Receptors Involved in Mental Disorders and the Use of Clozapine, Chlorpromazine, Olanzapine, and Aripiprazole to Treat Mental Disorders
Abstract
1. Introduction
2. Central Nervous System Receptors and Mental Disorders
2.1. Histamine Receptors’ Involvement in Mental Disorders
2.2. Trace Amines’ Receptors and Neurotransmitters Associated with Mental Illness
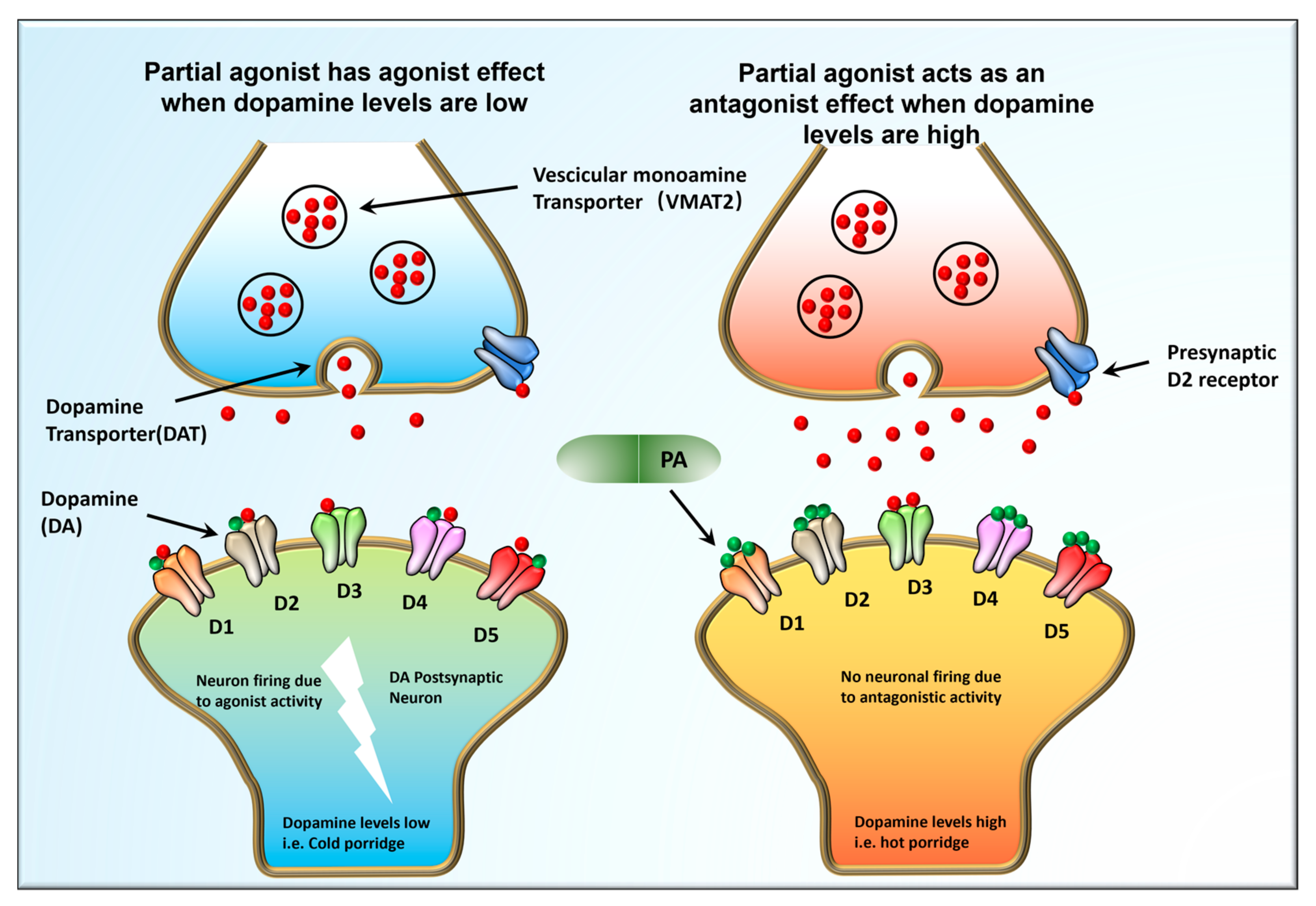
2.3. Mechanisms That Involve DA Receptors in Mental Diseases
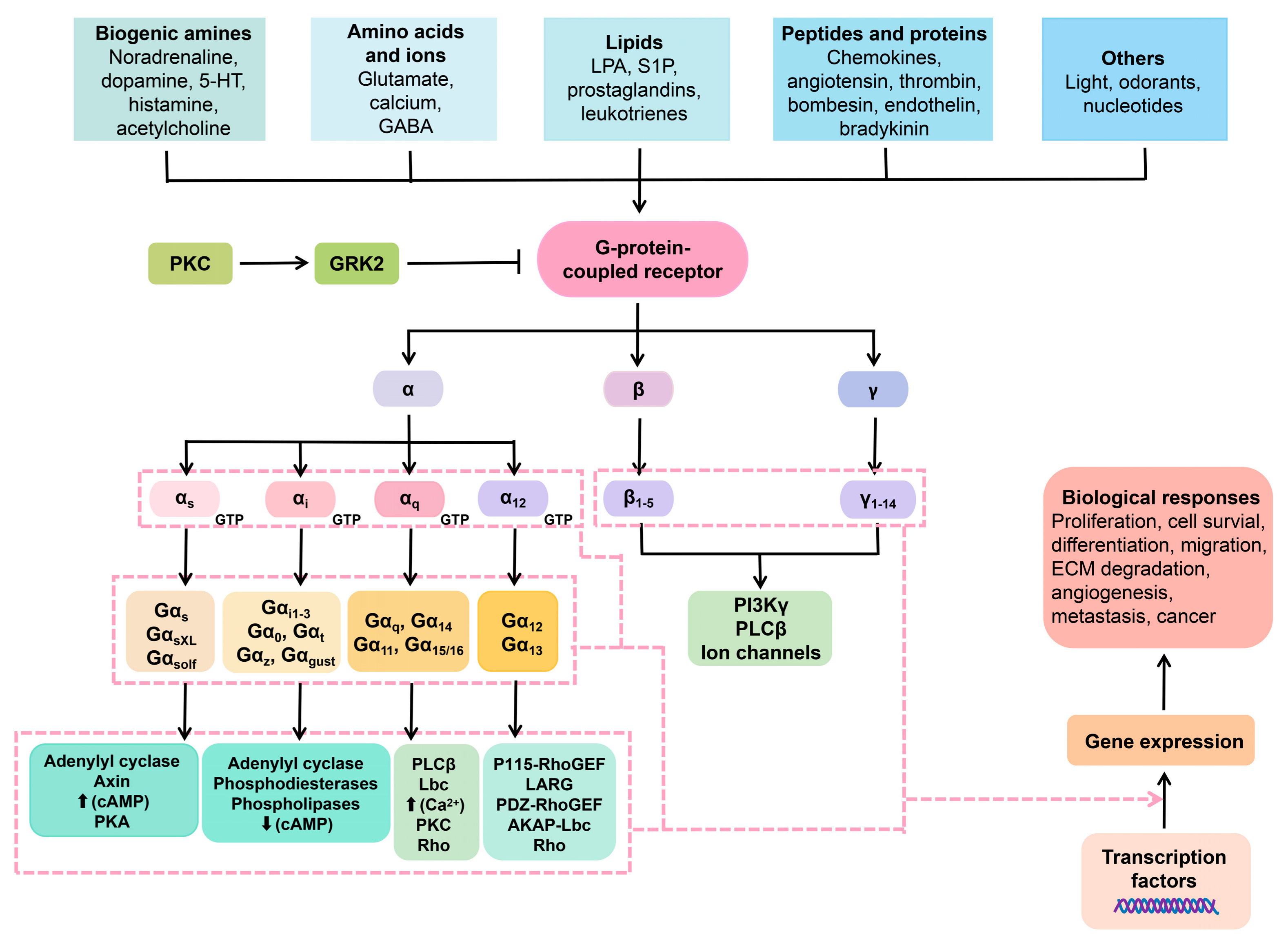
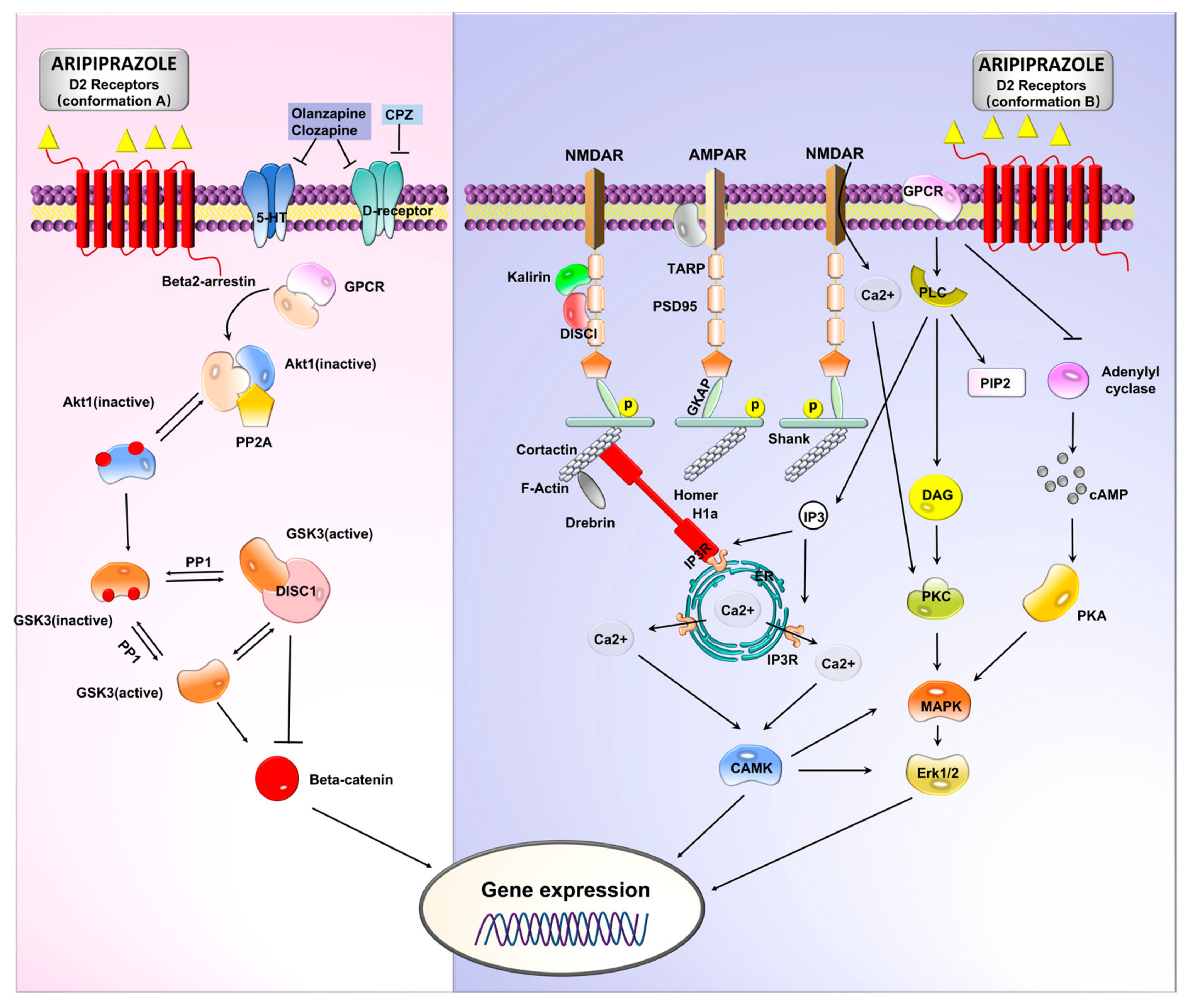
2.4. GPKs (G-Protein Coupled Receptor Kinases) and GPCRs Involvement in Psychoses Etiology
2.5. β-Arrestins and Psychoses
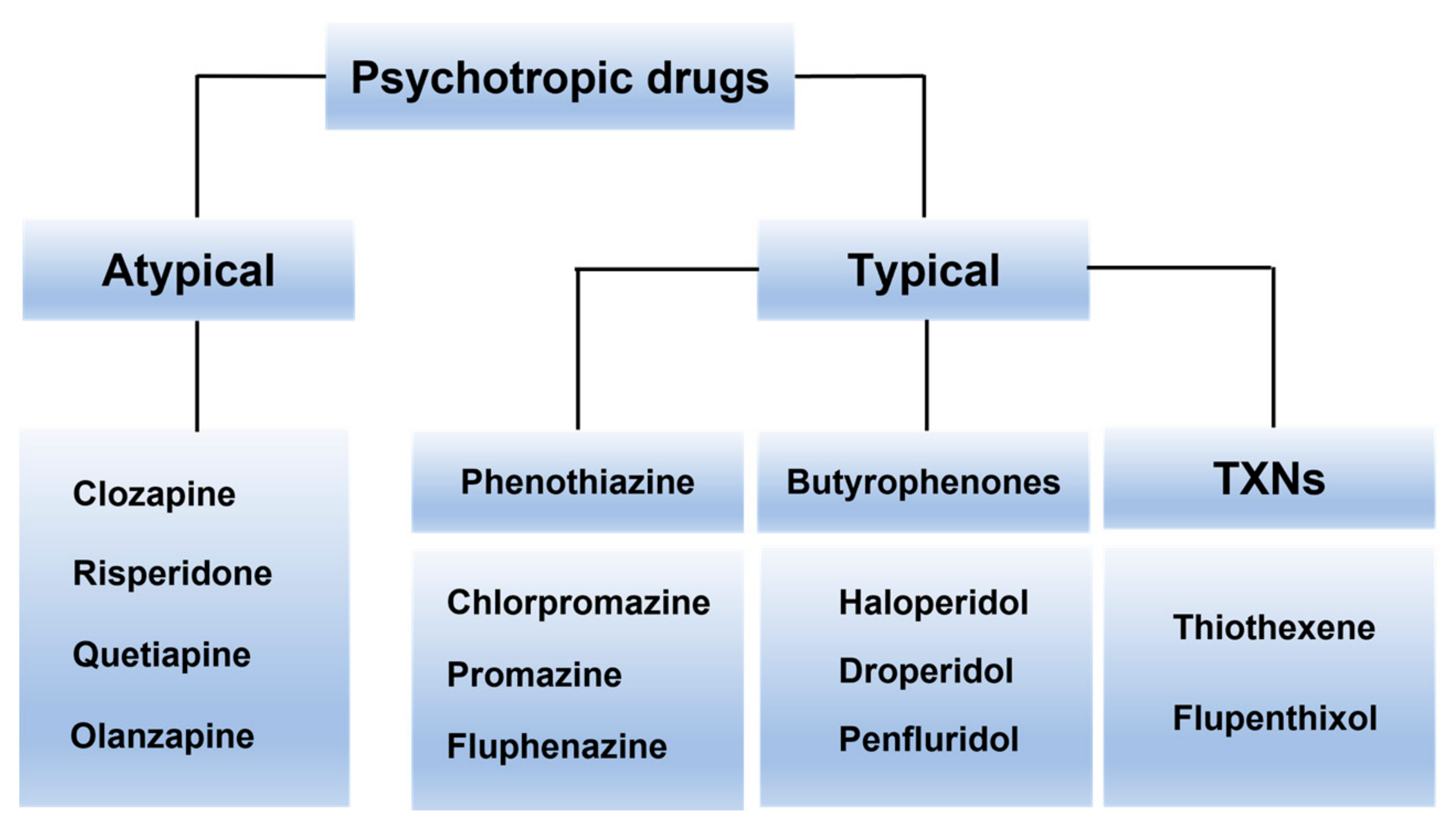
3. Psychotropic Drugs Usage
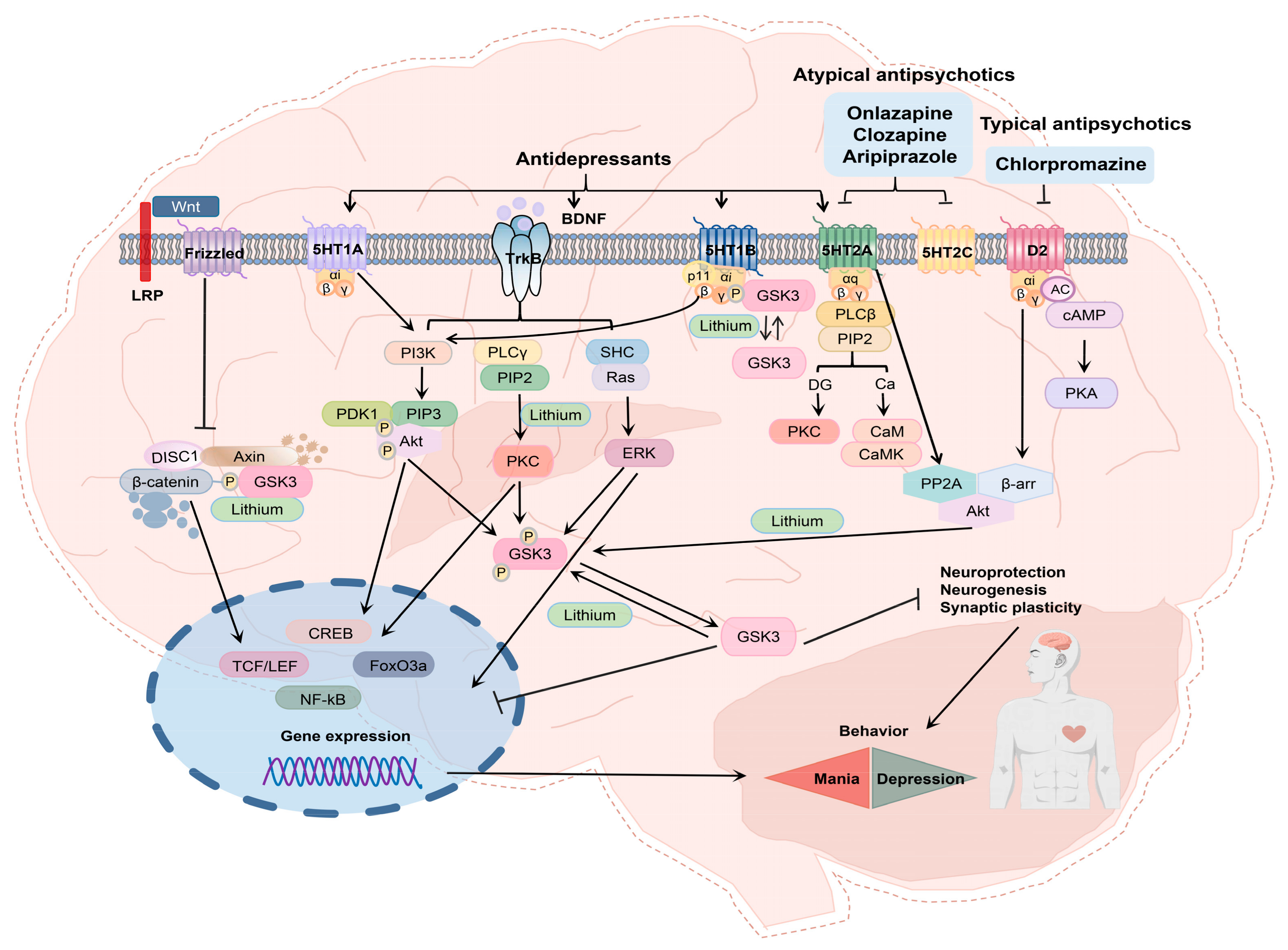
3.1. APYs as Adjuncts to Antidepressants Treat Schizophrenia and Many Other Mental Problems via Serotonin Receptors
3.2. CPZ
3.3. The Interaction of CPZ with Anti-COVIDS and Other Drugs
3.4. CZP
3.5. ARP
3.6. OZP
4. Knowledge Gap
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Effendi, W.I.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Focusing on Adenosine Receptors as a Potential Targeted Therapy in Human Diseases. Cells 2020, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Vahid-Ansari, F.; Albert, P.R. Rewiring of the Serotonin System in Major Depression. Front. Psychiatry 2021, 12, 802581. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Huang, C.; Lu, Q.; Lu, X. Therapeutic and Prophylactic Effects of Amphotericin B Liposomes on Chronic Social Defeat Stress-Induced Behavioral Abnormalities in Mice. Front. Pharmacol. 2022, 13, 918177. [Google Scholar] [CrossRef]
- Buckley, P.F. Neuroinflammation and Schizophrenia. Curr. Psychiatry Rep. 2019, 21, 72. [Google Scholar] [CrossRef]
- Howland, J.G.; Greenshaw, A.J.; Winship, I.R. Practical Aspects of Animal Models of Psychiatric Disorders. Can. J. Psychiatry 2019, 64, 3–4. [Google Scholar] [CrossRef]
- Nucifora, F.C., Jr.; Woznica, E.; Lee, B.J.; Cascella, N.; Sawa, A. Treatment resistant schizophrenia: Clinical, biological, and therapeutic perspectives. Neurobiol. Dis. 2018, 131, 104257. [Google Scholar] [CrossRef] [PubMed]
- Winship, I.R.; Dursun, S.M.; Baker, G.B.; Balista, P.A.; Kandratavicius, L.; Maia-De-Oliveira, J.P.; Hallak, J.; Howland, J.G. An Overview of Animal Models Related to Schizophrenia. Can. J. Psychiatry 2019, 64, 5–17. [Google Scholar] [CrossRef]
- Bartram, L.A.; Lozano, J.; Coury, D.L. Aripiprazole for treating irritability associated with autism spectrum disorders. Expert Opin. Pharmacother. 2019, 20, 1421–1427. [Google Scholar] [CrossRef]
- Chen, Y.; Sabatini, B.L. The Kinase Specificity of Protein Kinase Inhibitor Peptide. Front. Pharmacol. 2021, 12, 632815. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, L.; Wang, H.; Yu, J.; Lu, D.; Qi, J.; Nie, F.; Luo, Z.; Liu, Z.; Cheng, J.; et al. Structure-based design of a novel third-generation antipsychotic drug lead with potential antidepressant properties. Nat. Neurosci. 2022, 25, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Newcomer, J.W.; Silverman, B.; DiPetrillo, L.; Graham, C.; Jiang, Y.; Du, Y.; Simmons, A.; Hopkinson, C.; McDonnell, D.; et al. Effects of Olanzapine Combined with Samidorphan on Weight Gain in Schizophrenia: A 24-Week Phase 3 Study. Am. J. Psychiatry 2020, 177, 1168–1178. [Google Scholar] [CrossRef]
- Delgado, A.; Velosa, J.; Zhang, J.; Dursun, S.M.; Kapczinski, F.; Cardoso, T.D.A. Clozapine in bipolar disorder: A systematic review and meta-analysis. J. Psychiatr. Res. 2020, 125, 21–27. [Google Scholar] [CrossRef]
- Foletto, V.S.; da Rosa, T.F.; Serafin, M.B.; Hörner, R. Selective serotonin reuptake inhibitor (SSRI) antidepressants reduce COVID-19 infection: Prospects for use. Eur. J. Clin. Pharmacol. 2022, 78, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.H.; Schroder, S.; Toussaint, M.; Dukic-Stefanovic, S.; Kranz, M.; Ludwig, F.A.; Fischer, S.; Steinbach, J.; Deu-ther-Conrad, W.; Brust, P.; et al. Development of (18)F-Labeled Radiotracers for PET Imaging of the Adenosine A(2A) Receptor: Synthesis, Radiolabeling and Preliminary Biological Evaluation. Int. J. Mol. Sci. 2021, 22, 2285. [Google Scholar] [CrossRef]
- Hashimoto, K. Repurposing of CNS drugs to treat COVID-19 infection: Targeting the sigma-1 receptor. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Asada, H.; Inoue, A.; Kadji, F.M.N.; Im, D.; Mori, C.; Arakawa, T.; Hirata, K.; Nomura, Y.; Nomura, N.; et al. Structures of the 5-HT2A receptor in complex with the antipsychotics risperidone and zotepine. Nat. Struct. Mol. Biol. 2019, 26, 121–128. [Google Scholar] [CrossRef]
- Tavares, G.; Marques, D.; Barra, C.; Rosendo-Silva, D.; Costa, A.; Rodrigues, T.; Gasparini, P.; Melo, B.; Sacramento, J.; Seiça, R.; et al. Dopamine D2 receptor agonist, bromocriptine, remodels adipose tissue dopaminergic signalling and upregulates catabolic pathways, improving metabolic profile in type 2 diabetes. Mol. Metab. 2021, 51, 101241. [Google Scholar] [CrossRef]
- Zhuang, Y.; Xu, P.; Mao, C.; Wang, L.; Krumm, B.; Zhou, X.E.; Huang, S.; Liu, H.; Cheng, X.; Huang, X.-P.; et al. Structural insights into the human D1 and D2 dopamine receptor signaling complexes. Cell 2021, 184, 931–942.e18. [Google Scholar] [CrossRef]
- Wang, S.; Che, T.; Levit, A.; Shoichet, B.K.; Wacker, D.; Roth, B.L. Structure of the D2 dopamine receptor bound to the atypical antipsychotic drug risperidone. Nature 2018, 555, 269–273. [Google Scholar] [CrossRef]
- Ishibashi, K.; Miura, Y.; Wagatsuma, K.; Toyohara, J.; Ishiwata, K.; Ishii, K. Occupancy of adenosine A2A receptors by istradefylline in patients with Parkinson’s disease using 11C-preladenant PET. Neuropharmacology 2018, 143, 106–112. [Google Scholar] [CrossRef]
- Kishi, T.; Ikuta, T.; Matsuda, Y.; Sakuma, K.; Iwata, N. Aripiprazole vs. brexpiprazole for acute schizophrenia: A systematic review and network meta-analysis. Psychopharmacology 2020, 237, 1459–1470. [Google Scholar] [CrossRef]
- Kneller, L.A.; Zubiaur, P.; Koller, D.; Abad-Santos, F.; Hempel, G. Influence of CYP2D6 Phenotypes on the Pharmacokinetics of Aripiprazole and Dehydro-Aripiprazole Using a Physiologically Based Pharmacokinetic Approach. Clin. Pharmacokinet. 2021, 60, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- LeWitt, P.A.; Aradi, S.D.; Hauser, R.A.; Rascol, O. The challenge of developing adenosine A(2A) antagonists for Parkinson disease: Istradefylline, preladenant, and tozadenant. Park. Relat. Disord. 2020, 80 (Suppl. S1), S54–S63. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Chen, J.F.; Uchida, S.; Durlach, C.; King, S.M.; Jenner, P. The Pharmacological Potential of Adenosine A(2A) Receptor Antagonists for Treating Parkinson’s Disease. Molecules 2022, 27, 2366. [Google Scholar] [CrossRef] [PubMed]
- Sahlholm, K.; Valle-León, M.; Fernández-Dueñas, V.; Ciruela, F. Dopamine receptor heteromers: Biasing anti-psychotics. Future Med. Chem. 2018, 10, 2675–2677. [Google Scholar] [CrossRef]
- Salvan, P.; Fonseca, M.; Winkler, A.M.; Beauchamp, A.; Lerch, J.P.; Johansen-Berg, H. Serotonin regulation of behavior via large-scale neuromodulation of serotonin receptor networks. Nat. Neurosci. 2023, 26, 53–63. [Google Scholar] [CrossRef]
- Sargin, D.; Chottekalapanda, R.U.; Perit, K.E.; Yao, V.; Chu, D.; Sparks, D.W.; Kalik, S.; Power, S.K.; Troyanskaya, O.G.; Schmidt, E.F.; et al. Mapping the physiological and molecular markers of stress and SSRI antide-pressant treatment in S100a10 corticostriatal neurons. Mol. Psychiatry 2020, 25, 1112–1129. [Google Scholar] [CrossRef]
- Available online: https://psychopharmacologyinstitute.com/publication/mechanism-of-action-of-risperidone-2126 (accessed on 27 March 2023).
- Available online: https://psychopharmacologyinstitute.com/publication/mechanism-of-action-of-quetiapine-2109#:~:text=Quetiapine%20is%20a%20second-generation,1%20and%205-HT1A%20receptors (accessed on 28 March 2023).
- Available online: https://drugs.ncats.io/substance/O9M39HTM5W (accessed on 28 March 2023).
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Fluphenazine#:~:text=Fluphenazine%20exerts%20its%20actions%20by,cortical%20system%20and%20basal%20ganglia (accessed on 28 March 2023).
- Available online: https://go.drugbank.com/drugs/DB00502 (accessed on 28 March 2023).
- Available online: https://go.drugbank.com/drugs/DB00450 (accessed on 28 March 2023).
- Available online: https://www.selleckchem.com/datasheet/penfluridol-S415101-DataSheet.html#s_ref (accessed on 28 March 2023).
- Esbenshade, T.A.; Browman, K.E.; Bitner, R.S.; Strakhova, M.; Cowart, M.D.; Brioni, J.D. The histamine H3receptor: An attractive target for the treatment of cognitive disorders. Br. J. Pharmacol. 2008, 154, 1166–1181. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2483387/ (accessed on 28 March 2023).
- Grinchii, D.; Dremencov, E. Mechanism of Action of Atypical Antipsychotic Drugs in Mood Disorders. Int. J. Mol. Sci. 2020, 21, 9532. [Google Scholar] [CrossRef]
- Naguy, A.; Moodliar-Rensburg, S.; Alamiri, B. The long-acting injectable atypical antipsychotics-merits and demerits! CNS Spectr. 2021, 26, 442–443. [Google Scholar] [CrossRef]
- Orzelska-Górka, J.; Mikulska, J.; Wiszniewska, A.; Biała, G. New Atypical Antipsychotics in the Treatment of Schizophrenia and Depression. Int. J. Mol. Sci. 2022, 23, 10624. [Google Scholar] [CrossRef] [PubMed]
- Dedic, N.; Dworak, H.; Zeni, C.; Rutigliano, G.; Howes, O.D. Therapeutic Potential of TAAR1 Agonists in Schizophrenia: Evidence from Preclinical Models and Clinical Studies. Int. J. Mol. Sci. 2021, 22, 13185. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, W.; Duan, W.; Wüthrich, K.; Cheng, J. Tumor Immunotherapy Using A2A Adenosine Receptor Antagonists. Pharmaceuticals 2020, 13, 237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiang, Q.; Zhao, X.; Ma, L.; Cui, Y. Association between aripiprazole pharmacokinetics and CYP2D6 phenotypes: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2019, 44, 163–173. [Google Scholar] [CrossRef]
- Xin, Y.; Gao, L.; Tuo, Y.; Nie, G.; Mei, Y.; Chen, C.; Wang, J.; Li, S.; Sun, D.; Qian, Q.; et al. Understanding inter-individual variability in pharmacokinetics/pharmacodynamics of aripiprazole in children with tic disorders: Individualized administration based on physiological development and CYP2D6 genotypes. Front. Pharm. 2022, 13, 1048498. [Google Scholar] [CrossRef] [PubMed]
- Leucht, S.; Davis, J.M. Which first-generation antipsychotics should be “repurposed” for the treatment of schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 1–3. [Google Scholar] [CrossRef]
- Kesby, J.; Eyles, D.; McGrath, J.; Scott, J. Dopamine, psychosis and schizophrenia: The widening gap between basic and clinical neuroscience. Transl. Psychiatry 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Górska, N.; Słupski, J.; Cubała, W.J. Antipsychotic drugs in epilepsy. Neurol. Neurochir. Polska 2019, 53, 408–412. [Google Scholar] [CrossRef]
- Geyer, M.A. Developing translational animal models for symptoms of schizophrenia or bipolar mania. Neurotox. Res. 2008, 14, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Sellgren, C.M.; Imbeault, S.; Larsson, M.K.; Oliveros, A.; Nilsson, I.A.K.; Codeluppi, S.; Orhan, F.; Bhat, M.; Tufvesson-Alm, M.; Gracias, J.; et al. GRK3 deficiency elicits brain immune activation and psychosis. Mol. Psychiatry 2021, 26, 6820–6832. [Google Scholar] [CrossRef] [PubMed]
- Barch, D.M.; Ceaser, A. Cognition in schizophrenia: Core psychological and neural mechanisms. Trends Cogn. Sci. 2012, 16, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Laruelle, M.; Abi-Dargham, A.; Gil, R.; Kegeles, L.; Innis, R. Increased dopamine transmission in schizophrenia: Relationship to illness phases. Biol. Psychiatry 1999, 46, 56–72. [Google Scholar] [CrossRef] [PubMed]
- DeWire, S.M.; Ahn, S.; Lefkowitz, R.J.; Shenoy, S.K. Beta-arrestins and cell signaling. Annu. Rev. Physiol. 2007, 69, 483–510. [Google Scholar] [CrossRef]
- Ko, M.J.; Chiang, T.; Mukadam, A.A.; Mulia, G.E.; Gutridge, A.M.; Lin, A.; Chester, J.A.; van Rijn, R.M. Beta-Arrestin-dependent ERK signaling reduces anxiety-like and conditioned fear-related behaviors in mice. Sci. Signal. 2021, 14, 694. [Google Scholar] [CrossRef]
- Prado, M.; Francisco, P.; Barros, M.B.A. Use of psychotropic medications in adults and elderly living in Campinas, Sao Paulo, Brazil: Cross-sectional population-based study. Epidemiol. Serv. Saude 2017, 26, 747–758. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, X.; Zhao, Y.; Lu, C.Y.; Nie, X.; Shi, L. Trends in the utilization of psychotropic medications in China from 2018 to 2021. Front. Pharmacol. 2022, 13, 967826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luo, Z.; Duan, W.; Yang, K.; Ling, L.; Yan, W.; Liu, R.; Wuthrich, K.; Jiang, H.; Xie, C.; et al. Dual-acting antitumor agents targeting the A(2A) adenosine receptor and histone deacetylases: Design and synthesis of 4-(furan-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-6-amine derivatives. Eur. J. Med. Chem. 2022, 236, 114326. [Google Scholar] [CrossRef]
- Pai, N.; Acar, M.; Juneja, P.; Kouhkamari, M.H.; Siva, S.; Mullan, J. Antipsychotic prescribing patterns in Australia: A retro-spective analysis. BMC Psychiatry 2022, 22, 110. [Google Scholar] [CrossRef]
- Stelmach, A.; Guzek, K.; Rożnowska, A.; Najbar, I.; Sadakierska-Chudy, A. Antipsychotic drug—Aripiprazole against schizophrenia, its therapeutic and metabolic effects associated with gene polymorphisms. Pharmacol. Rep. 2023, 75, 19–31. [Google Scholar] [CrossRef]
- Albert, P.R.; Vahid-Ansari, F. The 5-HT1A receptor: Signaling to behavior. Biochimie 2019, 161, 34–45. [Google Scholar] [CrossRef]
- Sourbron, J.; Lagae, L. Serotonin receptors in epilepsy: Novel treatment targets? Epilepsia Open 2022, 7, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Schottle, D.; Janetzky, W.; Luedecke, D.; Beck, E.; Correll, C.U.; Wiedemann, K. Effectiveness of aripiprazole once-monthly in schizophrenia patients pretreated with oral aripiprazole: A 6-month, real-life non-interventional study. BMC Psychiatry 2018, 18, 365. [Google Scholar]
- Tveito, M.; Molden, E.; Hoiseth, G.; Correll, C.U.; Smith, R.L. Impact of age and CYP2D6 genetics on exposure of ari-piprazole and dehydroaripiprazole in patients using long-acting injectable versus oral formulation: Relevance of poor and intermediate metabolizer status. Eur. J. Clin. Pharm. 2020, 76, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; He, F.; Yacouba, M.B.M.; Zhou, H.; Li, J.; Xiong, Y.; Zhang, J.; Li, H.; Wang, Y.; Ke, J. Adenosine A2a Receptor Regulates Autophagy Flux and Apoptosis to Alleviate Ischemia-Reperfusion Injury via the cAMP/PKA Signaling Pathway. Front. Cardiovasc. Med. 2022, 9, 755619. [Google Scholar] [CrossRef]
- Skalbania, J.; Palasz, A.; Blaszczyk, I.; Suszka-Switek, A.; Krzystanek, M.; Tulcanaz, K.P.; Worthington, J.J.; Mordecka-Chamera, K. Chlorpromazine affects the numbers of Sox-2, Musashi1 and DCX-expressing cells in the rat brain sub-ventricular zone. Pharmacol. Rep. 2021, 73, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.; Martucci, N.; Kozlowska, A.; Gamal, W.; Brzeszczyński, F.; Treskes, P.; Samuel, K.; Hayes, P.; Nelson, L.; Bagnaninchi, P.; et al. Chlorpromazine toxicity is associated with disruption of cell membrane integrity and initiation of a pro-inflammatory response in the HepaRG hepatic cell line. Biomed. Pharmacother. 2019, 111, 1408–1416. [Google Scholar] [CrossRef]
- Plasencia-García, B.O.; Rodríguez-Menéndez, G.; Rico-Rangel, M.I.; Rubio-García, A.; Torelló-Iserte, J.; Crespo-Facorro, B. Drug-drug interactions between COVID-19 treatments and antipsychotics drugs: Integrated evidence from 4 databases and a systematic review. Psychopharmacology 2021, 238, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://go.drugbank.com/reactions/836 (accessed on 28 March 2023).
- Grajales, D.; Ferreira, V.; Valverde, M. Second-Generation Antipsychotics and Dysregulation of Glucose Metabolism: Beyond Weight Gain. Cells 2019, 8, 1336. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://https://go.drugbank.com/drugs/DB00477 (accessed on 28 March 2023).
- Andreea, T.; Petru, I.; Miron, A.A.; Paula-Simina, P.; Lorena, D. Clozapine for Treatment-Refractory Aggressive Behavior. Psychiatr. Q. 2021, 92, 721–733. [Google Scholar] [CrossRef]
- Albitar, O.; Harun, S.N.; Zainal, H.; Ibrahim, B.; Sheikh Ghadzi, S.M. Population Pharmacokinetics of Clozapine: A Systematic Review. Biomed. Res. Int. 2020, 2020, 9872936. [Google Scholar] [CrossRef]
- Stępnicki, P.; Kondej, M.; Kaczor, A.A. Current Concepts and Treatments of Schizophrenia. Molecules 2018, 23, 2087. [Google Scholar] [CrossRef] [PubMed]
- De Berardis, D.; Rapini, G.; Olivieri, L.; Di Nicola, D.; Tomasetti, C.; Valchera, A.; Fornaro, M.; Di Fabio, F.; Perna, G.; Di Nicola, M.; et al. Safety of an-tipsychotics for the treatment of schizophrenia: A focus on the adverse effects of clozapine. Ther. Adv. Drug Saf. 2018, 9, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Zuschlag, Z.D. Second-Generation Antipsychotics and Suicide: A Commentary. J. Clin. Psychiatry 2021, 82, 35372. [Google Scholar] [CrossRef]
- Xiao, T.; Wang, Z.; Li, G.; Huang, S.; Zhu, X.; Liu, S.; Li, X.; Hu, J.; Shang, D.; Wen, Y. What to Do About Missed Doses? A Retro-spective Study of Olanzapine in the Elderly. Drug Des. Dev. Ther. 2021, 15, 3411–3423. [Google Scholar] [CrossRef] [PubMed]
- Olmos, I.; Ibarra, M.; Vázquez, M.; Maldonado, C.; Fagiolino, P.; Giachetto, G. Population Pharmacokinetics of Clozapine and Norclozapine and Switchability Assessment between Brands in Uruguayan Patients with Schizophrenia. BioMed Res. Int. 2019, 2019, 3163502. [Google Scholar] [CrossRef] [PubMed]
- Preda, A.; Shapiro, B.B. A safety evaluation of aripiprazole in the treatment of schizophrenia. Expert Opin. Drug Saf. 2020, 19, 1529–1538. [Google Scholar] [CrossRef]
- Rodriguez, C.I.; Setaluri, V. EPAC mediates the dual role of cAMP signaling in melanoma. Oncoscience 2018, 6, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Ji, S.; Yue, W.; Yan, H.; Dong, F.; Ruan, C.; Li, W.; Lu, W.; Zhang, D.; Wang, C.-Y. Development of a population pharmacokinetic model of olanzapine for Chinese health volunteers and patients with schizophrenia. BMJ Open 2018, 8, e020070. [Google Scholar] [CrossRef]
- Moon, S.Y.; Lim, J.H.; Kim, E.H.; Nam, Y.; Yu, K.S.; Hong, K.T.; Choi, J.Y.; Hong, C.R.; Kim, H.; Kang, H.J.; et al. Quantification of Thiopurine Nucleotides in Erythrocytes and Clinical Application to Pediatric Acute Lymphoblastic Leukemia. Ther. Drug Monit. 2019, 41, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Fleischhacker, W.W. The long-term treatment of schizophrenia with antipsychotics: A perennial debate. World Psychiatry 2018, 17, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.; Schwartz, J.M. Why Psychotropic Drugs Don’t Cure Mental Illness—But Should They? Front. Psychiatry 2021, 12, 579566. [Google Scholar] [CrossRef] [PubMed]
| Drug | Target Receptor (s) | Description | Reference/Link |
|---|---|---|---|
| Risperidone | D2, H1, α1 and α2, and 5-HT2A. | Mechanism of action not fully understood. Current studies focus more on how the drug blocks D2 and 5-HT2A receptors, making it an antagonist. | [29] |
| Quetiapine | Has affinity for D2, 5-HT2A, H1, α1, and 5-HT1A receptors | It is a second-generation antipsychotic drug. Acts as an antagonist for 5HT2A, D2, α1, and H1 receptors. It is also a 5HT1A partial agonist. | [30] |
| Promazine | Has an affinity for D1, D2, D4, 5-HT2A, 5-HT2C, H1, M1, M2, M3, M4, M5, and α1 receptors. | It is an antagonist for all the listed receptors. | [31] |
| Fluphenazine | Fluphenazine exerts its actions by blocking postsynaptic D2 receptors in the limbic and cortical systems and the basal ganglia. | A D2 antagonist that prevents the actions of DA, thereby reducing the hallucinations and delusions that are associated with schizophrenia. | [32] |
| Haloperidol | A first-generation antipsychotic that exerts a strong antagonism effect on D2 receptors. | Highly effective for the management of the “positive” symptoms of schizophrenia, including hallucinations, hearing voices, aggression/hostility, disorganized speech, and psychomotor agitation. | [33] |
| Droperidol | A D2 and α1 antagonist. | Droperidol is a butyrophenone derivative and a DA antagonist used to prevent and treat postoperative nausea and vomiting. | [34] |
| Penfluridol | Penfluridol inhibits the binding of DA-to-DA receptors with a Ki of 1.6 μM. It exerts its antipsychotic activity by blocking the DA projections in the limbic system and in the mesocortical area. | First-generation diphenylbutylpiperidine antipsychotic drug. | [35] |
| Thioperamide | An H3 antagonist. | It is used for the treatment of psychiatric disorders and cognitive disorders. | [36] |
| Promazine | Has an affinity for D1, D2, D4, 5-HT2A, 5-HT2C, H1, M1, M2, M3, M4, M5, and α1 receptors. | It is an antagonist for all the listed receptors. | [37] |
| Drug | Effects |
|---|---|
| Abacavir | High serum levels of abacavir due to a decreased rate of elimination by CPZ. |
| 1,2-Benzodiazepine | It increases the antidepressant effect of CPZ. |
| Abametapir | It increases the CPZ serum levels. |
| Abemaciclib | Increased metabolism of abemaciclib may result when co-administered with CPZ. |
| Acetaminophen | Acetaminophen metabolism may slow down when co-administered with CPZ. |
| Amoxicillin | CPZ retards the excretion of amoxicillin. |
| Anakinra | CPZ metabolism increases. |
| Drug | Mode of action | Description |
|---|---|---|
| OZP | Blocks serotonin and DA receptors in the brain | A DA cum serotonin antagonist that is a new-generation medicine under APYs |
| CPZ | Blocks DA receptors | An old-generation medicine, which falls under typical antipsychotics |
| CZP | Blocks serotonin and DA receptors in the brain | A DA cum serotonin antagonist that is a new-generation medicine under APYs |
| ARP | It partially blocks DA receptors, and it also blocks serotonin receptors | Partial DA antagonist, serotonin antagonist, and a new-generation atypical antipsychotic drug |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlambo, R.; Liu, J.; Wang, Q.; Tan, S.; Chen, C. Receptors Involved in Mental Disorders and the Use of Clozapine, Chlorpromazine, Olanzapine, and Aripiprazole to Treat Mental Disorders. Pharmaceuticals 2023, 16, 603. https://doi.org/10.3390/ph16040603
Mlambo R, Liu J, Wang Q, Tan S, Chen C. Receptors Involved in Mental Disorders and the Use of Clozapine, Chlorpromazine, Olanzapine, and Aripiprazole to Treat Mental Disorders. Pharmaceuticals. 2023; 16(4):603. https://doi.org/10.3390/ph16040603
Chicago/Turabian StyleMlambo, Ronald, Jia Liu, Qian Wang, Songwen Tan, and Chuanpin Chen. 2023. "Receptors Involved in Mental Disorders and the Use of Clozapine, Chlorpromazine, Olanzapine, and Aripiprazole to Treat Mental Disorders" Pharmaceuticals 16, no. 4: 603. https://doi.org/10.3390/ph16040603
APA StyleMlambo, R., Liu, J., Wang, Q., Tan, S., & Chen, C. (2023). Receptors Involved in Mental Disorders and the Use of Clozapine, Chlorpromazine, Olanzapine, and Aripiprazole to Treat Mental Disorders. Pharmaceuticals, 16(4), 603. https://doi.org/10.3390/ph16040603







