Efficacy of Pregabalin and Duloxetine in Patients with Painful Diabetic Peripheral Neuropathy (PDPN): A Multi-Centre Phase IV Clinical Trial—BLOSSOM
Abstract
1. Introduction
2. Results
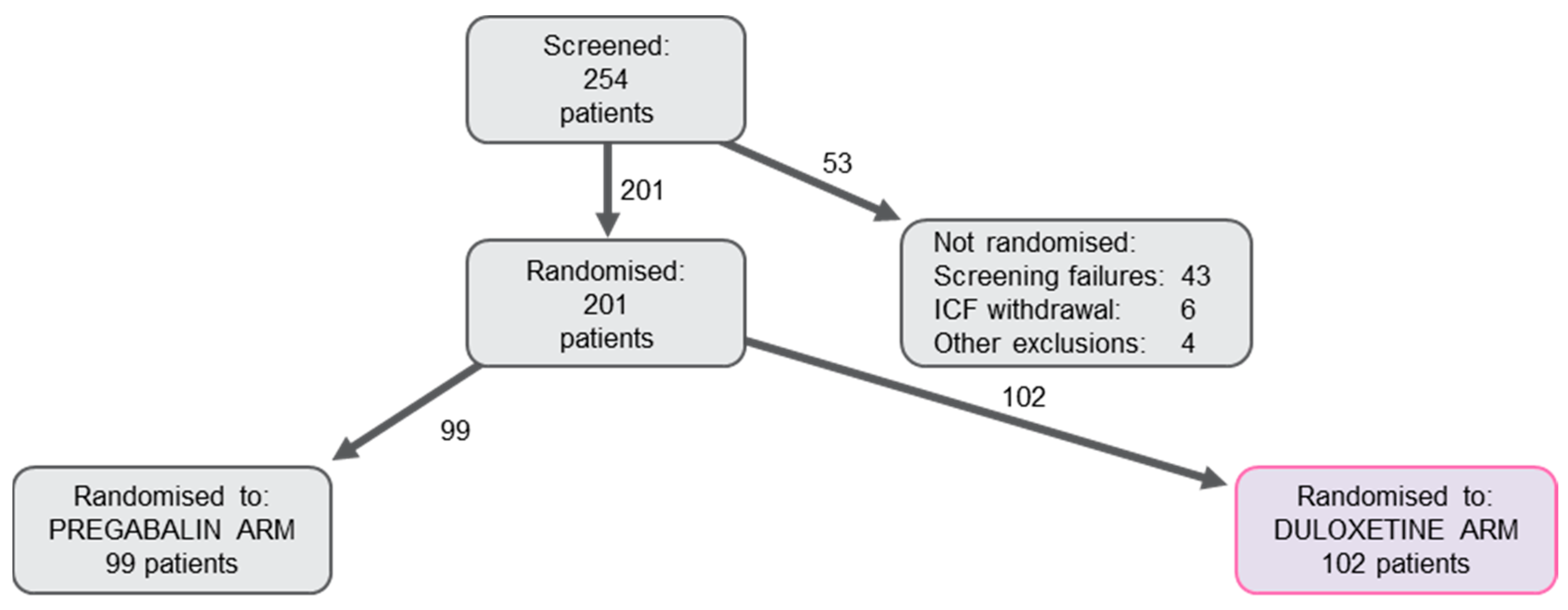
2.1. Population
2.2. Efficacy on Acute Pain
2.3. Efficacy on Subacute Pain
2.4. Adverse Events
3. Discussion
3.1. Efficacy on Acute Pain
3.2. Efficacy on Subacute Pain
3.3. Safety and Tolerability
3.4. Strengths and Limitations
4. Materials and Methods
4.1. Population
4.2. Protocol
4.3. Treatment Protocol
4.4. Efficacy and Safety Evaluation
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziegler, D.; Papanas, N.; Vinik, A.I.; Shaw, J.E. Chapter 1—Epidemiology of polyneuropathy in diabetes and prediabetes. In Diabetes and the Nervous System; Zochodne, D.W., Malik, R.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 126, pp. 3–22. ISBN 0072-9752. [Google Scholar]
- Peltier, A.; Goutman, S.A.; Callaghan, B.C. Painful diabetic neuropathy. BMJ 2014, 348, g1799. [Google Scholar] [CrossRef] [PubMed]
- Halawa, M.R.; Karawagh, A.; Zeidan, A.; Mahmoud, A.-E.-D.H.; Sakr, M.; Hegazy, A. Prevalence of painful diabetic peripheral neuropathy among patients suffering from diabetes mellitus in Saudi Arabia. Curr. Med. Res. Opin. 2010, 26, 337–343. [Google Scholar] [CrossRef]
- Truini, A.; Aleksovska, K.; Anderson, C.C.; Attal, N.; Baron, R.; Bennett, D.L.; Bouhassira, D.; Cruccu, G.; Eisenberg, E.; Enax-Krumova, E.; et al. Joint European Academy of Neurology—European Pain Federation–Neuropathic Pain Special Interest Group of the International Association for the Study of Pain guidelines on neuropathic pain assessment. Eur. J. Neurol. 2023, 30, 2177–2196. [Google Scholar] [CrossRef] [PubMed]
- Gylfadottir, S.S.; Christensen, D.H.; Nicolaisen, S.K.; Andersen, H.; Callaghan, B.C.; Itani, M.; Khan, K.S.; Kristensen, A.G.; Nielsen, J.S.; Sindrup, S.H.; et al. Diabetic polyneuropathy and pain, prevalence, and patient characteristics: A cross-sectional questionnaire study of 5,514 patients with recently diagnosed type 2 diabetes. Pain 2020, 161, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Galer, B.S.; Gianas, A.; Jensen, M.P. Painful diabetic polyneuropathy: Epidemiology, pain description, and quality of life. Diabetes Res. Clin. Pract. 2000, 47, 123–128. [Google Scholar] [CrossRef]
- Kim, S.S.; Won, J.C.; Kwon, H.S.; Kim, C.H.; Lee, J.H.; Park, T.S.; Ko, K.S.; Cha, B.Y. Prevalence and clinical implications of painful diabetic peripheral neuropathy in type 2 diabetes: Results from a nationwide hospital-based study of diabetic neuropathy in Korea. Diabetes Res. Clin. Pract. 2014, 103, 522–529. [Google Scholar] [CrossRef]
- Jende, J.M.E.; Groener, J.B.; Rother, C.; Kender, Z.; Hahn, A.; Hilgenfeld, T.; Juerchott, A.; Preisner, F.; Heiland, S.; Kopf, S.; et al. Association of Serum Cholesterol Levels With Peripheral Nerve Damage in Patients With Type 2 Diabetes. JAMA Netw. Open 2019, 2, e194798. [Google Scholar] [CrossRef]
- Zelman, D.C.; Brandenburg, N.A.; Gore, M. Sleep impairment in patients with painful diabetic peripheral neuropathy. Clin. J. Pain 2006, 22, 681–685. [Google Scholar] [CrossRef]
- Peltier, A.C.; Wood, D. Management of Neuropathic Pain in Polyneuropathy. Contin. Lifelong Learn. Neurol. 2020, 26, 1299–1322. [Google Scholar] [CrossRef]
- Parsons, B.; Li, C. The efficacy of pregabalin in patients with moderate and severe pain due to diabetic peripheral neuropathy. Curr. Med. Res. Opin. 2016, 32, 929–937. [Google Scholar] [CrossRef]
- Lunn, M.P.; Hughes, R.A.; Wiffen, P.J. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst. Rev. 2014, CD007115. [Google Scholar] [CrossRef] [PubMed]
- van Nooten, F.; Treur, M.; Pantiri, K.; Stoker, M.; Charokopou, M. Capsaicin 8% Patch Versus Oral Neuropathic Pain Medications for the Treatment of Painful Diabetic Peripheral Neuropathy: A Systematic Literature Review and Network Meta-analysis. Clin. Ther. 2017, 39, 787–803.e18. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Bell, R.F.; Straube, S.; Wiffen, P.J.; Aldington, D.; Moore, R.A. Pregabalin for neuropathic pain in adults. Cochrane Database Syst. Rev. 2019, 2019, CD007076. [Google Scholar] [CrossRef]
- Devi, P.; Madhu, K.; Ganapathy, B.; Sarma, G.; John, L.; Kulkarni, C. Evaluation of efficacy and safety of gabapentin, duloxetine, and pregabalin in patients with painful diabetic peripheral neuropathy. Indian J. Pharmacol. 2012, 44, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.C.; Lee, C.H.; Wu, C.S.; Huang, Y.J. Comparison of efficacy and safety of gabapentin and duloxetine in painful diabetic peripheral neuropathy: A systematic review and meta-analysis of randomised controlled trials. Int. J. Clin. Pract. 2021, 75, e14576. [Google Scholar] [CrossRef]
- Toth, C. Pregabalin: Latest safety evidence and clinical implications for the management of neuropathic pain. Ther. Adv. Drug Saf. 2014, 5, 38–56. [Google Scholar] [CrossRef]
- Lesser, H.; Sharma, U.; LaMoreaux, L.; Poole, R.M. Pregabalin relieves symptoms of painful diabetic neuropathy: A randomized controlled trial. Neurology 2004, 63, 2104–2110. [Google Scholar] [CrossRef]
- Ware, J.E., Jr. SF-36 Health Survey. In The Use of Psychological Testing for Treatment Planning and Outcomes Assessment, 2nd ed.; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 1999; pp. 1227–1246. ISBN 0-8058-2761-7. [Google Scholar]
- Raskin, J.; Pritchett, Y.L.; Wang, F.; D’Souza, D.N.; Waninger, A.L.; Iyengar, S.; Wernicke, J.F. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005, 6, 346–356. [Google Scholar] [CrossRef]
- Wernicke, J.F.; Pritchett, Y.L.; D’Souza, D.N.; Waninger, A.; Tran, P.; Iyengar, S.; Raskin, J. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology 2006, 67, 1411–1420. [Google Scholar] [CrossRef]
- Quilici, S.; Chancellor, J.; Löthgren, M.; Simon, D.; Said, G.; Le, T.K.; Garcia-Cebrian, A.; Monz, B. Meta-analysis of duloxetine vs. pregabalin and gabapentin in the treatment of diabetic peripheral neuropathic pain. BMC Neurol. 2009, 9, 6. [Google Scholar] [CrossRef]
- Ziegler, D.; Pritchett, Y.L.; Wang, F.; Desaiah, D.; Robinson, M.J.; Hall, J.A.; Chappell, A.S. Impact of disease characteristics on the efficacy of duloxetine in diabetic peripheral neuropathic pain. Diabetes Care 2007, 30, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.J.; Edwards, R.A.; Brodsky, M.; Savoldelli, A.; Manca, L.; Grugni, R.; Emir, B.; Whalen, E.; Watt, S.; Parsons, B. Assessing the Value of Time Series Real-World and Clinical Trial Data vs. Baseline-Only Data in Predicting Responses to Pregabalin Therapy for Patients with Painful Diabetic Peripheral Neuropathy. Clin. Drug Investig. 2019, 39, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.J.; Thomas, E.T.; Lee, J.J.; Goldacre, B.; Heneghan, C.J. Benefits and harms of pregabalin in the management of neuropathic pain: A rapid review and meta-analysis of randomised clinical trials. BMJ Open 2019, 9, e023600. [Google Scholar] [CrossRef]
- Hall, T.D.; Shah, S.; Ng, B.; Feberwee, H.M.; Dotchin, L.; Vandermost, M.; King, M.A. Changes in mood, depression and suicidal ideation after commencing pregabalin for neuropathic pain. Aust. Fam. Physician 2014, 43, 705–708. [Google Scholar] [PubMed]
- Molero, Y.; Larsson, H.; D’Onofrio, B.M.; Sharp, D.J.; Fazel, S. Associations between gabapentinoids and suicidal behaviour, unintentional overdoses, injuries, road traffic incidents, and violent crime: Population based cohort study in Sweden. BMJ 2019, 365, l2147. [Google Scholar] [CrossRef] [PubMed]
- Griebeler, M.L.; Morey-Vargas, O.L.; Brito, J.P.; Tsapas, A.; Wang, Z.; Carranza Leon, B.G.; Phung, O.J.; Montori, V.M.; Murad, M.H. Pharmacologic interventions for painful diabetic neuropathy: An umbrella systematic review and comparative effectiveness network meta-analysis. Ann. Intern. Med. 2014, 161, 639–649. [Google Scholar] [CrossRef]
- Goldstein, D.J.; Lu, Y.; Detke, M.J.; Lee, T.C.; Iyengar, S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain 2005, 116, 109–118. [Google Scholar] [CrossRef]
- Efficacy of Pregabalin and Duloxetine in Patients With PDPN: The Effect of Pain on Cognitive Function, Sleep and Quality of Life (BLOSSOM). Available online: https://beta.clinicaltrials.gov/study/NCT04246619?distance=50&titles=blossom&rank=1 (accessed on 8 July 2023).
- Bouhassira, D.; Attal, N.; Alchaar, H.; Boureau, F.; Brochet, B.; Bruxelle, J.; Cunin, G.; Fermanian, J.; Ginies, P.; Grun-Overdyking, A.; et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005, 114, 29–36. [Google Scholar] [CrossRef]
- Nasreddine, Z.; Phillips, N.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Colllin, I.; Cummings, J.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Thorndike, F.P.; Ritterband, L.M.; Saylor, D.K.; Magee, J.C.; Gonder-Frederick, L.A.; Morin, C.M. Validation of the Insomnia Severity Index as a Web-Based Measure. Behav. Sleep Med. 2011, 9, 216–223. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Hod, M.; Levy-Shiff, R.; Lerman, M.; Schindel, B.; Ben-Rafael, Z.; Bar, J. Developmental outcome of offspring of pregestational diabetic mothers. J. Pediatr. Endocrinol. Metab. 1999, 12, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Safety Working Party Recommendations Duration Contraception Following End Treatment Genotoxic Drug. Available online: https://www.ema.europa.eu/en/documents/other/safety-working-party-recommendations-duration-contraception-following-end-treatment-genotoxic-drug_en.pdf (accessed on 8 July 2023).
| Pregabalin Arm | Duloxetine Arm | ||
|---|---|---|---|
| n | 99 | 102 | |
| Sex | Female | 56 (56.6%) | 61 (59.8%) |
| Age (years) | Mean ± SD | 64.4 ± 9.1 | 63.4 ± 10.1 |
| Height (cm) | Mean ± SD | 168.3 ± 9.7 | 168.6 ± 9.6 |
| Weight (kg) | Mean ± SD | 85.7 ± 16.2 | 83 ± 13.4 |
| BMI (kg/m2) | Mean ± SD | 30.3 ± 5.6 | 29.3 ± 4.8 |
| Time from first diagnosis of type 2 DM (years) | Mean ± SD | 12.7 ± 8.8 | 12.7 ± 9.1 |
| Smoking | No | 72 (73%) | 71 (70%) |
| Yes | 27 (27%) | 31 (30%) | |
| Ex-smoker | 11 (11.1%) | 8 (7.8%) | |
| Occasional smoker | 2 (2%) | 4 (3.9%) | |
| Regular smoker | 14 (14.1%) | 19 (18.6%) | |
| Alcohol consumption | No | 82 (83%) | 89 (87%) |
| Yes | 17 (17%) | 13 (13%) | |
| Occasionally drinks | 16 (16.2%) | 11 (10.8%) | |
| Regularly drinks | 1 (1%) | 2 (2%) | |
| Other | 0 (0%) | 0 (0%) | |
| Drug abuse | No | 99 (100%) | 102 (100%) |
| Patients with comorbidities | 90 (90.9%) | 95 (93.1%) | |
| Vascular disorders | 72 (72.7%) | 78 (76.5%) | |
| Metabolism and nutrition disorders | 56 (56.6%) | 53 (52%) | |
| Cardiac disorders | 12 (12.1%) | 20 (19.6%) | |
| Endocrine disorders | 12 (12.1%) | 15 (14.7%) | |
| Gastrointestinal disorders | 13 (13.1%) | 13 (12.7%) | |
| Musculoskeletal and connective tissue disorders | 12 (12.1%) | 12 (11.8%) | |
| Eye disorders | 7 (7.1%) | 11 (10.8%) | |
| Reproductive system and breast disorders | 4 (4%) | 9 (8.8%) | |
| Nervous system disorders | 6 (6.1%) | 5 (4.9%) | |
| Respiratory, thoracic, and mediastinal disorders | 6 (6.1%) | 4 (3.9%) | |
| Renal and urinary disorders | 5 (5.1%) | 3 (2.9%) | |
| Surgical and medical procedures | 7 (7.1%) | 0 (0%) | |
| Blood and lymphatic system disorders | 4 (4%) | 1 (1%) | |
| Hepatobiliary disorders | 4 (4%) | 1 (1%) | |
| Psychiatric disorders | 0 (0%) | 3 (2.9%) | |
| Immune system disorders | 1 (1%) | 1 (1%) | |
| Infections and infestations | 2 (2%) | 0 (0%) | |
| Investigations | 1 (1%) | 1 (1%) | |
| Skin and subcutaneous tissue disorders | 1 (1%) | 1 (1%) | |
| Ear and labyrinth disorders | 1 (1%) | 0 (0%) | |
| Injury, poisoning, and procedural complications | 0 (0%) | 1 (1%) | |
| Neoplasms | 1 (1%) | 0 (0%) | |
| Pregabalin Arm | Duloxetine Arm | |
|---|---|---|
| (n = 99) | (n = 102) | |
| Patients with clinically meaningful improvement in 24 h-API 1 | 88.3%; [81.7%, 94.8%] | 86.9%; [76.7%, 97.1%] |
| Patients with clinically meaningful improvement (≥30%) in CPI 2, 24 h-API 1, and 24 h-WPI 3 | ||
| Week 1 | 14.7%; [7.5%, 22.0%] | 23.5%; [15.2%, 31.9%] |
| Week 2 | 31.9%; [22.5%, 41.3%] | 43.5%; [32.8%, 54.3%] |
| Week 6 | 58.4%; [47.7%, 69.1%] | 71.0%; [60.7%, 81.3%] |
| Week 8 | 65.1%; [54.9%, 75.2%] | 84.1%; [75.6%, 92.6%] |
| Week 12 | 83.2%; [73.1%, 93.3%] | 81.8%; [72.4%, 91.1%] |
| Patients who reached a reduction from baseline in CPI 2, 24 h-API 1, and 24 h-WPI 3 by ≥50% | ||
| Week 1 | 4.0%; [0.1%, 8.0%] | 6.9%; [1.9%, 11.9%] |
| Week 2 | 10.7%; [4.4%, 17.0%] | 20.4%; [12.2%, 28.6%] |
| Week 6 | 31.1%; [21.5%, 40.7%] | 44.9%; [33.9%, 55.9%] |
| Week 8 | 41.4%; [31.0%, 51.8%] | 60.2%; [48.7%, 71.7%] |
| Week 12 | 57.8%; [45.8%, 69.7%] | 67.6%; [57.8%, 77.5%] |
| Patients with CPI 2, 24 h-API 1, and 24 h-WPI 3 not exceeding 10 mm | ||
| Week 6 | 2.0%; [−0.8%, 4.8%] | 11.8%; [5.4%, 18.1%] |
| Week 8 | 15.2%; [7.8%, 22.5%] | 13.1%; [6.2%, 20.1%] |
| Week 12 | 28.9%; [19.6%, 38.2%] | 22.0%; [13.7%, 30.2%] |
| Patients with clinically meaningful improvement (≥30% and/or not exceeding 30 mm) in 4 wk-API 4 and 4 wk-WPI 5 | ||
| Week 8 | 63.0%; [52.6%, 73.5%] | 79.6%; [71.1%, 88.1%] |
| Week 12 | 79.6%; [71.0%, 88.2%] | 78.6%; [70.3%, 86.9%] |
| Patients who reached a reduction from baseline in 4 wk-API 4 and 4 wk-WPI 5 by ≥50% | ||
| Week 8 | 38.8%; [27.9%, 49.7%] | 53.1%; [42.9%, 63.4%] |
| Week 12 | 56.4%; [45.5%, 67.3%] | 67.3%; [57.3%, 77.2%] |
| Patients with 4 wk-API 4 and 4 wk-WPI 5 not exceeding 10 mm | ||
| Week 8 | 8.5%; [2.5%, 14.4%] | 7.8%; [2.3%, 13.4%] |
| Week 12 | 17.6%; [9.8%, 25.3%] | 14.9%; [7.6%, 22.2%] |
| DN-4 6 | ||
| Week 1 | 6.9 ± 1.6 | 6.9 ± 1.6 |
| Week 12 | 3.6 ± 2.4 | 3.6 ± 2.5 |
| Week 1 to Week 12 Mean Absolute Change | −3.3; [−3.8, −2.8] | −3.3; [−3.8, −2.8] |
| Week 1 to Week 12 Relative Difference | −47.5% | −48.0% |
| Time Point | Pregabalin Arm (n = 99) | Duloxetine Arm (n = 102) | |
|---|---|---|---|
| CPI 1 (mm) | Week 1 | −8.7; [−12.1, −5.2] | −9.4; [−13.3, −5.5] |
| Week 2 | −15.1; [−18.9, −11.3] | −17.8; [−21.5, −14.0] | |
| Week 6 | −22.9; [−27.1, −18.6] | −27.5; [−31.8, −23.3] | |
| Week 8 | −26.7; [−31.2, −22.2] | −32.8; [−37.2, −28.3] | |
| Week 12 | −35.3; [−40.5, −30.0] | −35.0; [−39.2, −30.7] | |
| 24 h-API 2 (mm) | Week 1 | −10.8; [−13.5, −8.1] | −10.8; [−13.7, −7.9] |
| Week 2 | −16.9; [−20.1, −13.8] | −19.0; [−22.8, −15.2] | |
| Week 6 | −25.5; [−29.1, −22.0] | −29.5; [−33.3, −25.8] | |
| Week 8 | −28.7; [−32.5, −24.8] | −34.8; [−38.5, −31.1] | |
| Week 12 | −37.0; [−41.4, −32.6] | −36.9; [−41.5, −32.3] | |
| 24 h-WPI 3 (mm) | Week 1 | −10.2; [−12.9, −7.5] | −10.7; [−14.1, −7.4] |
| Week 2 | −16.7; [−19.9, −13.6] | −18.7; [−23.0, −14.3] | |
| Week 6 | −24.5; [−28.5, −20.6] | −31.6; [−36.4, −26.8] | |
| Week 8 | −31.4; [−36.2, −26.6] | −38.2; [−43.5, −32.9] | |
| Week 12 | −41.6; [−46.6, −36.5] | −40.0; [−44.8, −35.2] | |
| 4 wk-API 4 (mm) | Week 8 | −26.2; [−30.0, −22.5] | −32.7; [−36.6, −28.8] |
| Week 12 | −32.2; [−35.8, −28.7] | −35.7; [−40.4, −31.0] | |
| 4 wk-WPI 5 (mm) | Week 8 | −30.9; [−35.3, −26.5] | −35.4; [−39.9, −30.9] |
| Week 12 | −38.5; [−42.9, −34.1] | −40.6; [−45.3, −35.8] |
| Non-Serious Adverse Events (Pregabalin Arm, n = 99) | Mild | Moderate | Severe | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Patients with non-serious adverse events | 21 (21.2%) | 19 (19.2%) | 1 (1%) | 32 (32.3%) | |||||
| SOC/PT | Related | Not related | Related | Not related | Related | Not related | Related | Not related | Total |
| Nervous system disorders | 10 (10.1%) | 0 (0%) | 10 (10.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 18 (18.2%) | 0 (0%) | 18 (18.2%) |
| Ear and labyrinth disorders | 2 (2%) | 0 (0%) | 6 (6.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 8 (8.1%) | 0 (0%) | 8 (8.1%) |
| General disorders and administration site conditions | 5 (5.1%) | 0 (0%) | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (7.1%) | 0 (0%) | 7 (7.1%) |
| Gastrointestinal disorders | 2 (2%) | 0 (0%) | 1 (1%) | 1 (1%) | 0 (0%) | 0 (0%) | 3 (3%) | 1 (1%) | 4 (4%) |
| Psychiatric disorders | 2 (2%) | 0 (0%) | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (4%) | 0 (0%) | 4 (4%) |
| Investigations | 0 (0%) | 2 (2%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | 2 (2%) | 3 (3%) |
| Metabolism and nutrition disorders | 1 (1%) | 1 (1%) | 1 (1%) | 0 (0%) | 1 (1%) | 0 (0%) | 2 (2%) | 1 (1%) | 3 (3%) |
| Musculoskeletal and connective tissue disorders | 1 (1%) | 1 (1%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 1 (1%) | 3 (3%) |
| Eye disorders | 1 (1%) | 0 (0%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 0 (0%) | 2 (2%) |
| Skin and subcutaneous tissue disorders | 0 (0%) | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 2 (2%) |
| Metabolism and nutrition disorders/gastrointestinal disorders | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | 0 (0%) | 1 (1%) |
| Vascular disorders | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | 0 (0%) | 1 (1%) |
| Non-serious adverse events (duloxetine arm, n = 102) | Mild | Moderate | Severe | Total | |||||
| Patients with non-serious adverse events | 20 (19.6%) | 22 (21.6%) | 4 (3.9%) | 33 (32.4%) | |||||
| SOC/PT | Related | Not related | Related | Not related | Related | Not related | Related | Not related | Total |
| Gastrointestinal disorders | 9 (8.8%) | 1 (1%) | 8 (7.8%) | 2 (2%) | 3 (2.9%) | 0 (0%) | 16 (15.7%) | 3 (2.9%) | 17 (16.7%) |
| Nervous system disorders | 8 (7.8%) | 1 (1%) | 5 (4.9%) | 1 (1%) | 1 (1%) | 0 (0%) | 14 (13.7%) | 2 (2%) | 16 (15.7%) |
| General disorders and administration site conditions | 0 (0%) | 1 (1%) | 4 (3.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (3.9%) | 1 (1%) | 5 (4.9%) |
| Psychiatric disorders | 2 (2%) | 0 (0%) | 3 (2.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (4.9%) | 0 (0%) | 5 (4.9%) |
| Ear and labyrinth disorders | 0 (0%) | 0 (0%) | 4 (3.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (3.9%) | 0 (0%) | 4 (3.9%) |
| Metabolism and nutrition disorders | 2 (2%) | 0 (0%) | 1 (1%) | 0 (0%) | 1 (1%) | 0 (0%) | 4 (3.9%) | 0 (0%) | 4 (3.9%) |
| Skin and subcutaneous tissue disorders | 1 (1%) | 0 (0%) | 2 (2%) | 0 (0%) | 1 (1%) | 0 (0%) | 4 (3.9%) | 0 (0%) | 4 (3.9%) |
| Cardiac disorders | 1 (1%) | 0 (0%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 0 (0%) | 2 (2%) |
| Eye disorders | 1 (1%) | 0 (0%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 0 (0%) | 2 (2%) |
| Injury, poisoning, and procedural complications | 1 (1%) | 0 (0%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 0 (0%) | 2 (2%) |
| Infections and infestations | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | 1 (1%) |
| Reproductive system and breast disorders | 0 (0%) | 0 (0%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | 0 (0%) | 1 (1%) |
| N | Mean ± SD | Median | [Min, Max] | Q1, Q3 | Patients with Compliance Exceeding 80% | ||
|---|---|---|---|---|---|---|---|
| Pregabalin (mg) | Week 1 | 99 | 69.44 ± 32.25 | 75 | [25, 150] | 50, 75 | / |
| Week 2 | 96 | 140.89 ± 33.07 | 150 | [50, 300] | 150, 150 | 97.8%; [94.7%, 100.9%] | |
| Week 6 | 95 | 224.21 ± 92.5 | 150 | [150, 600] | 150, 300 | / | |
| Week 8 | 90 | 256.67 ± 129.65 | 300 | [150, 600] | 150, 300 | 96.8%; [93.1%, 100.5%] | |
| Week 12 | 85 | 294.71 ± 162.76 | 300 | [150, 600] | 150, 300 | 89.3%; [81.7%, 96.9%] | |
| Duloxetine (mg) | Week 1 | 102 | 35 ± 11.24 | 30 | [30, 60] | 30, 30 | / |
| Week 2 | 99 | 54.55 ± 11.63 | 60 | [30, 60] | 60, 60 | 98.6%; [95.4%, 101.8%] | |
| Week 6 | 85 | 70.59 ± 19.48 | 60 | [60, 120] | 60, 90 | / | |
| Week 8 | 80 | 76.13 ± 22.36 | 60 | [60, 120] | 60, 90 | 94.5%; [89.9%, 99.2%] | |
| Week 12 | 76 | 80.13 ± 26.1 | 60 | [30, 120] | 60, 97.5 | 92.7%; [85.7%, 99.7%] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakusa, M.; Marolt, I.; Stevic, Z.; Rebrina, S.V.; Milenkovic, T.; Stepien, A. Efficacy of Pregabalin and Duloxetine in Patients with Painful Diabetic Peripheral Neuropathy (PDPN): A Multi-Centre Phase IV Clinical Trial—BLOSSOM. Pharmaceuticals 2023, 16, 1017. https://doi.org/10.3390/ph16071017
Rakusa M, Marolt I, Stevic Z, Rebrina SV, Milenkovic T, Stepien A. Efficacy of Pregabalin and Duloxetine in Patients with Painful Diabetic Peripheral Neuropathy (PDPN): A Multi-Centre Phase IV Clinical Trial—BLOSSOM. Pharmaceuticals. 2023; 16(7):1017. https://doi.org/10.3390/ph16071017
Chicago/Turabian StyleRakusa, Martin, Iris Marolt, Zorica Stevic, Sandra Vuckovic Rebrina, Tatjana Milenkovic, and Adam Stepien. 2023. "Efficacy of Pregabalin and Duloxetine in Patients with Painful Diabetic Peripheral Neuropathy (PDPN): A Multi-Centre Phase IV Clinical Trial—BLOSSOM" Pharmaceuticals 16, no. 7: 1017. https://doi.org/10.3390/ph16071017
APA StyleRakusa, M., Marolt, I., Stevic, Z., Rebrina, S. V., Milenkovic, T., & Stepien, A. (2023). Efficacy of Pregabalin and Duloxetine in Patients with Painful Diabetic Peripheral Neuropathy (PDPN): A Multi-Centre Phase IV Clinical Trial—BLOSSOM. Pharmaceuticals, 16(7), 1017. https://doi.org/10.3390/ph16071017







