Effect of Excipients on the Quality of Drug Formulation and Immediate Release of Generic Metformin HCl Tablets
Abstract
1. Introduction
2. Results
2.1. Quality Controlled Tablet Assessment
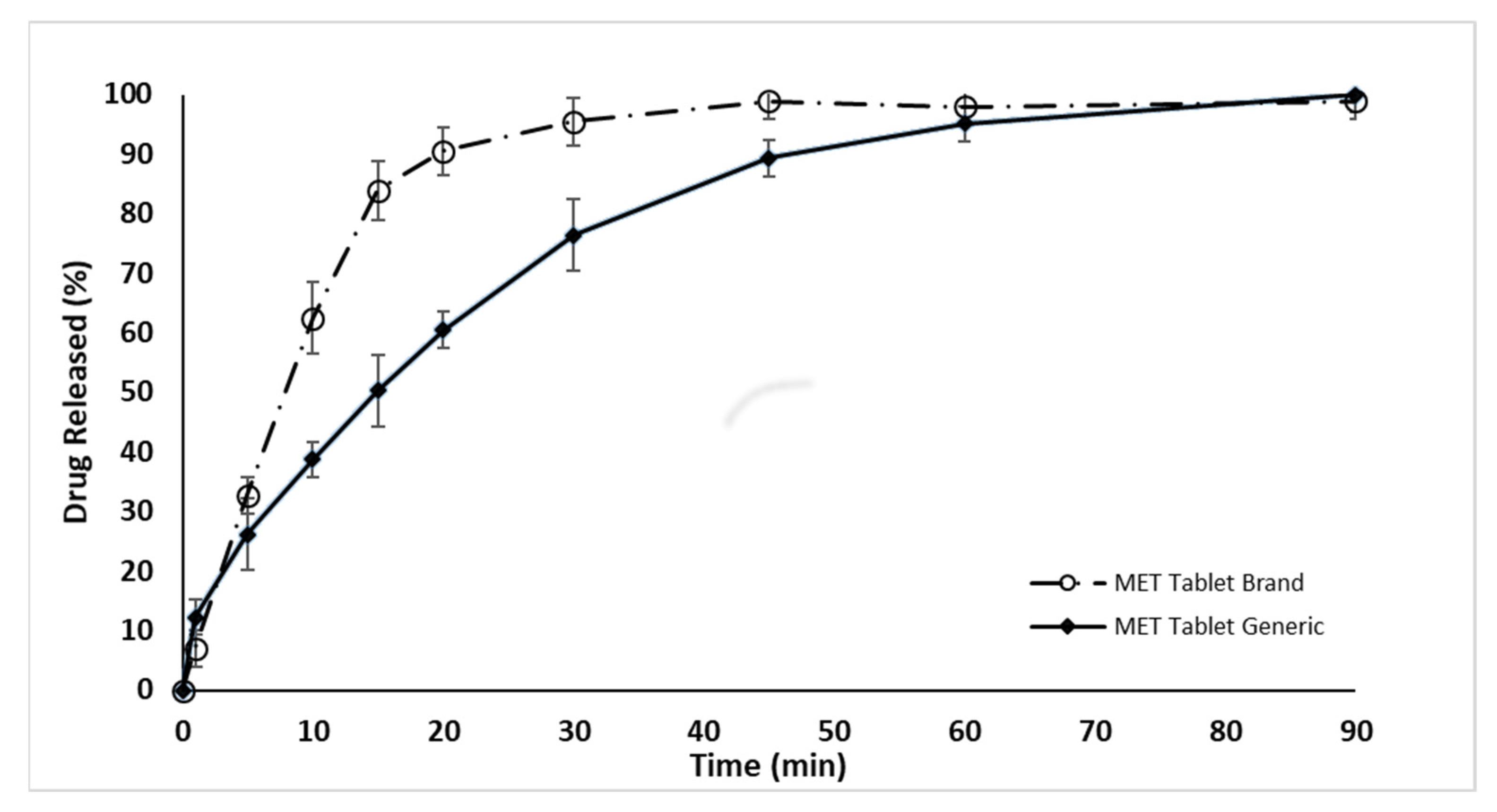
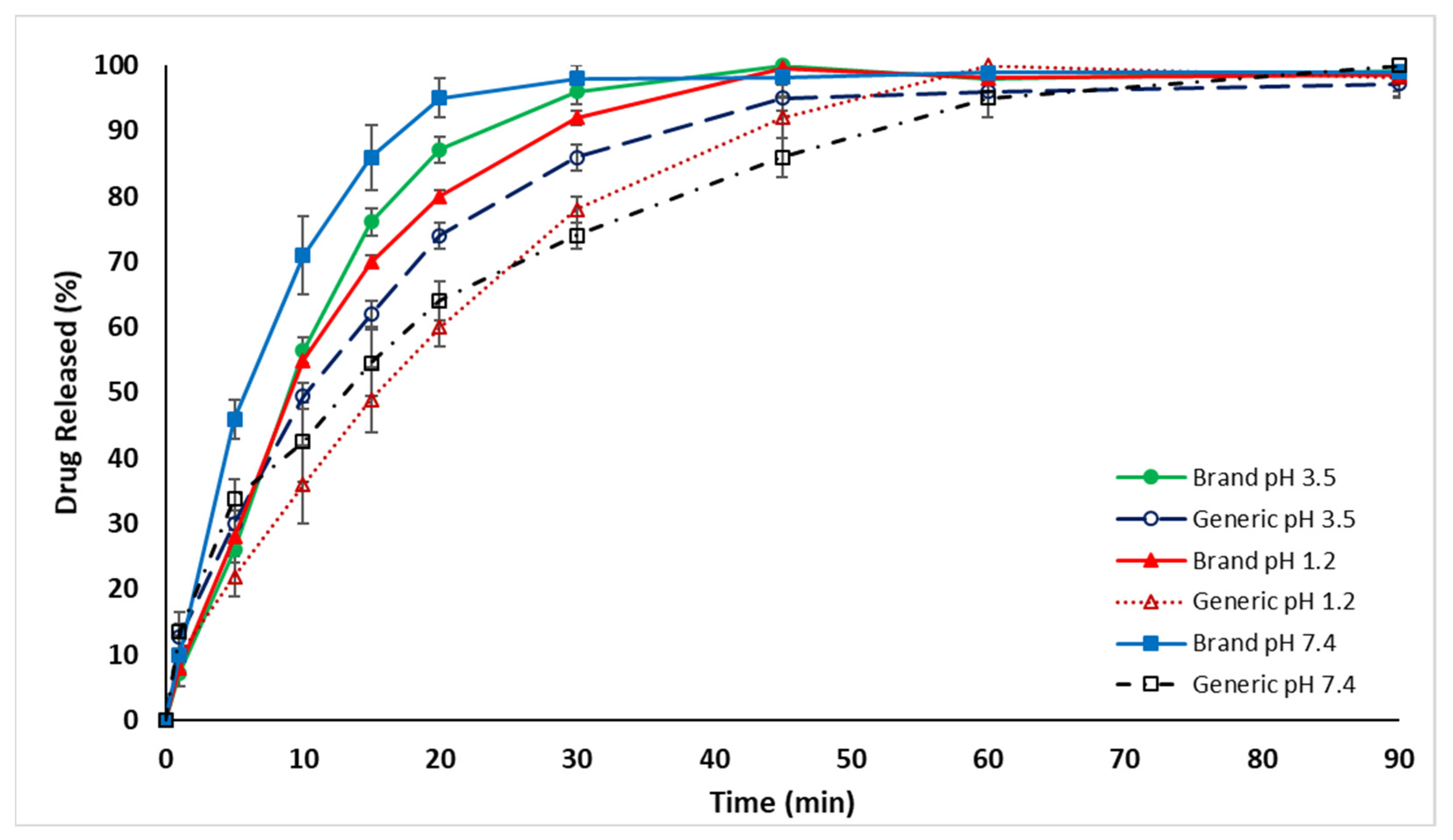
2.2. In Vitro Dissolution Drug Release Evaluation
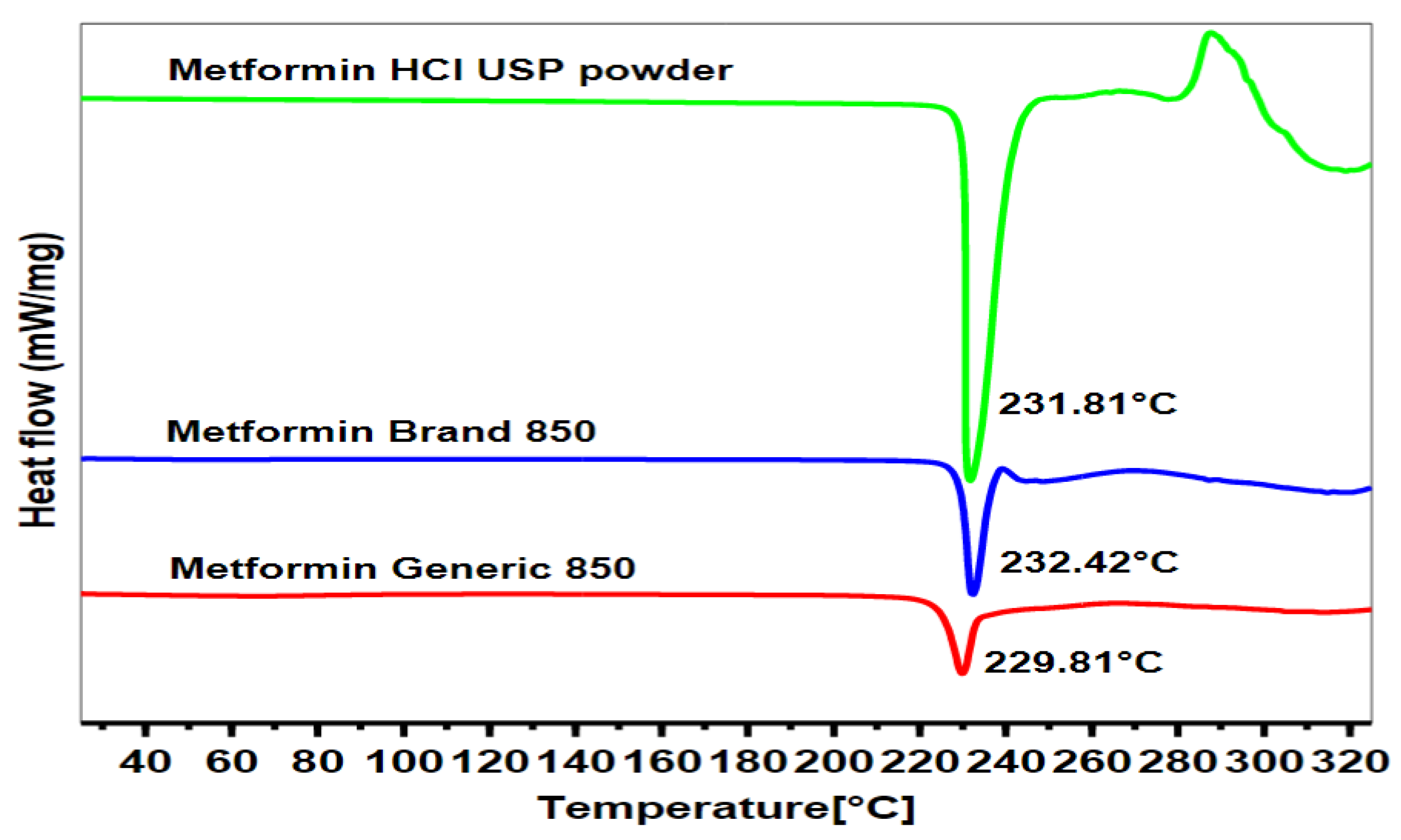
2.3. Differential Scanning Calorimetry
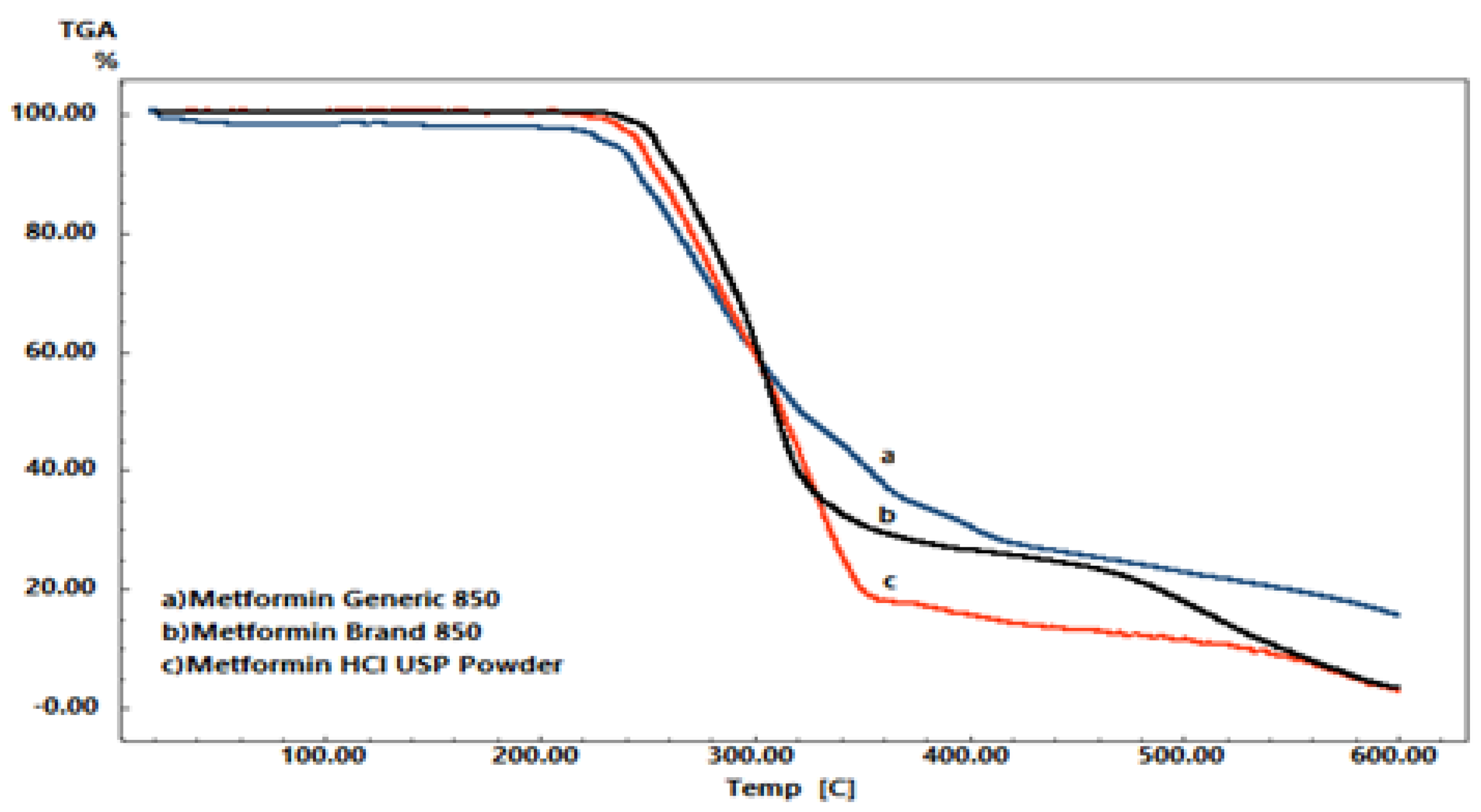
2.4. Thermal Gravimetric Analysis
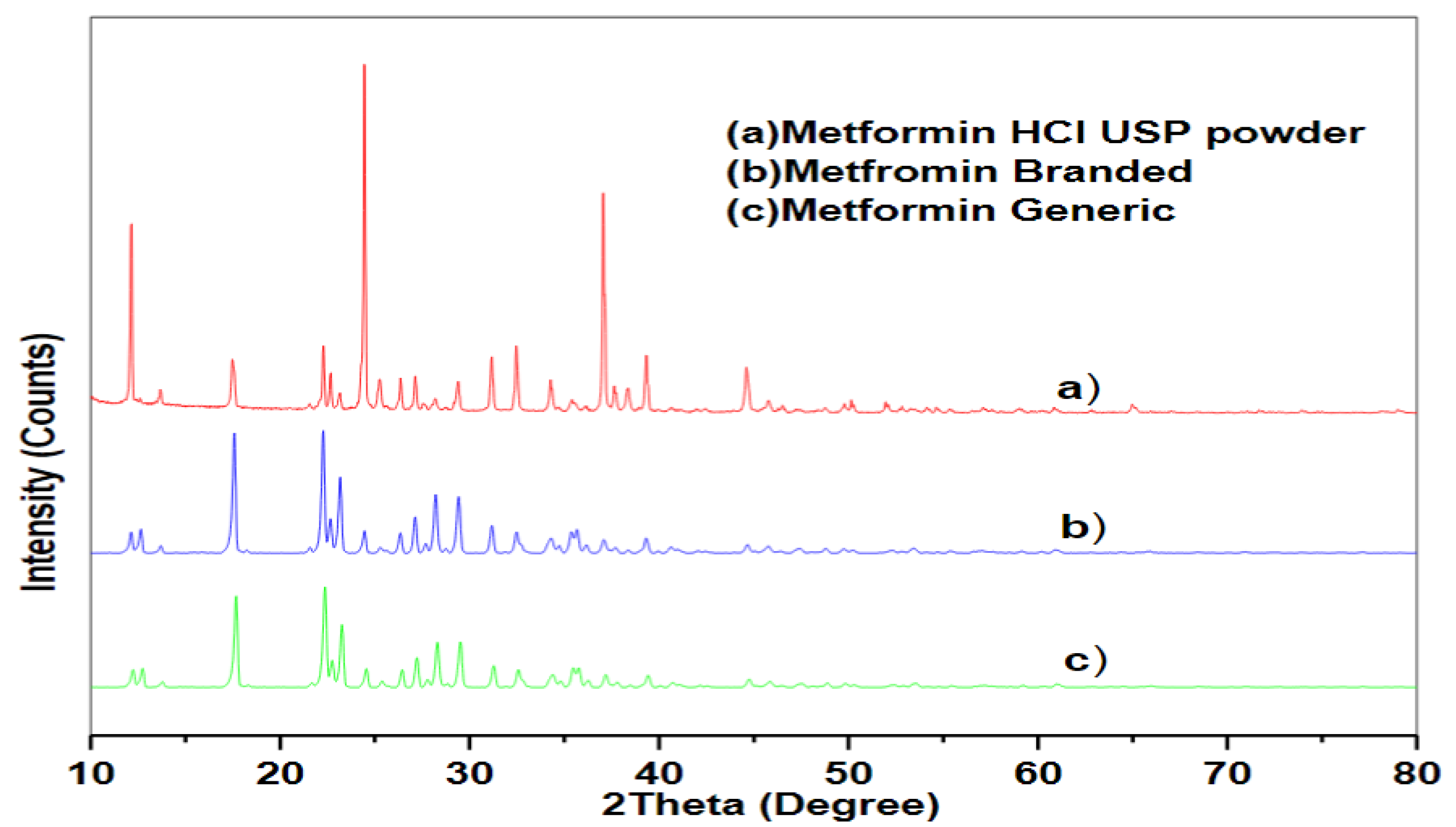
2.5. X-ray Diffraction
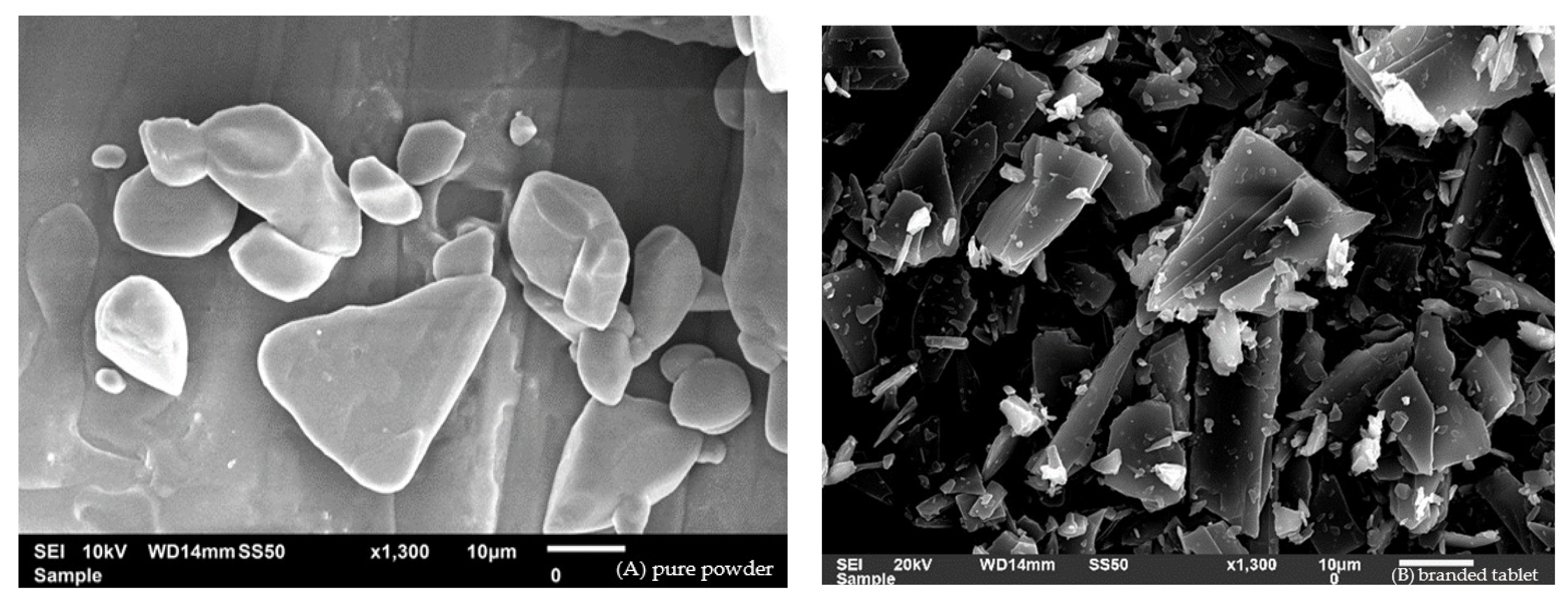
2.6. Scanning Electron Microscopy
2.7. Fourier-Transform Infrared Spectroscopy
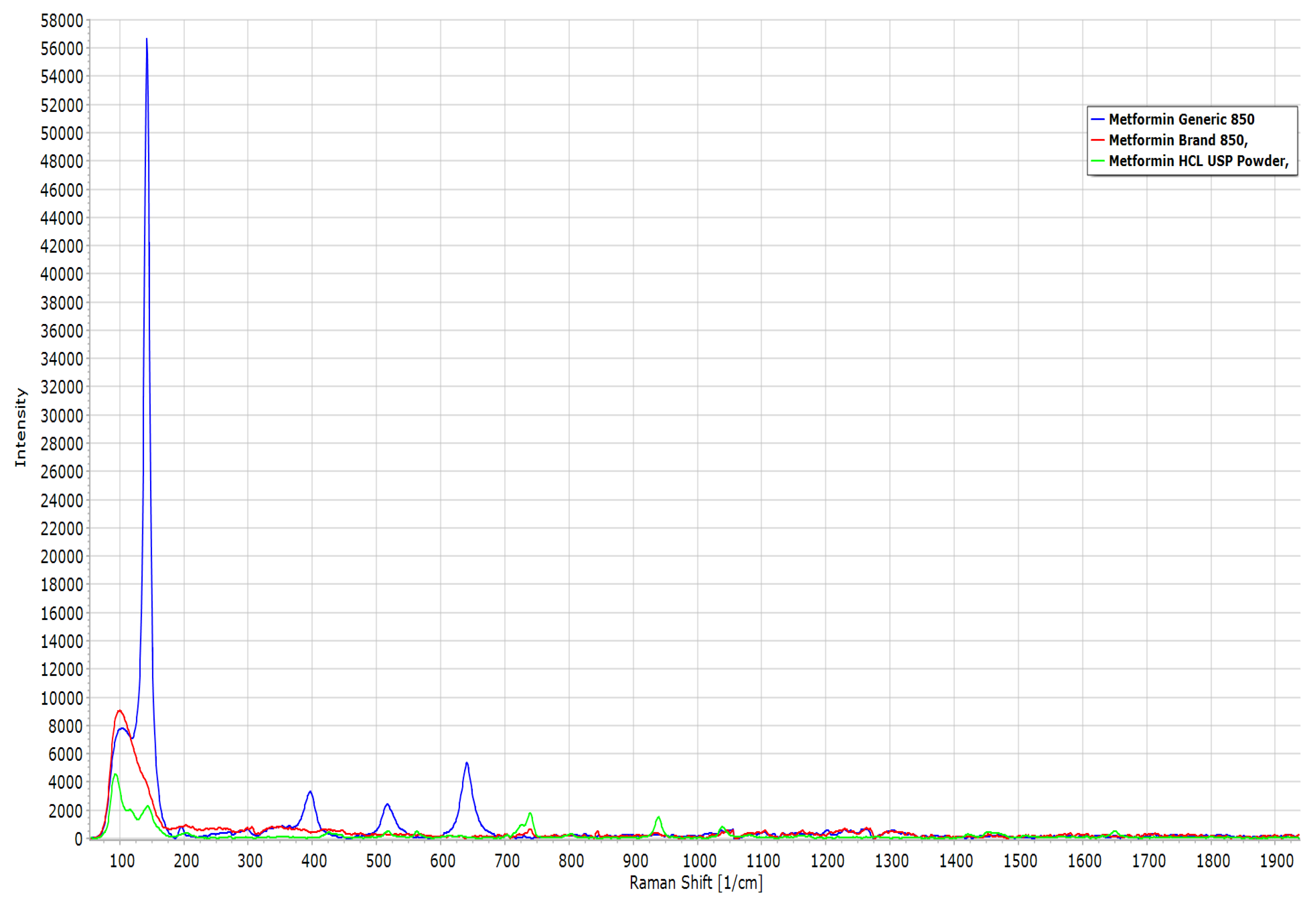
2.8. Raman Spectroscopy
3. Discussion
4. Material and Methods
4.1. Materials
4.2. Weight Variation of the Tablets
4.3. Tablet Friability Assessment
4.4. Mean Resistance Force of the Tablets
4.5. Chemical Content of the Drug
4.6. Tablet Disintegration
4.7. In Vitro Dissolution Evaluation
4.8. Differential Scanning Calorimetry
4.9. Thermogravimetric Analysis
4.10. X-ray Diffraction
4.11. Scanning Electron Microscopy
4.12. Fourier-Transform Infrared Spectroscopy
4.13. Confocal Raman Microscopy
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrade, C. Bioequivalence of Generic Drugs. J. Clin. Psychiatry 2015, 76, e1130–e1131. [Google Scholar] [CrossRef]
- Rayavarapu, S.; Braithwaite, E.; Dorsam, R.; Osterhout, J.; Furlong, L.-A.; Shetty, D.; Peters, J.R. Comparative Risk Assessment of Formulation Changes in Generic Drug Products: A Pharmacology/Toxicology Perspective. Toxicol. Sci. 2015, 146, 2–10. [Google Scholar] [CrossRef]
- Keire, D.A.; Bream, R.; Wollein, U.; Schmaler-Ripcke, J.; Burchardt, A.; Conti, M.; Zmysłowski, A.; Keizers, P.; Morin, J.; Poh, J.; et al. International Regulatory Collaboration on the Analysis of Nitrosamines in Metformin-Containing Medicines. AAPS J. 2022, 24, 56. [Google Scholar] [CrossRef]
- Bharate, S.S. Critical Analysis of Drug Product Recalls Due to Nitrosamine Impurities. J. Med. Chem. 2021, 64, 2923–2936. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Aurobindo Pharma USA, Inc. Initiates Voluntary Nationwide Recall of Two (2) Lots of Quinapril and Hydrochlorothiazide Tablets USP Due to the Potential Presence of Foreign Substance. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/aurobindo-pharma-usa-inc-initiates-voluntary-nationwide-recall-two-2-lots-quinapril-and (accessed on 20 February 2023).
- U.S. Food and Drug Administration. Torrent Pharmaceuticals Limited Issues Voluntary Nationwide Recall of Anagrelide Capsules USP Due to Dissolution Test Failure. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/torrent-pharmaceuticals-limited-issues-voluntary-nationwide-recall-anagrelide-capsules-usp-due#:~:text=Torrent%20Pharmaceuticals%20Limited%20is%20voluntarily,anagrelide%20available%20in%20the%20body (accessed on 9 December 2020).
- U.S. Food and Drug Administration. Teva Pharmaceuticals, USA Extends Voluntary Nationwide Recall to Consumer/User Level for One Lot of Paliperidone Extended-Release Tablets, 3 mg, 90 Count Bottles Distributed Under the Actavis Pharma Inc. Label Due to Dissolution Test Failure. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/teva-pharmaceuticals-usa-extends-voluntary-nationwide-recall-consumeruser-level-one-lot-paliperidone (accessed on 14 June 2017).
- U.S. Food and Drug Administration. Avet Pharmaceuticals Inc. Issues Voluntary Nationwide Recall of Tetracycline HCl Capsules USP, 250 mg and 500 mg Due to Failed Dissolution Specification. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/avet-pharmaceuticals-inc-issues-voluntary-nationwide-recall-tetracycline-hcl-capsules-usp-250-mg-an (accessed on 15 April 2020).
- U.S. Food and Drug Administration. RLC Labs, Inc., Issues Voluntary Nationwide Recall of All Lots of Nature-Throid® and WP Thyroid® with Current Expiry Due to Sub Potency. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/rlc-labs-inc-issues-voluntary-nationwide-recall-all-lots-nature-throidr-and-wp-thyroidr-current (accessed on 3 September 2020).
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Pfeiffer, A.F.H.; Klein, H.H. The Treatment of Type 2 Diabetes. Dtsch. Arztebl. Int. 2014, 111, 69–82. [Google Scholar] [CrossRef]
- Da Trindade, M.T.; Kogawa, A.C.; Salgado, H.R.N. Metformin: A Review of Characteristics, Properties, Analytical Methods and Impact in the Green Chemistry. Crit. Rev. Anal. Chem. 2018, 48, 66–72. [Google Scholar] [CrossRef]
- Rockville USP 39–NF 34|USP-NF. Available online: https://www.uspnf.com/official-text/proposal-statuscommentary/usp-39-nf-34 (accessed on 20 February 2023).
- Sun, C.K.; Weng, P.W.; Chang, J.Z.C.; Lin, Y.W.; Tsuang, F.Y.; Lin, F.H.; Tsai, T.H.; Sun, J.S. Metformin-Incorporated Gelatin/Hydroxyapatite Nanofiber Scaffold for Bone Regeneration. Tissue Eng. Part A 2022, 28, 1–12. [Google Scholar] [CrossRef]
- Arafat, M.; Sarfraz, M.; AbuRuz, S. Development and In Vitro Evaluation of Controlled Release Viagra® Containing Poloxamer-188 Using Gastroplus™ PBPK Modeling Software for In Vivo Predictions and Pharmacokinetic Assessments. Pharmaceuticals 2021, 14, 479. [Google Scholar] [CrossRef]
- Mady, O.Y.; Donia, A.A.; Aa, A.-S.; Qasim, W. Paracellular Pathway Enhancement of Metformin Hydrochloride via Molecular Dispersion in Span 60 Microparticles. Front. Pharmacol. 2019, 10, 713. [Google Scholar] [CrossRef]
- Darji, M.A.; Lalge, R.M.; Marathe, S.P.; Mulay, T.D.; Fatima, T.; Alshammari, A.; Lee, H.K.; Repka, M.A.; Narasimha Murthy, S. Excipient Stability in Oral Solid Dosage Forms: A Review. AAPS PharmSciTech 2018, 19, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Jain, A.; Pharm, S. In Vitro and In Vivo Study of Poly(Ethylene Glycol) Conjugated Ibuprofen to Extend the Duration of Action. Sci. Pharm. 2011, 79, 359–373. [Google Scholar] [CrossRef]
- Müller, R.; Mäder, K.S.G. Solid Lipid Nanoparticles (SLN) for Controlled Drug Delivery: A Review of the State of the Art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Al-Hanbali, O.A.; Hamed, R.; Arafat, M.; Bakkour, Y.; Al-Matubsi, H.; Mansour, R.; Al-Bataineh, Y.; Aldhoun, M.; Sarfraz, M.; Dardas, A.K.Y. Formulation and Evaluation of Diclofenac Controlled Release Matrix Tablets Made of HPMC and Poloxamer 188 Polymer: An Assessment on Mechanism of Drug Release. Pak. J. Pharm. Sci. 2018, 31, 345–351. [Google Scholar]
- Wu, Y.; Levons, J.; Narang, A.S.; Raghavan, K.; Rao, V.M. Reactive Impurities in Excipients: Profiling, Identification and Mitigation of Drug-Excipient Incompatibility. AAPS PharmSciTech 2011, 12, 1248–1263. [Google Scholar] [CrossRef] [PubMed]
- Ríos, Z.A.; Ghaly, E.S. The Effect of Formulation Excipients and Thermal Treatment on the Release Properties of Lisinopril Spheres and Tablets. BioMed Res. Int. 2015, 2015, 423615. [Google Scholar] [CrossRef]
- Flament, M.P.; Leterme, P.; Bizi, M.; Baudet, G.; Gayot, A. Study of Talcs as Antisticking Agents in the Production of Tablets. Eur. J. Pharm. Sci. 2002, 17, 239–245. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Owen, S.C. Handbook of Pharmaceutical Excipients, 6th ed.; Raymond, C., Rowe, P.J., Sheskey, M.E.Q., Eds.; APhA/Pharmaceutical Press: Chicago, IL, USA, 2009. [Google Scholar]
- Odeku, O.A.; Akinwande, B.L. Effect of the Mode of Incorporation on the Disintegrant Properties of Acid Modified Water and White Yam Starches. Saudi Pharm. J. 2012, 20, 171–175. [Google Scholar] [CrossRef]
- Arafat, M.; Sarfraz, M.; Bostanudin, M.F.; Esmaeil, A.; Salam, A.; AbuRuz, S. In Vitro and In Vivo Evaluation of Oral Controlled Release Formulation of BCS Class I Drug Using Polymer Matrix System. Pharmaceuticals 2021, 14, 929. [Google Scholar] [CrossRef]
- Reza, M.S.; Islam, S.N.; Afroze, S.; Bakar, M.S.A.; Taweekun, J.; Azad, A.K. Data on FTIR, TGA-DTG, DSC of Invasive Pennisetum Purpureum Grass. Data Br. 2020, 30, 105536. [Google Scholar] [CrossRef]
- Seçilmiş Canbay, H.; Doğantürk, M. Compatibility Studies of Sildenafil with Different Excipients by Using TGA, DSC, XRD and FTIR. Celal Bayar Üniversitesi Fen Bilim. Derg. 2019, 15, 401–407. [Google Scholar] [CrossRef]
- Mura, P.; Faucci, M.T.; Manderioli, A.; Bramanti, G.; Ceccarelli, L. Compatibility Study between Ibuproxam and Pharmaceutical Excipients Using Differential Scanning Calorimetry, Hot-Stage Microscopy and Scanning Electron Microscopy. J. Pharm. Biomed. Anal. 1998, 18, 151–163. [Google Scholar] [CrossRef]
- Tehsin, K.; Muhammad, S.; Mosab, A.; Momir, M.; Nisar, U.R. Rahman Cross-linked guar gum and sodium borate based microspheres as co-lon-targeted anticancer drug delivery systems for 5-fluorouracil. Pak. J. Pharm. Sci. 2017, 30, 2329–2336. [Google Scholar] [PubMed]
- Cheung, L.C.; Nguyen, M.; Tang, E.; von Ungern Sternberg, B.S.; Salman, S.; Tuleu, C.; Mohamed Ahmed, A.H.A.; Soto, J.; Lim, L.Y. Taste Evaluation of a Novel Midazolam Tablet for Pediatric Patients: In Vitro Drug Dissolution, in Vivo Animal Taste Aversion and Clinical Taste Perception Profiles. Int. J. Pharm. 2018, 535, 194–200. [Google Scholar] [CrossRef]
- Bostanudin, M.F.; Arafat, M.; Sarfraz, M.; Górecki, D.C.; Barbu, E. Butylglyceryl Pectin Nanoparticles: Synthesis, Formulation and Characterization. Polymers 2019, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Venkata Srikanth, M.; Songa, A.S.; Nali, S.R.; Battu, J.R.; Kolapalli, V.R.M. Thermal Sintering: A Novel Technique Used in the Design, Optimization and Biopharmaceutical Evaluation of Propranolol HCl Gastric Floating Tablets. Drug Dev. Ind. Pharm. 2014, 40, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Hertrampf, A.; Müller, H.; Menezes, J.C.; Herdling, T. Advanced Qualification of Pharmaceutical Excipient Suppliers by Multiple Analytics and Multivariate Analysis Combined. Int. J. Pharm. 2015, 495, 447–458. [Google Scholar] [CrossRef]
- Zafar, M.; Ahmad, M.; Sultana, S.; Lubna; Anjum, F.; Ozdemir, F.A.; Tariq, A.; Nazir, A.; Yaseen, G.; Aabidin, S.Z.U.; et al. Light Microscopy and Scanning Electron Microscopy: Implications for Authentication of Misidentified Herbal Drugs. Microsc. Res. Tech. 2019, 82, 1779–1786. [Google Scholar] [CrossRef]
- Jendrzejewska, I.; Goryczka, T.; Pietrasik, E.; Klimontko, J.; Jampilek, J. X-Ray and Thermal Analysis of Selected Drugs Containing Acetaminophen. Molecules 2020, 25, 5909. [Google Scholar] [CrossRef]
- Ramos, P. Application of Thermal Analysis to Evaluate Pharmaceutical Preparations Containing Theophylline. Pharmaceuticals 2022, 15, 1268. [Google Scholar] [CrossRef]
- Arafat, M.; Fahelelbom, K.; Sarfraz, M.; Bostanudin, M.; Sharif, Q.-A.; Esmaeil, A.; AL Hanbali, O.; Aburuz, S. Comparison between Branded and Generic Furosemide 40 Mg Tablets Using Thermal Gravimetric Analysis and Fourier Transform Infrared Spectroscopy. J. Pharm. Bioallied Sci. 2020, 12, 489. [Google Scholar] [CrossRef] [PubMed]
- Arafat, M.; Ahmed, Z.; Arafat, O. Comparison Between Generic Drugs and Brand Name Drugs from Bioequivalence and Thermoequivalence Prospective. Int. J. Pharm. Pharm. Sci. 2017, 9, 1. [Google Scholar] [CrossRef]
- Arafat, M.; Kirchhoefer, C.; Mikov, M.; Sarfraz, M.; Löbenberg, R. Nanosized Liposomes Containing Bile Salt: A Vesicular Nanocarrier for Enhancing Oral Bioavailability of BCS Class III Drug. J. Pharm. Pharm. Sci. 2017, 20, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Simionato, L.D.; Petrone, L.; Baldut, M.; Bonafede, S.L.; Segall, A.I. Comparison between the Dissolution Profiles of Nine Meloxicam Tablet Brands Commercially Available in Buenos Aires, Argentina. Saudi Pharm. J. 2018, 26, 578–584. [Google Scholar] [CrossRef]
- Medina-López, R.; Guillén-Moedano, S.; Hurtado, M. In Vitro Release Studies of Furosemide Reference Tablets: Influence of Agitation Rate, USP Apparatus and Dissolution Media. ADMET DMPK 2020, 8, 411–423. [Google Scholar] [CrossRef]
- Arafat, M.; Kirchhoefer, C.; Mikov, M. Mixed Micelles Loaded with Bile Salt: An Approach to Enhance Intestinal Transport of the BCS Class III Drug Cefotaxime in Rats. Eur. J. Drug Metab. Pharmacokinet. 2016, 42, 635–645. [Google Scholar] [CrossRef]
- Sarifudin, A.; Soontaranon, S.; Peerapattana, J.; Tongta, S. Mechanical Strength, Structural and Hydration Properties of Ethanol-Treated Starch Tablets and Their Impact on the Release of Active Ingredients. Int. J. Biol. Macromol. 2020, 149, 541–551. [Google Scholar] [CrossRef]
- Zheng, A.Y.; Heng, P.W.S.; Chan, L.W. Tablet Disintegratability: Sensitivity of Superdisintegrants to Temperature and Compaction Pressure. Pharmaceutics 2022, 14, 2725. [Google Scholar] [CrossRef]
- Manek, R.V.; Builders, P.F.; Kolling, W.M.; Emeje, M.; Kunle, O.O. Physicochemical and Binder Properties of Starch Obtained from Cyperus Esculentus. AAPS PharmSciTech 2012, 13, 379–388. [Google Scholar] [CrossRef]
- Paramakrishnan, N.; Jha, S.; Kumar, K.J. Physicochemical and Tablet Properties of Cyperus Alulatus Rhizomes Starch Granules. Int. J. Biol. Macromol. 2015, 76, 320–325. [Google Scholar] [CrossRef]
- Kinani, A.A.Y.; Taghi, H.S. Formulation and Characterization of Orodispersible Tablet of Glimepiride. J. Adv. Pharm. Technol. Res. 2022, 13, 252–260. [Google Scholar] [CrossRef]
- Kajiyama, A.; Takagi, H.; Moribe, K.; Yamamoto, K. Improvement of HPMC Tablet Disintegration by the Addition of Inorganic Salts. Chem. Pharm. Bull. 2008, 56, 598–601. [Google Scholar] [CrossRef]
- Li, J.Z.; Rekhi, G.S.; Augsburger, L.L.; Shangraw, R.F. The Role of Intra- and Extragranular Microcrystalline Cellulose in Tablet Dissolution. Pharm. Dev. Technol. 1996, 1, 343–355. [Google Scholar] [CrossRef]
- Bostanudin, M.F.; Salam, A.; Mahmood, A.; Arafat, M.; Kaharudin, A.N.; Sahudin, S.; Mat Lazim, A.; Azfaralariff, A. Formulation and In-Vitro Characterisation of Cross-Linked Amphiphilic Guar Gum Nanocarriers for Percutaneous Delivery of Arbutin. J. Pharm. Sci. 2021, 110, 3907–3918. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, A.L.P.; Wood, B.; Faisal, W.; Farag, F.; Garvie-Cook, H.; Glennon, B.; Vucen, S.; Crean, A.M. Application of Percolation Threshold to Disintegration and Dissolution of Ibuprofen Tablets with Different Microcrystalline Cellulose Grades. Int. J. Pharm. 2020, 589, 119838. [Google Scholar] [CrossRef] [PubMed]
- Motlaq, V.F.; Knudsen, K.D.; Nyström, B. Effect of PEGylation on the Stability of Thermoresponsive Nanogels. J. Colloid Interface Sci. 2018, 524, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Blundell, R.; Butterworth, P.; Charlier, A.; Daurio, D.; Degenhardt, M.; Harris, D.; Hancock, B.; Johnston, M.; Kasina, R.; Kaye, J.; et al. The Role of Titanium Dioxide (E171) and the Requirements for Replacement Materials in Oral Solid Dosage Forms: An IQ Consortium Working Group Review. J. Pharm. Sci. 2022, 111, 2943–2954. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaidi, H.; Majumder, M.; Bari, F. Amorphous and Crystalline Particulates: Challenges and Perspectives in Drug Delivery. Curr. Pharm. Des. 2017, 23, 350–361. [Google Scholar] [CrossRef]
- Dobrzyński, Ł.J.; Zgoda, M.M. Natural Biopolymers as Excipients in Medicinal Product Dosage Form. Part I. Soft Gelatin Capsules as a Modern and Elegant Pharmaceutical Dosage Form. Polim. Med. 2010, 40, 11–19. [Google Scholar]
- Bin Sintang, M.D.; Danthine, S.; Khalenkow, D.; Tavernier, I.; Tzompa Sosa, D.A.; Julmohammad, N.B.; Van de Walle, D.; Rimaux, T.; Skirtach, A.; Dewettinck, K. Modulating the Crystallization of Phytosterols with Monoglycerides in the Binary Mixture Systems: Mixing Behavior and Eutectic Formation. Chem. Phys. Lipids 2020, 230, 104912. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of Deep Eutectic Solvents in Biotechnology and Bioengineering—Promises and Challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Suo, Z.; Peng, X.; Gan, N.; Zhao, L.; Tang, P.; Wei, X.; Li, H. Microcrystalline Cellulose as an Effective Crystal Growth Inhibitor for the Ternary Ibrutinib Formulation. Carbohydr. Polym. 2020, 229, 115476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-W.; Yu, L.; Huang, J.; Hussain, M.A.; Derdour, L.; Qian, F.; de Villiers, M.M. A Method to Evaluate the Effect of Contact with Excipients on the Surface Crystallization of Amorphous Drugs. AAPS PharmSciTech 2014, 15, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Rashtbari, S.; Dehghan, G.; Khataee, S.; Amini, M.; Khataee, A. Dual Enzymes-Mimic Activity of Nanolayered Manganese-Calcium Oxide for Fluorometric Determination of Metformin. Chemosphere 2022, 291, 133063. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.; Sipaut, C.S. Synthesis of Hydroxyapatite/Bioglass Composite Nanopowder Using Design of Experiments. Nanomaterials 2022, 12, 2264. [Google Scholar] [CrossRef]
- Jones, R.N.; Ramsay, D.A.; Keir, D.S.; Dobriner, K. The Intensities of Carbonyl Bands in the Infrared Spectra of Steroids 1. J. Am. Chem. Soc. 1952, 74, 80–88. [Google Scholar] [CrossRef]
- Hambisa, S.; Belew, S.; Suleman, S. In Vitro Comparative Quality Assessment of Different Brands of Norfloxacin Tablets Available in Jimma, Southwest Ethiopia. Drug Des. Dev. Ther. 2019, 13, 1241–1249. [Google Scholar] [CrossRef]
- Minhas, M.U.; Khan, K.U.; Sarfraz, M.; Badshah, S.F.; Munir, A.; Barkat, K.; Basit, A.; Arafat, M. Polyvinylpyrrolidone K-30-Based Crosslinked Fast Swelling Nanogels: An Impeccable Approach for Drug’s Solubility Improvement. BioMed Res. Int. 2022, 2022, 5883239. [Google Scholar] [CrossRef]
- Rashid, M.; Hussain, T.; Sarfraz, M.; Arafat, M.; Hussain, A.; Abbas, N. Precise and Sensitive Ambient Temperature Based Analytical Colorimetric Method for Pregabalin. Braz. J. Pharm. Sci. 2022, 58. [Google Scholar] [CrossRef]
- Mahmood, A.; Haneef, R.; Al Meslamani, A.Z.; Bostanudin, M.F.; Sohail, M.; Sarfraz, M.; Arafat, M. Papain-Decorated Mucopenetrating SEDDS: A Tentative Approach to Combat Absorption Issues of Acyclovir via the Oral Route. Pharmaceutics 2022, 14, 1584. [Google Scholar] [CrossRef]
- Arafat, M. Simple HPLC Validated Method for the Determination of Diltiazem Hydrochloride in Human Plasma. Int. J. Pharm. Pharm. Sci. 2014, 6, 213–216. [Google Scholar]
- Arafat, M.; Ahmed, Z.; Mikov, M. Determination of nifedipine in rat plasma using HPLC-UV detector: A simple method for pharmacokinetics and oral bioavailability studies. Int. J. Pharma. Pharm. Sci. 2016, 8, 98–102. [Google Scholar]




| Measurements | Generic Tablet | Branded Tablet |
|---|---|---|
| Average Weight (mg) | 850 ± 0.2 | 850 ± 0.1 |
| Weight Variation Range (%) | 0.98 ± 0.02 | 0.91 ± 0.01 |
| Tablet Friability (%) | 0.16 ± 0.02 * | 0.12 ± 0.01 |
| Mean Resistance Force (N) | 450.90 ± 53.24 * | 299.10 ± 44.71 |
| Mean Tablet Length (mm) | 8.98 ± 0.32 * | 13.56 ± 0.16 |
| Chemical contents (%) | 1.39 ± 0.20 | 1.53 ± 0.13 |
| Disintegration time (min) | 15.25 ± 0.57 * | 10.30 ± 0.14 |
| Raman-Shift (cm−1) | MET Generic | MET Brand | MET Powder | Functional Group |
|---|---|---|---|---|
| 65 | 8000 | 9000 | 4500 | C-N-C deformation |
| 130 | 57,000 | 4100 | 2200 | C-N-C deformation |
| 400 | 3000 | 1000 | 500 | C-N-C deformation |
| 880 | 500 | 500 | 1900 | N-H wagging |
| 1040 | 1000 | 1000 | 1000 | C-N stretching |
| 1650 | 100 | 100 | 100 | C=N stretching |
| 2850 | 200 | 220 | 240 | CH3 sym. stretching |
| 2950 | 400 | 400 | 500 | CH3 asym. stretching |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arafat, M.; Sakkal, M.; Yuvaraju, P.; Esmaeil, A.; Poulose, V.; Aburuz, S. Effect of Excipients on the Quality of Drug Formulation and Immediate Release of Generic Metformin HCl Tablets. Pharmaceuticals 2023, 16, 539. https://doi.org/10.3390/ph16040539
Arafat M, Sakkal M, Yuvaraju P, Esmaeil A, Poulose V, Aburuz S. Effect of Excipients on the Quality of Drug Formulation and Immediate Release of Generic Metformin HCl Tablets. Pharmaceuticals. 2023; 16(4):539. https://doi.org/10.3390/ph16040539
Chicago/Turabian StyleArafat, Mosab, Molham Sakkal, Priya Yuvaraju, Anna Esmaeil, Vijo Poulose, and Salahdein Aburuz. 2023. "Effect of Excipients on the Quality of Drug Formulation and Immediate Release of Generic Metformin HCl Tablets" Pharmaceuticals 16, no. 4: 539. https://doi.org/10.3390/ph16040539
APA StyleArafat, M., Sakkal, M., Yuvaraju, P., Esmaeil, A., Poulose, V., & Aburuz, S. (2023). Effect of Excipients on the Quality of Drug Formulation and Immediate Release of Generic Metformin HCl Tablets. Pharmaceuticals, 16(4), 539. https://doi.org/10.3390/ph16040539








