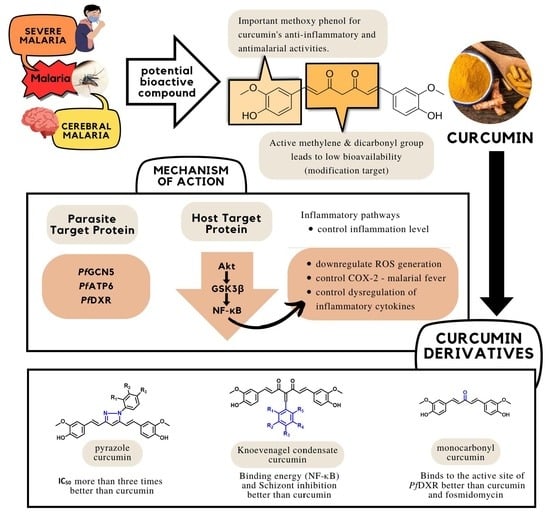
Curcumin and Its Derivatives as Potential Antimalarial and Anti-Inflammatory Agents: A Review on Structure–Activity Relationship and Mechanism of Action
Abstract
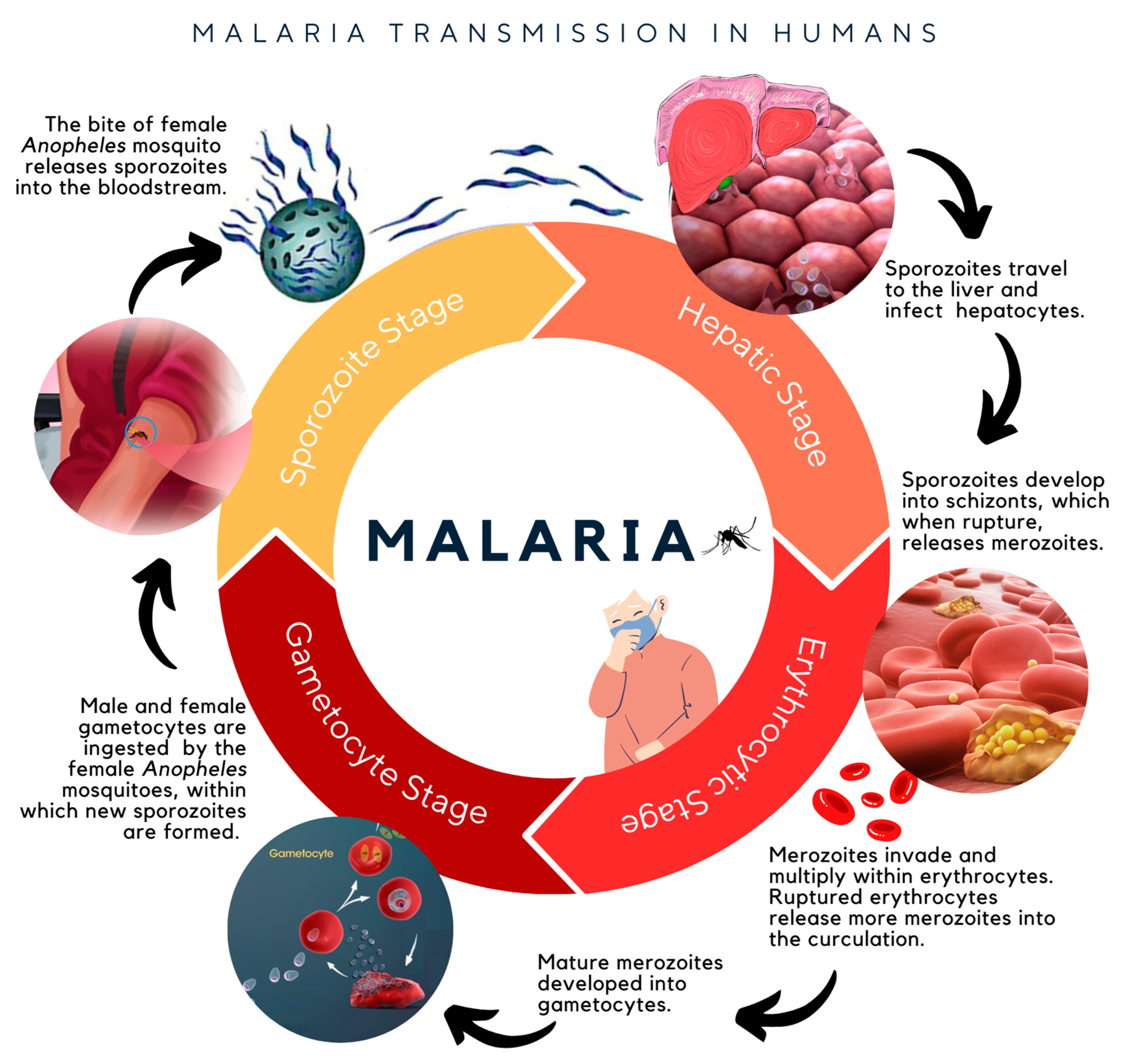
1. Introduction
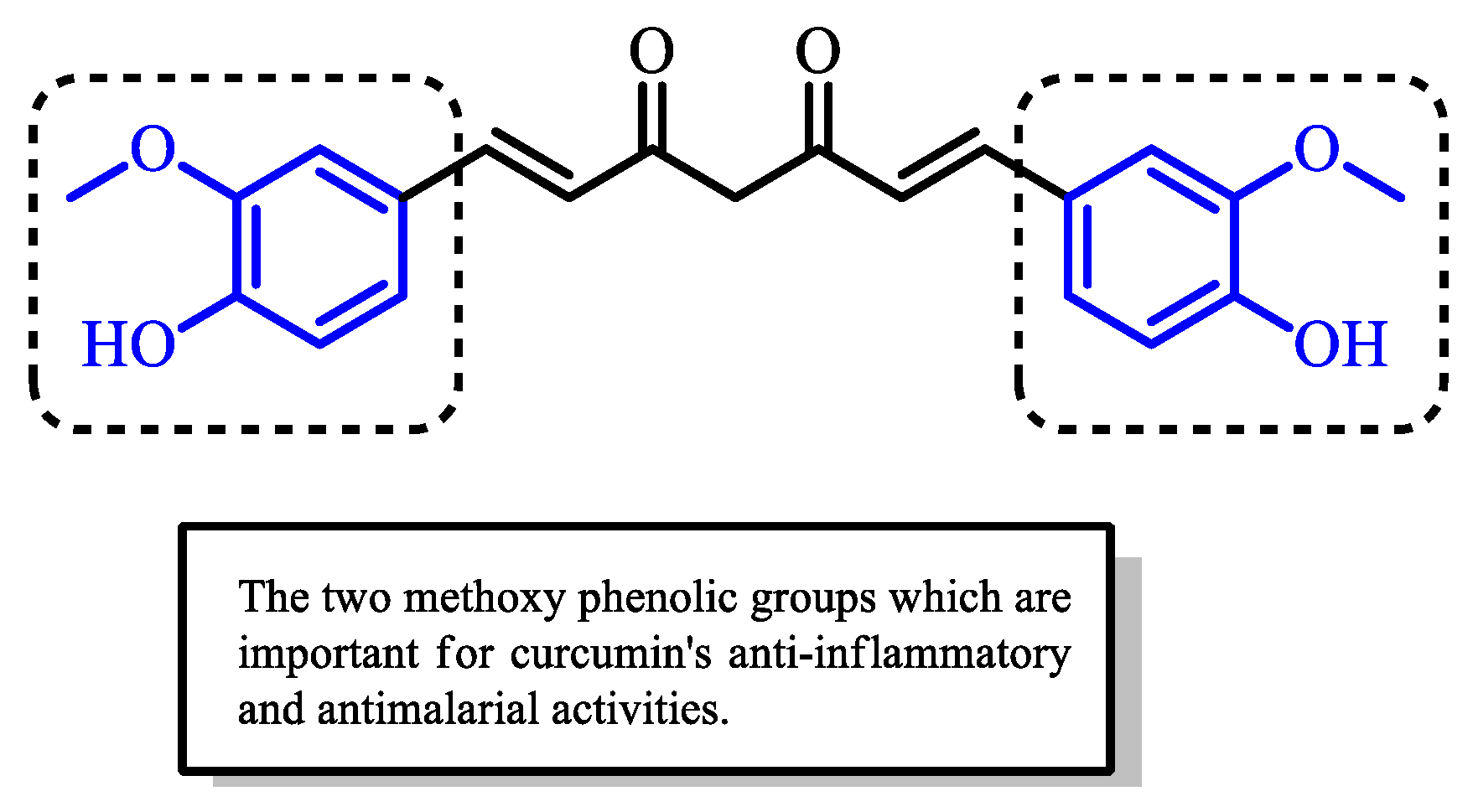
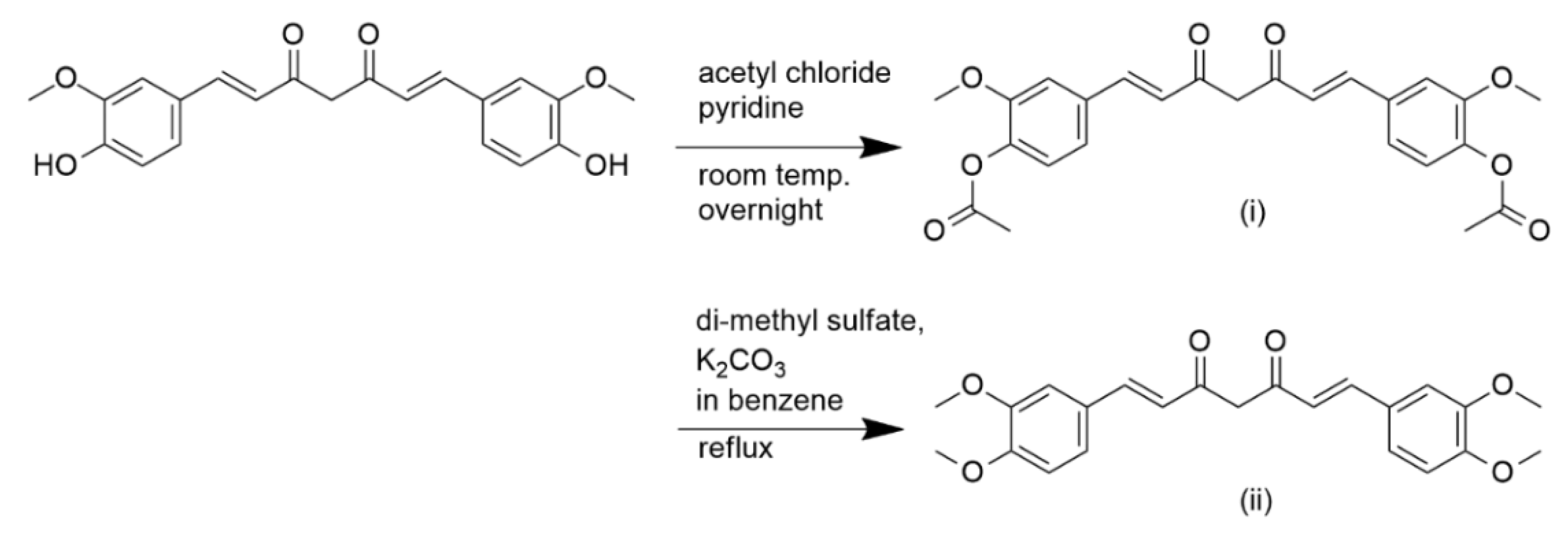
2. Antimalarial and Anti-Inflammatory Activities of Curcumin and Its Structural Modifications
3. Structure–Activity Relationship of Curcumin Derivatives as Antimalarial and Anti-Inflammatory Agents
4. Mechanism of Action of Curcumin
4.1. Host Proteins as Molecular Targets of Curcumin
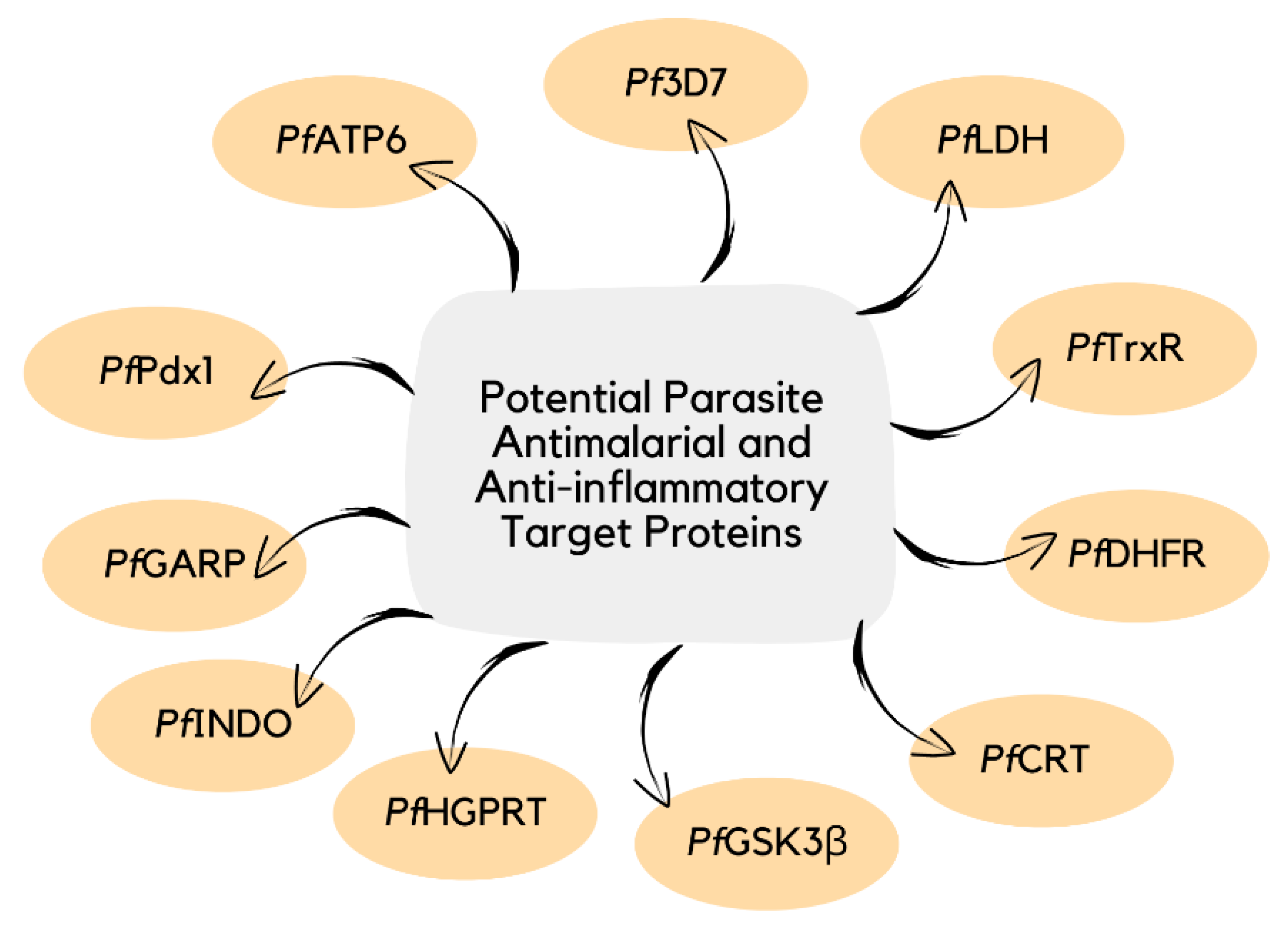
4.2. Parasite Proteins as Molecular Targets of Curcumin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC), 2022. Available online: https://www.cdc.gov/malaria (accessed on 21 March 2023).
- Habibi, P.; Grossi-de-Sa, M.F.; Shi, Y.; Khan, I. Plants as sources of natural and recombinant antimalaria agents. Mol. Biotechnol. 2022, 64, 1177–1197. [Google Scholar] [CrossRef] [PubMed]
- Kingston, D.G.I.; Cassera, M.B. Antimalarial natural products. Prog. Chem. Org. Nat. Prod. 2022, 117, 1–106. [Google Scholar] [PubMed]
- Moreno-García, M.; Recio-Tótoro, B.; Claudio-Piedras, F.; Lanz-Mendoza, H. Injury and immune response: Applying the danger theory to mosquitoes. Front. Plant Sci. 2014, 5, 451. [Google Scholar] [CrossRef] [PubMed]
- WHO, World Health Organisation. World Malaria Report, Geneva, 2022. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022 (accessed on 21 March 2023).
- Ridley, R.G. Medical need, scientific opportunity and the drive for antimalarial drugs. Nature 2002, 415, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B.; Mutabingwa, T. Malaria in 2002. Nature 2002, 415, 670–672. [Google Scholar] [CrossRef]
- Dasgupta, T.; Chitnumsub, P.; Kamchonwongpaisan, S.; Maneeruttanarungroj, C.; Swift, S.E.; Lyons, T.M.; Tirado-Rives, J.; Jorgensen, W.L.; Yuthavong, Y.; Anderson, K.S. Exploiting structural analysis, in silico screening, and serendipity to identify novel inhibitors of drug-resistant falciparum malaria. ACS Chem. Biol. 2009, 4, 29–40. [Google Scholar] [CrossRef]
- Alson, S.G.; Jansen, O.; Cieckiewicz, E.; Rakotoarimanana, H.; Rafatro, H.; Degotte, G.; Francotte, P.; Frederich, M. In-vitro and in-vivo antimalarial activity of caffeic acid and some of its derivatives. J. Pharm. Pharmacol. 2018, 70, 1349–1356. [Google Scholar] [CrossRef]
- Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: Biological activities and modern pharmaceutical forms. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Aggarwal, B.B.; Bhatt, I.D.; Ichikawa, H.; Ahn, K.S.; Sethi, G.; Sandur, S.K.; Natarajan, C.; Seeram, N.; Shishodia, S. 10 Curcumin—Biological and Medicinal Properties; Indsaff, Inc.: Batala, India, 2006; p. 297. [Google Scholar]
- Jayaprakasha, G.K.; Rao, L.J.; Sakariah, K.K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006, 98, 720–724. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef]
- Oglah, M.K.; Mustafa, Y.F.; Bashir, M.K.; Jasim, M.H.; Mustafa, Y.F. Curcumin and its derivatives: A review of their biological activities. Syst. Rev. Pharm. 2020, 11, 472. [Google Scholar]
- Lee, K.H.; Aziz, F.H.A.; Syahida, A.; Abas, F.; Shaari, K.; Israf, D.A.; Lajis, N.H. Synthesis and biological evaluation of curcumin-like diarylpentanoid analogues for anti-inflammatory, antioxidant and anti-tyrosinase activities. Eur. J. Med. Chem. 2009, 44, 3195–3200. [Google Scholar] [CrossRef]
- Liang, G.; Yang, S.; Jiang, L.; Zhao, Y.; Shao, L.; Xiao, J.; Ye, F.; Li, Y.; Li, X. Synthesis and anti-bacterial properties of mono-carbonyl analogues of curcumin. Chem. Pharm. Bull. 2008, 56, 162–167. [Google Scholar] [CrossRef]
- Dai, C.; Ciccotosto, G.D.; Cappai, R.; Tang, S.; Li, D.; Xie, S.; Xiao, X.; Velkov, T. Curcumin attenuates colistin-induced neurotoxicity in N2a cells via anti-inflammatory activity, suppression of oxidative stress, and apoptosis. Mol. Neurobiol. 2018, 55, 421–434. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, R.; Ma, Y.; Zhang, Z.; Xie, Z. Curcumin attenuates airway inflammation and airway remolding by inhibiting NF-κB signaling and COX-2 in cigarette smoke-induced COPD mice. Inflammation 2018, 41, 1804–1814. [Google Scholar] [CrossRef]
- Nandakumar, D.N.; Nagaraj, V.A.; Vathsala, P.G.; Rangarajan, P.; Padmanaban, G. Curcumin-artemisinin combination therapy for malaria. Antimicrob. Agents Chemother. 2006, 50, 1859–1860. [Google Scholar] [CrossRef]
- Martinelli, A.; Rodrigues, L.A.; Cravo, P. Plasmodium chabaudi: Efficacy of artemisinin+ curcumin combination treatment on a clone selected for artemisinin resistance in mice. Exp. Parasitol. 2008, 119, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.C.; Vatsala, P.G.; Keshamouni, V.G.; Padmanaban, G.; Rangarajan, P.N. Curcumin for malaria therapy. Biochem. Biophys. Res. Commun. 2004, 326, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Dovigo, L.N.; Pavarina, A.C.; Ribeiro, A.P.D.; Brunetti, I.L.; Costa, C.A.D.S.; Jacomassi, D.P.; Bagnato, V.S.; Kurachi, C. Investigation of the photodynamic effects of curcumin against Candida albicans. Photochem. Photobiol. 2011, 87, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gomes, A.S.; Curvelo, J.A.R.; Soares, R.A.; Ferreira-Pereira, A. Curcumin acts synergistically with fluconazole to sensitize a clinical isolate of Candida albicans showing a MDR phenotype. Med. Mycol. J. 2012, 50, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Gunes, H.; Gulen, D.; Mutlu, R.; Gumus, A.; Tas, T.; Topkaya, A.E. Antibacterial effects of curcumin: An in vitro minimum inhibitory concentration study. Toxicol. Ind. Health 2016, 32, 246–250. [Google Scholar] [CrossRef]
- Khudhayer, O.M.; Fakri Mustafa, Y. Curcumin analogs: Synthesis and biological activities. Med. Chem. Res. 2020, 29, 479–486. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Rajanikant, G.K. Curcumin stimulates the antioxidant mechanisms in mouse skin exposed to fractionated γ-irradiation. Antioxidants 2015, 4, 25–41. [Google Scholar] [CrossRef]
- Ma, Q.; Ren, Y.; Wang, L. Investigation of antioxidant activity and release kinetics of curcumin from tara gum/polyvinyl alcohol active film. Food Hydrocoll. 2017, 70, 286–292. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, Y.; Yun, X.; Ou, Y.; Zhang, W.; Li, J.X. Antinociceptive effects of curcumin in a rat model of postoperative pain. Sci. Rep. 2014, 4, 1–4. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Anand, P.; Aggarwal, B.B. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008, 269, 199–225. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.M.; Shen, Z.Z.; Liu, C.H.; Sartippour, M.R.; Go, V.L.; Heber, D.; Nguyen, M. Curcumin exerts multiple suppressive effects on human breast carcinoma cells. Int. J. Cancer 2002, 98, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Momtazi-Borojeni, A.A.; Mosafer, J.; Nikfar, B.; Ekhlasi-Hundrieser, M.; Chaichian, S.; Mehdizadehkashi, A.; Vaezi, A. Curcumin in advancing treatment for gynecological cancers with developed drug-and radiotherapy-associated resistance. Rev. Physiol. Biochem. Pharmacol. 2019, 176, 107–129. [Google Scholar]
- Dohutia, C.; Chetia, D.; Gogoi, K.; Bhattacharyya, D.R.; Sarma, K. Molecular docking, synthesis and in vitro antimalarial evaluation of certain novel curcumin analogues. Braz. J. Pharm. Sci. 2018, 53, 1–14. [Google Scholar] [CrossRef]
- Mishra, S.; Karmodiya, K.; Surolia, N.; Surolia, A. Synthesis and exploration of novel curcumin analogues as anti-malarial agents. Bioorg. Med. Chem. 2008, 16, 2894–2902. [Google Scholar] [CrossRef] [PubMed]
- Eckstein-Ludwig, U.; Webb, R.J.; Van Goethem, I.D.A.; East, J.M.; Lee, A.G.; Kimura, M.; O’neill, P.M.; Bray, P.G.; Ward, S.A.; Krishna, S. Artemisinins target the SERCA of Plasmodium falciparum. Nature 2003, 424, 957–961. [Google Scholar] [CrossRef]
- Hosseini-Zare, M.S.; Sarhadi, M.; Zarei, M.; Thilagavathi, R.; Selvam, C. Synergistic effects of curcumin and its analogs with other bioactive compounds: A comprehensive review. Eur. J. Med. Chem. 2021, 210, 113072. [Google Scholar] [CrossRef]
- Khairani, S.; Fauziah, N.; Wiraswati, H.L.; Panigoro, R.; Setyowati, E.Y.; Berbudi, A. The potential use of a curcumin-piperine combination as an antimalarial agent: A systematic review. J. Trop. Med. 2021, 2021, 9135617. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022. [Google Scholar] [CrossRef]
- Möhrle, J.J. Towards the next generation of antimalaria combination therapies. Lancet Infect. Dis. 2021, 21, 1620–1621. [Google Scholar] [CrossRef]
- Tjahjani, S.; Syafruddin; Tjokropranoto, R. Interaction of alphamangostin and curcumin with dihydroartemisinin as antimalaria in vitro. IOP Conf. Ser. Earth Environ. Sci. 2018, 125, 012017. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Liang, G.; Shao, L.; Wang, Y.; Zhao, C.; Chu, Y.; Xiao, J.; Zhao, Y.; Li, X.; Yang, S. Exploration and synthesis of curcumin analogues with improved structural stability both in vitro and in vivo as cytotoxic agents. Bioorg. Med. Chem. 2009, 17, 2623–2631. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Cheng, A.L. Clinical studies with curcumin. Adv. Exp. Med. Biol. 2007, 595, 471–480. [Google Scholar] [PubMed]
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar] [PubMed]
- Sharma, R.A.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and pharmacodynamics of curcumin. The molecular targets and therapeutic uses of curcumin in health and disease. AEMB 2007, 595, 453–470. [Google Scholar]
- da Silva, C.C.; Pacheco, B.S.; das Neves, R.N.; Alves, M.S.D.; Sena-Lopes, A.; Moura, S.; Borsuk, S.; de Pereira, C.M.P. Antiparasitic activity of synthetic curcumin monocarbonyl analogues against Trichomonas vaginalis. Biomed. Pharmacother. 2019, 111, 367–377. [Google Scholar] [CrossRef]
- Narlawar, R.; Pickhardt, M.; Leuchtenberger, S.; Baumann, K.; Krause, S.; Dyrks, T.; Weggen, S.; Mandelkow, E.; Schmidt, B. Curcumin-derived pyrazoles and isoxazoles: Swiss army knives or blunt tools for Alzheimer’s disease? ChemMedChem 2008, 3, 165–172. [Google Scholar] [CrossRef]
- Chakraborti, S.; Dhar, G.; Dwivedi, V.; Das, A.; Poddar, A.; Chakraborti, G.; Basu, G.; Chakrabarti, P.; Surolia, A.; Bhattacharyya, B. Stable and potent analogues derived from the modification of the dicarbonyl moiety of curcumin. Biochemistry 2013, 52, 7449–7460. [Google Scholar] [CrossRef]
- Zielińska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.F.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.V.; et al. Properties, extraction methods, and delivery systems for curcumin as a natural source of beneficial health effects. Medicina 2020, 56, 336. [Google Scholar] [CrossRef]
- Angelini, G.; Pasc, A.; Gasbarri, C. Curcumin in silver nanoparticles aqueous solution: Kinetics of keto-enol tautomerism and effects on AgNPs. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125235. [Google Scholar] [CrossRef]
- Thomachan, S.; Sindhu, S.; John, V.D. Synthesis, characterization, antibacterial, antifungal and cytotoxic activity of curcuminoid analogues with trisubstituted phenyl and anthracenyl ring and their zinc (II), copper (II) and vanadyl (IV) chelates. Int. J. Pharmac. Chem. 2016, 6, 78–86. [Google Scholar]
- Al-Hujaily, E.M.; Mohamed, A.G.; Al-Sharif, I.; Youssef, K.M.; Manogaran, P.S.; Al-Otaibi, B.; Al-Haza’a, A.; Al-Jammaz, I.; Al-Hussein, K.; Aboussekhra, A. PAC, a novel curcumin analogue, has anti-breast cancer properties with higher efficiency on ER-negative cells. Breast Cancer Res. Treat. 2011, 128, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cai, Z.; Wei, X.; Chen, M.; Ying, S.; Shi, L.; Xu, R.A.; He, F.; Liang, G.; Zhang, X. Anti-lung cancer activity of the curcumin analog JZ534 in vitro. Biomed. Res. Int. 2015, 2015, 504529. [Google Scholar] [CrossRef]
- Tan, X.; Sidell, N.; Mancini, A.; Huang, R.P.; Wang, S.; Horowitz, I.R.; Liotta, D.C.; Taylor, R.N.; Wieser, F. Multiple anticancer activities of EF24, a novel curcumin analog, on human ovarian carcinoma cells. Reprod. Sci. 2010, 17, 931–940. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Cai, L.; Cai, Y.; Hu, J.; Yu, C.; Li, J.; Feng, Z.; Yang, S.; Li, X.; et al. Inhibition of high glucose-induced inflammatory response and macrophage infiltration by a novel curcumin derivative prevents renal injury in diabetic rats. Br. J. Pharmacol. 2012, 166, 1169–1182. [Google Scholar] [CrossRef]
- Katsori, A.M.; Chatzopoulou, M.; Dimas, K.; Kontogiorgis, C.; Patsilinakos, A.; Trangas, T.; Hadjipavlou-Litina, D. Curcumin analogues as possible anti-proliferative & anti-inflammatory agents. Eur. J. Med. Chem. 2011, 46, 2722–2735. [Google Scholar]
- Chougala, M.B.; Bhaskar, J.J.; Rajan, M.G.R.; Salimath, P.V. Effect of curcumin and quercetin on lysosomal enzyme activities in streptozotocin-induced diabetic rats. Clin. Nutr. 2012, 31, 749–755. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Rodrigues, F.C.; Anil Kumar, N.V.; Thakur, G. The potency of heterocyclic curcumin analogues: An evidence-based review. Pharmacol. Res. 2021, 166, 105489. [Google Scholar] [CrossRef]
- Flynn, D.L.; Belliotti, T.R.; Boctor, A.M.; Connor, D.T.; Kostlan, C.R.; Nies, D.E.; Ortwine, D.F.; Schrier, D.J.; Sircar, J.C. Styrylpyrazoles, styrylisoxazoles, and styrylisothiazoles. Novel 5-lipoxygenase and cyclooxygenase inhibitors. J. Med. Chem. 1991, 34, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Selvam, C.; Jachak, S.M.; Thilagavathi, R.; Chakraborti, A.K. Design, synthesis, biological evaluation and molecular docking of curcumin analogues as antioxidant, cyclooxygenase inhibitory and anti-inflammatory agents. Bioorganic Med. Chem. Lett. 2005, 15, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, A.C.; Smith, J.W. The dipole moments of some aromatic nitro-compounds in relation to the steric inhibition of the mesomeric effect of the nitro-group. J. Chem. Soc. 1957, 1957, 2476–2482. [Google Scholar] [CrossRef]
- Jones, G. The Knoevenagel Condensation. Org. React. 2011, 15, 204–599. [Google Scholar]
- Zambre, A.P.; Kulkarni, V.M.; Padhye, S.; Sandur, S.K.; Aggarwal, B.B. Novel curcumin analogs targeting TNF-induced NF-κB proliferation in human leukemic KBM-5 cellsB activation. Bioorg. Med. Chem. 2006, 14, 7196–7204. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.; Shen, L. Interactions of curcumin with the PfATP6 model and the implications for its antimalarial mechanism. Bioorganic Med. Chem. Lett. 2009, 19, 2453–2455. [Google Scholar] [CrossRef]
- NCBI, National Center for Biotechnology Information. PubChem Compound Summary for CID 969516, Curcumin. PubChem 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/curcumin (accessed on 22 December 2022).
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Harrold, M.W.; Zavod, R.M. Functional Group Characteristics and Roles in Basic Concepts in Medicinal Chemistry; American Society of Health-System Pharmacists Publications: Bethesda, MD, USA, 2018; pp. 22–66. [Google Scholar]
- Yusuf, A.S.; Sada, I.; Hassan, Y.; Olomola, T.O.; Adeyemi, C.M.; Ajibade, S.O. Synthesis, antimalarial activity, and docking studies of monocarbonyl analogues of curcumin. Ovidius Univ. Ann. Chem. 2018, 29, 92–96. [Google Scholar] [CrossRef]
- Ziegler, H.L.; Stærk, D.; Christensen, J.; Hviid, L.; Hagerstrand, H.; Jaroszewski, J.W. In vitro Plasmodium falciparum drug sensitivity assay: Inhibition of parasite growth by incorporation of stomatocytogenic amphiphiles into the erythrocyte membrane. Antimicrob. Agents Chemother. 2002, 46, 1441–1446. [Google Scholar] [CrossRef]
- Mukhtar, M.D.; Bashir, M.; Arzai, A.H. Comparative in-vitro studies on antiplasmodial quality of some Nigerian and foreign brands of chloroquine oral formulations marketed in kano. Afr. J. Biotechnol. 2006, 5, 2464. [Google Scholar]
- Bodill, T.; Conibear, A.C.; Blatch, G.L.; Lobb, K.A.; Kaye, P.T. Synthesis and evaluation of phosphonated N-heteroarylcarboxamides as DOXP-reductoisomerase (DXR) inhibitors. Bioorg. Med. Chem. 2011, 19, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Jin, W.; Yuan, B.; Zhu, T.; Wang, J.; Jiang, J.; Liang, W.; Ma, Z. Curcumin inhibits the increase of labile zinc and the expression of inflammatory cytokines after traumatic spinal cord injury in rats. J. Surg. Res. 2014, 187, 646–652. [Google Scholar] [CrossRef]
- Schumann, R.R. Malarial fever: Hemozoin is involved but Toll-free. Proc. Natl. Acad. Sci. USA 2007, 104, 1743–1744. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.M.Y. Inhibition of tumor necrosis factor by curcumin, a phytochemical. Biochem. Pharmacol. 1995, 49, 1551–1556. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. The molecular targets and therapeutic uses of curcumin in health and disease. AEMB 2007, 595, 1–75. [Google Scholar]
- Siebenlist, U.; Franzoso, G.; Brown, K. Structure, regulation and function of NF-kappaB. Annu. Rev. Cell Dev. Biol. 1994, 10, 405–455. [Google Scholar] [CrossRef]
- Shishodia, S.; Singh, T.; Chaturvedi, M.M. Modulation of transcription factors by curcumin. The molecular targets and therapeutic uses of curcumin in health and disease. AEMB 2007, 595, 127–148. [Google Scholar]
- Xu, Y.X.; Pindolia, K.R.; Janakiraman, N.; Noth, C.J.; Chapman, R.A.; Gautam, S.C. Curcumin, a compound with anti-inflammatory and anti-oxidant properties, down-regulates chemokine expression in bone marrow stromal cells. Exp. Hematol. 1997, 25, 413–422. [Google Scholar]
- Surh, Y.J. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: A short review. Food Chem. Toxicol. 2002, 40, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Paulino, N.; Paulino, A.S.; Diniz, S.N.; de Mendonça, S.; Gonçalves, I.D.; Flores, F.F.; Santos, R.P.; Rodrigues, C.; Pardi, P.C.; Suarez, J.A.Q. Evaluation of the anti-inflammatory action of curcumin analog (DM1): Effect on iNOS and COX-2 gene expression and autophagy pathways. Bioorg. Med. Chem. 2016, 24, 1927–1935. [Google Scholar] [CrossRef] [PubMed]
- Perlmann, P.; Troye-Blomberg, M. Malaria blood-stage infection and its control by the immune system. Folia Biol. 2000, 46, 210–218. [Google Scholar]
- Jang, M.K.; Sohn, D.H.; Ryu, J.H. A curcuminoid and sesquiterpenes as inhibitors of macrophage TNF-α release from Curcuma zedoaria. Planta Med. 2001, 67, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Tewtrakul, S.; Morikawa, T.; Nakamura, A.; Yoshikawa, M. Anti-allergic principles from Thai zedoary: Structural requirements of curcuminoids for inhibition of degranulation and effect on the release of TNF-α and IL-4 in RBL-2H3 cells. Bioorg. Med. Chem. 2004, 12, 5891–5898. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y. Cell signaling pathways altered by natural chemopreventive agents. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2004, 555, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Aggarwal, B.B. Activation of transcription factor NF-κB is suppressed by curcumin (diferuloylmethane). J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar] [CrossRef]
- Ali, A.H.; Sudi, S.; Basir, R.; Embi, N.; Sidek, H.M. The antimalarial effect of curcumin is mediated by the inhibition of glycogen synthase kinase-3 β. J. Med. Food. 2017, 20, 152–161. [Google Scholar] [CrossRef]
- Mimche, P.N.; Taramelli, D.; Vivas, L. The plant-based immunomodulator curcumin as a potential candidate for the development of an adjunctive therapy for cerebral malaria. Malar. J. 2011, 10, 510. [Google Scholar] [CrossRef]
- Kaiser, K.; Texier, A.; Ferrandiz, J.; Buguet, A.; Meiller, A.; Latour, C.; Peyron, F.; Cespuglio, R.; Picot, S. Recombinant human erythropoietin prevents the death of mice during cerebral malaria. J. Infect. Dis. 2006, 193, 987–995. [Google Scholar] [CrossRef]
- Cui, L.; Miao, J.; Cui, L. Cytotoxic effect of curcumin on malaria parasite Plasmodium falciparum: Inhibition of histone acetylation and generation of reactive oxygen species. Antimicrob. Agents Chemother. 2007, 51, 488–494. [Google Scholar] [CrossRef]
- Shehzad, A.; Qureshi, M.; Anwar, M.N.; Lee, Y.S. Multifunctional curcumin mediate multitherapeutic effects. J. Food Sci. 2017, 82, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Banik, U.; Parasuraman, S.; Adhikary, A.K.; Othman, N.H. Curcumin: The spicy modulator of breast carcinogenesis. J. Exp. Clin. Cancer Res. 2017, 36, 98. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.U.; Rehman, M.S.U.; Khan, M.S.; Ali, M.A.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an alternative epigenetic modulator: Mechanism of action and potential effects. Front. Genet. 2019, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, S.; Anjum, R.; Rangaraj, N.; Pardhasaradhi, B.V.V.; Khar, A. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett. 1999, 456, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Atsumi, T.; Ishihara, M.; Kadoma, Y. Cytotoxicity, ROS-generation activity and radical-scavenging activity of curcumin and related compounds. Anticancer Res. 2004, 24, 563–570. [Google Scholar] [PubMed]
- Alumasa, J.N.; Gorka, A.P.; Casabiance, L.B.; Comstock, E.; de Dios, A.C.; Roepe, P.D. The hydroxyl functionality and a rigid proximal N are required for forming a novel non-covalent quinine-heme complex. J. Inorg. Biochem. 2011, 105, 467. [Google Scholar] [CrossRef]
- Hunt, N.H.; Golenser, J.; Chan-Ling, T.; Parekh, S.; Rae, C. Immunopathogenesis of cerebral malaria. Int. J. Parasitol. 2006, 36, 569–582. [Google Scholar] [CrossRef]
- Schofield, L.; Grau, G.E. Immunological processes in malaria pathogenesis. Nat. Rev. Immunol. 2005, 5, 722–735. [Google Scholar] [CrossRef]
- Balaji, S.N.; Ahsan, M.J.; Jadav, S.S.; Trivedi, V. Molecular modelling, synthesis, and antimalarial potentials of curcumin analogues containing heterocyclic ring. Arab. J. Chem. 2015, 12, 2492–2500. [Google Scholar] [CrossRef]
- Parveen, A.; Chakraborty, A.; Konreddy, A.K.; Chakravarty, H.; Sharon, A.; Trivedi, V.; Bal, C. Skeletal hybridization and PfRIO-2 kinase modeling for synthesis of α-pyrone analogs as anti-malarial agent. Eur. J. Med. Chem. 2013, 70, 607–612. [Google Scholar] [CrossRef]
- Gui, J.S.; Jalil, J.; Jubri, Z.; Kamisah, Y. Parkia speciosa empty pod extract exerts anti-inflammatory properties by modulating NFκB and MAPK pathways in cardiomyocytes exposed to tumor necrosis factor-α. Cytotechnology 2019, 71, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.B.; Dwivedi, S. Structural insight into binding mode of inhibitor with SAHH of Plasmodium and human: Interaction of curcumin with anti-malarial drug targets. J. Chem. Biol. 2016, 9, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Zahidah, A.F.; Faizah, O.; Nur Aqilah, K.; Taty Anna, K. Curcumin as an anti-arthritic agent in collagen-induced arthritic Sprague-Dawley rats. Sains Malays. 2012, 41, 591–595. [Google Scholar]
- Kevin, T.T.M.; Nur Idanis, A.S.; Anastasha, B.; Mohd Faris, M.R.; Faizah, O.; Taty Anna, K. Curcumin minimises histopathological and immunological progression in the ankle joints of collagen-induced arthritis rats. Med. Health 2020, 15, 26–36. [Google Scholar]
- Kamal, D.A.M.; Salamt, N.; Yusuf, A.N.M.; Kashim, M.I.A.M.; Mokhtar, M.H. Potential health benefits of curcumin on female reproductive disorders: A review. Nutrients 2021, 13, 3126. [Google Scholar] [CrossRef] [PubMed]
- Irfandi, R.; Ilham, M.; Erwing, R.; Arafah, M.; Rompegading, A.B.; Putri, S.E.; Sartika, S.D.; Fauziah, S.; Agustina, A.S.; Akbar, H.; et al. Review on curcumin compounds in turmeric plants for the treatment of COVID-19. Int. J. Des. Nat. Ecodyn. 2022, 17, 957–965. [Google Scholar] [CrossRef]
- Waknine-Grinberg, J.H.; McQuillan, J.A.; Hunt, N.; Ginsbur, J. Modulation of cerebral malaria by fasudil and other immune-modifying compounds. Exp. Parasitol. 2010, 125, 141–146. [Google Scholar] [CrossRef]
- Khairani, S.; Fauziah, N.; Wiraswati, H.L.; Panigoro, R.; Salleh, A.; Setyowati, E.Y.; Berbudi, A. Piperine enhances the antimalarial activity of curcumin in Plasmodium berghei ANKA-infected mice: A novel approach for malaria prophylaxis. Evid.-Based Complement. Altern. Med. 2022, 2022, 7897163. [Google Scholar] [CrossRef]
- Alkandahri, M.Y.; Berbudi, A.; Subarnas, A. Evaluation of experimental cerebral malaria of curcumin and kaempferol in Plasmodium berghei ANKA-infected mice. Pharmacogn. J. 2022, 14, 905–911. [Google Scholar] [CrossRef]
- Balasaheb, R.; Wanare, G.; Kawathekar, N.; Ranjan, R.; Kumar, N.; Sahal, D.; Singh, V. Dibenzylideneacetone analogues as novel Plasmodium falciparum inhibitors. Bioorganic Med. Chem. Lett. 2011, 21, 3034–3036. [Google Scholar]
- Munigunti, R.; Gathiaka, S.; Acevedo, O.; Sahu, R.; Tekwani, B.; Calderón, A.I. Determination of antiplasmodial activity and binding affinity of curcumin and demethoxycurcumin towards PfTrxR. Nat. Prod. Res. 2014, 28, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.B.; Gupta, M.K.; Singh, D.V.; Singh, S.K.; Misra, K. Docking and in silico ADMET studies of noraristeromycin, curcumin and its derivatives with Plasmodium falciparum SAH hydrolase: A molecular drug target against malaria. Interdiscip. Sci. Comput. Life Sci. 2013, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mimche, P.N.; Thompson, E.; Taramelli, D.; Vivas, L. Curcumin enhances non-opsonic phagocytosis of Plasmodium falciparum through up-regulation of cd36 surface expression on monocytes/macrophages. J. Antimicrob. Chemother. 2012, 67, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Montesino, N.L.; Kaiser, M.; Brun, R.; Schmidt, T.J. Search for antiprotozoal activity in herbal medicinal preparations; new natural leads against neglected tropical diseases. Molecules 2015, 20, 14118–14138. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Rehman, A.; Zafeer, M.F.; Rehman, L.; Khan, Y.A.; Khan, M.A.H.; Khan, S.N.; Khan, A.U.; Abidi, S.M.A. Anthelmintic potential of thymoquinone and curcumin on Fasciola gigantica. PLoS ONE 2017, 12, e0171267. [Google Scholar] [CrossRef]
- Busari, Z.A.; Dauda, K.A.; Morenikeji, O.A.; Afolayan, F.; Oyeyemi, O.T.; Meena, J.; Sahu, D.; Panda, A.K. Antiplasmodial activity and toxicological assessment of curcumin PLGA-encapsulated nanoparticles. Front. Pharmacol. 2017, 8, 622. [Google Scholar] [CrossRef]
- Memvanga, P.B.; Coco, R.; Préat, V. An oral malaria therapy: Curcumin-loaded lipid-based drug delivery systems combined with β-arteether. J. Control. Release 2013, 172, 904–913. [Google Scholar] [CrossRef]
- Olanlokun, J.O.; Abiodun, W.O.; Ebenezer, O.; Koorbanally, N.A.; Olorunsogo, O.O. Curcumin modulates multiple cell death, matrix metalloproteinase activation and cardiac protein release in susceptible and resistant Plasmodium berghei-infected mice. Biomed. Pharmacother. 2022, 146, 112454. [Google Scholar] [CrossRef]





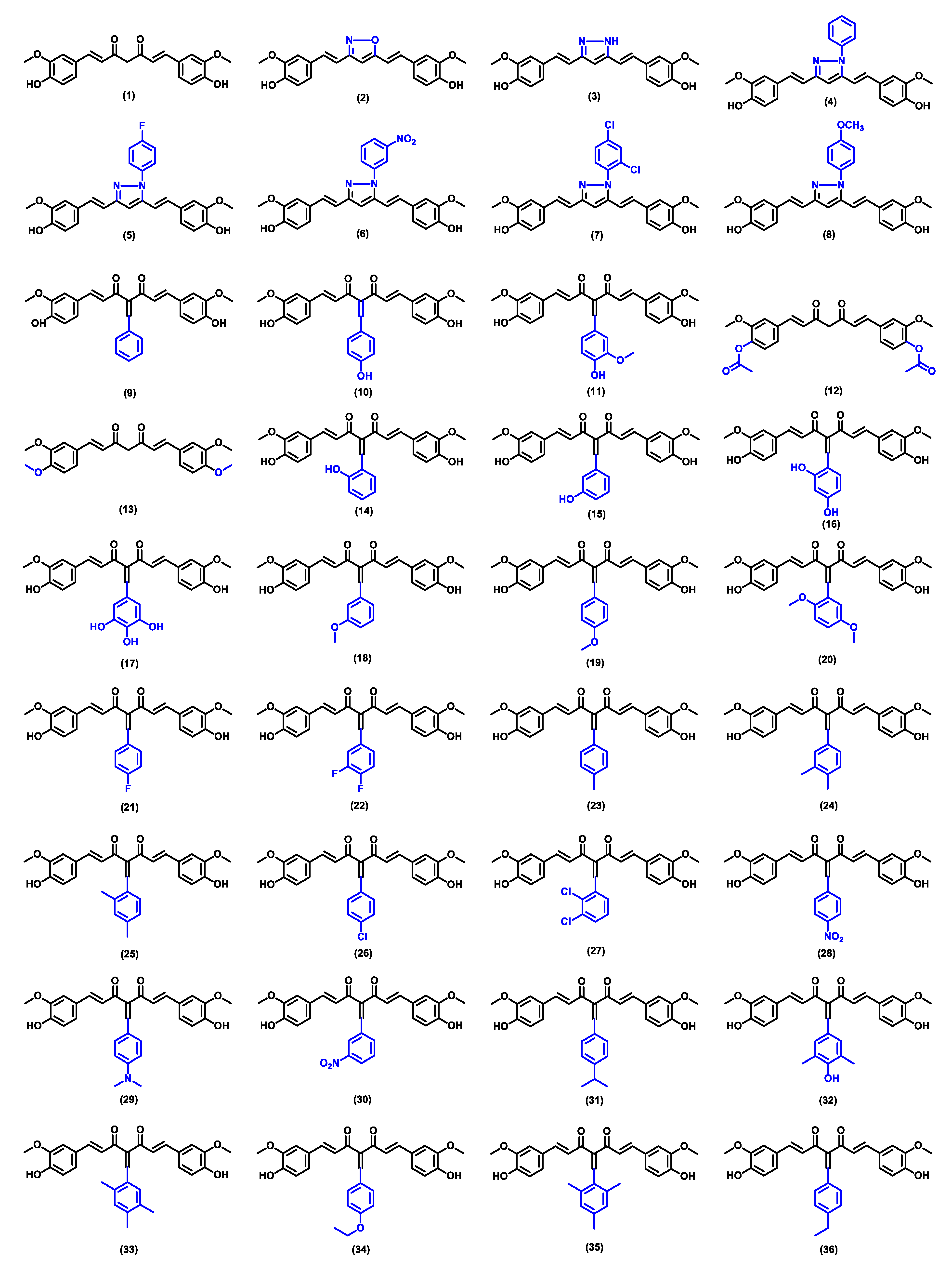
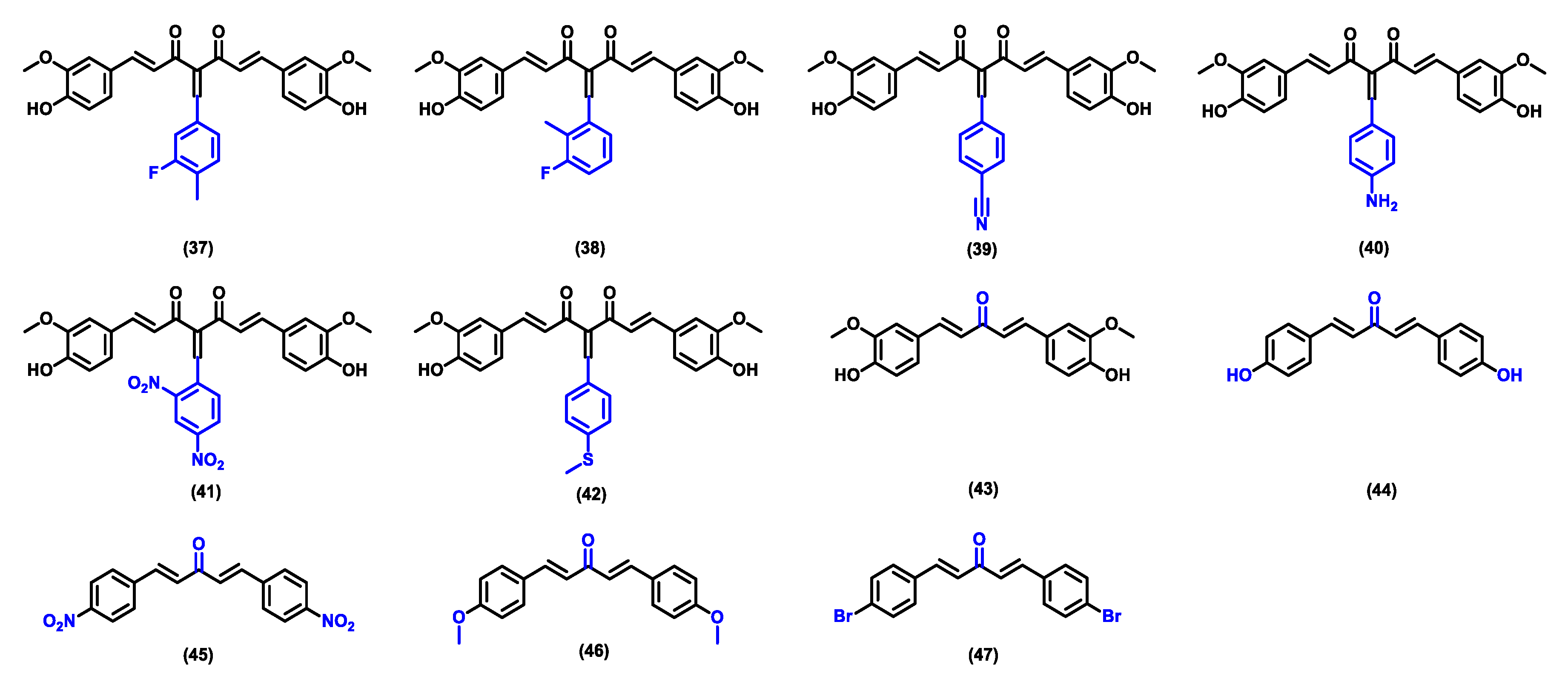
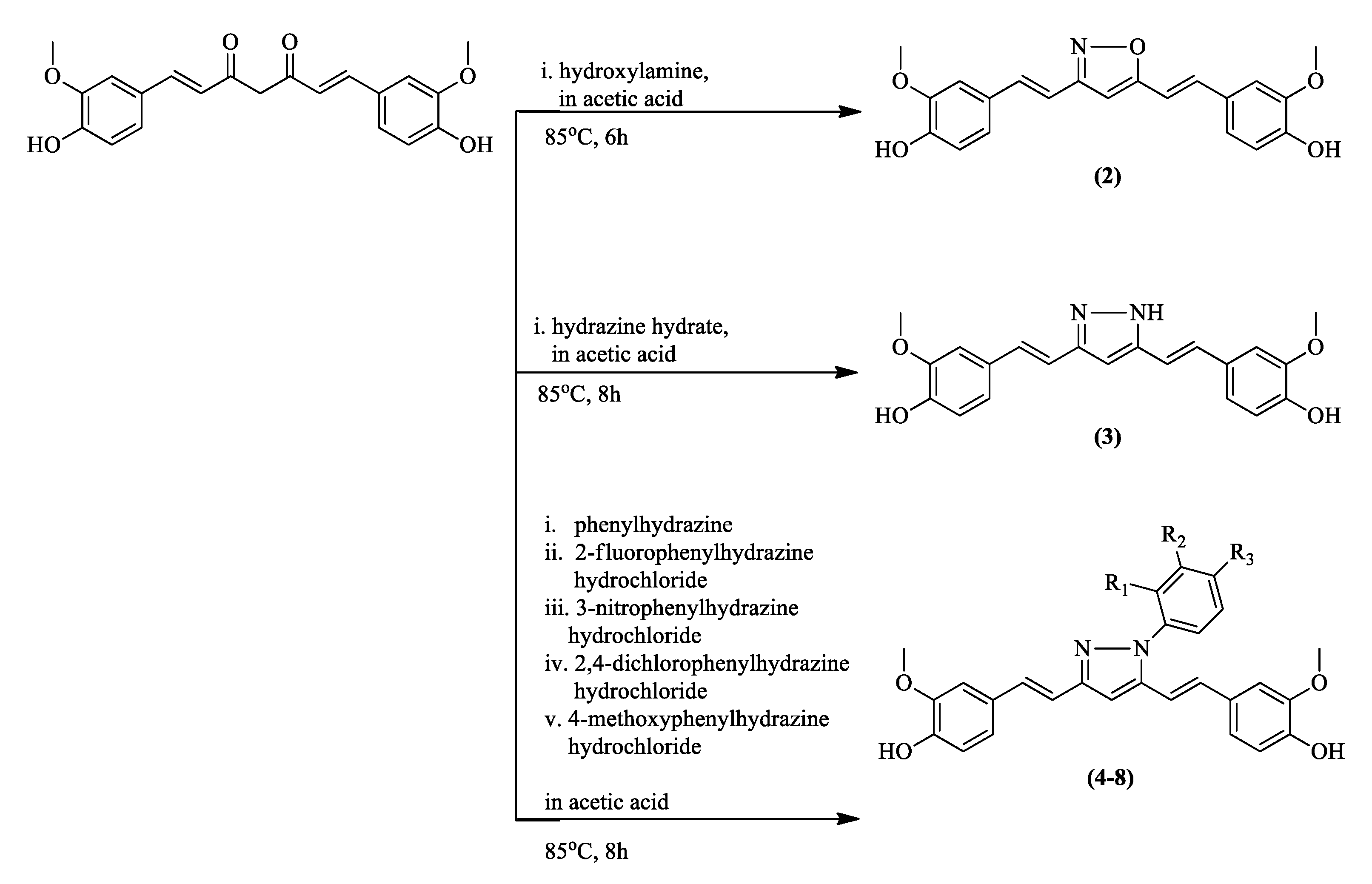



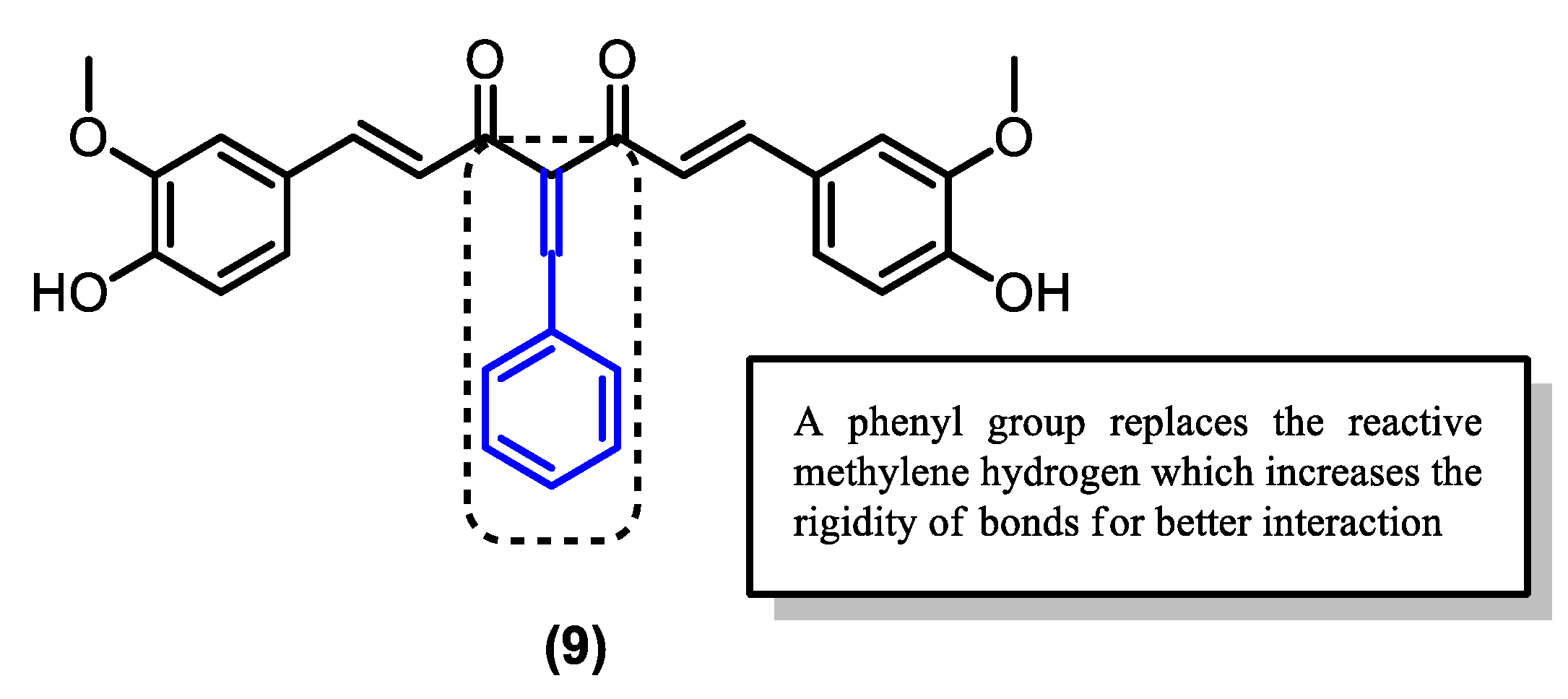

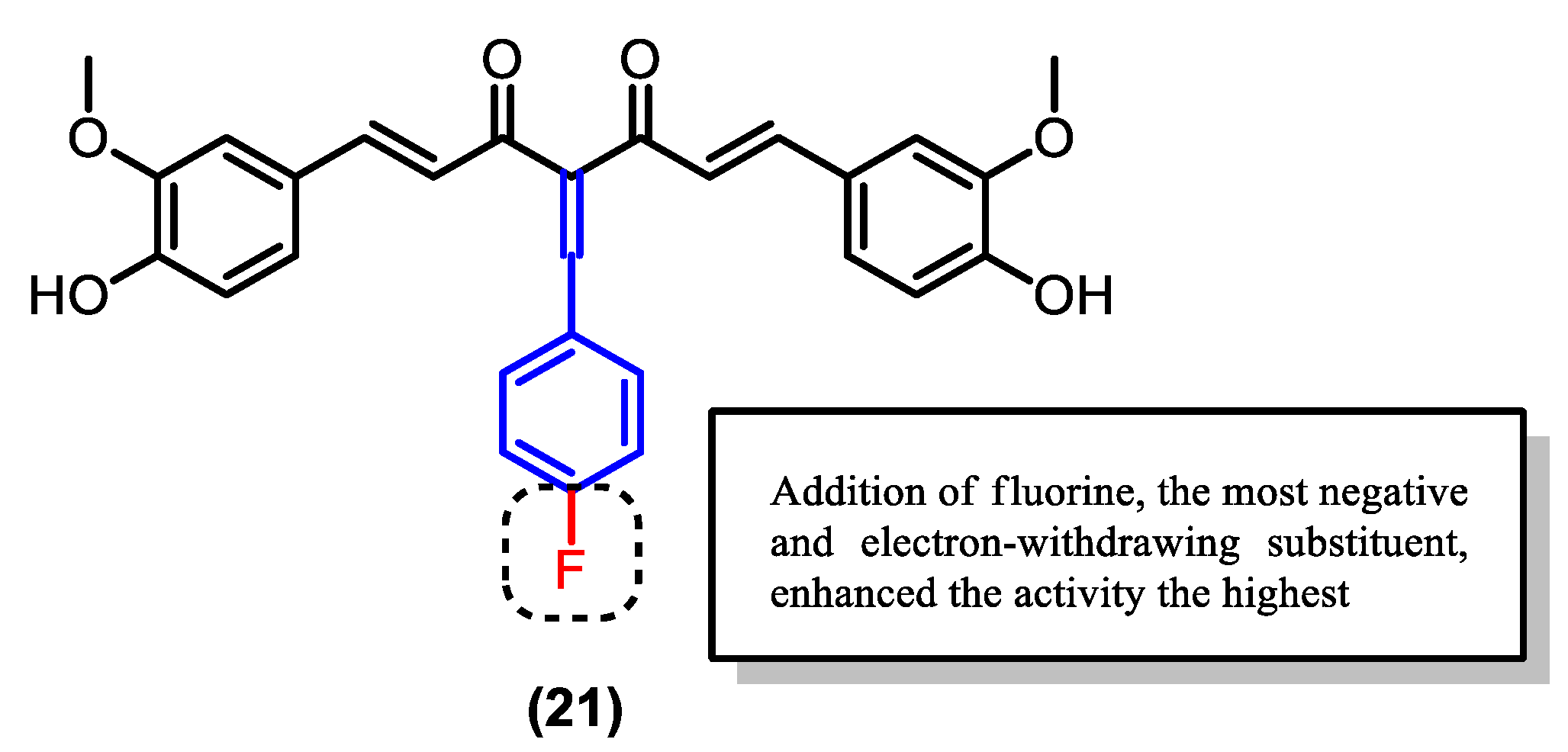



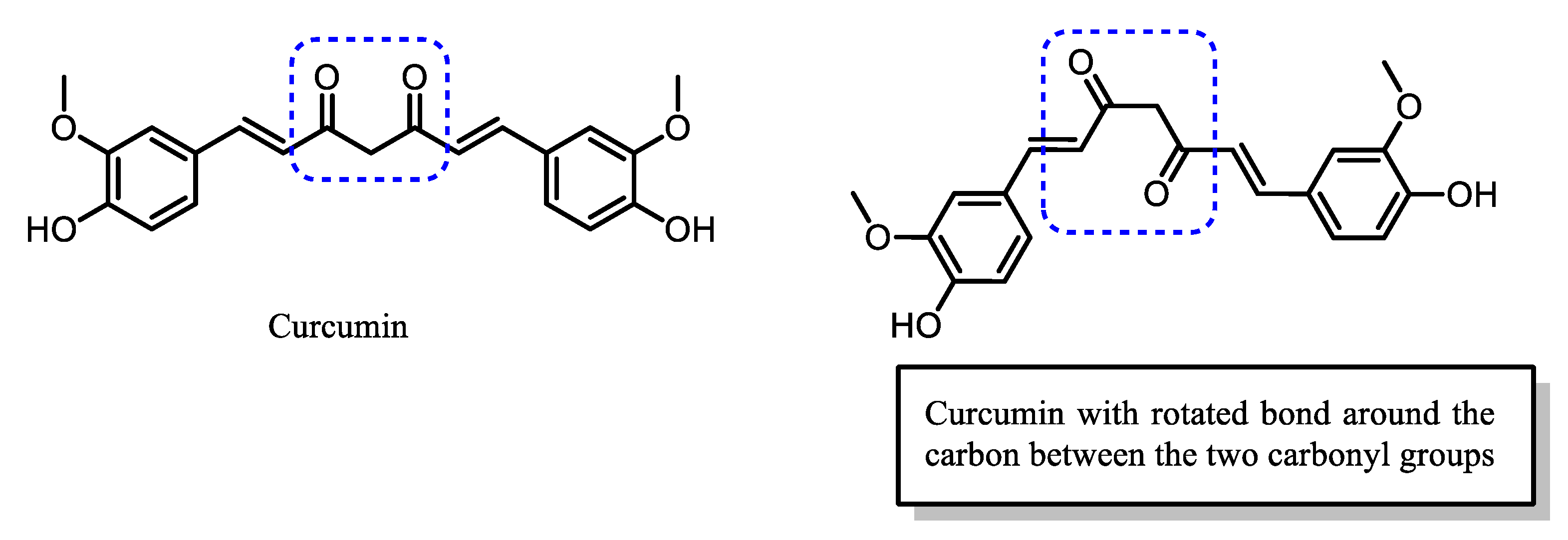
| Compound | Physicochemical Properties | Lipophilicity | Water Solubility | Drug-Likeness | Pharmacokinetics | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Weight (g/mol) | Heavy Atoms | Rotatable Bonds | H-Bond Acceptors | H-Bond Donors | Log P | Log S | Lipinski’s Rule Violation | GI Absorption | BBB Permeability | |
| 1 | 368.38 | 27 | 8 | 6 | 2 | 3.27 | 3.94 | No | High | No |
| 2 | 365.38 | 27 | 6 | 6 | 2 | 3.60 | 4.33 | No | High | No |
| 3 | 366.41 | 27 | 6 | 5 | 3 | 3.47 | 4.33 | No | High | No |
| 4 | 460.65 | 33 | 9 | 5 | 3 | 4.45 | 4.95 | No | High | Yes |
| 5 | 478.64 | 34 | 9 | 6 | 3 | 4.53 | 5.12 | No | High | Yes |
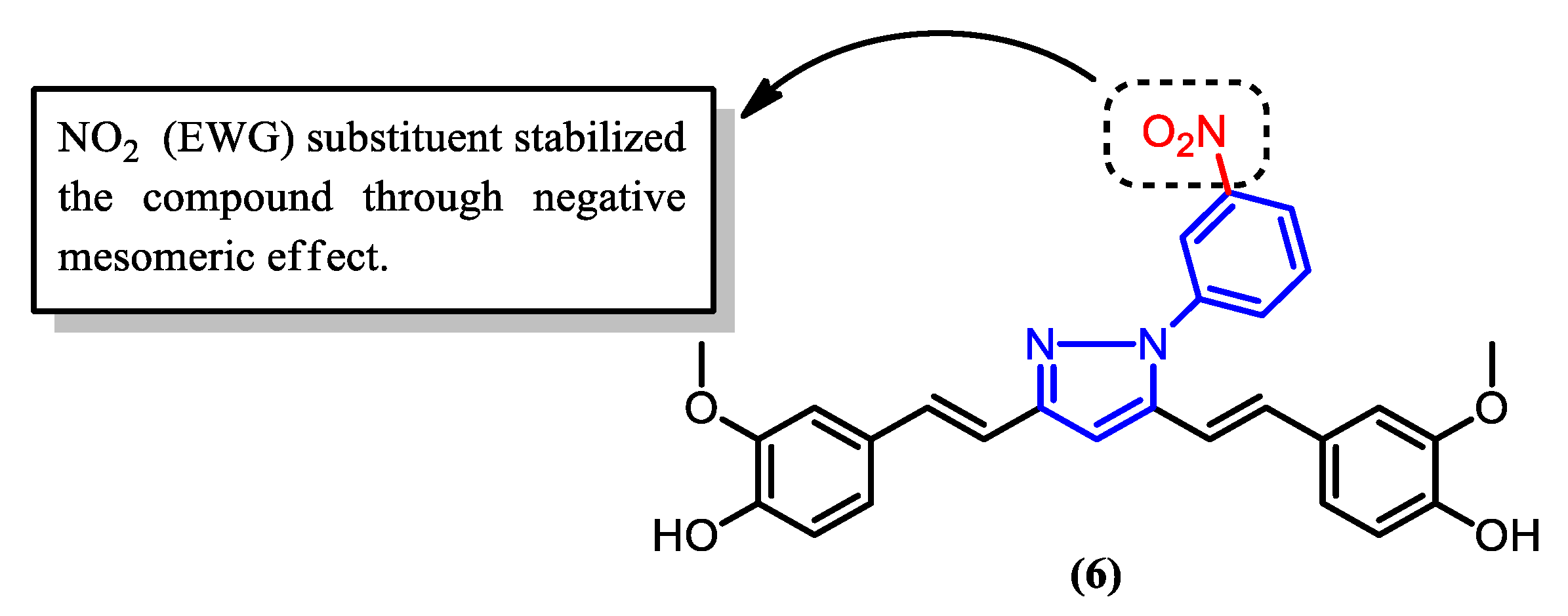
| 6 | 505.65 | 36 | 10 | 7 | 3 | 3.79 | 5.05 | 1 | High | No |
| 7 | 529.54 | 35 | 9 | 5 | 3 | 4.82 | 6.16 | 1 | High | No |
| 8 | 490.68 | 35 | 10 | 6 | 3 | 4.72 | 5.05 | No | High | No |
| 9 | 456.49 | 34 | 9 | 6 | 2 | 3.61 | 5.83 | No | High | No |
| 10 | 472.49 | 35 | 9 | 7 | 3 | 3.55 | 5.69 | No | High | No |
| 11 | 502.51 | 37 | 10 | 8 | 3 | 4.34 | 5.77 | 1 | Low | No |
| 12 | 452.45 | 33 | 12 | 8 | 0 | 3.83 | 4.28 | No | High | No |
| 13 | 396.43 | 29 | 10 | 6 | 0 | 3.59 | 4.37 | No | High | Yes |
| 14 | 472.49 | 35 | 9 | 7 | 3 | 3.56 | 5.69 | No | High | No |
| 15 | 472.49 | 35 | 9 | 7 | 3 | 3.37 | 5.69 | No | High | No |
| 16 | 488.49 | 36 | 9 | 8 | 4 | 3.44 | 5.56 | No | Low | No |
| 17 | 504.48 | 37 | 9 | 9 | 5 | 3.63 | 5.42 | 1 | Low | No |
| 18 | 486.51 | 36 | 10 | 7 | 2 | 4.11 | 5.91 | No | High | No |
| 19 | 486.51 | 36 | 10 | 7 | 2 | 3.95 | 5.91 | No | High | No |
| 20 | 516.54 | 38 | 11 | 8 | 2 | 3.86 | 5.99 | 1 | High | No |
| 21 | 474.48 | 35 | 9 | 7 | 2 | 3.76 | 5.99 | No | High | No |
| 22 | 492.47 | 36 | 9 | 8 | 2 | 3.65 | 6.15 | No | High | No |
| 23 | 470.51 | 35 | 9 | 6 | 2 | 3.62 | 6.13 | No | High | No |
| 24 | 484.54 | 36 | 9 | 6 | 2 | 3.90 | 6.44 | No | High | No |
| 25 | 484.54 | 36 | 9 | 6 | 2 | 3.97 | 6.44 | No | High | No |
| 26 | 490.93 | 35 | 9 | 6 | 2 | 3.82 | 6.42 | No | High | No |
| 27 | 525.38 | 36 | 9 | 6 | 2 | 3.48 | 7.02 | 1 | High | No |
| 28 | 501.48 | 37 | 10 | 8 | 2 | 2.99 | 5.89 | 1 | Low | No |
| 29 | 499.55 | 37 | 10 | 6 | 2 | 4.22 | 6.07 | No | High | No |
| 30 | 501.48 | 37 | 10 | 8 | 2 | 2.76 | 5.89 | 1 | Low | No |
| 31 | 498.57 | 37 | 10 | 6 | 2 | 3.89 | 6.69 | No | High | No |
| 32 | 500.54 | 37 | 9 | 7 | 3 | 3.67 | 6.30 | 1 | Low | No |
| 33 | 498.57 | 37 | 9 | 6 | 2 | 3.61 | 6.74 | No | High | No |
| 34 | 500.54 | 37 | 11 | 7 | 2 | 4.50 | 6.14 | 1 | High | No |
| 35 | 498.57 | 37 | 9 | 6 | 2 | 3.60 | 6.74 | No | High | No |
| 36 | 484.54 | 36 | 10 | 6 | 2 | 4.19 | 6.41 | No | High | No |
| 37 | 488.50 | 36 | 9 | 7 | 2 | 3.44 | 6.29 | No | High | No |
| 38 | 488.50 | 36 | 9 | 7 | 2 | 3.86 | 6.29 | No | High | No |
| 39 | 481.50 | 36 | 9 | 7 | 2 | 3.36 | 5.78 | No | High | No |
| 40 | 471.50 | 35 | 9 | 6 | 3 | 3.38 | 5.47 | No | High | No |
| 41 | 546.48 | 40 | 11 | 10 | 2 | 2.14 | 5.97 | 2 | Low | No |
| 42 | 502.58 | 36 | 10 | 6 | 2 | 4.66 | 6.35 | 1 | Low | No |
| 43 | 326.34 | 24 | 6 | 5 | 2 | 3.10 | 3.95 | No | High | Yes |
| 44 | 266.29 | 20 | 4 | 3 | 2 | 2.17 | 3.93 | No | High | Yes |
| 45 | 324.29 | 24 | 6 | 5 | 0 | 2.18 | 4.22 | No | High | No |
| 46 | 294.34 | 22 | 6 | 3 | 0 | 3.46 | 4.39 | No | High | Yes |
| 47 | 392.08 | 20 | 4 | 1 | 0 | 3.61 | 6.11 | 1 | High | Yes |
| Compound | P. falciparum In Vitro Analysis | |
|---|---|---|
| Chloroquine-Sensitive FCK2 | Chloroquine-Resistant MP-14 | |
| IC50 (μM) | IC50 (μM) | |
| 1 | 3.25 | 4.21 |
| 2 | 8.44 | 7.92 |
| 3 | 0.48 | 0.45 |
| 4 | 8.48 | 9.10 |
| 5 | 2.42 | 2.10 |
| 6 | 0.87 | 0.89 |
| 7 | 4.65 | 4.80 |
| 8 | 22.60 | 24.56 |
| 9 | 3.89 | 4.12 |
| 10 | 5.85 | 5.36 |
| 11 | 0.92 | 0.75 |
| 12 | 2.34 | 2.51 |
| 13 | 7.86 | 8.40 |
| Compound | MW (g/mol) | logP | H-Bond Donor | H-Bond Acceptor | Solubility (mg/L) | IC50 (M) | Free Binding Energy (kcal/mol) | % Schizont Inhibition | |
|---|---|---|---|---|---|---|---|---|---|
| 5 μg/mL | 50 μg/mL | ||||||||
| (21) | 474.48 | 5.43 | 2 | 6 | 1382.51 | - | −6.75 | 97.8 | 100 |
| (15) | 472.49 | 4.97 | 3 | 7 | 1869.61 | - | −5.89 | 89.5 | 100 |
| (9) | 456.49 | 5.33 | 2 | 6 | 1583.74 | 3.89 | −5.35 | 80.1 | 100 |
| Curcumin | 368.38 | 3.29 | 2 | 6 | 7.475 | 3.25 | −5.25 | 79.6 | 100 |
| (10) | 472.49 | 4.97 | 3 | 7 | 1869.61 | 5.85 | −3.87 | - | - |
| Activity | In Vitro/In Vivo/In Silico Evidence | References |
|---|---|---|
| Antiplasmodium PfATP6 | Curcumin (1) reduced P. falciparum viability, causing parasitic cell proliferation to decrease.
| [69] |
| Curcumin (1) and its derivatives (9, 14, 15, 19, 21, 23, 27, and 28) showed 100% inhibition of P. falciparum growth upon a 50 g/mL dose of treatment. | [37] | |
Molecular docking results validated binding of curcumin (1) and its derivatives to PfATP with favorable free binding energy.
(higher than both artemisinin (–6.73 kcal/mol) and curcumin (–5.25 kcal/mol), hence, better interaction with the protein).
| [37] | |
| In vitro study using CQR P. falciparum showed potent antimalarial activity of curcumin (1), with reported IC50 value of ~5 M. In vivo treatment of P. berghei-infected mice with 100 mg/kg curcumin showed:
| [23,25] | |
| Curcumin treatment on P. berghei-infected C57BI/6 mice delayed mice death by 10 days and prevented cerebral malaria. Dose: 50 mg/kg, twice daily for 6 days. | [112] | |
| Curcumin exhibited antimalarial activity in P. berghei-infected mice. Dose: 300 mg/kg daily for 4 days (60.22% parasitemia inhibition). Dose: 80 mg/kg daily for 4 days (60.21% chemosuppressive effect). | [113,114] | |
| Antiplasmodium Pf3D7 | Curcumin (1) showed potential inhibition of parasite transmission at the trophozoite stage. Curcumin derivative (monocarbonyl curcumin)
| [115] |
| Antiplasmodium PfDXR | In silico and in vitro studies validated synergistic binding of curcumin (1) to PfDXR protein with fosmidomycin.
(46)—57%. | [74] |
| Antiplasmodium PGCN5 HAT | In vitro study suggested curcumin (1) as a potent inhibitor of p300/CBP (CREB-binding protein) as tested on four P. falciparum strains.
| [92] |
| Antiplasmodium PfTrxR | In vitro study using CQS (D6 clone) and CQR (W2 clone) P. falciparum strains showed that curcumin (1) inhibited PfTrxR protein with an IC50 value of 2 M. | [116] |
| Antiplasmodium PfHGPRT PfSAHH | In silico simulation using Molegro Virtual Docker (MVD) and admetSAR showed high binding energy of curcumin (1) to the protein.
| [107,117] |
| Antimalaria ROS | In vitro study showed that curcumin (1) induced intracellular ROS production related to PPARɣ/Nrf2 activation.
| [95,118] |
| Antimalaria | In vitro study using NF54 intraerythrocytic-form P. falciparum strain reported highly potent antiparasitic activity of curcumin (1).
| [119] |
In vitro study using 3D7 clone strain of P. falciparum reported synergistic antimalarial effect of curcumin (1) with dihydroartemisinin and reduction in hemozoin formation upon several consecutive treatments of curcumin.
| [100] | |
| In vitro study showed the effectiveness of curcumin–artemisinin combination therapy with additive interaction in killing P. falciparum. In vivo study using P. berghei-infected mice showed 100% survival upon treatment. Dose: 750 µg. | [119] | |
| In vivo study on P. berghei ANKA-infected mice revealed treatment of curcumin (1) reduced parasitemia level and increased survival rate. Dose: 50 mg/kg daily. | [120] | |
In vitro study shows reported IC50:
Dose: 5 and 10 mg/kg. | [121] | |
| Anti-inflammatory COX-2 | In vitro study using DPPH radical-scavenging assay showed anti-inflammatory activity of curcumin and its derivatives. Reported IC50 value and % inhibition:
| [65] |
| Molecular docking using FlexX program validated COX-2 as a target protein and showed binding of curcumin (1) and curcumin derivatives (2, 3). Favorable interactions:
| [65] | |
| Anti-inflammatory NF-B | In vivo study on P. berghei ANKA-infected mice upon treatment of curcumin (1) showed inhibition of NF-B activation, which reduced expression of adhesion molecules and suppressed pro-inflammatory cytokines level. Dose: 100 mg/kg daily for 4 days. | [122] |
| Anti-inflammatory GSK3β | In vivo study on P. berghei NK65-infected rats upon treatment of curcumin (1) showed inhibition of host GSK3, leading to the phosphorylation of NF-B and regulation of pro- (decrease in serum TNF-α and IFN-γ levels) and anti-inflammatory (IL-10 and IL-4) cytokines. Dose: 3, 10, and 30 mg/kg. | [88] |
| Anti-inflammatory | In vivo study on P. berghei NK65- and ANKA-infected mice upon treatment of curcumin (1). Reported activity:
| [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamil, S.N.H.; Ali, A.H.; Feroz, S.R.; Lam, S.D.; Agustar, H.K.; Mohd Abd Razak, M.R.; Latip, J. Curcumin and Its Derivatives as Potential Antimalarial and Anti-Inflammatory Agents: A Review on Structure–Activity Relationship and Mechanism of Action. Pharmaceuticals 2023, 16, 609. https://doi.org/10.3390/ph16040609
Jamil SNH, Ali AH, Feroz SR, Lam SD, Agustar HK, Mohd Abd Razak MR, Latip J. Curcumin and Its Derivatives as Potential Antimalarial and Anti-Inflammatory Agents: A Review on Structure–Activity Relationship and Mechanism of Action. Pharmaceuticals. 2023; 16(4):609. https://doi.org/10.3390/ph16040609
Chicago/Turabian StyleJamil, Siti Nur Hidayah, Amatul Hamizah Ali, Shevin Rizal Feroz, Su Datt Lam, Hani Kartini Agustar, Mohd Ridzuan Mohd Abd Razak, and Jalifah Latip. 2023. "Curcumin and Its Derivatives as Potential Antimalarial and Anti-Inflammatory Agents: A Review on Structure–Activity Relationship and Mechanism of Action" Pharmaceuticals 16, no. 4: 609. https://doi.org/10.3390/ph16040609
APA StyleJamil, S. N. H., Ali, A. H., Feroz, S. R., Lam, S. D., Agustar, H. K., Mohd Abd Razak, M. R., & Latip, J. (2023). Curcumin and Its Derivatives as Potential Antimalarial and Anti-Inflammatory Agents: A Review on Structure–Activity Relationship and Mechanism of Action. Pharmaceuticals, 16(4), 609. https://doi.org/10.3390/ph16040609