Peptide-Based Vaccine against Breast Cancer: Recent Advances and Prospects
Abstract
1. Introduction
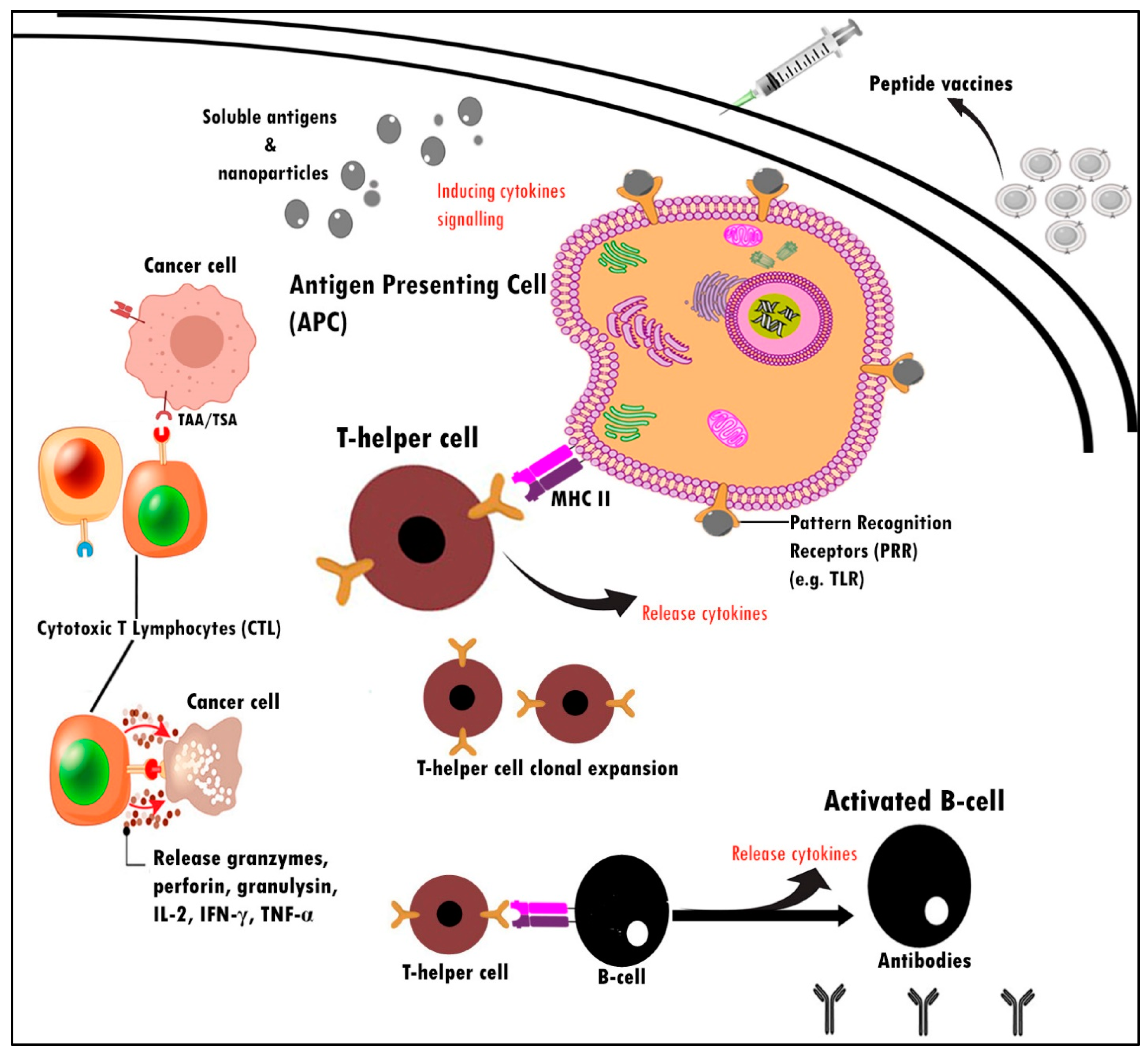
2. Cancer Vaccines
3. Peptide-Based Vaccine and Key Regulator in Breast Cancer Immunogenicity
4. Identified Tumor-Associated Antigens in Peptides Vaccine Development for Breast Cancer
4.1. HER2
4.2. MUC-1
4.3. EphA
4.4. Survivin
4.5. SART3
4.6. CEA
4.7. p53
4.8. WT1
5. Strategies to Improve the Immunogenicity of Peptide-Based Breast Cancer Vaccines
5.1. Multi-Epitope Peptide Vaccine Antigens
5.2. Immunostimulatory Adjuvants
5.2.1. Toll-Like Receptors (TLRs) Ligands Based
5.2.2. Granulocyte Macrophage-Colony Stimulating Factor (GM-CSF)
5.2.3. Keyhole Limpet Hemocyanin (KLH)
6. Selection of Main TAA-Derived Peptide Antigens
| Peptides | Mechanism of Action | Types of Study | Results | Ref. |
|---|---|---|---|---|
| GP2 Peptide | Stimulates helper T cells, cytotoxic T lymphocytes, and antibodies | In vivo study in xenograft mice using TUBO cells | GP2 peptide alone did not have a significant therapeutic and prophylactic effect in mice | [143] |
| Peptide I-6 | Targets MAGE-1 on breast cancer, thus, inducing the antitumor effect from CTLs | In vitro study: MDA-MB-231 cells In vivo study: MCF-7 cells | I-6 induced cytotoxic activity against MDA-MB-231 cells by activating CD8þ T lymphocytes | [32] |
| P5 peptide (HER-2 derived peptide) | P5 peptide releases a high amount of IFN-γ and IL-10, therefore, inducing a potent CTL immune response | In vivo: induce TUBO cells in BALB/c mice | P5 peptide conjugated with maleimide-PEG2000-DSPE incorporation into liposomes stimulate immunogenicity and anti-tumour activities more potent than P5 peptide alone | [109] |
| MUC1-specific peptide vaccine sequence APGSTAPPA and SAPDTRPAP | The peptide induces IFN-γ-producing T cells | In vitro MTag cell lines In vivo: Mammary gland tumors from PyV MT mice PyV MT mice | Immunosuppression within the tumor microenvironment hinders the immune response to anti-cancer vaccines | [144] |
| E75 Peptide, also known as p369 peptide | Ability to bind specific CD8+ TL clones that could lyse HER2-positive tumor cells | In vitro breast cancer cell lines; MCF-7, MDA-MB-231 In vivo mice model Clinical trial | Two Phase-II clinical trials on patients resulted in remission after breast cancer but were considered at high risk of recurrence. | [52,145,146,147] |
| p5 HER-2/neu derived peptide | Induce a high level of CD8+ CTL, which is capable of killing tumor cells via recognizing the TAAs epitopes presented on the surface of cancer cells in association with MHC I molecules. | In vitro: TUBO cell In vivo: Female BALB/c mice were subcutaneously administered at the right flank | Free p5 peptide showed weak antitumor and CTLs response activities compared to Liposome–DOPE–p5 + CpG-ODN formulation | [65] |
| Long peptide (conjugating SU18 peptide with SU22 peptide using glycine linker) | The long peptides (containing T helper and killer epitope) targeted the overexpression of Survivin antigens in breast cancer cells. | Clinical trial (Phase 1). The vaccine was given every two weeks for 4 times. | A customized peptide with multiple epitopes and containing a long sequence of amino acids provide superior and innovative cancer vaccine designs, which are capable of inducing both Th1 and Th2 immune responses in cancer patients. | [148] |
| Agent | Phase | Adjuvant | Enrolment | Regime of Treatment | Ref. |
|---|---|---|---|---|---|
| NeuVax™ (Nelipepimut-S or E75) | III | Leukine® [sargramostim, GM-CSF] | 758 patients | Once a month, for six consecutive months, and then booster for every six months total of 36 months | [52] |
| HER-2/neu ECD & ICD Peptides | I | Granulocyte-macrophage colony-stimulating factor (GM-CSF) | 8 patients | Once a month for 2–6 months, intradermally | [149] |
| Folate Receptor Alpha (FRα) peptide vaccine | II | GM-CSF | 80 patients | Single ID administration—monthly vaccinations repeated six times, followed by boosters every six months until recurrence. | [150,151,152] |
| MUC-1 peptide vaccine | I | poly-ICLC | 29 patients | Subcutaneous (SC) injection in weeks 0, 2, and 10. | [153] |
| AE37 Peptide Vaccine | II | GMCSF | 600 patients | Intradermally (ID) injection every 3–4 weeks for a total of up to 6 inoculations followed up every 3 months for the first 2 years. | [154] |
| E39 and J65 peptide vaccine | I | GMCSF | 39 patients | Receive six monthly injections of peptide + GM-CSF booster inoculation within 1–2 weeks of their 6-month period | [155] |
| hTERT/Survivin Multi-Peptide Vaccine | 1 | - | 11 patients | Receive subcutaneous injection every two weeks four times, then monthly up to 28 vaccinations, then every six months | [156] |
| WT1 peptide-based | I | Montanide ISA51 | 2 patients | Receive WT1 peptide intradermally three times at 2-week intervals | [157,158] |
| Ii-Key hybrid HER-2/neu peptide (AE37) vaccine | I | GM-CSF | 15 patients | Receive vaccine via intradermal injection for six months | [159] |
7. Nanoparticles as Peptide Vaccine Delivery Platform
8. Future Direction: Rational Vaccine Design
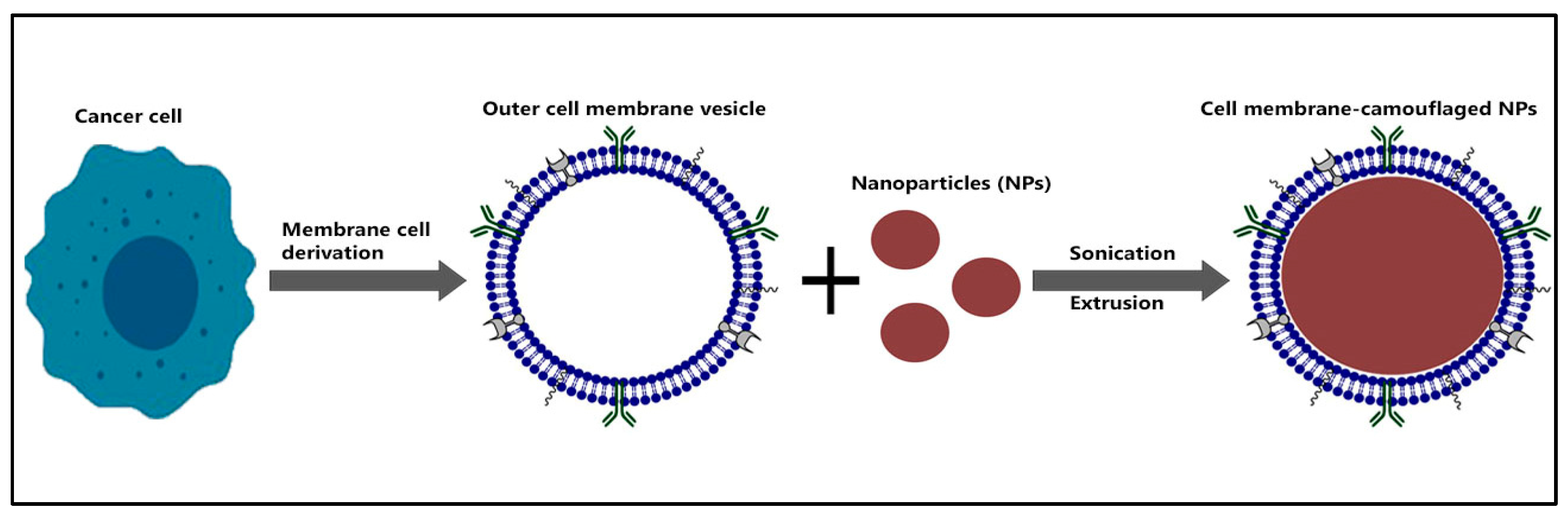
8.1. Biomimetic Nano-Peptide Vaccine
8.2. Combining Immune Checkpoint Blockade Agents with Peptide-Based Vaccine
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Breast Cancer: Prevention and Control. Available online: https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/ (accessed on 10 December 2020).
- Reddington, R.; Galer, M.; Hagedorn, A.; Liu, P.; Barrack, S.; Husain, E.; Sharma, R.; Speirs, V.; Masannat, Y. Incidence of Male Breast Cancer in Scotland over a Twenty-Five-Year Period (1992–2017). Eur. J. Surg. Oncol. 2020, 46, 1546–1550. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.H. Breast Cancer in Men. N. Engl. J. Med. 2018, 378, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, P.; Pensabene, M.; Milano, M.; Arpino, G.; Giuliano, M.; Forestieri, V.; Condello, C.; Lauria, R.; De Placido, S. Long-Term Survival and BRCA Status in Male Breast Cancer: A Retrospective Single-Center Analysis. BMC Cancer 2016, 16, 375. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, S.; Heller, S.L.; Babb, J.S.; Gao, Y. Male Breast Cancer Risk Assessment and Screening Recommendations in High-Risk Men Who Undergo Genetic Counseling and Multigene Panel Testing. Clin. Breast Cancer 2021, 21, e74–e79. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Shah, R.; Rosso, K.; David Nathanson, S. Pathogenesis, Prevention, Diagnosis and Treatment of Breast Cancer. World J. Clin. Oncol. 2014, 5, 283–298. [Google Scholar] [CrossRef]
- Ly, D.; Forman, D.; Ferlay, J.; Brinton, L.A.; Cook, M.B. An International Comparison of Male and Female Breast Cancer Incidence Rates. Int. J. Cancer 2013, 132, 1918–1926. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rahman, T. The Difficulties in Cancer Treatment. Ecancermedicalscience 2012, 6, ed16. [Google Scholar] [CrossRef]
- Ekici, S.; Jawzal, H. Breast Cancer Diagnosis Using Thermography and Convolutional Neural Networks. Med. Hypotheses 2020, 137, 109542. [Google Scholar] [CrossRef]
- Sánchez-Bermúdez, A.I.; Sarabia-Meseguer, M.D.; García-Aliaga, A.; Marín-Vera, M.; Macías-Cerrolaza, J.A.; Henaréjos, P.S.; Guardiola-Castillo, V.; la Peña, F.A.-D.; Alonso-Romero, J.L.; Noguera-Velasco, J.A.; et al. Mutational Analysis of RAD51C and RAD51D Genes in Hereditary Breast and Ovarian Cancer Families from Murcia (Southeastern Spain). Eur. J. Med. Genet. 2018, 61, 355–361. [Google Scholar] [CrossRef]
- Karami, F.; Mehdipour, P. A Comprehensive Focus on Global Spectrum of BRCA1 and BRCA2 Mutations in Breast Cancer. Biomed. Res. Int. 2013, 2013, 928562. [Google Scholar] [CrossRef]
- Ayala de la Peña, F.; Andrés, R.; Garcia-Sáenz, J.A.; Manso, L.; Margelí, M.; Dalmau, E.; Pernas, S.; Prat, A.; Servitja, S.; Ciruelos, E. SEOM Clinical Guidelines in Early Stage Breast Cancer (2018). Clin. Transl. Oncol. 2019, 21, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Suter, R.; Marcum, J.A. The Molecular Genetics of Breast Cancer and Targeted Therapy. Biologics 2007, 1, 241–258. [Google Scholar] [PubMed]
- Townsend, D.M.; Tew, K.D. The Role of Glutathione-S-Transferase in Anti-Cancer Drug Resistance. Oncogene 2003, 22, 7369–7375. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.; Ahmad, A. Epigenetic Basis of Cancer Drug Resistance. Cancer Drug Resist. 2020, 3, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel Immune Checkpoint Targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef]
- Ji, X.; Lu, Y.; Tian, H.; Meng, X.; Wei, M.; Cho, W.C. Chemoresistance Mechanisms of Breast Cancer and Their Countermeasures. Biomed. Pharmacother. 2019, 114, 108800. [Google Scholar] [CrossRef]
- Crespo, I.; Götz, L.; Liechti, R.; Coukos, G.; Doucey, M.A.; Xenarios, I. Identifying Biological Mechanisms for Favorable Cancer Prognosis Using Non-Hypothesis-Driven Iterative Survival Analysis. NPJ Syst. Biol. Appl. 2016, 2, 16037. [Google Scholar] [CrossRef]
- Anfray, C.; Ummarino, A.; Andón, F.T.; Allavena, P. Current Strategies to Target Tumor-Associated-Macrophages to Improve Anti-Tumor Immune Responses. Cells 2020, 9, 46. [Google Scholar] [CrossRef]
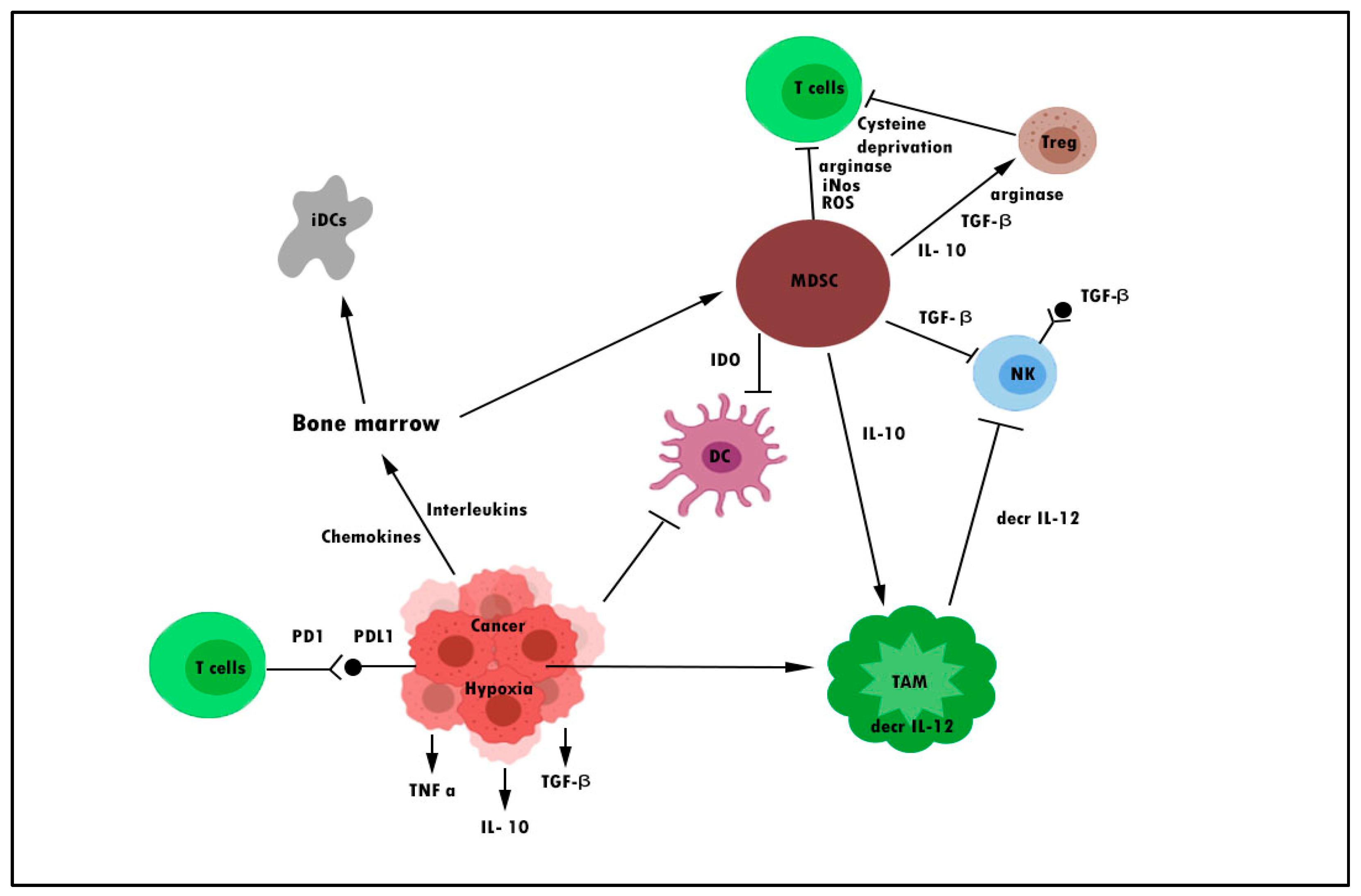
- Davis, R.J.; Van Waes, C.; Allen, C.T. Overcoming Barriers to Effective Immunotherapy: MDSCs, TAMs, and Tregs as Mediators of the Immunosuppressive Microenvironment in Head and Neck Cancer. Oral Oncol. 2016, 58, 59–70. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-Associated Macrophages in Tumor Metastasis: Biological Roles and Clinical Therapeutic Applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef]
- Schmitt, N.; Bustamante, J.; Bourdery, L.; Bentebibel, S.E.; Boisson-Dupuis, S.; Hamlin, F.; Tran, M.V.; Blankenship, D.; Pascual, V.; Savino, D.A.; et al. IL-12 Receptor Β1 Deficiency Alters in Vivo T Follicular Helper Cell Response in Humans. Blood 2013, 121, 3375–3385. [Google Scholar] [CrossRef]
- Lindau, D.; Gielen, P.; Kroesen, M.; Wesseling, P.; Adema, G.J. The Immunosuppressive Tumour Network: Myeloid-Derived Suppressor Cells, Regulatory T Cells and Natural Killer T Cells. Immunology 2013, 138, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yao, M.; Niu, C.; Liu, D.; Tang, Z.; Gu, C.; Zhao, H.; Ke, J.; Wu, S.; Wang, X.; et al. Inhibition of MCF-7 Breast Cancer Cell Proliferation by a Synthetic Peptide Derived from the C-Terminal Sequence of Orai Channel. Biochem. Biophys. Res. Commun. 2019, 516, 1066–1072. [Google Scholar] [CrossRef]
- Gatti-Mays, M.E.; Balko, J.M.; Gameiro, S.R.; Bear, H.D.; Prabhakaran, S.; Fukui, J.; Disis, M.L.; Nanda, R.; Gulley, J.L.; Kalinsky, K.; et al. If We Build It They Will Come: Targeting the Immune Response to Breast Cancer. NPJ Breast Cancer 2019, 5, 37. [Google Scholar] [CrossRef]
- Gun, S.Y.; Lee, S.W.L.; Sieow, J.L.; Wong, S.C. Targeting Immune Cells for Cancer Therapy. Redox Biol. 2019, 25, 101174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The History and Advances in Cancer Immunotherapy: Understanding the Characteristics of Tumor-Infiltrating Immune Cells and Their Therapeutic Implications. Cell Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; Viale, G.; Curigliano, G. Peptide Vaccines in Early Breast Cancer. Breast 2019, 44, 128–134. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Yamaue, H.; Okusaka, T.; Okuno, K.; Suzuki, H.; Fujioka, T.; Otsu, A.; Ohashi, Y.; Shimazawa, R.; Nishio, K.; et al. Guidance for Peptide Vaccines for the Treatment of Cancer. Cancer Sci. 2014, 105, 924–931. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA J. Am. Med. Assoc. 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Shi, W.; Qiu, Q.; Tong, Z.; Guo, W.; Zou, F.; Feng, Z.; Wang, Y.; Huang, W.; Qian, H. Synthetic Tumor-Specific Antigenic Peptides with a Strong Affinity to HLA-A2 Elicit Anti-Breast Cancer Immune Response through Activating CD8+ T Cells. Eur. J. Med. Chem. 2020, 189, 112051. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Choi, Y.; Kim, J. Mesoporous Silica as a Versatile Platform for Cancer Immunotherapy. Adv. Mater. 2019, 31, e1803953. [Google Scholar] [CrossRef]
- De Temmerman, M.L.; Rejman, J.; Demeester, J.; Irvine, D.J.; Gander, B.; De Smedt, S.C. Particulate Vaccines: On the Quest for Optimal Delivery and Immune Response. Drug Discov. Today 2011, 16, 569–582. [Google Scholar] [CrossRef]
- Nejad, A.E.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Javanmard, S.H.; Taherian, M.; Ahmadlou, M.; et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: A novel approach to developing treatment. Cancer Cell Intl. 2021, 21, 62. [Google Scholar] [CrossRef]
- Solinas, C.; Aiello, M.; Migliori, E.; Willard-Gallo, K.; Emens, L.A. Breast cancer vaccines: Heeding the lessons of the past to guide a path forward. Cancer Treat. Rev. 2020, 84, 101947. [Google Scholar] [CrossRef] [PubMed]
- de Paula Peres, L.; da Luz, F.A.C.; dos Anjos Pultz, B.; Brígido, P.C.; de Araújo, R.A.; Goulart, L.R.; Silva, M.J.B. Peptide Vaccines in Breast Cancer: The Immunological Basis for Clinical Response. Biotechnol. Adv. 2015, 33, 1868–1877. [Google Scholar] [CrossRef]
- Criscitiello, C. Tumor-Associated Antigens in Breast Cancer. Breast Care 2012, 7, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Thundimadathil, J. Cancer Treatment Using Peptides: Current Therapies and Future Prospects. J. Amino Acids 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of Antigen Processing. Annu. Rev. Immunol. 2013, 31, 443–473. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Lindstrom, A.R.; Lin, T.Y.; Lam, K.S.; Li, Y. Peptide-Based Materials for Cancer Immunotherapy. Theranostics 2019, 9, 7807–7825. [Google Scholar] [CrossRef]
- Santoni, D. Viral Peptides-MHC Interaction: Binding Probability and Distance from Human Peptides. J. Immunol. Methods 2018, 459, 35–43. [Google Scholar] [CrossRef]
- Jones, N.D.; Van Maurik, A.; Hara, M.; Spriewald, B.M.; Witzke, O.; Morris, P.J.; Wood, K.J. CD40-CD40 Ligand-Independent Activation of CD8+ T Cells Can Trigger Allograft Rejection. J. Immunol. 2000, 165, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Tay, N.Q.; Lee, D.C.P.; Chua, Y.L.; Prabhu, N.; Gascoigne, N.R.J.; Kemeny, D.M. CD40L Expression Allows CD8+ T Cells to Promote Their Own Expansion and Differentiation through Dendritic Cells. Front. Immunol. 2017, 8, 1484. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Tang, L.F.M.; Lew, F.C.; Wong, H.S.K.; Chua, Y.L.; MacAry, P.A.; Kemeny, D.M. CD44high Memory CD8 T Cells Synergize with CpG DNA to Activate Dendritic Cell IL-12p70 Production. J. Immunol. 2009, 183, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Shiku, H. Immunotherapy of Solid Tumor: Perspectives on Vaccine and Cell Therapy. Nihon Rinsho 2012, 70, 2043–2050. [Google Scholar]
- Costa, R.L.B.; Czerniecki, B.J. Clinical Development of Immunotherapies for HER2+ Breast Cancer: A Review of HER2-Directed Monoclonal Antibodies and Beyond. NPJ Breast Cancer 2020, 6, 10. [Google Scholar] [CrossRef]
- Lyu, H.; Huang, J.; He, Z.; Liu, B. Epigenetic Mechanism of Survivin Dysregulation in Human Cancer. Sci. China Life Sci. 2018, 61, 808–814. [Google Scholar] [CrossRef]
- Genitsch, V.; Zlobec, I.; Thalmann, G.N.; Fleischmann, A. MUC1 Is Upregulated in Advanced Prostate Cancer and Is an Independent Prognostic Factor. Prostate Cancer Prostatic. Dis. 2016, 19, 242–247. [Google Scholar] [CrossRef]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef]
- Kim, I.; Sanchez, K.; McArthur, H.L.; Page, D. Immunotherapy in Triple-Negative Breast Cancer: Present and Future. Curr. Breast Cancer Rep. 2019, 11, 259–271. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Lu, B.; Melisko, M.; Hiller, J.P.; Bondarenko, I.; Brunt, A.M.; Sergii, G.; Petrakova, K.; Peoples, G.E. Efficacy and Safety Analysis of Nelipepimut-S Vaccine to Prevent Breast Cancer Recurrence: A Randomized, Multicenter, Phase III Clinical Trial. Clin. Cancer Res. 2019, 25, 4248–4254. [Google Scholar] [CrossRef] [PubMed]
- Jackson Doreen, O.; Francois, T.A.; Travis, C.G.; Vreeland Timothy, J.; Peace Kaitlin, M.; Hale Diane, F.; Litton Jennifer, K.; Murray James, L.; Perez Sonia, A.; Michael, P.; et al. Effects of HLA Status and HER2 Status on Outcomes in Breast Cancer Patients at Risk for Recurrence—Implications for Vaccine Trial Design. Clin. Immunol. 2018, 195, 28–35. [Google Scholar] [CrossRef]
- Patil, R.; Clifton, G.T.; Holmes, J.P.; Amin, A.; Carmichael, M.G.; Gates, J.D.; Benavides, L.H.; Hueman, M.T.; Ponniah, S.; Peoples, G.E. Clinical and Immunologic Responses of HLA-A3+ Breast Cancer Patients Vaccinated with the HER2/Neu-Derived Peptide Vaccine, E75, in a Phase I/II Clinical Trial. J. Am. Coll. Surg. 2010, 210, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Gourley, C.; Thornton, C.; Massie, C.; Prescott, R.J.; Turner, M.; Leonard, R.C.F.; Kilpatrick, D.C. Is There a Relationship between HLA Type and Prognostic Factors in Breast Cancer? Anticancer Res. 2003, 23, 633–638. [Google Scholar]
- Mahjoubin-Tehran, M.; Rezaei, S.; Jalili, A.; Aghaee-Bakhtiari, S.H.; Orafai, H.M.; Jamialahmadi, T.; Sahebkar, A. Peptide Decoys: A New Technology Offering Therapeutic Opportunities for Breast Cancer. Drug Discov. Today 2020, 25, 593–598. [Google Scholar] [CrossRef]
- Farzad, N.; Barati, N.; Momtazi-Borojeni, A.A.; Yazdani, M.; Arab, A.; Razazan, A.; Shariat, S.; Mansourian, M.; Abbasi, A.; Saberi, Z.; et al. P435 HER2/Neu-Derived Peptide Conjugated to Liposomes Containing DOPE as an Effective Prophylactic Vaccine Formulation for Breast Cancer. Artif. Cells Nanomed. Biotechnol. 2019, 47, 664–672. [Google Scholar] [CrossRef]
- Furrer, D.; Sanschagrin, F.; Jacob, S.; Diorio, C. Advantages and Disadvantages of Technologies for HER2 Testing in Breast Cancer Specimens: Table 1. Am. J. Clin. Pathol. 2015, 144, 686–703. [Google Scholar] [CrossRef]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human Breast Cancer: Correlation of Relapse and Survival with Amplification of the HER-2/Neu Oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef]
- Ayoub, N.M.; Al-Shami, K.M.; Yaghan, R.J. Immunotherapy for HER2-Positive Breast Cancer: Recent Advances and Combination Therapeutic Approaches. Breast Cancer Targets Ther. 2019, 11, 53–69. [Google Scholar] [CrossRef]
- Krasniqi, E.; Barchiesi, G.; Pizzuti, L.; Mazzotta, M.; Venuti, A.; Maugeri-Saccà, M.; Sanguineti, G.; Massimiani, G.; Sergi, D.; Carpano, S.; et al. Immunotherapy in HER2-Positive Breast Cancer: State of the Art and Future Perspectives. J. Hematol. Oncol. 2019, 12, 1–26. [Google Scholar] [CrossRef]
- Katzorke, N.; Rack, B.K.; Haeberle, L.; Neugebauer, J.K.; Melcher, C.A.; Hagenbeck, C.; Forstbauer, H.; Ulmer, H.U.; Soeling, U.; Kreienberg, R.; et al. Prognostic Value of HER2 on Breast Cancer Survival. J. Clin. Oncol. 2013, 31, 640. [Google Scholar] [CrossRef]
- Clynes, R.A.; Towers, T.L.; Presta, L.G.; Ravetch, J.V. Inhibitory Fc Receptors Modulate in Vivo Cytoxicity against Tumor Targets. Nat. Med. 2000, 6, 443–446. [Google Scholar] [CrossRef]
- Wang, J.; Xu, B. Targeted Therapeutic Options and Future Perspectives for Her2-Positive Breast Cancer. Signal Transduct. Target. Ther. 2019, 4, 3305. [Google Scholar] [CrossRef]
- Mansourian, M.; Badiee, A.; Jalali, S.A.; Shariat, S.; Yazdani, M.; Amin, M.; Jaafari, M.R. Effective Induction of Anti-Tumor Immunity Using P5 HER-2/Neu Derived Peptide Encapsulated in Fusogenic DOTAP Cationic Liposomes Co-Administrated with CpG-ODN. Immunol. Lett. 2014, 162, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.L.B.; Soliman, H.; Czerniecki, B.J. The Clinical Development of Vaccines for HER2+ Breast Cancer: Current Landscape and Future Perspectives. Cancer Treat. Rev. 2017, 61, 107–115. [Google Scholar] [CrossRef]
- Richman, C.M.; DeNardo, S.J. Systemic Radiotherapy in Metastatic Breast Cancer Using 90Y-Linked Monoclonal MUC-1 Antibodies. Crit. Rev. Oncol. Hematol. 2001, 38, 25–35. [Google Scholar] [CrossRef]
- Nath, S.; Mukherjee, P. MUC1: A Multifaceted Oncoprotein with a Key Role in Cancer Progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef]
- Kovjazin, R.; Horn, G.; Smorodinsky, N.I.; Shapira, M.Y.; Carmon, L. Cell Surface-Associated Anti-MUC1-Derived Signal Peptide Antibodies: Implications for Cancer Diagnostics and Therapy. PLoS ONE 2014, 9, e85400. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayanan, V.; Thompson, P.; Wolfert, M.A.; Buskas, T.; Bradley, J.M.; Pathangey, L.B.; Madsen, C.S.; Cohen, P.A.; Gendler, S.J.; Boons, G.J. Immune Recognition of Tumor-Associated Mucin MUC1 Is Achieved by a Fully Synthetic Aberrantly Glycosylated MUC1 Tripartite Vaccine. Proc. Natl. Acad. Sci. USA 2012, 109, 261–266. [Google Scholar] [CrossRef]
- Xing, P.X.; Michael, M.; Apostolopoulos, V.; Prenzoska, J.; Marshall, C.; Bishop, J.; McKenzie, I.F.C. Phase I Study of Synthetic MUC1 Peptides in Breast Cancer. Int. J. Oncol. 1995, 6, 1283–1289. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Pietersz, G.A.; Tsibanis, A.; Tsikkinis, A.; Drakaki, H.; Loveland, B.E.; Piddlesden, S.J.; Plebanski, M.; Pouniotis, D.S.; Alexis, M.N.; et al. Pilot Phase III Immunotherapy Study in Early-Stage Breast Cancer Patients Using Oxidized Mannan-MUC1 [ISRCTN71711835]. Breast Cancer Res. 2006, 8, R27. [Google Scholar] [CrossRef]
- Jeong, S.; Park, M.J.; Song, W.; Kim, H.S. Current Immunoassay Methods and Their Applications to Clinically Used Biomarkers of Breast Cancer. Clin. Biochem. 2020, 78, 43–57. [Google Scholar] [CrossRef]
- Brantley-Sieders, D.M.; Zhuang, G.; Hicks, D.; Wei, B.F.; Hwang, Y.; Cates, J.M.M.; Coffman, K.; Jackson, D.; Bruckheimer, E.; Muraoka-Cook, R.S.; et al. The Receptor Tyrosine Kinase EphA2 Promotes Mammary Adenocarcinoma Tumorigenesis and Metastatic Progression in Mice by Amplifying ErbB2 Signaling. J. Clin. Investig. 2008, 118, 64–78. [Google Scholar] [CrossRef]
- Gökmen-Polar, Y.; Toroni, R.A.; Hocevar, B.A.; Badve, S.; Zhao, Q.; Shen, C.; Bruckheimer, E.; Kinch, M.S.; Miller, K.D. Dual Targeting of EphA2 and ER Restores Tamoxifen Sensitivity in ER/EphA2-Positive Breast Cancer. Breast Cancer Res. Treat. 2011, 127, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Scarberry, K.E.; Dickerson, E.B.; McDonald, J.F.; Zhang, Z.J. Magnetic Nanoparticle-Peptide Conjugates for in Vitro and in Vivo Targeting and Extraction of Cancer Cells. J. Am. Chem. Soc. 2008, 130, 10258–10262. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.F.; Wang, S.; Billet, S.; Chen, J.F.; Udompholkul, P.; Gambini, L.; Baggio, C.; Tseng, H.R.; Posadas, E.M.; Bhowmick, N.A.; et al. Reduction of Circulating Cancer Cells and Metastases in Breast-Cancer Models by a Potent EphA2-Agonistic Peptide-Drug Conjugate. J. Med. Chem. 2018, 61, 2052–2061. [Google Scholar] [CrossRef]
- Guo, Z.; He, B.; Yuan, L.; Dai, W.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Dual Targeting for Metastatic Breast Cancer and Tumor Neovasculature by EphA2-Mediated Nanocarriers. Int. J. Pharm. 2015, 493, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Jha, K.; Shukla, M.; Pandey, M. Survivin Expression and Targeting in Breast Cancer. Surg. Oncol. 2012, 21, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C. Survivin, Cancer Networks and Pathway-Directed Drug Discovery. Nat. Rev. Cancer. 2008, 8, 61–70. [Google Scholar] [CrossRef]
- Ryan, B.M.; Konecny, G.E.; Kahlert, S.; Wang, H.J.; Untch, M.; Meng, G.; Pegram, M.D.; Podratz, K.C.; Crown, J.; Slamon, D.J.; et al. Survivin Expression in Breast Cancer Predicts Clinical Outcome and Is Associated with HER2, VEGF, Urokinase Plasminogen Activator and PAI-1. Ann. Oncol. 2006, 17, 597–604. [Google Scholar] [CrossRef]
- Tanaka, K.; Kanazawa, T.; Horiuchi, S.; Ando, T.; Sugawara, K.; Takashima, Y.; Seta, Y.; Okada, H. Cytoplasm-responsive nanocarriers conjugated with a functional cell-penetrating peptide for systemic siRNA delivery. Intl. J. Pharm. 2013, 455, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Rodel, F.; Sprenger, T.; Kaina, B.; Liersch, T.; Rodel, C.; Fulda, S.; Hehlgans, S. Survivin as a Prognostic/Predictive Marker and Molecular Target in Cancer Therapy. Curr. Med. Chem. 2012, 19, 3679–3688. [Google Scholar] [CrossRef]
- Garg, H.; Suri, P.; Gupta, J.C.; Talwar, G.P.; Dubey, S. Survivin: A Unique Target for Tumor Therapy. Cancer Cell Int. 2016, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, N.; Murayama, K.; Ishida, H.; Matsunaga, K.; Komiya, S.; Itoh, K.; Yamada, A. Expression of a Newly Defined Tumor-Rejection Antigen SART3 in Musculoskeletal Tumors and Induction of HLA Class I-Restricted Cytotoxic T Lymphocytes by SART3-Derived Peptides. J. Orthop. Res. 2001, 19, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, Y.; Sasatomi, T.; Mine, T.; Isomoto, H.; Shirouzu, K.; Yamana, H.; Imai, N.; Yamada, A.; Katagiri, K.; Muto, A.; et al. Induction of Cellular Immune Responses to Tumor Cells and Peptides in Colorectal Cancer Patients by Vaccination with SART3 Peptides. Clin. Cancer Res. 2001, 7, 3950–3962. [Google Scholar] [PubMed]
- Sherman, E.J.; Mitchell, D.C.; Garner, A.L. The RNA-Binding Protein SART3 Promotes MiR-34a Biogenesis and G1 Cell Cycle Arrest in Lung Cancer Cells. J. Biol. Chem. 2019, 294, 17188–17196. [Google Scholar] [CrossRef]
- Timani, K.A.; Gyorffy, B.; Liu, Y.; Mohammad, K.S.; He, J.J. Tip110/SART3 Regulates IL-8 Expression and Predicts the Clinical Outcomes in Melanoma. Mol. Cancer 2018, 17, 124. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.W. The Roles of Carcinoembryonic Antigen in Liver Metastasis and Therapeutic Approaches. Gastroenterol. Res. Pract. 2017, 2017, 7521987. [Google Scholar] [CrossRef]
- Turriziani, M.; Fantini, M.; Benvenuto, M.; Izzi, V.; Masuelli, L.; Sacchetti, P.; Modesti, A.; Bei, R. Carcinoembryonic Antigen (CEA)-Based Cancer Vaccines: Recent Patents and Antitumor Effects from Experimental Models to Clinical Trials. Recent. Pat. Anticancer Drug Discov. 2012, 7, 265–296. [Google Scholar] [CrossRef]
- Ojima, T.; Iwahashi, M.; Nakamura, M.; Matsuda, K.; Nakamori, M.; Ueda, K.; Naka, T.; Ishida, K.; James Primus, F.; Yamaue, H. Successful Cancer Vaccine Therapy for Carcinoembryonic Antigen (CEA)-Expressing Colon Cancer Using Genetically Modified Dendritic Cells That Express CEA and T Helper-Type 1 Cytokines in CEA Transgenic Mice. Int. J. Cancer 2007, 120, 585–593. [Google Scholar] [CrossRef]
- Gulley, J.L.; Arlen, P.M.; Tsang, K.-Y.; Yokokawa, J.; Palena, C.; Poole, D.J.; Remondo, C.; Cereda, V.; Jones, J.L.; Pazdur, M.P.; et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin. Cancer Res. 2008, 14, 3060–3069. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Guo, P.; McCarthy, C.; Wang, B.; Tao, Y.; Auguste, D. Peptide Density Targets and Impedes Triple Negative Breast Cancer Metastasis. Nat. Commun. 2018, 9, 2612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bergman, J.; Wiman, K.G.; Bykov, V.J.N. Role of Thiol Reactivity for Targeting Mutant P53. Cell Chem. Biol. 2018, 25, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.R.; Joerger, A.C.; Fersht, A.R. 2-Sulfonylpyrimidines: Mild Alkylating Agents with Anticancer Activity toward P53-Compromised Cells. Proc. Natl. Acad. Sci. USA 2016, 113, E5271–E5280. [Google Scholar] [CrossRef] [PubMed]
- Synnott, N.C.; Bauer, M.R.; Madden, S.; Murray, A.; Klinger, R.; O’Donovan, N.; O’Connor, D.; Gallagher, W.M.; Crown, J.; Fersht, A.R.; et al. Mutant P53 as a Therapeutic Target for the Treatment of Triple-Negative Breast Cancer: Preclinical Investigation with the Anti-P53 Drug, PK11007. Cancer Lett. 2018, 414, 99–106. [Google Scholar] [CrossRef]
- Nijman, H.W.; Vermeij, R.; Leffers, N.; Van Der Burg, S.H.; Melief, C.J.; Daemen, T. Immunological and Clinical Effects of Vaccines Targeting P53-Overexpressing Malignancies. J. Biomed. Biotechnol. 2011, 2011, 702146. [Google Scholar] [CrossRef]
- Vijayan, V.; Mohapatra, A.; Uthaman, S.; Park, I.K. Recent Advances in Nanovaccines Using Biomimetic Immunomodulatory Materials. Pharmaceutics 2019, 11, 534. [Google Scholar] [CrossRef]
- Chianese-Bullock, K.A.; Lewis, S.T.; Sherman, N.E.; Shannon, J.D.; Slingluff, C.L. Multi-Peptide Vaccines Vialed as Peptide Mixtures Can Be Stable Reagents for Use in Peptide-Based Immune Therapies. Vaccine 2009, 27, 1764–1770. [Google Scholar] [CrossRef]
- Oka, Y.; Tsuboi, A.; Nakata, J.; Nishida, S.; Hosen, N.; Kumanogoh, A.; Oji, Y.; Sugiyama, H. Wilms’ Tumor Gene 1 (WT1) Peptide Vaccine Therapy for Hematological Malignancies: From CTL Epitope Identification to Recent Progress in Clinical Studies Including a Cure-Oriented Strategy. Oncol. Res. Treat. 2017, 40, 682–690. [Google Scholar] [CrossRef]
- Qi, X.W.; Zhang, F.; Wu, H.; Liu, J.L.; Zong, B.G.; Xu, C.; Jiang, J. Wilms’ Tumor 1 (WT1) Expression and Prognosis in Solid Cancer Patients: A Systematic Review and Meta-Analysis. Sci. Rep. 2015, 5, srep08924. [Google Scholar] [CrossRef]
- Coosemans, A.; Moerman, P.; Verbist, G.; Maes, W.; Neven, P.; Vergote, I.; Van Gool, S.W.; Amant, F. Wilms’ Tumor Gene 1 (WT1) in Endometrial Carcinoma. Gynecol. Oncol. 2008, 111, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, F.; Wang, L.; Zhao, W.; Zhang, D.; Yang, H.; Yu, J.; Niu, L.; Yang, F.; Zheng, S.; et al. Screening and Identification of Non-Inflammatory Specific Protein Markers in Wilms’ Tumor Tissues. Arch. Biochem. Biophys. 2019, 676, 108112. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, N.S.; Uzieblo, A. WT1 Immunoreactivity in Uterine Papillary Serous Carcinomas Is Different from Ovarian Serous Carcinomas. Am. J. Clin. Pathol. 2002, 117, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Iiyama, T.; Udaka, K.; Takeda, S.; Takeuchi, T.; Adachi, Y.C.; Ohtsuki, Y.; Tsuboi, A.; Nakatsuka, S.I.; Elisseeva, O.A.; Oji, Y.; et al. WT1 (Wilms’ Tumor 1) Peptide Immunotherapy for Renal Cell Carcinoma. Microbiol. Immunol. 2007, 51, 519–530. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Valiante, N.M. Recent Advances in the Discovery and Delivery of Vaccine Adjuvants. Nat. Rev. Drug Discov. 2003, 2, 727–735. [Google Scholar] [CrossRef]
- Ghaffari-Nazari, H.; Tavakkol-Afshari, J.; Jaafari, M.R.; Tahaghoghi-Hajghorbani, S.; Masoumi, E.; Jalali, S.A. Improving Multi-Epitope Long Peptide Vaccine Potency by Using a Strategy That Enhances CD4+ T Help in BALB/c Mice. PLoS ONE 2015, 10, e0142563. [Google Scholar] [CrossRef]
- Wu, C.Y.; Monie, A.; Pang, X.; Hung, C.F.; Wu, T.C. Improving Therapeutic HPV Peptide-Based Vaccine Potency by Enhancing CD4+ T Help and Dendritic Cell Activation. J. Biomed. Sci. 2010, 17, 88. [Google Scholar] [CrossRef]
- Zamani, P.; Teymouri, M.; Nikpoor, A.R.; Navashenaq, J.G.; Gholizadeh, Z.; Darban, S.A.; Jaafari, M.R. Nanoliposomal Vaccine Containing Long Multi-Epitope Peptide E75-AE36 Pulsed PADRE-Induced Effective Immune Response in Mice TUBO Model of Breast Cancer. Eur. J. Cancer 2020, 129, 80–96. [Google Scholar] [CrossRef]
- Yazdani, Z.; Rafiei, A.; Yazdani, M.; Valadan, R. Design an Efficient Multi-Epitope Peptide Vaccine Candidate against SARS-CoV-2: An in Silico Analysis. Infect. Drug Resist. 2020, 13, 3007–3022. [Google Scholar] [CrossRef]
- Nezafat, N.; Ghasemi, Y.; Javadi, G.; Khoshnoud, M.J.; Omidinia, E. A Novel Multi-Epitope Peptide Vaccine against Cancer: An in Silico Approach. J. Theor. Biol. 2014, 349, 121–134. [Google Scholar] [CrossRef]
- Bijker, M.S.; van den Eeden, S.J.F.; Franken, K.L.; Melief, C.J.M.; van der Burg, S.H.; Offringa, R. Superior Induction of Anti-Tumor CTL Immunity by Extended Peptide Vaccines Involves Prolonged, DC-Focused Antigen Presentation. Eur. J. Immunol. 2008, 38, 1033–1042. [Google Scholar] [CrossRef]
- Slingluff, C.L. The Present and Future of Peptide Vaccines for Cancer: Single or Multiple, Long or Short, Alone or in Combination? Cancer J. 2011, 17, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, S.; Guerin, T.; Toth, I.; Stephenson, R.J. Recent Advances in Self-Assembled Peptides: Implications for Targeted Drug Delivery and Vaccine Engineering. Adv. Drug Deliv. Rev. 2017, 110–111, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Mohit, E.; Hashemi, A.; Allahyari, M. Breast Cancer Immunotherapy: Monoclonal Antibodies and Peptide-Based Vaccines. Expert Rev. Clin. Immunol. 2014, 10, 927–961. [Google Scholar] [CrossRef] [PubMed]
- Mufson, R.A. Tumor Antigen Targets and Tumor Immunotherapy. Front. Biosci. 2006, 11, 337–343. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Hos, B.J.; Tondini, E.; van Kasteren, S.I.; Ossendorp, F. Approaches to Improve Chemically Defined Synthetic Peptide Vaccines. Front. Immunol. 2018, 9, 884. [Google Scholar] [CrossRef]
- Bartnik, A.; Nirmal, A.J.; Yang, S.-Y. Peptide Vaccine Therapy in Colorectal Cancer. Vaccines 2012, 1, 1–16. [Google Scholar] [CrossRef]
- Tsuruma, T.; Hata, F.; Furuhata, T.; Ohmura, T.; Katsuramaki, T.; Yamaguchi, K.; Kimura, Y.; Torigoe, T.; Sato, N.; Hirata, K. Peptide-Based Vaccination for Colorectal Cancer. Expert Opin. Biol. Ther. 2005, 5, 799–807. [Google Scholar] [CrossRef]
- Calvo Tardón, M.; Allard, M.; Dutoit, V.; Dietrich, P.-Y.; Walker, P.R. Peptides as Cancer Vaccines. Curr. Opin. Pharmacol. 2019, 47, 20–26. [Google Scholar] [CrossRef]
- Azmi, F.; Fuaad, A.A.H.A.; Skwarczynski, M.; Toth, I. Recent Progress in Adjuvant Discovery for Peptide-Based Subunit Vaccines. Hum. Vaccin. Immunother. 2014, 10, 778–796. [Google Scholar] [CrossRef] [PubMed]
- Chatzikleanthous, D.; Schmidt, S.T.; Buffi, G.; Paciello, I.; Cunliffe, R.; Carboni, F.; Romano, M.R.; O’Hagan, D.T.; D’Oro, U.; Woods, S.; et al. Design of a Novel Vaccine Nanotechnology-Based Delivery System Comprising CpGODN-Protein Conjugate Anchored to Liposomes. J. Control. Release 2020, 323, 125–137. [Google Scholar] [CrossRef]
- Melssen, M.M.; Petroni, G.R.; Chianese-Bullock, K.A.; Wages, N.A.; Grosh, W.W.; Varhegyi, N.; Smolkin, M.E.; Smith, K.T.; Galeassi, N.V.; Deacon, D.H.; et al. A Multipeptide Vaccine plus Toll-like Receptor Agonists LPS or PolyICLC in Combination with Incomplete Freund’s Adjuvant in Melanoma Patients. J. Immunother. Cancer 2019, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Feng, R.; Wang, Y.Z.; Sun, H.W.; Zou, Q.M.; Li, H.B. Toll-like Receptors: Triggers of Regulated Cell Death and Promising Targets for Cancer Therapy. Immunol. Lett. 2020, 223, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Temizoz, B.; Kuroda, E.; Ishii, K.J. Combination and Inducible Adjuvants Targeting Nucleic Acid Sensors. Curr. Opin. Pharmacol. 2018, 41, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, E.; Liu, H.; Huckriede, A.; Hak, E. Safety and Tolerability Evaluation of the Use of Montanide ISATM51 as Vaccine Adjuvant: A Systematic Review. Hum. Vaccin. Immunother. 2016, 12, 159–169. [Google Scholar] [CrossRef]
- Belnoue, E.; Di Berardino-Besson, W.; Gaertner, H.; Carboni, S.; Dunand-Sauthier, I.; Cerini, F.; Suso-Inderberg, E.M.; Wälchli, S.; König, S.; Salazar, A.M.; et al. Enhancing Antitumor Immune Responses by Optimized Combinations of Cell-Penetrating Peptide-Based Vaccines and Adjuvants. Mol. Ther. 2016, 24, 1675–1685. [Google Scholar] [CrossRef]
- Ponomarev, E.D.; Shriver, L.P.; Maresz, K.; Pedras-Vasconcelos, J.; Verthelyi, D.; Dittel, B.N. GM-CSF Production by Autoreactive T Cells Is Required for the Activation of Microglial Cells and the Onset of Experimental Autoimmune Encephalomyelitis. J. Immunol. 2007, 178, 39–48. [Google Scholar] [CrossRef]
- Yu, T.W.; Chueh, H.Y.; Tsai, C.C.; Lin, C.T.; Qiu, J.T. Novel GM-CSF-Based Vaccines: One Small Step in GM-CSF Gene Optimization, One Giant Leap for Human Vaccines. Hum. Vaccin. Immunother. 2016, 12, 3020–3028. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Budnick, I.; Singh, M.; Thiruppathi, M.; Alharshawi, K.; Elshabrawy, H.; Holterman, M.J.; Prabhakar, B.S. Dual Role of GM-CSF as a Pro-Inflammatory and a Regulatory Cytokine: Implications for Immune Therapy. J. Interferon Cytokine Res. 2015, 35, 585–599. [Google Scholar] [CrossRef]
- Borriello, F.; Galdiero, M.R.; Varricchi, G.; Loffredo, S.; Spadaro, G.; Marone, G. Innate Immune Modulation by GM-CSF and IL-3 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 834. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, G.; Wang, B. Revisiting GM-CSF as an Adjuvant for Therapeutic Vaccines. Cell. Mol. Immunol. 2018, 15, 187–189. [Google Scholar] [CrossRef]
- Shiomi, A.; Usui, T. Pivotal Roles of GM-CSF in Autoimmunity and Inflammation. Mediat. Inflamm. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Di Gregoli, K.; Johnson, J.L. Role of Colony-Stimulating Factors in Atherosclerosis. Curr. Opin. Lipidol. 2012, 23, 412–421. [Google Scholar] [CrossRef]
- Kim, I.K.; Koh, C.H.; Jeon, I.; Shin, K.S.; Kang, T.S.; Bae, E.A.; Seo, H.; Ko, H.J.; Kim, B.S.; Chung, Y.; et al. GM-CSF Promotes Antitumor Immunity by Inducing Th9 Cell Responses. Cancer Immunol. Res. 2019, 7, 498–509. [Google Scholar] [CrossRef]
- Decker, W.K.; Safdar, A. Cytokine Adjuvants for Vaccine Therapy of Neoplastic and Infectious Disease. Cytokine Growth Factor Rev. 2011, 22, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Wimmers, F.; De Haas, N.; Scholzen, A.; Schreibelt, G.; Simonetti, E.; Eleveld, M.J.; Brouwers, H.M.L.M.; Beldhuis-Valkis, M.; Joosten, I.; De Jonge, M.I.; et al. Monitoring of Dynamic Changes in Keyhole Limpet Hemocyanin (KLH)-Specific B Cells in KLHvaccinated Cancer Patients. Sci. Rep. 2017, 7, srep43486. [Google Scholar] [CrossRef]
- Bi, S.; Bailey, W.; Brisson, C. Performance of Keyhole Limpet Hemocyanin (KLH) as an Antigen Carrier for Protein Antigens Depends on KLH Property and Conjugation Route. J. Immunol. 2016, 196, 76.16. [Google Scholar] [CrossRef]
- Aarntzen, E.H.J.G.; De Vries, I.J.M.; GöErtz, J.H.; Beldhuis-Valkis, M.; Brouwers, H.M.L.M.; Van De Rakt, M.W.M.M.; Van Der Molen, R.G.; Punt, C.J.A.; Adema, G.J.; Tacken, P.J.; et al. Humoral Anti-KLH Responses in Cancer Patients Treated with Dendritic Cell-Based Immunotherapy Are Dictated by Different Vaccination Parameters. Cancer Immunol. Immunother. 2012, 61, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Swanson, M.A.; Schwartz, R.S. Immunosuppressive Therapy. N. Engl. J. Med. 1967, 277, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Slovin, S.F.; Ragupathi, G.; Musselli, C.; Fernandez, C.; Diani, M.; Verbel, D.; Danishefsky, S.; Livingston, P.; Scher, H.I. Thomsen-Friedenreich (TF) Antigen as a Target for Prostate Cancer Vaccine: Clinical Trial Results with TF Cluster (c)-KLH plus QS21 Conjugate Vaccine in Patients with Biochemically Relapsed Prostate Cancer. Cancer Immunol. Immunother. 2005, 54, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Razazan, A.; Behravan, J.; Arab, A.; Barati, N.; Arabi, L.; Gholizadeh, Z.; Jaafari, M.R. Conjugated nanoliposome with the HER2/neu-derived peptide GP2 as an effective vaccine against breast cancer in mice xenograft model. PLoS ONE 2017, 12, e0185099. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.M.; Besmer, D.M.; Erick, T.K.; Steuerwald, N.; Das Roy, L.; Grover, P.; Rao, S.; Nath, S.; Ferrier, J.W.; Reid, R.W.; et al. Indomethacin Enhances Anti-Tumor Efficacy of a MUC1 Peptide Vaccine against Breast Cancer in MUC1 Transgenic Mice. PLoS ONE 2019, 14, e0224309. [Google Scholar] [CrossRef] [PubMed]
- Ladjemi, M.Z.; Jacot, W.; Chardès, T.; Pèlegrin, A.; Navarro-Teulon, I. Anti-HER2 Vaccines: New Prospects for Breast Cancer Therapy. Cancer Immunol. Immunother. 2010, 59, 1295–1312. [Google Scholar] [CrossRef]
- Knutson, K.L.; Schiffman, K.; Cheever, M.A.; Disis, M.L. Immunization of Cancer Patients with a HER-2/Neu, HLA-A2 Peptide, P369-377, Results in Short-Lived Peptide-Specific Immunity. Clin. Cancer Res. 2002, 8, 1014–1018. [Google Scholar]
- Anderson, B.W.; Peoples, G.E.; Murray, J.L.; Gillogly, M.A.; Gershenson, D.M.; Ioannides, C.G. Peptide Priming of Cytolytic Activity to HER-2 Epitope 369-377 in Healthy Individuals. Clin. Cancer Res. 2000, 6, 4192–4200. [Google Scholar]
- Ohtake, J.; Ohkuri, T.; Togashi, Y.; Kitamura, H.; Okuno, K.; Nishimura, T. Identification of Novel Helper Epitope Peptides of Survivin Cancer-Associated Antigen Applicable to Developing Helper/Killer-Hybrid Epitope Long Peptide Cancer Vaccine. Immunol. Lett. 2014, 161, 20–30. [Google Scholar] [CrossRef]
- Disis, M.L.; Gooley, T.A.; Rinn, K.; Davis, D.; Piepkorn, M.; Cheever, M.A.; Knutson, K.L.; Schiffman, K. Generation of T-Cell Immunity to the HER-2/Neu Protein after Active Immunization with HER-2/Neu Peptide-Based Vaccines. J. Clin. Oncol. 2002, 20, 2624–2632. [Google Scholar] [CrossRef]
- Kalli, K.R.; Block, M.S.; Kasi, P.M.; Erskine, C.L.; Hobday, T.J.; Dietz, A.; Padley, D.; Gustafson, M.P.; Shreeder, B.; Puglisi-Knutson, D.; et al. Folate Receptor Alpha Peptide Vaccine Generates Immunity in Breast and Ovarian Cancer Patients. Clin. Cancer Res. 2018, 24, 3014–3025. [Google Scholar] [CrossRef]
- Farran, B.; Pavitra, E.; Kasa, P.; Peela, S.; Rama Raju, G.S.; Nagaraju, G.P. Folate-Targeted Immunotherapies: Passive and Active Strategies for Cancer. Cytokine Growth Factor Rev. 2019, 45, 45–52. [Google Scholar] [CrossRef]
- Folate Receptor Alpha Peptide Vaccine with GM-CSF in Patients with Triple Negative Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02593227 (accessed on 3 December 2020).
- MUC1 Vaccine for Triple-Negative Breast Cancer. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00986609 (accessed on 3 December 2020).
- Vaccine Therapy in Treating Patients with Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00524277 (accessed on 3 December 2020).
- Phase Ib Trial of Two Folate Binding Protein Peptide Vaccines (E39 and J65) in Breast and Ovarian Cancer Patients (J65). Available online: https://clinicaltrials.gov/ct2/show/NCT02019524 (accessed on 3 December 2020).
- Multipeptide Vaccine for Advanced Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00573495 (accessed on 3 December 2020).
- Oka, Y.; Tsuboi, A.; Taguchi, T.; Osaki, T.; Kyo, T.; Nakajima, H.; Elisseeva, O.A.; Oji, Y.; Kawakami, M.; Ikegame, K.; et al. Induction of WT1 (Wilms’ Tumor Gene)-Specific Cytotoxic T Lymphocytes by WT1 Peptide Vaccine and the Resultant Cancer Regression. Proc. Natl. Acad. Sci. USA 2004, 101, 13885–13890. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Tsuboi, A.; Oji, Y.; Kawase, I.; Sugiyama, H. WT1 Peptide Vaccine for the Treatment of Cancer. Curr. Opin. Immunol. 2008, 20, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J.P.; Benavides, L.C.; Gates, J.D.; Carmichael, M.G.; Hueman, M.T.; Mittendorf, E.A.; Murray, J.L.; Amin, A.; Craig, D.; Von Hofe, E.; et al. Results of the First Phase I Clinical Trial of the Novel Ii-Key Hybrid Preventive HER-2/Neu Peptide (AE37) Vaccine. J. Clin. Oncol. 2008, 26, 3426–3433. [Google Scholar] [CrossRef]
- Nevagi, R.J.; Toth, I.; Skwarczynski, M. Peptide-Based Vaccines. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 327–358. [Google Scholar]
- Roces, C.B.; Khadke, S.; Christensen, D.; Perrie, Y. Scale-Independent Microfluidic Production of Cationic Liposomal Adjuvants and Development of Enhanced Lymphatic Targeting Strategies. Mol. Pharm. 2019, 16, 4372–4386. [Google Scholar] [CrossRef] [PubMed]
- Salvador, A.; Igartua, M.; Hernández, R.M.; Pedraz, J.L. An Overview on the Field of Micro- and Nanotechnologies for Synthetic Peptide-Based Vaccines. J. Drug Deliv. 2011, 2011, 181646. [Google Scholar] [CrossRef]
- Ohta, S.; Kikuchi, E.; Ishijima, A.; Azuma, T.; Sakuma, I.; Ito, T. Investigating the Optimum Size of Nanoparticles for Their Delivery into the Brain Assisted by Focused Ultrasound-Induced Blood–Brain Barrier Opening. Sci. Rep. 2020, 10, 18220. [Google Scholar] [CrossRef]
- Koerner, J.; Horvath, D.; Groettrup, M. Harnessing Dendritic Cells for Poly (D,L-Lactide-Co-Glycolide) Microspheres (PLGA MS)-Mediated Anti-Tumor Therapy. Front. Immunol. 2019, 10, 707. [Google Scholar] [CrossRef]
- Durán, V.; Yasar, H.; Becker, J.; Thiyagarajan, D.; Loretz, B.; Kalinke, U.; Lehr, C.M. Preferential Uptake of Chitosan-Coated PLGA Nanoparticles by Primary Human Antigen Presenting Cells. Nanomedicine 2019, 21, 102073. [Google Scholar] [CrossRef]
- Kroll, A.V.; Fang, R.H.; Jiang, Y.; Zhou, J.; Wei, X.; Yu, C.L.; Gao, J.; Luk, B.T.; Dehaini, D.; Gao, W.; et al. Nanoparticulate Delivery of Cancer Cell Membrane Elicits Multiantigenic Antitumor Immunity. Adv. Mater. 2017, 29, 1–9. [Google Scholar] [CrossRef]
- Gu, P.; Liu, Z.; Sun, Y.; Ou, N.; Hu, Y.; Liu, J.; Wu, Y.; Wang, D. Angelica Sinensis Polysaccharide Encapsulated into PLGA Nanoparticles as a Vaccine Delivery and Adjuvant System for Ovalbumin to Promote Immune Responses. Int. J. Pharm. 2019, 554, 72–80. [Google Scholar] [CrossRef]
- Boraschi, D.; Italiani, P. From Antigen Delivery System to Adjuvanticy: The Board Application of Nanoparticles in Vaccinology. Vaccines 2015, 3, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef]
- Pandey, A.; Jain, D.S. Poly Lactic-Co-Glycolic Acid (PLGA) Copolymer and Its Pharmaceutical Application. In Handbook of Polymers for Pharmaceutical Technologies; Scrivener Publishing LLC.: Beverly, MA, USA, 2015; Volume 2, pp. 151–172. [Google Scholar]
- Ma, W.; Chen, M.; Kaushal, S.; McElroy, M.; Zhang, Y.; Ozkan, C.; Bouvet, M.; Kruse, C.; Grotjahn, D.; Ichim, T.; et al. PLGA Nanoparticle-Mediated Delivery of Tumor Antigenic Peptides Elicits Effective Immune Responses. Int. J. Nanomed. 2012, 7, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal Therapy with Immune-Adjuvant Nanoparticles Together with Checkpoint Blockade for Effective Cancer Immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef]
- Chu, B.Y.; Al Kobiasi, M.; Zeng, W.; Mainwaring, D.; Jackson, D.C. Chitosan-Based Particles as Biocompatible Delivery Vehicles for Peptide and Protein-Based Vaccines. Procedia Vaccinol. 2012, 6, 74–79. [Google Scholar] [CrossRef]
- Singh, B.; Maharjan, S.; Sindurakar, P.; Cho, K.H.; Choi, Y.J.; Cho, C.S. Needle-Free Immunization with Chitosan-Based Systems. Int. J. Mol. Sci. 2018, 19, 3639. [Google Scholar] [CrossRef] [PubMed]
- Singla, A.K.; Chawla, M. Chitosan: Some Pharmaceutical and Biological Aspects—An Update. J. Pharm. Pharmacol. 2010, 53, 1047–1067. [Google Scholar] [CrossRef]
- Jadidi-Niaragh, F.; Atyabi, F.; Rastegari, A.; Kheshtchin, N.; Arab, S.; Hassannia, H.; Ajami, M.; Mirsanei, Z.; Habibi, S.; Masoumi, F.; et al. CD73 Specific SiRNA Loaded Chitosan Lactate Nanoparticles Potentiate the Antitumor Effect of a Dendritic Cell Vaccine in 4T1 Breast Cancer Bearing Mice. J. Control. Release 2017, 246, 46–59. [Google Scholar] [CrossRef]
- Pei, M.; Liang, J.; Zhang, C.; Wang, X.; Zhang, C.; Ma, G.; Sun, H. Chitosan/Calcium Phosphates Nanosheet as a Vaccine Carrier for Effective Cross-Presentation of Exogenous Antigens. Carbohydr. Polym. 2019, 224, 115172. [Google Scholar] [CrossRef]
- Fang, R.H.; Hu, C.M.J.; Luk, B.T.; Gao, W.; Copp, J.A.; Tai, Y.; O’Connor, D.E.; Zhang, L. Cancer Cell Membrane-Coated Nanoparticles for Anticancer Vaccination and Drug Delivery. Nano Lett. 2014, 14, 2181–2188. [Google Scholar] [CrossRef]
- Harris, J.C.; Scully, M.A.; Day, E.S. Cancer Cell Membrane-Coated Nanoparticles for Cancer Management. Cancers 2019, 11, 1836. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Targeting of Drugs and Nanoparticles to Tumors. J. Cell Biol. 2010, 188, 759–768. [Google Scholar] [CrossRef]
- Xuan, M.; Shao, J.; Li, J. Cell Membrane-Covered Nanoparticles as Biomaterials. Natl. Sci. Rev. 2019, 6, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Krishnamachary, B.; Barnett, J.D.; Chatterjee, S.; Chang, D.; Mironchik, Y.; Wildes, F.; Jaffee, E.M.; Nimmagadda, S.; Bhujwalla, Z.M. Human Cancer Cell Membrane-Coated Biomimetic Nanoparticles Reduce Fibroblast-Mediated Invasion and Metastasis and Induce T-Cells. ACS Appl. Mater. Interfaces 2019, 11, 7850–7861. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef]
- Nelde, A.; Rammensee, H.G.; Walz, J.S. The Peptide Vaccine of the Future. Mol. Cell. Proteom. 2021, 20, 100022. [Google Scholar] [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.M.C.S.; et al. Immune Evasion in Cancer: Mechanistic Basis and Therapeutic Strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef]
- Ahmadzadeh, M. OR.93. Tumor Antigen-Specific CD8 T Cells Infiltrating the Tumor Express High Levels of PD-1 and Are Functionally Impaired. Clin. Immunol. 2009, 131, 1537–1544. [Google Scholar] [CrossRef]
- Colozza, M.; de Azambuja, E.; Personeni, N.; Lebrun, F.; Piccart, M.J.; Cardoso, F. Achievements in Systemic Therapies in the Pregenomic Era in Metastatic Breast Cancer. Oncologist 2007, 12, 253–270. [Google Scholar] [CrossRef]
- Planes-Laine, G.; Rochigneux, P.; Bertucci, F.; Chrétien, A.S.; Viens, P.; Sabatier, R.; Gonçalves, A. PD-1/PD-L1 Targeting in Breast Cancer: The First Clinical Evidences Are Emerging. a Literature Review. Cancers 2019, 11, 1033. [Google Scholar] [CrossRef]
- Kamphorst, A.O.; Pillai, R.N.; Yang, S.; Nasti, T.H.; Akondy, R.S.; Wieland, A.; Sica, G.L.; Yu, K.; Koenig, L.; Patel, N.T.; et al. Proliferation of PD-1+ CD8 T Cells in Peripheral Blood after PD-1-Targeted Therapy in Lung Cancer Patients. Proc. Natl. Acad. Sci. USA 2017, 114, 4993–4998. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Nishimura, Y. The Present Status and Future Prospects of Peptide-Based Cancer Vaccines. Int. Immunol. 2016, 28, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Kleponis, J.; Skelton, R.; Zheng, L. Fueling the Engine and Releasing the Break: Combinational Therapy of Cancer Vaccines and Immune Checkpoint Inhibitors. Cancer Biol. Med. 2015, 12, 201–208. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nordin, M.L.; Azemi, A.K.; Nordin, A.H.; Nabgan, W.; Ng, P.Y.; Yusoff, K.; Abu, N.; Lim, K.P.; Zakaria, Z.A.; Ismail, N.; et al. Peptide-Based Vaccine against Breast Cancer: Recent Advances and Prospects. Pharmaceuticals 2023, 16, 923. https://doi.org/10.3390/ph16070923
Nordin ML, Azemi AK, Nordin AH, Nabgan W, Ng PY, Yusoff K, Abu N, Lim KP, Zakaria ZA, Ismail N, et al. Peptide-Based Vaccine against Breast Cancer: Recent Advances and Prospects. Pharmaceuticals. 2023; 16(7):923. https://doi.org/10.3390/ph16070923
Chicago/Turabian StyleNordin, Muhammad Luqman, Ahmad Khusairi Azemi, Abu Hassan Nordin, Walid Nabgan, Pei Yuen Ng, Khatijah Yusoff, Nadiah Abu, Kue Peng Lim, Zainul Amiruddin Zakaria, Noraznawati Ismail, and et al. 2023. "Peptide-Based Vaccine against Breast Cancer: Recent Advances and Prospects" Pharmaceuticals 16, no. 7: 923. https://doi.org/10.3390/ph16070923
APA StyleNordin, M. L., Azemi, A. K., Nordin, A. H., Nabgan, W., Ng, P. Y., Yusoff, K., Abu, N., Lim, K. P., Zakaria, Z. A., Ismail, N., & Azmi, F. (2023). Peptide-Based Vaccine against Breast Cancer: Recent Advances and Prospects. Pharmaceuticals, 16(7), 923. https://doi.org/10.3390/ph16070923







