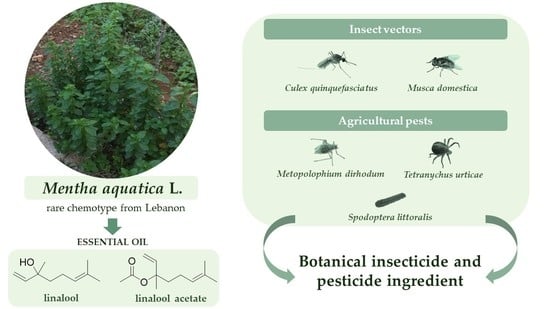
Efficacy of Mentha aquatica L. Essential Oil (Linalool/Linalool Acetate Chemotype) against Insect Vectors and Agricultural Pests
Abstract
1. Introduction
2. Results
2.1. EO Chemical Composition
2.2. Insecticidal and Acaricidal Efficacy
3. Discussion
4. Materials and Methods
4.1. Plant Material and EO Extraction
4.2. GC–MS Analysis of Essential Oils
4.3. Target Insects and Mites
4.4. Insecticidal and Acaricidal Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deschamps, C.; Zanatta, J.L.; Bizzo, H.R.; Oliveira, M.D.C.; Roswalka, L.C. Seasonal evaluation of essential oil yield of mint species. Ciênc. Agrotecnol. 2008, 32, 725–730. [Google Scholar] [CrossRef]
- Ferhat, M.; Erol, E.; Beladjila, K.A.; Çetintaş, Y.; Duru, M.E.; Öztürk, M.; Kabouche, A.; Kabouche, Z. Antioxidant, anticholinesterase and antibacterial activities of Stachys guyoniana and Mentha aquatica. Pharm. Biol. 2017, 55, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Gethaun, Z.; Asres, K.; Mazumder, A.; Bucar, F. Essential oil composition, antibacterial and antioxidant activities of Mentha aquatica growing in Ethiopia. Ethiop. Pharm. J. 2008, 26, 9–16. [Google Scholar]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Insecticidal properties of Mentha species: A review. Ind. Crops Prod. 2011, 34, 802–817. [Google Scholar] [CrossRef]
- Andro, A.R.; Boz, I.; Zamfirache, M.M.; Burzo, I. Chemical composition of essential oils from Mentha aquatica L. at different moments of the ontogenetic cycle. J. Med. Plant Res. 2013, 7, 470–473. [Google Scholar]
- Jäger, A.K.; Almqvist, J.P.; Vangsøe, S.A.K.; Stafford, G.I.; Adsersen, A.; van Staden, J. Compounds from Mentha aquatica with affinity to the GABA-benzodiazepine receptor. S. Afr. J. Bot. 2007, 73, 518–521. [Google Scholar] [CrossRef]
- De Oliveira Braga, L.E.; da Silva, G.G.; de Oliveira Sousa, I.M.; de Oliveira, E.C.S.; Jorge, M.P.; Monteiro, K.M.; Sedano, T.C.; Foglio, M.A.; Ruiz, A.L.T.G. Gastrointestinal effects of Mentha aquatica L. essential oil. Inflammopharmacology 2022, 30, 2127–2137. [Google Scholar] [CrossRef]
- Olsen, H.T.; Stafford, G.I.; van Staden, J.; Christensen, S.B.; Jäger, A.K. Isolation of the MAO-inhibitor naringenin from Mentha aquatica L. J. Ethnopharmacol. 2008, 117, 500–502. [Google Scholar] [CrossRef]
- Nouri, A.; Yaraki, M.T.; Lajevardi, A.; Rezaei, Z.; Ghorbanpour, M.; Tanzifi, M. Ultrasonic-assisted green synthesis of silver nanoparticles using Mentha aquatica leaf extract for enhanced antibacterial properties and catalytic activity. Colloid Interface Sci. Commun. 2020, 35, 100252. [Google Scholar] [CrossRef]
- Pereira, O.R.; Macias, R.I.; Domingues, M.R.; Marin, J.J.; Cardoso, S.M. Hepatoprotection of Mentha aquatica L., Lavandula dentata L. and Leonurus cardiaca L. Antioxidants 2019, 8, 267. [Google Scholar] [CrossRef]
- Dhifi, W.; Litaiem, M.; Jelali, N.; Hamdi, N.; Mnif, W. Identification of a new chemotye of the plant Mentha aquatica grown in Tunisia: Chemical composition, antioxidant and biological activities of its essential oil. J. Essent. Oil Bear. Plants 2011, 14, 320–328. [Google Scholar] [CrossRef]
- Djamila, B.; Zohra, K.F.; Lahcene, K.; Zohra, R.F. Drying methods affect the extracts and essential oil of Mentha aquatica L. Food Biosci. 2021, 41, 101007. [Google Scholar] [CrossRef]
- Esmaeili, A.; Rustaiyan, A.; Masoudi, S.; Nadji, K. Composition of the essential oils of Mentha aquatica L. and Nepeta meyeri Benth. from Iran. J. Essent. Oil Res. 2006, 18, 263–265. [Google Scholar] [CrossRef]
- Karami, H.; Rasekh, M.; Darvishi, Y.; Khaledi, R. Effect of drying temperature and air velocity on the essential oil content of Mentha aquatica L. J. Essent. Oil Bear. Plants 2017, 20, 1131–1136. [Google Scholar] [CrossRef]
- Zaks, A.; Davidovich-Rikanati, R.; Bar, E.; Inbar, M.; Lewinsohn, E. Biosynthesis of linalyl acetate and other terpenes in lemon mint (Mentha aquatica var. citrata, Lamiaceae) glandular trichomes. Isr. J. Plant Sci. 2008, 56, 233–244. [Google Scholar] [CrossRef]
- Chengala, L.; Singh, N. Botanical pesticides—A major alternative to chemical pesticides: A review. Int. J. Life Sci 2017, 5, 722–729. [Google Scholar]
- Pavela, R.; Morshedloo, M.R.; Mumivand, H.; Khorsand, G.J.; Karami, A.; Maggi, F.; Desneux, N.; Benelli, G. Phenolic monoterpene-rich essential oils from Apiaceae and Lamiaceae species: Insecticidal activity and safety evaluation on non-target earthworms. Entomol. Gen. 2020, 40, 421–435. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Boukouvala, M.C.; Ntalaka, C.T.; Skourti, A.; Nika, E.P.; Maggi, F.; Spinozzi, E.; Mazzara, E.; Petrelli, R.; Lupidi, G.; et al. Efficacy of 12 commercial essential oils as wheat protectants against stored-product beetles, and their acetylcholinesterase inhibitory activity. Entomol. Gen. 2021, 41, 385–414. [Google Scholar] [CrossRef]
- Ricupero, M.; Biondi, A.; Cincotta, F.; Condurso, C.; Palmeri, V.; Verzera, A.; Zappalà, L.; Campolo, O. Bioactivity and physico-chemistry of garlic essential oil nanoemulsion in tomato. Entomol. Gen. 2022, 1127, 921–930. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Menail, A.H.; Boutefnouchet-Bouchema, W.F.; Haddad, N.; Taning, N.T.C.; Smagghe, G.; Loucif-Ayad, W. Effects of thiamethoxam and spinosad on the survival and hypopharyngeal glands of the African honey bee (Apis mellifera intermissa). Entomol. Gen. 2020, 40, 207–215. [Google Scholar] [CrossRef]
- Yadav, I.C.; Devi, N.L. Pesticides classification and its impact on human and environment. Environ. Sci. Eng. 2017, 6, 140–158. [Google Scholar]
- Baweja, P.; Kumar, S.; Kumar, G. Fertilizers and pesticides: Their impact on soil health and environment. In Soil Health; Cham, P., Kumar, S., Kumar, G., Eds.; Springer: Cham, Switzerland, 2020; pp. 265–285. [Google Scholar]
- Giunti, G.; Benelli, G.; Palmeri, V.; Laudani, F.; Ricupero, M.; Ricciardi, R.; Maggi, F.; Lucchi, A.; Guedes, R.N.C.; Desneux, N.; et al. Non-target effects of essential oil-based biopesticides for crop protection: Impact on natural enemies, pollinators, and soil invertebrates. Biol. Control 2022, 176, 105071. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Pavela, R.; Bonacucina, G.; Baldassarri, C.; Spinozzi, E.; Torresi, J.; Petrelli, R.; Morshedloo, M.R.; Maggi, F.; Benelli, G.; et al. Development, characterization, insecticidal and sublethal effects of Bunium persicum and Ziziphora clinopodioides-based essential oil nanoemulsions on Culex quinquefasciatus. Ind. Crops Prod. 2022, 186, 115249. [Google Scholar] [CrossRef]
- Giordani, C.; Spinozzi, E.; Baldassarri, C.; Ferrati, M.; Cappellacci, L.; Santibañez Nieto, D.; Pavela, R.; Ricciardi, R.; Benelli, G.; Petrelli, R.; et al. Insecticidal Activity of Four Essential Oils Extracted from Chilean Patagonian Plants as Potential Organic Pesticides. Plants 2022, 11, 2012. [Google Scholar] [CrossRef]
- Wandjou, J.G.N.; Baldassarri, C.; Ferrati, M.; Maggi, F.; Pavela, R.; Tsabang, N.; Petrelli, R.; Ricciardi, R.; Desneux, N.; Benelli, G. Essential Oils from Cameroonian Aromatic Plants as Effective Insecticides against Mosquitoes, Houseflies, and Moths. Plants 2022, 11, 2353. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Giordani, C.; Casettari, L.; Curzi, G.; Cappellacci, L.; Petrelli, R.; Maggi, F. Acute and sub-lethal toxicity of eight essential oils of commercial interest against the filariasis mosquito Culex quinquefasciatus and the housefly Musca domestica. Ind. Crops Prod. 2018, 112, 668–680. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Nika, E.P.; Skourti, A.; Xefteri, D.N.; Cianfaglione, K.; Perinelli, D.R.; Spinozzi, E.; Bonacucina, G.; Canale, A.; Benelli, G.; et al. Piperitenone oxide-rich Mentha longifolia essential oil and its nanoemulsion to manage different developmental stages of insect and mite pests attacking stored wheat. Ind. Crops Prod. 2022, 178, 114600. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chrom. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Taheri-Garavand, A.; Mumivand, H.; Fatahi, S.; Nasiri, A.; Omid, M. Modeling the kinetics of essential oil content and main constituents of mint (Mentha aquatica L.) leaves during thin-layer drying process using response surface methodology. J. Food Process. 2021, 45, e15515. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Anackov, G.; Zlatkovic, B.; Igic, R. Variability of Content and Composition of Mentha aquatica L. (Lamiaceae) Essential Oil in Different Phenophases. J. Essent. Oil-Bear. Plants. 2006, 9, 223–229. [Google Scholar] [CrossRef]
- Jerkovic, I.; Mastelic, J. Composition of free and glycosidically bound volatiles of Mentha aquatica L. Croat. Chem. Acta 2001, 74, 431–439. [Google Scholar]
- Malingré, T.M.; Maarse, H. Composition of the essential oil of Mentha aquatica. Phytochemistry 1974, 13, 1531–1535. [Google Scholar] [CrossRef]
- Mimica-Dukić, N.; Božin, B.; Soković, M.; Mihajlović, B.; Matavulj, M. Antimicrobial and antioxidant activities of three Mentha species essential oils. Planta Med. 2003, 69, 413–419. [Google Scholar]
- Atsbaha Zebelo, S.; Bertea, C.M.; Bossi, S.; Occhipinti, A.; Gnavi, G.; Maffei, M.E. Chrysolina herbacea modulates terpenoid biosynthesis of Mentha aquatica L. PLoS ONE 2011, 6, e17195. [Google Scholar] [CrossRef]
- Murray, M.J.; Hefendehl, F.W. Changes in monoterpene composition of Mentha aquatica produced by gene substitution from M. arvensis. Phytochemistry 1972, 11, 2469–2474. [Google Scholar] [CrossRef]
- Murray, M.J.; Lincoln, D.E. Oil composition of Mentha aquatica-M. longifolia F1 hybrids and M. dumetorum. Euphytica 1972, 21, 337–343. [Google Scholar] [CrossRef]
- Pavela, R.; Kaffkova, K.; Kumšta, M. Chemical composition and larvicidal activity of essential oils from different Mentha L. and Pulegium species against Culex quinquefasciatus say (Diptera: Culicidae). Plant Prot. Sci. 2014, 50, 36–42. [Google Scholar] [CrossRef]
- Pavela, R. Insecticidal properties of several essential oils on the house fly (Musca domestica L.). Phytother. Res. 2008, 22, 274–278. [Google Scholar] [CrossRef]
- Choi, W.I.; Lee, S.G.; Park, H.M.; Ahn, Y.J. Toxicity of plant essential oils to Tetranychus urticae (Acari: Tetranychidae) and Phytoseiulus persimilis (Acari: Phytoseiidae). J. Econ. Entomol. 2004, 97, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Tandon, S.; Ahmad, A.; Singh, A.K.; Tripathi, A.K. Structure–activity relationships of monoterpenes and acetyl derivatives against Aedes aegypti (Diptera: Culicidae) larvae. Pest Manag. Sci. 2013, 69, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Li, A.S.; Iijima, A.; Huang, J.; Li, Q.X.; Chen, Y. Putative mode of action of the monoterpenoids linalool, methyl eugenol, estragole, and citronellal on ligand-gated ion channels. Engineering 2020, 6, 541–545. [Google Scholar] [CrossRef]
- Pajaro-Castro, N.; Caballero-Gallardo, K.; Olivero-Verbel, J. Neurotoxic effects of linalool and β-pinene on Tribolium castaneum Herbst. Molecules 2017, 22, 2052. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.; Proença, P.L.; Oliveira, J.L.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Use of botanical insecticides for sustainable agriculture: Future perspectives. Ecol. Indic. 2019, 105, 483–495. [Google Scholar] [CrossRef]
- Praveena, A.; Sanjayan, K.P. Inhibition of acetylcholinesterase in three insects of economic importance by linalool, a monoterpene phytochemical. Insect Pest Manag. Curr. Scenario 2011, 2010, 240–345. [Google Scholar]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological Activities of Lavender Essential Oil. Phyther. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, M. Essential oils and fragrance compounds: Bioactivity and mechanisms of action. Flavour Fragr. J. 2004, 19, 159–165. [Google Scholar] [CrossRef]
- Tong, F.; Coats, J.R. Effects of monoterpenoid insecticides on [3H]-TBOB binding in house fly GABA receptor and 36Cl− uptake in American cockroach ventral nerve cord. Pestic. Biochem. Phys. 2010, 98, 317–324. [Google Scholar] [CrossRef]
- Ottai, M.E.S.; Ahmed, S.S.; Din, M.M.E. Genetic variability among some quantitative characters, insecticidal activity and essential oil composition of two Egyptian and French sweet basil varieties. Aust. J. Basic Appl. Sci. 2012, 6, 185–192. [Google Scholar]
- Hossain, F.; Lacroix, M.; Salmieri, S.; Vu, K.; Follett, P.A. Basil oil fumigation increases radiation sensitivity in adult Sitophilus oryzae (Coleoptera: Curculionidae). J. Stored Prod. Res. 2014, 59, 108–112. [Google Scholar] [CrossRef]
- Ling Chang, C.; Kyu Cho, I.; Li, Q.X. Insecticidal activity of basil oil, trans-anethole, estragole, and linalool to adult fruit flies of Ceratitis capitata, Bactrocera dorsalis, and Bactrocera cucurbitae. J. Econ. Entomol. 2009, 102, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Vicenço, C.B.; Silvestre, W.P.; Lima, T.S.; Pauletti, G.F. Insecticidal activity of Cinnamomum camphora Ness and Eberm var. linaloolifera Fujita leaf essential oil and linalool against Anticarsia gemmatalis. J. Essent. Oil Res. 2021, 33, 601–609. [Google Scholar] [CrossRef]
- Vicenço, C.B.; Silvestre, W.P.; Pauletti, G.F.; de Barros, N.M.; Schwambach, J. Cinnamomum camphora var. linaloolifera essential oil on pest control: Its effect on Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Res. Soc. Dev. 2021, 10, e45710716216. [Google Scholar] [CrossRef]
- Khani, A.; Rahdari, T. Chemical composition and insecticidal activity of essential oil from Coriandrum sativum seeds against Tribolium confusum and Callosobruchus maculatus. Int. Sch. Res. Notices 2012, 2012, 263517. [Google Scholar] [CrossRef]
- Ayvaz, A.; Sagdic, O.; Karaborklu, S.; Ozturk, I. Insecticidal activity of the essential oils from different plants against three stored-product insects. J. Insect Sci. 2010, 10, 21. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, K.; Zhao, N.N.; Wang, X.G.; Wang, S.Y.; Liu, Z.L. Composition and insecticidal activity of the essential oil of Cananga odorata leaves against Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). J. Med. Plant Res. 2012, 6, 3568–3572. [Google Scholar]
- Pavela, R.; Ferrati, M.; Spinozzi, E.; Maggi, F.; Petrelli, R.; Rakotosaona, R.; Ricciardi, R.; Benelli, G. The Essential Oil from the Resurrection Plant Myrothamnus moschatus Is Effective against Arthropods of Agricultural and Medical Interest. Pharmaceuticals 2022, 15, 1511. [Google Scholar] [CrossRef]
- WHO. Report of the WHO Informal Consultation on the Evaluation and Testing of Insecticides; CTD/WHOPES/IC/96.1; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis; Cambridge University Press: London, UK, 1971. [Google Scholar]
| No | Component a | RI b | RI Lit c | Area % d |
|---|---|---|---|---|
| 1 | α-thujene | 921 | 924 | Tr |
| 2 | α-pinene | 925 | 932 | 0.2 ± 0.0 |
| 3 | sabinene | 966 | 969 | 0.5 ± 0.1 |
| 4 | β-pinene | 968 | 974 | 0.6 ± 0.1 |
| 5 | myrcene | 989 | 988 | 2.0 ± 0.3 |
| 6 | α-terpinene | 1013 | 1014 | 0.1 ± 0.0 |
| 7 | ρ-cymene | 1022 | 1020 | Tr e |
| 8 | limonene | 1024 | 1024 | 0.9 ± 0.2 |
| 9 | 1,8-cineole | 1025 | 1026 | 6.7 ± 1.0 |
| 10 | (Z)-β-ocimene | 1036 | 1032 | 0.7 ± 0.1 |
| 11 | (E)-β-ocimene | 1046 | 1044 | 0.9 ± 0.2 |
| 12 | γ-terpinene | 1054 | 1054 | 0.2 ± 0.0 |
| 13 | cis-sabinene hydrate | 1063 | 1065 | Tr |
| 14 | terpinolene | 1084 | 1086 | 0.2 ± 0.0 |
| 15 | linalool | 1100 | 1095 | 26.8 ± 2.5 |
| 16 | isopentyl 2-methyl butanoate | 1104 | 1100 | 0.4 ± 0.1 |
| 17 | 2-methyl butyl isovalerate | 1109 | 1103 | 0.1 ± 0.0 |
| 18 | 1-octen-3-yl acetate | 1114 | 1110 | 0.1 ± 0.0 |
| 19 | 3-octanol acetate | 1126 | 1120 | 0.3 ± 0.1 |
| 20 | δ-terpineol | 1163 | 1162 | Tr |
| 21 | terpinen-4-ol | 1172 | 1174 | 0.2 ± 0.0 |
| 22 | α-terpineol | 1185 | 1186 | 5.1 ± 0.9 |
| 23 | nerol | 1227 | 1227 | 0.2 ± 0.0 |
| 24 | linalool acetate | 1256 | 1254 | 34.9 ± 3.1 |
| 25 | α-terpinyl acetate | 1345 | 1346 | 12.3 ± 1.9 |
| 26 | neryl acetate | 1365 | 1359 | 0.7 ± 0.1 |
| 27 | β-bourbonene | 1374 | 1387 | Tr |
| 28 | (E)-caryophyllene | 1412 | 1417 | 1.3 ± 0.3 |
| 29 | α-humulene | 1446 | 1452 | Tr |
| 30 | (E)-β-farnesene | 1455 | 1454 | Tr |
| 31 | germacrene D | 1470 | 1484 | 1.8 ± 0.3 |
| 32 | hedycaryol | 1542 | 1546 | 0.7 ± 0.1 |
| Total identified (%) | 98.1 ± 0.5 | |||
| Grouped compounds (%) | ||||
| Monoterpene hydrocarbons | 6.2 ± 0.3 | |||
| Oxygenated monoterpenes | 86.9 ± 0.7 | |||
| Sesquiterpene hydrocarbons | 3.2 ± 0.2 | |||
| Oxygenated sesquiterpenes | 0.7 ± 0.1 | |||
| Esters | 1.0 ± 0.1 |
| Target Insect Species | Unit | LD50/LC50 | CI95 a | LD90/LC90 | CI95 a | Chi | p-Level | Df |
|---|---|---|---|---|---|---|---|---|
| Musca domestica—adults female Musca domestica—adults male | µg adult−1 | 71.4 ± 7.2 | 58.2–85.9 | 329.8 ± 15.5 | 298.5–522.7 | 3.678 | 0.321 | 4 |
| µg adult−1 | 50.5 ± 5.9 | 48.2–62.8 | 462.6 ± 25.7 | 398.8–552.1 | 3.781 | 0.203 | 5 | |
| Culex quinquefasciatus 2nd instar larvae | µl L−1 | 31.5 ± 2.2 | 22.8–36.7 | 80.9 ± 6.7 | 72.8–91.5 | 1.512 | 0.896 | 4 |
| Culex quinquefasciatus 3rd instar larvae | µl L−1 | 79.4 ± 5.2 | 62.5–98.7 | 307.2 ± 26.4 | 285.7–332.5 | 3.219 | 0.124 | 4 |
| Spodoptera littoralis 2nd instar larvae | µg larva−1 | 18.5 ± 2.1 | 15.2–22.9 | 41.9 ± 2.9 | 33.8–47.7 | 0.845 | 0.985 | 4 |
| Spodoptera littoralis 3rd instar larvae | µg larva−1 | 44.2 ± 5.8 | 36.9–53.2 | 117.8 ± 5.1 | 98.7–123.8 | 1.169 | 0.760 | 3 |
| Metopolophium dirhodum adult | mL L−1 | 4.9 ± 0.8 | 4.5–5.2 | 7.1 ± 0.3 | 6.5–8.9 | 0.891 | 0.598 | 3 |
| Tetranychus urticae adults | mL L−1 | 3.3 ± 0.5 | 2.9–3.9 | 6.2 ± 0.8 | 5.7–7.3 | 1.258 | 0.722 | 3 |
| No | Origin | Major Compound | Reference |
|---|---|---|---|
| 1 | South of Tunisia, Region of Sfax | Pulegone | [11] |
| 2 | Vojvodina, Serbia | Menthofuran | [33] |
| Submediterranean region of south Croatia | [34] | ||
| Ethiopia | [3] | ||
| Pisa, Italy | [35] | ||
| South-east Romania | [5] | ||
| 3 | North of Iran, Mazandaran province | Piperitenone oxide | [13] |
| 4 | West of Iran, Kermanshah province | Menthol | [14] |
| 5 | Israel | Linalool | [14] |
| Western Iran, Lorestan region | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrati, M.; Spinozzi, E.; Baldassarri, C.; Maggi, F.; Pavela, R.; Canale, A.; Petrelli, R.; Cappellacci, L. Efficacy of Mentha aquatica L. Essential Oil (Linalool/Linalool Acetate Chemotype) against Insect Vectors and Agricultural Pests. Pharmaceuticals 2023, 16, 633. https://doi.org/10.3390/ph16040633
Ferrati M, Spinozzi E, Baldassarri C, Maggi F, Pavela R, Canale A, Petrelli R, Cappellacci L. Efficacy of Mentha aquatica L. Essential Oil (Linalool/Linalool Acetate Chemotype) against Insect Vectors and Agricultural Pests. Pharmaceuticals. 2023; 16(4):633. https://doi.org/10.3390/ph16040633
Chicago/Turabian StyleFerrati, Marta, Eleonora Spinozzi, Cecilia Baldassarri, Filippo Maggi, Roman Pavela, Angelo Canale, Riccardo Petrelli, and Loredana Cappellacci. 2023. "Efficacy of Mentha aquatica L. Essential Oil (Linalool/Linalool Acetate Chemotype) against Insect Vectors and Agricultural Pests" Pharmaceuticals 16, no. 4: 633. https://doi.org/10.3390/ph16040633
APA StyleFerrati, M., Spinozzi, E., Baldassarri, C., Maggi, F., Pavela, R., Canale, A., Petrelli, R., & Cappellacci, L. (2023). Efficacy of Mentha aquatica L. Essential Oil (Linalool/Linalool Acetate Chemotype) against Insect Vectors and Agricultural Pests. Pharmaceuticals, 16(4), 633. https://doi.org/10.3390/ph16040633