Therapeutic Potential of Chinese Medicine for Endogenous Neurogenesis: A Promising Candidate for Stroke Treatment
Abstract
1. Introduction
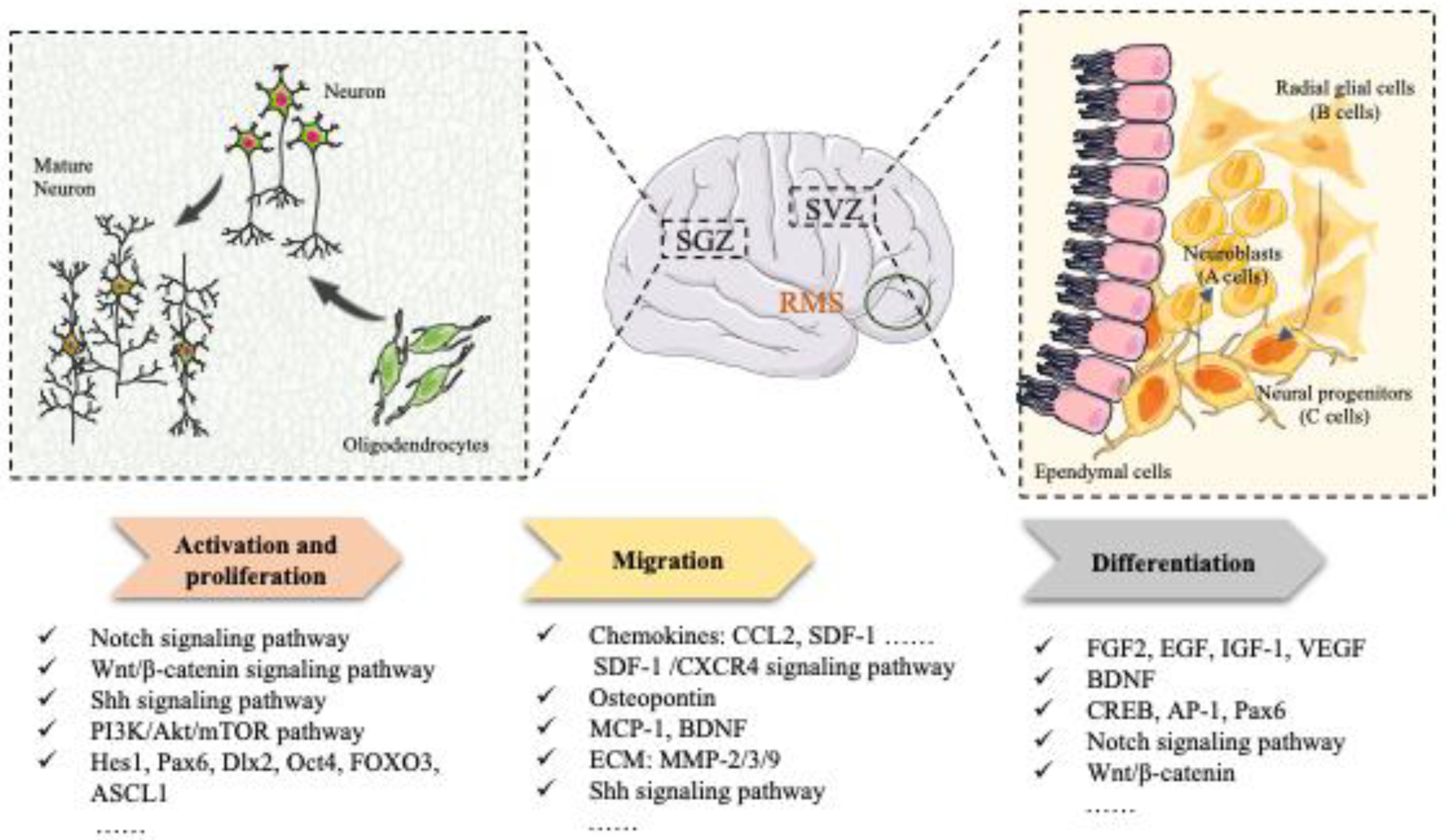
Endogenous Neurogenesis
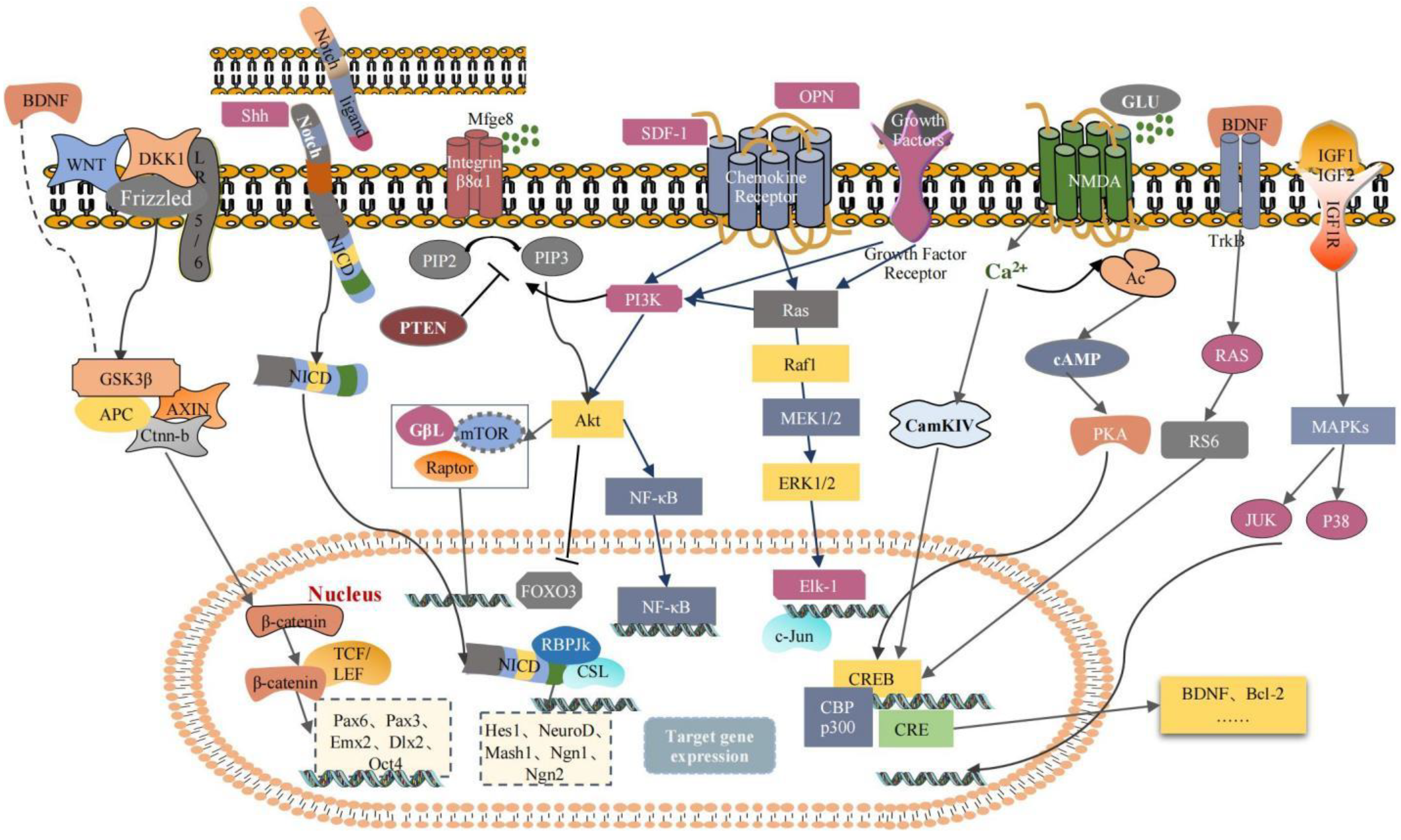
2. Endogenous Neurogenesis Mechanism: An Approach to Restoring Neurological Function
2.1. Activation and Proliferation
2.2. Migration
2.3. Differentiation
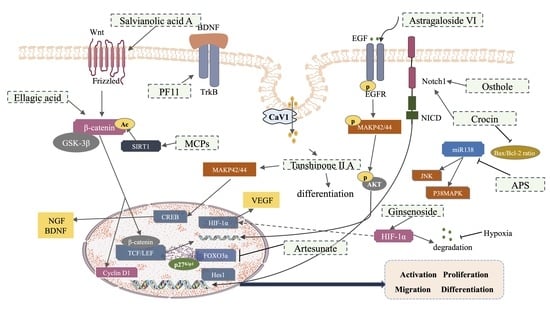
3. Effects and Mechanism of Traditional Chinese Medicine (TCM) in Promoting NSCs Involved in Neurogenesis after an Ischemic Stroke
4. Discussion and Conclusions
| Source | Classification | Species | Dosage | Treatment Route | Neurogenic Region | Model | Mechanism | Phenotype | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Momordica charantia (Ku Gua) | M.charantia polysaccharides | Rat | 200 mg/kg | Intragastric administration | SVZ/SGZ | MCAO | SIRT1, cytoplasmic, β-catenin, deacetylation | Rescue the memory and learning abilities of rats; enhance NSC proliferation | [125,148] |
| Panax pseudoginseng subsp (San Qi) | Pseudoginsenoside-F11 | Mice | 16, 32 mg/kg | Orally treated | DG | tMCAO | pro-BDNF, TrkB-T; ↑m-BDNF, TrkB-FL, p-AkT, p-CREB | Reduce brain infarction and brain edema; attenuate the mortality, sensorimotor dysfunction, cognitive impairment, and hippocampal atrophy | [126] |
| ginseng (Ren Shen) | Ginsenoside Rb1 | Mice | 50 mg/kg | Intraperitoneal injection | SVZ/SGZ | dMCAO | ↑cAMP, ↑PKA, ↑p-CREB | Improve functional recovery; stimulate axonal regeneration and brain repair | [132] |
| Radix Astragali (Huang Qi) | Astragaloside VI | Rat | 2 μg/kg | Intravenous injection | SVZ/DG | MCAO, | Nestin, p-EGFR, p-MAPK | Promote spatial learning and memory; improve impaired motor function | [134] |
| Radix Astragali (Huang Qi) | Astragaloside IV | Mice | 200 mg/kg | Intravenous injection | Hippocampus | IL-17 KO mice, Photochemical brain ischemia model | p-Akt, p-GSK-3β, Wnt2, β-catenin, Nestin, IL-17, Wnt | Ameliorate stroke-induced cognitive deficits; repair spines of apical dendrites in the hippocampus; stimulate hippocampal neurogenesis; inhibite neural apoptosis; relieve anxiety after stroke | [135,149] |
| Salvia miltiorrhiza Bge (Dan Shen) | Salvianolic acid A | Rat | 10 mg/kg | Intragastric administration | SVZ/ Hippocampus | Electrocoagulation-induced autologous thrombus stroke model | Wnt3a, p-GSK3β/GSK3β, β-catenin, TCF-4 | Decrease infarction volume and vascular embolism; ameliorate pathological injury; promote NSPC proliferation, migration and differentiation; enhance axonal regeneration and diminish neuronal apoptosis | [136] |
| artemisinin (Qing Hao Su) | Artesunate | Mice | 150 mg/kg | Intraperitoneal administration | SVZ | MCAO | Penumbra damage, white matter injury, FOXO3a, p27Kip1; DCX | Rescue ischemia damage; alleviate white matter injury; promote functional recovery; promote neurogenesis and proliferation of endogenous NSPCs | [138] |
| Cnidium monnieri (L.) (She Chuang Zi) | Osthole | Mice | 30 mg/kg | Intraperitoneal administration | SVZ/SGZ/DG/ CA3 | Model of stab wound injury is created to mimic the neuroendoscopy procedure | Notch-1, Hes-1, Nestin, NICD | Improve learning and memory function; promote the proliferation of endogenous NSCs; improve neuronal restoration; increase the number of neurons in the regions of brain injury | [137] |
| Crocus sativus L. (Fan Hong Hua) | Crocin | Rat | 10, 50 mg/kg | Intragastric administration | SVZ/DG | MCAO/R | Bax/bcl-2; Notch1 | Inhibit the release of inflammatory factors; reduce the apoptosis of nerve cells | [140] |
| Pomegranates (Shi Liu) | Ellagic acid | Rat | 10, 30, 90 mg/kg | Intragastric administration | SVZ/SGZ | Photothrombotic nerve injury model | nestin, β-catenin, Cyclin D1 | Improve the rats’ nerve-related abilities; remedy infarct volumes and morphological changes in the brain | [141] |
| Source | Classification | Species | Dosage | Model | Mechanism | Phenotype | Reference |
|---|---|---|---|---|---|---|---|
| Momordica charantia (Ku Gua) | M.charantia polysaccharides | C17.2 cells, primary cortical neural stem cells | 5 μg/mL | OGD, IRI | SIRT1, cytoplasmic, β-catenin, deacetylation | Change intracellular redox state; stimulate the proliferation | [125,148] |
| Panax pseudoginseng subsp (San Qi) | Pseudoginsenoside-F11 | Primary cultured NSCs | 100 μm | OGD/R | pro-BDNF, TrkB-T; ↑m-BDNF, TrkB-FL, p-AkT, p-CREB | Promote proliferation and differentiation | [126] |
| ginseng (Ren Shen) | Ginsenoside | Primary cultured NSCs | 1 μg/mL | OGD/R | HIF-1α, VEGF | Maintain NSC replication; promote NSC proliferation; promote NSC differentiation into neurons and astrocytes | [127] |
| Radix Astragali (Huang Qi) | Astragaloside VI | C17.2 cells or primary cultured NSCs | 10, 100 nM | DMEM/F12 media deprived of EGF or normal DMEM/F12 media stimulated for 2 h | Nestin, p-EGFR, p-MAPK | Enhance NSCs self-renewal and proliferation without affecting NSCs | [134] |
| Radix Astragali (Huang Qi) | Astragaloside IV | Primary cultured NSCs | 10 nM, 100 nM, 20 μmM | / | p-Akt, p-GSK-3β, Wnt2, β-catenin, Nestin, IL-17, Wnt | Promote hippocampal neurogenesis and NSC proliferation | [135,149] |
| Salvia miltiorrhiza Bge (Dan Shen) | Tanshinone II A | C17.2 cells, primary culture of embryonic cortical NSCs or PC12 cells | 0.1–3 μM | TIIA stimulated for 7 d | p-MAPK42/44, p-CREB, BDNF, NGF, GAP-43 | Promote neuronal differentiation; facilitate endocytosis and transportation across the cell membrane | [133] |
| Crocus sativus L. (Fan Hong Hua) | Crocin | NSCs | 10, 50 μM | Bax/bcl-2; Notch1 | Promote cell proliferation; increase cell migration; inhibit cell apoptosis; and promote neural regeneration | [140] | |
| Pomegranates (Shi Liu) | Ellagic acid | Primary cultured NSCs | 1, 3, 9 μg/mL | OGD/R | nestin, β-catenin, Cyclin D1 | Increase proliferation of NSCs | [141] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herpich, F.; Rincon, F. Management of Acute Ischemic Stroke. Crit. Care Med. 2020, 48, 1654–1663. [Google Scholar] [CrossRef]
- Fisher, M. New approaches to neuroprotective drug development. Stroke 2011, 42, S24–S27. [Google Scholar] [CrossRef]
- Mendelson, S.J.; Prabhakaran, S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: A Review. JAMA 2021, 325, 1088–1098. [Google Scholar] [CrossRef]
- Li, L.; Fan, X.; Zhang, X.-T.; Yue, S.-Q.; Sun, Z.-Y.; Zhu, J.-Q.; Zhang, J.-H.; Gao, X.-M.; Zhang, H. The effects of Chinese medicines on cAMP/PKA signaling in central nervous system dysfunction. Brain Res. Bull. 2017, 132, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Cao, L.; Kalionis, B.; Xia, S.; Tai, X. Applications of Induced Pluripotent Stem Cells in Studying the Neurodegenerative Diseases. Stem Cells Int. 2015, 2015, 382530. [Google Scholar] [CrossRef]
- Verheyden, G.; Nieuwboer, A.; De Wit, L.; Thijs, V.; Dobbelaere, J.; Devos, H.; Severijns, D.; Vanbeveren, S.; De Weerdt, W. Time course of trunk, arm, leg, and functional recovery after ischemic stroke. Neurorehabilit. Neural Repair 2008, 22, 173–179. [Google Scholar] [CrossRef]
- Campbell, B.C.; Mitchell, P.J.; Kleinig, T.J.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.J.; Parsons, M.W.; Oxley, T.J.; et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Berkhemer, O.A.; Fransen, P.S.S.; Beumer, D.; Berg, L.A.V.D.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.H.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef]
- Feliciano, D.; Zhang, S.; Nasrallah, C.M.; Lisgo, S.; Bordey, A. Embryonic cerebrospinal fluid nanovesicles carry evolutionarily conserved molecules and promote neural stem cell amplification. PLoS ONE 2014, 9, e88810. [Google Scholar] [CrossRef] [PubMed]
- Thored, P.; Wood, J.; Arvidsson, A.; Cammenga, J.; Kokaia, Z.; Lindvall, O. Long-Term Neuroblast Migration along Blood Vessels in an Area with Transient Angiogenesis and Increased Vascularization after Stroke. Stroke 2007, 38, 3032–3039. [Google Scholar] [CrossRef]
- Macas, J.; Nern, C.; Plate, K.H.; Momma, S. Increased Generation of Neuronal Progenitors after Ischemic Injury in the Aged Adult Human Forebrain. J. Neurosci. 2006, 26, 13114–13119. [Google Scholar] [CrossRef]
- Picard-Riera, N.; Nait-Oumesmar, B.; Evercooren, A.B.-V. Endogenous adult neural stem cells: Limits and potential to repair the injured central nervous system. J. Neurosci. Res. 2004, 76, 223–231. [Google Scholar] [CrossRef]
- Zhang, Z.; Chopp, M. Neural Stem Cells and Ischemic Brain. J. Stroke 2016, 18, 267–272. [Google Scholar] [CrossRef]
- Barker, R.A.; Götz, M.; Parmar, M. New approaches for brain repair—From rescue to reprogramming. Nature 2018, 557, 329–334. [Google Scholar] [CrossRef]
- Baker, E.W.; Platt, S.R.; Lau, V.W.; Grace, H.E.; Holmes, S.P.; Wang, L.; Duberstein, K.J.; Howerth, E.W.; Kinder, H.A.; Stice, S.L.; et al. Induced Pluripotent Stem Cell-Derived Neural Stem Cell Therapy Enhances Recovery in an Ischemic Stroke Pig Model. Sci. Rep. 2017, 7, 10075. [Google Scholar] [CrossRef]
- Eckert, A.; Huang, L.; Gonzalez, R.; Kim, H.-S.; Hamblin, M.H.; Lee, J.-P. Bystander Effect Fuels Human Induced Pluripotent Stem Cell-Derived Neural Stem Cells to Quickly Attenuate Early Stage Neurological Deficits after Stroke. Stem. Cells Transl. Med. 2015, 4, 841–851. [Google Scholar] [CrossRef]
- Tornero, D.; Wattananit, S.; Madsen, M.G.; Koch, P.; Wood, J.; Tatarishvili, J.; Mine, Y.; Ge, R.; Monni, E.; Devaraju, K.; et al. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain 2013, 136, 3561–3577. [Google Scholar] [CrossRef]
- Kawabori, M.; Shichinohe, H.; Kuroda, S.; Houkin, K. Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7380. [Google Scholar] [CrossRef]
- Sinden, J.D.; Hicks, C.; Stroemer, P.; Vishnubhatla, I.; Corteling, R. Human Neural Stem Cell Therapy for Chronic Ischemic Stroke: Charting Progress from Laboratory to Patients. Stem Cells Dev. 2017, 26, 933–947. [Google Scholar] [CrossRef]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef]
- Dibajnia, P.; Morshead, C.M. Role of neural precursor cells in promoting repair following stroke. Acta Pharmacol. Sin. 2013, 34, 78–90. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Tucker, D.; Dong, Y.; Lu, Y.; Zhao, N.; Wang, R.; Zhang, Q. Methylene Blue promotes cortical neurogenesis and ameliorates behavioral deficit after photothrombotic stroke in rats. Neuroscience 2016, 336, 39–48. [Google Scholar] [CrossRef]
- Zhao, Y.; Guan, Y.-F.; Zhou, X.-M.; Li, G.-Q.; Li, Z.-Y.; Zhou, C.-C.; Wang, P.; Miao, C.-Y. Regenerative Neurogenesis After Ischemic Stroke Promoted by Nicotinamide Phosphoribosyltransferase–Nicotinamide Adenine Dinucleotide Cascade. Stroke 2015, 46, 1966–1974. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, L. Neural stem cell therapies and hypoxic-ischemic brain injury. Prog. Neurobiol. 2019, 173, 1–17. [Google Scholar] [CrossRef]
- Bassi, M.S.; Iezzi, E.; Gilio, L.; Centonze, D.; Buttari, F. Synaptic Plasticity Shapes Brain Connectivity: Implications for Network Topology. Int. J. Mol. Sci. 2019, 20, 6193. [Google Scholar] [CrossRef]
- Farah, M.; Marshall, K. Axonal regeneration and sprouting as a potential therapeutic target for nervous system disorders. Neural Regen. Res. 2021, 16, 1901–1910. [Google Scholar] [CrossRef]
- Li, F.; Sami, A.; Noristani, H.N.; Slattery, K.; Qiu, J.; Groves, T.; Wang, S.; Veerasammy, K.; Chen, Y.X.; Morales, J.; et al. Glial Metabolic Rewiring Promotes Axon Regeneration and Functional Recovery in the Central Nervous System. Cell Metab. 2020, 32, 767–785.e7. [Google Scholar] [CrossRef]
- Liu, T.; Ding, Y.; Wen, A. Traditional Chinese medicine for ischaemic stroke. Lancet Neurol. 2018, 17, 745. [Google Scholar] [CrossRef]
- Wang, J.; Hu, J.; Chen, X.; Lei, X.; Feng, H.; Wan, F.; Tan, L. Traditional Chinese Medicine Monomers: Novel Strategy for Endogenous Neural Stem Cells Activation after Stroke. Front. Cell Neurosci. 2021, 15, 628115. [Google Scholar] [CrossRef]
- Haas, S.; Weidner, N.; Winkler, J. Adult stem cell therapy in stroke. Curr. Opin. Neurol. 2005, 18, 59–64. [Google Scholar] [CrossRef]
- Richards, L.J.; Kilpatrick, T.J.; Bartlett, P.F. De novo generation of neuronal cells from the adult mouse brain. Proc. Natl. Acad. Sci. USA 1992, 89, 8591–8595. [Google Scholar] [CrossRef]
- Reynolds, B.A.; Weiss, S. Generation of Neurons and Astrocytes from Isolated Cells of the Adult Mammalian Central Nervous System. Science 1992, 255, 1707–1710. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, C.; Zheng, X.; Wu, L.; Liu, Z.; Liao, B.; Shi, Y.; Li, X.; Xu, J.; Chen, S. Full-Length Multi-Barcoding: DNA Barcoding from Single Ingredient to Complex Mixtures. Genes 2019, 10, 343. [Google Scholar] [CrossRef]
- Temple, S. The development of neural stem cells. Nature 2001, 414, 112–117. [Google Scholar] [CrossRef]
- Breunig, J.J.; Haydar, T.F.; Rakic, P. Neural stem cells: Historical perspective and future prospects. Neuron 2011, 70, 614–625. [Google Scholar] [CrossRef]
- Yadirgi, G.; Marino, S. Adult neural stem cells and their role in brain pathology. J. Pathol. 2009, 217, 242–253. [Google Scholar] [CrossRef]
- Li, Y.; Guo, W. Neural Stem Cell Niche and Adult Neurogenesis. The Neuroscientist: A Review Journal Bringing Neurobiology. Neurol. Psychiatry 2021, 27, 235–245. [Google Scholar]
- Shihabuddin, L.S.; Horner, P.J.; Ray, J.; Gage, F.H. Adult Spinal Cord Stem Cells Generate Neurons after Transplantation in the Adult Dentate Gyrus. J. Neurosci. 2000, 20, 8727–8735. [Google Scholar] [CrossRef]
- Mehler, M.F.; Gokhan, S. Postnatal Cerebral Cortical Multipotent Progenitors: Regulatory Mechanisms and Potential Role in the Development of Novel Neural Regenerative Strategies. Brain Pathol. 1999, 9, 515–526. [Google Scholar] [CrossRef]
- Huang, L.; Wang, G. The Effects of Different Factors on the Behavior of Neural Stem Cells. Stem Cells Int. 2017, 2017, 1–16. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Georgi, R.; Kolberg, B.; Yang, L. Effects of acupuncture on angiogenesis-associated factor expression in ischemic brain tissue following cerebral infarction in rats. Acupunct. Herb. Med. 2023, 3, 46–54. [Google Scholar] [CrossRef]
- Song, M.; Yu, S.P.; Mohamad, O.; Cao, W.; Wei, Z.Z.; Gu, X.; Jiang, M.Q.; Wei, L. Optogenetic stimulation of glutamatergic neuronal activity in the striatum enhances neurogenesis in the subventricular zone of normal and stroke mice. Neurobiol. Dis. 2017, 98, 9–24. [Google Scholar] [CrossRef]
- Kaneko, N.; Sawada, M.; Sawamoto, K. Mechanisms of neuronal migration in the adult brain. J. Neurochem. 2017, 141, 835–847. [Google Scholar] [CrossRef]
- Ihrie, R.A.; Alvarez-Buylla, A. Cells in the astroglial lineage are neural stem cells. Cell Tissue Res. 2008, 331, 179–191. [Google Scholar] [CrossRef]
- Li, W.L.; Chu, M.W.; Wu, A.; Suzuki, Y.; Imayoshi, I.; Komiyama, T. Adult-born neurons facilitate olfactory bulb pattern separation during task engagement. eLife 2018, 7, e33006. [Google Scholar] [CrossRef]
- Cameron, H.A.; Mckay, R.D. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001, 435, 406–417. [Google Scholar] [CrossRef]
- Ming, G.-L.; Song, H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Robel, S.; Berninger, B.; Götz, M. The stem cell potential of glia: Lessons from reactive gliosis. Nat. Rev. Neurosci. 2011, 12, 88–104. [Google Scholar] [CrossRef]
- Kleiderman, S.; Gutbier, S.; Tufekci, K.U.; Ortega, F.; Sá, J.V.; Teixeira, A.P.; Brito, C.; Glaab, E.; Berninger, B.; Alves, P.M.; et al. Conversion of Nonproliferating Astrocytes into Neurogenic Neural Stem Cells: Control by FGF2 and Interferon-γ. Stem Cells 2016, 34, 2861–2874. [Google Scholar] [CrossRef]
- Tan, L.; Zhong, J.; Li, R.-W.; Wang, J.; Wang, Y.; Ge, H.-F.; Xian, J.-S.; Feng, H. Neuroprotection by cattle encephalon glycoside and ignotin beyond the time window of thrombolysis in ischemic stroke. Neural Regen. Res. 2021, 16, 312. [Google Scholar] [CrossRef]
- Nakayama, D.; Matsuyama, T.; Ishibashi-Ueda, H.; Nakagomi, T.; Kasahara, Y.; Hirose, H.; Kikuchi-Taura, A.; Stern, D.M.; Mori, H.; Taguchi, A. Injury-induced neural stem/progenitor cells in post-stroke human cerebral cortex. Eur. J. Neurosci. 2010, 31, 90–98. [Google Scholar] [CrossRef]
- Marti-Fabregas, J.; Romaguera-Ros, M.; Gomez-Pinedo, U.; Martinez-Ramirez, S.; Jimenez-Xarrie, E.; Marin, R.; Marti-Vilalta, J.L.; Garcia-Verdugo, J.M. Proliferation in the human ipsilateral subventricular zone after ischemic stroke. Neurology 2010, 74, 357–365. [Google Scholar] [CrossRef]
- Quiroz, E.N.; Quiroz, R.N.; Ahmad, M.; Escorcia, L.G.; Villarreal, J.L.; Ponce, C.F.; Martinez, G.A. Cell Signaling in Neuronal Stem Cells. Cells 2018, 7, 75. [Google Scholar] [CrossRef]
- Bain, J.M.; Moore, L.; Ren, Z.; Simonishvili, S.; Levison, S.W. Vascular Endothelial Growth Factors A and C are Induced in the SVZ following Neonatal Hypoxia–Ischemia and Exert Different Effects on Neonatal Glial Progenitors. Transl. Stroke Res. 2013, 4, 158–170. [Google Scholar] [CrossRef]
- Yu, J.H.; Seo, J.-H.; Lee, J.Y.; Lee, M.-Y.; Cho, S.-R. Induction of Neurorestoration from Endogenous Stem Cells. Cell Transplant. 2016, 25, 863–882. [Google Scholar] [CrossRef]
- Yagita, Y.; Kitagawa, K.; Ohtsuki, T.; Takasawa, K.-I.; Miyata, T.; Okano, H.; Hori, M.; Matsumoto, M. Neurogenesis by Progenitor Cells in the Ischemic Adult Rat Hippocampus. Stroke 2001, 32, 1890–1896. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Zhang, L.; Chopp, M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience 2001, 105, 33–41. [Google Scholar] [CrossRef]
- Jin, K.; Wang, X.; Xie, L.; Mao, X.O.; Zhu, W.; Wang, Y.; Shen, J.; Mao, Y.; Banwait, S.; Greenberg, D.A. Evidence for stroke-induced neurogenesis in the human brain. Proc. Natl. Acad. Sci. USA 2006, 103, 13198–13202. [Google Scholar] [CrossRef]
- Jin, K.; Wang, X.; Xie, L.; Mao, X.O.; Greenberg, D.A. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc. Natl. Acad. Sci. USA 2010, 107, 7993–7998. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kageyama, R. Lysosomes and signaling pathways for maintenance of quiescence in adult neural stem cells. FEBS J. 2021, 288, 3082–3093. [Google Scholar] [CrossRef]
- Grandbarbe, L.; Bouissac, J.; Rand, M.; de Angelis, M.H.; Artavanis-Tsakonas, S.; Mohier, E. Delta-Notch signaling controls the generation of neurons/glia from neural stem cells in a stepwise process. Development 2003, 130, 1391–1402. [Google Scholar] [CrossRef]
- Bray, S.; Bernard, F. Notch Targets and Their Regulation. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 253–275. [Google Scholar]
- Qin, W.; Chen, S.; Yang, S.; Xu, Q.; Xu, C.; Cai, J. The Effect of Traditional Chinese Medicine on Neural Stem Cell Proliferation and Differentiation. Aging Dis. 2017, 8, 792–811. [Google Scholar] [CrossRef]
- Sivakumar, K.C.; Dhanesh, S.B.; Shobana, S.; James, J.; Mundayoor, S.; Song, K.; Ge, D.; Guan, S.; Sun, C.; Liu, T.; et al. A Systems Biology Approach to Model Neural Stem Cell Regulation by Notch, Shh, Wnt, and EGF Signaling Pathways. OMICS: A J. Integr. Biol. 2011, 15, 729–737. [Google Scholar] [CrossRef]
- Alexson, T.O.; Hitoshi, S.; Coles, B.L.; Bernstein, A.; van der Kooy, D. Notch signaling is required to maintain all neural stem cell populations—Irrespective of spatial or temporal niche. Dev. Neurosci. 2006, 28, 34–48. [Google Scholar] [CrossRef]
- Wang, X.; Mao, X.; Xie, L.; Greenberg, D.A.; Jin, K. Involvement of notch1 signaling in neurogenesis in the subventricular zone of normal and ischemic rat brain in vivo. J. Cereb. Blood Flow Metab. 2009, 29, 1644–1654. [Google Scholar] [CrossRef]
- Nyfeler, Y.; Kirch, R.D.; Mantei, N.; Leone, D.P.; Radtke, F.; Suter, U.; Taylor, V. Jagged1 signals in the postnatal subventricular zone are required for neural stem cell self-renewal. EMBO J. 2005, 24, 3504–3515. [Google Scholar] [CrossRef]
- Androutsellis-Theotokis, A.; Leker, R.R.; Soldner, F.; Hoeppner, D.J.; Ravin, R.; Poser, S.W.; Rueger, M.A.; Bae, S.-K.; Kittappa, R.; McKay, R.D.G. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 2006, 442, 823–826. [Google Scholar] [CrossRef]
- Wang, L.; Chopp, M.; Zhang, R.; Zhang, L.; LeTourneau, Y.; Feng, Y.; Jiang, A.; Morris, D.; Zhang, Z. The Notch pathway mediates expansion of a progenitor pool and neuronal differentiation in adult neural progenitor cells after stroke. Neuroscience 2009, 158, 1356–1363. [Google Scholar] [CrossRef]
- Sun, F.; Mao, X.; Xie, L.; Ding, M.; Shao, B.; Jin, K. Notch1 signaling modulates neuronal progenitor activity in the subventricular zone in response to aging and focal ischemia. Aging Cell 2013, 12, 978–987. [Google Scholar] [CrossRef]
- Jessell, T.M. Neuronal specification in the spinal cord: Inductive signals and transcriptional codes. Nat. Rev. Genet. 2000, 1, 20–29. [Google Scholar] [CrossRef]
- Ferent, J.; Cochard, L.; Faure, H.; Taddei, M.; Hahn, H.; Ruat, M.; Traiffort, E. Genetic activation of Hedgehog signaling unbalances the rate of neural stem cell renewal by increasing symmetric divisions. Stem Cell Rep. 2014, 3, 312–323. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Guo, C.Y.; Shi, D.Z. Effect of shengmai injection on the fatality rate of patients with acute myocardial in-farction: A systematic review. Zhongguo Zhong Xi Yi Jie He Za Zhi 2008, 28, 1069–1073. [Google Scholar]
- Merson, T.D.; Bourne, J.A. Endogenous neurogenesis following ischaemic brain injury: Insights for therapeutic strategies. Int. J. Biochem. Cell Biol. 2014, 56, 4–19. [Google Scholar] [CrossRef]
- Banerjee, S.B.; Rajendran, R.; Dias, B.G.; Ladiwala, U.; Tole, S.; Vaidya, V.A. Recruitment of the Sonic hedgehog signalling cascade in electroconvulsive seizure-mediated regulation of adult rat hippocampal neurogenesis. Eur. J. Neurosci. 2005, 22, 1570–1580. [Google Scholar] [CrossRef]
- Machold, R.; Hayashi, S.; Rutlin, M.; Muzumdar, M.D.; Nery, S.; Corbin, J.G.; Gritli-Linde, A.; Dellovade, T.; Porter, J.A.; Rubin, L.L.; et al. Sonic Hedgehog Is Required for Progenitor Cell Maintenance in Telencephalic Stem Cell Niches. Neuron 2003, 39, 937–950. [Google Scholar] [CrossRef]
- Lai, K.; Kaspar, B.K.; Gage, F.H.; Schaffer, D.V. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci. 2003, 6, 21–27. [Google Scholar] [CrossRef]
- Webb, A.E.; Pollina, E.A.; Vierbuchen, T.; Urbán, N.; Ucar, D.; Leeman, D.S.; Martynoga, B.; Sewak, M.; Rando, T.A.; Guillemot, F.; et al. FOXO3 Shares Common Targets with ASCL1 Genome-wide and Inhibits ASCL1-Dependent Neurogenesis. Cell Rep. 2013, 4, 477–491. [Google Scholar] [CrossRef]
- Bonaguidi, M.A.; Wheeler, M.A.; Shapiro, J.S.; Stadel, R.P.; Sun, G.J.; Ming, G.-L.; Song, H. In Vivo Clonal Analysis Reveals Self-Renewing and Multipotent Adult Neural Stem Cell Characteristics. Cell 2011, 145, 1142–1155. [Google Scholar] [CrossRef]
- Pilz, G.A.; Bottes, S.; Betizeau, M.; Jörg, D.J.; Carta, S.; April, S.; Simons, B.D.; Helmchen, F.; Jessberger, S. Live imaging of neurogenesis in the adult mouse hippocampus. Science 2018, 359, 658–662. [Google Scholar] [CrossRef]
- Khalifeh-Soltani, A.; Ha, A.; Podolsky, M.J.; McCarthy, D.A.; McKleroy, W.; Azary, S.; Sakuma, S.; Tharp, K.M.; Wu, N.; Yokosaki, Y.; et al. α8β1 integrin regulates nutrient absorption through an Mfge8-PTEN dependent mechanism. Elife 2016, 5, e13063. [Google Scholar] [CrossRef]
- Paik, J.-H.; Ding, Z.; Narurkar, R.; Ramkissoon, S.; Muller, F.; Kamoun, W.S.; Chae, S.-S.; Zheng, H.; Ying, H.; Mahoney, J.; et al. FoxOs Cooperatively Regulate Diverse Pathways Governing Neural Stem Cell Homeostasis. Cell Stem Cell 2009, 5, 540–553. [Google Scholar] [CrossRef]
- Schäffner, I.; Minakaki, G.; Khan, M.A.; Balta, E.-A.; Schlötzer-Schrehardt, U.; Schwarz, T.J.; Beckervordersandforth, R.; Winner, B.; Webb, A.E.; DePinho, R.A.; et al. FoxO Function Is Essential for Maintenance of Autophagic Flux and Neuronal Morphogenesis in Adult Neurogenesis. Neuron 2018, 99, 1188–1203.e6. [Google Scholar] [CrossRef]
- Renault, V.M.; Rafalski, V.A.; Morgan, A.A.; Salih, D.A.; Brett, J.O.; Webb, A.E.; Villeda, S.A.; Thekkat, P.U.; Guillerey, C.; Denko, N.C.; et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell 2009, 5, 527–539. [Google Scholar] [CrossRef]
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002, 8, 963–970. [Google Scholar] [CrossRef]
- Lee, J.-P.; Jeyakumar, M.; Gonzalez, R.; Takahashi, H.; Lee, P.-J.; Baek, R.C.; Clark, D.; Rose, H.; Fu, G.; Clarke, J.; et al. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nat. Med. 2007, 13, 439–447. [Google Scholar] [CrossRef]
- Lee, J.-P.; Zhang, R.; Yan, M.; Duggineni, S.; Wakeman, D.R.; Niles, W.L.; Feng, Y.; Chen, J.; Hamblin, M.H.; Han, E.B.; et al. Chemical mutagenesis of a GPCR ligand: Detoxifying “inflammo-attraction” to direct therapeutic stem cell migration. Proc. Natl. Acad. Sci. USA 2020, 117, 31177–31188. [Google Scholar] [CrossRef]
- Boese, A.C.; Eckert, A.; Hamblin, M.H.; Lee, J.-P. Human neural stem cells improve early stage stroke outcome in delayed tissue plasminogen activator-treated aged stroke brains. Exp. Neurol. 2020, 329, 113275. [Google Scholar] [CrossRef]
- Coviello, S.; Benedetti, B.; Jakubecova, D.; Belles, M.; Klimczak, P.; Gramuntell, Y.; Couillard-Despres, S.; Nacher, J. PSA Depletion Induces the Differentiation of Immature Neurons in the Piriform Cortex of Adult Mice. Int. J. Mol. Sci. 2021, 22, 5733. [Google Scholar] [CrossRef]
- Cipriani, S.; Ferrer, I.; Aronica, E.; Kovacs, G.G.; Verney, C.; Nardelli, J.; Khung, S.; Delezoide, A.-L.; Milenkovic, I.; Rasika, S.; et al. Hippocampal Radial Glial Subtypes and Their Neurogenic Potential in Human Fetuses and Healthy and Alzheimer’s Disease Adults. Cereb. Cortex 2018, 28, 2458–2478. [Google Scholar] [CrossRef]
- Imitola, J.; Comabella, M.; Chandraker, A.K.; Dangond, F.; Sayegh, M.H.; Snyder, E.Y.; Khoury, S.J. Neural Stem/Progenitor Cells Express Costimulatory Molecules That Are Differentially Regulated by Inflammatory and Apoptotic Stimuli. Am. J. Pathol. 2004, 164, 1615–1625. [Google Scholar] [CrossRef]
- Robin, A.M.; Zhang, Z.G.; Wang, L.; Zhang, R.L.; Katakowski, M.; Zhang, L.; Wang, Y.; Zhang, C.; Chopp, M. Stromal Cell-Derived Factor 1α Mediates Neural Progenitor Cell Motility after Focal Cerebral Ischemia. J. Cereb. Blood Flow Metab. 2006, 26, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Tiveron, M.C.; Rossel, M.; Moepps, B.; Zhang, Y.L.; Seidenfaden, R.; Favor, J.; König, N.; Cremer, H. Molecular Interaction between Projection Neuron Precursors and Invading Interneurons via Stromal-Derived Factor 1 (CXCL12)/CXCR4 Signaling in the Cortical Subventricular Zone/Intermediate Zone. J. Neurosci. 2006, 26, 13273–13278. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Y.; Gao, S.; Fu, X.; Cao, Y.; Peng, Y.; Zhuang, J.; Hu, J.; Shao, A.; Wang, L. Potential Mechanisms and Perspectives in Ischemic Stroke Treatment Using Stem Cell Therapies. Front. Cell Dev. Biol. 2021, 9, 646927. [Google Scholar] [CrossRef]
- Rabenstein, M.; Hucklenbroich, J.; Willuweit, A.; Ladwig, A.; Fink, G.R.; Schroeter, M.; Langen, K.-J.; Rueger, M.A. Osteopontin mediates survival, proliferation and migration of neural stem cells through the chemokine receptor CXCR4. Stem Cell Res. Ther. 2015, 6, 99. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Cai, J.; Qiu, Y.; Zheng, H.; Lai, X.; Sui, X.; Wang, Y.; Lu, Q.; Zhang, Y.; et al. Targeted homing of CCR2-overexpressing mesenchymal stromal cells to ischemic brain enhances post-stroke recovery partially through PRDX4-mediated blood-brain barrier preservation. Theranostics 2018, 8, 5929–5944. [Google Scholar] [CrossRef]
- Lee, S.; Kim, O.J.; Lee, K.O.; Jung, H.; Oh, S.-H.; Kim, N.K. Enhancing the Therapeutic Potential of CCL2-Overexpressing Mesenchymal Stem Cells in Acute Stroke. Int. J. Mol. Sci. 2020, 21, 7795. [Google Scholar] [CrossRef]
- Lee, S.-R.; Kim, H.-Y.; Rogowska, J.; Zhao, B.-Q.; Bhide, P.; Parent, J.M.; Lo, E.H. Involvement of Matrix Metalloproteinase in Neuroblast Cell Migration from the Subventricular Zone after Stroke. J. Neurosci. 2006, 26, 3491–3495. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.G.; Zhang, R.L.; Gregg, S.R.; Hozeska-Solgot, A.; LeTourneau, Y.; Wang, Y.; Chopp, M. Matrix Metalloproteinase 2 (MMP2) and MMP9 Secreted by Erythropoietin-Activated Endothelial Cells Promote Neural Progenitor Cell Migration. J. Neurosci. 2006, 26, 5996–6003. [Google Scholar] [CrossRef]
- Lindvall, O.; Kokaia, Z. Neurogenesis following Stroke Affecting the Adult Brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a019034. [Google Scholar] [CrossRef]
- Navlakha, S.; Bar-Joseph, Z.; Barth, A.L. Network Design and the Brain. Trends Cogn. Sci. 2018, 22, 64–78. [Google Scholar] [CrossRef]
- Park, H.-J.; Friston, K. Structural and Functional Brain Networks: From Connections to Cognition. Science 2013, 342, 1238411. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.A.; Jin, K. Growth factors and stroke. Neurorx 2006, 3, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Kirby, E.D.; Muroy, S.E.; Sun, W.G.; Covarrubias, D.; Leong, M.J.; Barchas, L.A.; Kaufer, D. Acute stress enhances adult rat hippocampal neurogenesis and activation of newborn neurons via secreted astrocytic FGF2. eLife 2013, 2, e00362. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, D.; Shimazu, K.; Zhou, Y.-X.; Lu, B.; Deng, C.-X. Fibroblast Growth Factor Receptor-1 is Required for Long-Term Potentiation, Memory Consolidation, and Neurogenesis. Biol. Psychiatry 2007, 62, 381–390. [Google Scholar] [CrossRef]
- Sun, J.-Q.; Sha, B.; Zhou, W.-H.; Yang, Y. Basic fibroblast growth factor stimulates the proliferation and differentiation of neural stem cells in neonatal rats after ischemic brain injury. Brain Dev. 2009, 31, 331–340. [Google Scholar] [CrossRef]
- Talbot, K.; Wang, H.Y.; Kazi, H.; Han, L.Y.; Bakshi, K.P.; Stucky, A.; Fuino, R.L.; Kawaguchi, K.R.; Samoyedny, A.J.; Wilson, R.S.; et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Investig. 2012, 122, 1316–1338. [Google Scholar] [CrossRef]
- Åberg, M.A.; Åberg, N.D.; Palmer, T.D.; Alborn, A.M.; Carlsson-Skwirut, C.; Bang, P.; Rosengren, L.E.; Olsson, T.; Gage, F.H.; Eriksson, P.S. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol. Cell Neurosci. 2003, 24, 23–40. [Google Scholar] [CrossRef]
- Hsieh, J.; Aimone, J.; Kaspar, B.K.; Kuwabara, T.; Nakashima, K.; Gage, F.H. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J. Cell Biol. 2004, 164, 111–122. [Google Scholar] [CrossRef]
- Greenberg, D.A.; Jin, K. Vascular endothelial growth factors (VEGFs) and stroke. Cell Mol. Life Sci. 2013, 70, 1753–1761. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, W.; Sha, B.; Yang, Y. Ischemia induced neural stem cell proliferation and differentiation in neonatal rat involved vascular endothelial growth factor and transforming growth factor-beta pathways. Brain Dev. 2010, 32, 191–200. [Google Scholar] [CrossRef]
- Takahashi, T.; Shibuya, M. The 230 kDa mature form of KDR/Flk-1 (VEGF receptor-2) activates the PLC-gamma pathway and partially induces mitotic signals in NIH3T3 fibroblasts. Oncogene 1997, 14, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.B.; Ren, D.; Veldhouse, T.J.; Miller, R.J. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J. Neurosci. Res. 2004, 76, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, J. Conditional knockout of brain-derived neurotrophic factor in the hippocampus increases death of adult-born immature neurons following traumatic brain injury. J. Neurotrauma 2009, 26, 1325–1335. [Google Scholar] [CrossRef]
- Waterhouse, E.G.; An, J.J.; Orefice, L.L.; Baydyuk, M.; Liao, G.-Y.; Zheng, K.; Lu, B.; Xu, B. BDNF Promotes Differentiation and Maturation of Adult-born Neurons through GABAergic Transmission. J. Neurosci. 2012, 32, 14318–14330. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Sawada, M.; Huang, S.-H.; Ogino, T.; Ohata, S.; Kubo, A.; Sawamoto, K. Roles of Wnt Signaling in the Neurogenic Niche of the Adult Mouse Ventricular–Subventricular Zone. Neurochem. Res. 2016, 41, 222–230. [Google Scholar] [CrossRef]
- Lambert, C.; Cisternas, P.; Inestrosa, N.C. Role of Wnt Signaling in Central Nervous System Injury. Mol. Neurobiol. 2016, 53, 2297–2311. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.-L.; Wang, W.; Zuo, W.; Xue, J.-L.; Xu, J.-D.; Ai, H.-X.; Zhang, L.; Wang, X.-M.; Ji, X.-M. Promoting neurogenesis via Wnt/β-catenin signaling pathway accounts for the neurorestorative effects of morroniside against cerebral ischemia injury. Eur. J. Pharmacol. 2014, 738, 214–221. [Google Scholar] [CrossRef]
- Martínez-Cerdeño, V.; Cunningham, C.L.; Camacho, J.; Keiter, J.A.; Ariza, J.; Lovern, M.; Noctor, S.C. Evolutionary origin of Tbr2-expressing precursor cells and the subventricular zone in the developing cortex: Tbr2-expressing precursor cells. J. Comp. Neurol. 2016, 524, 433–447. [Google Scholar] [CrossRef]
- Tanaka, T.; Abe, H.; Kimura, M.; Onda, N.; Mizukami, S.; Yoshida, T.; Shibutani, M. Developmental exposure to T-2 toxin reversibly affects postnatal hippocampal neurogenesis and reduces neural stem cells and progenitor cells in mice. Arch. Toxicol. 2016, 90, 2009–2024. [Google Scholar] [CrossRef]
- Jami, A.; Gadi, J.; Lee, M.J.; Kim, E.J.; Lee, M.J.; Jung, H.-S.; Kim, H.-H.; Lim, S.-K. Pax6 expressed in osteocytes inhibits canonical Wnt signaling. Mol. Cells 2013, 35, 305–312. [Google Scholar] [CrossRef]
- Singh, S.; Mishra, A.; Srivastava, N.; Shukla, S. MK-801 (Dizocilpine) Regulates Multiple Steps of Adult Hippocampal Neurogenesis and Alters Psychological Symptoms via Wnt/β-Catenin Signaling in Parkinsonian Rats. ACS Chem. Neurosci. 2017, 8, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Shruster, A.; Ben-Zur, T.; Melamed, E.; Offen, D. Wnt signaling enhances neurogenesis and improves neurological function after focal ischemic injury. PLoS ONE 2012, 7, e40843. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.Z.; Zhang, J.Y.; Taylor, T.M.; Gu, X.; Zhao, Y.; Wei, L. Neuroprotective and regenerative roles of intranasal Wnt-3a administration after focal ischemic stroke in mice. J. Cereb. Blood Flow Metab. 2018, 38, 404–421. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Fan, H.; Cai, H.; Hu, Z.; Zhou, X.; Li, F.; Chen, H.; Shen, J.; Qi, S. Promotion of Momordica Charantia polysaccharides on neural stem cell proliferation by increasing SIRT1 activity after cerebral ischemia/reperfusion in rats. Brain Res. Bull. 2021, 170, 254–263. [Google Scholar] [CrossRef]
- Yuan, L.; Sun, S.; Pan, X.; Zheng, L.; Li, Y.; Yang, J.; Wu, C. Pseudoginsenoside-F11 improves long-term neurological function and promotes neurogenesis after transient cerebral ischemia in mice. Neurochem. Int. 2020, 133, 104586. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Bai, H.; Li, Q.; Li, J.; Wan, F.; Tian, M.; Li, Y.; Song, Y.; Zhang, J.; Si, Y. In vitro investigation of the mechanism underlying the effect of ginsenoside on the proliferation and differentiation of neural stem cells subjected to oxygen-glucose deprivation/reperfusion. Int. J. Mol. Med. 2018, 41, 353–363. [Google Scholar] [CrossRef]
- Giachino, C.; De Marchis, S.; Giampietro, C.; Parlato, R.; Perroteau, I.; Schütz, G.; Fasolo, A.; Peretto, P. cAMP response element-binding protein regulates differentiation and survival of newborn neurons in the olfactory bulb. J. Neurosci. 2005, 25, 10105–10118. [Google Scholar] [CrossRef]
- Hong, H.; Lu, X.; Lu, Q.; Huang, C.; Cui, Z. Potential therapeutic effects and pharmacological evidence of sinomenine in central nervous system disorders. Front. Pharmacol. 2022, 13, 1015035. [Google Scholar] [CrossRef]
- Egeland, M.; Zunszain, P.; Pariante, C.M. Molecular mechanisms in the regulation of adult neurogenesis during stress. Nat. Rev. Neurosci. 2015, 16, 189–200. [Google Scholar] [CrossRef]
- Herold, S.; Jagasia, R.; Merz, K.; Wassmer, K.; Lie, D. CREB signalling regulates early survival, neuronal gene expression and morphological development in adult subventricular zone neurogenesis. Mol. Cell Neurosci. 2011, 46, 79–88. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, X.; Cui, L.; Chen, R.; Zhang, C.; Xue, J.; Zhang, L.; He, W.; Li, J.; Wei, S.; et al. Ginsenoside Rb1 Promotes Motor Functional Recovery and Axonal Regeneration in Post-stroke Mice through cAMP/PKA/CREB Signaling Pathway. Brain Res. Bull. 2020, 154, 51–60. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, P.; Hu, S.; Du, L.; Xu, Z.; Zhang, H.; Cui, W.; Mak, S.; Xu, D.; Shen, J.; et al. Tanshinone II A, a multiple target neuroprotectant, promotes caveolae-dependent neuronal differentiation. Eur. J. Pharmacol. 2015, 765, 437–446. [Google Scholar] [CrossRef]
- Chen, X.; Wu, H.; Chen, H.; Wang, Q.; Xie, X.-J.; Shen, J. Astragaloside VI Promotes Neural Stem Cell Proliferation and Enhances Neurological Function Recovery in Transient Cerebral Ischemic Injury via Activating EGFR/MAPK Signaling Cascades. Mol. Neurobiol. 2019, 56, 3053–3067. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, H.; Wang, W.; Chen, Z.; Wang, S.; Li, J.; Li, G.; Gao, C.; Sun, X. Astragaloside IV Exerts Cognitive Benefits and Promotes Hippocampal Neurogenesis in Stroke Mice by Downregulating Interleukin-17 Expression via Wnt Pathway. Front. Pharmacol. 2020, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kong, D.W.; Ma, G.D.; Liu, C.D.; Yang, Y.J.; Liu, S.; Jiang, N.; Pan, Z.R.; Zhang, W.; Kong, L.L.; et al. Long-term administration of salvianolic acid A promotes endogenous neurogenesis in ischemic stroke rats through activating Wnt3a/GSK3beta/beta-catenin signaling pathway. Acta Pharmacol. Sin. 2022, 43, 2212–2225. [Google Scholar] [CrossRef]
- Yan, Y.; Kong, L.; Xia, Y.; Liang, W.; Wang, L.; Song, J.; Yao, Y.; Lin, Y.; Yang, J. Osthole promotes endogenous neural stem cell proliferation and improved neurological function through Notch signaling pathway in mice acute mechanical brain injury. Brain, Behav. Immun. 2018, 67, 118–129. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, Y.; Ge, H.; Wang, J.; Lei, X.; Chen, X.; Wan, F.; Feng, H.; Tan, L. Neurogenesis and Proliferation of Neural Stem/Progenitor Cells Conferred by Artesunate via FOXO3a/p27Kip1 Axis in Mouse Stroke Model. Mol. Neurobiol. 2022, 59, 4718–4729. [Google Scholar] [CrossRef]
- Kong, L.; Hu, Y.; Yao, Y.; Jiao, Y.; Li, S.; Yang, J. The Coumarin Derivative Osthole Stimulates Adult Neural Stem Cells, Promotes Neurogenesis in the Hippocampus, and Ameliorates Cognitive Impairment in APP/PS1 Transgenic Mice. Biol. Pharm. Bull. 2015, 38, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Ma, Y.; Xu, Y.; Liu, X.; Zhang, X.; Zhang, J.; Yang, C. Crocin regulates the proliferation and migration of neural stem cells after cerebral ischemia by activating the Notch1 pathway. Folia Neuropathol. 2020, 58, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-S.; Li, S.-R.; Li, K.; Li, X.; Yin, X.; Pang, Z. Ellagic acid improves endogenous neural stem cells proliferation and neurorestoration through Wnt/β-catenin signaling in vivo and in vitro. Mol. Nutr. Food Res. 2017, 61, 1600587. [Google Scholar] [CrossRef]
- Tu, W.-J.; Zhao, Z.; Yin, P.; Cao, L.; Zeng, J.; Chen, H.; Fan, D.; Fang, Q.; Gao, P.; Gu, Y.; et al. Estimated Burden of Stroke in China in 2020. JAMA Netw. Open 2023, 6, e231455. [Google Scholar] [CrossRef] [PubMed]
- Kalladka, D.; Sinden, J.; Pollock, K.; Haig, C.; McLean, J.; Smith, W.; McConnachie, A.; Santosh, C.; Bath, P.M.; Dunn, L.; et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. Lancet 2016, 388, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Boese, A.C.; Le, Q.-S.E.; Pham, D.; Hamblin, M.H.; Lee, J.-P. Neural stem cell therapy for subacute and chronic ischemic stroke. Stem Cell Res. Ther. 2018, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Parent, J.M.; Vexler, Z.S.; Gong, C.; Ma, N.D.; Ferriero, D.M. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann. Neurol. 2002, 52, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Kreuzberg, M.; Kanov, E.; Timofeev, O.; Schwaninger, M.; Monyer, H.; Khodosevich, K. Increased subventricular zone-derived cortical neurogenesis after ischemic lesion. Exp. Neurol. 2010, 226, 90–99. [Google Scholar] [CrossRef]
- Zhang, G.; Cunningham, M.; Zhang, H.; Dai, Y.; Zhang, P.; Ge, G.; Wang, B.; Bai, M.; Hazel, T.; Johe, K.; et al. First Human Trial of Stem Cell Transplantation in Complex Arrays for Stroke Patients Using the Intracerebral Microinjection Instrument. Neurosurg. 2020, 18, 503–510. [Google Scholar] [CrossRef]
- Hu, Z.; Li, F.; Zhou, X.; Zhang, F.; Huang, L.; Gu, B.; Shen, J.; Qi, S. Momordica charantia polysaccharides modulate the differentiation of neural stem cells via SIRT1/Beta-catenin axis in cerebral ischemia/reperfusion. Stem. Cell Res. Ther. 2020, 11, 485. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Han, R.; Guo, F.; Chen, H.; Wang, W.; Chen, Z.; Liu, W.; Sun, X.; Gao, C. Antagonistic effects of IL-17 and Astragaloside IV on cortical neurogenesis and cognitive behavior after stroke in adult mice through Akt/GSK-3beta pathway. Cell Death Discov. 2020, 6, 74. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Li, X.; Han, R.; Wu, M.; Ma, Y.; Chen, Y.; Zhang, H.; Li, Y. Therapeutic Potential of Chinese Medicine for Endogenous Neurogenesis: A Promising Candidate for Stroke Treatment. Pharmaceuticals 2023, 16, 706. https://doi.org/10.3390/ph16050706
Li L, Li X, Han R, Wu M, Ma Y, Chen Y, Zhang H, Li Y. Therapeutic Potential of Chinese Medicine for Endogenous Neurogenesis: A Promising Candidate for Stroke Treatment. Pharmaceuticals. 2023; 16(5):706. https://doi.org/10.3390/ph16050706
Chicago/Turabian StyleLi, Lin, Xiao Li, Rui Han, Meirong Wu, Yaolei Ma, Yuzhao Chen, Han Zhang, and Yue Li. 2023. "Therapeutic Potential of Chinese Medicine for Endogenous Neurogenesis: A Promising Candidate for Stroke Treatment" Pharmaceuticals 16, no. 5: 706. https://doi.org/10.3390/ph16050706
APA StyleLi, L., Li, X., Han, R., Wu, M., Ma, Y., Chen, Y., Zhang, H., & Li, Y. (2023). Therapeutic Potential of Chinese Medicine for Endogenous Neurogenesis: A Promising Candidate for Stroke Treatment. Pharmaceuticals, 16(5), 706. https://doi.org/10.3390/ph16050706