Assessment of Physicochemical Parameters and Contaminants in Herbal Dietary Supplements Used in the Treatment of Inflammatory Bowel Disease
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization
2.2. Assessment of Contaminants
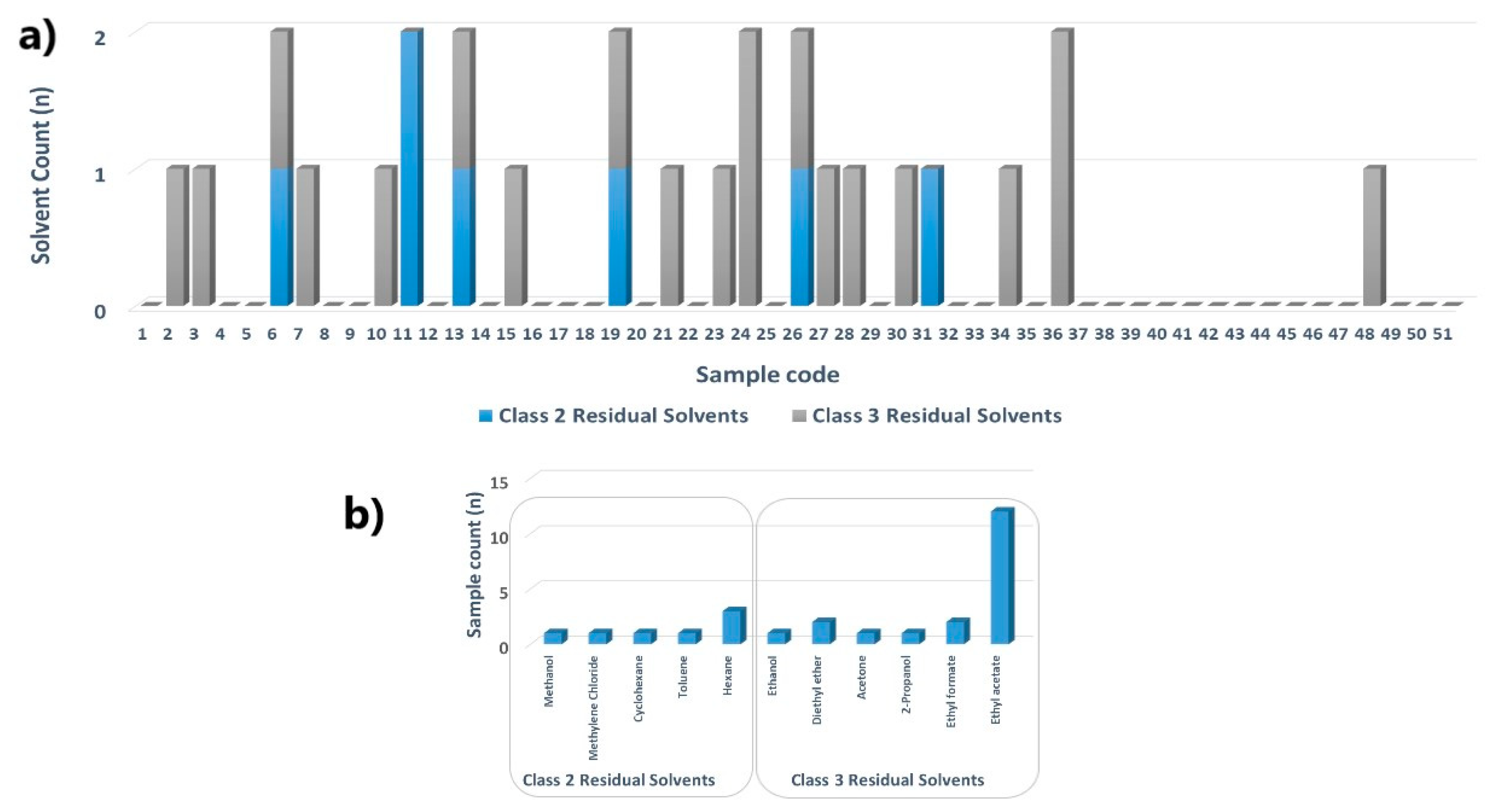
2.2.1. Volatile Matter Content
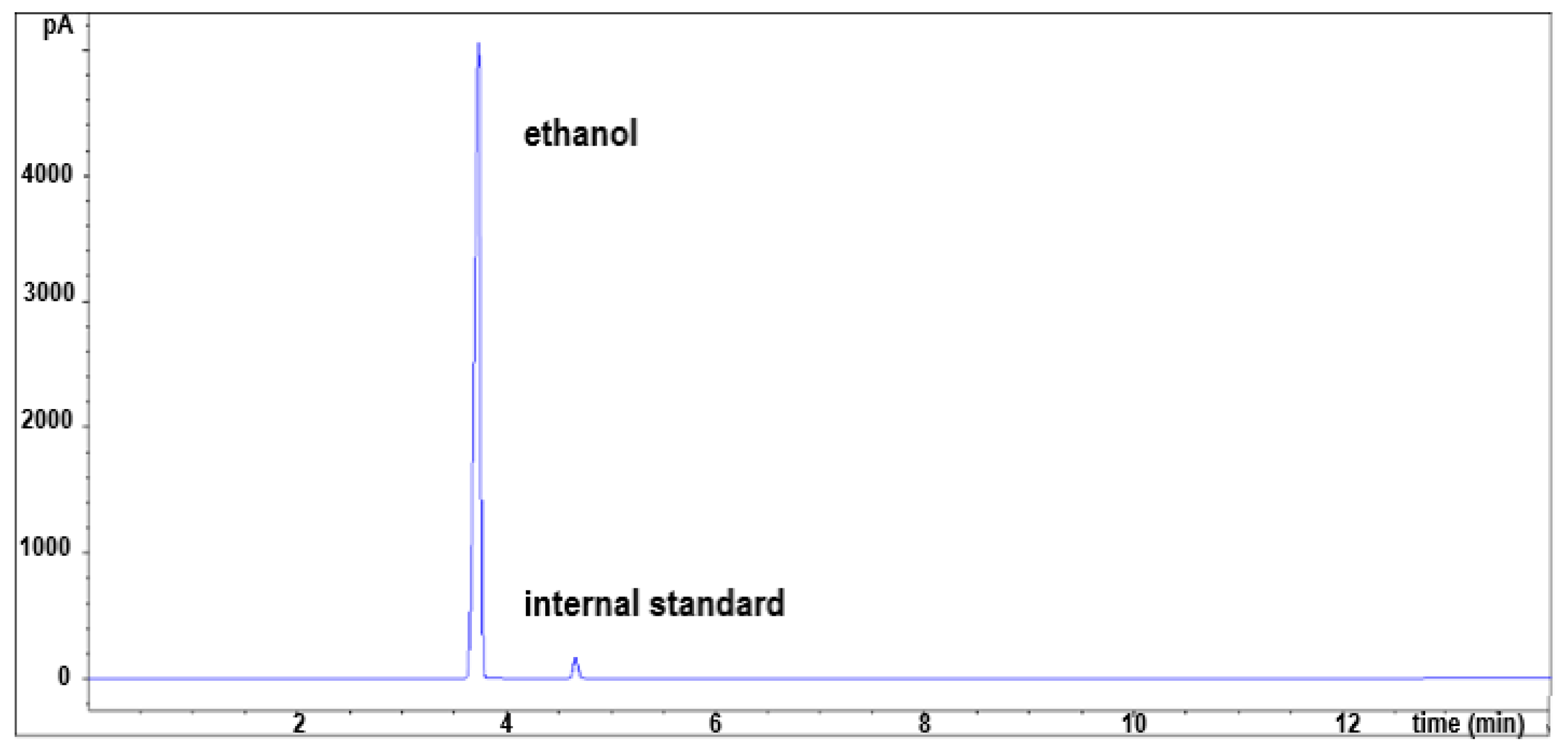
2.2.2. Ethanol and Its Impurities Content
2.2.3. Ethylene Oxide Content
2.2.4. Gluten Content
3. Materials and Methods
3.1. Chemicals
3.2. Samples
3.3. Physicochemical Characterization
3.3.1. Weight Variation of the Dosage Forms
3.3.2. Friability Test
3.3.3. Tablet Breaking Force
3.3.4. Disintegration Time
3.3.5. Rupture Test for Soft-Shell Capsule
3.3.6. Bulk and Tapped Density of Powders
3.3.7. Loss on Drying
3.4. Assessment of Residual Solvents by HSS-GC-FID Method
3.4.1. Standard Solutions and Sample Preparation
3.4.2. Assay Protocol
3.5. Assessment of Ethanol and Its Impurities Content in Liquid Extracts using the HSS-GC-FID Method
3.5.1. Standard Solutions and Sample Preparation
3.5.2. Assay Protocol
3.6. Assessment of Ethylene Oxide using GC/MS/MS Method
3.6.1. Standard Solutions and Sample Preparation
3.6.2. Assay Protocol
3.7. Assessment of Gluten using Enzyme-Linked Immunosorbent Assay
3.7.1. Reagents and Sample Preparation
3.7.2. Assay Protocol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zion Market Research. Available online: https://www.zionmarketresearch.com/news/dietary-supplements-market (accessed on 15 April 2023).
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohns Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnár, T.; Raine, T.; Sebastian, S.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohns Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Cheifetz, A.S. The Use of Complementary and Alternative Medicine in Patients with Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2018, 14, 415–425. [Google Scholar]
- Picardo, S.; Altuwaijri, M.; Devlin, S.M.; Seow, C.H. Complementary and Alternative Medications in the Management of Inflammatory Bowel Disease. Ther. Adv. Gastroenterol. 2020, 13, 1756284820927550. [Google Scholar] [CrossRef] [PubMed]
- De Conno, B.; Pesce, M.; Chiurazzi, M.; Andreozzi, M.; Rurgo, S.; Corpetti, C.; Seguella, L.; Del Re, A.; Palenca, I.; Esposito, G.; et al. Nutraceuticals and Diet Supplements in Crohn’s Disease: A General Overview of the Most Promising Approaches in the Clinic. Foods 2022, 11, 1044. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.E.; Whyand, T.; Murray, C.D.; Hamilton, M.I.; Conte, D.; Caplin, M.E. The role of Dietary Supplements in Inflammatory Bowel Disease: A Systematic Review. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- White, C.M. Dietary Supplements Pose Real Dangers to Patients. Ann. Pharmacother. 2020, 54, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Sarma, N.; Giancaspro, G.; Venema, J. Dietary Supplements Quality Analysis Tools from the United States Pharmacopeia. Drug Test. Anal. 2016, 8, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L. Current Regulatory Guidelines and Resources to Support Research of Dietary Supplements in the United States. Crit. Rev. Food Sci. Nutr. 2020, 60, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Mornar, A.; Buhač, T.; Amidžić Klarić, D.; Klarić, I.; Sertić, M.; Nigović, B. Multi-targeted Screening of Phytoestrogens in Food, Raw Material, and Dietary Supplements by Liquid Chromatography with Tandem Mass Spectrometry. Food Anal. Methods 2020, 13, 482–495. [Google Scholar] [CrossRef]
- Buhač, T.; Amidžić Klarić, D.; Klarić, I.; Nigović, B.; Brusač, E.; Jeličić, M.-L.; Mornar, A. Assessment of Active Ingredients and Metal Impurities in Phytoestrogen-containing Food and Dietary Supplements. J. Food Nutr. Res. 2020, 59, 87–97. [Google Scholar]
- Mornar, A.; Sertić, M.; Nigović, B. Development of a Rapid LC/DAD/FLD/MSn Method for the Simultaneous Determination of Monacolins and Citrinin in Red Fermented Rice Products. J. Agric. Food Chem. 2013, 61, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Mornar, A.; Sertić, M.; Amidžić Klarić, D.; Klarić, I.; Stipanović, K.; Nigović, B. Evaluation of Alcohol Content and Metal Impurities in Liquid Dietary Supplements by sHSS-GC-FID and GFAAS Techniques. Food Chem. 2016, 211, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Brzezińska, J.; Szewczyk, A.; Brzezicha, J.; Prokopowicz, M.; Grembecka, M. Evaluation of Physicochemical Properties of Beetroot-Based Dietary Supplements. Foods 2021, 10, 1693. [Google Scholar] [CrossRef] [PubMed]
- Ośko, J.; Szewczyk, A.; Berk, P.; Prokopowicz, M.; Grembecka, M. Assessment of the Mineral Composition and the Selected Physicochemical Parameters of Dietary Supplements Containing Green Tea Extracts. Foods 2022, 11, 3580. [Google Scholar] [CrossRef] [PubMed]
- Brusač, E.; Jeličić, M.-L.; Nigović, B.; Amidžić Klarić, D.; Mornar, A. Determination of Curcuminoids, Piperine, Boswellic Acids and Andrographolides in Food and Dietary Supplements by HPLC. Food Technol. Biotechnol. 2022, 60, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Size, Shape, and Other Physical Attributes of Generic Tablets and Capsules. Available online: https://www.fda.gov/media/87344/download (accessed on 15 April 2023).
- Bachour, G.; Bou-Chacra, N.; Löbenberg, R. Evaluation of the Rupture Test for Stability Studies of Soft-Shell Capsules. Dissolution Technol. 2017, 24, 16–19. [Google Scholar] [CrossRef]
- Reflection Paper on Ethanol Content in Herbal Medicinal Products and Traditional Herbal Medicinal Products Used in Children. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-reflection-paper-ethanol-content-herbal-medicinal-products-traditional-herbal-medicinal_en.pdf (accessed on 15 April 2023).
- Commission Regulation (EU) 2022/1396 of 11 August 2022 Amending the Annex to Regulation (EU) No 231/2012 Laying Down Specifications for Food Additives Listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as Regards the Presence of Ethylene oxide in Food Additives. Available online: https://eur-lex.europa.eu/eli/reg/2022/1396 (accessed on 15 April 2023).
- Weaver, K.N.; Herfarth, H. Gluten-Free Diet in IBD: Time for a Recommendation? Mol. Nutr. Food Res. 2021, 65, e1901274. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, R.O.; Nowatzke, W.L.; Cho, C.Y.; Oliver, K.G.; Garber, E.A.E. Cross-reactivity by Botanicals Used in Dietary Supplements and Spices Using the Multiplex xMAP Food Allergen Detection Assay (xMAP FADA). Anal. Bioanal. Chem. 2018, 410, 5791–5806. [Google Scholar] [CrossRef] [PubMed]
- Cenni, S.; Sesenna, V.; Boiardi, G.; Casertano, M.; Russo, G.; Reginelli, A.; Esposito, S.; Strisciuglio, C. The Role of Gluten in Gastrointestinal Disorders: A Review. Nutrients 2023, 15, 1615. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeial Convention. Weight variation of dietary supplement. In United States Pharmacopeia and National Formulary (USP 43-NF 38); United States Pharmacopeial Convention: Rockville, MD, USA, 2021. [Google Scholar]
- United States Pharmacopeial Convention. Tablet friability. In United States Pharmacopeia and National Formulary (USP 43-NF 38); United States Pharmacopeial Convention: Rockville, MD, USA, 2021. [Google Scholar]
- United States Pharmacopeial Convention. Tablet Breaking Force. In United States Pharmacopeia and National Formulary (USP 43-NF 38); United States Pharmacopeial Convention: Rockville, MD, USA, 2021. [Google Scholar]
- United States Pharmacopeial Convention. Disintegration and dissolution of dietary supplements. In United States Pharmacopeia and National Formulary (USP 43-NF 38); United States Pharmacopeial Convention: Rockville, MD, USA, 2021. [Google Scholar]
- United States Pharmacopeial Convention. Bulk Density and Tapped Density of Powders. In United States Pharmacopeia and National Formulary (USP 43-NF 38); United States Pharmacopeial Convention: Rockville, MD, USA, 2021. [Google Scholar]
- United States Pharmacopeial Convention. Loss on drying. In United States Pharmacopeia and National Formulary (USP 43-NF 38); United States Pharmacopeial Convention: Rockville, MD, USA, 2021. [Google Scholar]
- United States Pharmacopeial Convention. Residual Solvents. In United States Pharmacopeia and National Formulary (USP 43-NF 38); United States Pharmacopeial Convention: Rockville, MD, USA, 2021. [Google Scholar]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Validation of Analytical Procedures: Text and Methodology Q2(R1). Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 15 April 2023).
| Sample Code | Weight Variation | Disintegration | ||||||
|---|---|---|---|---|---|---|---|---|
| Labeled Weight (mg) | Average Weight (mg, n = 20) | Percentage of Labeled Weight (%) | Minimum Percentage of Average Weight (%) | Maximum Percentage of Average Weight (%) | USP Criteria | Disintegration Time | USP Criteria | |
| S1 | 400 | 496.8 | 124.2 | 95.6 | 104.4 | passed | 6 capsules within 15 min | passed |
| S2 | 500 | 601.1 | 120.2 | 93.6 | 105.4 | passed | 6 capsules within 15 min | passed |
| S3 | 600 | 600.3 | 100.1 | 94.7 | 106.9 | passed | 6 capsules within 15 min | passed |
| S4 | 633 | 649.8 | 102.6 | 93.3 | 106.5 | passed | 6 capsules within 30 min | passed |
| S5 | 587 | 597.8 | 101.8 | 95.5 | 104.4 | passed | 6 capsules within 15 min | passed |
| S6 | N/D 1 | 1101.0 | N/A 2 | 94.3 | 105.3 | passed | 6 capsules within 30 min | passed |
| S7 | 1167 | 830.2 | 71.1 | 98.6 | 101.6 | passed | 6 capsules within 15 min | passed |
| S8 | 500 | 499.6 | 99.9 | 97.7 | 104.2 | passed | 6 capsules within 15 min | passed |
| S9 | N/D | 579.4 | N/A | 92.6 | 107.1 | passed | 6 capsules within 30 min | passed |
| S10 | N/D | 507.8 | N/A | 89.5 | 106.5 | passed | 6 capsules within 30 min | passed |
| S11 | 500 | 400.7 | 80.1 | 91.1 | 105.5 | passed | 6 capsules within 30 min | passed |
| S12 | N/D | 527.7 | N/A | 97.6 | 108.4 | passed | 6 capsules within 30 min | passed |
| S13 | N/D | 563.8 | N/A | 100.0 | 100.0 | passed | 6 capsules within 30 min | passed |
| S14 | N/D | 408.9 | N/A | 98.0 | 102.8 | passed | 6 capsules within 30 min | passed |
| S15 | N/D | 486.5 | N/A | 98.3 | 101.7 | passed | 6 capsules within 15 min | passed |
| S16 | N/D | 1191.9 | N/A | 94.6 | 102.9 | passed | 6 capsules within 15 min | passed |
| S17 | N/D | 484.1 | N/A | 95.3 | 108.5 | passed | 6 capsules within 30 min | passed |
| S18 | N/D | 594.5 | N/A | 96.0 | 104.9 | passed | 6 capsules within 15 min | passed |
| S19 | N/D | 532.1 | N/A | 94.2 | 105.9 | passed | 6 capsules within 15 min | passed |
| S20 | N/D | 542.7 | N/A | 94.4 | 107.7 | passed | 6 capsules within 30 min | passed |
| S21 | 500 | 597.7 | 119.5 | 95.6 | 105.2 | passed | 6 capsules within 15 min | passed |
| S22 | 980 | 985.8 | 100.6 | 96.7 | 105.2 | passed | 6 capsules within 15 min | passed |
| S23 | N/D | 606.0 | N/A | 94.8 | 105.9 | passed | 6 capsules within 15 min | passed |
| S24 | N/D | 995.0 | N/A | 97.4 | 101.8 | passed | 6 capsules within 15 min | passed |
| S25 | N/D | 814.6 | N/A | 97.5 | 101.6 | passed | 6 capsules within 15 min | passed |
| Sample Code | Weight Variation | Disintegration | Rupture Test | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Labeled Weight (mg) | Average Weight (mg, n = 20) | Percentage of Labeled Weight (%) | Minimum Percentage of Average Weight (%) | Maximum Percentage of Average Weight (%) | USP Criteria | Disintegration Time | USP Criteria | Rupture Time | USP Criteria | |
| S26 | N/D 1 | 1581.8 | N/A 2 | 99.0 | 101.3 | passed | 6 capsules within 15 min | passed | 6 capsules within 1 min | passed |
| S27 | 916.7 | 959.6 | 104.7 | 95.4 | 102.7 | passed | 6 capsules within 15 min | passed | 6 capsules within 1 min | passed |
| S28 | 1280 | 1303.0 | 101.8 | 97.8 | 101.6 | passed | 6 capsules within 15 min | passed | 6 capsules within 3 min | passed |
| S29 | 596 | 589.8 | 99.0 | 99.0 | 101.0 | passed | 6 capsules within 15 min | passed | 6 capsules within 7 min | passed |
| Sample code | Weight Variation | Disintegration | Tablet Breaking Force | Friability | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Labeled Weight (mg) | Average Weight (mg, n = 20) | Percentage of Labeled Weight (%) | Minimum Percentage of Average Weight (%) | Maximum Percentage of Average Weight (%) | USP Criteria | Disintegration Time | USP Criteria | Hardness (N, n = 10) | RSD (%) 1 | Friability (%, n = 3) | USP Criteria | |
| S30 | N/D 2 | 224.5 | N/A 3 | 94.1 | 103.1 | passed | 6 tablets disintegrate completely within 15 min | passed | 46.0 | 0.1 | ≤0.01 | passed |
| S31 | 1633.3 | 1621.2 | 99.3 | 98.4 | 102.9 | passed | 6 tablets disintegrate completely within 30 min | passed | 248.5 | 7.7 | ≤0.01 | passed |
| S32 | N/D | 335.7 | N/A | 97.7 | 102.1 | passed | 6 tablets disintegrate completely within 1 min | passed | 73.7 | 12.4 | ≤0.01 | passed |
| S33 | 300 | 294.0 | 98.0 | 95.0 | 107.7 | passed (1 unit > 7.5%) | 6 tablets disintegrate completely within 5 min | passed | 27.7 | 6.5 | 0.14–0.38 | passed |
| S34 | 1680 | 1617.5 | 96.3 | 97.9 | 103.8 | passed | 6 tablets disintegrate completely within 25 min | passed | 241.0 | 10.2 | ≤0.01 | passed |
| S35 | 1584.5 | 1958.7 | 123.6 | 98.8 | 101.0 | passed | none of the tablets disintegrate completely within 30 min | failed | 138.2 | 7.2 | 0.1–0.28 | passed |
| Botanical Source | Turmeric | Indian Frankincense | Green Chiretta | Black Pepper |
|---|---|---|---|---|
| bulk density (g/mL) | ||||
| Average | 0.4778 | 0.4201 | 0.3172 | 0.4555 |
| Median | 0.4698 | 0.4196 | 0.3236 | N/A 1 |
| Range | 0.4070–0.5525 | 0.2958–0.5329 | 0.3040–0.3240 | N/A |
| tapped density (g/mL) | ||||
| Average | 0.6569 | 0.6159 | 0.5847 | 0.6263 |
| Median | 0.6664 | 0.61946 | 0.5892 | N/A |
| Range | 0.5644–0.7375 | 0.4986–0.7190 | 0.5779–0.5892 | N/A |
| Hausner ratio | ||||
| Average | 1.38 | 1.50 | 1.84 | 1.38 |
| Median | 1.39 | 1.45 | 1.81 | N/A |
| Range | 1.32–1.45 | 1.35–1.69 | 1.79–1.92 | N/A |
| Compressibility Index (%) | ||||
| Average | 27.5 | 32.7 | 45.7 | 27.3 |
| Median | 28.0 | 33.0 | 44.8 | N/A |
| Range | 24.3–31.0 | 25.9–40.7 | 44.0–48.4 | N/A |
| USP category | passable—poor | poor—very poor | very, very poor | poor |
| Sample Type | Loss on Drying (%, n = 3) | RSD 1 (%) | USP Criteria |
|---|---|---|---|
| hard-shell capsule | |||
| dry extract | 1.05–34.67 | ≤11.01 | 17 passed, 4 failed |
| extract | 6.32–8.04 | 0.21–5.54 | 4 passed |
| tablet | |||
| dry extract | 2.55–12.14 | 0.29–9.33 | 2 passed, 3 failed |
| extract | 6.20 | 7.53 | passed |
| dietary supplement ingredient (powder) | |||
| dry extract | 1.05–5.15 | 12.10–10.94 | 2 passed, 3 failed |
| extract | 2.40–8.65 | 0.92–47.64 | 12 passed |
| Sample Code | Labeled Ethanol Content (%) | Ethanol (n = 3, %,/ RSD 1, %) | Amount of Ethanol Per Daily Serving (mL) | Methanol | Acetone | Isopropanol | Tert-Butanol | 1-Propanol | Isobutanol | 1-Butanol |
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 3, ppm/RSD, %) | ||||||||||
| S52 | 22 | 16.21/4.56 | 0.19 | 10.64/1.99 | <LOQ | 8.59/2.53 | <LOQ | <LOQ | <LOD | <LOD |
| S53 | 40–50 | 47.08/2.41 | 1.41 | 18.12/1.07 | 3.59/2.00 | 15.66/1.44 | <LOQ | <LOQ | <LOD | <LOD |
| Sample Type | Incidence [%] 1 (Number of QUANTIFIED Samples) | Mean (mg/kg) | Median (mg/kg) | Range of Quantified Values (mg/kg) |
|---|---|---|---|---|
| hard-shell capsule | 52.00 (13) | 0.61 | 0.14 | 0.02–3.29 |
| soft-shell capsule | 25.00 (1) | 0.05 | ||
| tablet | 66.66 (4) | 0.42 | 0.04 | 0.02–1.58 |
| dietary supplement ingredient | 43.75 (7) | 0.37 | 0.02 | 0.02–0.09 |
| tincture | 0 (0) | N/A 2 | N/A | N/A |
| Sample Type | Incidence (%) 1 (Number of Quantified Samples) | Mean (ppm) | Median (ppm) | Range of Quantified Values (ppm) |
|---|---|---|---|---|
| Classification by dosage form | ||||
| hard-shell capsule | 32.00 (8) | 1.36 | 1.20 | 0.69–2.31 |
| soft-shell capsule | 25.00 (1) | N/A 2 | N/A | 5.97 |
| tablet | 50.00 (3) | 1.11 | 1.06 | 0.57–1.70 |
| dietary supplement ingredient | 13.33 (2) | 1.62 | 1.62 | 0.69–2.54 |
| tincture | 0 (0) | N/A | N/A | N/A |
| Classification by product label | ||||
| gluten-free products with “Cross Grain” symbol | 0 (0) | N/A | N/A | N/A |
| products labeled as gluten-free | 27.27 (3) | 1.43 | 1.79 | 0.69–2.31 |
| naturally (by origin) gluten-free products | 0 (0) | N/A | N/A | N/A |
| products with unknown gluten content | 28.95 (11) | 1.74 | 1.11 | 0.57–5.97 |
| products labeled that they may contain traces of gluten | 0 (0) | N/A | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amidžić Klarić, D.; Kovačić, J.; Jeličić, M.-L.; Zubčić, S.; Stankov, V.; Gulan Čičak, M.; Bučar, B.; Klarić, I.; Mornar, A. Assessment of Physicochemical Parameters and Contaminants in Herbal Dietary Supplements Used in the Treatment of Inflammatory Bowel Disease. Pharmaceuticals 2023, 16, 893. https://doi.org/10.3390/ph16060893
Amidžić Klarić D, Kovačić J, Jeličić M-L, Zubčić S, Stankov V, Gulan Čičak M, Bučar B, Klarić I, Mornar A. Assessment of Physicochemical Parameters and Contaminants in Herbal Dietary Supplements Used in the Treatment of Inflammatory Bowel Disease. Pharmaceuticals. 2023; 16(6):893. https://doi.org/10.3390/ph16060893
Chicago/Turabian StyleAmidžić Klarić, Daniela, Jelena Kovačić, Mario-Livio Jeličić, Snježana Zubčić, Vladimir Stankov, Marija Gulan Čičak, Boris Bučar, Ilija Klarić, and Ana Mornar. 2023. "Assessment of Physicochemical Parameters and Contaminants in Herbal Dietary Supplements Used in the Treatment of Inflammatory Bowel Disease" Pharmaceuticals 16, no. 6: 893. https://doi.org/10.3390/ph16060893
APA StyleAmidžić Klarić, D., Kovačić, J., Jeličić, M.-L., Zubčić, S., Stankov, V., Gulan Čičak, M., Bučar, B., Klarić, I., & Mornar, A. (2023). Assessment of Physicochemical Parameters and Contaminants in Herbal Dietary Supplements Used in the Treatment of Inflammatory Bowel Disease. Pharmaceuticals, 16(6), 893. https://doi.org/10.3390/ph16060893