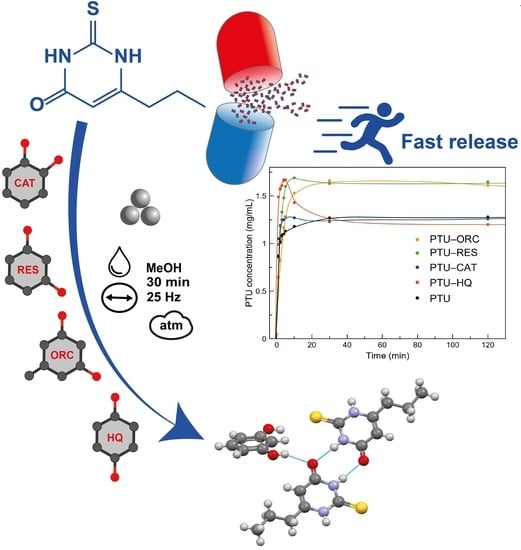
Rational Coformer Selection in the Development of 6-Propyl-2-thiouracil Pharmaceutical Cocrystals
Abstract
1. Introduction
2. Results and Discussion
2.1. Cocrystals Containing PTU
2.2. Virtual Cocrystal Screening
2.3. Cocrystal Synthesis
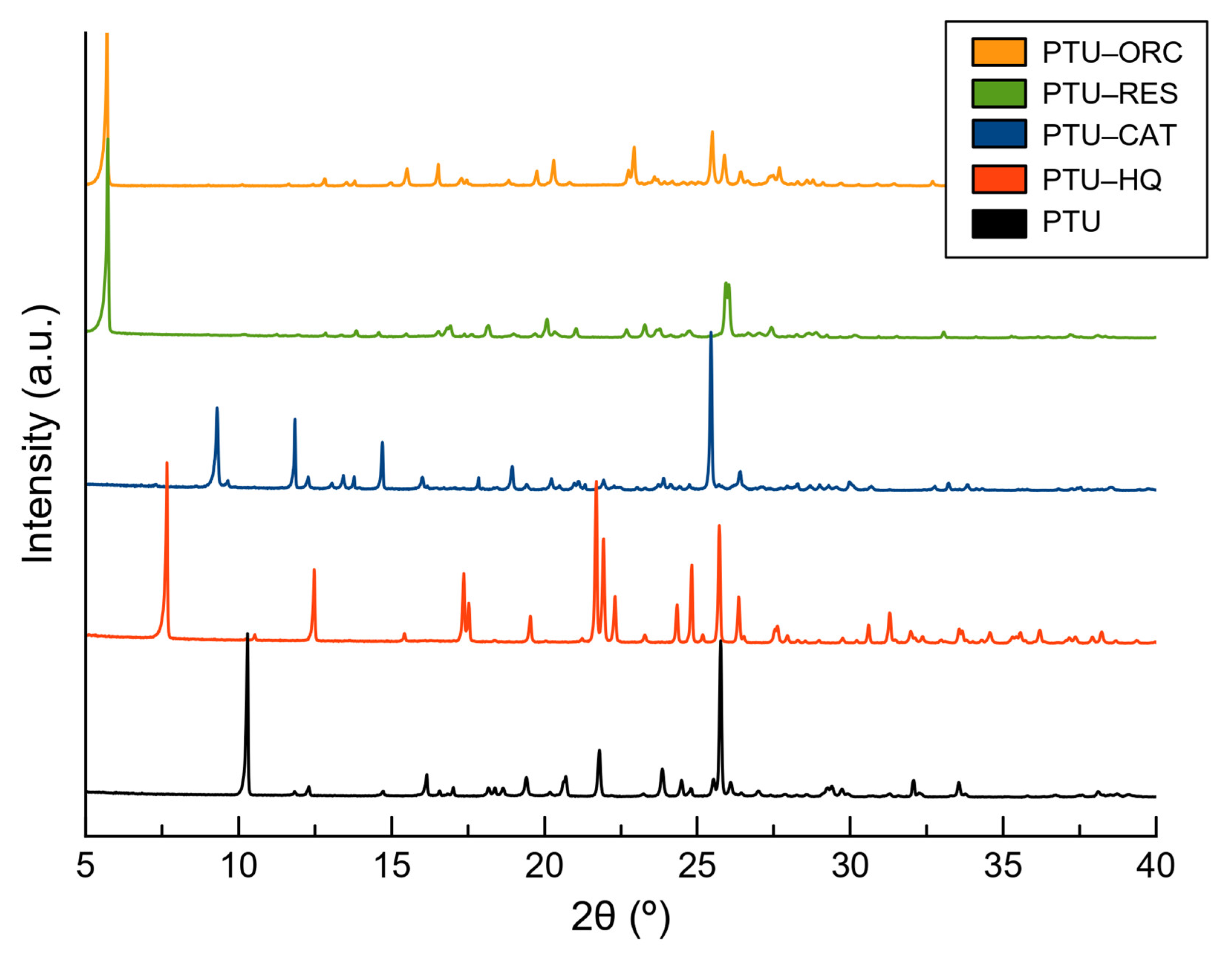
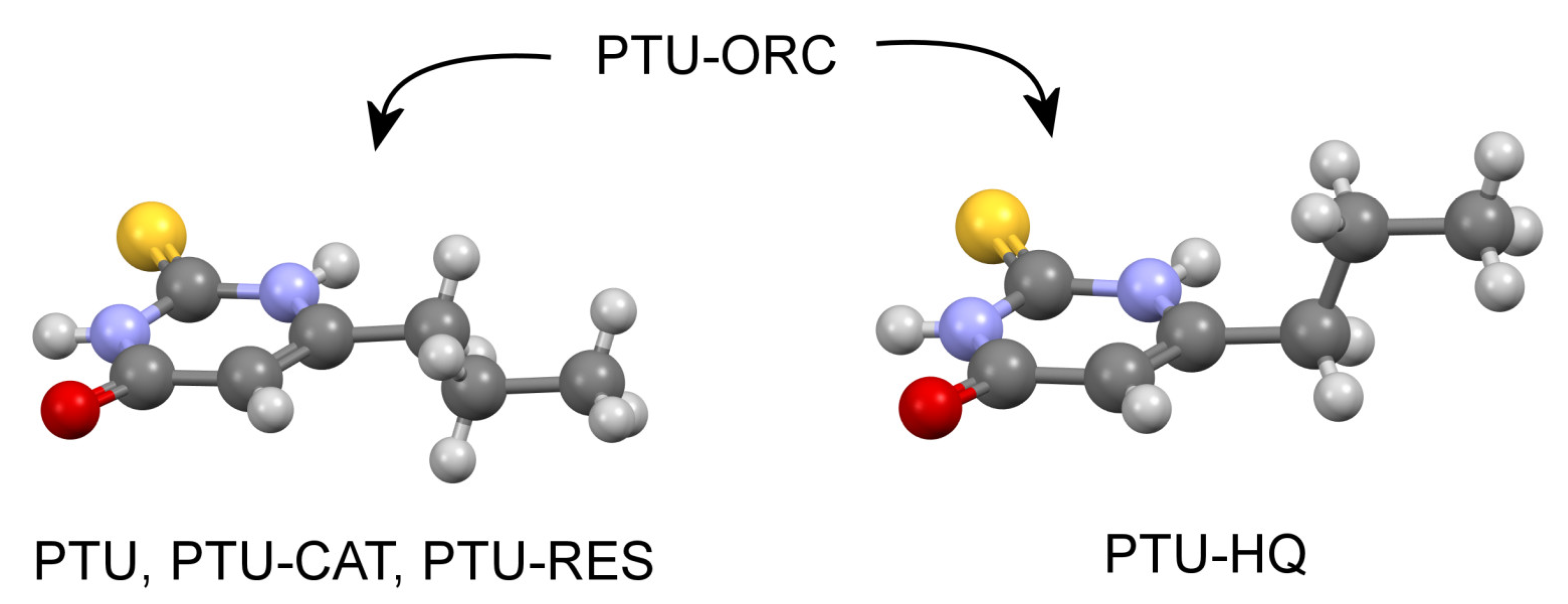
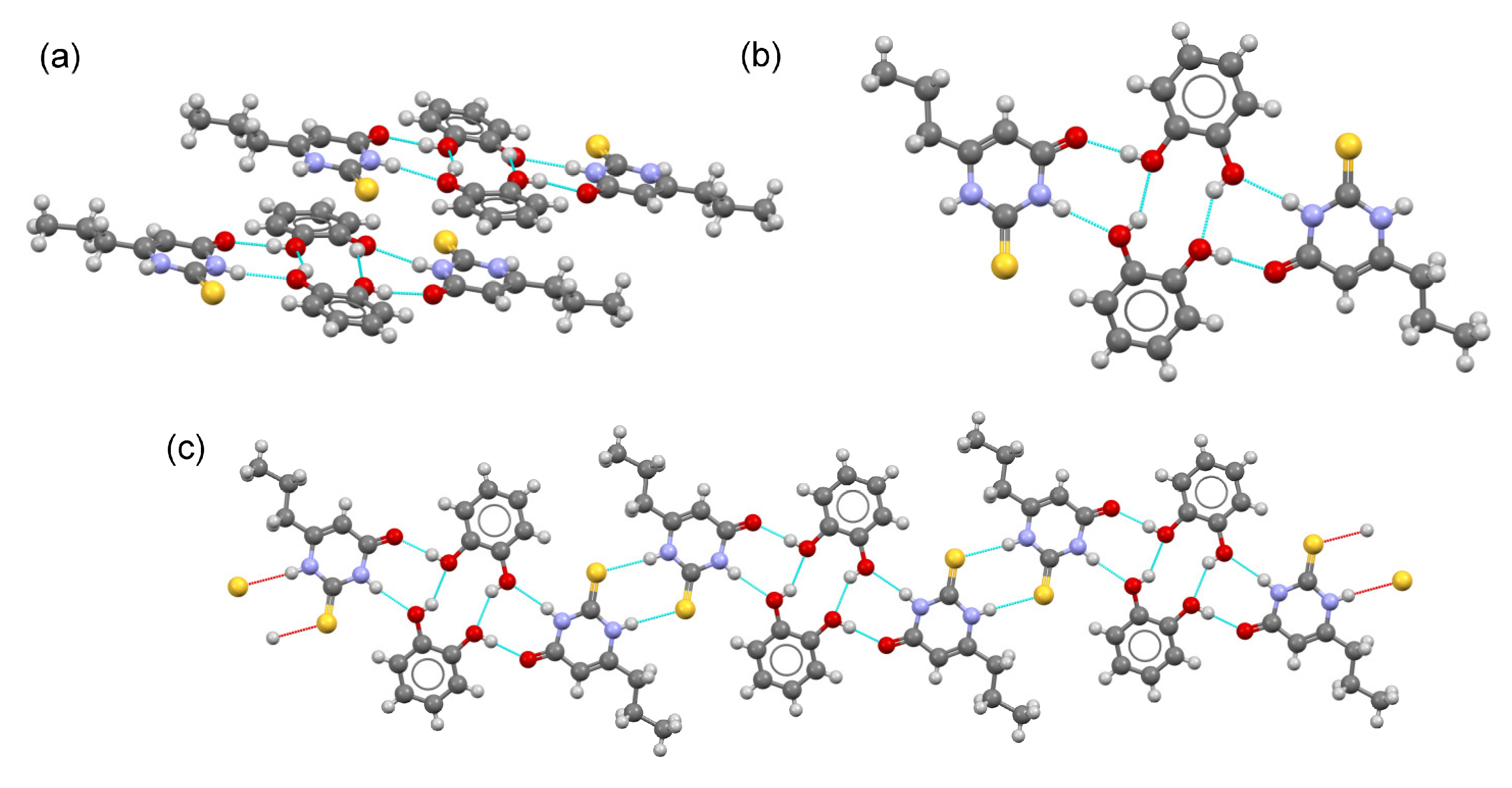
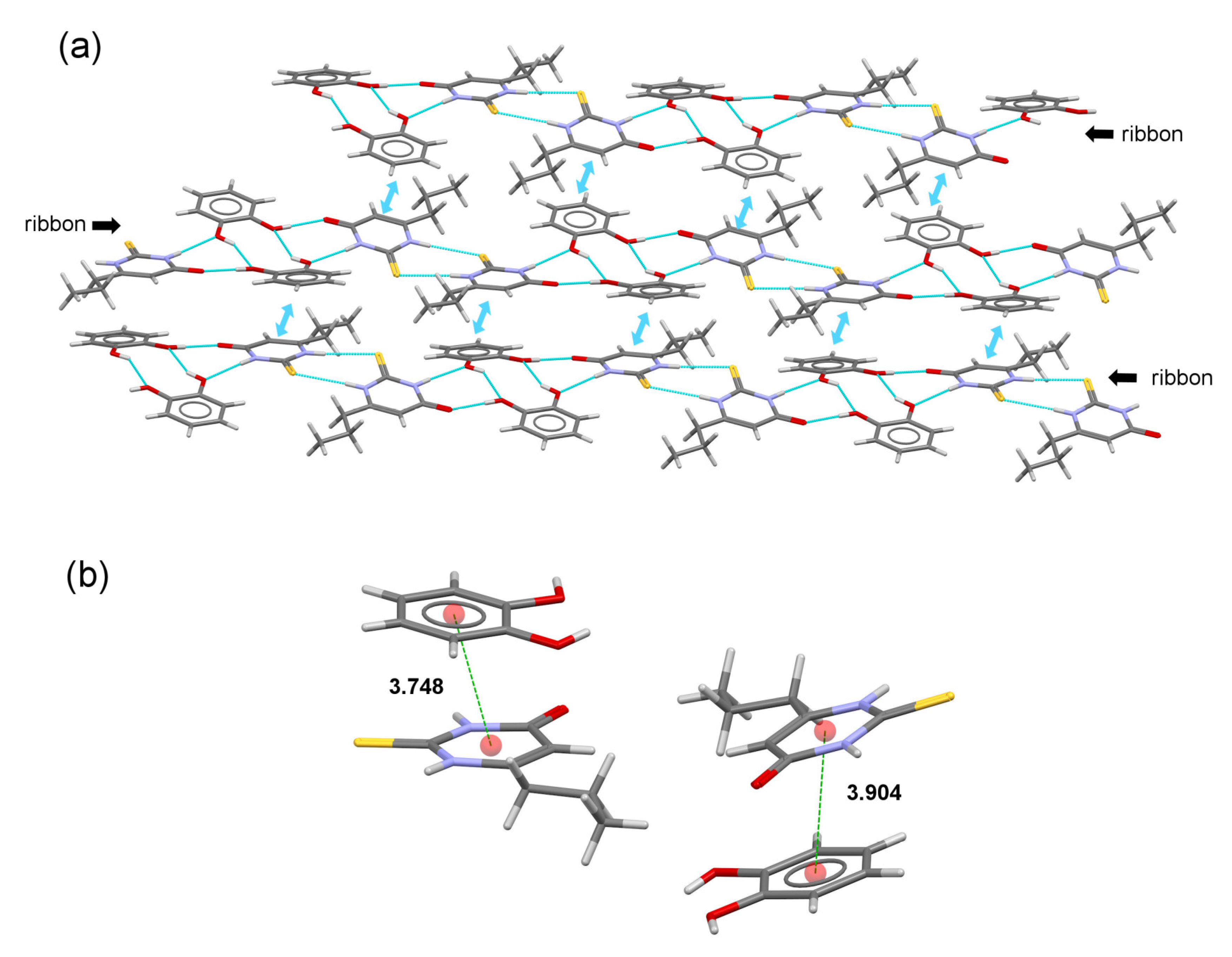
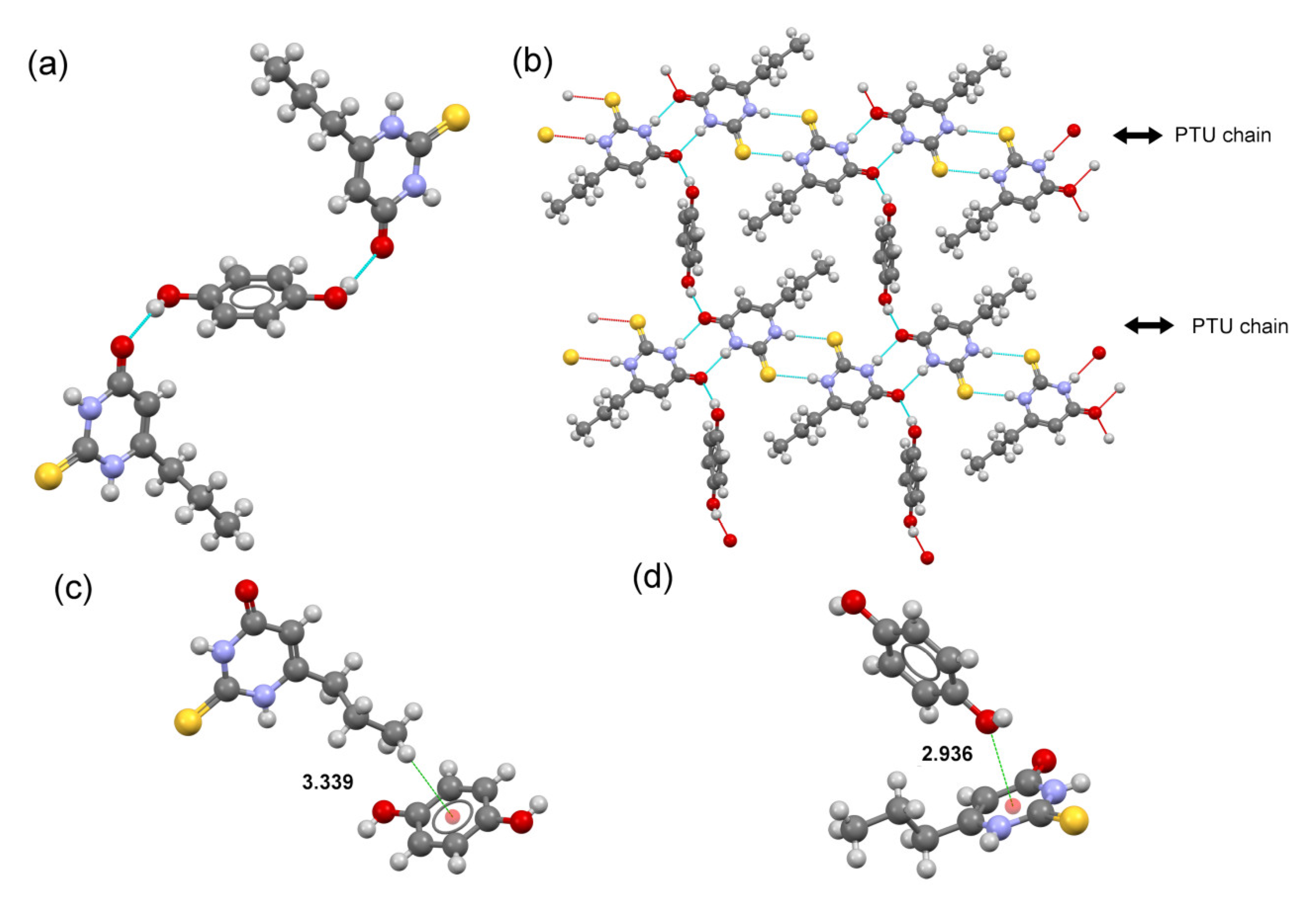
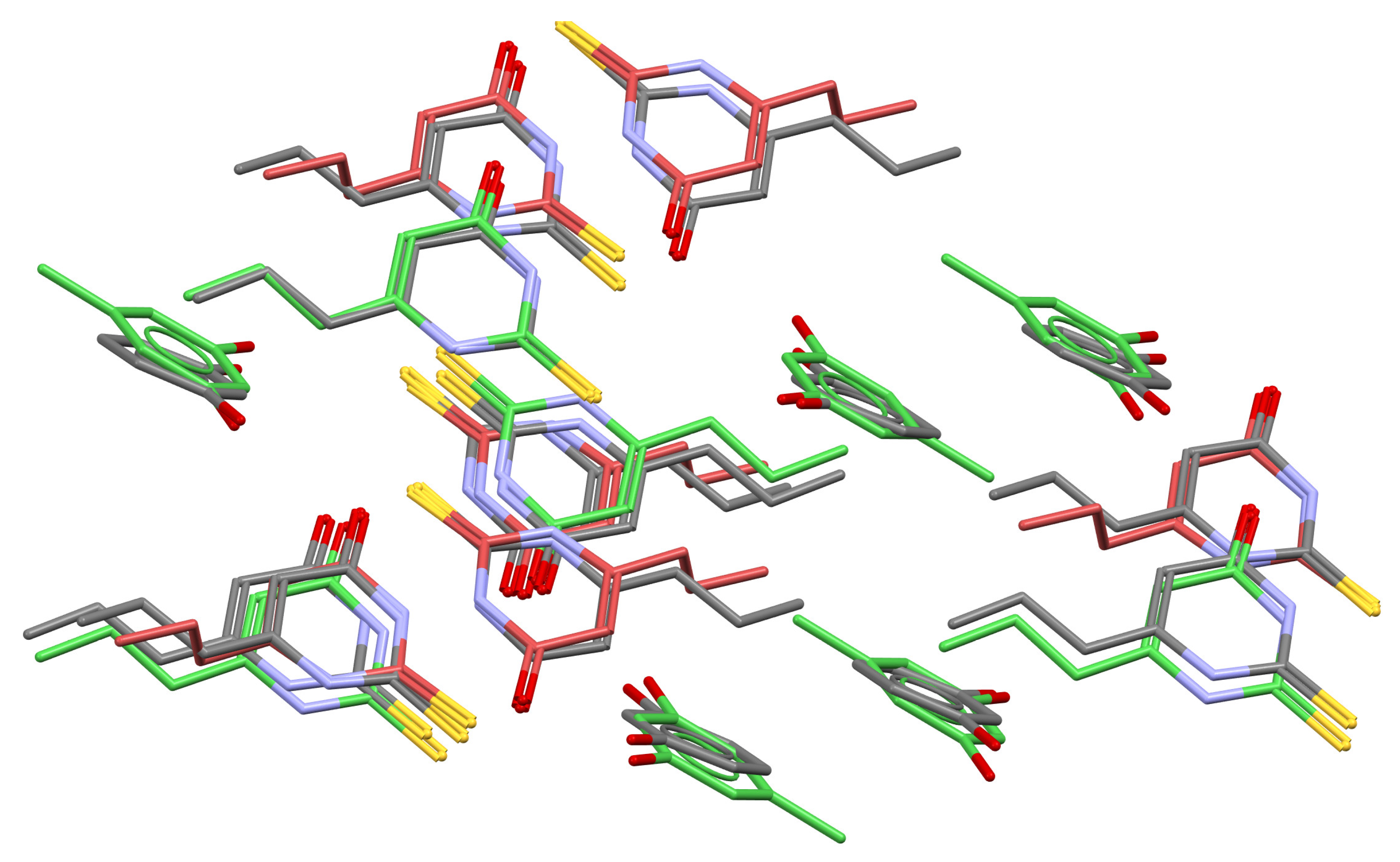
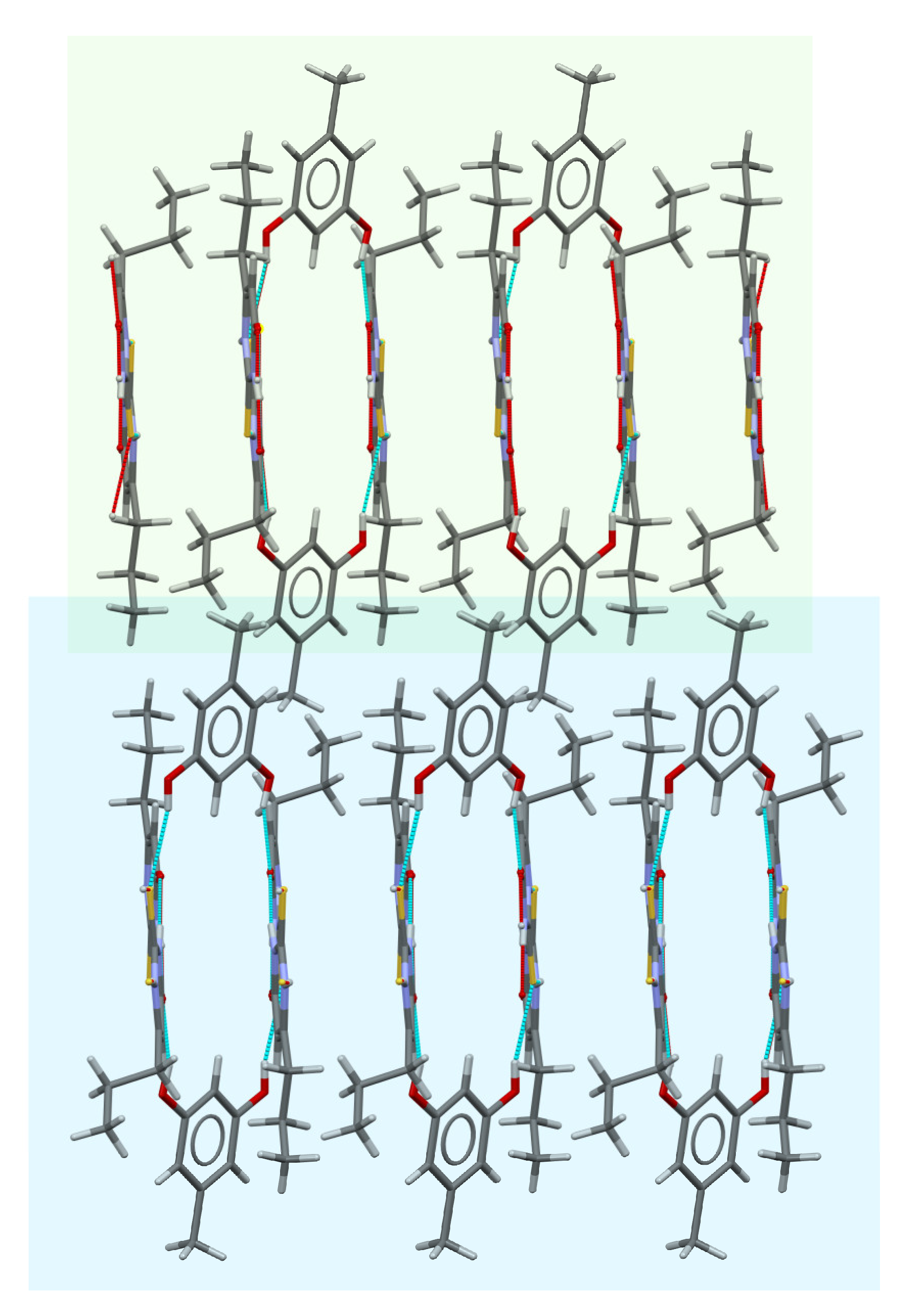
2.4. Crystal Structure Analysis
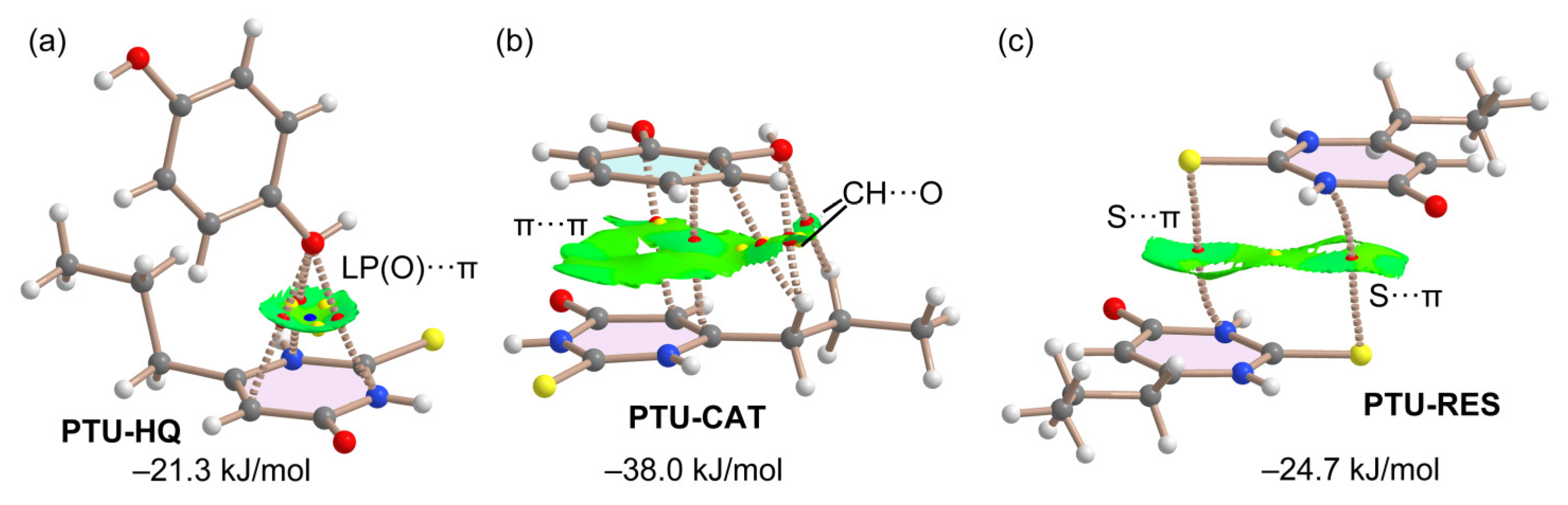
2.5. DFT Study of Non-Covalent Interactions
2.6. FT-IR Spectroscopy
2.7. Thermal Analysis
2.8. Stability
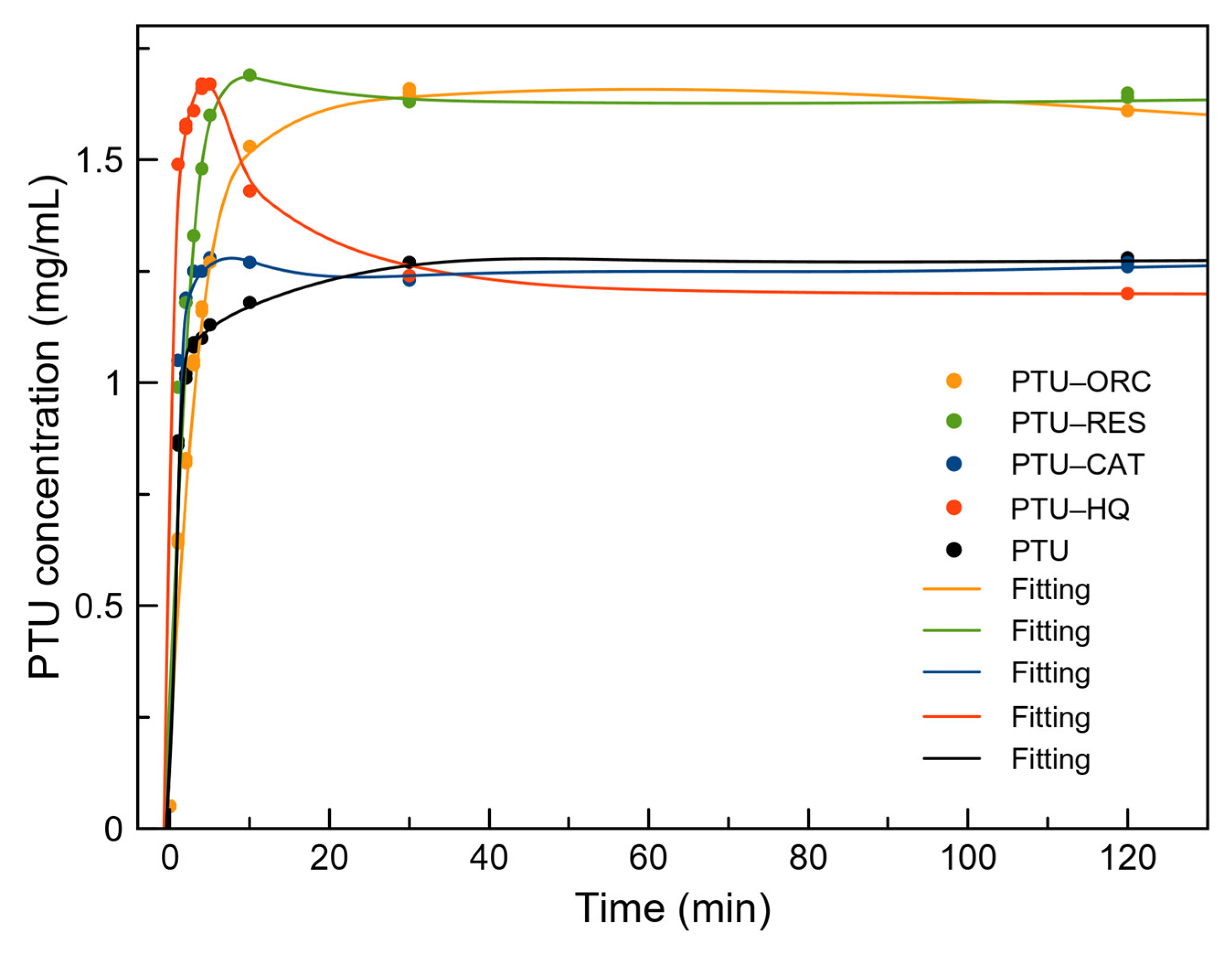
2.9. Powder Dissolution Profile
3. Materials and Methods
3.1. Materials
3.2. Cocrystal Synthesis
3.3. X-ray Diffraction Analysis
3.4. Theoretical Methods
3.5. FT-IR Analysis
3.6. Thermal Analysis
3.7. Stability Studies
3.8. Powder Dissolution Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goth, A. Medical Pharmacology, 9th ed.; The C.V. Company Mosby: Saint Louis, MO, USA, 1978. [Google Scholar]
- U.S. Food and Drug Administration. Propylthiouracil Information. Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/propylthiouracil-information (accessed on 18 January 2023).
- Okabe, N.; Fujiwara, T.; Yamagata, Y.; Tomita, K. Structure of a Potent Antithyroid Drug, 6-Propyl-2-thiouracil. Bull. Chem. Soc. Jpn. 1983, 56, 1543–1544. [Google Scholar] [CrossRef]
- Shiroozu, A.; Taurog, A.; Engler, H.; Dorris, M.L. Mechanism of Action of Thioureylene Antithyroid Drugs in the Rat: Possible Inactivation of Thyroid Peroxidase by Propylthiouracil. Endocrinology 1983, 113, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, A.; Luo, Y.; Ishido, Y.; Usukura, K.; Oda, K.; Sue, M.; Kawashima, A.; Hiroi, N.; Suzuki, K. Inhibitory Effects of Methimazole and Propylthiouracil on Iodotyrosine Deiodinase 1 in Thyrocytes. Endocr. J. 2019, 66, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.W.; Galanko, J.A.; Shrestha, R.; Fried, M.W.; Watkins, P. Liver Transplantation for Acute Liver Failure from Drug Induced Liver Injury in the United States. Liver Transplant. 2004, 10, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Malozowski, S.; Chiesa, A. Propylthiouracil-Induced Hepatotoxicity and Death. Hopefully, Never More. J. Clin. Endocrinol. Metab. 2010, 95, 3161. [Google Scholar] [CrossRef]
- Rivkees, S.A. The Treatment of Graves’ Disease in Children. J. Pediatr. Endocrinol. Metab. 2006, 19, 1095–1111. [Google Scholar] [CrossRef]
- American Thyroid Association|ATA. Available online: https://www.thyroid.org/ (accessed on 18 January 2023).
- Andersen, S.L.; Knøsgaard, L.; Olsen, J.; Vestergaard, P.; Andersen, S. Maternal Thyroid Function, Use of Antithyroid Drugs in Early Pregnancy, and Birth Defects. J. Clin. Endocrinol. Metab. 2019, 104, 6040–6048. [Google Scholar] [CrossRef]
- Hudzik, B.; Zubelewicz-Szkodzinska, B. Antithyroid Drugs during Breastfeeding. Clin. Endocrinol. 2016, 85, 827–830. [Google Scholar] [CrossRef]
- Wisher, D. Martindale: The Complete Drug Reference. 37th Ed. J. Med. Libr. Assoc. 2012, 100, 75–76. [Google Scholar] [CrossRef]
- Del Corso, A.; Cappiello, M.; Mura, U. Thiol Dependent Oxidation of Enzymes: The Last Chance against Oxidative Stress. Int. J. Biochem. 1994, 26, 745–750. [Google Scholar] [CrossRef]
- Berry, D.J.; Steed, J.W. Pharmaceutical Cocrystals, Salts and Multicomponent Systems; Intermolecular Interactions and Property Based Design. Adv. Drug Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Blagden, N.; de Matas, M.; Gavan, P.T.; York, P. Crystal Engineering of Active Pharmaceutical Ingredients to Improve Solubility and Dissolution Rates. Adv. Drug Deliv. Rev. 2007, 59, 617–630. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysøgu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Wiley Online Libr. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and Beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Saeed, F.; Anjum, M.; Afzaal, M.; Tufail, T.; Shakeel Bashir, M.; Ishtiaq, A.; Hussain, S.; Ansar, H.; Suleria, R. Natural Polyphenols: An Overview. Taylor Fr. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Substances Added to Food (Formerly EAFUS)|FDA. Available online: https://www.fda.gov/food/food-additives-petitions/substances-added-food-formerly-eafus (accessed on 30 January 2023).
- Bolla, G.; Sanphui, P.; Nangia, A. Solubility Advantage of Tenoxicam Phenolic Cocrystals Compared to Salts. Cryst. Growth Des. 2013, 13, 1988–2003. [Google Scholar] [CrossRef]
- Magaña-Vergara, N.E.; de La Cruz-Cruz, P.; Peraza-Campos, A.L.; Martínez-Martínez, F.J.; González-González, J.S. Mechanochemical Synthesis and Crystal Structure of the Lidocaine-Phloroglucinol Hydrate 1:1:1 Complex. Crystals 2018, 8, 130. [Google Scholar] [CrossRef]
- González-González, J.S.; Martínez-Santiago, A.M.M.; Martínez-Martínez, F.J.; Emparán-Legaspi, M.J.; Pineda-Contreras, A.; Flores-Alamo, M.; García-Ortega, H. Cocrystals of Isoniazid with Polyphenols: Mechanochemical Synthesis and Molecular Structure. Crystals 2020, 10, 569. [Google Scholar] [CrossRef]
- Dias, J.L.; Lanza, M.; Ferreira, S.R.S. Cocrystallization: A Tool to Modulate Physicochemical and Biological Properties of Food-Relevant Polyphenols. Trends Food Sci. Technol. 2021, 110, 13–27. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, L.; Hao, H.; Bao, Y.; Yin, Q.; Xie, C. Cocrystals of Propylthiouracil and Nutraceuticals toward Sustained-Release: Design, Structure Analysis, and Solid-State Characterization. Cryst. Growth Des. 2021, 21, 1202–1217. [Google Scholar] [CrossRef]
- Verdugo-Escamilla, C.; Alarcón-Payer, C.; Acebedo-Martínez, F.J.; Fernández-Penas, R.; Domínguez-Martín, A.; Choquesillo-Lazarte, D. Lidocaine Pharmaceutical Multicomponent Forms: A Story about the Role of Chloride Ions on Their Stability. Crystals 2022, 12, 798. [Google Scholar] [CrossRef]
- Sinha, A.S.; Rao Khandavilli, U.B.; O’Connor, E.L.; Deadman, B.J.; Maguire, A.R.; Lawrence, S.E. Novel Co-Crystals of the Nutraceutical Sinapic Acid. CrystEngComm 2015, 17, 4832–4841. [Google Scholar] [CrossRef]
- Tutughamiarso, M.; Egert, E. Cocrystals of 6-Propyl-2-thio-uracil: N-H⋯O versus N-H⋯S Hydrogen Bonds. Acta Crystallogr. C 2011, 67, o439–o445. [Google Scholar] [CrossRef] [PubMed]
- Hützler, W.M.; Egert, E.; Bolte, M. 6-Propyl-2-thiouracil versus 6-Methoxymethyl-2-Thiouracil: Enhancing the Hydrogen-Bonded Synthon Motif by Replacement of a Methylene Group with an O Atom. Acta Crystallogr. C Struct. Chem. 2016, 72, 634–646. [Google Scholar] [CrossRef]
- Isaia, F.; Aragoni, M.C.; Arca, M.; Caltagirone, C.; Castellano, C.; Demartin, F.; Garau, A.; Lippolis, V.; Pintus, A. Oxidative Properties of Iodine-Adducts of Propylthiouracil and Methimazole: Direct Synthesis of Mercury(II) Complexes from the Reaction with Liquid Mercury. Dalton Trans. 2011, 40, 4505–4513. [Google Scholar] [CrossRef]
- Paizanos, K.; Charalampou, D.; Kourkoumelis, N.; Kalpogiannaki, D.; Hadjiarapoglou, L.; Spanopoulou, A.; Lazarou, K.; Manos, M.J.; Tasiopoulos, A.J.; Kubicki, M.; et al. Synthesis and Structural Characterization of New Cu(I) Complexes with the Antithyroid Drug 6-n-Propyl-Thiouracil. Study of the Cu(I)-Catalyzed Intermolecular Cycloaddition of Iodonium Ylides toward Benzo[b]Furans with Pharmaceutical Implementations. Inorg. Chem. 2012, 51, 12248–12259. [Google Scholar] [CrossRef]
- Ferreira, F.F.; Trindade, A.C.; Antonio, S.G.; de Oliveira Paiva-Santos, C. Crystal Structure of Propylthiouracil Determined Using High-Resolution Synchrotron X-Ray Powder Diffraction. CrystEngComm 2011, 13, 5474–5479. [Google Scholar] [CrossRef]
- Abramov, Y.A.; Loschen, C.; Klamt, A. Rational Coformer or Solvent Selection for Pharmaceutical Cocrystallization or Desolvation. J. Pharm. Sci. 2012, 101, 3687–3697. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Peterson, M.L.; Klein, D. Continuously Substituted Solid Solutions of Organic Co-Crystals. Cryst. Growth Des. 2008, 8, 4487–4493. [Google Scholar] [CrossRef]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Heinz, A.; Strachan, C.J.; Gordon, K.C.; Rades, T. Analysis of Solid-State Transformations of Pharmaceutical Compounds Using Vibrational Spectroscopy. J. Pharm. Pharmacol. 2009, 61, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Perlovich, G. Melting Points of One- and Two-Component Molecular Crystals as Effective Characteristics for Rational Design of Pharmaceutical Systems. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2020, 76, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Tanaka, T. Crystallization Behavior of Polymorphous Ni-Complex Clathrate in the Presence of 2-Methylnaphthalene. J. Cryst. Growth 1994, 142, 165–170. [Google Scholar] [CrossRef]
- Kitamura, M.; Abe, T.; Kishida, M. Mechanism for the Release of the Industrial Biocide CMI from Clathrate Crystal. Chem. Eng. Res. Des. 2008, 86, 1053–1058. [Google Scholar] [CrossRef]
- Yamashita, H.; Sun, C.C. Expedited Tablet Formulation Development of a Highly Soluble Carbamazepine Cocrystal Enabled by Precipitation Inhibition in Diffusion Layer. Pharm. Res. 2019, 36, 1–10. [Google Scholar] [CrossRef]
- Bruker-AXS. Bruker APEX4. In APEX4 V2022.1; Bruker-AXS: Madison, WI, USA, 2022. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.v.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. Structure Validation in Chemical Crystallography. Acta Crystallogr. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian16 (Revision A.03); Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. A Quantum Theory of Molecular Structure and Its Applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Bader, R.F.W. A Bond Path: A Universal Indicator of Bonded Interactions. J. Phys. Chem. A 1998, 102, 7314–7323. [Google Scholar] [CrossRef]
- Keith, T.A. AIMALL (Version 19.10.12); TK Gristmill Software: Overland Park, KS, USA, 2019. [Google Scholar]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen Bond Strengths Revealed by Topological Analyses of Experimentally Observed Electron Densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Efimenko, Z.M.; Eliseeva, A.A.; Ivanov, D.M.; Galmés, B.; Frontera, A.; Bokach, N.A.; Kukushkin, V.Y. Bifurcated μ 2 -I···(N,O) Halogen Bonding: The Case of (Nitrosoguanidinate)Ni II Cocrystals with Iodine(I)-Based σ-Hole Donors. Cryst. Growth Des. 2021, 21, 588–596. [Google Scholar] [CrossRef]
- Zelenkov, L.E.; Ivanov, D.M.; Sadykov, E.K.; Bokach, N.A.; Galmés, B.; Frontera, A.; Kukushkin, V.Y. Semicoordination Bond Breaking and Halogen Bond Making Change the Supramolecular Architecture of Metal-Containing Aggregates. Cryst. Growth Des. 2020, 20, 6956–6965. [Google Scholar] [CrossRef]
- Soldatova, N.S.; Postnikov, P.S.; Suslonov, V.v.; Kissler, T.Y.; Ivanov, D.M.; Yusubov, M.S.; Galmés, B.; Frontera, A.; Kukushkin, V.Y. Diaryliodonium as a Double σ-Hole Donor: The Dichotomy of Thiocyanate Halogen Bonding Provides Divergent Solid State Arylation by Diaryliodonium Cations. Org. Chem. Front. 2020, 7, 2230–2242. [Google Scholar] [CrossRef]
- Katlenok, E.A.; Haukka, M.; Levin, O.v.; Frontera, A.; Kukushkin, V.Y. Supramolecular Assembly of Metal Complexes by (Aryl)I⋯d[Pt II ] Halogen Bonds. Chem. –A Eur. J. 2020, 26, 7692–7701. [Google Scholar] [CrossRef]
- Rozhkov, A.v.; Eliseeva, A.A.; Baykov, S.v.; Galmés, B.; Frontera, A.; Kukushkin, V.Y. One-Pot Route to X-Perfluoroarenes (X = Br, I) Based on Fe III -Assisted C–F Functionalization and Utilization of These Arenes as Building Blocks for Crystal Engineering Involving Halogen Bonding. Cryst. Growth Des. 2020, 20, 5908–5921. [Google Scholar] [CrossRef]
- Rozhkov, A.v.; Ananyev, I.v.; Gomila, R.M.; Frontera, A.; Kukushkin, V.Y. π-Hole··· d z 2 [Pt II ] Interactions with Electron-Deficient Arenes Enhance the Phosphorescence of Pt II -Based Luminophores. Inorg. Chem. 2020, 59, 9308–9314. [Google Scholar] [CrossRef]
- Verdugo-Escamilla, C.; Alarcón-Payer, C.; Frontera, A.; Acebedo-Martínez, F.J.; Domínguez-Martín, A.; Gómez-Morales, J.; Choquesillo-Lazarte, D. Interconvertible Hydrochlorothiazide–Caffeine Multicomponent Pharmaceutical Materials: A Solvent Issue. Crystals 2020, 10, 1088. [Google Scholar] [CrossRef]
- Barbas, R.; Kumar, V.; Vallcorba, O.; Prohens, R.; Frontera, A. Sildenafil–Resorcinol Cocrystal: XRPD Structure and DFT Calculations. Crystals 2020, 10, 1126. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Glomme, A.; März, J.; Dressman, J.B. Comparison of a Miniaturized Shake-Flask Solubility Method with Automated Potentiometric Acid/Base Titrations and Calculated Solubilities. J. Pharm. Sci. 2005, 94, 1–16. [Google Scholar] [CrossRef] [PubMed]


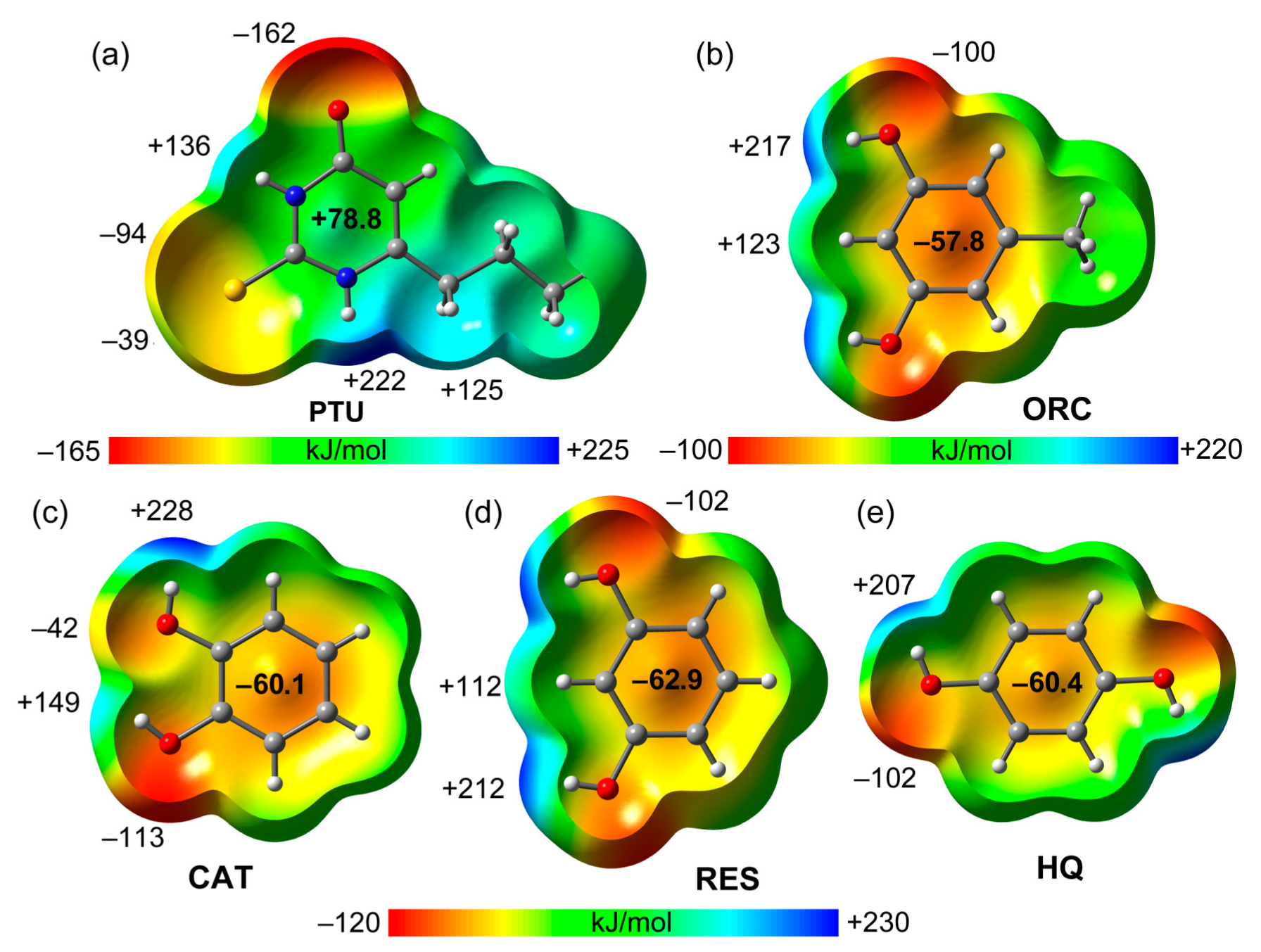

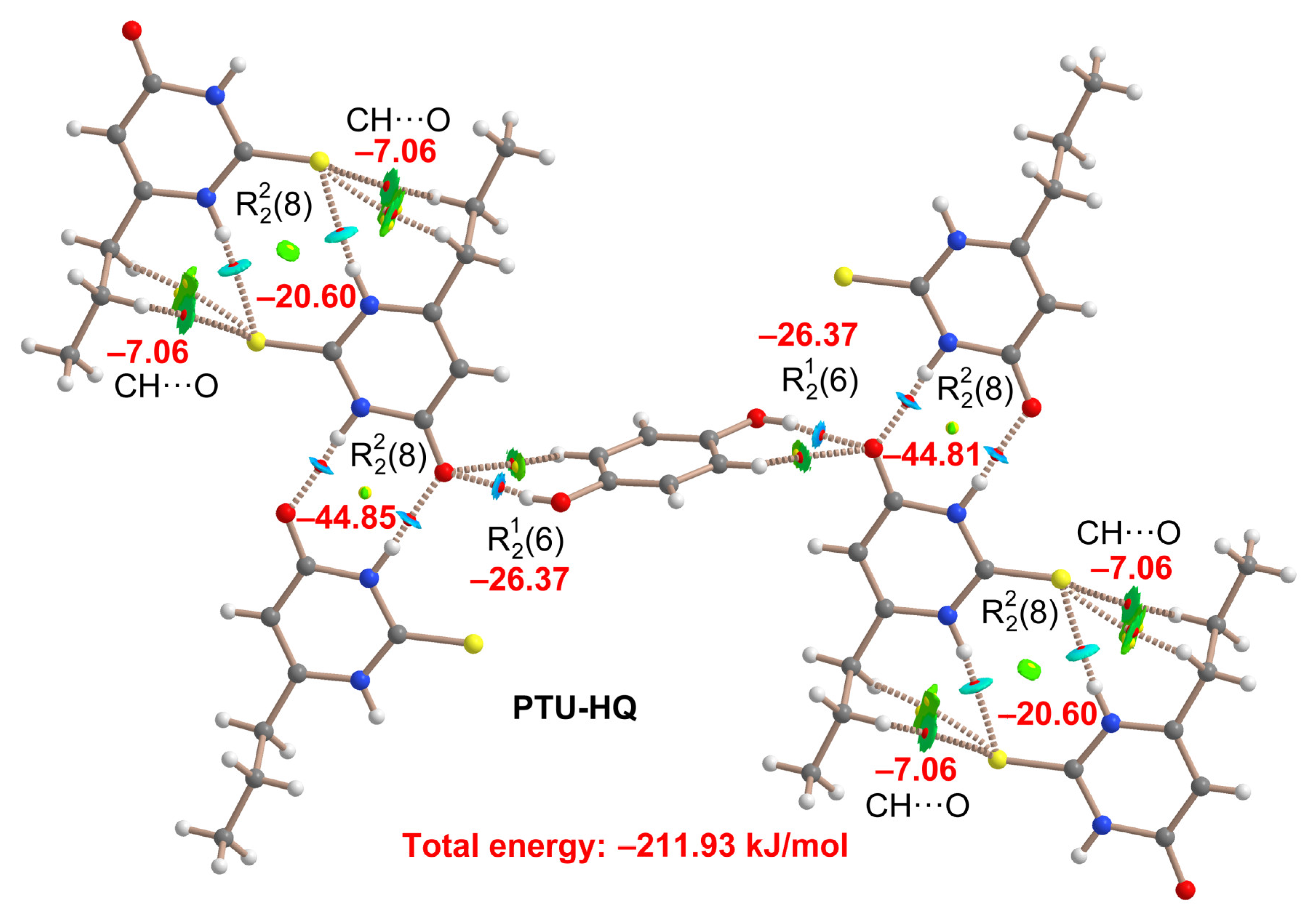
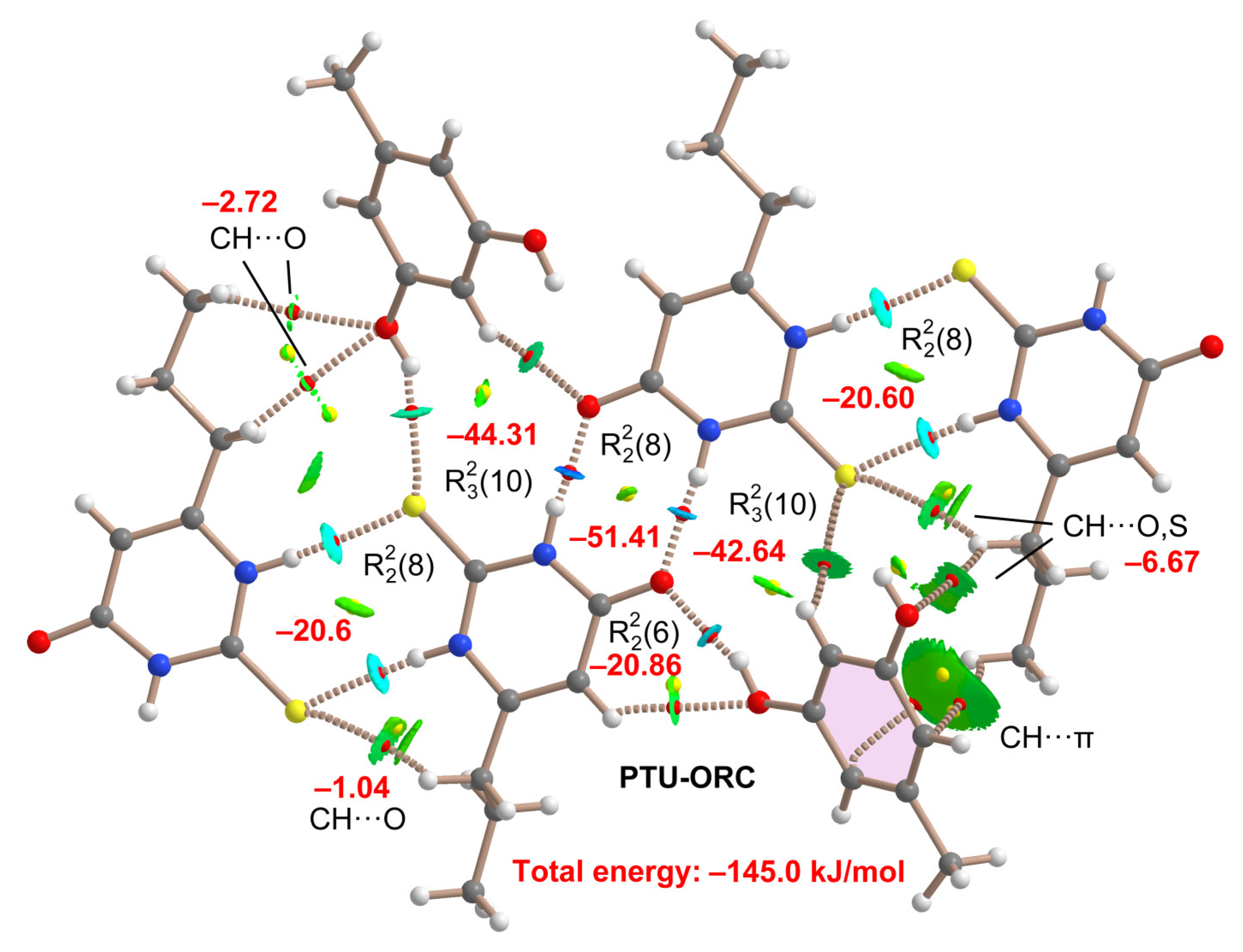
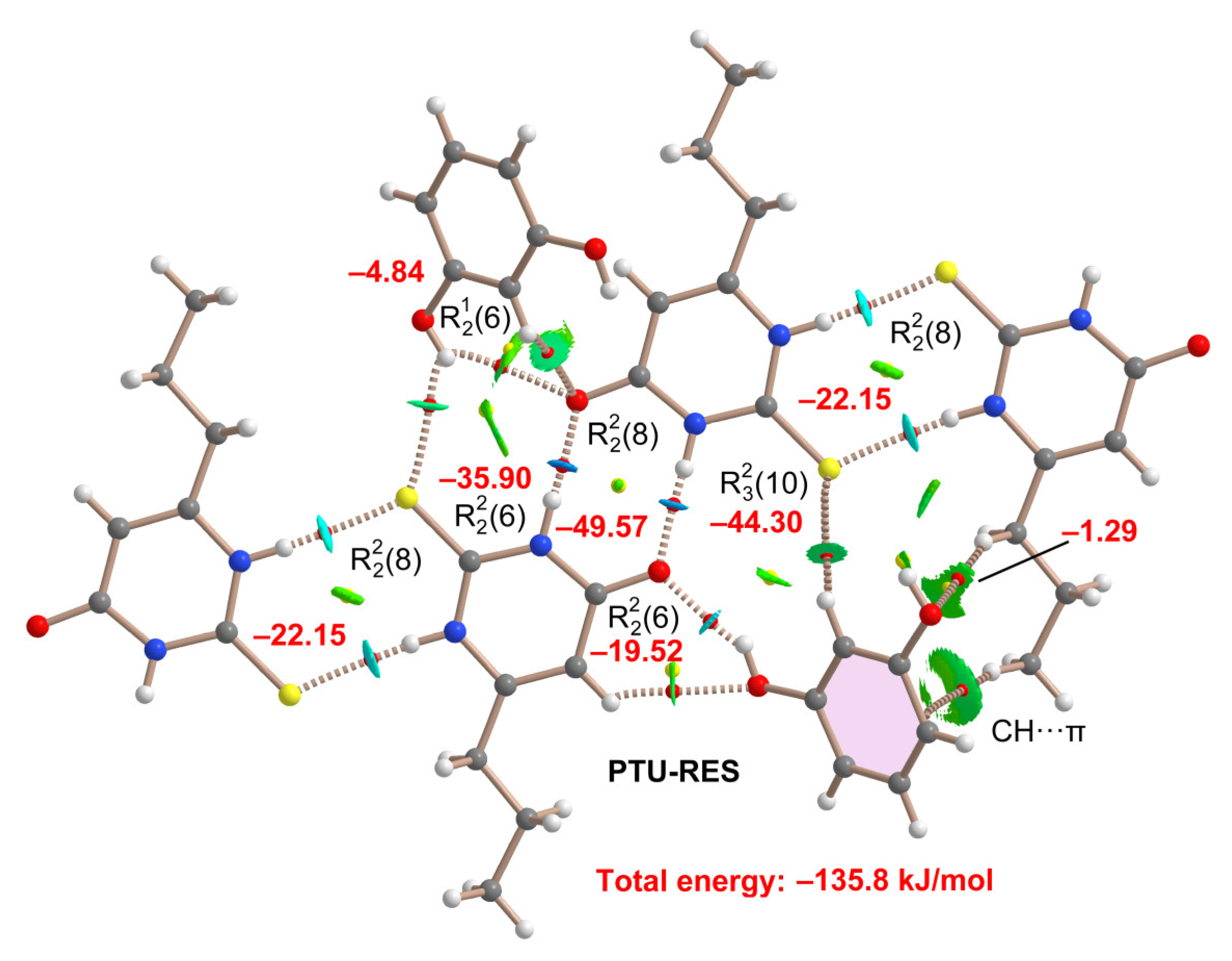

| Coformer | Hex [kJ/mol] | Reference |
|---|---|---|
| Catechol | −2.081625 | This work |
| Resorcinol | −2.04305 | This work |
| Kaempferol | −1.910405 | [25] |
| Gentisic acid | −1.78116 | [25] |
| Orcinol | −1.77036 | This work |
| Ellagic acid | −1.717475 | [25] |
| Hydroquinone | −1.477705 | This work |
| 2,4-Diaminopyrimidine | −0.294005 | [29] |
| N-(6-acetamidopyridin-2-yl)acetamide | −0.25925 | [28] |
| 2-Aminopyrimidine | −0.20317 | [28] |
| Sinapic acid | −0.17387 | [27] |
| Cinnamic acid | −0.14058 | [25] |
| Wavenumber (cm–1) | |||
|---|---|---|---|
| Compound | C=O Stretching | N-H Bending | C=S Stretching |
| PTU | 1651.94 | 1557.44 | 1190.98 |
| PTU–CAT | 1660.61 | 1557.44 | 1184.23 |
| PTU–RES | 1646.16 | 1558.40 | 1180.37 |
| PTU–HQ | 1651.95 | 1563.22 | 1189.05 |
| PTU–ORC | 1651.94 | 1547.43 | 1201.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acebedo-Martínez, F.J.; Alarcón-Payer, C.; Verdugo-Escamilla, C.; Martín, J.; Frontera, A.; Domínguez-Martín, A.; Gómez-Morales, J.; Choquesillo-Lazarte, D. Rational Coformer Selection in the Development of 6-Propyl-2-thiouracil Pharmaceutical Cocrystals. Pharmaceuticals 2023, 16, 370. https://doi.org/10.3390/ph16030370
Acebedo-Martínez FJ, Alarcón-Payer C, Verdugo-Escamilla C, Martín J, Frontera A, Domínguez-Martín A, Gómez-Morales J, Choquesillo-Lazarte D. Rational Coformer Selection in the Development of 6-Propyl-2-thiouracil Pharmaceutical Cocrystals. Pharmaceuticals. 2023; 16(3):370. https://doi.org/10.3390/ph16030370
Chicago/Turabian StyleAcebedo-Martínez, Francisco Javier, Carolina Alarcón-Payer, Cristóbal Verdugo-Escamilla, Jesús Martín, Antonio Frontera, Alicia Domínguez-Martín, Jaime Gómez-Morales, and Duane Choquesillo-Lazarte. 2023. "Rational Coformer Selection in the Development of 6-Propyl-2-thiouracil Pharmaceutical Cocrystals" Pharmaceuticals 16, no. 3: 370. https://doi.org/10.3390/ph16030370
APA StyleAcebedo-Martínez, F. J., Alarcón-Payer, C., Verdugo-Escamilla, C., Martín, J., Frontera, A., Domínguez-Martín, A., Gómez-Morales, J., & Choquesillo-Lazarte, D. (2023). Rational Coformer Selection in the Development of 6-Propyl-2-thiouracil Pharmaceutical Cocrystals. Pharmaceuticals, 16(3), 370. https://doi.org/10.3390/ph16030370