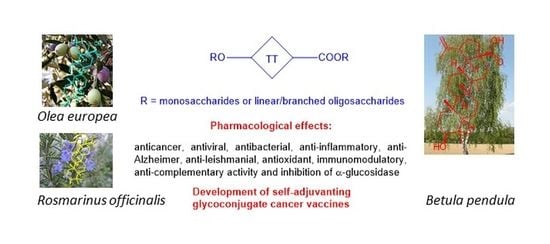
Saponins of Selected Triterpenoids as Potential Therapeutic Agents: A Review
Abstract
1. Introduction
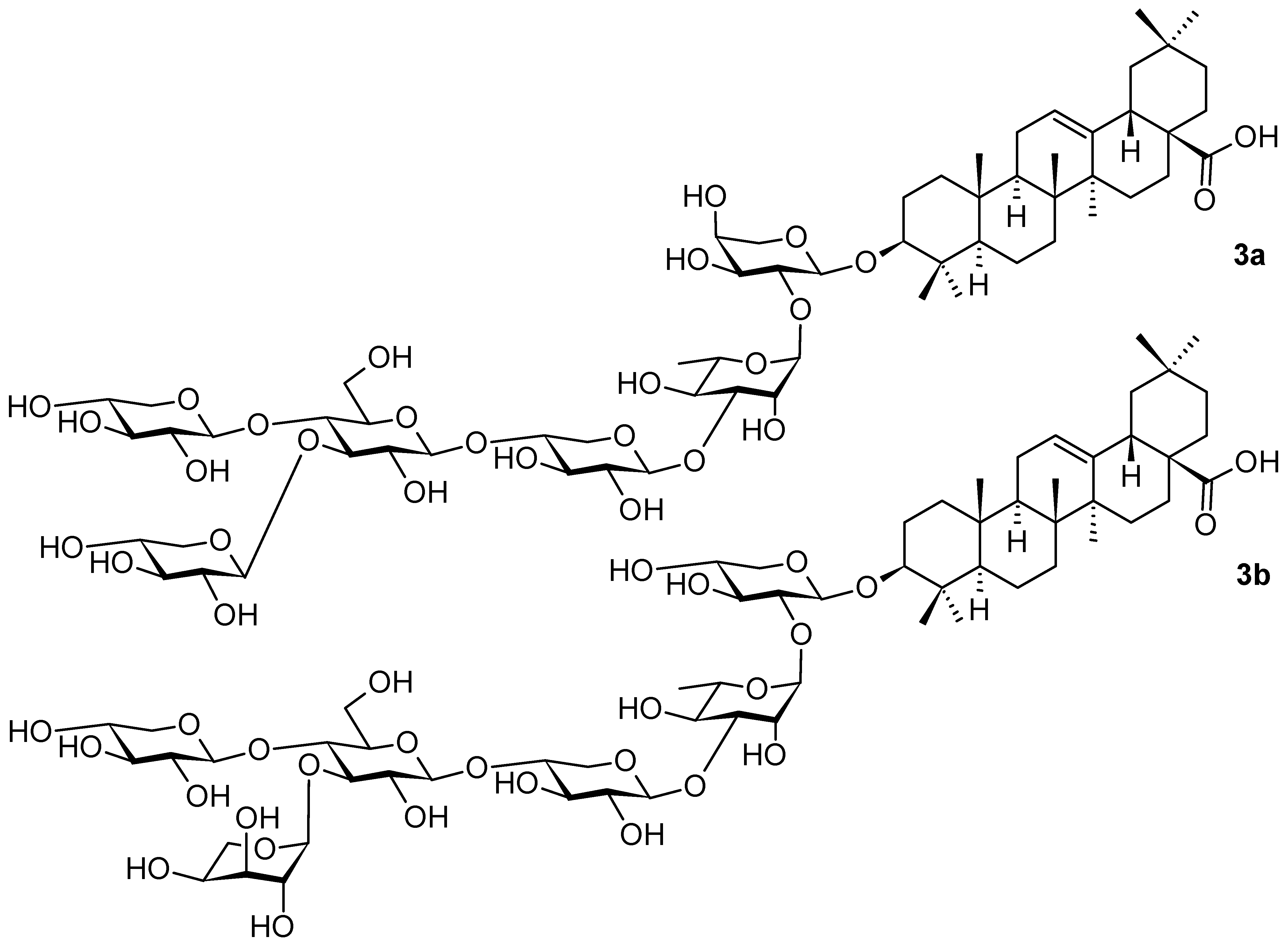
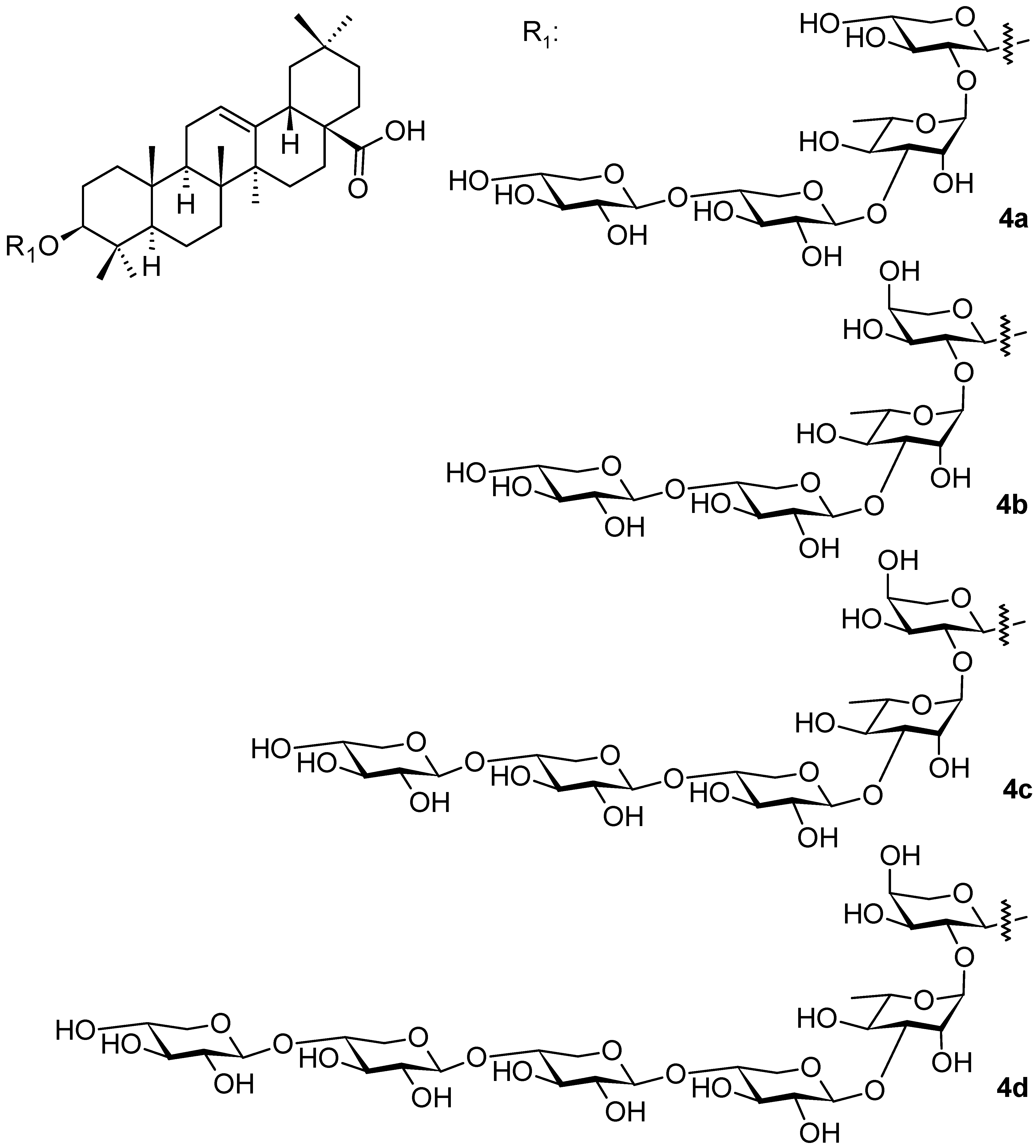
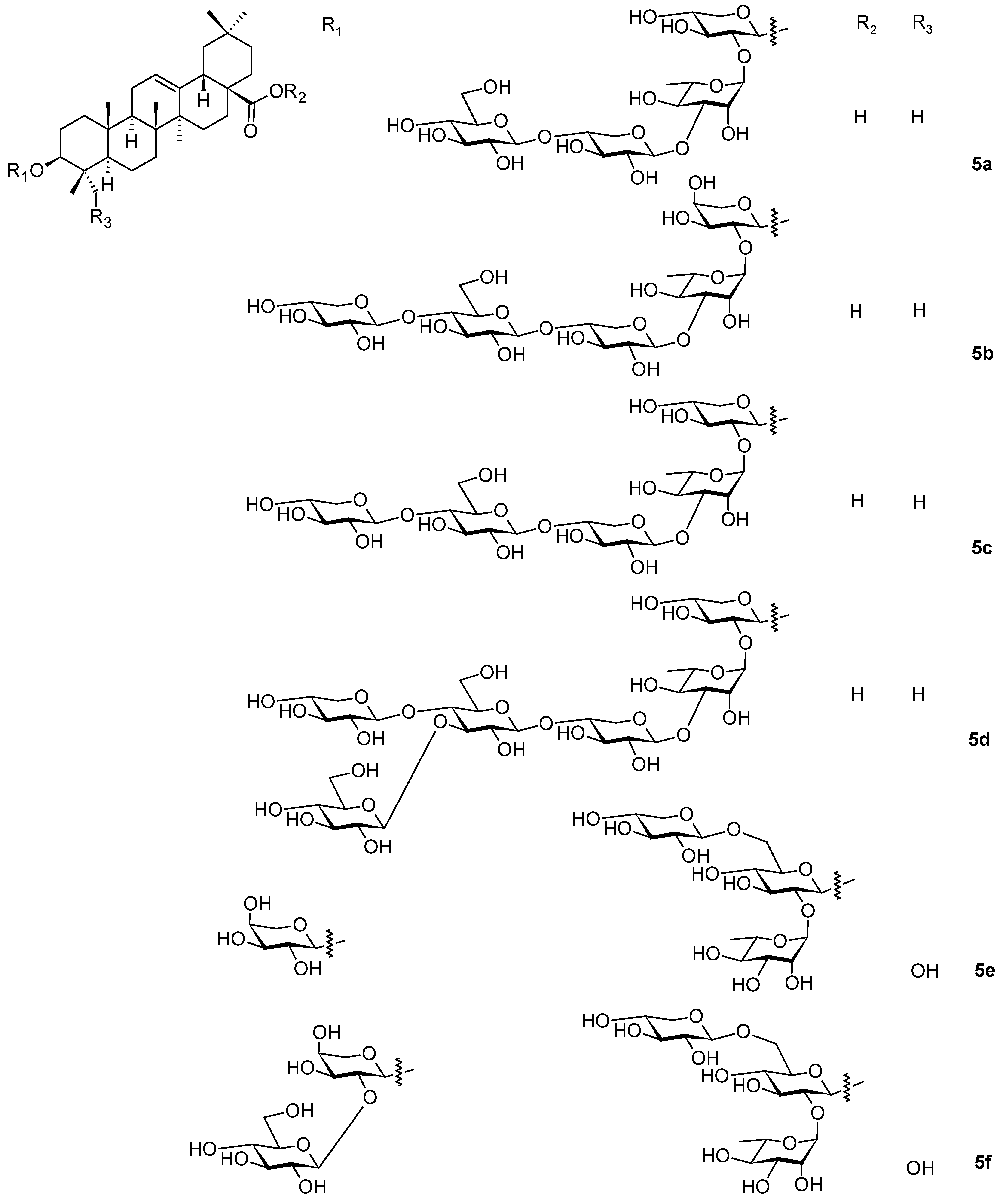
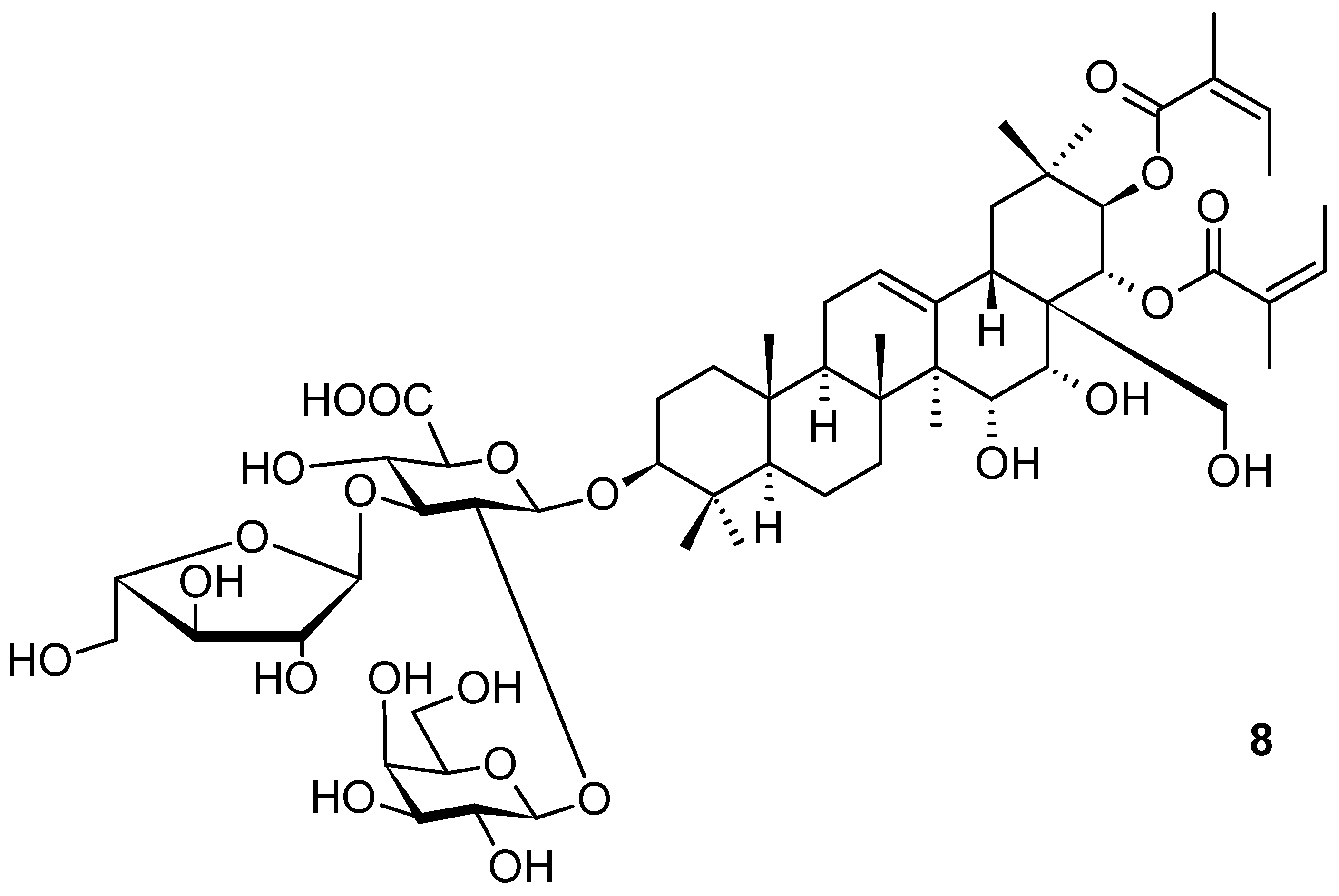
2. Triterpenoids with the Oleanane Skeleton
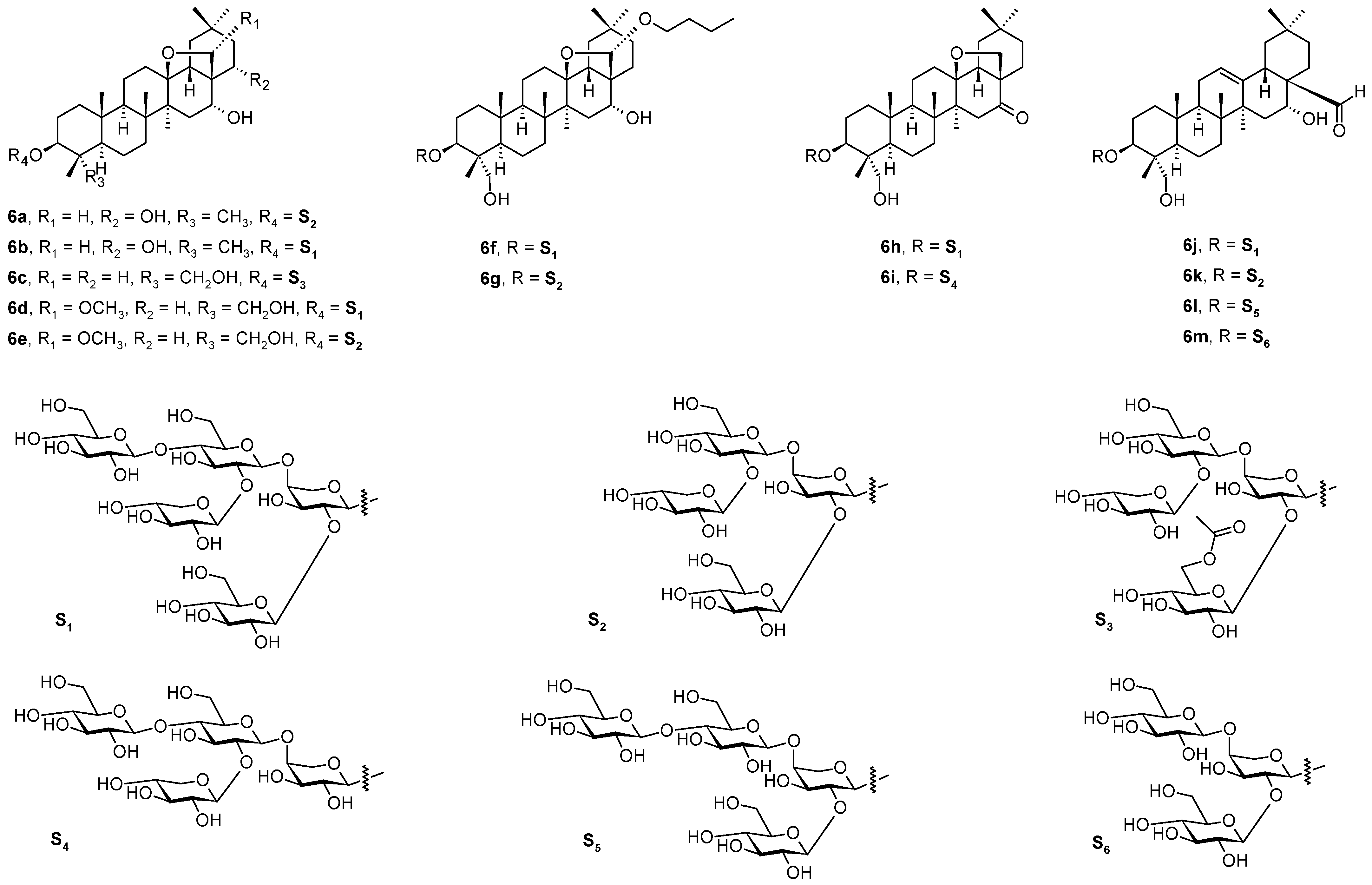
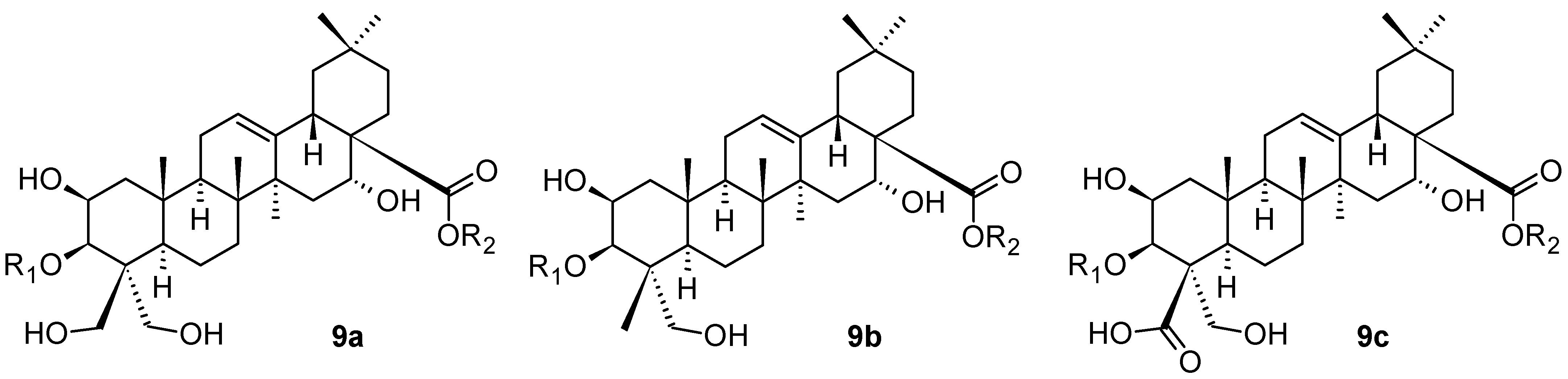
3. Triterpenoids with the Ursane Skeleton
4. Triterpenoids with the Lupane Skeleton
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gudoityte, E.; Arandarcikaite, O.; Mazeikiene, I.; Bendokas, V.; Liobikas, J. Ursolic and Oleanolic Acids: Plant metabolites with neuroprotective potential. Int. J. Mol. Sci. 2021, 22, 4599. [Google Scholar] [CrossRef] [PubMed]
- De Souza Miranda, R.; da Silva Mascarenhas de Jesus, B.; da Silva Luiz, S.R.; Viana, C.B.; Malafaia, C.R.A.; de Souza Figueiredo, F.; Carvalho, T.S.C.; Silva, M.L.; Londero, V.S.; da Costa-Silva, T.A.; et al. Antiinflammatory activity of natural triterpenes—An overview from 2006 to 2021. Phytother. Res. 2022, 36, 1459–1506. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, Z.; Wimmer, Z. Selected plant triterpenoids and their amide derivatives in cancer treatment: A review. Phytochemistry 2022, 203, 113340. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Van, D.; Deibert, L.; Bishop, G.; Balsevich, J. Antiproliferative quillaic acid and gypsogenin saponins from Saponaria officinalis L. roots. Phytochemistry 2015, 113, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Arslan, I. Quillaic acid–Containing saponin-based immunoadjuvants trigger early immune responses. Rev. Brasil. Farmacognosia 2020, 30, 467–473. [Google Scholar] [CrossRef]
- Mohammed, E.A.H.; Peng, Y.; Wang, Z.; Qiang, X.; Zhao, Q. Synthesis, antiviral, and antibacterial activity of the glycyrrhizic acid and glycyrrhetinic acid derivatives. Russ. J. Bioorg. Chem. 2022, 48, 906–918. [Google Scholar] [CrossRef]
- Tan, D.; Tseng, H.H.L.; Zhong, Z.; Wang, S.; Vong, C.T.; Wang, Y. Glycyrrhizic acid and its derivatives: Promising candidates for the management of type 2 diabetes mellitus and its complications. Int. J. Mol. Sci. 2022, 23, 10988. [Google Scholar] [CrossRef]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef]
- Wozniak, L.; Skapska, S.; Marszalek, K. Ursolic acid–A pentacyclic triterpenoid with a wide spectrum of pharmacological activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef]
- Muru, K.; Gauthier, C. Glycosylation and protecting group strategies towards the synthesis of saponins and bacterial oligosaccharides: A personal account. Chem. Rec. 2021, 21, 2990–3004. [Google Scholar] [CrossRef]
- Li, W.; Yu, B. Gold-catalyzed glycosylation in the synthesis of complex carbohydrate-containing natural products. Chem. Soc. Rev. 2018, 47, 7954–7984. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-H.; Dai, S.-Y.; Deng, F.-H.; Peng, L.-H.; Li, C.; Pei, Y.-H. Recent advances in medicinal chemistry of oleanolic acid derivatives. Phytochemistry 2022, 203, 113397. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.; Hull, J. The anti-cancer effect of Olea europaea L. products: A review. Curr. Nutr. Rep. 2021, 10, 99–124. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants-Rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: Preclinical and clinical evidence. Cancer Lett. 2014, 346, 206–216. [Google Scholar] [CrossRef]
- Patocka, J.; Bhardwaj, K.; Klimova, B.; Nepovimova, E.; Wu, Q.; Landi, M.; Kuca, K.; Valis, M.; Wu, W. Malus domestica: A review on nutritional features, chemical composition, traditional and medicinal value. Plants 2020, 9, 1408. [Google Scholar] [CrossRef]
- Raal, A. Birch (Betula spp.). In Drug Plants II, Recent Progress in Medicinal Plants; Awaad, A.S., Singh, V.K., Govil, J.N., Eds.; Studium Press/NHBS: Totnes, UK, 2010; Volume 28, pp. 121–142. [Google Scholar]
- Cichewicz, R.H.; Kouzi, S.A. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med. Res. Rev. 2004, 24, 90–114. [Google Scholar] [CrossRef]
- Bag, B.G.; Majumdar, R. Self-assembly of renewable nano-sized triterpenoids. Chem. Rec. 2017, 17, 841–873. [Google Scholar] [CrossRef]
- Bag, B.G.; Hasan, S.N.; Ghorai, S.; Panja, S.K. First self-assembly of dihydroxy triterpenoid maslinic acid yielding vesicles. ACS Omega 2019, 4, 7684–7690. [Google Scholar] [CrossRef]
- Bag, B.G.; Garai, C.; Ghorai, S. Vesicular self-assembly of a natural ursane-type dihydroxy-triterpenoid corosolic acid. RSC Adv. 2019, 9, 15190–15195. [Google Scholar] [CrossRef]
- Bag, B.G.; Barai, A.C.; Hasan, S.N.; Panja, S.K.; Ghorai, S.; Patra, S. Terpenoids, nano-entities and molecular self-assembly. Pure Appl. Chem. 2020, 92, 567–577. [Google Scholar] [CrossRef]
- Wimmerová, M.; Siglerová, V.; Šaman, D.; Šlouf, M.; Kaletová, E.; Wimmer, Z. Improved enzyme-mediated synthesis and supramolecular self-assembly of naturally occurring conjugates of β-sitosterol. Steroids 2017, 117, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Bildziukevich, U.; Kaletová, E.; Šaman, D.; Sievänen, E.; Kolehmainen, E.T.; Šlouf, M.; Wimmer, Z. Spectral and microscopic study of self-assembly of novel cationic spermine amides of betulinic acid. Steroids 2017, 117, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Bildziukevich, U.; Malík, M.; Özdemir, Z.; Rárová, L.; Janovská, L.; Šlouf, M.; Šaman, D.; Šarek, J.; Nonappa; Wimmer, Z. Spermine amides of selected triterpenoid acids: Dynamic supramolecular system formation influences the cytotoxicity of the drugs. J. Mater. Chem. B 2020, 8, 484–491. [Google Scholar] [CrossRef]
- Özdemir, Z.; Šaman, D.; Bednárová, L.; Pazderková, M.; Janovská, L.; Nonappa; Wimmer, Z. Aging-induced structural transition of nanoscale oleanolic acid amphiphiles and selectivity against Gram-positive bacteria. ACS Appl. Nano Mater. 2022, 5, 3799–3810. [Google Scholar] [CrossRef]
- Bildziukevich, U.; Özdemir, Z.; Šaman, D.; Vlk, M.; Šlouf, M.; Rárová, L.; Wimmer, Z. Novel cytotoxic 1,10-phenanthroline–triterpenoid amphiphiles with supramolecular characteristics capable of coordinating 64Cu(II) labels. Org. Biomol. Chem. 2022, 20, 8157–8163. [Google Scholar] [CrossRef]
- Özdemir, Z.; Nonappa; Wimmer, Z. Triterpenoid building blocks for functional nanoscale assemblies: A review. ACS Appl. Nano Mater. 2022, 5, 16264–16277. [Google Scholar] [CrossRef]
- Ramos-Soriano, J.; Ghirardello, M.; Galan, M.C. Recent advances in multivalent carbon nanoform-based glycoconjugates. Curr. Med. Chem. 2022, 29, 1232–1257. [Google Scholar] [CrossRef]
- Schijns, V.; Majhen, D.; van der Ley, P.; Thakur, A.; Summerfield, A.; Berisio, R.; Nativi, C.; Fernandez-Tejada, A.; Alvarez-Dominguez, C.; Gizurarson, S.; et al. Rational vaccine design in times of emerging diseases: The critical choices of immunological correlates of protection, vaccine antigen and immunomodulation. Pharmaceutics 2021, 13, 501. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 1–13. [Google Scholar] [CrossRef]
- Mioc, M.; Milan, A.; Malita, D.; Mioc, A.; Prodea, A.; Rakoviceanu, R.; Ghiulai, R.; Cristea, A.; Caruntu, F.; Soica, C. Recent advances regarding the molecular mechanisms of triterpenic acids: A review (part I). Int. J. Mol. Sci. 2022, 23, 7740. [Google Scholar] [CrossRef] [PubMed]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Juang, Y.-P.; Liang, P.-H. Biological and pharmacological effects of synthetic saponins. Molecules 2020, 25, 4974. [Google Scholar] [CrossRef] [PubMed]
- Thu, H.N.T.; Huong, D.N.H.; Dieu, T.N.T.; Ngoc, H.T.T.; Van, H.P.; Ngoc, A.H.T.; Xuan, H.N.; Pham, N.K.; Manh, C.N.; Toan, P.N.H. In vitro and in silico cytotoxic activities of triterpenoids from the leaves of Aralia dasyphylla Miq. and the assessment of their ADMET properties. J. Biomol. Struct. Dyn. 2022. [Google Scholar] [CrossRef]
- Nguyen, H.D. Two new triterpenoid saponins from the underground parts of Weigela x “Bristol Ruby”. J. Asian Nat. Prod. Res. 2022. [Google Scholar] [CrossRef]
- Rezgui, A.; Mitaine-Offer, A.C.; Miyamoto, T.; Tanaka, C.; Delemasure, S.; Dutartre, P.; Lacaille-Dubois, M.A. Oleanolic acid and hederagenin glycosides from Weigela stelzneri. Phytochemistry 2016, 123, 40–47. [Google Scholar] [CrossRef]
- Lim, H.J.; Jie, E.Y.; Park, I.S.; Kim, S.J.; Ahn, W.S.; Jeong, S.I.; Kim, S.W.; Jung, C.H. Anti-inflammatory effects of Weigela subsessilis callus extract via suppression of MAPK and NF-κB signaling. Plants 2021, 10, 1635. [Google Scholar] [CrossRef]
- Thuong, P.T.; Min, B.-S.; Jin, W.; Na, M.; Lee, J.; Seong, R.; Lee, Y.-M.; Song, K.; Seong, Y.; Lee, H.-K.; et al. Anti-complementary activity of ursane-type triterpenoids from Weigela subsessilis. Biol. Pharm. Bull. 2006, 29, 830–833. [Google Scholar] [CrossRef]
- Won, Y.M.; Seong, Z.K.; Kim, J.L.; Kim, H.S.; Song, H.H.; Kim, D.Y.; Kim, J.H.; Oh, S.R.; Cho, H.W.; Cho, J.H.; et al. Triterpene glycosides with stimulatory activity on melanogenesis from the aerial parts of Weigela subsessilis. Arch. Pharm. Res. 2015, 38, 1541–1551. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Mitaine-Offer, A.C.; Miyamoto, T.; Tanaka, C.; Bellaye, P.S.; Collin, B.; Chambin, O.; Lacaille-Dubois, M.A. Phytochemical analysis of two Weigela florida cultivars, “Pink Poppet” and “Jean’s Gold”. Phytochem. Lett. 2020, 37, 85–89. [Google Scholar] [CrossRef]
- Andriamisaina, N.; Mitaine-Offer, A.C.; Pruvot, B.; Chluba, J.; Miyamoto, T.; Tanaka, C.; Lacaille-Dubois, M.A. Phytochemistry of Weigela x “kosteriana variegata” (Caprifoliaceae). Nat. Prod. Commun. 2018, 13, 403–406. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Mitaine-Offer, A.C.; Maroso, S.; Papini, A.M.; Paululat, T.; Bellaye, P.S.; Collin, B.; Chambin, O.; Lacaille-Dubois, M.A. Cytotoxic glycosides from the roots of Weigela x “Bristol Ruby”. Fitoterapia 2019, 137, 104242. [Google Scholar] [CrossRef] [PubMed]
- Champy-Tixier, A.S.; Mitaine-Offer, A.C.; Fernandez, F.R.; Miyamoto, T.; Tanaka, C.; Papini, A.M.; Lacaille-Dubois, M.A. Oleanane-type glycosides from the roots of Weigela florida “rumba” and evaluation of their antibody recognition. Fitoterapia 2018, 128, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Hobloss, S.; Bruguiere, A.; Champy-Tixier, A.-S.; Miyamoto, T.; Tanaka, C.; Dessertaine, S.; Sautour, M.; Lacaille-Dubois, M.-A.; Mitaine-Offer, A.-C. Oleanane-type glycosides from Weigela x Styriaca and two cultivars of W. florida: “Minor black” and “Brigela”. Phytochemistry Lett. 2022, 50, 77–84. [Google Scholar] [CrossRef]
- Aouane, C.; Kabouche, A.; Voutquenne-Nazabadioko, L.; Sayagh, C.; Martinez, A.; Magid, A.A.; Kabouche, Z. Triterpenoid saponins from Anagallis monelli ssp. linifolia (L.) Maire and their chemotaxonomic significance. Phytochemistry 2022, 202, 113305. [Google Scholar] [PubMed]
- Zulkifli, S.Z.; Ab Ghani, N.; Rasol, N.E.; Salleh, W.M.N.H.W.; Ismail, N.H. Lepiginosides A-D: Three new triterpenoid saponins and a new farnesyl glycoside from the stembarks of Lepisanthes rubiginosa (roxb.) Leenh. Nat. Prod. Res. 2022. [Google Scholar] [CrossRef]
- Tran, L.V.; Thi, N.; Thi, L.; Van Tran, C.; Vo, N.T.Q.; Ho, A.N.; Tran, T.T.P. Two new glycosides, farnesyl pentaglycoside and oleanane triglycoside from Lepisanthes rubiginosa, a mangrove plant collected from Thua Thien-Hue province, Vietnam. Nat. Prod. Res. 2022, 36, 1774–1780. [Google Scholar] [CrossRef]
- Hasan, M.M.; Hossain, A.; Shamim, A.; Rahmam, M.M. Phytochemical and pharmacological evaluation of ethanolic extract of Lepisanthes rubiginosa leaves. BMC Complement. Altern. Med. 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Yang, Y.; Mouri, A.; Lu, Q.; Kunisawa, K.; Kubota, H.; Hasegawa, M.; Hirakawa, M.; Mori, Y.; Libo, Z.; Saito, K.; et al. Loureirin C and xanthoceraside prevent abnormal behaviors associated with downregulation of brain derived neurotrophic factor and AKT/mTOR/CREB signaling in the prefrontal cortex induced by chronic corticosterone exposure in mice. Neurochem. Res. 2022, 47, 2865–2879. [Google Scholar] [CrossRef]
- Pereira, G.C.; Roversi, K.; Trevisan, G.; Burger, M.E.; Bochi, G.V. Glucocorticoid and brain-derived neurotrophic factor relationship: A brief investigation into the model of depression by chronic administration of corticosterone. Behav. Pharmacol. 2020, 31, 407–412. [Google Scholar] [CrossRef]
- Yau, L.-F.; Huang, H.; Tong, T.-T.; Bai, L.-B.; Zhu, G.-Y.; Hou, Y.; Bai, G.; Jiang, Z.-H. Characterization of deglycosylated metabolites of platycosides reveals their biotransformation after oral administration. Food Chem. 2022, 393, 133383. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Y.; Ren, G.; Jiang, D.; Liu, C. Functional characterization and substrate promiscuity analysis of UDP-glucose dehydrogenases from licorice (Glycyrrhiza uralensis). J. Mol. Struct. 2022, 1265, 133355. [Google Scholar] [CrossRef]
- Qiao, X.; Wang, Q.; Wang, S.; Kuang, Y.; Li, K.; Song, W.; Ye, M. A 42-markers pharmacokinetic study reveals interactions of berberine and glycyrrhizic acid in the anti-diabetic Chinese medicine formula Gegen-Qinlian decoction. Front. Pharmacol. 2018, 9, 622. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, L.; Shan, L.; Fan, G.; Gao, X. Liquorice, a unique guide drug of traditional Chinese medicine: A review of its role in drug interactions. J. Ethnopharmacol. 2013, 150, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.C.; Wu, C.H.; Yen, G.C. Bioactivity and potential health benefits of licorice. J. Agric. Food Chem. 2014, 62, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.Z.; Wang, B.; Liang, Y.K.; Bao, Y.Y.; Gu, Y. Hepatoprotective and Antioxidant effects of licorice extract against Ccl (4)-induced oxidative damage in rats. Int. J. Mol. Sci. 2011, 12, 6529–6543. [Google Scholar] [CrossRef] [PubMed]
- Simayi, Z.; Rozi, P.; Yang, X.; Ababaikeri, G.; Maimaitituoheti, W.; Bao, X.; Yadikar, N. Isolation, structural characterization, biological activity, and application of Glycyrrhiza polysaccharides: Systematic review. Int. J. Biol. Macromol. 2021, 183, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, R.; Yuan, B.; Liu, Y.; Liu, C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B 2015, 5, 310–315. [Google Scholar] [CrossRef]
- Wang, Z.F.; Liu, J.; Yang, Y.A.; Zhu, H.L. A review: The anti-inflammatory, anticancer and antibacterial properties of four kinds of licorice flavonoids isolated from licorice. Curr. Med. Chem. 2020, 27, 1997–2011. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Herrera-Bravo, J.; Belen, L.H.; Kaur, R.; Kregiel, D.; Suleria, H.A.R. Glycyrrhiza genus: Enlightening phytochemical components for pharmacological and health-promoting abilities. Oxid. Med. Cell. Longev. 2021, 7571132. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, J.H.; Yang, W.K.; Geum, J.H.; Kim, H.R.; Choi, S.Y.; Lee, Y.C. Herbal combinational medication of Glycyrrhiza glabra, Agastache rugosa containing glycyrrhizic acid, tilianin inhibits neutrophilic lung inflammation by affecting Cxcl2, interleukin-17/Stat3 signal pathways in a murine model of COPD. Nutrients 2020, 12, 926. [Google Scholar] [CrossRef] [PubMed]
- Rani, K.; Devi, N.; Saharan, V.; Kharb, P. Glycyrrhiza glabra: An insight to nanomedicine. J. Nanosci. Nanotechnol. 2021, 21, 3367–3378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Huang, H.Z.; Qiu, M.; Wu, Z.F.; Xin, Z.C.; Cai, X.F.; Han, L. Traditional uses, pharmacological effects, and molecular mechanisms of licorice in potential therapy of Covid-19. Front. Pharmacol. 2021, 12, 719758. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yuan, B.C.; Ma, Y.S.; Zhou, S.; Liu, Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 2017, 55, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Si, L.; Ji, S.; Wang, H.; Fang, X.M.; Yu, L.Y.; Li, R.Y.; Liang, L.N.; Zhou, D.; Ye, M. Uralsaponins M-Y, Antiviral triterpenoid saponins from the roots of Glycyrrhiza uralensis. J. Nat. Prod. 2014, 77, 1632–1643. [Google Scholar] [CrossRef]
- Marciani, D.J. Effects of N-acylation on the immune adjuvanticity of analogs of the Quillaja saponins derivative GPI-0100. Vaccine 2022, 40, 4169–4173. [Google Scholar] [CrossRef]
- Fuentes, R.; Aguinagalde, L.; Sacristan, N.; Fernandez-Tejada, A. Design, synthesis, and initial immunological evaluation of glycoconjugates based on saponin adjuvants and the Tn antigen. Chem. Commun. 2021, 57, 11382–11385. [Google Scholar] [CrossRef]
- Oda, K.; Matsuda, H.; Murakami, T.; Katayama, S.; Ohgitani, T.; Yoshikawa, M. Relationship between adjuvant activity and amphipathic structure of soyasaponins. Vaccine 2003, 21, 2145–2151. [Google Scholar] [CrossRef]
- Su, X.-D.; Jang, H.-J.; Wang, C.-Y.; Lee, S.W.; Rho, M.-C.; Kim, Y.H.; Yang, S.Y. Anti-inflammatory potential of saponins from Aster tataricus via NF-κB/MAPK activation. J. Nat. Prod. 2019, 82, 1139–1148. [Google Scholar] [CrossRef]
- Bera, M.; Mukhopadhyay, B. Chemical synthesis of the pentasaccharide related to the anti-inflammatory oleanane type saponins isolated from medicinal plant Aster tataricus L. f. Carbohydr. Res. 2022, 516, 108563. [Google Scholar] [CrossRef]
- Li, H.; Cheng, C.; Shi, S.; Wu, Y.; Gao, Y.; Liu, Z.; Liu, M.; Li, Z.; Huo, L.; Pan, X.; et al. Identification, optimization, and biological evaluation of 3-O-β-chacotriosyl ursolic acid derivatives as novel SARS-CoV-2 entry inhibitors by targeting the prefusion state of spike protein. Eur. J. Med. Chem. 2022, 238, 114426. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cao, D.; Jiang, S.; Liu, X.; Pan, W.; Cui, H.; Yang, W.; Liu, Z.; Jin, J.; Zhao, Z. Triterpenoid saponins from Ilex pubescens promote blood circulation in blood stasis syndrome by regulating sphingolipid metabolism and the PI3K/AKT/eNOS signaling pathway. Phytomedicine 2022, 104, 154242. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Acankoreagenin and acankoreosides, a family of lupane triterpenoids with anti-inflammatory properties: An overview. Ann. N. Y. Acad. Sci. 2021, 1502, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Tsepaeva, O.V.; Nemtarev, A.V.; Kundina, A.V.; Grigoreva, L.B.; Mironov, V.F. Synthesis of novel mannopyranosyl betulinic acid phosphoniohexyl ester. Mendeleev Commun. 2021, 31, 110–112. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, T.; Yuan, H.; Li, D.; Lou, H.; Fan, P. Mitochondria-targeted lupane triterpenoid derivatives and their selective apoptosis-inducing anticancer mechanisms. J. Med. Chem. 2017, 60, 6353–6363. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, C.; Legault, J.; Lebrun, M.; Dufour, P.; Pichette, A. Glycosidation of lupane-type triterpenoids as potent in vitro cytotoxic agents. Bioorg. Med. Chem. 2006, 14, 6713–6725. [Google Scholar] [CrossRef]
- Smith, P.F.; Ogundele, A.; Forrest, A.; Wilton, J.; Salzwedel, K.; Doto, J.; Allaway, G.P.; Martin, D.E. Phase I and II study of safety, virologic effect, and pharmacokinetics/pharmacodynamics of single-dose 3-O-(3’,3’-dimethylsuccinyl) betulinic acid (bevirimat) against human immunodeficiency virus infection. Antimicrob. Agents Chemother. 2007, 51, 3574–3581. [Google Scholar] [CrossRef]
- Margot, N.A.; Gibbs, C.S.; Miller, M.D. Phenotypic susceptibility to bevirimat in isolates from HIV-1-infected patients without prior exposure to bevirimat. Antimicrob. Agents Chemother. 2010, 54, 2345–2353. [Google Scholar] [CrossRef]
- Özdemir, Z.; Yang, M.; Kim, G.; Bildziukevich, U.; Šaman, D.; Li, X.; Yoon, J.; Wimmer, Z. Redox-responsive nanoparticles self-assembled from porphyrin-betulinic acid conjugates for chemo- and photodynamic therapy. Dyes Pigments 2021, 190, 109307. [Google Scholar] [CrossRef]
- Yang, M.; Özdemir, Z.; Kim, H.; Nah, S.; Andris, E.; Li, X.; Wimmer, Z.; Yoon, J. Acid-responsive nanoporphyrin evolution for near-infrared fluorescence-guided photo-ablation of biofilm. Adv. Healthc. Mater. 2022, 11, 2200529. [Google Scholar] [CrossRef]

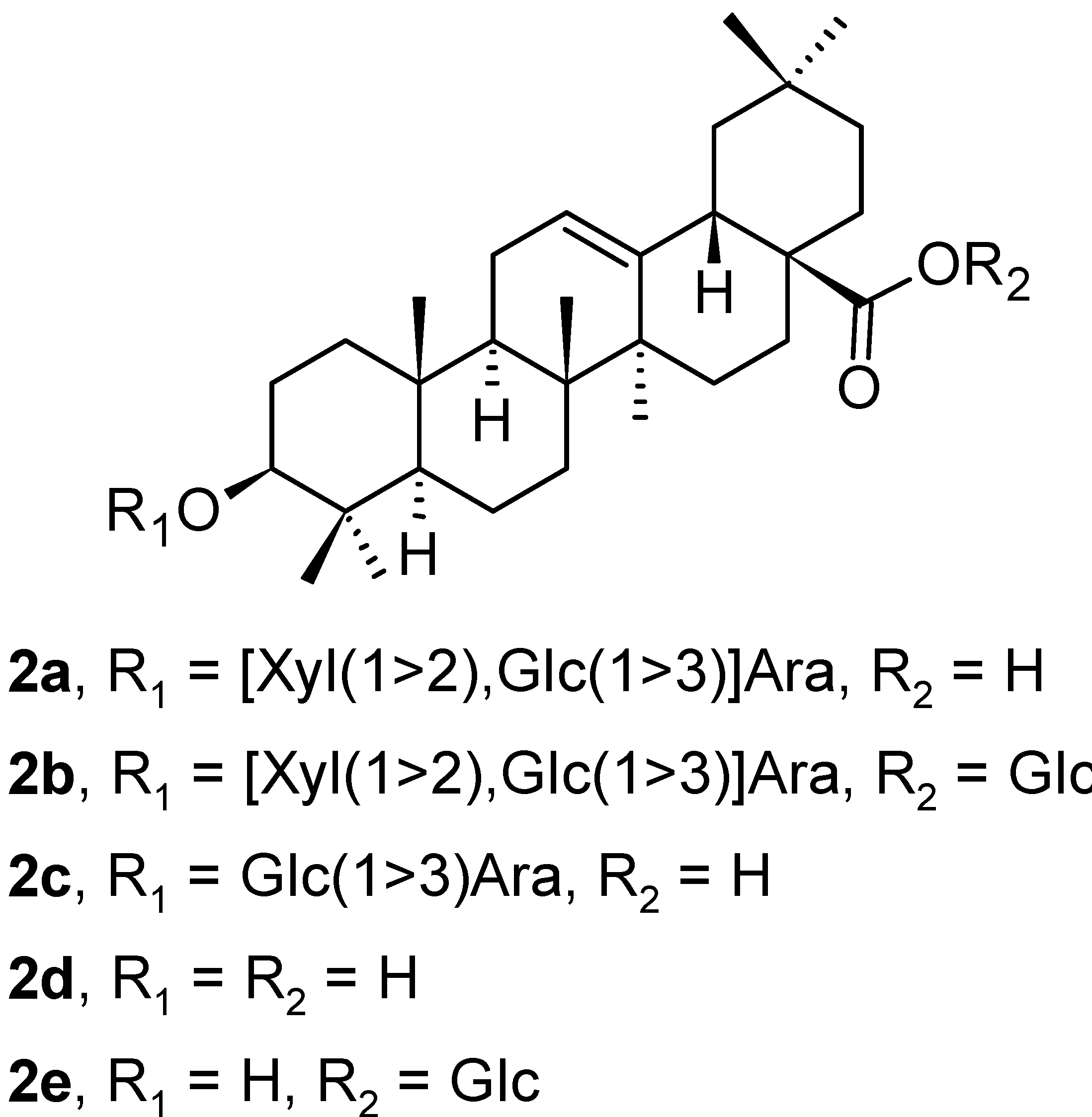

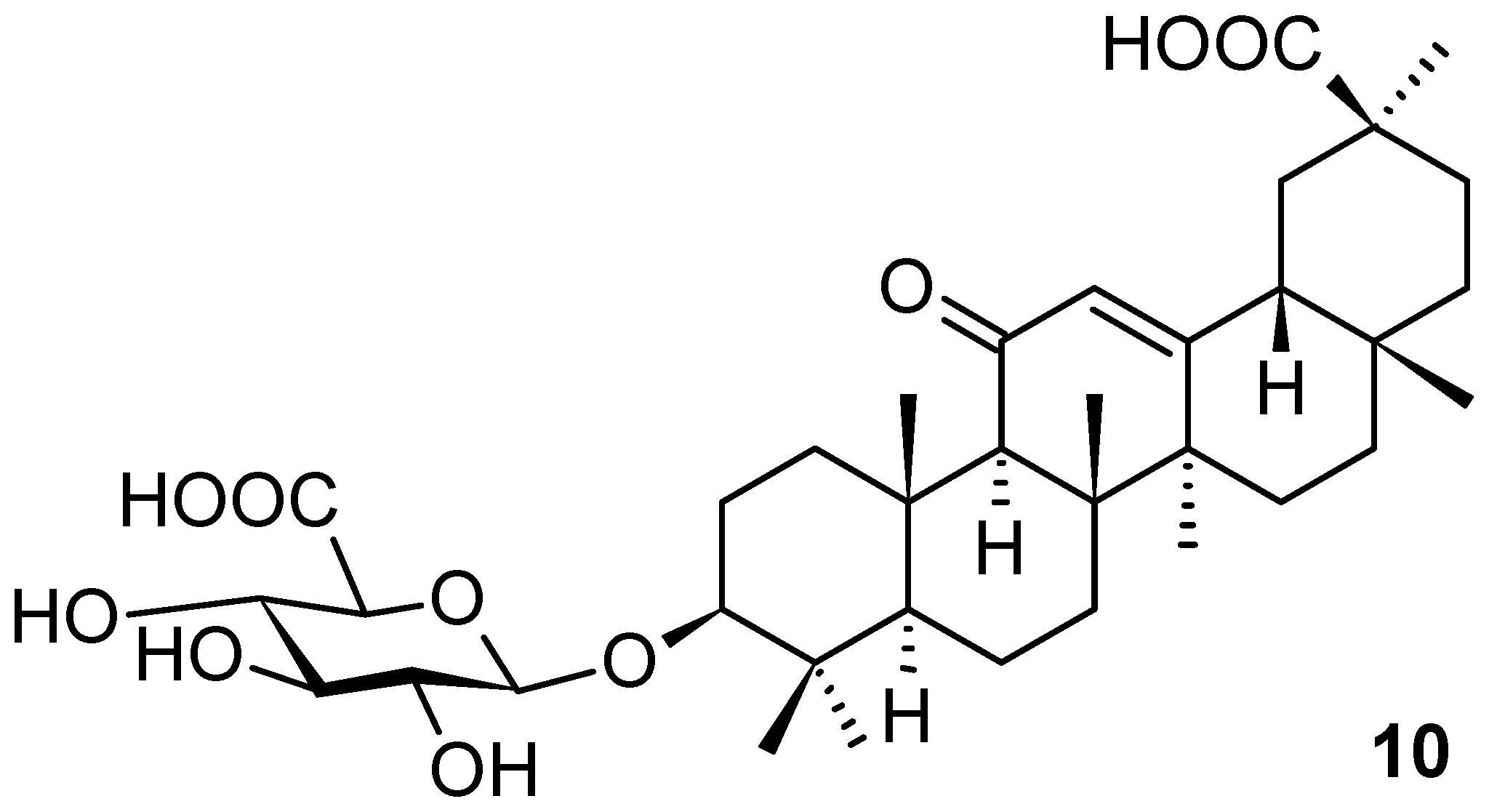
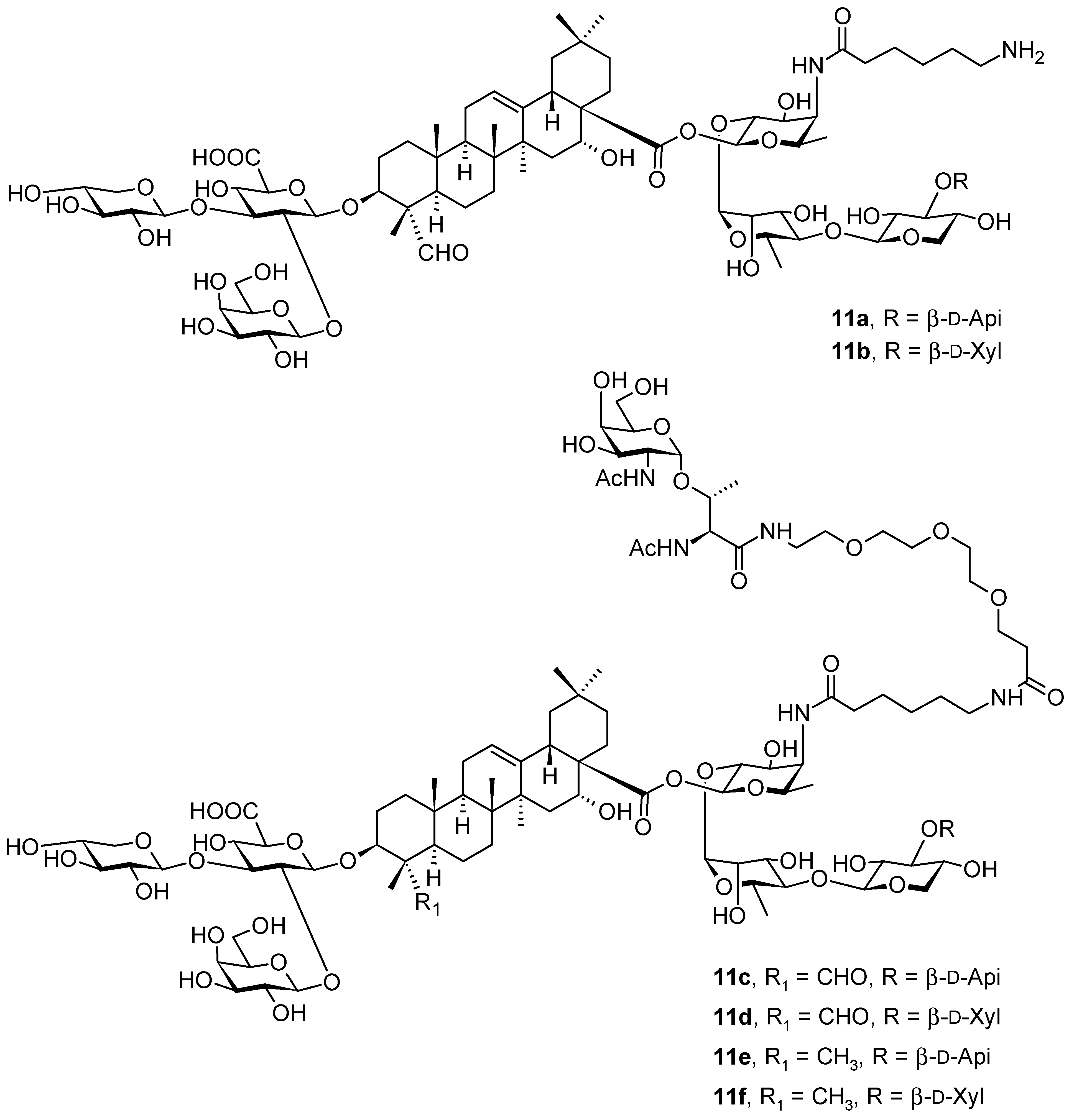
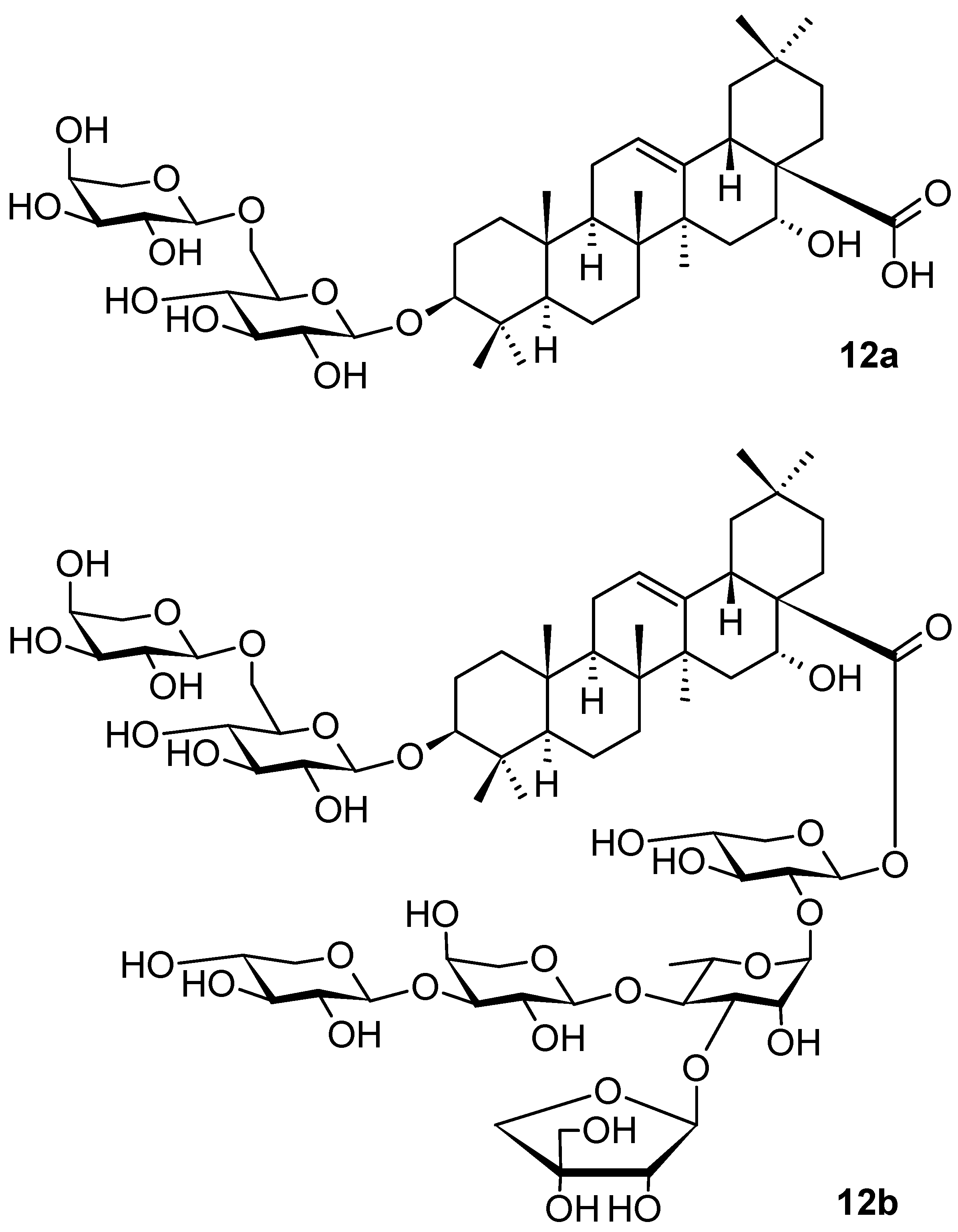
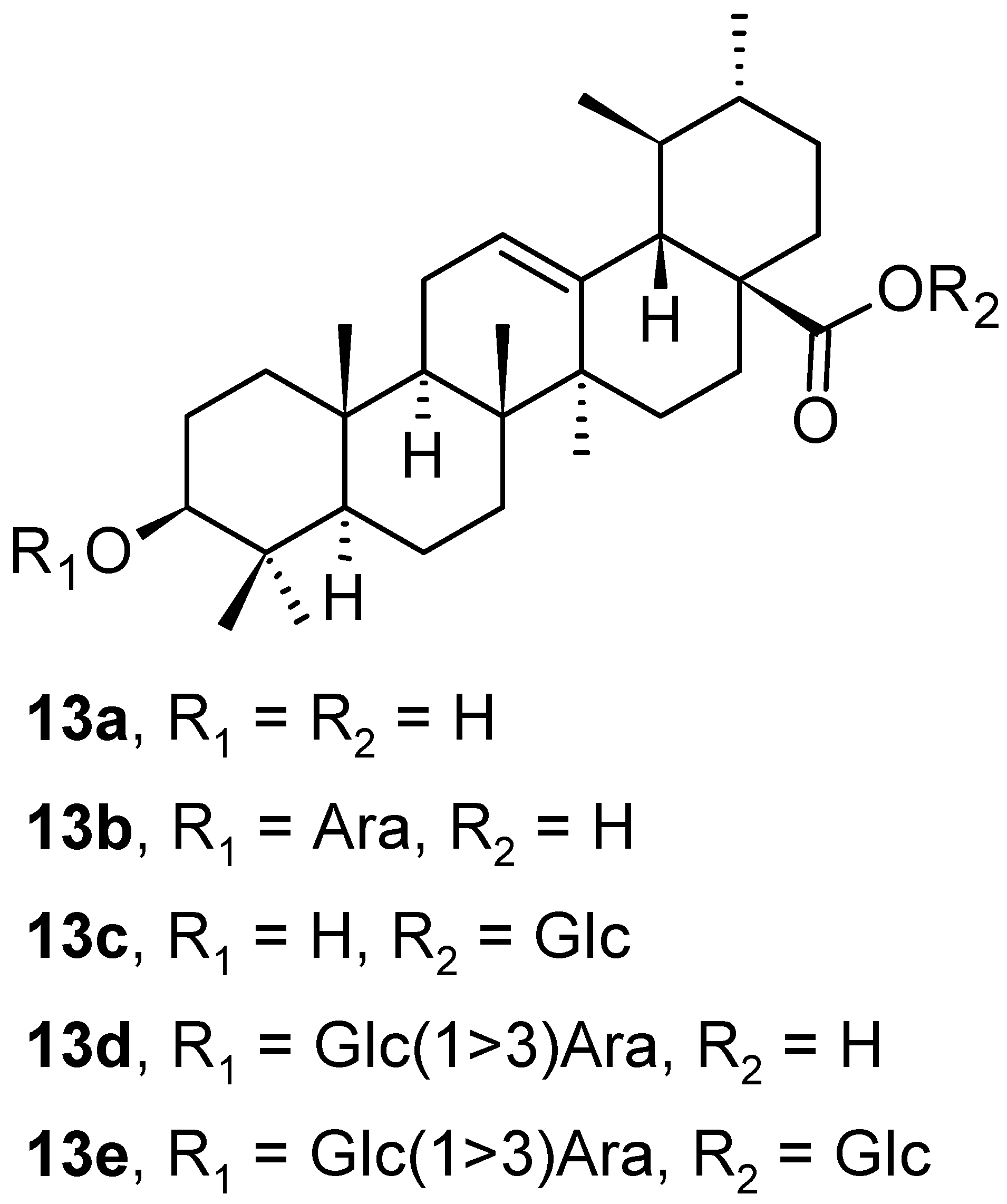
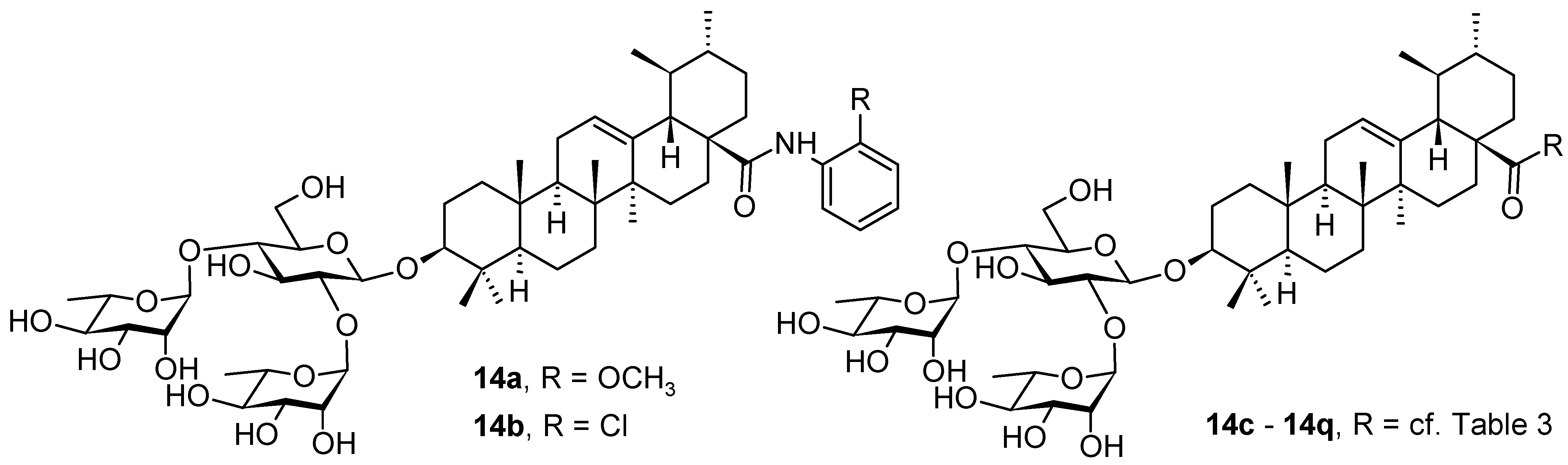
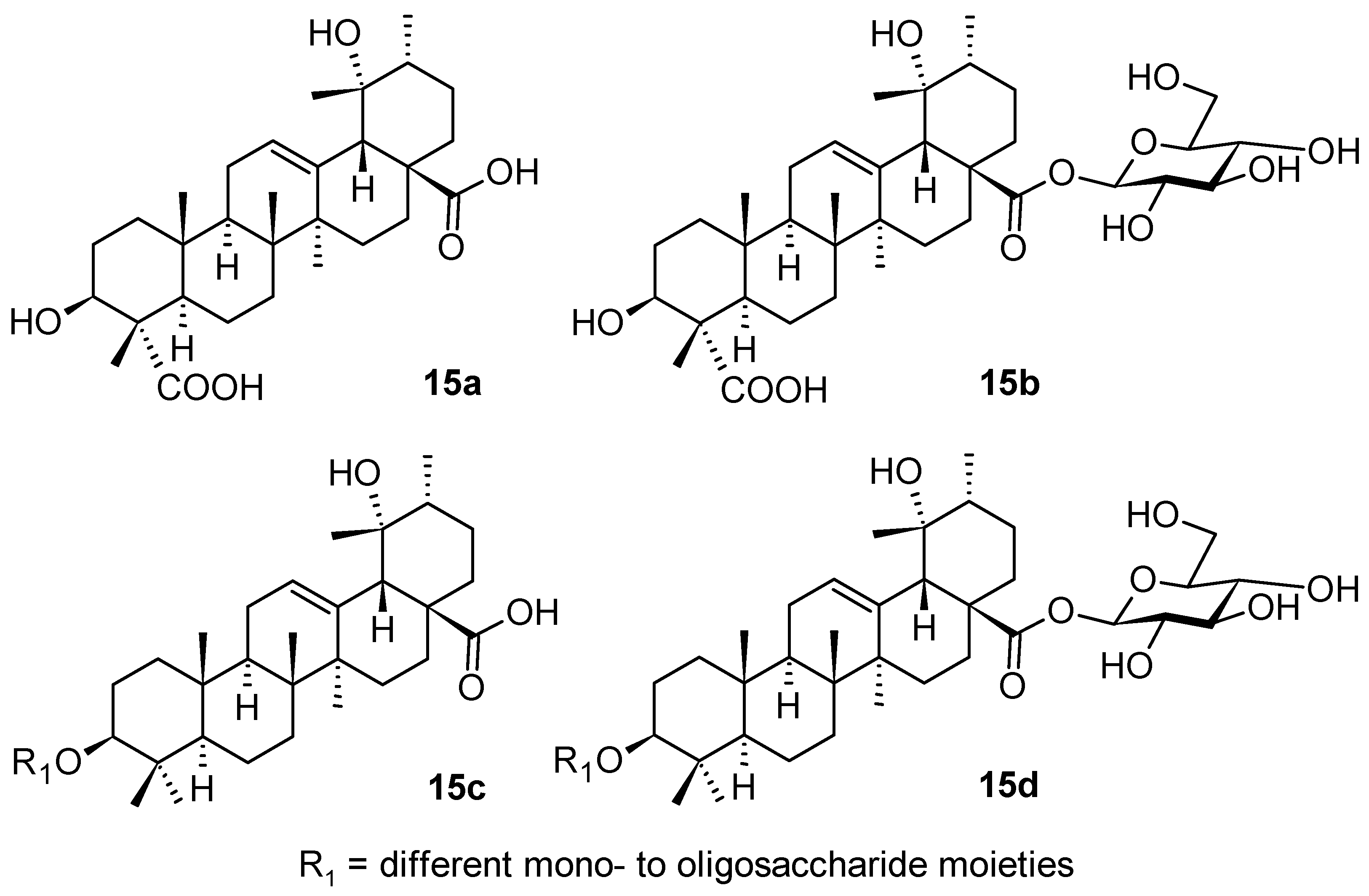
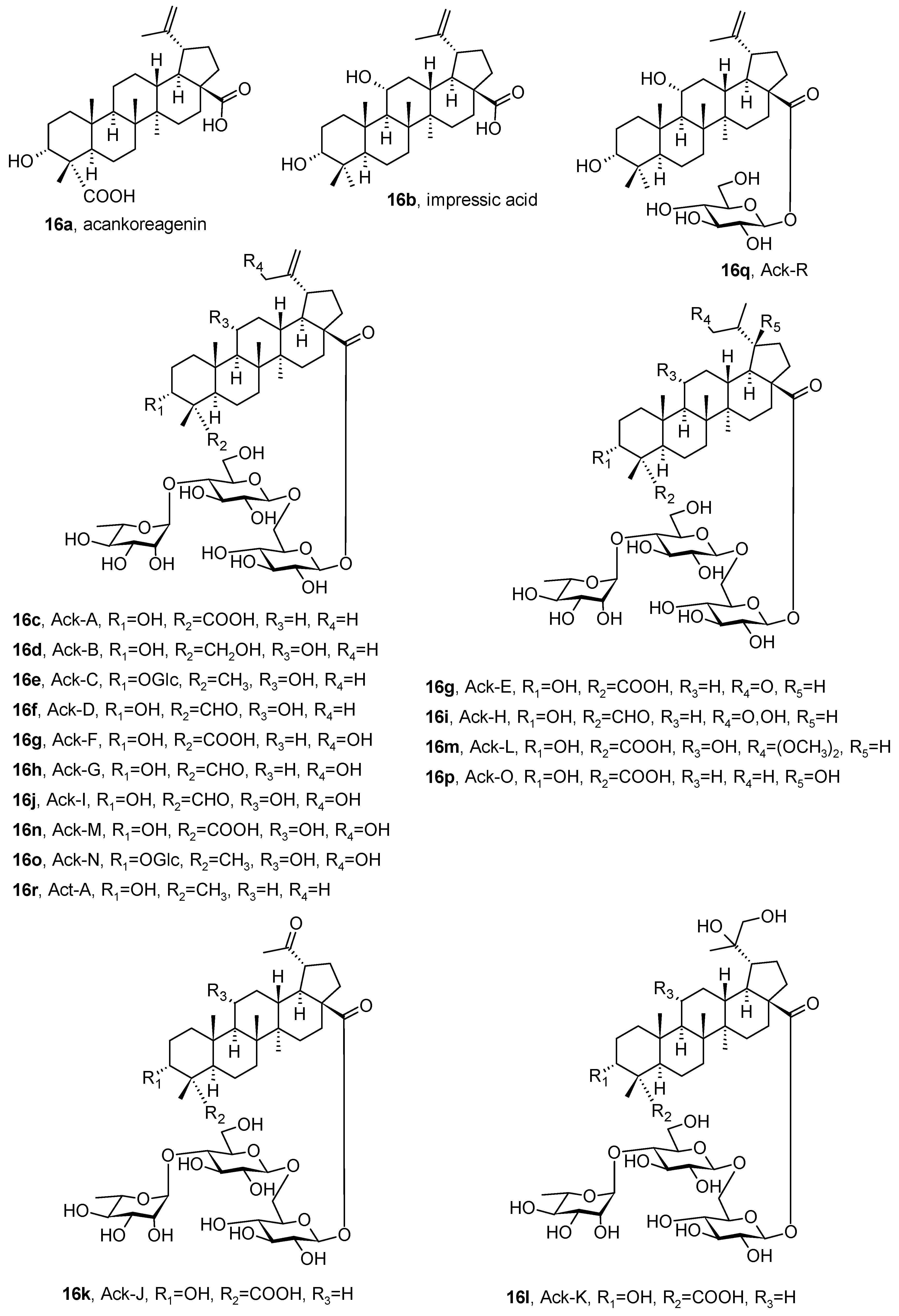
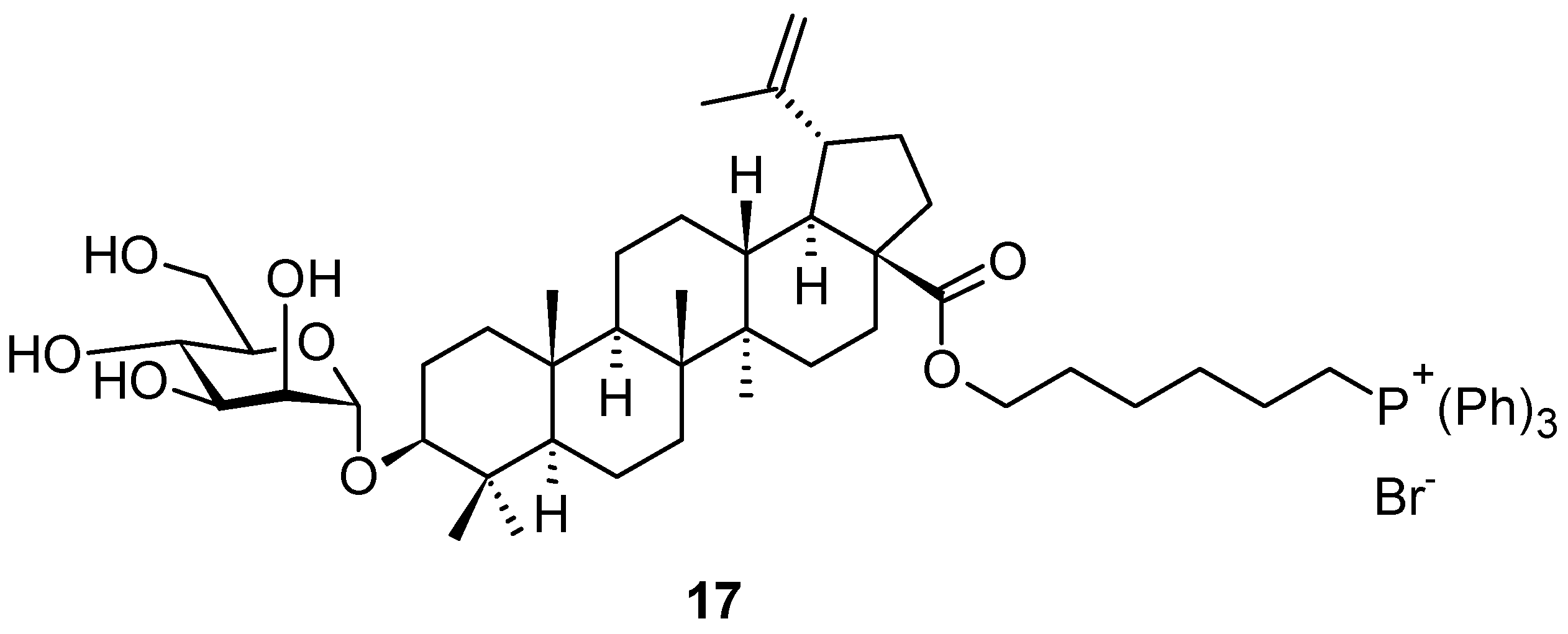
| Compound | CS Values [%] (Mean ± SD) a | IC50 [μM] (Mean ± SD) b | ||||
|---|---|---|---|---|---|---|
| HepG2 c | LU-1 d | RD e | HepG2 c | LU-1 d | RD e | |
| 2a | 0 | 18.51 ± 1.20 | 67.13 ± 2.17 | 3.24 ± 0.22 | 2.55 ± 0.12 | >100 ± 1.45 |
| 2b | 97.01 ± 0.90 | 76.47 ± 2.00 | 95.57 ± 1.90 | >100 ± 1.32 | >100 ± 0.97 | >100 ± 0.58 |
| 2c | 5.98 ± 0.39 | 0 | 0 | 2.73 ± 0.12 | 1.76 ± 0.11 | 2.63 ± 0.10 |
| 2d | 43.98 ± 1.46 | 25.21 ± 1.34 | 72.82 ± 1.26 | 7.21 ± 0.57 | 4.56 ± 0.24 | >100 ± 1.28 |
| 2e | 60.88 ± 1.80 | 67.52 ± 2.33 | 65.52 ± 1.54 | >100 ± 2.10 | >100 ± 1.43 | >100 ± 1.45 |
| ellipticine f | 1.25 ± 0.30 | 1.87 ± 0.20 | 0 | 1.22 ± 0.09 | 1.30 ± 0.10 | 1.18 ± 0.08 |
| Compound | CS Values [%] (Mean ± SD) a | IC50 [μM] (Mean ± SD) b | ||||
|---|---|---|---|---|---|---|
| HepG2 c | LU-1 d | RD e | HepG2 c | LU-1 d | RD e | |
| 13a | 68.42 ± 0.96 | 29.61 ± 0.15 | 66.79 ± 1.51 | >100 ± 1.45 | 7.04 ± 0.64 | >100 ± 1.16 |
| 13b | 45.98 ± 1.45 | 25.11 ± 1.54 | 72.81 ± 1.56 | 7.21 ± 0.60 | 4.56 ± 0.18 | >100 ± 0.65 |
| 13c | 59.88 ± 1.80 | 65.52 ± 2.53 | 64.52 ± 1.34 | >100 ± 1.80 | >100 ± 0.84 | >100 ± 0.35 |
| 13d | 37.20 ± 2.30 | 15.12 ± 0.60 | 70.00 ± 2.19 | 5.36 ± 0.47 | 2.85 ± 0.20 | >100 ± 1.42 |
| 13e | 98.28 ± 0.95 | 78.70 ± 1.15 | 98.42 ± 1.47 | >100 ± 1.36 | >100 ± 1.34 | >100 ± 1.89 |
| ellipticine f | 1.25 ± 0.30 | 1.87 ± 0.20 | 0 | 1.22 ± 0.09 | 1.30 ± 0.10 | 1.18 ± 0.08 |
| Compound | R | Inhibition Rate [%] | |
|---|---|---|---|
| c = 10 μM | c = 40 μM | ||
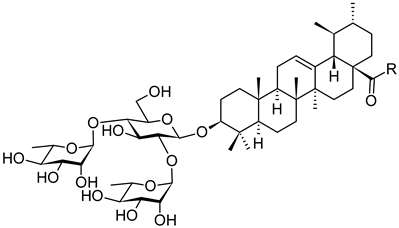 | |||
| 14c |  | toxic a | toxic a |
| 14d | 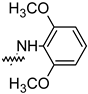 | 96.84 ± 5.40 | 46.81 ± 3.55 |
| 14e |  | 30.45 ± 2.13 | −2.49 ± 0.24 |
| 14f | 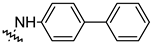 | 27.44 ± 4.11 | 16.94 ± 2.35 |
| 14g | 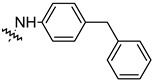 | 19.25 ± 1.03 | 10.32 ± 2.10 |
| 14h | 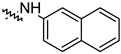 | 14.51 ± 1.06 | −7.27 ± 1.03 |
| 14i | 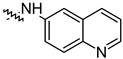 | 33.08 ± 1.02 | 27.35 ± 2.51 |
| 14j |  | −21.66 ± 1.20 | −31.75 ± 4.32 |
| 14k | 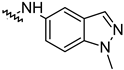 | 51.87 ± 2.23 | 41.08 ± 1.89 |
| 14l | 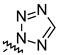 | toxic a | toxic a |
| 14m | 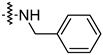 | 92.49 ± 3.78 | 59.23 ± 4.21 |
| 14n | 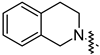 | 58.74 ± 5.13 | 32.52 ± 2.59 |
| 14o | 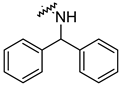 | 1.58 ± 0.34 | 0.47 ± 0.08 |
| 14p |  | 85.69 ± 1.33 | 36.26 ± 2.45 |
| 14q |  | 49.47 ± 3.08 | 28.16 ± 2.22 |
| salvianolic acid C b | 98.30 ± 2.62 | 80.25 ± 2.56 | |
| Plant [Reference] | Saponin Found | Type of Pharmacological Application |
|---|---|---|
| Aralia dasyphylla Miq. (Araliaceae) [35] | Oleanane-type | Cytotoxicity |
| Weigela Thunb. (Caprifoliaceae) [36,45] | Oleanane-type | Cytotoxicity, antifungal activity, antibacterial activity |
| Anagallis monelli ssp. linifolia (L.) Maire (Primulaceae) [46] | Oleanane-type: monellosides | Cytotoxicity, antibacterial, antifungal, antioxidant, antihyperglycemic and antipruritic activity |
| Lepisanthes rubiginosa Roxb. (Sapindaceae) [47] | Oleanane-type | Antibacterial activity |
| Xanthoceras sorbifolium Bunge (Sapindaceae) [50] | Oleanane-type: xanthoceraside | Major depressive disorders treatment |
| Platycodon grandiflorus Jacq. (Campanulaceae) [52] | Oleanane-type: platycosides | Dietary supplements absorbed into the bloodstream |
| Glycyrrhiza uralensis Fisch. (Fabaceae) [53] a | Oleanane-type: glycyrrhizic acid, glycyrrhetinic acid glucuronide | Anti-inflammatory activity, liver protection, immune regulation, antiviral activity, anticancer activity |
| Quillaja saponaria Molina (Quillajaceae) [67,68] | Oleanane-type | Saponin adjuvants, carbohydrate antigen |
| Aster tataricus L. f. (Asteraceae) [70,71] | Oleanane-type | Anti-inflammatory activity |
| Aralia dasyphylla Miq. (Araliaceae) [35] | Ursane-type | Cytotoxicity |
| Ilex pubescens Hook. & Arn. (Aquifoliaceae) [73] | Ursane-type | Regulation of lipid level, improving blood biochemical function |
| Acanthopanax spp. (Eleutherococcus) Decne. & Planch. (Araliaceae) [74] | Lupane-type: acankoreagenin, impressic acid, acankoreosides | Antinociceptive activity, anti-inflammatory activity |
| Schefflera spp. J.R. Forst. & G. Forst. (Araliaceae) [74] | Lupane-type: acankoreagenin, impressic acid, acankoreosides | Antinociceptive activity, anti-inflammatory activity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bildziukevich, U.; Wimmerová, M.; Wimmer, Z. Saponins of Selected Triterpenoids as Potential Therapeutic Agents: A Review. Pharmaceuticals 2023, 16, 386. https://doi.org/10.3390/ph16030386
Bildziukevich U, Wimmerová M, Wimmer Z. Saponins of Selected Triterpenoids as Potential Therapeutic Agents: A Review. Pharmaceuticals. 2023; 16(3):386. https://doi.org/10.3390/ph16030386
Chicago/Turabian StyleBildziukevich, Uladzimir, Martina Wimmerová, and Zdeněk Wimmer. 2023. "Saponins of Selected Triterpenoids as Potential Therapeutic Agents: A Review" Pharmaceuticals 16, no. 3: 386. https://doi.org/10.3390/ph16030386
APA StyleBildziukevich, U., Wimmerová, M., & Wimmer, Z. (2023). Saponins of Selected Triterpenoids as Potential Therapeutic Agents: A Review. Pharmaceuticals, 16(3), 386. https://doi.org/10.3390/ph16030386