Abstract
The macrolide erythromycin (ERM) inhibits excessive neutrophil accumulation and bone resorption in inflammatory tissues. We previously reported that the expression of developmental endothelial locus-1 (DEL-1), an endogenous anti-inflammatory factor induced by ERM, is involved in ERM action. Furthermore, DEL-1 is involved in the induction of bone regeneration. Therefore, in this study, we investigated whether ERM exerts an osteoblastogenic effect by upregulating DEL-1 under inflammatory conditions. We performed in vitro cell-based mechanistic analyses and used a model of Porphyromonas gingivalis lipopolysaccharide (LPS)-induced periodontitis to evaluate how ERM restores osteoblast activity. In vitro, P. gingivalis LPS stimulation suppressed osteoblast differentiation and bone formation. However, ERM treatment combined with P. gingivalis LPS stimulation upregulated osteoblast differentiation-related factors and Del1, indicating that osteoblast differentiation was restored. Alveolar bone resorption and gene expression were evaluated in a periodontitis model, and the results confirmed that ERM treatment increased DEL-1 expression and suppressed bone loss by increasing the expression of osteoblast-associated factors. In conclusion, ERM restores bone metabolism homeostasis in inflammatory environments possibly via the induction of DEL-1.
1. Introduction
Periodontal disease is an infectious disease of the tissues surrounding the teeth and is characterized by alveolar bone resorption owing to inflammation caused by dysbiosis of the oral microflora, which ultimately leads to tooth loss [1]. Periodontopathogenic bacteria stimulate excessive migration of neutrophils into periodontal tissues and the secretion of inflammatory cytokines, thereby triggering inflammatory responses [2]. Furthermore, the local immune response to periodontal disease promotes bone loss by disrupting the homeostasis between bone formation and bone resorption [3]. Therefore, in periodontitis treatment, in addition to eliminating the infection, inflammation and bone metabolism mediated by osteoclasts and osteoblasts must be controlled. Accordingly, several new candidate substances, including flavonoids, rice peptides, and hinokitiol, have been reported to exert therapeutic effects on inflammation and bone resorption in periodontal disease [4,5,6,7]. However, no established approach exists for the treatment of periodontal disease that restores homeostasis in bone metabolism, including effects on bone formation and osteoblasts.
Macrolides are antibiotics that inhibit protein synthesis by targeting the bacterial ribosome and exhibit a broad spectrum of activity against bacteria [8]. Therefore, they are used to treat various infections such as pneumonia, skin infections, infectious enteritis, Helicobacter pylori infection, and periodontal disease [9,10,11,12,13]. In addition, macrolides have a wide range of immunomodulatory properties beyond suppression and stimulation [14,15]. Macrolide-induced immunomodulation represents a well-established therapeutic approach for chronic respiratory diseases, including chronic obstructive pulmonary disease, cystic fibrosis, and bronchiectasis [16]. Furthermore, several studies have reported that macrolides affect bone metabolism. Among macrolides, azithromycin (AZM) promotes wound healing by suppressing inflammation via immunomodulation in a mouse apical periodontitis model [17], and clarithromycin promotes bone formation in a rabbit cranial crown defect model when used in combination with β-tricalcium phosphate [18]. Furthermore, rapamycin, a macrolide used as an anticancer drug, has been suggested to promote osteoblast differentiation and new bone formation in a lipopolysaccharide (LPS)-induced inflammatory environment [19]. Thus, macrolides may contribute to the restoration of bone metabolic homeostasis by promoting bone formation. However, the mechanism by which macrolides affect bone metabolism, especially osteoblast activity, remains unclear.
We previously showed that developmental endothelial locus-1 (DEL-1) is induced by the immunomodulatory effects of macrolides and inhibits alveolar bone resorption by suppressing excessive neutrophil infiltration and osteoclast differentiation [20,21]. In addition, DEL-1 affects both osteoclasts and osteoblasts, thereby affecting bone metabolism. DEL-1 suppresses osteoclast differentiation by activating B-cell lymphoma 6 via interaction with Mac-1 integrin in osteoclasts and by downregulating nuclear factor of activated T-cells, cytoplasmic 1, which is a master transcription factor for osteoclast differentiation [22]. Furthermore, for osteoblastic progenitor cells, DEL-1 can activate the β3 integrin–focal adhesion kinase (FAK)–extracellular signal-regulated kinase 1 (ERK1)/2–runt-related transcription factor 2 (RUNX2) pathway and promote new bone formation in mice [23]. Based on these findings, we hypothesized that erythromycin (ERM) may not only inhibit osteoclast differentiation, but also promote osteoblast differentiation by inducing DEL-1, thereby restoring the homeostasis of bone metabolism that is lost in inflammatory conditions. The effects of ERM on osteoblast differentiation and bone formation activity were investigated both in vitro and in vivo.
The purpose of this study was to analyze ERM-mediated immunomodulatory effects on bone metabolism in periodontal inflammatory conditions. Periodontopathogenic bacteria have strong pathogenic properties that induce inflammatory reactions in periodontal tissues. A major periodontopathogenic bacterium is Porphyromonas gingivalis, a Gram-negative anaerobic bacterium [24]. P. gingivalis encodes various virulence factors such as LPS, fimbriae, hemagglutinin, and protease gingipains [25,26]. Among these, LPS exerts inhibitory effects on osteoblast differentiation and bone formation [27]. Furthermore, P. gingivalis-derived LPS promotes bone loss by suppressing osteoblast differentiation via TLR2-mediated and Notch1 signaling activation [28,29]. Hence, in this study, we investigated whether ERM could restore the inflammation-induced suppression of osteoblast differentiation and bone formation induced by P. gingivalis-derived LPS.
2. Results
2.1. ERM Ameliorated P. gingivalis LPS-Induced Decrease in Mineral Nodule Formation in MC3T3 Cells by Promoting Osteoblast Differentiation
First, we examined the effect of ERM on osteogenesis in vitro by culturing MC-3T3 cells in osteoblast differentiation medium containing P. gingivalis LPS. MC-3T3 cells have been cloned as osteoprogenitor murine cell lines and are used in several osteoblast differentiation and characterization experiments [30,31]. Furthermore, the properties of the mineral and matrix phases of MC3T3-E1 osteoblast cultures have been reported to be highly similar to those of mouse bones in terms of mineral structure and composition [32]. After MC3T3 cells were cultured with P. gingivalis LPS (100 ng/mL) for 15 days, Alizarin Red staining was performed to measure the amount of mineralized nodule formation. The amount of mineral nodule formation was substantially decreased in all P. gingivalis LPS-supplemented groups except the ERM (10 μg/mL) treatment group. In the LPS + ERM treatment group, nodule formation was restored to a level similar to that in the control group (Figure 1A,B). P. gingivalis LPS and the antibiotics did not affect the viability of MC-3T3 cells at the concentrations tested, as shown in the MTT assay (Figure 1C).
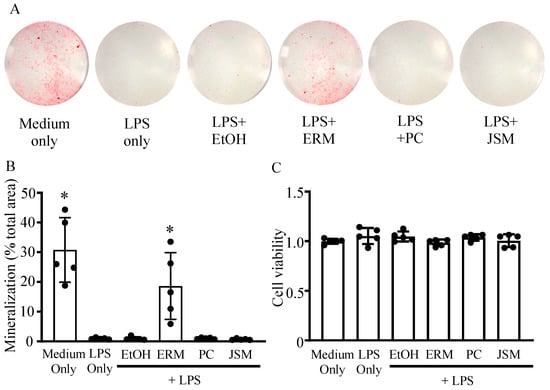
Figure 1.
Erythromycin rescued osteoblast mineralization inhibited by Porphyromonas gingivalis LPS. MC3T3-E1 osteoblastic progenitors were incubated in growth medium with P. gingivalis LPS (100 ng/mL) and 20% ethanol (EtOH), erythromycin (ERM, 10 μg/mL), penicillin (PC, 5 unit/mL), or josamycin (JSM, 10 μg/mL). (A) Representative images (entire bottom of each well of a 96-well plate) of mineralized nodule formation detected by Alizarin Red S staining after 15 days. (B) The total mineralization area in each culture was quantified and expressed in % relative to the total area. (C) Cell viability was assessed using the MTT assay. * p < 0.05 compared to the EtOH + LPS group; values are shown as the mean ± SD (n = 5 per group).
We measured the mRNA expression levels of osteoblast differentiation-related factors and Del-1 on day 12 of culture. The mRNA expression levels of Sp7 and Bglap were decreased in all LPS (100 ng/mL) groups compared to those in the control group (Figure 2B,C). In addition, Runx2 and Sp7 were markedly upregulated in the LPS + ERM (10 μg/mL) group compared to the EtOH + LPS group (Figure 2A,B). Notably, Del1 was also strongly upregulated in the LPS + ERM group (Figure 2D). Furthermore, when the protein expression of RUNX2 in each group was analyzed by western blotting, it was found to be markedly decreased in the LPS-supplemented group, except in the LPS + ERM group (Figure 2E). These results suggest that P. gingivalis LPS severely suppressed the expression of factors essential for osteoblast differentiation and that ERM might attenuate this effect and restore osteoblast differentiation activity.
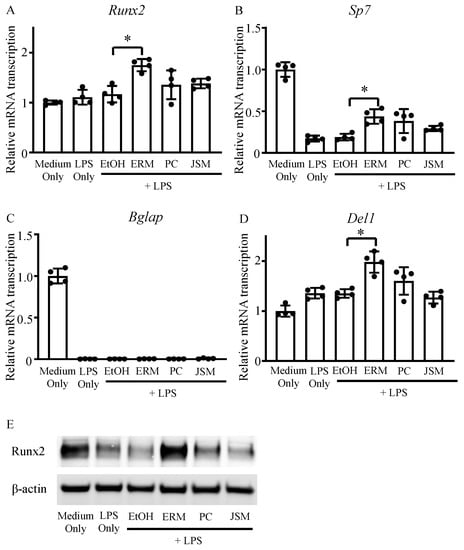
Figure 2.
Erythromycin promoted the expression of DEL-1 and osteoblast differentiation-related factors. MC3T3-E1 osteoblastic progenitors were incubated in growth medium with P. gingivalis LPS (100 ng/mL) and EtOH, ERM (10 μg/mL), PC (5 unit/mL), or JSM (10 μg/mL). (A–D) The mRNA transcription levels of osteoblast differentiation-related factors and Del1 were quantified using real-time qPCR on day 12; * p < 0.05 compared to the EtOH + LPS group; values are shown as the mean ± SD (n = 4 per group). (E) Intracellular expression of Runx2 protein was determined using western blot analysis on day 12.
2.2. ERM Significantly Suppressed Periodontal Bone Loss Induced by P. gingivalis LPS
Based on the aforementioned in vitro results, we analyzed the effect of ERM on P. gingivalis LPS-induced inflammatory alveolar bone resorption in vivo. To measure the immunomodulatory effects of antimicrobial agents, a mouse periodontitis model using P. gingivalis LPS was established. Several studies have reported the induction of alveolar bone resorption via local administration of P. gingivalis LPS to the gingiva of mice [33,34,35]. In the present study, considerable alveolar bone resorption was similarly induced via administration of P. gingivalis LPS (500 μg/kg/d). Analysis of the suppression of alveolar bone resorption in the antimicrobial treatment group showed that alveolar bone resorption was potently suppressed in the ERM (100 mg/kg/d) treatment group alone. The PC (10,000 unit/kg/d) and JSM (100 mg/kg/d) treatments did not suppress alveolar bone resorption (Figure 3A,B).
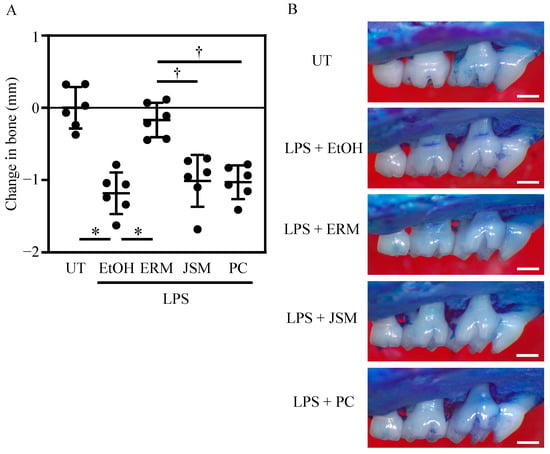
Figure 3.
Peritoneal injection of antibiotics inhibits bone resorption in Porphyromonas gingivalis LPS-induced periodontitis. Periodontal bone loss was induced by the administration of P. gingivalis LPS to maxillary molars. The untreated (UT) group was set as a baseline control. The groups of mice were administered intraperitoneally 20% ethanol (EtOH; control), erythromycin (ERM), josamycin (JSM), or penicillin (PC). (A) The distance from the cement–enamel junction to the pinnacle of the alveolar bone was measured. Negative values (in mm) indicate bone loss relative to the UT control. (B) Representative images of the mouse maxillary bone from the indicated groups (scale bar of 0.5 mm). * p < 0.05 compared to the EtOH group; † p < 0.05 compared to the ERM group; values are shown as the mean ± SD (n = 5 per group).
2.3. ERM Recovered the Expression of Osteoblast Differentiation-Related Factors Suppressed by P. gingivalis LPS
Runx2, Sp7 (osterix), and Bglap are typically expressed as osteogenic markers in the early, middle, and late stages of osteoblast differentiation. Several studies have reported that LPS inhibits osteoblast differentiation via gene regulation [27,36,37]. Therefore, we measured gene expression levels in the palatal gingiva of mouse molars via qPCR. We found that Sp7 and Bglap were substantially downregulated in all the P. gingivalis LPS-supplemented (500 μg/kg/d) groups except the ERM (100 mg/kg/d) treatment group. In contrast, the expression levels of Runx2 and Del1 were unaffected by the addition of P. gingivalis LPS. Furthermore, ERM treatment markedly upregulated Runx2 and Del1 compared to the levels measured in the EtOH group (Figure 4A–D). Therefore, ERM may facilitate the recovery from the downregulation of Sp7 and Bglap induced by P. gingivalis LPS. In addition, osteoclast differentiation-related factors, which are crucial for bone resorption in periodontitis, were also analyzed. The results showed no significant differences in Nfatc1 and RANK mRNA transcription between the groups (Figure 4E,F). These results of samples collected 2 weeks after LPS injection are consistent with the fact that in the periodontitis model with LPS administration, the strongest bone loss occurred within the first week of administration, followed by a further loss within one to two weeks, whereas there was little difference in bone levels between two and three weeks (Figure S1).
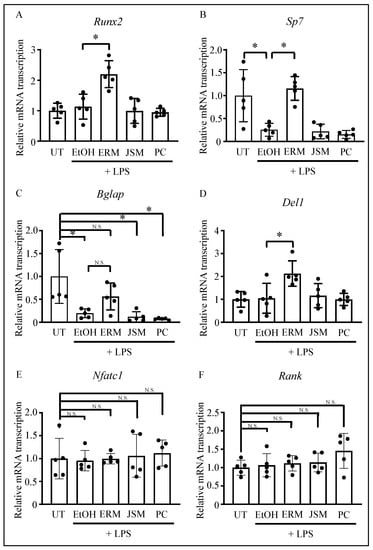
Figure 4.
Effect of antibacterial drugs on the transcription of osteoblast-related factors in the gingiva, using the Porphyromonas gingivalis LPS-induced periodontitis model. (A–F) Real-time qPCR was performed to quantify the mRNA transcription levels of osteoblast-related factors, DEL-1, and osteoclast-related factors. * p < 0.05 compared to the indicated group; values are shown as the mean ± SD (n = 5 per group). N.S., not significant.
2.4. ERM Rescued DEL-1 Expression Reduced by P. gingivalis LPS
DEL-1 protein expression in periodontal ligament tissues was examined via immunohistochemical analysis. The EtOH + LPS (500 μg/kg/d) group showed a decrease in DEL-1 protein expression, whereas the LPS + ERM (100 mg/kg/d) treatment group showed a rescue of DEL-1 protein expression in periodontal ligament tissues (Figure 5). This result was similar to that of the experiment in which ERM treatment was performed in a mouse model of tooth-ligated periodontitis [21].
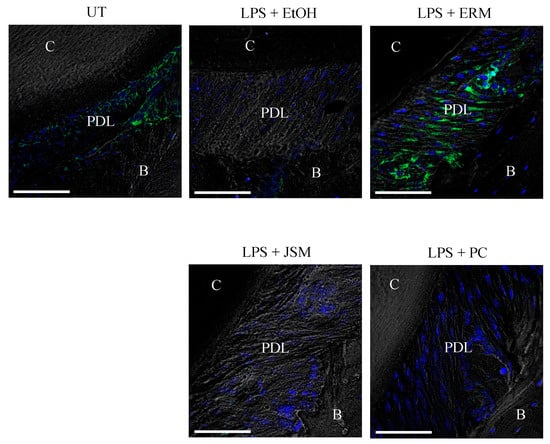
Figure 5.
Erythromycin increases DEL-1 expression in the periodontal ligament. Frozen maxillae sections were stained for DEL-1 (green) and nuclei using DAPI (blue). Representative images obtained by optical microscopy are shown. C: cementum; PDL: periodontal ligament; B: alveolar bone. Scale bars, 50 µm.
2.5. ERM Enhanced Alkaline Phosphatase (ALP) Activity Attenuated by P. gingivalis LPS
ALP staining was performed to histologically evaluate the osteoblasts in periodontal tissues. In the EtOH + LPS (500 μg/kg/d) group, ALP activity in periodontal ligament tissues was attenuated when compared to that in the control group. In contrast, ALP activity was partially enhanced in periodontal ligament tissues in the LPS + ERM (100 mg/kg/d) treatment group (Figure 6).
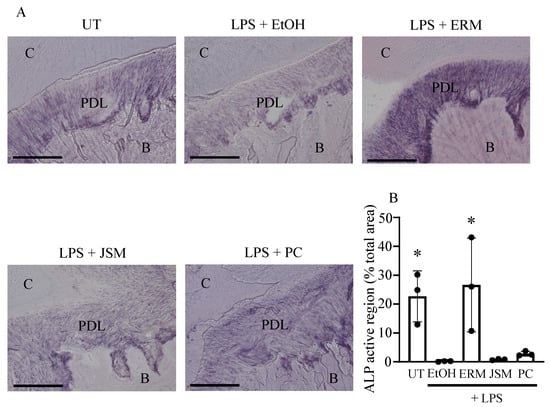
Figure 6.
Erythromycin increases the alkaline phosphatase activity of cells in periodontal ligament tissue. Alkaline phosphatase staining was performed on frozen maxillary sections. (A) Representative images obtained by optical microscopy are shown. C: cementum; PDL: periodontal ligament; B: alveolar bone. Scale bars, 100 µm. (B) The total active region of alkaline phosphatase in each field was quantified and expressed as % relative to the total area. * p < 0.05 as compared to EtOH + LPS group, means ± SD (n = 3 per group).
3. Discussion
In this study, ERM upregulated Del1 in vivo and in vitro and recovered osteoblast differentiation that was attenuated by P. gingivalis LPS-induced inflammation. Our results suggest that ERM has the ability to restore the homeostasis of bone metabolism in inflammatory environments by inducing DEL-1. Several studies have reported that macrolides are effective in the treatment of periodontal disease [13,38]. This has been attributed to the immunomodulatory effects of macrolides in addition to their antibacterial effects [39,40,41]. Macrolides have the characteristic structure of a macrocyclic lactone ring and are classified into several types based on differences in the structure [42]. Macrolides classified based on 14-membered rings (such as erythromycin and clarithromycin) and 15-membered rings (such as AZM) have immunomodulatory and anti-inflammatory properties, which are absent in 16-membered-ring macrolides (such as JSM) [15,43]. The results of the present study showed that ERM, a 14-membered-ring macrolide, has a strong inhibitory effect on inflammatory bone resorption induced by LPS, independent of bacterial infection. In addition, previous studies using a model of periodontitis with ligated teeth have shown similar results, which indicate that ERM has a strong inhibitory effect on bone resorption [20,21]. These findings suggest that macrolides suppress inflammation via immunomodulatory effects, at least in the entire tissue. In contrast, macrolides may elicit different immune responses in different cell types. AZM may alter the inflammatory response in human gingival fibroblasts by increasing the expression of interleukin 6 (IL-6) and IL-8 under LPS stimulation [44,45]. Thus, to elucidate the mechanism of macrolide-mediated immunomodulation, the effects of macrolides on different cell types must be analyzed. In the analysis of alveolar bone resorption due to the periodontitis, it is important to analyze both bone resorption by osteoclasts and bone formation by osteoblasts. In particular, only a few macrolide-derived effects on osteoblasts are known. Therefore, we focused on the effects of ERM on osteoblasts and collected samples 2 weeks after LPS and ERM injection, the optimal time to confirm osteoblast differentiation. Furthermore, at this point, the present experiment suggested that strong osteoclast differentiation did not occur, at least at the genetic level. Consequently, the present analysis focused more on osteoblasts. Considering the results of previous experiments, it is possible that erythromycin mainly acts on osteoclasts up to 1 week after the induction of periodontitis and then shifts its action to osteoblasts, contributing to the regulation of bone metabolism. Our study shows for the first time that ERM increases or restores the expression of osteoblast differentiation-related factors and ALP activity. ERM likely restored the homeostasis of bone metabolism by promoting osteoblast differentiation and bone formation under P. gingivalis LPS-induced inflammatory conditions.
The effect of LPS on osteoblasts has been analyzed in various aspects. LPS has been found to induce apoptosis when added to MC-3T3 cells at high concentrations [46]. In the present study, we focused on the effect of ERM on LPS-induced inhibition of osteoblast differentiation and therefore used a concentration of LPS that did not affect cell viability. The expression of Runx2, Sp7, and Bglap, which are essential for osteoblast differentiation, is repressed by E. coli LPS in vitro [47,48]. In the present study, the addition of P. gingivalis LPS to MC-3T3 cells markedly decreased the expression of Runx2, Sp7, and Bglap. These findings suggest that P. gingivalis and E. coli LPSs have an inhibitory effect on osteoblast differentiation and can contribute to the deterioration of tissues in periodontal pathology. P. gingivalis LPS treatment suppresses ALP activity and osteocalcin expression in human periodontal ligament cells and promotes the transition from bone formation to bone resorption in experiments using mouse osteoblasts and osteoclasts [49,50]. In addition, P. gingivalis LPS is known to have a characteristic toxic effect, inducing inflammatory responses via TLR2 in addition to inducing inflammation via TLR4 [51]. Furthermore, TLR2-mediated stimulation activates osteoclast differentiation by increasing receptor activator of nuclear factor kappa-Β ligand expression in osteoblasts [52], suggesting that P. gingivalis LPS stimulation activates bone resorption via its action on osteoblasts [53]. Subsequently, based on these molecular findings, the effects of ERM were analyzed in an animal model using P. gingivalis LPS, which also has unique toxic effects on bone metabolism. Several animal periodontal disease models using LPS have been established previously, including models using P. gingivalis LPS and Aggregatibacter actinomycetemcomitans LPS [54,55]. Currently, the tooth ligation model is commonly used as a mouse model for studying periodontal disease, and several studies have applied this model to analyze the molecular pathology of the disease and to investigate new treatment approaches [56,57]. However, in the tooth ligation model, alveolar bone resorption is induced by dysbiosis caused by the biofilm attached to the ligature threads [58,59]. Hence, the analysis of the inhibitory effect of antimicrobial agents on inflammatory alveolar bone resorption using the tooth ligation model cannot completely exclude the effect of antimicrobial properties. Therefore, in this study, we attempted to analyze the effects of ERM on bone formation based on its immunomodulatory effects using the P. gingivalis LPS-induced alveolar bone resorption model.
One mechanism by which ERM positively regulates osteoblast differentiation may involve DEL-1, which is a 52 kDa protein secreted by various tissue-resident cells, including endothelial cells, osteolineage cells, and certain macrophage subsets [60,61]. In addition, DEL-1 is a standard local regulator of tissue immunoplasticity and inflammatory disease, with its ability to promote macrophage efferocytosis to clear inflammation and activate regulatory T-cell function [62,63,64,65]. Bone healing and remodeling require the proper regulation of the inflammatory response, and while severe inflammation obviously inhibits bone regeneration, an appropriate level of inflammation-related factors is essential [66,67]. Furthermore, an anti-inflammatory treatment to restore the homeostasis of bone metabolism requires an immunomodulatory action, as studies indicate that the administration of anti-inflammatory drugs has a negative effect on bone healing [68,69].
Because DEL-1 has an important immunomodulatory effect on bone healing, DEL-1 may positively regulate bone metabolism in osteoclasts and osteoblasts as an effect on osteolineage cells. Particularly, DEL-1 inhibits osteoclast differentiation and bone resorption activity [22], whereas it promotes bone formation in osteoblasts; thus, DEL-1-deficient mice fail to regenerate bone unless recombinant DEL-1 is administered locally [23]. Hence, ERM might have strongly suppressed bone resorption by increasing DEL-1 expression in the periodontal tissue in this study. We previously reported that resolvin D1 and ERM suppress inflammatory bone resorption by inducing DEL-1 [70]. In addition, DEL-1 promotes osteogenic differentiation of osteoblastic cells in a β3 integrin-dependent manner. The ability of DEL-1 to promote in vitro osteogenesis, indicated by the induction of osteogenic genes such as the master transcription factor Runx2 and by mineralized nodule formation, depends on its capacity to induce the phosphorylation of FAK and ERK1/2 [23]. Consequently, DEL-1 positively regulates osteoblast differentiation.
Certain limitations were noted in our current study. In this experiment, LPS injection and antimicrobial treatment were started at the same time. In other words, periodontitis was induced, and treatment was initiated simultaneously. However, because periodontal disease treatment in clinical practice is usually performed after the disease becomes apparent in the patient, not all of the results of this experiment can be considered translatable to actual clinical situations. Periodontal disease is a chronic inflammatory disease, and after the onset of the disease, the pathological process progresses through a series of quiescent and active phases [71]. In this experiment, LPS injection was used to induce acute inflammation, which can be regarded as a model that mimics the pathophysiology of periodontitis during the active phase of the disease. Therefore, the results of this study suggest that treatment with erythromycin during the active phase of periodontal disease could suppress the progression of the disease. In addition, there is a challenge in applying the results of this study to clinical practice. That is, the development of resistance to macrolide antimicrobial agents, including erythromycin, is a problem in the treatment of many infectious diseases [72,73,74,75]. In oral bacteria, the erythromycin resistance genes erm (B) and erm (F) were detected using PCR in oral Prevotella strains [76]. If macrolide antibiotics are used for a certain long period of time in anticipation of their bone metabolism-regulatory action, they may promote the growth of drug-resistant bacteria. Therefore, the development of macrolide molecules without antimicrobial activity is expected [77,78]. We expect that a more detailed clarification of the bone metabolism modulatory mechanism and regulatory molecules by erythromycin will lead to the development of macrolide-based bone metabolism agents that do not produce drug-resistant bacteria.
4. Materials and Methods
4.1. Reagents
The following antibacterial drugs were obtained: ERM (FUJIFILM Wako Pure Chemical Corporation, Tokyo, Japan), josamycin (JSM) (Sigma-Aldrich, St. Louis, MO, USA), and penicillin (PC) (Meiji Seika Pharma Co., Ltd., Tokyo, Japan). Each substance was dissolved in 80% phosphate-buffered saline (PBS) and 20% ethanol (EtOH).
4.2. Murine Model
C57BL/6Ncrl mice (age, 10 weeks; Charles River Laboratories Japan, Inc., Yokohama, Japan) were maintained in individually ventilated cages and provided sterile food and water ad libitum under specific pathogen-free conditions. All animal experiments were approved by the Institutional Animal Care and Use Committee of Niigata University (SA00181). The mice were housed at ambient temperature and humidity in ventilated caging systems on a 12 h/12 h light/dark cycle. Periodontitis was induced in mice via microinjection of P. gingivalis-derived LPS (500 μg/kg/d; InvivoGen, San Diego, CA, USA) into the palatal gingiva once a day for 2 weeks. Antibacterial drugs (ERM, 100 mg/kg body weight; PC, 10,000 unit/kg body weight; JSM, 100 mg/kg body weight) or 20% EtOH were administered intraperitoneally once a day for 14 days in the intervention experiments. The mice were euthanized 14 days after the start of P. gingivalis LPS administration. The gingiva was dissected and processed for performing real-time quantitative polymerase chain reaction (qPCR) determination of osteoblast-related factors and Del1 mRNA expression levels. Periodontal bone loss was morphometrically assessed in defleshed maxillae using a stereoscopic microscope (Leica Microsystems, Wetzlar, Germany) (35×). The distance from the cement–enamel junction to the alveolar bone crest (CEJ-ABC) was measured in 13 sites at predetermined points from the first molar to the third molar. Bone loss was calculated by subtracting the sum of the values at the 13 sites from CEJ-ABC in the untreated regions. Negative values (mm) indicated bone loss relative to the baseline (untreated control).
4.3. Histological Analysis
For the standard histological and subsequent quantitative histomorphometric analyses, the maxillae were fixed in 4% paraformaldehyde (PFA)-containing PBS (Wako Pure Chemical Industries, Osaka, Japan) for 24 h, followed by decalcification in Decalcifying Solution B (Wako Pure Chemical Industries) for 1 week at 4 °C. The specimens were then embedded in optimal cutting temperature compound (Sakura Finetek, Torrance, CA, USA) and frozen in liquid nitrogen, and coronal sections were cut using a cryostat (Leica Biosystems, Wetzlar, Germany).
4.4. Osteoblastic Progenitors
The murine osteoblastic progenitor cell line MC3T3-E1 was obtained from the RIKEN Bioresource Center (RCB1126). MC3T3-E1 cells were maintained in minimum essential Eagle medium, alpha modification (α-MEM; FUJIFILM Wako Pure Chemical CorporationOsaka, Japan), supplemented with 10% fetal bovine serum (FBS; SERANA Brandenburg, Germany), a penicillin–streptomycin solution (×100) (FUJIFILM Wako Pure Chemical Corporation) at 37 °C, and 5% CO2.
4.5. Osteogenic Differentiation Assay
For osteogenic differentiation, osteoblast progenitor cells were cultured in α-MEM supplemented with 10% FBS and an osteoblast-inducer reagent (Takara Bio Inc., Kusatsu, Japan) for up to 15 days. Antibiotics and P. gingivalis-derived LPS were added to the aforementioned osteoblast differentiation medium and replaced every 3 days. Mineralized bone nodules were detected by staining with Alizarin Red S (FUJIFILM Wako Pure Chemical Corporation). Briefly, the cultures were washed twice with PBS, fixed with 4% PFA in PBS for 10 min, and washed again with distilled water. Staining was performed by immersing the cells in a 2% Alizarin Red S solution for 30 min. After washing with distilled water to remove the unbound dye, the entire well containing the calcified nodule was photographed. The calcified nodules were quantified as a percentage of area covered relative to the total area using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Cell viability was assessed by MTT assay, in which MTT (400 µg/mL) was directly added to the cultures, followed by incubation at 37 °C and 5% CO2 in humidified air. Subsequently, the supernatant was aspirated and 200 µL of lysis solution (90% isopropanol, 0.5% SDS, 0.04 N HCl, DW) was added to dissolve the formazan dye. Optical density (OD) was measured at 570 nm using a microplate reader. The mean OD of the control group was set as 1, and the experimental groups were compared to the control.
4.6. Quantitative Real-Time PCR
Total RNA was extracted from MC3T3-E1 cells cultured in osteoblast differentiation medium for 12 days using the same method as described above and mouse maxillary palatal gingiva using the TRI reagent (Molecular Research Center, Inc., Cincinnati, OH, USA); RNA was quantified via spectrophotometry at 260 and 280 nm. RNA was reverse-transcribed using SuperScript VILO Ⅳ Master Mix (Thermo Fisher Scientific, Waltham, MA, USA). qPCR was performed using the cDNA in a StepOnePlus real-time PCR system (Thermo Fisher Scientific) according to the manufacturer’s protocol. The data were analyzed using the comparative CT (ΔΔCt) method. TaqMan probes, sense primers, and antisense primers for the expression of a housekeeping gene (glyceraldehyde 3-phosphate dehydrogenase, assay ID: 4331182 Mm99999915_g1), along with Runx2 (assay ID: 4331182 Mm00501584_m1), Sp7 (osterix, assay ID: 4331182 Mm00504574_m1), bone γ-carboxyglutamic acid (Bglap, assay ID: 4331182 Mm00649782_gH), epidermal growth factor-like repeats and discoidin domains 3 (Edil3; Del1, encoded DEL-1, assay ID: 4331182 Mm01291247_m1), nuclear factor of activated T cells 1 (Nfatc1, assay ID: 4331182 Mm01265944_m1), and tumor necrosis factor receptor superfamily member 11A (Tnfrsf11a; Rank, encoded RANK, assay ID: 4331182 Mm00437132_m1) were purchased from Thermo Fisher Scientific.
4.7. Western Blot Analysis
Proteins were extracted from lysates of MC3T3-E1 cells cultured in osteoblast differentiation medium for 12 days via the same method described above using M-PER Mammalian Protein Extraction Reagent (Thermo Fisher Scientific) with Halt Protease and Phosphatase Inhibitor Single-Use Cocktail (100×) (Thermo Fisher Scientific). The protein solution was centrifuged, and the supernatant was mixed with 4× Bolt LDS Sample Buffer (Thermo Fisher Scientific), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis) on a Bolt 4–12% Bis-Tris Plus gel (Thermo Fisher Scientific), and transferred to a polyvinylidene difluoride Membrane Filter Paper Sandwich (Thermo Fisher Scientific). The membranes were probed using an anti-RUNX2 antibody (Abcam, Cambridge, UK) and incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody (Cell Signaling Technology, Danvers, MA, USA) or an anti-β-actin HRP-conjugated antibody (Cell Signaling Technology), followed by incubation with StartingBlock (TBS) Blocking Buffer (Thermo Fisher Scientific). Subsequently, the membranes were incubated with ECL Select reagent (GE Healthcare, Little Chalfont, UK) and analyzed using an Image Quant LAS 4000 system (GE Healthcare Bio-Sciences AB, Uppsala, Sweden).
4.8. Statistical Analysis
The data were evaluated using analysis of variance (ANOVA) and one-way ANOVA with Tukey’s multiple comparisons test using GraphPad Software version 7.03 (GraphPad Software Inc., La Jolla, CA, USA). Statistical significance was set at p < 0.05.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph16020303/s1, Figure S1: Bone resorption in Porphyromonas gingivalis LPS-induced periodontitis.
Author Contributions
Conceptualization, T.M. (Tomoki Maekawa); methodology, T.M. (Tomoki Maekawa); software, H.T. and K.S. (Kridtapat Sirisereephap).; validation, H.T., T.M. (Tomoki Maekawa) and H.D.; formal analysis, T.H. and K.S. (Kridtapat Sirisereephap); investigation, H.T., K.S. (Kridtapat Sirisereephap), T.H., S.H., T.I., K.S. (Karin Sasagawa), and F.T.; resources, T.M. (Tomoki Maekawa), T.M. (Takeyasu Maeda) and Y.T.; data curation, H.D. and K.T.; writing—original draft preparation, H.T.; writing—review and editing, T.M. (Tomoki Maekawa) and H.D.; supervision, K.T. and Y.T.; project administration, T.M. (Tomoki Maekawa); funding acquisition, T.M. (Tomoki Maekawa), H.D. and Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Japan Society for the Promotion of Science KAKENHI to Tomoki Maekawa [grant numbers 19H03828 and 22H03267] and to H.T. [grant number 20J15490A]. This work was also funded by the Takeda Science Foundation, the Uehara Memorial Foundation, the Terumo Life Science Foundation, Kowa Life Science Foundation, and AMED-Interstellar Initiative to Tomoki Maekawa.
Institutional Review Board Statement
The genetic recombination-related mouse procedures were approved by the Genetic Recombination Experiment Safety Committee of Niigata University (SD00861). All animal experiments were approved by the Institutional Animal Care and Use Committee of Niigata University (SA00181).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. New developments in neutrophil biology and periodontitis. Periodontology 2020, 82, 78–92. [Google Scholar] [CrossRef]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.S.; Ramadan, D.; de Paiva Goncalves, V.; Maquera-Huacho, P.M.; Assis, R.P.; Lima, T.F.O.; Brunetti, I.L.; Spolidorio, D.M.P.; Cesar, T.; Manthey, J.A.; et al. Impact of citrus flavonoid supplementation on inflammation in lipopolysaccharide-induced periodontal disease in mice. Food Funct. 2021, 12, 5007–5017. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Ji, W.; Wang, F.; Zhang, F.; Xue, P.; Cheng, M.; Sun, Y.; Wang, X.; Zhang, T. Quercetin Inhibits Inflammatory Response Induced by LPS from Porphyromonas gingivalis in Human Gingival Fibroblasts via Suppressing NF-kappaB Signaling Pathway. BioMed Res. Int. 2019, 2019, 6282635. [Google Scholar] [CrossRef]
- Tamura, H.; Maekawa, T.; Domon, H.; Hiyoshi, T.; Yonezawa, D.; Nagai, K.; Ochiai, A.; Taniguchi, M.; Tabeta, K.; Maeda, T.; et al. Peptides from rice endosperm protein restrain periodontal bone loss in mouse model of periodontitis. Arch. Oral Biol. 2019, 98, 132–139. [Google Scholar] [CrossRef]
- Hiyoshi, T.; Domon, H.; Maekawa, T.; Yonezawa, D.; Kunitomo, E.; Tabeta, K.; Terao, Y. Protective effect of hinokitiol against periodontal bone loss in ligature-induced experimental periodontitis in mice. Arch. Oral Biol. 2020, 112, 104679. [Google Scholar] [CrossRef]
- Vazquez-Laslop, N.; Mankin, A.S. How Macrolide Antibiotics Work. Trends Biochem. Sci. 2018, 43, 668–684. [Google Scholar] [CrossRef]
- Restrepo, M.I.; Sole-Violan, J.; Martin-Loeches, I. Macrolide therapy of pneumonia: Is it necessary, and how does it help? Curr. Opin. Infect. Dis. 2016, 29, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Parsad, D.; Pandhi, R.; Dogra, S. A guide to selection and appropriate use of macrolides in skin infections. Am. J. Clin. Dermatol. 2003, 4, 389–397. [Google Scholar] [CrossRef]
- Jin, C.; Gibani, M.M.; Pennington, S.H.; Liu, X.; Ardrey, A.; Aljayyoussi, G.; Moore, M.; Angus, B.; Parry, C.M.; Biagini, G.A.; et al. Treatment responses to Azithromycin and Ciprofloxacin in uncomplicated Salmonella Typhi infection: A comparison of Clinical and Microbiological Data from a Controlled Human Infection Model. PLoS Negl. Trop. Dis. 2019, 13, e0007955. [Google Scholar] [CrossRef] [PubMed]
- Zagari, R.M.; Frazzoni, L.; Marasco, G.; Fuccio, L.; Bazzoli, F. Treatment of Helicobacter pylori infection: A clinical practice update. Minerva Med. 2021, 112, 281–287. [Google Scholar] [CrossRef]
- O’Rourke, V.J. Azithromycin as an adjunct to non-surgical periodontal therapy: A systematic review. Aust. Dent. J. 2017, 62, 14–22. [Google Scholar] [CrossRef]
- Reijnders, T.D.Y.; Saris, A.; Schultz, M.J.; van der Poll, T. Immunomodulation by macrolides: Therapeutic potential for critical care. Lancet Respir. Med. 2020, 8, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Ziesenitz, V.C.; Curtis, N.; Ritz, N. The Immunomodulatory Effects of Macrolides—A Systematic Review of the Underlying Mechanisms. Front. Immunol. 2018, 9, 302. [Google Scholar] [CrossRef]
- Kanoh, S.; Rubin, B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef] [PubMed]
- Andrada, A.C.; Azuma, M.M.; Furusho, H.; Hirai, K.; Xu, S.; White, R.R.; Sasaki, H. Immunomodulation Mediated by Azithromycin in Experimental Periapical Inflammation. J. Endod. 2020, 46, 1648–1654. [Google Scholar] [CrossRef]
- Alenezi, A.; Naito, Y.; Terukina, T.; Prananingrum, W.; Jinno, Y.; Tagami, T.; Ozeki, T.; Galli, S.; Jimbo, R. Controlled release of clarithromycin from PLGA microspheres enhances bone regeneration in rabbit calvaria defects. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chang, B.; Wang, B.; Bu, W.; Zhao, L.; Liu, J.; Meng, L.; Wang, L.; Xin, Y.; Wang, D.; et al. Rapamycin promotes osteogenesis under inflammatory conditions. Mol. Med. Rep. 2017, 16, 8923–8929. [Google Scholar] [CrossRef]
- Tamura, H.; Maekawa, T.; Domon, H.; Hiyoshi, T.; Hirayama, S.; Isono, T.; Sasagawa, K.; Yonezawa, D.; Takahashi, N.; Oda, M.; et al. Effects of Erythromycin on Osteoclasts and Bone Resorption via DEL-1 Induction in Mice. Antibiotics 2021, 10, 312. [Google Scholar] [CrossRef]
- Maekawa, T.; Tamura, H.; Domon, H.; Hiyoshi, T.; Isono, T.; Yonezawa, D.; Hayashi, N.; Takahashi, N.; Tabeta, K.; Maeda, T.; et al. Erythromycin inhibits neutrophilic inflammation and mucosal disease by upregulating DEL-1. JCI Insight 2020, 5, e136706. [Google Scholar] [CrossRef]
- Shin, J.; Maekawa, T.; Abe, T.; Hajishengallis, E.; Hosur, K.; Pyaram, K.; Mitroulis, I.; Chavakis, T.; Hajishengallis, G. DEL-1 restrains osteoclastogenesis and inhibits inflammatory bone loss in nonhuman primates. Sci. Transl. Med. 2015, 7, 307ra155. [Google Scholar] [CrossRef] [PubMed]
- Yuh, D.Y.; Maekawa, T.; Li, X.; Kajikawa, T.; Bdeir, K.; Chavakis, T.; Hajishengallis, G. The secreted protein DEL-1 activates a beta3 integrin-FAK-ERK1/2-RUNX2 pathway and promotes osteogenic differentiation and bone regeneration. J. Biol. Chem. 2020, 295, 7261–7273. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, N.; Belibasakis, G.N. Porphyromonas gingivalis: An invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 2012, 333, 1–9. [Google Scholar] [CrossRef]
- Lunar Silva, I.; Cascales, E. Molecular Strategies Underlying Porphyromonas gingivalis Virulence. J. Mol. Biol. 2021, 433, 166836. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, W.; Wang, H.; Liang, S. Roles of Porphyromonas gingivalis and its virulence factors in periodontitis. Adv. Protein Chem. Struct. Biol. 2020, 120, 45–84. [Google Scholar] [CrossRef] [PubMed]
- Bandow, K.; Maeda, A.; Kakimoto, K.; Kusuyama, J.; Shamoto, M.; Ohnishi, T.; Matsuguchi, T. Molecular mechanisms of the inhibitory effect of lipopolysaccharide (LPS) on osteoblast differentiation. Biochem. Biophys. Res. Commun. 2010, 402, 755–761. [Google Scholar] [CrossRef]
- Wang, Y.H.; Nemati, R.; Anstadt, E.; Liu, Y.; Son, Y.; Zhu, Q.; Yao, X.; Clark, R.B.; Rowe, D.W.; Nichols, F.C. Serine dipeptide lipids of Porphyromonas gingivalis inhibit osteoblast differentiation: Relationship to Toll-like receptor 2. Bone 2015, 81, 654–661. [Google Scholar] [CrossRef]
- Xing, Q.; Ye, Q.; Fan, M.; Zhou, Y.; Xu, Q.; Sandham, A. Porphyromonas gingivalis lipopolysaccharide inhibits the osteoblastic differentiation of preosteoblasts by activating Notch1 signaling. J. Cell. Physiol. 2010, 225, 106–114. [Google Scholar] [CrossRef]
- Quarles, L.D.; Yohay, D.A.; Lever, L.W.; Caton, R.; Wenstrup, R.J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: An in vitro model of osteoblast development. J. Bone Miner. Res. 1992, 7, 683–692. [Google Scholar] [CrossRef]
- Kartsogiannis, V.; Ng, K.W. Cell lines and primary cell cultures in the study of bone cell biology. Mol. Cell. Endocrinol. 2004, 228, 79–102. [Google Scholar] [CrossRef] [PubMed]
- Addison, W.N.; Nelea, V.; Chicatun, F.; Chien, Y.C.; Tran-Khanh, N.; Buschmann, M.D.; Nazhat, S.N.; Kaartinen, M.T.; Vali, H.; Tecklenburg, M.M.; et al. Extracellular matrix mineralization in murine MC3T3-E1 osteoblast cultures: An ultrastructural, compositional and comparative analysis with mouse bone. Bone 2015, 71, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, C.; Hu, Y.; Xia, X.; Liao, Y.; Zhang, J.; Chen, H.; Lu, W.; Zhou, W.; Song, Z. New Application of Psoralen and Angelicin on Periodontitis With Anti-bacterial, Anti-inflammatory, and Osteogenesis Effects. Front. Cell. Infect. Microbiol. 2018, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Qiu, C.; Shen, H.; Zhang, H.; He, Z.; Song, Z.; Zhou, W. The imbalance of Th17/Treg via STAT3 activation modulates cognitive impairment in P. gingivalis LPS-induced periodontitis mice. J. Leukoc. Biol. 2021, 110, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.; Lai, C.H.; Kuo, C.H.; Chang, B.I.; Wu, H.L.; Cheng, T.L. Recombinant thrombomodulin lectin-like domain attenuates Porphyromonas gingivalis lipopolysaccharide-induced osteoclastogenesis and periodontal bone resorption. J. Periodontol. 2021, 92, 1622–1634. [Google Scholar] [CrossRef]
- Guo, C.; Yuan, L.; Wang, J.G.; Wang, F.; Yang, X.K.; Zhang, F.H.; Song, J.L.; Ma, X.Y.; Cheng, Q.; Song, G.H. Lipopolysaccharide (LPS) induces the apoptosis and inhibits osteoblast differentiation through JNK pathway in MC3T3-E1 cells. Inflammation 2014, 37, 621–631. [Google Scholar] [CrossRef]
- Liu, Y.H.; Huang, D.; Li, Z.J.; Li, X.H.; Wang, X.; Yang, H.P.; Tian, S.P.; Mao, Y.; Liu, M.F.; Wang, Y.F.; et al. Toll-like receptor-4-dependence of the lipopolysaccharide-mediated inhibition of osteoblast differentiation. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Muniz, F.W.; de Oliveira, C.C.; de Sousa Carvalho, R.; Moreira, M.M.; de Moraes, M.E.; Martins, R.S. Azithromycin: A new concept in adjuvant treatment of periodontitis. Eur. J. Pharm. 2013, 705, 135–139. [Google Scholar] [CrossRef]
- Hirsch, R.; Deng, H.; Laohachai, M.N. Azithromycin in periodontal treatment: More than an antibiotic. J. Periodontal. Res. 2012, 47, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; du Bois, A.H.; Gannon, S.; Haynes, D.R.; Hirsch, R.S. Antibacterial and immunomodulatory properties of azithromycin treatment implications for periodontitis. Inflammopharmacology 2013, 21, 321–338. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, Y.S.; Choi, E.Y.; Choi, J.I.; Choi, I.S.; Kim, S.J. Subantibiotic dose of azithromycin attenuates alveolar bone destruction and improves trabecular microarchitectures in a rat model of experimental periodontitis: A study using micro-computed tomography. Int. Immunopharmacol. 2017, 47, 212–217. [Google Scholar] [CrossRef]
- Steel, H.C.; Theron, A.J.; Cockeran, R.; Anderson, R.; Feldman, C. Pathogen- and host-directed anti-inflammatory activities of macrolide antibiotics. Mediat. Inflamm. 2012, 2012, 584262. [Google Scholar] [CrossRef] [PubMed]
- Arsic, B.; Barber, J.; Cikos, A.; Mladenovic, M.; Stankovic, N.; Novak, P. 16-membered macrolide antibiotics: A review. Int. J. Antimicrob. Agents 2018, 51, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Kamemoto, A.; Ara, T.; Hattori, T.; Fujinami, Y.; Imamura, Y.; Wang, P.L. Macrolide antibiotics like azithromycin increase lipopolysaccharide-induced IL-8 production by human gingival fibroblasts. Eur. J. Med. Res. 2009, 14, 309–314. [Google Scholar] [CrossRef]
- Nagano, T.; Yamaguchi, T.; Kajiyama, S.; Suzuki, T.; Matsushima, Y.; Yashima, A.; Shirakawa, S.; Gomi, K. Effect of Azithromycin on Proinflammatory Cytokine Production in Gingival Fibroblasts and the Remodeling of Periodontal Tissue. J. Clin. Med. 2020, 10, 99. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Zhao, J.; Miao, W.; Ye, T.; Chen, A. Resveratrol Attenuates Lipopolysaccharides (LPS)-Induced Inhibition of Osteoblast Differentiation in MC3T3-E1 Cells. Med. Sci. Monit. 2018, 24, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, R.J.; Jang, K.; Zhou, X.L.; Liu, Y.Z. Protective Effects of Pretreatment with Quercetin Against Lipopolysaccharide-Induced Apoptosis and the Inhibition of Osteoblast Differentiation via the MAPK and Wnt/beta-Catenin Pathways in MC3T3-E1 Cells. Cell. Physiol. Biochem. 2017, 43, 1547–1561. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hao, W.; Wang, X.; Su, H. miR-23b targets Smad 3 and ameliorates the LPS-inhibited osteogenic differentiation in preosteoblast MC3T3-E1 cells. J. Toxicol. Sci. 2016, 41, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Taguchi, Y.; Tominaga, K.; Umeda, M.; Tanaka, A. Porphyromonas gingivalis LPS inhibits osteoblastic differentiation and promotes pro-inflammatory cytokine production in human periodontal ligament stem cells. Arch. Oral Biol. 2014, 59, 167–175. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.C.; Bao, X.F.; Hu, M.; Yu, W.X. Effects of Porphyromonas gingivalis lipopolysaccharide on osteoblast-osteoclast bidirectional EphB4-EphrinB2 signaling. Exp. Ther. Med. 2014, 7, 80–84. [Google Scholar] [CrossRef]
- Ogawa, T.; Asai, Y.; Hashimoto, M.; Takeuchi, O.; Kurita, T.; Yoshikai, Y.; Miyake, K.; Akira, S. Cell activation by Porphyromonas gingivalis lipid A molecule through Toll-like receptor 4- and myeloid differentiation factor 88-dependent signaling pathway. Int. Immunol. 2002, 14, 1325–1332. [Google Scholar] [CrossRef]
- Kassem, A.; Lindholm, C.; Lerner, U.H. Toll-Like Receptor 2 Stimulation of Osteoblasts Mediates Staphylococcus Aureus Induced Bone Resorption and Osteoclastogenesis through Enhanced RANKL. PLoS ONE 2016, 11, e0156708. [Google Scholar] [CrossRef]
- Kassem, A.; Henning, P.; Lundberg, P.; Souza, P.P.; Lindholm, C.; Lerner, U.H. Porphyromonas gingivalis Stimulates Bone Resorption by Enhancing RANKL (Receptor Activator of NF-kappaB Ligand) through Activation of Toll-like Receptor 2 in Osteoblasts. J. Biol. Chem. 2015, 290, 20147–20158. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, H.; Zhang, J.; Zhang, X.; Xia, X.; Qiu, C.; Liao, Y.; Chen, H.; Song, Z.; Zhou, W. Periodontitis Induced by P. gingivalis-LPS Is Associated With Neuroinflammation and Learning and Memory Impairment in Sprague-Dawley Rats. Front. Neurosci. 2020, 14, 658. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Z.; Zhang, L.; Kirkwood, K.L.; Lopes-Virella, M.F.; Huang, Y. Acid sphingomyelinase deficiency exacerbates LPS-induced experimental periodontitis. Oral Dis. 2020, 26, 637–646. [Google Scholar] [CrossRef]
- Lin, P.; Niimi, H.; Ohsugi, Y.; Tsuchiya, Y.; Shimohira, T.; Komatsu, K.; Liu, A.; Shiba, T.; Aoki, A.; Iwata, T.; et al. Application of Ligature-Induced Periodontitis in Mice to Explore the Molecular Mechanism of Periodontal Disease. Int. J. Mol. Sci. 2021, 22, 8900. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Maekawa, T.; Hiyoshi, T.; Terao, Y. Analysis of Experimental Ligature-Induced Periodontitis Model in Mice. Methods Mol. Biol. 2021, 2210, 237–250. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J.; Graves, D.T. The enduring importance of animal models in understanding periodontal disease. Virulence 2015, 6, 229–235. [Google Scholar] [CrossRef]
- Graves, D.T.; Fine, D.; Teng, Y.T.A.; Van Dyke, T.E.; Hajishengallis, G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J. Clin. Periodontol. 2008, 35, 89–105. [Google Scholar] [CrossRef]
- Hidai, C.; Zupancic, T.; Penta, K.; Mikhail, A.; Kawana, M.; Quertermous, E.E.; Aoka, Y.; Fukagawa, M.; Matsui, Y.; Platika, D.; et al. Cloning and characterization of developmental endothelial locus-1: An embryonic endothelial cell protein that binds the alphavbeta3 integrin receptor. Genes Dev. 1998, 12, 21–33. [Google Scholar] [CrossRef]
- Chavakis, E.; Choi, E.Y.; Chavakis, T. Novel aspects in the regulation of the leukocyte adhesion cascade. Thromb. Haemost. 2009, 102, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Colamatteo, A.; Kalafati, L.; Kajikawa, T.; Wang, H.; Lim, J.H.; Bdeir, K.; Chung, K.J.; Yu, X.; Fusco, C.; et al. The DEL-1/beta3 integrin axis promotes regulatory T cell responses during inflammation resolution. J. Clin. Investig. 2020, 130, 6261–6277. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Chavakis, T. DEL-1: A potential therapeutic target in inflammatory and autoimmune disease? Expert Rev. Clin. Immunol. 2021, 17, 549–552. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. DEL-1-Regulated Immune Plasticity and Inflammatory Disorders. Trends Mol. Med. 2019, 25, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Kourtzelis, I.; Li, X.; Mitroulis, I.; Grosser, D.; Kajikawa, T.; Wang, B.; Grzybek, M.; von Renesse, J.; Czogalla, A.; Troullinaki, M.; et al. DEL-1 promotes macrophage efferocytosis and clearance of inflammation. Nat. Immunol. 2019, 20, 40–49. [Google Scholar] [CrossRef]
- Loi, F.; Cordova, L.A.; Pajarinen, J.; Lin, T.H.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Mountziaris, P.M.; Spicer, P.P.; Kasper, F.K.; Mikos, A.G. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng. Part B Rev. 2011, 17, 393–402. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng. Part B Rev. 2008, 14, 179–186. [Google Scholar] [CrossRef]
- Aspenberg, P. Drugs and fracture repair. Acta Orthop. 2005, 76, 741–748. [Google Scholar] [CrossRef]
- Maekawa, T.; Hosur, K.; Abe, T.; Kantarci, A.; Ziogas, A.; Wang, B.; Van Dyke, T.E.; Chavakis, T.; Hajishengallis, G. Antagonistic effects of IL-17 and D-resolvins on endothelial Del-1 expression through a GSK-3beta-C/EBPbeta pathway. Nat. Commun. 2015, 6, 8272. [Google Scholar] [CrossRef]
- Page, R.C.; Altman, L.C.; Ebersole, J.L.; Vandesteen, G.E.; Dahlberg, W.H.; Williams, B.L.; Osterberg, S.K. Rapidly progressive periodontitis. A distinct clinical condition. J. Periodontol. 1983, 54, 197–209. [Google Scholar] [CrossRef]
- Silva-Costa, C.; Friaes, A.; Ramirez, M.; Melo-Cristino, J. Macrolide-resistant Streptococcus pyogenes: Prevalence and treatment strategies. Expert Rev. Anti-Infect. Ther. 2015, 13, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Lanata, M.M.; Wang, H.; Everhart, K.; Moore-Clingenpeel, M.; Ramilo, O.; Leber, A. Macrolide-Resistant Mycoplasma pneumoniae Infections in Children, Ohio, USA. Emerg. Infect. Dis. 2021, 27, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Belanger, A.E.; Shryock, T.R. Macrolide-resistant Campylobacter: The meat of the matter. J. Antimicrob. Chemother. 2007, 60, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Kimura, O.; Domon, H.; Maekawa, T.; Yonezawa, D.; Terao, Y. Antimicrobial susceptibility of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis clinical isolates from children with acute otitis media in Japan from 2014 to 2017. J. Infect. Chemother. 2019, 25, 229–232. [Google Scholar] [CrossRef]
- Arredondo, A.; Blanc, V.; Mor, C.; Nart, J.; Leon, R. Azithromycin and erythromycin susceptibility and macrolide resistance genes in Prevotella from patients with periodontal disease. Oral Dis. 2019, 25, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Otsu, K.; Ishinaga, H.; Suzuki, S.; Sugawara, A.; Sunazuka, T.; Omura, S.; Jono, H.; Takeuchi, K. Effects of a novel nonantibiotic macrolide, EM900, on cytokine and mucin gene expression in a human airway epithelial cell line. Pharmacology 2011, 88, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Sadamatsu, H.; Takahashi, K.; Tashiro, H.; Kato, G.; Noguchi, Y.; Kurata, K.; Omura, S.; Kimura, S.; Sunazuka, T.; Sueoka-Aragane, N. The non-antibiotic macrolide EM900 attenuates HDM and poly(I:C)-induced airway inflammation with inhibition of macrophages in a mouse model. Inflamm. Res. 2020, 69, 139–151. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).