Add-On Treatment with Passiflora incarnata L., herba, during Benzodiazepine Tapering in Patients with Depression and Anxiety: A Real-World Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
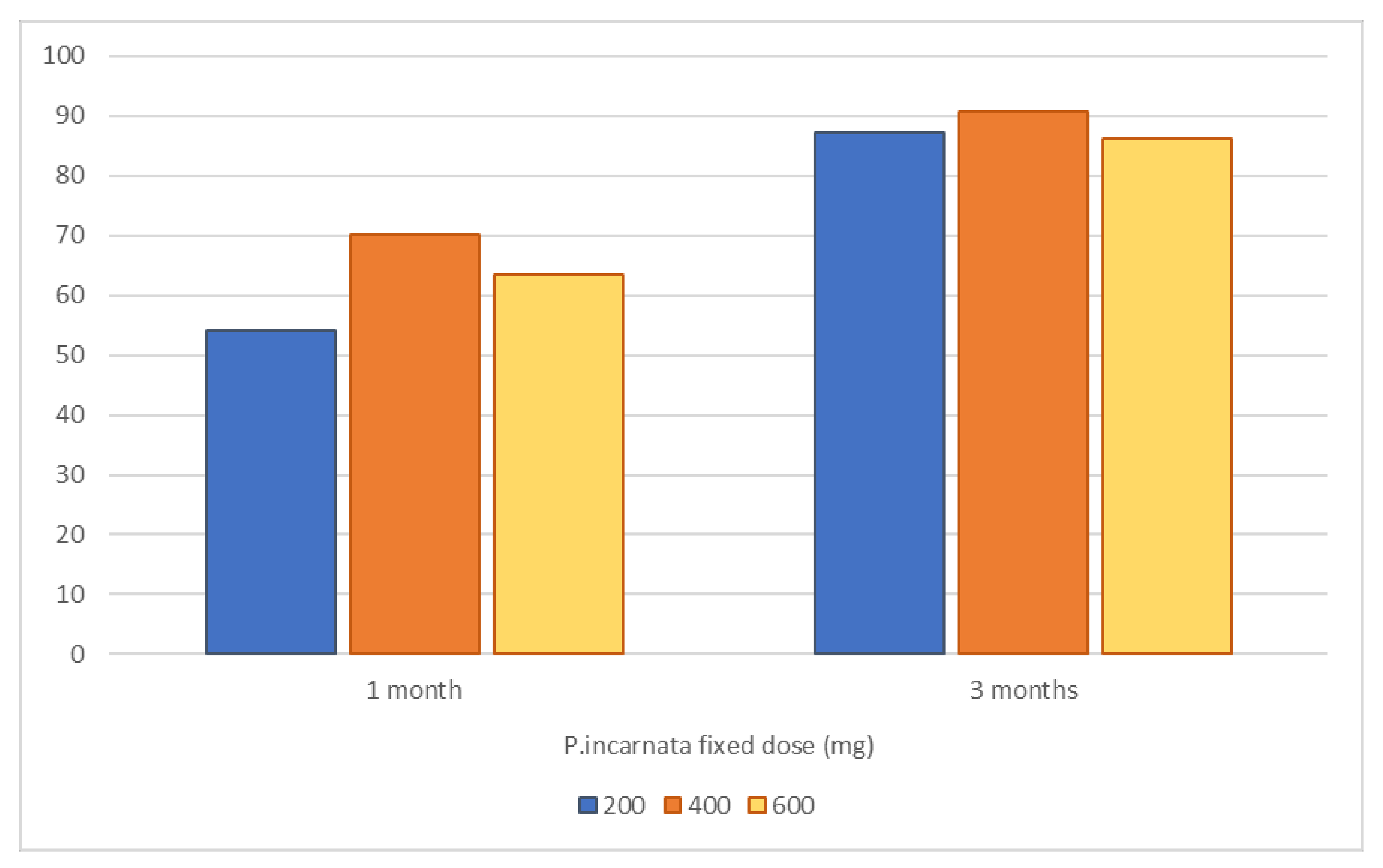
2.2. Passiflora incarnata L., herba
2.3. Data Collection
2.4. Statistical Analyses
3. Results
4. Discussion
5. Limitations and Strengths
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FDA Drug Safety Communication. FDA Drug Safety Communication on Benzodiazepine, 9/23/2020. Available online: https://www.fda.gov/media/142368/download (accessed on 9 February 2023).
- Luscher, B.; Shen, Q.; Sahir, N. The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiatry 2011, 16, 383–406. [Google Scholar] [CrossRef]
- Zaric Kontic, M.; Dragic, M.; Martinovic, J.; Mihajlovic, K.; Brkic, Z.; Mitrovic, N.; Grkovic, I. Prolonged Alprazolam Treatment Alters Components of Glutamatergic Neurotransmission in the Hippocampus of Male Wistar Rats—The Neuroadaptive Changes following Long-Term Benzodiazepine (Mis) Use. Pharmaceuticals 2023, 16, 331. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Editorial of Special Issue ‘Dissecting Neurological and Neuropsychiatric Diseases: Neurodegeneration and Neuroprotection’. Int. J. Mol. Sci. 2022, 23, 6991. [Google Scholar] [CrossRef] [PubMed]
- Institute of Health Metrics and Evaluation. Global Health Data Exchange (GHDx). Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 14 May 2022).
- Taylor, S.; McCracken, C.F.; Wilson, K.C.; Copeland, J.R. Extent and appropriateness of benzodiazepine use. Results from an elderly urban community. Br. J. Psychiatry 1998, 173, 433–438. [Google Scholar] [CrossRef]
- Maust, D.T.; Lin, L.A.; Blow, F.C. Benzodiazepine Use and Misuse Among Adults in the United States. Psychiatr. Serv. 2019, 70, 97–106. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Nix, C.A.; Odisho, A.S.; Babin, C.P.; Derouen, A.G.; Lutfallah, S.C.; Cornett, E.M.; Murnane, K.S.; Kaye, A.M.; Kaye, A.D. Novel Designer Benzodiazepines: Comprehensive Review of Evolving Clinical and Adverse Effects. Neurol. Int. 2022, 14, 648–663. [Google Scholar] [CrossRef]
- Panes, A.; Verdoux, H.; Fourrier-Réglat, A.; Berdaï, D.; Pariente, A.; Tournier, M. Misuse of benzodiazepines: Prevalence and impact in an inpatient population with psychiatric disorders. Br. J. Clin. Pharmacol. 2020, 86, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Edinoff, A.N.; Nix, C.A.; Hollier, J.; Sagrera, C.E.; Delacroix, B.M.; Abubakar, T.; Cornett, E.M.; Kaye, A.M.; Kaye, A.D. Benzodiazepines: Uses, Dangers, and Clinical Considerations. Neurol. Int. 2021, 13, 594–607. [Google Scholar] [CrossRef]
- Reid Finlayson, A.J.; Macoubrie, J.; Huff, C.; Foster, D.E.; Martin, P.R. Experiences with benzodiazepine use, tapering, and discontinuation: An Internet survey. Ther. Adv. Psychopharmacol. 2022, 12, 20451253221082386. [Google Scholar] [CrossRef] [PubMed]
- Lader, M. Benzodiazepine harm: How can it be reduced? Br. J. Clin. Pharmacol. 2014, 77, 295–301. [Google Scholar] [CrossRef]
- O’brien, C.P. Benzodiazepine use, abuse, and dependence. J. Clin. Psychiatry 2005, 66 (Suppl. S2), 28–33. [Google Scholar] [PubMed]
- Fernandes, M.; Neves, I.; Oliveira, J.; Santos, O.; Aguiar, P.; Atalaia, P.; Matos, F.; Freitas, M.C.; Alvim, A.; Maria, V. Discontinuation of chronic benzodiazepine use in primary care: A nonrandomized intervention. Fam. Pract. 2022, 39, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B. Current and Novel Psychopharmacological Drugs for Anxiety Disorders. Adv. Exp. Med. Biol. 2020, 1191, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Breilmann, J.; Girlanda, F.; Guaiana, G.; Barbui, C.; Cipriani, A.; Castellazzi, M.; Bighelli, I.; Davies, S.J.; Furukawa, T.; Koesters, M. Benzodiazepines versus placebo for panic disorder in adults. Cochrane Database Syst. Rev. 2019, 3, CD010677. [Google Scholar] [CrossRef]
- Quagliato, L.A.; Freire, R.C.; Nardi, A.E. Risks and benefits of medications for panic disorder: A comparison of SSRIs and benzodiazepines. Expert Opin. Drug Saf. 2018, 17, 315–324. [Google Scholar] [CrossRef]
- Balon, R.; Starcevic, V. Role of Benzodiazepines in Anxiety Disorders. Adv. Exp. Med. Biol. 2020, 1191, 367–388. [Google Scholar] [CrossRef]
- Zandstra, S.M.; Van Rijswijk, E.; Rijnders, C.A.; van de Lisdonk, E.; Bor, J.; van Weel, C.; Zitman, F. Long-term benzodiazepine users in family practice: Differences from short-term users in mental health, coping behaviour and psychological characteristics. Fam. Pract. 2004, 21, 266–269. [Google Scholar] [CrossRef]
- Garakani, A.; Murrough, J.W.; Freire, R.C.; Thom, R.P.; Larkin, K.; Buono, F.D.; Iosifescu, D.V. Pharmacotherapy of Anxiety Disorders: Current and Emerging Treatment Options. Front. Psychiatry 2020, 11, 595584. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.F.; Barthel, A.L.; Hofmann, S.G. Comparing the efficacy of benzodiazepines and serotonergic anti-depressants for adults with generalized anxiety disorder: A meta-analytic review. Expert Opin. Pharmacother. 2018, 19, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Vikander, B.; Koechling, U.M.; Borg, S.; Tönne, U.; Hiltunen, A.J. Benzodiazepine tapering: A prospective study. Nord. J. Psychiatry 2010, 64, 273–282. [Google Scholar] [CrossRef]
- Reeve, E.; Ong, M.; Wu, A.; Jansen, J.; Petrovic, M.; Gnjidic, D. A systematic review of interventions to deprescribe benzodiazepines and other hypnotics among older people. Eur. J. Clin. Pharmacol. 2017, 73, 927–935. [Google Scholar] [CrossRef]
- Votaw, V.R.; Geyer, R.; Rieselbach, M.M.; McHugh, R.K. The epidemiology of benzodiazepine misuse: A systematic review. Drug Alcohol Depend. 2019, 200, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.; Han, B.; Jones, C.M.; Johnson, K.; Compton, W.M. Prevalence and Correlates of Benzodiazepine Use, Misuse, and Use Disorders Among Adults in the United States. J. Clin. Psychiatry 2018, 79, 18m12174. [Google Scholar] [CrossRef] [PubMed]
- McHugh, R.K.; Peckham, A.D.; Björgvinsson, T.; Korte, F.M.; Beard, C. Benzodiazepine misuse among adults receiving psychiatric treatment. J. Psychiatr. Res. 2020, 128, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Sproule, B.A.; Zahra, Z.; Sunderji, N.; Kennedy, S.H.; Rizvi, S.J. Understanding the effects of chronic benzodiazepine use in depression: A focus on neuropharmacology. Int. Clin. Psychopharmacol. 2020, 35, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Elbe, D. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications, Third Edition. J. Can. Acad. Child Adolesc. Psychiatry 2010, 19, 230. [Google Scholar]
- Sarangi, A.; McMahon, T.; Gude, J. Benzodiazepine Misuse: An Epidemic Within a Pandemic. Cureus 2021, 13, e15816. [Google Scholar] [CrossRef]
- Peng, L.; Meeks, T.W.; Blazes, C.K. Complex Persistent Benzodiazepine Dependence-When Benzodiazepine Deprescribing Goes Awry. JAMA Psychiatry 2022, 79, 639–640. [Google Scholar] [CrossRef]
- Pergolizzi, J.V., Jr.; LeQuang, J.A.; Raffa, R.B. Benzodiazepines: Thinking outside the black box. J. Clin. Pharm. Ther. 2021, 46, 554–559. [Google Scholar] [CrossRef]
- Takeshima, M.; Aoki, Y.; Ie, K.; Katsumoto, E.; Tsuru, E.; Tsuboi, T.; Inada, K.; Kise, M.; Watanabe, K.; Mishima, K.; et al. Attitudes and Difficulties Associated with Benzodiazepine Discontinuation. Int. J. Environ. Res. Public Health 2022, 19, 15990. [Google Scholar] [CrossRef]
- Reeves, R.R.; Kamal, A. Complicated Withdrawal Phenomena During Benzodiazepine Cessation in Older Adults. J. Osteopath. Med. 2019, 119, 327–331. [Google Scholar] [CrossRef]
- Felice, D.; Cryan, J.F.; O’Leary, O.F. GABAB Receptors: Anxiety and Mood Disorders. Curr. Top. Behav. Neurosci. 2022, 52, 241–265. [Google Scholar] [CrossRef]
- Caniff, K.; Telega, E.; Bostwick, J.R.; Gardner, K.N. Pregabalin as adjunctive therapy in benzodiazepine discontinuation. Am. J. Health Syst. Pharm. 2018, 75, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Bobes, J.; Rubio, G.; Terán, A.; Cervera, G.; Gómez, V.L.; Vilardaga, I.; Pérez, M. Pregabalin for the discontinuation of long-term benzodiazepines use: An assessment of its effectiveness in daily clinical practice. Eur. Psychiatry 2012, 27, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Mariani, J.J.; Malcolm, R.J.; Mamczur, A.K.; Choi, J.C.; Brady, R.; Nunes, E.; Levin, F.R. Pilot trial of gabapentin for the treatment of benzodiazepine abuse or dependence in methadone maintenance patients. Am. J. Drug Alcohol Abus. 2016, 42, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Baandrup, L.; Ebdrup, B.H.; Rasmussen, J.Ø.; Lindschou, J.; Gluud, C.; Glenthøj, B.Y. Pharmacological interventions for benzodiazepine discontinuation in chronic benzodiazepine users. Cochrane Database Syst. Rev. 2018, 3, CD011481. [Google Scholar] [CrossRef]
- Rickels, K.; DeMartinis, N.; Rynn, M.; Mandos, L. Pharmacologic strategies for discontinuing benzodiazepine treatment. J. Clin. Psychopharmacol. 1999, 19 (Suppl. S2), 12S–16S. [Google Scholar] [CrossRef]
- Tyrer, P.; Rutherford, D.; Huggett, T. Benzodiazepine withdrawal symptoms and propranolol. Lancet 1981, 1, 520–522. [Google Scholar] [CrossRef]
- Schweizer, E.; Case, W.G.; Garcia-Espana, F.; Greenblatt, D.J.; Rickels, K. Progesterone co-administration in patients discontinuing long-term benzodiazepine therapy: Effects on withdrawal severity and taper outcome. Psychopharmacology 1995, 117, 424–429. [Google Scholar] [CrossRef]
- Romach, M.K.; Kaplan, H.L.; Busto, U.E.; Somer, G.; Sellers, E.M. A controlled trial of ondansetron, a 5-HT3 antagonist, in benzodiazepine discontinuation. J. Clin. Psychopharmacol. 1998, 18, 121–131. [Google Scholar] [CrossRef]
- Tyrer, P.; Ferguson, B.; Hallström, C.; Michie, M.; Tyrer, S.; Cooper, S.; Caplan, R.; Barczak, P. A controlled trial of dothiepin and placebo in treating benzodiazepine withdrawal symptoms. Br. J. Psychiatry 1996, 168, 457–461. [Google Scholar] [CrossRef]
- Lader, M.; Olajide, D. A comparison of buspirone and placebo in relieving benzodiazepine withdrawal symptoms. J. Clin. Psychopharmacol. 1987, 7, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, E.; Rickels, K. Failure of buspirone to manage benzodiazepine withdrawal. Am. J. Psychiatry 1986, 143, 1590–1592. [Google Scholar] [CrossRef] [PubMed]
- Rickels, K.; DeMartinis, N.; García-España, F.; Greenblatt, D.J.; Mandos, L.A.; Rynn, M. Imipramine and buspirone in treatment of patients with generalized anxiety disorder who are discontinuing long-term benzodiazepine therapy. Am. J. Psychiatry 2000, 157, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.W.; Tretyak, V.; McHugh, R.K.; Weiss, R.D.; Bogunovic, O. Review: Adjunctive pharmacologic approaches for benzodiazepine tapers. Drug Alcohol Depend. 2018, 189, 96–107. [Google Scholar] [CrossRef]
- Rickels, K.; Schweizer, E.; Garcia España, F.; Case, G.; DeMartinis, N.; Greenblatt, D. Trazodone and valproate in patients discontinuing long-term benzodiazepine therapy: Effects on withdrawal symptoms and taper outcome. Psychopharmacology 1999, 141, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Shinjyo, N.; Waddell, G.; Green, J. Valerian Root in Treating Sleep Problems and Associated Disorders—A Systematic Review and Meta-Analysis. J. Evid.-Based Integr. Med. 2020, 25, 2515690X20967323. [Google Scholar] [CrossRef]
- Janda, K.; Wojtkowska, K.; Jakubczyk, K.; Antoniewicz, J.; Skonieczna-Żydecka, K. Passiflora incarnata in Neuropsychiatric Disorders—A Systematic Review. Nutrients 2020, 12, 3894. [Google Scholar] [CrossRef]
- Sigel, E.; Ernst, M. The Benzodiazepine Binding Sites of GABAA Receptors. Trends Pharmacol. Sci. 2018, 39, 659–671. [Google Scholar] [CrossRef]
- Appel, K.; Rose, T.; Fiebich, B.; Kammler, T.; Hoffmann, C.; Weiss, G. Modulation of the γ-aminobutyric acid (GABA) system by Passiflora incarnata L. Phytother. Res. 2011, 25, 838–843. [Google Scholar] [CrossRef]
- Bruni, O.; Ferini-Strambi, L.; Giacomoni, E.; Pellegrino, P. Herbal Remedies and Their Possible Effect on the GABAergic System and Sleep. Nutrients 2021, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- HMPC. “Assessment Report on Passiflora incarnata L., herba,” Ema/Hmpc/669738/2013, No. November. 2014. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-passiflora-incarnata-l-herba_en.pdf (accessed on 2 March 2023).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing, Inc.: Washington, DC, USA, 2013. [Google Scholar]
- Zimmerman, M.; Martinez, J.H.; Young, D.; Chelminski, I.; Dalrymple, K. Severity classification on the Hamilton Depression Rating Scale. J. Affect. Disord. 2013, 150, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E. Hamilton Rating Scale for Anxiety (HAM-A). Occup. Med. 2015, 65, 601. [Google Scholar] [CrossRef] [PubMed]
- Julian, L.J. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res. 2011, 63 (Suppl. S11), S467–S472. [Google Scholar] [CrossRef]
- Richter, P.; Werner, J.; Heerlein, A.; Kraus, A.; Sauer, H. On the validity of the Beck Depression Inventory: A review. Psychopathology 1998, 31, 160–168. [Google Scholar] [CrossRef]
- JASP Team. JASP (Version 0.17.1) [Computer Software]; Free Software Foundation, Inc.: Boston, MA, USA, 2023. [Google Scholar]
- Microsoft Corporation. Microsoft Excel. 2018. Available online: https://office.microsoft.com/excel (accessed on 9 February 2023).
- Coll, S.; Walsh, M.E.; Fahey, T.; Moriarty, F. Hospital initiation of benzodiazepines and Z-drugs in older adults and discontinuation in primary care. Res. Soc. Adm. Pharm. 2022, 18, 2670–2674. [Google Scholar] [CrossRef]
- Nardi, A.E.; Freire, R.C.; Valença, A.M.; Amrein, R.; De Cerqueira, A.C.R.; Lopes, F.L.; Nascimento, I.; Mezzasalma, M.A.; Veras, A.B.; Sardinha, A.; et al. Tapering clonazepam in patients with panic disorder after at least 3 years of treatment. J. Clin. Psychopharmacol. 2010, 30, 290–293. [Google Scholar] [CrossRef]
- Lolli, L.F.; Sato, C.M.; Romanini, C.V.; Villas-Boas Lde, B.; Santos, C.A.; de Oliveira, R.M. Possible involvement of GABA A-benzodiazepine receptor in the anxiolytic-like effect induced by Passiflora actinia extracts in mice. J. Ethnopharmacol. 2007, 111, 308–314. [Google Scholar] [CrossRef]
- Kumar, K.; Sharma, S.; Kumar, P.; Deshmukh, R. Therapeutic potential of GABA (B) receptor ligands in drug addiction, anxiety, depression and other CNS disorders. Pharmacol. Biochem. Behav. 2013, 110, 174–184. [Google Scholar] [CrossRef]
- Dhawan, K.; Dhawan, S.; Chhabra, S. Attenuation of benzodiazepine dependence in mice by a tri-substituted benzoflavone moiety of Passiflora incarnata Linneaus: A non-habit forming anxiolytic. J. Pharm. Pharm. Sci. 2003, 6, 215–222. [Google Scholar]
- Ngan, A.; Conduit, R. A double-blind, placebo-controlled investigation of the effects of Passiflora incarnata (passionflower) herbal tea on subjective sleep quality. Phytother. Res. 2011, 25, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Kashani, L.; Mobaseri, M.; Hosseini, S.H.; Nikzad, S.; Khani, M. Passionflower in the treatment of opiates withdrawal: A double-blind randomized controlled trial. J. Clin. Pharm. Ther. 2001, 26, 369–373. [Google Scholar] [CrossRef] [PubMed]
| Group A (P. incarnata) (n = 93) | Group B (n = 93) | |
|---|---|---|
| Age (years) | 54.6 ± 16.4 | 51.7 ± 13.1 |
| Sex ratio (M/F) | 27/66 (29.0%/71.0%) | 35/58 (37.6%/62.4%) |
| Education (years) | 13.4 ± 3.55 | 12.6 ± 3.43 |
| Occupation (U/E) | 13/80 (13.9%/86.1%) | 17/76 (18.3%/81.7%) |
| Status (single/coupled) | 23/70 (24.7%/75.3%) | 24/69 (25.8%/74.2%) |
| Diagnosis (depression/anxiety) | 43/50 (46.2%/53.8%) | 48/45 (51.6%/48.4) |
| Baseline clinical measurements | ||
| HARS | 6.43 ± 4.76 | 6.34 ± 4.77 |
| BAI | 5.75 ± 4.68 | 5.59 ± 4.63 |
| HDRS | 4.69 ± 3.73 | 4.64 ± 3.68 |
| BDI | 4.89 ± 3.85 | 4.85 ± 3.83 |
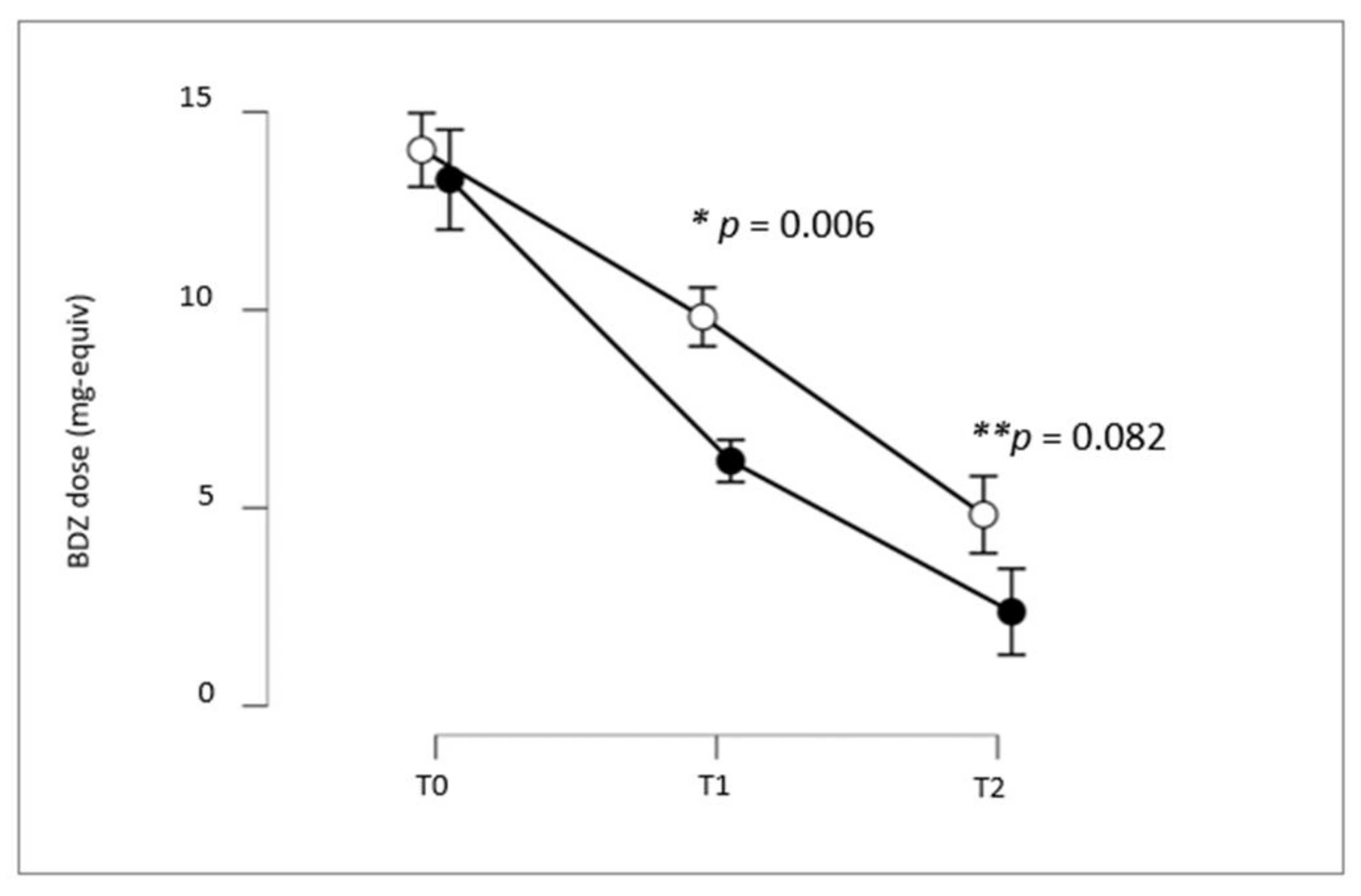
| Group A P. incarnata L., herba | Group B No Add-On Treatment | p-Value * | |
|---|---|---|---|
| 50% BDZ reduction | |||
| 1 month | 76.35% | 26.88% | <0.001 |
| 3 months | 95.69% | 72.04% | <0.001 |
| Complete BDZ discontinuation | |||
| 1 month | 32.26% | 12.90% | 0.002 |
| 3 months | 69.89% | 52.69% | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanardi, R.; Carminati, M.; Fazio, V.; Maccario, M.; Verri, G.; Colombo, C. Add-On Treatment with Passiflora incarnata L., herba, during Benzodiazepine Tapering in Patients with Depression and Anxiety: A Real-World Study. Pharmaceuticals 2023, 16, 426. https://doi.org/10.3390/ph16030426
Zanardi R, Carminati M, Fazio V, Maccario M, Verri G, Colombo C. Add-On Treatment with Passiflora incarnata L., herba, during Benzodiazepine Tapering in Patients with Depression and Anxiety: A Real-World Study. Pharmaceuticals. 2023; 16(3):426. https://doi.org/10.3390/ph16030426
Chicago/Turabian StyleZanardi, Raffaella, Matteo Carminati, Valentina Fazio, Melania Maccario, Greta Verri, and Cristina Colombo. 2023. "Add-On Treatment with Passiflora incarnata L., herba, during Benzodiazepine Tapering in Patients with Depression and Anxiety: A Real-World Study" Pharmaceuticals 16, no. 3: 426. https://doi.org/10.3390/ph16030426
APA StyleZanardi, R., Carminati, M., Fazio, V., Maccario, M., Verri, G., & Colombo, C. (2023). Add-On Treatment with Passiflora incarnata L., herba, during Benzodiazepine Tapering in Patients with Depression and Anxiety: A Real-World Study. Pharmaceuticals, 16(3), 426. https://doi.org/10.3390/ph16030426







