Calcifediol: Why, When, How Much?
Abstract
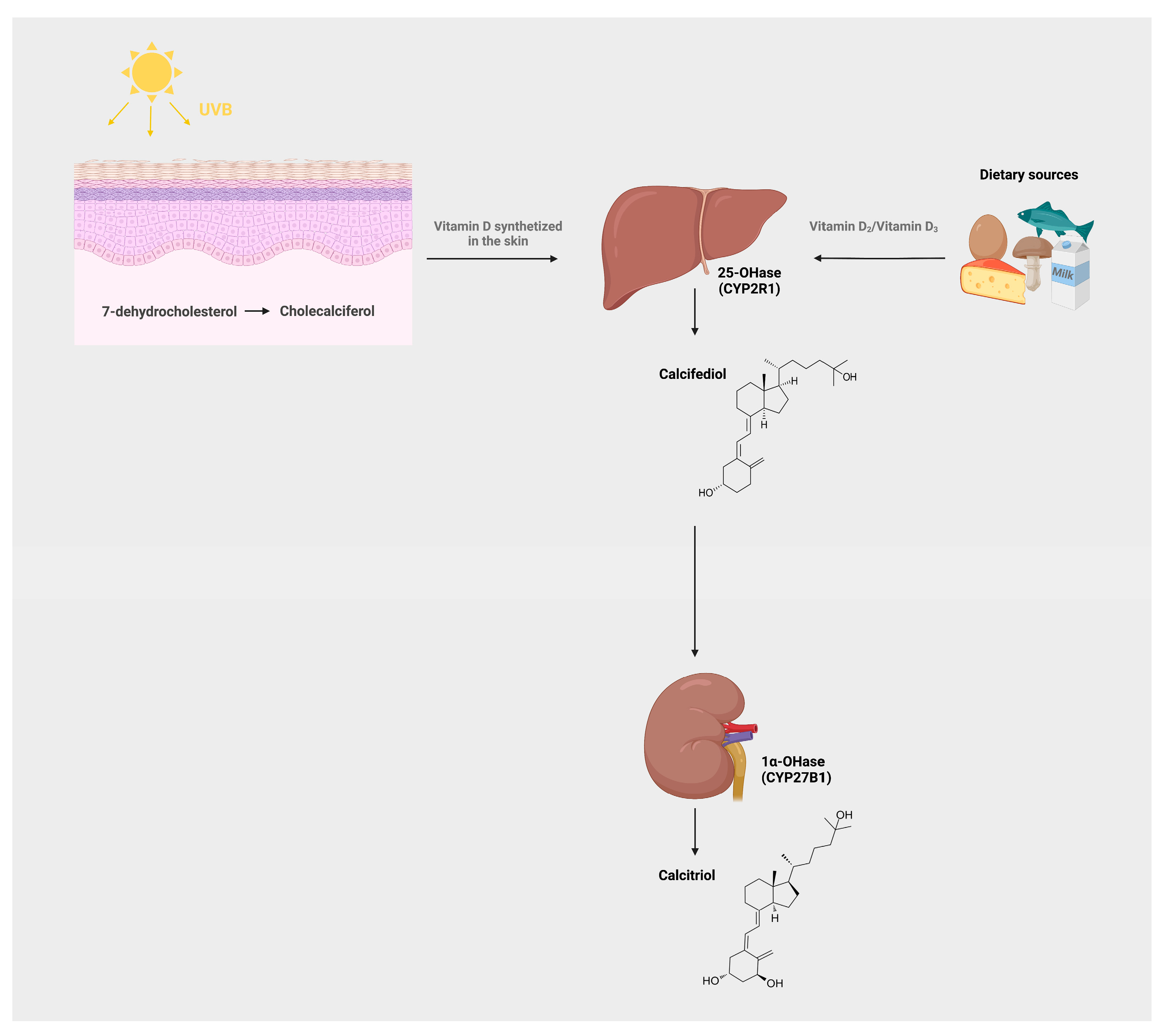
1. Introduction
2. Definition of Hypovitaminosis D Status
3. Vitamin D Deficiency and Related Disorders
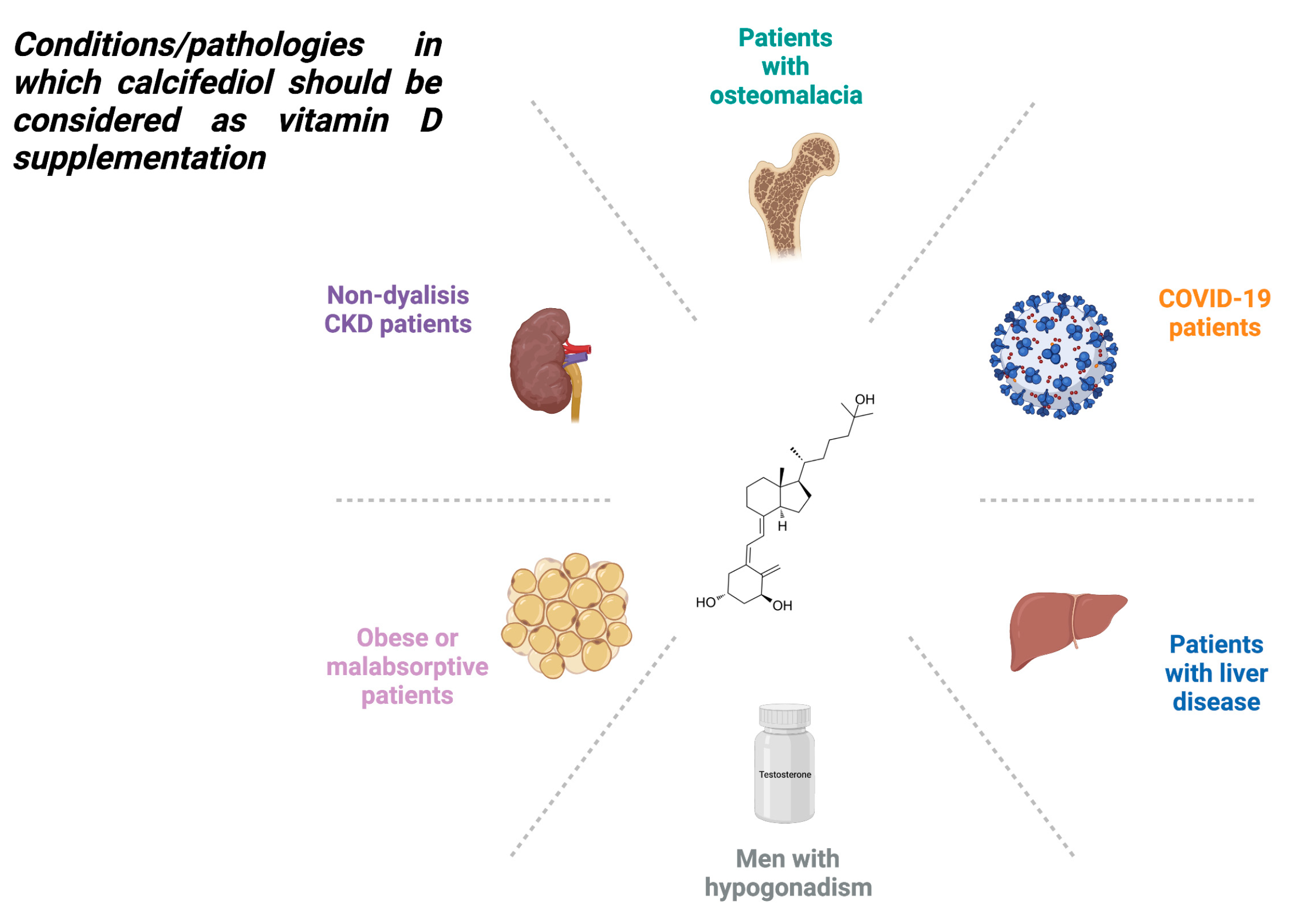
4. Treatment of Vitamin D Deficiency
5. Biological Activity of Calcifediol: Rapid Non-Genomic Responses
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wimalawansa, S.J. Vitamin D in the New Millennium. Curr. Osteoporos. Rep. 2012, 10, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: Importance in the Prevention of Cancers, Type 1 Diabetes, Heart Disease, and Osteoporosis. Am. J. Clin. Nutr. 2004, 79, 362–371. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A Global Perspective for Health. Dermato-Endocrinology 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-M.; Shin, E.-A. Exploring Vitamin D Metabolism and Function in Cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight and Vitamin D for Bone Health and Prevention of Autoimmune Diseases, Cancers, and Cardiovascular Disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [CrossRef]
- Quesada-Gomez, J.M.; Bouillon, R. Is Calcifediol Better than Cholecalciferol for Vitamin D Supplementation? Osteoporos. Int. 2018, 29, 1697–1711. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Farruggia, M.; Veronese, N.; Barbagallo, M. Vitamin D Sources, Metabolism, and Deficiency: Available Compounds and Guidelines for Its Treatment. Metabolites 2021, 11, 255. [Google Scholar] [CrossRef]
- Spiro, A.; Buttriss, J.L. Vitamin D: An Overview of Vitamin D Status and Intake in Europe. Nutr. Bull. 2014, 39, 322–350. [Google Scholar] [CrossRef]
- Plum, L.A.; DeLuca, H.F. The Functional Metabolism and Molecular Biology of Vitamin D Action. In Vitamin D: Physiology, Molecular Biology, and Clinical Applications; Holick, M.F., Ed.; Nutrition and Health; Humana Press: Totowa, NJ, USA, 2010; pp. 61–97. ISBN 978-1-60327-303-9. [Google Scholar]
- DeLuca, H.F. The Metabolism and Functions of Vitamin D. In Steroid Hormone Resistance: Mechanisms and Clinical Aspects; Chrousos, G.P., Loriaux, D.L., Lipsett, M.B., Eds.; Advances in Experimental Medicine and Biology; Springer US: Boston, MA, USA, 1986; pp. 361–375. ISBN 978-1-4684-5101-6. [Google Scholar]
- Chun, R.F.; Peercy, B.E.; Orwoll, E.S.; Nielson, C.M.; Adams, J.S.; Hewison, M. Vitamin D and DBP: The Free Hormone Hypothesis Revisited. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 132–137. [Google Scholar] [CrossRef]
- Nykjaer, A.; Fyfe, J.C.; Kozyraki, R.; Leheste, J.R.; Jacobsen, C.; Nielsen, M.S.; Verroust, P.J.; Aminoff, M.; de la Chapelle, A.; Moestrup, S.K.; et al. Cubilin Dysfunction Causes Abnormal Metabolism of the Steroid Hormone 25(OH) Vitamin D(3). Proc. Natl. Acad. Sci. USA 2001, 98, 13895–13900. [Google Scholar] [CrossRef]
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F.; Holick, M.F.; Schnoes, H.K.; Suda, T.; Cousins, R.J. Isolation and Identification of 1,25-Dihydroxycholecalciferol. A Metabolite of Vitamin D Active in Intestine. Biochemistry 1971, 10, 2799–2804. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; Malaguarnera, M.; Nicoletti, F.; Malaguarnera, L. Vitamin D3: A Helpful Immuno-Modulator. Immunology 2011, 134, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and Human Health: Lessons from Vitamin D Receptor Null Mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Evolution of Our Understanding of Vitamin D. Nutr. Rev. 2008, 66, S73–S87. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Meyer, M.B. The Vitamin D Receptor: New Paradigms for the Regulation of Gene Expression by 1,25-Dihydroxyvitamin D3. Endocrinol. Metab. Clin. N. Am. 2010, 39, 255–269. [Google Scholar] [CrossRef]
- Gallieni, M.; Cozzolino, M.; Fallabrino, G.; Pasho, S.; Olivi, L.; Brancaccio, D. Vitamin D: Physiology and Pathophysiology. Int. J. Artif. Organs 2009, 32, 87–94. [Google Scholar] [CrossRef]
- Lou, Y.-R.; Molnár, F.; Peräkylä, M.; Qiao, S.; Kalueff, A.V.; St-Arnaud, R.; Carlberg, C.; Tuohimaa, P. 25-Hydroxyvitamin D(3) Is an Agonistic Vitamin D Receptor Ligand. J. Steroid Biochem. Mol. Biol. 2010, 118, 162–170. [Google Scholar] [CrossRef]
- DeLuca, H.F. Overview of General Physiologic Features and Functions of Vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Donati, S.; Palmini, G.; Aurilia, C.; Falsetti, I.; Miglietta, F.; Iantomasi, T.; Brandi, M.L. Rapid Nontranscriptional Effects of Calcifediol and Calcitriol. Nutrients 2022, 14, 1291. [Google Scholar] [CrossRef] [PubMed]
- Valdivielso, J.M. The Physiology of Vitamin D Receptor Activation. Contrib. Nephrol. 2009, 163, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, I. Vitamin D Metabolism and Guidelines for Vitamin D Supplementation. Clin. Biochem. Rev. 2020, 41, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Dietary Reference Values for Vitamin D. EFSA J. 2016, 14, e04547. [CrossRef]
- Nowson, C.A.; McGrath, J.J.; Ebeling, P.R.; Haikerwal, A.; Daly, R.M.; Sanders, K.M.; Seibel, M.J.; Mason, R.S. Working Group of Australian and New Zealand Bone and Mineral Society, Endocrine Society of Australia and Osteoporosis Australia Vitamin D and Health in Adults in Australia and New Zealand: A Position Statement. Med. J. Aust. 2012, 196, 686–687. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Sempos, C.T.; Binkley, N. 25-Hydroxyvitamin D Assay Standardisation and Vitamin D Guidelines Paralysis. Public Health Nutr. 2020, 23, 1153–1164. [Google Scholar] [CrossRef]
- Hilger, J.; Friedel, A.; Herr, R.; Rausch, T.; Roos, F.; Wahl, D.A.; Pierroz, D.D.; Weber, P.; Hoffmann, K. A Systematic Review of Vitamin D Status in Populations Worldwide. Br. J. Nutr. 2014, 111, 23–45. [Google Scholar] [CrossRef]
- Feng, Y.; Cheng, G.; Wang, H.; Chen, B. The Associations between Serum 25-Hydroxyvitamin D Level and the Risk of Total Fracture and Hip Fracture. Osteoporos. Int. 2017, 28, 1641–1652. [Google Scholar] [CrossRef]
- Lv, Q.-B.; Gao, X.; Liu, X.; Shao, Z.-X.; Xu, Q.-H.; Tang, L.; Chi, Y.-L.; Wu, A.-M. The Serum 25-Hydroxyvitamin D Levels and Hip Fracture Risk: A Meta-Analysis of Prospective Cohort Studies. Oncotarget 2017, 8, 39849–39858. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.-T.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Camargo, C.A.; Scragg, R. Effect of Monthly High-Dose Vitamin D Supplementation on Falls and Non-Vertebral Fractures: Secondary and Post-Hoc Outcomes from the Randomised, Double-Blind, Placebo-Controlled ViDA Trial. Lancet Diabetes Endocrinol. 2017, 5, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Grey, A.; Avenell, A. Effects of Vitamin D Supplementation on Musculoskeletal Health: A Systematic Review, Meta-Analysis, and Trial Sequential Analysis. Lancet Diabetes Endocrinol. 2018, 6, 847–858. [Google Scholar] [CrossRef]
- Kupisz-Urbańska, M.; Płudowski, P.; Marcinowska-Suchowierska, E. Vitamin D Deficiency in Older Patients-Problems of Sarcopenia, Drug Interactions, Management in Deficiency. Nutrients 2021, 13, 1247. [Google Scholar] [CrossRef] [PubMed]
- Chun, R.F.; Liu, P.T.; Modlin, R.L.; Adams, J.S.; Hewison, M. Impact of Vitamin D on Immune Function: Lessons Learned from Genome-Wide Analysis. Front. Physiol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Cyprian, F.; Lefkou, E.; Varoudi, K.; Girardi, G. Immunomodulatory Effects of Vitamin D in Pregnancy and Beyond. Front. Immunol. 2019, 10, 2739. [Google Scholar] [CrossRef]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.-M.; Manson, J.E.; Costenbader, K.H. Vitamin D and Marine Omega 3 Fatty Acid Supplementation and Incident Autoimmune Disease: VITAL Randomized Controlled Trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef] [PubMed]
- Chandler, P.D.; Chen, W.Y.; Ajala, O.N.; Hazra, A.; Cook, N.; Bubes, V.; Lee, I.-M.; Giovannucci, E.L.; Willett, W.; Buring, J.E.; et al. Effect of Vitamin D3 Supplements on Development of Advanced Cancer. JAMA Netw. Open 2020, 3, e2025850. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Vitamin D Supplements and Cancer Incidence and Mortality: A Meta-Analysis. Br. J. Cancer 2014, 111, 976–980. [Google Scholar] [CrossRef]
- Peixoto, R.D.; Oliveira, L.J.D.C.; Passarini, T.D.M.; Andrade, A.C.; Diniz, P.H.; Prolla, G.; Amorim, L.C.; Gil, M.; Lino, F.; Garicochea, B.; et al. Vitamin D and Colorectal Cancer—A Practical Review of the Literature. Cancer Treat. Res. Commun. 2022, 32, 100616. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. Vitamin D Baseline Levels at Diagnosis of Breast Cancer: A Systematic Review and Meta-Analysis. Hematol. Oncol. Stem Cell Ther. 2021, 14, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Alcala-Diaz, J.F.; Limia-Perez, L.; Gomez-Huelgas, R.; Martin-Escalante, M.D.; Cortes-Rodriguez, B.; Zambrana-Garcia, J.L.; Entrenas-Castillo, M.; Perez-Caballero, A.I.; López-Carmona, M.D.; Garcia-Alegria, J.; et al. Calcifediol Treatment and Hospital Mortality Due to COVID-19: A Cohort Study. Nutrients 2021, 13, 1760. [Google Scholar] [CrossRef]
- Oristrell, J.; Oliva, J.C.; Casado, E.; Subirana, I.; Domínguez, D.; Toloba, A.; Balado, A.; Grau, M. Vitamin D Supplementation and COVID-19 Risk: A Population-Based, Cohort Study. J. Endocrinol. Investig. 2022, 45, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Sabico, S.; Enani, M.A.; Sheshah, E.; Aljohani, N.J.; Aldisi, D.A.; Alotaibi, N.H.; Alshingetti, N.; Alomar, S.Y.; Alnaami, A.M.; Amer, O.E.; et al. Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial. Nutrients 2021, 13, 2170. [Google Scholar] [CrossRef] [PubMed]
- Khan, Q.J.; Fabian, C.J. How I Treat Vitamin D Deficiency. J. Oncol. Pract. 2010, 6, 97–101. [Google Scholar] [CrossRef]
- Cesareo, R.; Falchetti, A.; Attanasio, R.; Tabacco, G.; Naciu, A.M.; Palermo, A. Hypovitaminosis D: Is It Time to Consider the Use of Calcifediol? Nutrients 2019, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Sosa Henríquez, M.; Gómez de Tejada Romero, M.J. Cholecalciferol or Calcifediol in the Management of Vitamin D Deficiency. Nutrients 2020, 12, 1617. [Google Scholar] [CrossRef]
- Jones, G. Pharmacokinetics of Vitamin D Toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S. [Google Scholar] [CrossRef]
- Adami, S.; Romagnoli, E.; Carnevale, V.; Scillitani, A.; Giusti, A.; Rossini, M.; Gatti, D.; Nuti, R.; Minisola, S. Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS) [Guidelines on prevention and treatment of vitamin D deficiency. Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS)]. Reumatismo 2011, 63, 129–147. [Google Scholar] [CrossRef]
- Rossini, M.; Adami, S.; Bertoldo, F.; Diacinti, D.; Gatti, D.; Giannini, S.; Giusti, A.; Malavolta, N.; Minisola, S.; Osella, G.; et al. Guidelines for the Diagnosis, Prevention and Management of Osteoporosis. Reumatismo 2016, 68, 1–39. [Google Scholar] [CrossRef]
- Selye, H. Correlations between the chemical structure and the pharmacological actions of the steroids. Endocrinology 1942, 30, 437–453. [Google Scholar] [CrossRef]
- Spach, C.; Streeten, D.H.P. Retardation of Sodium Exchange in Dog Erythrocytes by Physiological Concentrations of Aldosterone, in Vitro. J. Clin. Investig. 1964, 43, 217–227. [Google Scholar] [CrossRef]
- Nemere, I.; Yoshimoto, Y.; Norman, A.W. Calcium Transport in Perfused Duodena from Normal Chicks: Enhancement within Fourteen Minutes of Exposure to 1,25-Dihydroxyvitamin D3. Endocrinology 1984, 115, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W. Vitamin D Receptor: New Assignments for an Already Busy Receptor. Endocrinology 2006, 147, 5542–5548. [Google Scholar] [CrossRef]
- Dormanen, M.C.; Bishop, J.E.; Hammond, M.W.; Okamura, W.H.; Nemere, I.; Norman, A.W. Nonnuclear Effects of the Steroid Hormone 1 Alpha,25(OH)2-Vitamin D3: Analogs Are Able to Functionally Differentiate between Nuclear and Membrane Receptors. Biochem. Biophys. Res. Commun. 1994, 201, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, M.A.; Carlberg, C. Vitamin D Receptor(s): In the Nucleus but Also at Membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C. Rapid, Membrane-Initiated Actions of 1,25 Dihydroxyvitamin D: What Are They and What Do They Mean? J. Nutr. 2004, 134, 3215–3218. [Google Scholar] [CrossRef] [PubMed]
- Doroudi, M.; Schwartz, Z.; Boyan, B.D. Membrane-Mediated Actions of 1,25-Dihydroxy Vitamin D3: A Review of the Roles of Phospholipase A2 Activating Protein and Ca(2+)/Calmodulin-Dependent Protein Kinase II. J. Steroid Biochem. Mol. Biol. 2015, 147, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, P.P.; Hii, C.S.T.; Ferrante, A.; Tan, J.; Der, C.J.; Omdahl, J.L.; Morris, H.A.; May, B.K. Role of MAP Kinases in the 1,25-Dihydroxyvitamin D3-Induced Transactivation of the Rat Cytochrome P450C24 (CYP24) Promoter. Specific Functions for ERK1/ERK2 and ERK5. J. Biol. Chem. 2002, 277, 29643–29653. [Google Scholar] [CrossRef]
- Nutchey, B.K.; Kaplan, J.S.; Dwivedi, P.P.; Omdahl, J.L.; Ferrante, A.; May, B.K.; Hii, C.S.T. Molecular Action of 1,25-Dihydroxyvitamin D3 and Phorbol Ester on the Activation of the Rat Cytochrome P450C24 (CYP24) Promoter: Role of MAP Kinase Activities and Identification of an Important Transcription Factor Binding Site. Biochem. J. 2005, 389, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, P.; Gao, X.; Tan, J.; Evdokiou, A.; Ferrante, A.; Morris, H.; May, B.; Hii, C. A Role for the Phosphatidylinositol 3-Kinase--Protein Kinase C Zeta--Sp1 Pathway in the 1,25-Dihydroxyvitamin D3 Induction of the 25-Hydroxyvitamin D3 24-Hydroxylase Gene in Human Kidney Cells. Cell. Signal. 2010, 22, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Jiang, Y.; Nguyen, T.; Oda, Y.; Tu, C. Disruption of Vitamin D and Calcium Signaling in Keratinocytes Predisposes to Skin Cancer. Front. Physiol. 2016, 7, 296. [Google Scholar] [CrossRef] [PubMed]
- Bandera Merchan, B.; Morcillo, S.; Martin-Nuñez, G.; Tinahones, F.J.; Macías-González, M. The Role of Vitamin D and VDR in Carcinogenesis: Through Epidemiology and Basic Sciences. J. Steroid Biochem. Mol. Biol. 2017, 167, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Hadden, M.K. Hedgehog and Vitamin D Signaling Pathways in Development and Disease. Vitam. Horm. 2016, 100, 231–253. [Google Scholar] [CrossRef]
- Lisse, T.S.; Saini, V.; Zhao, H.; Luderer, H.F.; Gori, F.; Demay, M.B. The Vitamin D Receptor Is Required for Activation of CWnt and Hedgehog Signaling in Keratinocytes. Mol. Endocrinol. 2014, 28, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Teichert, A.E.; Elalieh, H.; Elias, P.M.; Welsh, J.; Bikle, D.D. Overexpression of Hedgehog Signaling Is Associated with Epidermal Tumor Formation in Vitamin D Receptor-Null Mice. J. Investig. Dermatol. 2011, 131, 2289–2297. [Google Scholar] [CrossRef]
- Teichert, A.; Elalieh, H.; Bikle, D. Disruption of the Hedgehog Signaling Pathway Contributes to the Hair Follicle Cycling Deficiency in Vdr Knockout Mice. J. Cell. Physiol. 2010, 225, 482–489. [Google Scholar] [CrossRef]
- Tapia, C.; Suares, A.; De Genaro, P.; González-Pardo, V. In Vitro Studies Revealed a Downregulation of Wnt/β-Catenin Cascade by Active Vitamin D and TX 527 Analog in a Kaposi’s Sarcoma Cellular Model. Toxicol. In Vitro 2020, 63, 104748. [Google Scholar] [CrossRef]
- Muralidhar, S.; Filia, A.; Nsengimana, J.; Poźniak, J.; O’Shea, S.J.; Diaz, J.M.; Harland, M.; Randerson-Moor, J.A.; Reichrath, J.; Laye, J.P.; et al. Vitamin D–VDR Signaling Inhibits Wnt/β-Catenin–Mediated Melanoma Progression and Promotes Antitumor Immunity. Cancer Res. 2019, 79, 5986–5998. [Google Scholar] [CrossRef]
- Tang, L.; Fang, W.; Lin, J.; Li, J.; Wu, W.; Xu, J. Vitamin D Protects Human Melanocytes against Oxidative Damage by Activation of Wnt/β-Catenin Signaling. Lab. Investig. 2018, 98, 1527–1537. [Google Scholar] [CrossRef]
- Larriba, M.J.; González-Sancho, J.M.; Bonilla, F.; Muñoz, A. Interaction of Vitamin D with Membrane-Based Signaling Pathways. Front. Physiol. 2014, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, X.; Xu, L.; Zhang, J.; Cao, H. A Molecular Sub-Cluster of Colon Cancer Cells with Low VDR Expression Is Sensitive to Chemotherapy, BRAF Inhibitors and PI3K-MTOR Inhibitors Treatment. Aging 2019, 11, 8587–8603. [Google Scholar] [CrossRef]
- Olsson, K.; Saini, A.; Strömberg, A.; Alam, S.; Lilja, M.; Rullman, E.; Gustafsson, T. Evidence for Vitamin D Receptor Expression and Direct Effects of 1α,25(OH)2D3 in Human Skeletal Muscle Precursor Cells. Endocrinology 2016, 157, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Raghavan, S. Getting under the Skin of Epidermal Morphogenesis. Nat. Rev. Genet. 2002, 3, 199–209. [Google Scholar] [CrossRef]
- Lou, Y.-R.; Laaksi, I.; Syvälä, H.; Bläuer, M.; Tammela, T.L.J.; Ylikomi, T.; Tuohimaa, P. 25-Hydroxyvitamin D3 Is an Active Hormone in Human Primary Prostatic Stromal Cells. FASEB J. 2004, 18, 332–334. [Google Scholar] [CrossRef]
- Peng, X.; Hawthorne, M.; Vaishnav, A.; St-Arnaud, R.; Mehta, R.G. 25-Hydroxyvitamin D3 Is a Natural Chemopreventive Agent against Carcinogen Induced Precancerous Lesions in Mouse Mammary Gland Organ Culture. Breast Cancer Res. Treat. 2009, 113, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Verone-Boyle, A.R.; Shoemaker, S.; Attwood, K.; Morrison, C.D.; Makowski, A.J.; Battaglia, S.; Hershberger, P.A. Diet-Derived 25-Hydroxyvitamin D3 Activates Vitamin D Receptor Target Gene Expression and Suppresses EGFR Mutant Non-Small Cell Lung Cancer Growth in Vitro and in Vivo. Oncotarget 2016, 7, 995–1013. [Google Scholar] [CrossRef] [PubMed]
- Deluca, H.F.; Prahl, J.M.; Plum, L.A. 1,25-Dihydroxyvitamin D Is Not Responsible for Toxicity Caused by Vitamin D or 25-Hydroxyvitamin D. Arch. Biochem. Biophys. 2011, 505, 226–230. [Google Scholar] [CrossRef]
- Donati, S.; Palmini, G.; Romagnoli, C.; Aurilia, C.; Miglietta, F.; Falsetti, I.; Marini, F.; Zonefrati, R.; Galli, G.; Marcucci, G.; et al. In Vitro Non-Genomic Effects of Calcifediol on Human Preosteoblastic Cells. Nutrients 2021, 13, 4227. [Google Scholar] [CrossRef]
- Asano, L.; Watanabe, M.; Ryoden, Y.; Usuda, K.; Yamaguchi, T.; Khambu, B.; Takashima, M.; Sato, S.-I.; Sakai, J.; Nagasawa, K.; et al. Vitamin D Metabolite, 25-Hydroxyvitamin D, Regulates Lipid Metabolism by Inducing Degradation of SREBP/SCAP. Cell Chem. Biol. 2017, 24, 207–217. [Google Scholar] [CrossRef]
- Cesareo, R.; Attanasio, R.; Caputo, M.; Castello, R.; Chiodini, I.; Falchetti, A.; Guglielmi, R.; Papini, E.; Santonati, A.; Scillitani, A.; et al. Italian Association of Clinical Endocrinologists (AME) and Italian Chapter of the American Association of Clinical Endocrinologists (AACE) Position Statement: Clinical Management of Vitamin D Deficiency in Adults. Nutrients 2018, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Lips, P.; de Jongh, R.T.; van Schoor, N.M. Trends in Vitamin D Status Around the World. JBMR Plus 2021, 5, e10585. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.A.; Cooper, C.; Ebeling, P.R.; Eggersdorfer, M.; Hilger, J.; Hoffmann, K.; Josse, R.; Kanis, J.A.; Mithal, A.; Pierroz, D.D.; et al. A Global Representation of Vitamin D Status in Healthy Populations. Arch. Osteoporos. 2012, 7, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Van Schoor, N.; Lips, P. Global Overview of Vitamin D Status. Endocrinol. Metab. Clin. N. Am. 2017, 46, 845–870. [Google Scholar] [CrossRef]
- Pérez-Castrillón, J.L.; Dueñas-Laita, A.; Brandi, M.L.; Jódar, E.; Del Pino-Montes, J.; Quesada-Gómez, J.M.; Cereto Castro, F.; Gómez-Alonso, C.; Gallego López, L.; Olmos Martínez, J.M.; et al. Calcifediol Is Superior to Cholecalciferol in Improving Vitamin D Status in Postmenopausal Women: A Randomized Trial. J. Bone Miner. Res. 2021, 36, 1967–1978. [Google Scholar] [CrossRef]
- Chevalley, T.; Brandi, M.L.; Cashman, K.D.; Cavalier, E.; Harvey, N.C.; Maggi, S.; Cooper, C.; Al-Daghri, N.; Bock, O.; Bruyère, O.; et al. Role of Vitamin D Supplementation in the Management of Musculoskeletal Diseases: Update from an European Society of Clinical and Economical Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) Working Group. Aging Clin. Exp. Res. 2022, 34, 2603–2623. [Google Scholar] [CrossRef]
- Authority (EFSA), E.F.S. Opinion of the Scientific Panel on Additives and Products or Substances Used in Animal Feed (FEEDAP) on the Evaluation of Safety and Efficacy of “Hy•D” (Calcifediol), Based on 25-Hydroxylcholecalciferol/25-Hydroxy-Pre-Cholecalciferol, as Feed Additive in Accordance with Council Directive 70/524/EEC. EFSA J. 2005, 3, 224. [Google Scholar] [CrossRef]
- Navarro-Valverde, C.; Sosa-Henríquez, M.; Alhambra-Expósito, M.R.; Quesada-Gómez, J.M. Vitamin D3 and Calcidiol Are Not Equipotent. J. Steroid Biochem. Mol. Biol. 2016, 164, 205–208. [Google Scholar] [CrossRef]
- Olmos, J.M.; Arnaiz, F.; Hernández, J.L.; Olmos-Martínez, J.M.; González Macías, J.; Olmos, J.M.; Arnaiz, F.; Hernández, J.L.; Olmos-Martínez, J.M.; González Macías, J. Calcifediol Mensual Frente a Calcifediol Quincenal En El Tratamiento de Pacientes Osteoporóticos. Estudio En La Vida Real. Rev. Osteoporos. Metab. Miner. 2018, 10, 89–95. [Google Scholar] [CrossRef]
- García Doladé, N.; Cereza García, G.; Madurga Sanz, M.; Montero Corominas, D. Risk of hypercalcemia and hipervitaminosis D induced by calcifediol. Review of cases reported to the Spanish Pharmacovigilance System. Med. Clin. 2013, 141, 88–89. [Google Scholar] [CrossRef]

| Serum 25(OH)D3 Levels (ng/mL) | IOM | ES | EFSA | Working Group of the Australian and New Zealand Bone and Mineral Society | SACN | ESE |
|---|---|---|---|---|---|---|
| <10 | Vitamin D deficiency | Vitamin D deficiency | <5 ng/mL (severe vitamin D deficiency) 5–11.6 ng/mL (moderate vitamin D deficiency) | Vitamin D deficiency | Vitamin D deficiency | |
| 10–20 | Vitamin D insufficiency | Vitamin D deficiency | 12–19.6 (mild vitamin D deficiency) | Sufficient | Vitamin D deficiency | |
| 20–30 | Sufficient | Vitamin D insufficiency | Sufficient | Sufficient | Sufficient | Vitamin D insufficiency |
| >30 | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donati, S.; Marini, F.; Giusti, F.; Palmini, G.; Aurilia, C.; Falsetti, I.; Iantomasi, T.; Brandi, M.L. Calcifediol: Why, When, How Much? Pharmaceuticals 2023, 16, 637. https://doi.org/10.3390/ph16050637
Donati S, Marini F, Giusti F, Palmini G, Aurilia C, Falsetti I, Iantomasi T, Brandi ML. Calcifediol: Why, When, How Much? Pharmaceuticals. 2023; 16(5):637. https://doi.org/10.3390/ph16050637
Chicago/Turabian StyleDonati, Simone, Francesca Marini, Francesca Giusti, Gaia Palmini, Cinzia Aurilia, Irene Falsetti, Teresa Iantomasi, and Maria Luisa Brandi. 2023. "Calcifediol: Why, When, How Much?" Pharmaceuticals 16, no. 5: 637. https://doi.org/10.3390/ph16050637
APA StyleDonati, S., Marini, F., Giusti, F., Palmini, G., Aurilia, C., Falsetti, I., Iantomasi, T., & Brandi, M. L. (2023). Calcifediol: Why, When, How Much? Pharmaceuticals, 16(5), 637. https://doi.org/10.3390/ph16050637







