRef]
- Kusuma, I.W.; Arung, E.T.; Rosamah, E.; Purwatiningsih, S.; Kuspradini, H.; Syafrizal Astuti, J.; Kim, Y.U.; Shimizu, K. Antidermatophyte and antimelanogenesis compound from Eleutherine americana grown in Indonesia. J. Nat. Med. 2010, 64, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Insanu, M.; Kusmardiyani, S.; Hartati, R. Recent studies on phytochemicals and pharmacological effects of Eleutherine americana Merr. Procedia Chem. 2014, 13, 221–228. [Google Scholar] [CrossRef]
- Padhi, L.; Panda, S.K. Antibacterial activity of Eleutherine bulbosa against multidrug-resistant bacteria. J. Acute Med. 2015, 5, 53–61. [Google Scholar] [CrossRef]
- Couto, C.L.L.; Moraes, D.F.C.; Cartágenes, M.d.S.S.; do Amaral, F.M.M.; Guerra, R.N. Eleutherine bulbous (Mill.) Urb.: A review study. J. Med. Plants Res. 2016, 10, 286–297. [Google Scholar]
- Rani, V.S.; Bindu, R.N. Pharmacognostic and physicochemical evaluation of bulbs of Eleutherine bulbosa (Miller) Urban, a medicinal plant. J. Pharmacogn. Phytochem. 2015, 4, 273–277. [Google Scholar]
- Yuswi, N.C.R. Ekstraksi antioksidan bawang Dayak (Eleutherine palmifolia) dengan metode ultrasonic bath (Antioxidant extraction of dayak onion (Eleutherine palmifolia) using ultrasonic bath method). J. Pangan Agroindustri 2018, 5, 71–78. (In Indonesian) [Google Scholar]
- Lubis, I.A.; Ichwan, M.F.; Mustofa, M.; Satria, D. Anticancer activity of Eleutherine bulbosa (Mill.) Urb. extract on WiDr cell line in vitro. In Proceedings of the 2nd Public Health International Conference (PHICo 2017), Medan, Indonesia, 18–19 December 2017; Atlantis Press: Amsterdam, The Netherlands, 2018; Volume 9, pp. 123–127. [Google Scholar]
- Kamarudin, A.A.; Sayuti, N.H.; Saad, N.; Razak, N.A.; Esa, N.M. Induction of apoptosis by Eleutherine bulbosa (mill.) urb. bulb extracted under optimised extraction condition on human retinoblastoma cancer cells (WERI-RB-1). J. Ethnopharmacol. 2022, 284, 114770. [Google Scholar] [CrossRef]
- Kamarudin, A.A.; Sayuti, N.H.; Saad, N.; Razak, N.A.; Esa, N.M. Eleutherine bulbosa (Mill.) urb. bulb: Review of the pharmacological activities and its prospects for application. Int. J. Mol. Sci. 2021, 22, 6747. [Google Scholar] [CrossRef]
- Järvinen, P.; Bonabi, A.; Jokinen, V.; Sikanen, T. Simultaneous culturing of cell monolayers and spheroids on a single microfluidic device for bridging the gap between 2D and 3D cell assays in drug research. Adv. Funct. Mater. 2020, 30, 2000479. [Google Scholar] [CrossRef]
- Ballav, S.; Jaywant Deshmukh, A.; Siddiqui, S.; Aich, J.; Basu, S. Two-dimensional and three-dimensional cell culture and their applications. In Cell Culture—Advanced Technology and Applications in Medical and Life Sciences; IntechOpen: London, UK, 2020. [Google Scholar]
- Muthia, R.; Wati, H.; Jamaludin, W.B.; Kartini, K.K.; Setiawan, F.; Fikri, M.; Wahhab, A. Standardization of Eleutherine bulbosa Urb. bulbs and total flavonoid content from three locations in Kalimantan, Indonesia. Pharmacogn. J. 2021, 13, 73–80. [Google Scholar] [CrossRef]
- Vajrabhaya, L.; Korsuwannawong, S. Cytotoxicity evaluation of a Thai herb using tetrazolium (MTT) and sulforhodamine B (SRB) assays. J. Anal. Sci. Technol. 2018, 9, 15. [Google Scholar] [CrossRef]
- Fithrotunnisa, Q.; Arsianti, A.; Kurniawan, G.; Qorina, F.; Tejaputri, N.A.; Azizah, N.N. In vitro cytotoxicity of hibiscus SABDARIFFA Linn extracts on A549 lung cancer cell line. Pharmacogn. J. 2020, 12, 14–19. [Google Scholar] [CrossRef]
- Berrouet, C.; Dorilas, N.; Rejniak, K.A.; Tuncer, N. Comparison of drug inhibitory effects (IC50) in monolayer and spheroid cultures. Bull. Math. Biol. 2020, 82, 68. [Google Scholar] [CrossRef] [PubMed]
- Atjanasuppat, K.; Wongkham, W.; Meepowpan, P.; Kittakoop, P.; Sobhon, P.; Bartlett, A.; Whitfield, P.J. In vitro screening for anthelmintic and antitumour activity of ethnomedicinal plants from Thailand. J. Ethnopharmacol. 2009, 123, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Ogbole, O.O.; Segun, P.A.; Adeniji, A.J. In vitro cytotoxic activity of medicinal plants from Nigeria ethnomedicine on Rhabdomyosarcoma cancer cell line and HPLC analysis of active extracts. BMC Complement. Altern. Med. 2017, 17, 494. [Google Scholar] [CrossRef]
- Mutiah, R.; Hadya, C.M.; Burhan Ma’arif, Z.A.; Bhagawan, W.S.; Annisa, R.; Indrawijaya, Y.Y.A.; Huwaida, F.I.; Ria Ramadhani, D.A.; Susilowati, R.; Taufik, I. Metabolite profiling of Eleutherine palmifolia (L.) Merr. by HPTLC-densitometry and its correlation with anticancer activities and in vitro toxicity. Indones. J. Pharm. 2019, 30, 157. [Google Scholar] [CrossRef]
- Elias, A.; Shebaby, W.N.; Nehme, B.; Faour, W.; Bassil, B.S.; Hakim, J.E.; Iskandar, R.; Dib-Jalbout, N.; Mroueh, M.; Daher, C.; et al. In vitro and in vivo evaluation of the anticancer and anti-inflammatory activities of 2-Himachelen-7-ol isolated from Cedrus Libani. Sci. Rep. 2019, 9, 12855. [Google Scholar] [CrossRef]
- Shaghayegh, G.; Alabsi, A.M.; Ali-Saeed, R.; Ali, A.M.; Vincent-Chong, V.K.; Ismail, N.H.; Choon, Y.F.; Zain, R.B. Effects of damnacanthal and nordamnacanthal on proliferation, apoptosis, and migration of oral squamous cell carcinoma cells. Asian Pac. J. Cancer Prev. 2017, 18, 3333–3341. [Google Scholar]
- Florea, A.M.; Büsselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef]
- Navanesan, S.; Abdul Wahab, N.; Manickam, S.; Sim, K.S. Leptospermum flavescens constituent-LF1 causes cell death through the induction of cell cycle arrest and apoptosis in human lung carcinoma cells. PLoS ONE 2015, 10, e0135995. [Google Scholar] [CrossRef]
- Živković, M.D.; Kljun, J.; Ilic-Tomic, T.; Pavic, A.; Veselinović, A.; Manojlović, D.D.; Nikodinovic-Runic, J.; Turel, I. A new class of platinum(ii) complexes with the phosphine ligand PTA which show potent anticancer activity. Inorg. Chem. Front. 2018, 5, 39–53. [Google Scholar] [CrossRef]
- Mrid, R.B.; Bouchmaa, N.; Bouargalne, Y.; Ramdan, B.; Karrouchi, K.; Kabach, I.; El Karbane, M.; Idir, A.; Zyad, A.; Nhiri, M. Phytochemical characterization, antioxidant and in vitro cytotoxic activity evaluation of juniperus oxycedrus subsp. oxycedrus needles and berries. Molecules 2019, 24, 502. [Google Scholar] [CrossRef] [PubMed]
- Fedr, R.; Pernicová, Z.; Slabáková, E.; Straková, N.; Bouchal, J.; Grepl, M.; Kozubík, A.; Souček, K. Automatic cell cloning assay for determining the clonogenic capacity of cancer and cancer stem-like cells. Cytom. Part A 2013, 83A, 472–482. [Google Scholar] [CrossRef]
- Backer, M.V.; Backer, J.M.; Chinnaiyan, P. Targeting the unfolded protein response in cancer therapy. Methods Enzymol. 2011, 491, 37–56. [Google Scholar] [PubMed]
- Franco, M.S.; Roque, M.C.; Oliveira, M.C. Short and long-term effects of the exposure of breast cancer cell lines to different ratios of free or co-encapsulated liposomal paclitaxel and doxorubicin. Pharmaceutics 2019, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Farghadani, R.; Rajarajeswaran, J.; Mohd Hashim, N.B.; Abdulla, M.A.; Muniandy, S. A novel β-diiminato manganeseiii complex as the promising anticancer agent induces G0/g1 cell cycle arrest and triggers apoptosis via mitochondrial-dependent pathways in MCF-7 and MDA-MB-231 human breast cancer cells. RSC Adv. 2017, 7, 24387–24398. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Y.; Guan, W.; Huang, L.; Xu, X.; Zhang, C.; Chen, X.; Wu, Y.; Zeng, G.; Zhong, N. Antitumor effect of para-toluenesulfonamide against lung cancer xenograft in a mouse model. J. Thorac. Dis. 2013, 5, 472–483. [Google Scholar]
- Lema, C.; Varela-Ramirez, A.; Aguilera, R.J. Differential nuclear staining assay for high-throughput screening to identify cytotoxic compounds. Curr. Cell. Biochem. 2011, 1, 1–14. [Google Scholar]
- De Oliveira Fonseca, M.; Soares da Silva, N.; Pacheco Soares, C. Effect of cortisol on K562 leukemia cells. Mundo Saúde 2019, 43, 854–869. [Google Scholar] [CrossRef]
- Kuswanti, N.; Widyarti, S.; Widodo, W.; Rifa’i, M. Apoptotic and necrotic lymphocytes after treatment of stem bark extract of plumeria rubra l in vitro. IOP Conf. Ser. Earth Environ. Sci. 2019, 391, 012031. [Google Scholar] [CrossRef]
- Grusch, M.; Polgar, D.; Gfatter, S.; Leuhuber, K.; Huettenbrenner, S.; Leisser, C.; Fuhrmann, G.; Kassie, F.; Steinkellner, H.; Smid, K.; et al. Maintenance of ATP favours apoptosis over necrosis triggered by benzamide riboside. Cell Death Differ. 2002, 9, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Hiramoto, A.; Kim, H.S.; Wataya, Y. Anticancer strategy targeting cell death regulators: Switching the mechanism of anticancer floxuridine-induced cell death from necrosis to apoptosis. Int. J. Mol. Sci. 2020, 21, 5876. [Google Scholar] [CrossRef] [PubMed]
- Saleh, K.A.; Aldulmani, S.A.; Awwad, N.S.; Ibrahium, H.A.; Asiri, T.H.; Hamdy, M.S. Utilization of lithium incorporated mesoporous silica for preventing necrosis and increase apoptosis in different cancer cells. BMC Chem. 2019, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Lestari, D.; Kartika, R.; Marliana, E. Antioxidant and anticancer activity of Eleutherine bulbosa (Mill.) Urb on leukemia cells L1210. J. Phys. Conf. Ser. 2019, 1277, 012022. [Google Scholar] [CrossRef]
- Gemantari, B.M.; Romadhonsyah, F.; Nurrochmad, A.; Wahyuono, S.; Astuti, P. Bioactivity screening of endophytic fungus eutypa linearis isolated from coleus amboinicus (Lour.). Indones. J. Pharm. 2021, 32, 86–95. [Google Scholar] [CrossRef]
- Kamarudin, A.A.; Esa, N.M.; Saad, N.; Sayuti, N.H.; Razak, N.A. Heat assisted extraction of phenolic compounds from Eleutherine bulbosa (Mill.) bulb and its bioactive profiles using response surface methodology. Ind. Crops Prod. 2020, 144, 112064. [Google Scholar] [CrossRef]
- Malheiros, L.C.; de Mello, J.C.; Barbosa, W.L. Eleutherine plicata—quinones and antioxidant activity. In Phytochemicals—Isolation Characterisation and Role in Human Health; IntechOpen: London, UK, 2015; pp. 323–338. [Google Scholar]
- Syamsul, E.S.; Apriliana, A.; Supomo Saleh, C.; Erwin. Thrombocyte Counts in Mice After the Administration of Chloroform Fraction of Eleutherine palmifolia L (Merr). In Proceedings of the International Conference on Pharmaceutical Research and Practice 2018, Universitas Islam Indonesia, Yogyakarta, Indonesia, 27 December 2018. [Google Scholar]
- Chou, H.L.; Fong, Y.; Wei, C.K.; Tsai, E.M.; Chen JY, F.; Chang, W.T.; Wu, C.Y.; Huang, H.W.; Chiu, C.C. A quinone-containing compound enhances camptothecin-induced apoptosis of lung cancer through modulating endogenous ROS and Erk Signaling. Arch. Immunol. Ther. Exp. 2017, 65, 241–252. [Google Scholar] [CrossRef]
- Zanoaga, O.; Braicu, C.; Jurj, A.; Rusu, A.; Buiga, R.; Berindan-Neagoe, I. Progress in research on the role of flavonoids in lung cancer. Int. J. Mol. Sci. 2019, 20, 4291. [Google Scholar] [CrossRef]
- Bakun, P.; Czarczynska-Goslinska, B.; Goslinski, T.; Lijewski, S. In vitro and in vivo biological activities of azulene derivatives with potential applications in medicine. Med. Chem. Res. 2021, 30, 834–846. [Google Scholar] [CrossRef]
- Sindhoor, S.M.; Naveen, N.R.; Rao GS, N.K.; Gopan, G.; Chopra, H.; Park, M.N.; Alshahrani, M.M.; Jose, J.; Emran, T.B.; Kim, B. A spotlight on alkaloid nanoformulations for the treatment of lung cancer. Front. Oncol. 2022, 12, 994155. [Google Scholar]
- Tofani, L.B.; Abriata, J.P.; Luiz, M.T.; Marchetti, J.M.; Swiech, K. Establishment and characterization of an in vitro 3D ovarian cancer model for drug screening assays. Biotechnol. Prog. 2020, 36, e3034. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Russo, A.; Vidal, G.; Demange, E.; Pannetier, P.; Souguir, Z.; Lagarde, J.-M.; Ducommun, B.; Lobjois, V. Evaluation by quantitative image analysis of anticancer drug activity on multicellular spheroids grown in 3D matrices. Oncol. Lett. 2016, 12, 4371–4376. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.; Gage, J.A.; Shen, T.; Haisler, W.L.; Neeley, S.K.; Shiao, S.; Chen, J.; Desai, P.K.; Liao, A.; Hebel, C.; et al. A spheroid toxicity assay using magnetic 3D bioprinting and real-time mobile device-based imaging. Sci. Rep. 2015, 5, 13987. [Google Scholar] [CrossRef]
- Mittler, F.; Obeïd, P.; Rulina, A.V.; Haguet, V.; Gidrol, X.; Balakirev, M.Y. High-content monitoring of drug effects in a 3D spheroid model. Front. Oncol. 2017, 7, 293. [Google Scholar] [CrossRef] [PubMed]
- Baek, N.; Seo, O.W.; Lee, J.; Hulme, J.; An, S.S.A. Real-time monitoring of cisplatin cytotoxicity on three-dimensional spheroid tumor cells. Drug Design. Dev. Ther. 2016, 10, 2155–2165. [Google Scholar]
- Thakuri, P.S.; Luker, G.D.; Tavana, H. Cyclical treatment of colorectal tumor spheroids induces resistance to MEK inhibitors. Transl. Oncol. 2019, 12, 404–416. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Seo, T.S. A 3D printed size-tunable flow-focusing droplet microdevice to produce cell-laden hydrogel microspheres. Anal. Chim. Acta 2022, 1192, 339344. [Google Scholar] [CrossRef]
- Tu, V.T.K.; Le, H.T.N.; To, X.H.V.; Nguyen, P.D.N.; Huynh, P.D.; Le, T.M.; Vu, N.B. Method for in vitro production of cartilage microtissues from scaffold-free spheroids composed of human adipose-derived Stem Cells. Biomed. Res. Ther. 2020, 7, 3697–3708. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncotarget 2016, 7, 45745–45756. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, J.; Miao, Y.; Sun, Z.; Zhang, Q. Effects of microvesicles on cell apoptosis under hypoxia. Oxidative Med. Cell. Longev. 2019, 2019, 5972152. [Google Scholar] [CrossRef]
- Baniahmad, A. Tumor spheroids and organoids as preclinical model systems. Med. Genet. 2021, 33, 229–234. [Google Scholar] [CrossRef]
- Martincuks, A.; Li, P.C.; Zhao, Q.; Zhang, C.; Li, Y.J.; Yu, H.; Rodriguez-Rodriguez, L. CD44 in ovarian cancer progression and therapy resistance—A critical role for STAT3. Front. Oncol. 2020, 10, 589601. [Google Scholar] [CrossRef] [PubMed]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Niu, M.; Yuan, X.; Wu, K.; Liu, A. CD44 as a tumor biomarker and therapeutic target. Exp. Hematol. Oncol. 2020, 9, 36. [Google Scholar] [CrossRef]
- Porres, J.M.; Wu, M.; Cheng, W.H. Legumes, Genome Maintenance, and Optimal Health. In Bioactive Food as Dietary Interventions for the Aging Population; Elsevier: Amsterdam, The Netherlands, 2013; pp. 321–334. [Google Scholar]
- Choe, C.; Kim, H.; Min, S.; Park, S.; Seo, J.; Roh, S. SOX2, a stemness gene, induces progression of NSCLC A549 cells toward anchorage-independent growth and chemoresistance to vinblastine. OncoTargets Ther. 2018, 11, 6197–6207. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.C.; Shi, L.H.; Wang, X.J.; Wang, S.X.; Wan, X.Q.; Liu, S.R.; Wang, Y.F.; Lu, Z.; Wang, L.H.; Ding, Y. STAT3/Oct-4/c-Myc Signal Circuit for regulating stemness-mediated doxorubicin resistance of triple-negative breast cancer cells and inhibitory effects of WP1066. Int. J. Oncol. 2018, 53, 339–348. [Google Scholar] [CrossRef]
- Park, S.B.; Seo, K.W.; So, A.Y.; Seo, M.S.; Yu, K.R.; Kang, S.K.; Kang, K.S. SOX2 has a crucial role in the lineage determination and proliferation of mesenchymal stem cells through dickkopf-1 and c-myc. Cell Death Differ. 2012, 19, 534–545. [Google Scholar] [CrossRef]
- Kuo, H.Y.; Hsu, H.T.; Chen, Y.C.; Chang, Y.W.; Liu, F.T.; Wu, C.W. Galectin-3 modulates the EGFR signalling-mediated regulation of SOX2 expression via c-myc in lung cancer. Glycobiology 2016, 26, 155–165. [Google Scholar] [CrossRef]
- Park, M.; Kim, M. Analysis of antioxidant and anti-inflammatory activities of solvent fractions from Rhynchosia nulubilis cultivated with Ganoderma lucidum mycelium. Prev. Nutr. Food Sci. 2017, 22, 365–371. [Google Scholar] [CrossRef]
- Moritz, M.; Marostica, L.; Bianco, É.; Almeida, M.; Carraro, J.; Cabrera, G.; Palermo, J.; Simões, C.; Schenkel, E. Polyoxygenated steroids from the octocoral Leptogorgia Punicea and in vitro evaluation of their cytotoxic activity. Mar. Drugs 2014, 12, 5864–5880. [Google Scholar] [CrossRef]
- Yong, W.K.; Ho, Y.F.; Abd Malek, S.N. Xanthohumol induces apoptosis and S phase cell cycle arrest in A549 non-small cell lung cancer cells. Pharmacogn. Mag. 2015, 11, S275–S283. [Google Scholar] [PubMed]
- Rani, V.S.; Bindu, R.N. GC-MS analysis of ethyl acetate extract of Eleutherine bulbosa (Urban) Miller (iridaceae). Int. J. Pharm. Sci. Res. 2016, 7, 1729–1733. [Google Scholar]
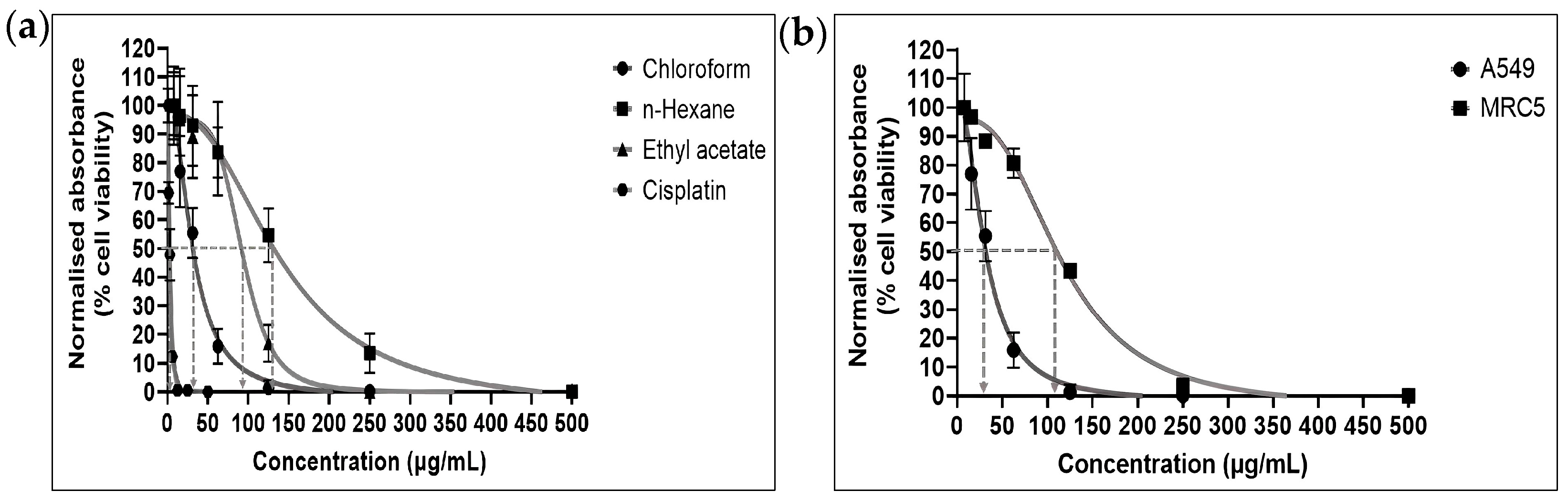
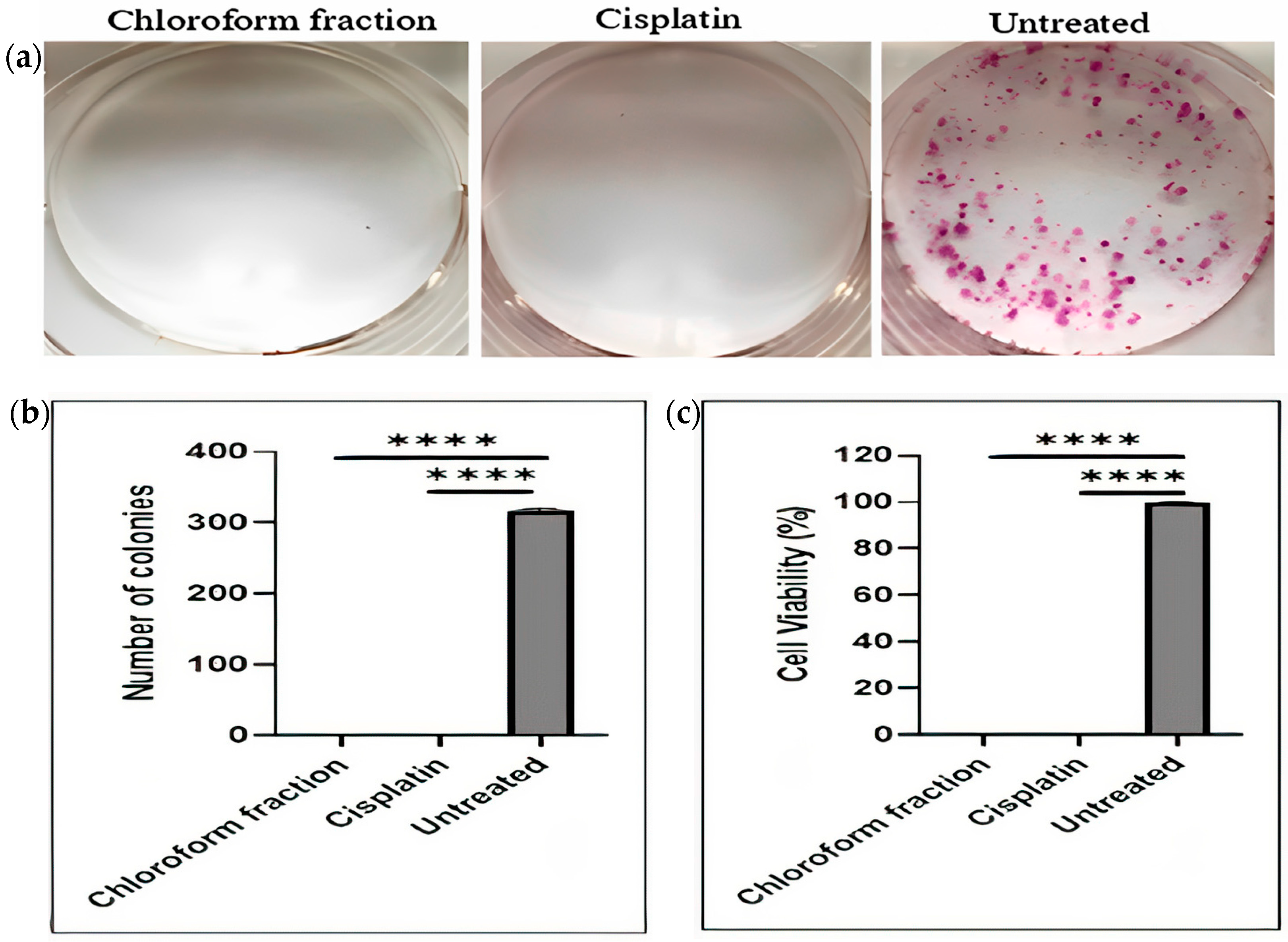
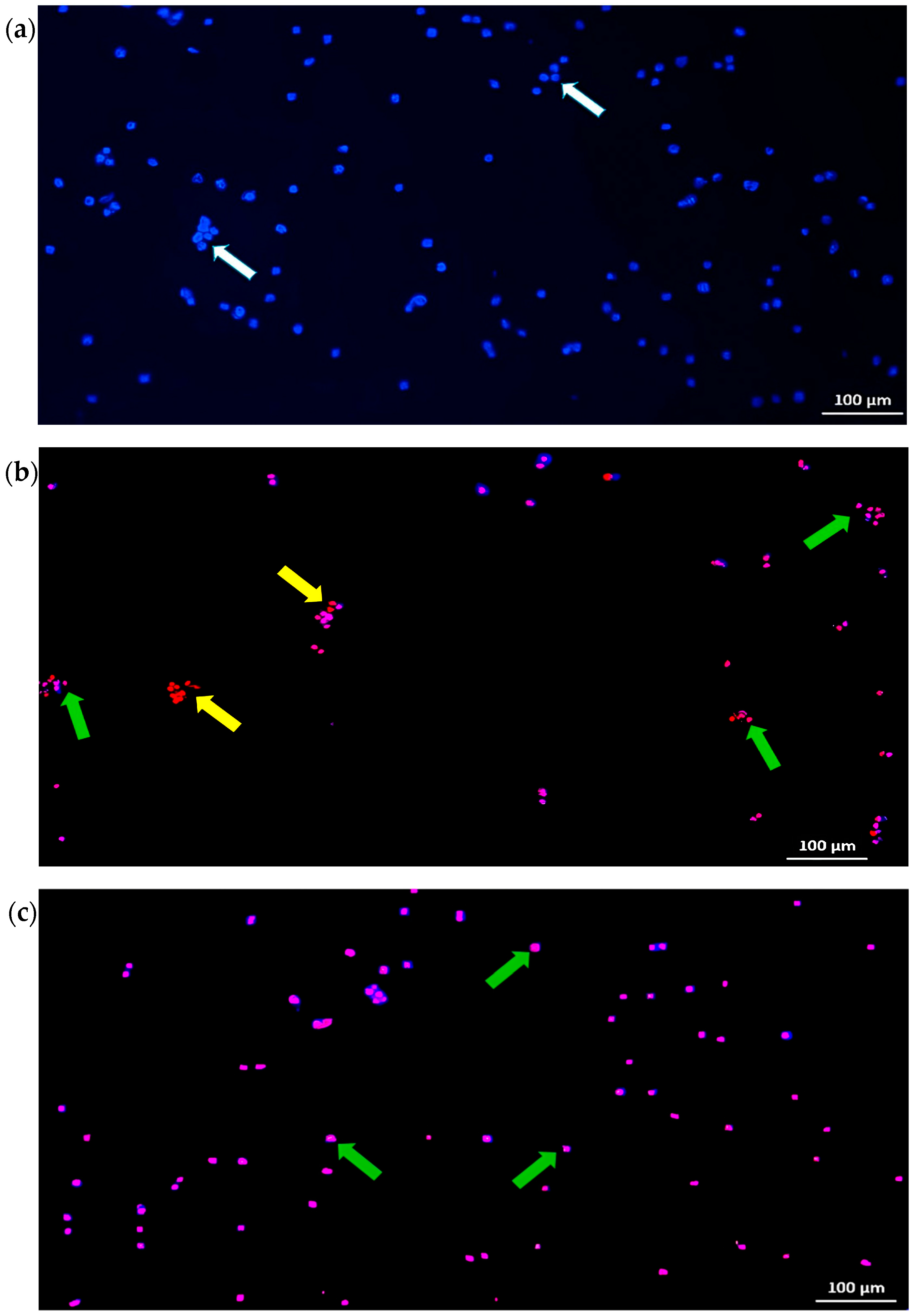
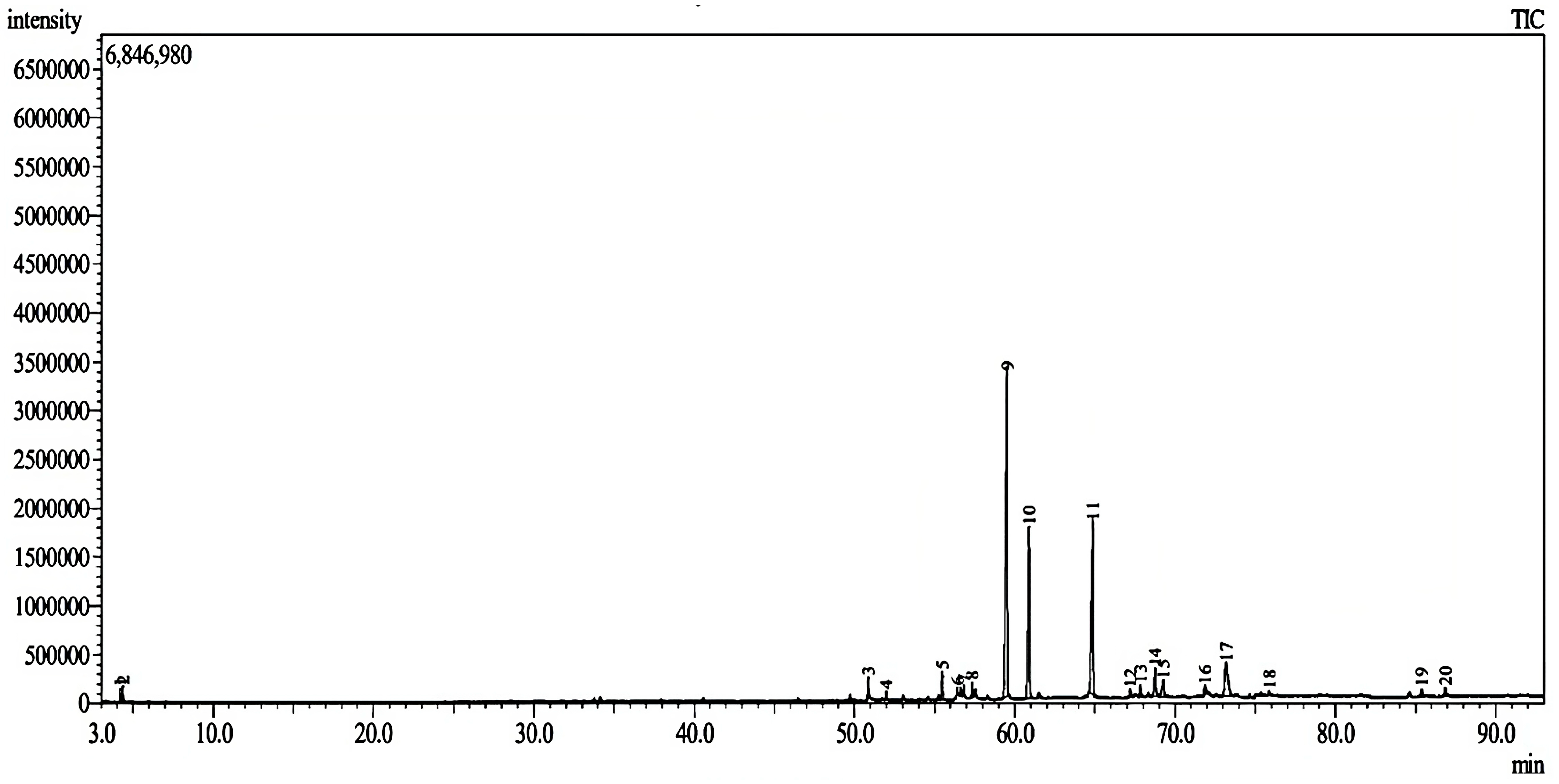

| Fraction | IC50 (μg/mL) | Selectivity Index (SI) | |
|---|---|---|---|
| A549 Cells | MRC-5 Cells | ||
| Chloroform | 30.01 ± 2.14 | 102.2 ± 1.78 | 3.4 |
| n-Hexane | 126.60 ± 4.15 | - | - |
| Ethyl acetate | 83.44 ± 2.14 | - | - |
| Cisplatin | 2.61 ± 0.09 | - | - |
| No | Retention Time (min) | Compound Name | Molecular Formula | Molecular Weight (MW) | Peak Area (%) | Similarity Index (%) |
|---|---|---|---|---|---|---|
| 1 | 4.1663 | 2,3-butanediol | C4H10O2 | 90 | 0.38 | 97 |
| 2 | 4.3425 | 2,3-butanediol, [R-R *, R *] | C4H10O2 | 90 | 0.47 | 97 |
| 3 | 50.8417 | n-hexadecanoic acid | C16H32O2 | 256 | 1.49 | 94 |
| 4 | 51.9708 | Hexadecanoic acid, ethyl ester | C18H36O2 | 284 | 0.42 | 78 |
| 5 | 55.4617 | Unknown | N/A * | N/A | 2.36 | N/A |
| 6 | 56.3982 | 9,12-octadecadienoic acid | C18H32O2 | 280 | 0.56 | 83 |
| 7 | 56.8070 | Benzeneethanal, 4-[1,1-dimethylethyl] | C12H16O | 176 | 0.78 | 83 |
| 8 | 57.3080 | 14-methyl-8-hexadecyn-1-ol | C17H32O | 252 | 1.02 | 89 |
| 9 | 59.5095 | Unknown | N/A | N/A | 36.69 | N/A |
| 10 | 60.8740 | Unknown | N/A | N/A | 17.25 | N/A |
| 11 | 64.8472 | Propanedinitrile, [(3,4,5-trimethoxyphenyl) methylene] | C13H12N2O3 | 244 | 20.92 | 78 |
| 12 | 67.1923 | Hexadecanoic acid, 2-hydroxy-1-(hydroxyethyl) ethyl ester | C19H38O4 | 330 | 0.65 | 83 |
| 13 | 67.8215 | Unknown | N/A | N/A | 1.02 | N/A |
| 14 | 68.7435 | Unknown | N/A | N/A | 2.91 | N/A |
| 15 | 69.2533 | Unknown | N/A | N/A | 2.42 | N/A |
| 16 | 71.8457 | Ethyl linoleate | C20H36O2 | 308 | 0.97 | 81 |
| 17 | 73.1697 | Unknown | N/A | N/A | 7.49 | N/A |
| 18 | 75.8435 | Unknown | N/A | N/A | 0.42 | N/A |
| 19 | 85.3685 | Stigma sterol | C29H48O | 412 | 0.83 | 80 |
| 20 | 86.8377 | Stigmast-5-en-3-ol, (3.beta.,24s)-(CAS) clionasterol | C29H50O | 414 | 0.95 | 84 |
| Cell Line | Average Size of Spheroid (μm) (Mean ± SD) | |
|---|---|---|
| Untreated | Treated | |
| A549 | 492.36 ± 10.62 | 422.58 ± 9.43 |
| Genes | Accession Number | 5′-3′ Sequence | |
|---|---|---|---|
| GAPDH (control) | NM_002046.7 | Forward Reverse | GTCATCCCTGAGCTGAACGG CCACCTGGTGCTCAGTGTAG |
| MYC | NM_002467.6 | Forward Reverse | CATCAGCACAACTACGCAGC GCTGGTGCATTTTCGGTTGT |
| SOX2 | NM_003106.4 | Forward Reverse | GCCCTGCAGTACAACTCCAT GACTTGACCACCGAACCCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakaria, N.H.; Saad, N.; Che Abdullah, C.A.; Mohd. Esa, N. The Antiproliferative Effect of Chloroform Fraction of Eleutherine bulbosa (Mill.) Urb. on 2D- and 3D-Human Lung Cancer Cells (A549) Model. Pharmaceuticals 2023, 16, 936. https://doi.org/10.3390/ph16070936
Zakaria NH, Saad N, Che Abdullah CA, Mohd. Esa N. The Antiproliferative Effect of Chloroform Fraction of Eleutherine bulbosa (Mill.) Urb. on 2D- and 3D-Human Lung Cancer Cells (A549) Model. Pharmaceuticals. 2023; 16(7):936. https://doi.org/10.3390/ph16070936
Chicago/Turabian StyleZakaria, Nur Hannan, Norazalina Saad, Che Azurahanim Che Abdullah, and Norhaizan Mohd. Esa. 2023. "The Antiproliferative Effect of Chloroform Fraction of Eleutherine bulbosa (Mill.) Urb. on 2D- and 3D-Human Lung Cancer Cells (A549) Model" Pharmaceuticals 16, no. 7: 936. https://doi.org/10.3390/ph16070936
APA StyleZakaria, N. H., Saad, N., Che Abdullah, C. A., & Mohd. Esa, N. (2023). The Antiproliferative Effect of Chloroform Fraction of Eleutherine bulbosa (Mill.) Urb. on 2D- and 3D-Human Lung Cancer Cells (A549) Model. Pharmaceuticals, 16(7), 936. https://doi.org/10.3390/ph16070936







