Abstract
The amyloid concept of Alzheimer’s disease (AD) assumes the β-amyloid peptide (Aβ) as the main pathogenic factor, which injures neural and other brain cells, causing their malfunction and death. Although Aβ has been documented to exert its cytotoxic effect in a solitary manner, there is much evidence to claim that its toxicity can be modulated by other proteins. The list of such Aβ co-factors or interactors includes tau, APOE, transthyretin, and others. These molecules interact with the peptide and affect the ability of Aβ to form oligomers or aggregates, modulating its toxicity. Thus, the list of potential substances able to reduce the harmful effects of the peptide should include ones that can prevent the pathogenic interactions by specifically binding Aβ and/or its partners. In the present review, we discuss the data on Aβ-based complexes in AD pathogenesis and on the compounds directly targeting Aβ or the destructors of its complexes with other polypeptides.
1. Introduction
Alzheimer’s disease (AD) and similar pathologies stemming from cytotoxic polypeptide species cover a wide range of knowledge and methods, from the molecular organization of a single cell to the behavioral reactions of the whole organism. Although it is hard to imagine, thousands of papers, monographs, and doctoral theses support the idea that only a few species of amyloid precursor protein (APP) demonstrate pathogenic effects when outside or inside a neural cell [1]. The amyloid hypothesis has a firmly established basis, and the meaningfulness of APP-derived peptides as pathogenic triggers seems to have dominated in the last few decades [2,3,4]. APP peptides, such as Aβ1–42 or Aβ1–40, occurring intracellularly or exported in excess by brain cells, may form oligomers, small aggregates, or fibrils and damage neurons by inducing oxidative stress, suppressing the function of membrane channels, or affecting transport/sorting mechanisms (Figure 1). Some Aβ molecules are localized to the structures called senile plaques, which, according to some data, may have cytotoxic activity [3]. Notably, the amount of Aβ accumulating in the extracellular matrix, cerebrospinal fluid, and blood may indicate the progress of AD. Gradually, such techniques are used in prostheses for diagnosing AD [5,6].
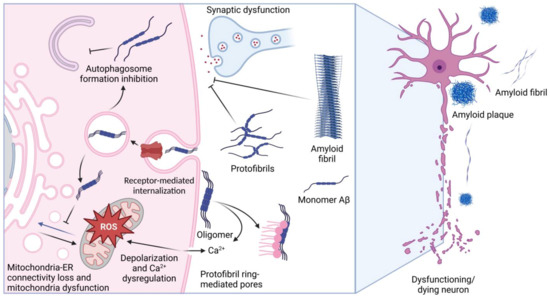
Figure 1.
Targets of Aβ pathogenicity. The figure schematically shows the main known mechanisms of the pathogenic action of Aβ and amyloid fibrils, which cause dysfunction of neurons and their death. The figure was created with BioRender.com, accessed on 28 December 2022.
The physical properties and biological activity of Aβ depend on its amino acid composition and on its post-translational modifications, features that confine the aggregation ability and toxicity of the peptides [7]. As a solitary factor, Aβ can attack neurons, damaging their synapses or other structures and causing a pathologic chain of events including the disruption of intercellular contacts; examples of such deleterious effects come from investigations performed on glial and microglial cells [8]. It is also noteworthy that Aβ was found to bind some low-molecular-weight compounds, metabolites, and metals that are also able to modulate the pathogenicity of the peptide [9,10]. Lastly, several proteins were found to specifically bind Aβ, and their activity may be modulated by the peptide, or vice versa, the peptide’s activity may be affected by the interactors. These phenomena form a complex picture of the Aβ-based effect on brain tissue, as shown in Figure 1. In this review, we present the data on Aβ-binding proteins and on the effects of such complexes in the context of AD pathology. It seems that the role of such interactions is underestimated in modern neurobiology, while it is likely that it is precisely such interprotein bonds that determine the course of disease pathogenesis. In the present review, special attention is paid to small molecules able to affect the above interactions and, therefore, to be employed for AD therapy.
2. Aβ and Its Protein Interactors
2.1. Structural Features of Aβ
Aβ is the proteolytic fragment of APP protein, the product of the APP gene, localized to the 21st chromosome [11]. Having several isoforms, this transmembrane protein interacts with heparan sulfate, type I collagen, fibronectin, and other components of the cell matrix, making APP an important player in the regulation of cell adhesion. In addition, APP is involved in neuronal development, particularly neurite growth and differentiation [12]. It was shown that APP plays an important role in excitatory as well as inhibitory synaptic transmission. In particular, APP interacts with glutamatergic [13], cholinergic [14], and gamma-aminobutyric acid B receptor (GABAB) [15]. APP was found to participate in the regulation of Ca2+ homeostasis by interacting with NMDA receptors and VGC channels [16].
Amyloid precursor protein has a long N-terminal extracellular domain, a smaller C-terminal cytoplasmic domain, and a single-span transmembrane domain [17]. There are two paths of APP processing, where non-pathogenic fragment 1–40 is produced by the consecutive cleavage of α- and γ-secretases, and the amyloidogenic 1–42 fragment of precursor protein is processed by β- and γ-secretases [9]. The resulting Aβ structure has a sequence of 12 aliphatic amino acids at the C-terminus and two hydrophobic stretches, one of which is situated C-terminally and the second lies in the interim of 12–25 residues. Furthermore, the peptide has several sites of interaction with metal ions [18].
In the normal brain, secreted amyloids, primarily in monomeric form, participate in synaptic transmission, protection against oxidative stress, and signaling [19,20]. In conditions when the concentration of Aβ increases up to 10−7 M, it can form oligomers and demonstrate neurotoxicity [11]. Having a sequence of five positively charged amino acids at the N-terminus, Aβ can interact with negatively charged cell surface phospholipids, such as sphingomyelin and phosphoethanolamine, and integrate into the membrane [9]. It was also demonstrated that binding with Aβ triggers the internalization of NMDA receptors and facilitates Aβ penetration [21]. Moreover, Aβ impairs mitochondrial function [22] by increasing the contact between sub-areas of mitochondria and ER membranes, thereby causing mitochondrial dysfunction and alterations in autophagosome assembly [23].
The amyloid peptide structure defines its ability to self-assemble; monomers are considered non-toxic, but so are oligomers. Some authors use the term “smallest neurotoxic species” to refer to soluble Aβ oligomers. Many previous investigations confirm this point of view; for example, Shankar et al. showed that the injection of Aβ oligomers collected directly from the brains of AD patients into rat hippocampus impairs synaptic plasticity and causes memory deterioration [24]. It was shown that Aβ oligomers have an even higher ability to be incorporated into lipid membrane than their monomeric form, and they may form protofibril rings, which are able to create pores within a plasma membrane. This causes ion influx and the disruption of Ca2+ homeostasis, followed by mitochondrial disfunction and neuronal death [25].
The assembly of Aβ into higher-order molecular forms proceeds by the mechanism of nucleated polymerization. This mechanism suggests that nucleation components, including the extracellular matrix, lipid surfaces, such as plasma membranes, and metal ions serve as the “scaffold” for different forms of Aβ [18]. Protofibrils demonstrate high neurotoxicity; e.g., in a transgenic model of AD, many protofibrils were detected, and the progressing neuronal dysfunction appeared before the formation of fibrils and plaques [26]. In addition, Yasumoto et al. reported that protofibrils induce reactive oxygen species generation, lipid peroxidation, leading to calcium dysregulation, and the depolarization of iPSC-generated human cortical neuron plasma membrane [27]. Contrary to protofibrils, the fibrils and plaques formed by Aβ may not exert neurotoxicity [28], but many data prove the causative role of the latter in synaptic dysfunction [3].
Similar to other cellular polypeptides, Aβ undergoes post-translational modifications, including phosphorylation, nitrosylation, pyroglutamination, glycosylation, etc.
For example, Aβ phosphorylation at Ser8 is traditionally associated with an increase in the ability of the peptide to form aggregates, as well as an increase in its toxic effect [29]. Interestingly, the interaction of phosphorylated Aβ with zinc has the opposite effect; the formation of stable dimers in the presence of zinc prevents aggregation and toxicity of the peptide [30]. Moreover, it was shown that administration of the phosphorylated Aβ to B6C3-Tg transgenic mice prevented the formation of amyloid plaques in the hippocampus of the animals [31]. Likewise, the isomerization of Asp7 was shown to induce Aβ toxicity. When studying the response of cellular models of AD to Aβ with isoAsp7, the latter was found to be more toxic than its naïve form and to induce apoptosis [32,33], while in the case of intact Aβ peptides, necrosis dominated [34]. Another well-known modification of Aβ—pyroglutamate—increases the hydrophobicity of the peptide, prevents its degradation, and increases the tendency to form aggregates and toxicity [35]. It was also shown in transgenic mice of the TBA2 (expresses Aβ with N-terminal glutamine) line that this modification of Aβ accelerates the progression of AD in vivo [36]. Indeed, there are clearance mechanisms that withstand the toxicity of Aβ oligomers, including the glymphatic system, perivascular drainage, and blood–brain barrier, but their function is often impaired in the aged brain [5,37]. Another way of amyloid removal that is associated with its internalization, packing into endosomes, and lysosomal degradation can also exhibit negative alterations with aging. Impaired lysosomal function in older brain tissue may cause the local condensation of amyloids into the lumen of late endosomes, forming cytotoxic structures in brain cells; this compartment becomes a suitable site for Aβ to aggregate [38]. It can be assumed that Aβ is released from endosomes into the cytoplasm, proving that the intracellular location of the peptide may provide a cytotoxic effect [39].
Thus, the structural (including isomerization, oligomerization, and aggregation) and chemical (including phosphorylation) modifications of Aβ can greatly affect its toxic properties. Moreover, such changes in toxicity may depend on Aβ partner proteins.
2.2. Protein Interactome of Aβ
Various forms of Aβ are neurotoxic by themselves; however, in many cases, amyloid-mediated pathogenicity is due to its effects on the plasma membrane, synaptic space, cytosol, endosomes, etc., where Aβ interacts with a great number of cellular proteins, some of which are implicated in cell damage.
The proteomic analysis of amyloid plaques shows approximately 2000 proteins with significant variation in samples of AD patients with a distinct clinical story. Liao et al. found that the level of at least 26 proteins is higher in plaques compared with healthy brain samples. Among these proteins are tau, proteins involved in vesicular transport proteolysis (cathepsin D, lysosomal ATPase, ubiquitin-activating enzyme E1) and inflammation (GFAP and vimentin), and HSP90 chaperone [40]. Data from the proteomic analysis of brain- and CSF-derived extracellular vesicles from AD patients show that heat shock protein 70 (HSP70), puromycin-sensitive aminopeptidase (NPEPPS), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), transthyretin, cystatin C, and prostaglandin F2 receptor negative regulator (PTGFRN) can also be implicated in AD pathogenesis, since their expression varies during the pathogenic process [41]. Many of the above proteins found to be involved in the progression of Alzheimer’s disease are potential interactors of the amyloid (Table 1). The list of Aβ interactors including K(lysine) acetyltransferase 5 (Tip60) [42], fibulin 1 [43], solute carrier family 25 (mitochondrial carrier; adenine nucleotide translocator), member 4 (SLC25A4) [44], and essential meiotic structure-specific endonuclease 1 (EME1) [45] as the most abundant, according to the Biogrid database, are provided below (Table 1).

Table 1.
Potential interactors of Aβ and their function in AD progression.
Taking into consideration the data presented above, there are plenty of Aβ partner proteins, and some of them have attracted the attention of investigators in the search for new targets of anti-AD therapy.
2.3. Proteins Whose Function Is Affected by Aβ
During the progression of AD in the intercellular space, as well as in the cytoplasm of brain cells, a significant accumulation of the Aβ peptide is observed. Accordingly, greater amounts of the peptide interactors could be recruited to impact the pathogenic function of the peptide. Conversely, in conditions of enhanced protein–protein interaction probability, Aβ itself may affect the function of its binders, and in following chapters we discuss the outcomes of such interactions.
2.3.1. Tau-Protein
Microtubule-associated protein tau (MAPT, tau) is one of Aβ’s best known partners. The normal function of tau is to regulate the formation of tubulin microtubules, predominantly in neuronal axons [48] and, to a lesser extent, in dendritic cells [49]. Other functions of tau have been discovered in the past decade, including translational regulation, insulin signaling, and others [50]. In AD pathogenesis, tau is known to form protofibrils, which convert into fibrillar cytotoxic structures, and the process can occur both in the cerebrospinal fluid and inside neural cells [51].
It is believed that Aβ causes the hyperphosphorylation of tau, which, on the one hand, disrupts the normal function of the latter, and, on the other hand, leads to fibrillation and neurotoxicity [52]. Notably, tau phosphorylation is an ordinary event in cell physiology [53,54], and eight possible phosphorylation sites have been discovered on all six isoforms of tau [55,56]. The mechanisms of Aβ’s effect on tau hyperphosphorylation are not yet clear, despite the fact that there is much evidence of the two polypeptides’ coaggregation in the brains of AD patients [57,58]. In a recent report, the effect of Aβ on the kinases phosphorylating tau was evidenced [59], while earlier data suggest the possibility of targeting by Aβ available tau phosphorylation sites [60] (Figure 2A).
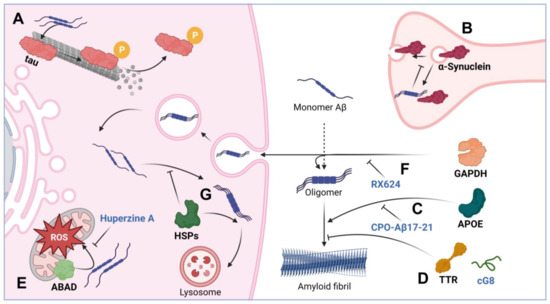
Figure 2.
Modulation of Aβ toxicity with interactor proteins and their dissociators. The figure schematically shows the interaction: (A) Aβ with tau causing microtubule disruption. (B) Aβ interaction with α-synuclein causing vesicle trafficking disorder. (C) APOE promotes the formation of amyloid fibrils; CPO-Aβ17–21 peptide may block this interaction. (D) TTR and its cG8 mimetic prevent the formation of toxic amyloid fibrils. (E) ABAD interacts with Aβ, activates ROS in mitochondria; this process can be prevented with the help of Huperzine A. (F) GAPDH activates the formation of amyloid fibrils due to direct interaction with Aβ and promotes horizontal transfer of the amyloid; the hydrocortisone derivative RX624 is able to block the pathogenic action of GAPDH. (G) Chaperones prevent the formation of toxic amyloid fibrils and are involved in the lysosomal and proteolytic degradation of Aβ. The figure was created with BioRender.com, accessed on 28 December 2022.
2.3.2. α-Synuclein
α-synuclein is a neuronal protein which is thought to be involved in the regulation of synaptic transmission through vesicle trafficking [61]. During the progression of AD, and in other neurodegenerative pathologies, including Parkinson’s disease, Aβ and synuclein may interact to increase each other’s amyloidogenic potential [62]. It has recently been shown in vitro and in vivo in Tg2576 transgenic mice that Aβ can serve as a trigger for α-synuclein aggregation [63]. It was also established that Aβ is able to influence α-synuclein functioning via phosphorylation at Ser129, which has been proven in vitro in SH-SY5Y cells and in brain tissue homogenates [64]; the enhanced phosphorylation corroborated with the formation of insoluble aggregates of α-synuclein [65], and a violation of its synaptic function [66,67] (Figure 2B). Interestingly, the formation of synuclein aggregates (Lewy bodies) is associated with the indications of synucleopathies, thus suggesting that the effect of Aβ on α-synuclein in some way blurs the line between Alzheimer’s and Parkinson’s disease. However, the exact mechanism of the effect of Aβ on α-synuclein phosphorylation is still elusive; as a possible option, death-associated protein kinase 1 is worth mentioning, for which the ability to phosphorylate α-synuclein at Ser129 with subsequent synuclein aggregation has also been demonstrated [65,68].
Thus, the interaction of Aβ with normal proteins and peptides often leads to a violation of the physiological functions of the latter, and even, in some cases, to their involvement in cytotoxic complexes.
2.4. Proteins Affecting Aβ Toxicity
The cytotoxicity of Aβ1–42, being the basis of its pathogenicity, can be mediated through a variety of mechanisms based on polypeptides, whose role and interactions with Aβ have been proven (Figure 2); some of these proteins are considered in the following chapter.
2.4.1. Apolipoprotein E
Apolipoprotein E (APOE) is a protein with a well-established role in AD pathogenesis. Normally, it is an extracellular protein, whose major function is the transport of cholesterol and lipids between neuronal and glial cells [69]. According to the generally accepted view, isoform 4 of the APOE protein is associated with a high risk of rapid progression of AD, while isoform 2, on the contrary, is associated with a low risk [70]. The interaction between APOE and Aβ is traditionally considered in two aspects: (i) in the pro-survival context of Aβ clearance [71,72], it has been repeatedly demonstrated that APOE binding Aβ ensures its transport across the blood–brain barrier [73], as well as its uptake and utilization by cellular proteolytic systems [74]; and (ii) in the pro-aggregation context, APOE may induce Aβ oligomerization and aggregation (Figure 2C). Thus, the interaction of extracellular APOE with Aβ in a number of experiments initiated the formation of toxic aggregates based on Aβ [75,76]. Interestingly, the opposite statement is also correct—the interaction of Aβ with APOE causes the formation of neurotoxic oligomers based on APOE—which was convincingly demonstrated in SK-N-SH cells [77].
2.4.2. Transthyretin
Transthyretin is a protein necessary for the transport of thyroxine and retinol; its concentration is highest in the cerebrospinal fluid, where its share can be up to 20% of the total protein content [78]. Mutations in the gene encoding transthyretin are commonly associated with amyloidosis [79]. Transthyretin was first described as an Aβ-binding protein in 1994 [80]. Probably due to its ability to sequester Aβ, transthyretin may decrease the amplitude of the protopathic stress induced by amyloids, which has been repeatedly demonstrated, including in APPswe/PS1A246E transgenic mice [81]. The results of experiments in vivo and in AD patients indicate that, being in the blood plasma or in the cerebrospinal fluid, transthyretin is able to prevent the formation of Aβ-based amyloid fibrils (Figure 2D) [82]. The protective mechanism of transthyretin is associated with its ability to directly bind Aβ and thus prevent the processes of primary and secondary nucleation [83]. This action reduces not only the toxicity of amyloid-based fibrils, but also their ability to grow further.
2.4.3. ABAD
Another protein that has been shown to influence Aβ-mediated cytotoxicity is Aβ-binding alcohol dehydrogenase (ABAD). ABAD is a mitochondrial protein that can bind Aβ predominantly in the mitochondria. In AD pathogenesis, the level of ABAD expression is increased, primarily in the hippocampus and cerebral cortex [84]. We can consider the relationship between the functioning of mitochondria and the progression of AD to be convincing and repeatedly proven [85]. Mitochondrial dysfunction is known to contribute to a reduction in energy metabolism, the elevation of free radical formation, and the violation of cellular calcium homeostasis, all being relevant to the pathogenesis of AD [86]. One of the possible mechanisms of mitochondrial dysfunction in AD is associated with the accumulation of Aβ in the mitochondria; in particular, in mitochondrial cristae [87]. At the same time, many researchers agree that the accumulation of the pathogenic peptide in mitochondria occurs through its anchoring with ABAD [88] (Figure 2E). Since the Aβ–ABAD interaction is believed to promote Aβ-mediated mitochondrial and neuronal dysfunction, this complex can become a therapeutic target in the treatment of AD [89].
2.4.4. GAPDH
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), another protein essential for normal cell function, is known as one of the basic enzymes driving glycolysis; however, it is also implied in numerous crucial events in cell physiology, particularly in response to oxidative stress [90]. GAPDH can be covalently crosslinked with Aβ [91,92,93] and can significantly increase the toxicity of its complex with Aβ occurring in the intercellular space (Figure 2F) [94]. Using the method of enzyme immunoassay, we established that tissue transglutaminase (tTG) enhances the interaction between GAPDH and Aβ1–42; moreover, tTG accelerated the formation of co-aggregates of GAPDH and Aβ1–42 by covalent crosslinking through the 15th glutamine on the Aβ molecule and lysines on the GAPDH molecule, [95]. According to atomic force microscopy data, GAPDH with Aβ in the presence of tTG may form amyloid-like structures. Notably, the level of GAPDH expression correlated with AD progression in a chemically induced AD model in rats—a high level of GAPDH expression led to severe memory dysfunction and the formation of intensive amyloid plaques. Finally, we found that in patients with AD, the GAPDH–Aβ complex is present in the cerebrospinal fluid, and the amount of the complex correlates well with the severity of disease [94]. These data suggest that GAPDH can be a target for AD therapy, which was proved in our work: the use of the GAPDH binder, a hydrocortisone derivative RX624, in the 5xFAD transgenic mice model led to a significant slowdown in the progress of memory impairment and the formation of amyloid plaques.
2.4.5. Chaperones
Chaperones comprise a group of proteins that are able to bind Aβ and reduce the level of the peptide-initiated pathological processes (Figure 2G). Chaperones provide cell protection under stressful conditions, prevent the development of apoptosis, participate in the processes of protein folding and renaturation, and are necessary for the recognition and labeling of damaged proteins to be utilized through autophagy [96] and proteasomal machineries [97].
The ability of Hsp70 and Hsp90 to bind Aβ oligomers and prevent their further growth was demonstrated in vitro [98]. Moreover, the concentration of chaperones sufficient for the effective blocking of the processes of aggregation was 50 times lower than the concentration of Aβ, suggesting that the inhibitory activity is most effective in the early stages of oligomerization.
Recent data demonstrate that the interaction of the Hsp110 co-chaperone with Aβ can also prevent the formation of Aβ fibrils [99]. Using a Drosophila AD model, Yakubu et al. showed that the β-subunit of the Hsp110 substrate-binding domain is required to provide a protective interaction with amyloid.
Interestingly, the cognate form of the Hsp70 protein, Hsc70, is able to bind the amyloid precursor protein via the KFERQ-motif and ensure its utilization by the mechanism of chaperone-mediated autophagy [100]. It is believed that such utilization of the precursor reduces the potential toxicity of Aβ. Based on the mechanism of chaperone-mediated autophagy, a new approach for AD therapy has recently been proposed, based on labeling toxic Aβ oligomers with KFERQ-motifs, which ensured the utilization of Aβ by lysosomes [101].
In general, the ability of the chaperone machine to bind Aβ and prevent the formation of amyloid aggregates and their toxicity in vitro is well-established [102,103], but experimental confirmation of this hypothesis in vivo has not yet been presented.
Notably, the data obtained from the clinical analysis of the chaperones and their interactors, together called the epichaperome, led to the conclusion that the state of this united interactome is tightly associated with the pathogenesis of many neurological diseases, including AD [104]. In this case, the therapeutic effect can be obtained with the drug correction of the epichaperome—networks of interprotein intra- and extracellular interactions, including those involving chaperones and Aβ [105].
2.4.6. Cystatin C
Cystatin C is a protein capable of forming amyloid structures on its own under certain circumstances [106,107,108]. Nevertheless, Selenica et al. recently established in vitro that the direct binding of cystatin to Aβ in a 1:1 molar ratio prevented the further formation of oligomers and aggregates of the latter [109]. Moreover, using transgenic mice with the overexpression of cystatin, it was found that increasing the concentration of cystatin C prevented the deposition and formation of Aβ-based amyloid plaques [110,111]. Later, using cultured primary hippocampal neurons, it was demonstrated that the addition of purified recombinant human cystatin C significantly reduced the cytotoxicity of growing Aβ oligomers [112]. Moreover, for a significant decrease in the toxicity of amyloid, near-physiological concentrations of cystatin C are sufficient. Despite such results, attempts to use cystatin C as a target for AD therapy have not yet been undertaken.
From the presented data, it follows that normal non-pathogenic proteins can affect the cytotoxic properties of Aβ in completely diverse ways (Figure 2). The complexes formed with the peptide in some cases have a higher toxicity compared with Aβ oligomers, as exemplified by GAPDH; sometimes, on the contrary, the interaction of proteins with Aβ significantly reduces the toxic potential of the complex (such as cystatin C).
3. Chemicals Targeting Aβ and Its Intermolecular Complexes
Aβ is one of the most ubiquitous drug targets in neurology, and to date, approximately 100 different substances remain in different stages of clinical trials; 28 of them were claimed in 2019 to target the Aβ or APP cleavage mechanism [ClinicalTrials.gov; Alzforum.org]. Generally, an efficient anti-amyloid drug must recognize and bind oligomeric and protofibrillar amyloid forms to prevent their conversion to cytotoxic fibrils (Table 2). As such, the most well-known anti-AD drug, aducanumab, is based on a monoclonal antibody generated against Aβ aggregates and humanized for therapeutic use. In the experiments performed on transgenic mice, the antibody was reported to ameliorate cognitive functions, to clean the brain tissue of the peptide, and to activate microglia phagocytosis [113]. However, the ability of aducanumab to recognize toxic species of Aβ is limited [114]; this and problems with the clinical use of the medicine have cast doubt on its future. Hence, the approval of aducanumab by the Food and Drug Administration in 2021 was one of the most criticized FDA decisions in recent years [115]. Very recently, Lecanemab, another humanized monoclonal antibody that binds to Aβ soluble protofibrils, demonstrated reduced markers of amyloid in persons with early AD in Phase III clinical trials while the authors assume that longer trials are warranted to estimate the effectiveness. It is likely that the new drug Lecanemab (also based on antibodies) will be more effective than aducanumab, but so far it has certain side effects [116].
The list of compounds able to reduce Aβ pathogenicity includes a number of synthetic or natural peptides, some of which share an amino acid sequence similar to the hydrophobic domains of Aβ (aa16–20, aa11–23, or aa32–37), and were reported to decrease the formation of fibrils consisting of the protein target [117]. An example of such compounds is the peptide-targeting amyloid fragment 13–23 designed using the molecular dynamics approach; the peptide was found to block amyloid–amyloid binding, the first step of the formation of toxic Aβ species [118]. More recently, Kim et al. presented a collection of synthetic peptides able to recognize various domains of Aβ molecules and to serve as diagnostic tools, if their clinical use is not excluded [119]. Overall, it is likely that compounds modifying or binding amyloid-β would influence its multiple interactions with other molecules.
Indeed, during the last decade, a few novel small molecules able to inhibit Aβ misfolding and enhance its clearance were reported, one of which, the LS-4 amphiphilic compound, demonstrated the extremely high binding affinity toward various Aβ forms, especially for soluble Aβ oligomers. In the 5xFAD mouse model, LS-4 reduced the amount of amyloid plaques and phosphorylated tau aggregates, the most-known manifestations of AD [120].
Proteins binding Aβ may impact its pathogenicity, and therefore their complexes with the polypeptide have become essential druggable targets for AD therapy [121]. One of the key Aβ-binding proteins is ApoE. Liu et al. found that the inhibition of its interaction with Aβ using CPO-Aβ17–21 peptide led to a decrease in cognitive impairment and neuroprotection in an AD APP/PS1 transgenic mouse model [122]. Recent studies have shown that blocking the ability of APOE to initiate Aβ oligomerization also has a therapeutic effect—a similar approach has been successfully tested in imipramine and olanzapine preparations on 5xFAD transgenic mice, TgF344-AD transgenic rats, and a primary neuronal culture obtained from these animals [123].
Transthyretin was also demonstrated to bind Aβ in cell and animal AD models; this binding leads to a reduction in the toxicity of Aβ-based fibrillar structures, and several approaches have been proposed to establish such an effect. First, it was shown that a cyclic peptide cG8 comprising the Aβ-binding domain of transthyretin reduced the deposits of fibrillar Aβ; the authors stated that success was achieved, and believed that the optimization of the mimetic should be continued [124]. A similar approach was used in another study, in which an Aβ-binding peptide fragment of transthyretin was able to prevent the toxic effect of Aβ on SH-SY5Y and PC-12 cells [125].
The ability of ABAD to bind Aβ was firmly attributed to AD pathogenesis, and a few attempts have been made to prevent the interaction between the two proteins. First, the prevention of the interaction between Aβ and ABAD in mAPP mice using a specific ABAD-decoy peptide protected neurons from Aβ-mediated toxicity [126]. Secondly, Huperzine A, an alkaloid isolated from lycopodium, was found to reduce the deposition of Aβ and the ABAD level, as well to weaken Aβ–ABAD interaction and finally to ameliorate cerebral mitochondrial function in APP/PS1 mice [127]. Evidence of the neuroprotective effect of ABAD blocking comes also from the recent data obtained in experiments on human SH-SY5Y cells and the culture of primary neurons of 5xFAD transgenic mice; ABAD inhibition was performed with allopurinol derivatives and resulted in a reduction in Aβ-induced mitochondrial dysfunction [128].
Another potential therapeutic approach is to block the formation of the GAPDH–Aβ complex, which was found to be extremely cytotoxic to neural cells. Thus, it was shown that the hydrocortisone derivative RX624, capable of binding GAPDH, in a 5xFAD transgenic mice model of Alzheimer’s disease, not only inhibited the formation of the GAPDH–Aβ complex, but also prevented memory impairment [94]. Additionally, an extensive panel of GAPDH inhibitors was presented that prevented the aggregation of oxidized protein and reduced its intra- and extracellular cytoxicity; the list includes deprenyl, PGL-135, N-phenoxyacetyl-L-cysteine [129], RX409, RX426, and RX648 [130]. It is believed that such compounds may be useful in preventing the formation of the GAPDH–Aβ complex in the context of AD pathogenesis.
Inducers of chaperone synthesis are worth mentioning as a separate therapeutic approach aimed at blocking the formation of Aβ complexes with other proteins [131]. Since the molecular chaperones, in particular Hsp70, act as strong factors dissociating protein complexes with the peptide by binding Aβ [98], the induction of the chaperone can impact the therapeutic development of a variety of neurodegenerative diseases, including AD. Among the inducers with demonstrated efficacy in in vitro models of Alzheimer’s disease are celastrol [132], geranylgeranylacetone [133], 17-AAG Hsp90 inhibitor [134,135], and the recently discovered PQ-29 and other pyrrolyl- and indolylazine derivatives [136,137].

Table 2.
Therapeutical agents with action based on prevention of Aβ-containing pathological complexes formation.
Table 2.
Therapeutical agents with action based on prevention of Aβ-containing pathological complexes formation.
| Therapeutical Agent (Class of Agents) | Potential Function | Reference |
|---|---|---|
| Aducanumab (specific antibodies) and other agents preventing formation of Aβ fibrils formation | Prevention of Aβ assemblage into cytotoxic fibrils | [113] |
| Synthetic and natural peptides that may block amyloid–amyloid binding | Interaction conditioned by the similarity to the hydrophobic domains of Aβ | [117] |
| Small molecules able to inhibit Aβ misfolding and enhance its clearance (LS4, for example) | Specifically binding to different soluble forms of Aβ | [120] |
| CPO-Aβ17–21 peptide | Blocking the ability of APOE to initiate Aβ oligomerization | [122] |
| Cyclic peptide cG8 | TTR-mimetic peptide comprising its Aβ-binding domain | [124] |
| Huperzine A and other ABAD blocking compounds | ABAD inhibition reduces Aβ-induced mitochondrial dysfunction | [127] |
| GAPDH–Aβ complex inhibitors | Blocking the formation of the GAPDH–Aβ complex and reduction of its cytotoxicity | [94,138] |
| Chaperone synthesis inducers | Newly synthesized chaperones block the formation of Aβ complexes with other proteins | [131,136,137] |
4. Conclusions
Proteins interacting by any way with various forms of Aβ can become markers of AD and there are samples where they are employed so. Aβ was found to kill neurons or other cells by influencing function of a few of important proteins appearing in extracellular space and particularly concentrating in certain locations, such as in interstitial liquids. In such domains the concentration of the latter complexes may increase to values becoming very toxic and this may be a reason for neuronal death. We further hypothesize that the process of cell death once initiated would be expanded and result in the release of more Aβ interactors from dying cells, including alarmins and activation of microglia response, as established in many instances. This mortality circle can be interrupted by using molecules specifically dissociating Aβ complexes. To date, extensive information has been accumulated on possible dissociators of Aβ-containing complexes. In most cases, these are small molecules of completely different chemical nature. Probably, the modulation of the toxic activity of Aβ-containing aggregates with the participation of various normal proteins is a biologically significant process that plays a decisive role in the pathogenesis of Alzheimer’s disease. Thus, blocking the formation of numerous protein complexes involving Aβ may be a more effective therapeutic strategy than inhibiting the formation of oligomers and aggregates formed only with Aβ.
Author Contributions
Conceptualization, B.A.M.; writing—original draft preparation, B.A.M., V.F.L., E.A.D. and I.E.K.; writing—review and editing, B.A.M., I.V.G. and V.F.L.; visualization, E.A.D.; supervision, B.A.M.; project administration, I.V.G.; funding acquisition, I.V.G. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the Ministry of Science and Higher Education of Russia, Research Project N 075-15-2020-795, local identifier 13.1902.21.0027.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Briggs, C.A.; Chakroborty, S.; Stutzmann, G.E. Emerging pathways driving early synaptic pathology in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2017, 483, 988–997. [Google Scholar] [CrossRef]
- Pistollato, F.; Cano, S.S.; Elio, I.; Vergara, M.M.; Giampieri, F.; Battino, M. Associations between Sleep, Cortisol Regulation, and Diet: Possible Implications for the Risk of Alzheimer Disease. Adv. Nutr. 2016, 7, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Luehmann, M.; Spires-Jones, T.L.; Prada, C.; Garcia-Alloza, M.; De Calignon, A.; Rozkalne, A.; Koenigsknecht-Talboo, J.; Holtzman, D.M.; Bacskai, B.J.; Hyman, B.T. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature 2008, 451, 720–724. [Google Scholar] [CrossRef]
- Knowles, R.B.; Wyart, C.; Buldyrev, S.V.; Cruz, L.; Urbanc, B.; Hasselmo, M.E.; Stanley, H.E.; Hyman, B.T. Plaque-induced neurite abnormalities: Implications for disruption of neural networks in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 1999, 96, 5274–5279. [Google Scholar] [CrossRef] [PubMed]
- Bateman, G.A.; Levi, C.R.; Schofield, P.; Wang, Y.; Lovett, E.C. Quantitative measurement of cerebral haemodynamics in early vascular dementia and Alzheimer’s disease. J. Clin. Neurosci. 2006, 13, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers. Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Meyer, H.E.; Egensperger, R.; Marcus, K. The amyloid precursor protein intracellular domain (AICD) as modulator of gene expression, apoptosis, and cytoskeletal dynamics-relevance for Alzheimer’s disease. Prog. Neurobiol. 2008, 85, 393–406. [Google Scholar] [CrossRef]
- Pereira, J.B.; Janelidze, S.; Ossenkoppele, R.; Kvartsberg, H.; Brinkmalm, A.; Mattsson-Carlgren, N.; Stomrud, E.; Smith, R.; Zetterberg, H.; Blennow, K.; et al. Untangling the association of amyloid-β and tau with synaptic and axonal loss in Alzheimer’s disease. Brain 2021, 144, 310–324. [Google Scholar] [CrossRef]
- Wiatrak, B.; Piasny, J.; Kuźniarski, A.; Gąsiorowski, K. Interactions of Amyloid-β with Membrane Proteins. Int. J. Mol. Sci. 2021, 22, 6075. [Google Scholar] [CrossRef]
- Bush, A.I.; Pettingell, W.H.; Multhaup, G.; Paradis, M.D.; Vonsattel, J.P.; Gusella, J.F.; Beyreuther, K.; Masters, C.L.; Tanzi, R.E. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science 1994, 265, 1464–1467. [Google Scholar] [CrossRef]
- Villaflores, O.B.; Chen, Y.J.; Chen, C.P.; Yeh, J.M.; Wu, T.Y. Curcuminoids and resveratrol as anti-Alzheimer agents. Taiwan. J. Obstet. Gynecol. 2012, 51, 515–525. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar]
- Hoe, H.S.; Fu, Z.; Makarova, A.; Lee, J.Y.; Lu, C.; Feng, L.; Pajoohesh-Ganji, A.; Matsuoka, Y.; Hyman, B.T.; Ehlers, M.D.; et al. The effects of amyloid precursor protein on postsynaptic composition and activity. J. Biol. Chem. 2009, 284, 8495–8506. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.C.; Ludewig, S.; Winschel, A.; Abel, T.; Bold, C.; Salzburger, L.R.; Klein, S.; Han, K.; Weyer, S.W.; Fritz, A.; et al. Distinct in vivo roles of secreted APP ectodomain variants APPsα and APPsβ in regulation of spine density, synaptic plasticity, and cognition. EMBO J. 2018, 37, e98335. [Google Scholar] [CrossRef] [PubMed]
- Dinamarca, M.C.; Raveh, A.; Schneider, A.; Fritzius, T.; Früh, S.; Rem, P.D.; Stawarski, M.; Lalanne, T.; Turecek, R.; Choo, M.; et al. Complex formation of APP with GABAB receptors links axonal trafficking to amyloidogenic processing. Nat. Commun. 2019, 10, 1331. [Google Scholar] [CrossRef]
- Hefter, D.; Ludewig, S.; Draguhn, A.; Korte, M. Amyloid, APP, and Electrical Activity of the Brain. Neuroscientist 2020, 26, 231–251. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Pittman, J.M.; Zerweck, J.; Venkata, B.S.; Moore, P.C.; Sachleben, J.R.; Meredith, S.C. β-Amyloid aggregation and heterogeneous nucleation. Protein Sci. 2019, 28, 1567–1581. [Google Scholar] [CrossRef] [PubMed]
- Sadleir, K.R.; Kandalepas, P.C.; Buggia-Prévot, V.; Nicholson, D.A.; Thinakaran, G.; Vassar, R. Presynaptic dystrophic neurites surrounding amyloid plaques are sites of microtubule disruption, BACE1 elevation, and increased Aβ generation in Alzheimer’s disease. Acta Neuropathol. 2016, 132, 235–256. [Google Scholar] [CrossRef]
- Rice, H.C.; De Malmazet, D.; Schreurs, A.; Frere, S.; Van Molle, I.; Volkov, A.N.; Creemers, E.; Vertkin, I.; Nys, J.; Ranaivoson, F.M.; et al. Secreted amyloid-β precursor protein functions as a GABABR1a ligand to modulate synaptic transmission. Science 2019, 363, eaao4827. [Google Scholar] [CrossRef]
- McLaurin, J.; Lai, A.Y. Mechanisms of amyloid-Beta Peptide uptake by neurons: The role of lipid rafts and lipid raft-associated proteins. Int. J. Alzheimers. Dis. 2010, 2011, 548380. [Google Scholar]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Leal, N.S.; Dentoni, G.; Schreiner, B.; Naia, L.; Piras, A.; Graff, C.; Cattaneo, A.; Meli, G.; Hamasaki, M.; Nilsson, P.; et al. Amyloid Β-Peptide Increases Mitochondria-Endoplasmic Reticulum Contact Altering Mitochondrial Function and Autophagosome Formation in Alzheimer’s Disease-Related Models. Cells 2020, 9, 2552. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Canevari, L.; Abramov, A.Y.; Duchen, M.R. Toxicity of amyloid beta peptide: Tales of calcium, mitochondria, and oxidative stress. Neurochem. Res. 2004, 29, 637–650. [Google Scholar] [CrossRef]
- Lord, A.; Englund, H.; Söderberg, L.; Tucker, S.; Clausen, F.; Hillered, L.; Gordon, M.; Morgan, D.; Lannfelt, L.; Pettersson, F.E.; et al. Amyloid-beta protofibril levels correlate with spatial learning in Arctic Alzheimer’s disease transgenic mice. FEBS J. 2009, 276, 995–1006. [Google Scholar] [CrossRef]
- Yasumoto, T.; Takamura, Y.; Tsuji, M.; Watanabe-Nakayama, T.; Imamura, K.; Inoue, H.; Nakamura, S.; Inoue, T.; Kimura, A.; Yano, S.; et al. High molecular weight amyloid β1-42 oligomers induce neurotoxicity via plasma membrane damage. FASEB J. 2019, 33, 9220–9234. [Google Scholar] [CrossRef]
- Penke, B.; Szucs, M.; Bogár, F. Oligomerization and Conformational Change Turn Monomeric β-Amyloid and Tau Proteins Toxic: Their Role in Alzheimer’s Pathogenesis. Molecules 2020, 25, 1659. [Google Scholar] [CrossRef]
- Jamasbi, E.; Separovic, F.; Hossain, M.A.; Ciccotosto, G.D. Phosphorylation of a full length amyloid-β peptide modulates its amyloid aggregation, cell binding and neurotoxic properties. Mol. Biosyst. 2017, 13, 1545–1551. [Google Scholar] [CrossRef]
- Mezentsev, Y.V.; Medvedev, A.E.; Kechko, O.I.; Makarov, A.A.; Ivanov, A.S.; Mantsyzov, A.B.; Kozin, S.A. Zinc-induced heterodimer formation between metal-binding domains of intact and naturally modified amyloid-beta species: Implication to amyloid seeding in Alzheimer’s disease? J. Biomol. Struct. Dyn. 2016, 34, 2317–2326. [Google Scholar] [CrossRef]
- Barykin, E.P.; Petrushanko, I.Y.; Kozin, S.A.; Telegin, G.B.; Chernov, A.S.; Lopina, O.D.; Radko, S.P.; Mitkevich, V.A.; Makarov, A.A. Phosphorylation of the Amyloid-Beta Peptide Inhibits Zinc-Dependent Aggregation, Prevents Na, K-ATPase Inhibition, and Reduces Cerebral Plaque Deposition. Front. Mol. Neurosci. 2018, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Kummer, M.P.; Heneka, M.T. Truncated and modified amyloid-beta species. Alzheimers. Res. Ther. 2014, 6, 28–29. [Google Scholar] [CrossRef]
- Yurinskaya, M.M.; Mitkevich, V.A.; Kozin, S.A.; Evgenev, M.B.; Makarov, A.A.; Vinokurov, M.G. HSP70 protects human neuroblastoma cells from apoptosis and oxidative stress induced by amyloid peptide isoAsp7-Aβ(1-42). Cell Death Dis. 2015, 6, e1977. [Google Scholar] [CrossRef] [PubMed]
- Mitkevich, V.A.; Petrushanko, I.Y.; Yegorov, Y.E.; Simonenko, O.V.; Vishnyakova, K.S.; Kulikova, A.A.; Tsvetkov, P.O.; Makarov, A.A.; Kozin, S.A. Isomerization of Asp7 leads to increased toxic effect of amyloid-β42 on human neuronal cells. Cell Death Dis. 2013, 4, e939. [Google Scholar] [CrossRef]
- Bayer, T.A. Pyroglutamate Aβ cascade as drug target in Alzheimer’s disease. Mol. Psychiatry 2022, 27, 1880. [Google Scholar] [CrossRef]
- Wirths, O.; Breyhan, H.; Cynis, H.; Schilling, S.; Demuth, H.U.; Bayer, T.A. Intraneuronal pyroglutamate-Abeta 3-42 triggers neurodegeneration and lethal neurological deficits in a transgenic mouse model. Acta Neuropathol. 2009, 118, 487–496. [Google Scholar] [CrossRef]
- Engelhardt, B.; Carare, R.O.; Bechmann, I.; Flügel, A.; Laman, J.D.; Weller, R.O. Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol. 2016, 132, 317–338. [Google Scholar] [CrossRef]
- Forester, B.P.; Berlow, Y.A.; Harper, D.G.; Jensen, J.E.; Lange, N.; Froimowitz, M.P.; Ravichandran, C.; Iosifescu, D.V.; Lukas, S.E.; Renshaw, P.F.; et al. Age-related changes in brain energetics and phospholipid metabolism. NMR Biomed. 2010, 23, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Hossain, M.S.; Bilkis, T.; Islam, M.I.; Park, I.S. Evidence for a Strong Relationship between the Cytotoxicity and Intracellular Location of β-Amyloid. Life 2022, 12, 577. [Google Scholar] [CrossRef]
- Liao, L.; Cheng, D.; Wang, J.; Duong, D.M.; Losik, T.G.; Gearing, M.; Rees, H.D.; Lah, J.J.; Levey, A.I.; Peng, J. Proteomic characterization of postmortem amyloid plaques isolated by laser capture microdissection. J. Biol. Chem. 2004, 279, 37061–37068. [Google Scholar] [CrossRef]
- Muraoka, S.; Jedrychowski, M.P.; Yanamandra, K.; Ikezu, S.; Gygi, S.P.; Ikezu, T. Proteomic Profiling of Extracellular Vesicles Derived from Cerebrospinal Fluid of Alzheimer’s Disease Patients: A Pilot Study. Cells 2020, 9, 1959. [Google Scholar] [CrossRef] [PubMed]
- Oláh, J.; Vincze, O.; Virók, D.; Simon, D.; Bozsó, Z.; Tokési, N.; Horváth, I.; Hlavanda, E.; Kovács, J.; Magyar, A.; et al. Interactions of pathological hallmark proteins: Tubulin polymerization promoting protein/p25, beta-amyloid, and alpha-synuclein. J. Biol. Chem. 2011, 286, 34088–34100. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, L.; Kakuyama, H.; Möllert, A.; Ito, A.; Winblad, B.; Tjernberg, L.O.; Näslund, J. Characterization of the Alzheimer’s disease-associated CLAC protein and identification of an amyloid beta-peptide-binding site. J. Biol. Chem. 2005, 280, 1007–1015. [Google Scholar] [CrossRef]
- Amadoro, G.; Corsetti, V.; Atlante, A.; Florenzano, F.; Capsoni, S.; Bussani, R.; Mercanti, D.; Calissano, P. Interaction between NH(2)-tau fragment and Aβ in Alzheimer’s disease mitochondria contributes to the synaptic deterioration. Neurobiol. Aging 2012, 33, 833.e1–833.e25. [Google Scholar] [CrossRef] [PubMed]
- Huttlin, E.L.; Bruckner, R.J.; Navarrete-Perea, J.; Cannon, J.R.; Baltier, K.; Gebreab, F.; Gygi, M.P.; Thornock, A.; Zarraga, G.; Tam, S.; et al. Dual proteome-scale networks reveal cell-specific remodeling of the human interactome. Cell 2021, 184, 3022–3040. [Google Scholar] [CrossRef]
- Gerber, H.; Mosser, S.; Boury-Jamot, B.; Stumpe, M.; Piersigilli, A.; Goepfert, C.; Dengjel, J.; Albrecht, U.; Magara, F.; Fraering, P.C. The APMAP interactome reveals new modulators of APP processing and beta-amyloid production that are altered in Alzheimer’s disease. Acta Neuropathol. Commun. 2019, 7, 13. [Google Scholar] [CrossRef]
- Panikker, P.; Xu, S.J.; Zhang, H.; Sarthi, J.; Beaver, M.; Sheth, A.; Akhter, S.; Elefant, F. Restoring Tip60 HAT/HDAC2 Balance in the Neurodegenerative Brain Relieves Epigenetic Transcriptional Repression and Reinstates Cognition. J. Neurosci. 2018, 38, 4569–4583. [Google Scholar] [CrossRef]
- Combs, B.; Mueller, R.L.; Morfini, G.; Brady, S.T.; Kanaan, N.M. Tau and Axonal Transport Misregulation in Tauopathies. Adv. Exp. Med. Biol. 2019, 1184, 81–95. [Google Scholar]
- Ittner, L.M.; Ke, Y.D.; Delerue, F.; Bi, M.; Gladbach, A.; van Eersel, J.; Wölfing, H.; Chieng, B.C.; Christie, M.J.; Napier, I.A.; et al. Dendritic function of tau mediates amyloid-β toxicity in alzheimer’s disease mouse models. Cell 2010, 142, 387–397. [Google Scholar] [CrossRef]
- Sotiropoulos, I.; Galas, M.C.; Silva, J.M.; Skoulakis, E.; Wegmann, S.; Maina, M.B.; Blum, D.; Sayas, C.L.; Mandelkow, E.M.; Mandelkow, E.; et al. Atypical, non-standard functions of the microtubule associated Tau protein. Acta Neuropathol. Commun. 2017, 5, 1–11. [Google Scholar] [CrossRef]
- Pichet Binette, A.; Franzmeier, N.; Spotorno, N.; Ewers, M.; Brendel, M.; Biel, D.; Weiner, M.; Aisen, P.; Petersen, R.; Jack, C.R.; et al. Amyloid-associated increases in soluble tau relate to tau aggregation rates and cognitive decline in early Alzheimer’s disease. Nat. Commun. 2022, 13, 6635. [Google Scholar] [CrossRef] [PubMed]
- Aliakbari, F.; Attar, F.; Movahedi, M.; Falahati, M. Human tau fibrillization and neurotoxicity in the presence of magnesium oxide nanoparticle fabricated through laser ablation method. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2022, 278, 121372. [Google Scholar] [CrossRef] [PubMed]
- Scholz, T.; Mandelkow, E. Transport and diffusion of Tau protein in neurons. Cell Mol. Life Sci. 2014, 71, 3139–3150. [Google Scholar] [CrossRef]
- Fan, Q.W.; Yu, W.; Senda, T.; Yanagisawa, K.; Michikawa, M. Cholesterol-dependent modulation of tau phosphorylation in cultured neurons. J. Neurochem. 2001, 76, 391–400. [Google Scholar] [CrossRef]
- Aragão Gomes, L.; Uytterhoeven, V.; Lopez-Sanmartin, D.; Tomé, S.O.; Tousseyn, T.; Vandenberghe, R.; Vandenbulcke, M.; von Arnim, C.A.F.; Verstreken, P.; Thal, D.R. Maturation of neuronal AD-tau pathology involves site-specific phosphorylation of cytoplasmic and synaptic tau preceding conformational change and fibril formation. Acta Neuropathol. 2021, 141, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Tomé, S.O. The central role of tau in Alzheimer’s disease: From neurofibrillary tangle maturation to the induction of cell death. Brain Res. Bull. 2022, 190, 204–217. [Google Scholar] [CrossRef]
- Mukherjee, S.; Dubois, C.; Perez, K.; Varghese, S.; Birchall, I.E.; Leckey, M.; Davydova, N.; McLean, C.; Nisbet, R.M.; Roberts, B.R.; et al. Quantitative proteomics of tau and Aβ in detergent fractions from Alzheimer’s disease brains. J. Neurochem. 2022. [Google Scholar] [CrossRef]
- Lam, S.; Hérard, A.S.; Boluda, S.; Petit, F.; Eddarkaoui, S.; Cambon, K.; Letournel, F.; Martin-Négrier, M.L.; Faisant, M.; Godfraind, C.; et al. Pathological changes induced by Alzheimer’s brain inoculation in amyloid-beta plaque-bearing mice. Acta Neuropathol. Commun. 2022, 10, 112. [Google Scholar] [CrossRef]
- Ikezu, S.; Ingraham Dixie, K.L.; Koro, L.; Watanabe, T.; Kaibuchi, K.; Ikezu, T. Tau-tubulin kinase 1 and amyloid-β peptide induce phosphorylation of collapsin response mediator protein-2 and enhance neurite degeneration in Alzheimer disease mouse models. Acta Neuropathol. Commun. 2020, 8, 12. [Google Scholar] [CrossRef]
- Manczak, M.; Reddy, P.H. Abnormal interaction of oligomeric amyloid-β with phosphorylated tau: Implications to synaptic dysfunction and neuronal damage. J. Alzheimers. Dis. 2013, 36, 285–295. [Google Scholar] [CrossRef]
- Atias, M.; Tevet, Y.; Sun, J.; Stavsky, A.; Tal, S.; Kahn, J.; Roy, S.; Gitler, D. Synapsins regulate α-synuclein functions. Proc. Natl. Acad. Sci. USA 2019, 166, 11116–11118. [Google Scholar] [CrossRef]
- Williams, D.M.; Thorn, D.C.; Dobson, C.M.; Meehan, S.; Jackson, S.E.; Woodcock, J.M.; Carver, J.A. The Amyloid Fibril-Forming β-Sheet Regions of Amyloid β and α-Synuclein Preferentially Interact with the Molecular Chaperone 14-3-3ζ. Molecules 2021, 26, 6120. [Google Scholar] [CrossRef] [PubMed]
- Köppen, J.; Schulze, A.; Machner, L.; Wermann, M.; Eichentopf, R.; Guthardt, M.; Hähnel, A.; Klehm, J.; Kriegeskorte, M.C.; Hartlage-Rübsamen, M.; et al. Amyloid-Beta Peptides Trigger Aggregation of Alpha-Synuclein In Vitro. Molecules 2020, 25, 580. [Google Scholar] [CrossRef] [PubMed]
- Swirski, M.; Miners, J.S.; De Silva, R.; Lashley, T.; Ling, H.; Holton, J.; Revesz, T.; Love, S. Evaluating the relationship between amyloid-β and α-synuclein phosphorylated at Ser129 in dementia with Lewy bodies and Parkinson’s disease. Alzheimers. Res. Ther. 2014, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.H.; Chung, K.C. Death-associated Protein Kinase 1 Phosphorylates α-Synuclein at Ser129 and Exacerbates Rotenone-induced Toxic Aggregation of α-Synuclein in Dopaminergic SH-SY5Y Cells. Exp. Neurobiol. 2020, 29, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Colom-Cadena, M.; Pegueroles, J.; Herrmann, A.G.; Henstridge, C.M.; Muñoz, L.; Querol-Vilaseca, M.; Martín-Paniello, C.S.; Luque-Cabecerans, J.; Clarimon, J.; Belbin, O.; et al. Synaptic phosphorylated α-synuclein in dementia with Lewy bodies. Brain 2017, 140, 3204–3214. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli-Daley, L.A.; Luk, K.C.; Patel, T.P.; Tanik, S.A.; Riddle, D.M.; Stieber, A.; Meaney, D.F.; Trojanowski, J.Q.; Lee, V.M.Y. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011, 72, 57–71. [Google Scholar] [CrossRef]
- Su, Y.; Deng, M.F.; Xiong, W.; Xie, A.J.; Guo, J.; Liang, Z.H.; Hu, B.; Chen, J.G.; Zhu, X.; Man, H.Y.; et al. MicroRNA-26a/Death-Associated Protein Kinase 1 Signaling Induces Synucleinopathy and Dopaminergic Neuron Degeneration in Parkinson’s Disease. Biol. Psychiatry 2019, 85, 769–781. [Google Scholar] [CrossRef]
- Mahley, R.W. Apolipoprotein E: Cholesterol Transport Protein with Expanding Role in Cell Biology. Science 1988, 240, 622–630. [Google Scholar] [CrossRef]
- Troutwine, B.R.; Hamid, L.; Lysaker, C.R.; Strope, T.A.; Wilkins, H.M. Apolipoprotein E and Alzheimer’s disease. Acta Pharm. Sin. B 2022, 12, 496–510. [Google Scholar] [CrossRef]
- Huynh, T.P.V.; Davis, A.A.; Ulrich, J.D.; Holtzman, D.M. Apolipoprotein E and Alzheimer’s disease: The influence of apolipoprotein E on amyloid-β and other amyloidogenic proteins. J. Lipid Res. 2017, 58, 824–836. [Google Scholar] [CrossRef]
- Sadowski, M.J.; Pankiewicz, J.; Scholtzova, H.; Mehta, P.D.; Prelli, F.; Quartermain, D.; Wisniewski, T. Blocking the apolipoprotein E/amyloid-beta interaction as a potential therapeutic approach for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2006, 103, 18787–18792. [Google Scholar] [CrossRef]
- Deane, R.; Sagare, A.; Hamm, K.; Parisi, M.; Lane, S.; Finn, M.B.; Holtzman, D.M.; Zlokovic, B.V. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J. Clin. Investig. 2008, 118, 4002–4013. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Lee, C.Y.D.; Mandrekar, S.; Wilkinson, B.; Cramer, P.; Zelcer, N.; Mann, K.; Lamb, B.; Willson, T.M.; Collins, J.L.; et al. ApoE promotes the proteolytic degradation of Abeta. Neuron 2008, 58, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ji, Y.; Mehta, P.; Bates, K.A.; Sun, Y.; Wisniewski, T. Blocking the apolipoprotein E/amyloid-β interaction reduces fibrillar vascular amyloid deposition and cerebral microhemorrhages in TgSwDI mice. J. Alzheimers. Dis. 2011, 24, 269–285. [Google Scholar] [CrossRef]
- Folin, M.; Baiguera, S.; Guidolin, D.; Di Liddo, R.; Grandi, C.; De Carlo, E.; Nussdorfer, G.G.; Parnigotto, P.P. Apolipoprotein-E modulates the cytotoxic effect of beta-amyloid on rat brain endothelium in an isoform-dependent specific manner. Int. J. Mol. Med. 2006, 17, 821–826. [Google Scholar]
- Dafnis, I.; Argyri, L.; Chroni, A. Amyloid-peptide β 42 Enhances the Oligomerization and Neurotoxicity of apoE4: The C-terminal Residues Leu279, Lys282 and Gln284 Modulate the Structural and Functional Properties of apoE4. Neuroscience 2018, 394, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J.; Wilcox, J.N.; Pham, K.T.C.; Fremeau, R.T.; Zeviani, M.; Dwork, A.; Soprano, D.R.; Makover, A.; Goodman, D.S.; Zimmerman, E.A. Transthyretin: A choroid plexus-specific transport protein in human brain. The 1986 S. Weir Mitchell award. Neurology 1986, 36, 900–911. [Google Scholar] [CrossRef]
- Saraiva, M.J.M. Transthyretin mutations in hyperthyroxinemia and amyloid diseases. Hum. Mutat. 2001, 17, 493–503. [Google Scholar] [CrossRef]
- Schwarzman, A.L.; Gregori, L.; Vitek, M.P.; Lyubski, S.; Strittmatter, W.J.; Enghilde, J.J.; Bhasin, R.; Silverman, J.; Weisgraber, K.H.; Coyle, P.K.; et al. Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proc. Natl. Acad. Sci. USA 1994, 91, 8368–8372. [Google Scholar] [CrossRef]
- Ribeiro, C.A.; Oliveira, S.M.; Guido, L.F.; Magalhães, A.; Valencia, G.; Arsequell, G.; Saraiva, M.J.; Cardoso, I. Transthyretin stabilization by iododiflunisal promotes amyloid-β peptide clearance, decreases its deposition, and ameliorates cognitive deficits in an Alzheimer’s disease mouse model. J. Alzheimers. Dis. 2014, 39, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Jung, E.S.; Sohn, J.H.; Hong, H.J.; Hong, H.S.; Kim, J.W.; Na, D.L.; Kim, M.; Kim, H.; Ha, H.J.; et al. Human serum transthyretin levels correlate inversely with Alzheimer’s disease. J. Alzheimers. Dis. 2011, 25, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ghadami, S.A.; Chia, S.; Ruggeri, F.S.; Meisl, G.; Bemporad, F.; Habchi, J.; Cascella, R.; Dobson, C.M.; Vendruscolo, M.; Knowles, T.P.J.; et al. Transthyretin Inhibits Primary and Secondary Nucleations of Amyloid-β Peptide Aggregation and Reduces the Toxicity of Its Oligomers. Biomacromolecules 2020, 21, 1112–1125. [Google Scholar] [CrossRef]
- Yao, J.; Irwin, R.W.; Zhao, L.; Nilsen, J.; Hamilton, R.T.; Brinton, R.D. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2009, 106, 14670–14675. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Song, X.; Zhu, C.; Patrick, R.; Skurla, M.; Santangelo, I.; Green, M.; Harper, D.; Ren, B.; Forester, B.P.; et al. Mitochondrial dysfunction, oxidative stress, neuroinflammation, and metabolic alterations in the progression of Alzheimer’s disease: A meta-analysis of in vivo magnetic resonance spectroscopy studies. Ageing Res. Rev. 2021, 72, 101503. [Google Scholar] [CrossRef]
- Olajide, O.J.; La Rue, C.; Bergdahl, A.; Chapman, C.A. Inhibiting amyloid beta (1–42) peptide-induced mitochondrial dysfunction prevents the degradation of synaptic proteins in the entorhinal cortex. Front. Aging Neurosci. 2022, 14, 960314. [Google Scholar] [CrossRef]
- Hansson Petersen, C.A.; Alikhani, N.; Behbahani, H.; Wiehager, B.; Pavlov, P.F.; Alafuzoff, I.; Leinonen, V.; Ito, A.; Winblad, B.; Glaser, E.; et al. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc. Natl. Acad. Sci. USA 2008, 105, 13145–13150. [Google Scholar] [CrossRef]
- Morsy, A.; Trippier, P.C. Amyloid-Binding Alcohol Dehydrogenase (ABAD) Inhibitors for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2019, 62, 4252–4264. [Google Scholar] [CrossRef]
- Borger, E.; Aitken, L.; Muirhead, K.E.A.; Allen, Z.E.; Ainge, J.A.; Conway, S.J.; Gunn-Moore, F.J. Mitochondrial β-amyloid in Alzheimer’s disease. Biochem. Soc. Trans. 2011, 39, 868–873. [Google Scholar] [CrossRef]
- Sirover, M.A. Moonlighting glyceraldehyde-3-phosphate dehydrogenase: Posttranslational modification, protein and nucleic acid interactions in normal cells and in human pathology. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 354–371. [Google Scholar] [CrossRef]
- Naletova, I.; Schmalhausen, E.; Kharitonov, A.; Katrukha, A.; Saso, L.; Caprioli, A.; Muronetz, V. Non-native glyceraldehyde-3-phosphate dehydrogenase can be an intrinsic component of amyloid structures. Biochim. Biophys. Acta-Proteins Proteomics 2008, 1784, 2052–2058. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Li, H.; Gao, Z. Amyloid beta modulated the selectivity of heme-catalyzed protein tyrosine nitration: An alternative mechanism for selective protein nitration. J. Biol. Inorg. Chem. 2012, 17, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Verdier, Y.; Földi, I.; Sergeant, N.; Fülöp, L.; Penke, Z.; Janáky, T.; Szücs, M.; Penke, B. Characterization of the interaction between Abeta 1-42 and glyceraldehyde phosphodehydrogenase. J. Pept. Sci. 2008, 14, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Lazarev, V.F.; Tsolaki, M.; Mikhaylova, E.R.; Benken, K.A.; Shevtsov, M.A.; Nikotina, A.D.; Lechpammer, M.; Mitkevich, V.A.; Makarov, A.A.; Moskalev, A.A.; et al. Extracellular GAPDH Promotes Alzheimer Disease Progression by Enhancing Amyloid-β Aggregation and Cytotoxicity. Aging Dis. 2021, 12, 1223–1237. [Google Scholar] [CrossRef]
- Orru, S.; Ruoppolo, M.; Francese, S.; Vitagliano, L.; Marino, G.; Esposito, C. Identification of tissue transglutaminase-reactive lysine residues in glyceraldehyde-3-phosphate dehydrogenase. Protein Sci. 2002, 11, 137–146. [Google Scholar] [CrossRef]
- Wang, Y.T.; Lu, J.H. Chaperone-Mediated Autophagy in Neurodegenerative Diseases: Molecular Mechanisms and Pharmacological Opportunities. Cells 2022, 11, 2250. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.R.; Gragera, M.; Ochoa-Ibarrola, L.; Quintana-Gallardo, L.; Valpuesta, J.M. Hsp70—A master regulator in protein degradation. FEBS Lett. 2017, 591, 2648–2660. [Google Scholar] [CrossRef]
- Evans, C.G.; Wisén, S.; Gestwicki, J.E. Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1-42) aggregation in vitro. J. Biol. Chem. 2006, 281, 33182–33191. [Google Scholar] [CrossRef]
- Yakubu, U.M.; Morano, K.A. Suppression of aggregate and amyloid formation by a novel intrinsically disordered region in metazoan Hsp110 chaperones. J. Biol. Chem. 2021, 296, 100567. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, D.H.; Yoon, S.Y. Regulation of amyloid precursor protein processing by its KFERQ motif. BMB Rep. 2016, 49, 337–342. [Google Scholar] [CrossRef]
- Dou, J.; Su, P.; Xu, C.; Wen, Z.; Mao, Z.; Li, W. Targeting Hsc70-based autophagy to eliminate amyloid β oligomers. Biochem. Biophys. Res. Commun. 2020, 524, 923. [Google Scholar] [CrossRef]
- Beretta, G.; Shala, A.L. Impact of Heat Shock Proteins in Neurodegeneration: Possible Therapeutical Targets. Ann. Neurosci. 2022, 29, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Sinnige, T.; Yu, A.; Morimoto, R.I. Challenging Proteostasis: Role of the Chaperone Network to Control Aggregation-Prone Proteins in Human Disease. Adv. Exp. Med. Biol. 2020, 1243, 53–68. [Google Scholar] [PubMed]
- Ginsberg, S.D.; Neubert, T.A.; Sharma, S.; Digwal, C.S.; Yan, P.; Timbus, C.; Wang, T.; Chiosis, G. Disease-specific interactome alterations via epichaperomics: The case for Alzheimer’s disease. FEBS J. 2022, 289, 2047–2066. [Google Scholar] [CrossRef] [PubMed]
- Inda, M.C.; Joshi, S.; Wang, T.; Bolaender, A.; Gandu, S.; Koren, J.; Che, A.Y.; Taldone, T.; Yan, P.; Sun, W.; et al. The epichaperome is a mediator of toxic hippocampal stress and leads to protein connectivity-based dysfunction. Nat. Commun. 2020, 11, 319. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, P.; Groves, P.; Szymanska, A.; Rodziewicz-Motowidlo, S. Human cystatin C monomer, dimer, oligomer, and amyloid structures are related to health and disease. FEBS Lett. 2016, 590, 4192–4201. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Jaskolski, M.; Grubb, A. The role of cystatin C in cerebral amyloid angiopathy and stroke: Cell biology and animal models. Brain Pathol. 2006, 16, 60–70. [Google Scholar] [CrossRef]
- Perlenfein, T.J.; Murphy, R.M. Expression, purification, and characterization of human cystatin C monomers and oligomers. Protein Expr. Purif. 2016, 117, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Selenica, M.L.; Wang, X.; Ostergaard-Pedersen, L.; Westlind-Danielsson, A.; Grubb, A. Cystatin C reduces the in vitro formation of soluble Abeta1-42 oligomers and protofibrils. Scand. J. Clin. Lab. Investig. 2007, 67, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Mi, W.; Pawlik, M.; Sastre, M.; Jung, S.S.; Radvinsky, D.S.; Klein, A.M.; Sommer, J.; Schmidt, S.D.; Nixon, R.A.; Mathews, P.M.; et al. Cystatin C inhibits amyloid-beta deposition in Alzheimer’s disease mouse models. Nat. Genet. 2007, 39, 1440–1442. [Google Scholar] [CrossRef]
- Kaeser, S.A.; Herzig, M.C.; Coomaraswamy, J.; Kilger, E.; Selenica, M.L.; Winkler, D.T.; Staufenbiel, M.; Levy, E.; Grubb, A.; Jucker, M. Cystatin C modulates cerebral beta-amyloidosis. Nat. Genet. 2007, 39, 1437–1439. [Google Scholar] [CrossRef] [PubMed]
- Tizon, B.; Ribe, E.M.; Mi, W.; Troy, C.M.; Levy, E. Cystatin C protects neuronal cells from amyloid-beta-induced toxicity. J. Alzheimers. Dis. 2010, 19, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Frost, C.V.; Zacharias, M. From monomer to fibril: Abeta-amyloid binding to Aducanumab antibody studied by molecular dynamics simulation. Proteins 2020, 88, 1592–1606. [Google Scholar] [CrossRef]
- Jeremic, D.; Jiménez-Díaz, L.; Navarro-López, J.D. Past, present and future of therapeutic strategies against amyloid-β peptides in Alzheimer’s disease: A systematic review. Ageing Res. Rev. 2021, 72, 101496. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Baig, M.H.; Ahmad, K.; Rabbani, G.; Choi, I. Use of Peptides for the Management of Alzheimer’s Disease: Diagnosis and Inhibition. Front. Aging Neurosci. 2018, 10, 21. [Google Scholar] [CrossRef]
- Mehrazma, B.; Robinson, M.; Opare, S.K.A.; Petoyan, A.; Lou, J.; Hane, F.T.; Rauk, A.; Leonenko, Z. Pseudo-peptide amyloid-β blocking inhibitors: Molecular dynamics and single molecule force spectroscopy study. Biochim. Biophys. Acta. Proteins Proteom. 2017, 1865, 1707–1718. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, E.H.; Lee, S.C.; Kim, A.R.; Park, H.H.; Son, J.W.; Koh, S.H.; Yoon, M.Y. Development of peptide aptamers as alternatives for antibody in the detection of amyloid-beta 42 aggregates. Anal. Biochem. 2020, 609, 113921. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cho, H.J.; Sen, S.; Arango, A.S.; Huynh, T.T.; Huang, Y.; Bandara, N.; Rogers, B.E.; Tajkhorshid, E.; Mirica, L.M. Amphiphilic Distyrylbenzene Derivatives as Potential Therapeutic and Imaging Agents for Soluble and Insoluble Amyloid β Aggregates in Alzheimer’s Disease. J. Am. Chem. Soc. 2021, 143, 10462–10476. [Google Scholar] [CrossRef]
- Medvedev, A.; Buneeva, O.; Kopylov, A.; Gnedenko, O.; Ivanov, A.; Zgoda, V.; Makarov, A.A. Amyloid-binding proteins: Affinity-based separation, proteomic identification, and optical biosensor validation. Methods Mol. Biol. 2015, 1295, 465–477. [Google Scholar] [PubMed]
- Liu, S.; Park, S.; Allington, G.; Prelli, F.; Sun, Y.; Martá-Ariza, M.; Scholtzova, H.; Biswas, G.; Brown, B.; Verghese, P.B.; et al. Targeting Apolipoprotein E/Amyloid β Binding by Peptoid CPO_Aβ17-21 P Ameliorates Alzheimer’s Disease Related Pathology and Cognitive Decline. Sci. Rep. 2017, 7, 8009. [Google Scholar] [CrossRef]
- Johnson, N.R.; Wang, A.C.J.; Coughlan, C.; Sillau, S.; Lucero, E.; Viltz, L.; Markham, N.; Allen, C.; Dhanasekaran, A.R.; Chial, H.J.; et al. Imipramine and olanzapine block apoE4-catalyzed polymerization of Aβ and show evidence of improving Alzheimer’s disease cognition. Alzheimers. Res. Ther. 2022, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Pate, K.M.; Kim, B.J.; Shusta, E.V.; Murphy, R.M. Transthyretin Mimetics as Anti-β-Amyloid Agents: A Comparison of Peptide and Protein Approaches. ChemMedChem 2018, 13, 968–979. [Google Scholar] [CrossRef]
- Cao, Q.; Anderson, D.H.; Liang, W.Y.; Chou, J.; Saelices, L. The inhibition of cellular toxicity of amyloid-β by dissociated transthyretin. J. Biol. Chem. 2020, 295, 14015–14024. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Du, H.; Yan, S.; Fang, F.; Wang, C.; Lue, L.F.; Guo, L.; Chen, D.; Stern, D.M.; Gunn Moore, F.J.; et al. Inhibition of amyloid-beta (Abeta) peptide-binding alcohol dehydrogenase-Abeta interaction reduces Abeta accumulation and improves mitochondrial function in a mouse model of Alzheimer’s disease. J. Neurosci. 2011, 31, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Chen, Q.; Zhu, X.; Wang, Y. ABAD/17β-HSD10 reduction contributes to the protective mechanism of huperzine a on the cerebral mitochondrial function in APP/PS1 mice. Neurobiol. Aging 2019, 81, 77–87. [Google Scholar] [CrossRef]
- Morsy, A.; Maddeboina, K.; Gao, J.; Wang, H.; Valdez, J.; Dow, L.F.; Wang, X.; Trippier, P.C. Functionalized Allopurinols Targeting Amyloid-Binding Alcohol Dehydrogenase Rescue Aβ-Induced Mitochondrial Dysfunction. ACS Chem. Neurosci. 2022, 13, 2176–2190. [Google Scholar] [CrossRef]
- Lazarev, V.F.; Benken, K.A.; Semenyuk, P.I.; Sarantseva, S.V.; Bolshakova, O.I.; Mikhaylova, E.R.; Muronetz, V.I.; Guzhova, I.V.; Margulis, B.A. GAPDH binders as potential drugs for the therapy of polyglutamine diseases: Design of a new screening assay. FEBS Lett. 2015, 589, 581–587. [Google Scholar] [CrossRef]
- Lazarev, V.F.; Nikotina, A.D.; Semenyuk, P.I.; Evstafyeva, D.B.; Mikhaylova, E.R.; Muronetz, V.I.; Shevtsov, M.A.; Tolkacheva, A.V.; Dobrodumov, A.V.; Shavarda, A.L.; et al. Small molecules preventing GAPDH aggregation are therapeutically applicable in cell and rat models of oxidative stress. Free Radic. Biol. Med. 2016, 92, 29–38. [Google Scholar] [CrossRef]
- Zatsepina, O.G.; Evgen’ev, M.B.; Garbuz, D.G. Role of a Heat Shock Transcription Factor and the Major Heat Shock Protein Hsp70 in Memory Formation and Neuroprotection. Cells 2021, 10, 1638. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, H.; Lobo, N.; Guo, X.; Gentleman, S.M.; Ma, D. Celastrol enhances cell viability and inhibits amyloid-β production induced by lipopolysaccharide in vitro. J. Alzheimers. Dis. 2014, 41, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, J.R.; Chen, S. Suppression of Alzheimer’s disease-related phenotypes by the heat shock protein 70 inducer, geranylgeranylacetone, in APP/PS1 transgenic mice via the ERK/p38 MAPK signaling pathway. Exp. Ther. Med. 2017, 14, 5267–5274. [Google Scholar] [CrossRef] [PubMed]
- Ortega, L.; Calvillo, M.; Luna, F.; Pérez-Severiano, F.; Rubio-Osornio, M.; Guevara, J.; Limón, I.D. 17-AAG improves cognitive process and increases heat shock protein response in a model lesion with Aβ25-35. Neuropeptides 2014, 48, 221–232. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Liu, D.; Li, J.J.; Xue, Y.; Sakata, K.; Zhu, L.Q.; Heldt, S.A.; Xu, H.; Liao, F.F. Hsp90 chaperone inhibitor 17-AAG attenuates Aβ-induced synaptic toxicity and memory impairment. J. Neurosci. 2014, 34, 2464–2470. [Google Scholar] [CrossRef]
- Dutysheva, E.A.; Utepova, I.A.; Trestsova, M.A.; Anisimov, A.S.; Charushin, V.N.; Chupakhin, O.N.; Margulis, B.A.; Guzhova, I.V.; Lazarev, V.F. Synthesis and approbation of new neuroprotective chemicals of pyrrolyl- and indolylazine classes in a cell model of Alzheimer’s disease. Eur. J. Med. Chem. 2021, 222, 113577. [Google Scholar] [CrossRef]
- Lazarev, V.F.; Dutysheva, E.A.; Mikhaylova, E.R.; Trestsova, M.A.; Utepova, I.A.; Chupakhin, O.N.; Margulis, B.A.; Guzhova, I.V. Indolylazine Derivative Induces Chaperone Expression in Aged Neural Cells and Prevents the Progression of Alzheimer’s Disease. Molecules 2022, 27, 8950. [Google Scholar] [CrossRef]
- Lazarev, V.F.; Guzhova, I.V.; Margulis, B.A. Glyceraldehyde-3-phosphate dehydrogenase is a multifaceted therapeutic target. Pharmaceutics 2020, 12, 416. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).