1. Introduction
Dengue virus (DENV) is an important arthropod-borne human pathogen that can cause life-threatening diseases, mainly in tropical and subtropical regions [
1]. As one member of the Flaviviridae family, DENV is divided into four widely known serotypes (DENV-1–4) based on its antigenic diversity. DENV-5 is the latest serotype, which was announced in October 2013. DENV consists of a positive-sense, single-stranded RNA genome (11 kb in length). Upon virus entry into the host cell, the genetic material is translated into a polyprotein that is subsequently cleaved by both viral and host proteases into seven non-structural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) and three structural proteins (capsid, precursor membrane, and envelope (E)) [
2]. Clinically, infection with any of the four DENVs can either be asymptomatic or manifest in two increasingly severe diseases, known as mild dengue fever and severe dengue diseases [
3]. It is estimated that the annual incidence of DENV infections is approximately 400 million each year, of which about 25% are clinically symptomatic and account for a heavy socioeconomic burden [
4]. However, no broadly protective vaccines or specific antiviral agents to treat dengue infection are currently available. Due to the challenges encountered in developing a safe vaccine that provides durable protection against all four DENV serotypes, there is an urgent need to develop antivirals that can treat dengue infection.
Direct-acting antiviral (DAA), low-toxicity compounds with a wide treatment window interact with viral proteins to exert antiviral functions [
5]. E protein, the major structural protein exposed on the surface of mature DENV, is responsible for attachment to the cellular receptor present on the surface of the host cell [
6]. Studies have shown that the E protein consists of three distinct domains, termed ED I, ED II, and ED III. Of these, ED III has been proposed to function as the binding site for cellular receptors [
7]. Currently, the E protein is the most extensively studied structural protein as an antiviral target [
5].
Glycyrrhizae Radix et Rhizoma, the dried root and rhizome of
Glycyrrhiza uralensis Fisch.,
Glycyrrhiza inflata Bat., or
Glycyrrhiza glabra L., is widely exploited in traditional medicine for nourishing qi, alleviating pain, tonifying the spleen and stomach, eliminating phlegm, and relieving coughing [
8,
9]. With the increasing study of Glycyrrhizae Radix et Rhizoma, substantial efforts have demonstrated that it exerts broad-spectrum antiviral activities against HIV, severe acute respiratory syndrome coronavirus, HSV, and so on [
8]. Recently, Glycyrrhizae Radix et Rhizoma was reported to prevent or treat COVID-19, which was due to its anti-inflammatory and anti-allergic activities [
8]. The purpose of this study was to identify the best inhibitory effects of four different polar extracts from Glycyrrhizae Radix et Rhizome on DENV infection and explore the underlying mechanism.
3. Discussion
Glycyrrhizae Radix et Rhizoma, a widely used traditional Chinese medicine, has been known for its antiviral activities against several viruses, including hepatitis B, hepatitis C, influenza, H1N1, and HIV [
8,
21]. Previous studies have shown that glycyrrhizic acid identified from Glycyrrhizae Radix et Rhizoma had inhibitory effects on DENV infection [
22]. However, whether Glycyrrhizae Radix et Rhizoma could inhibit DENV replication has not been elucidated yet.
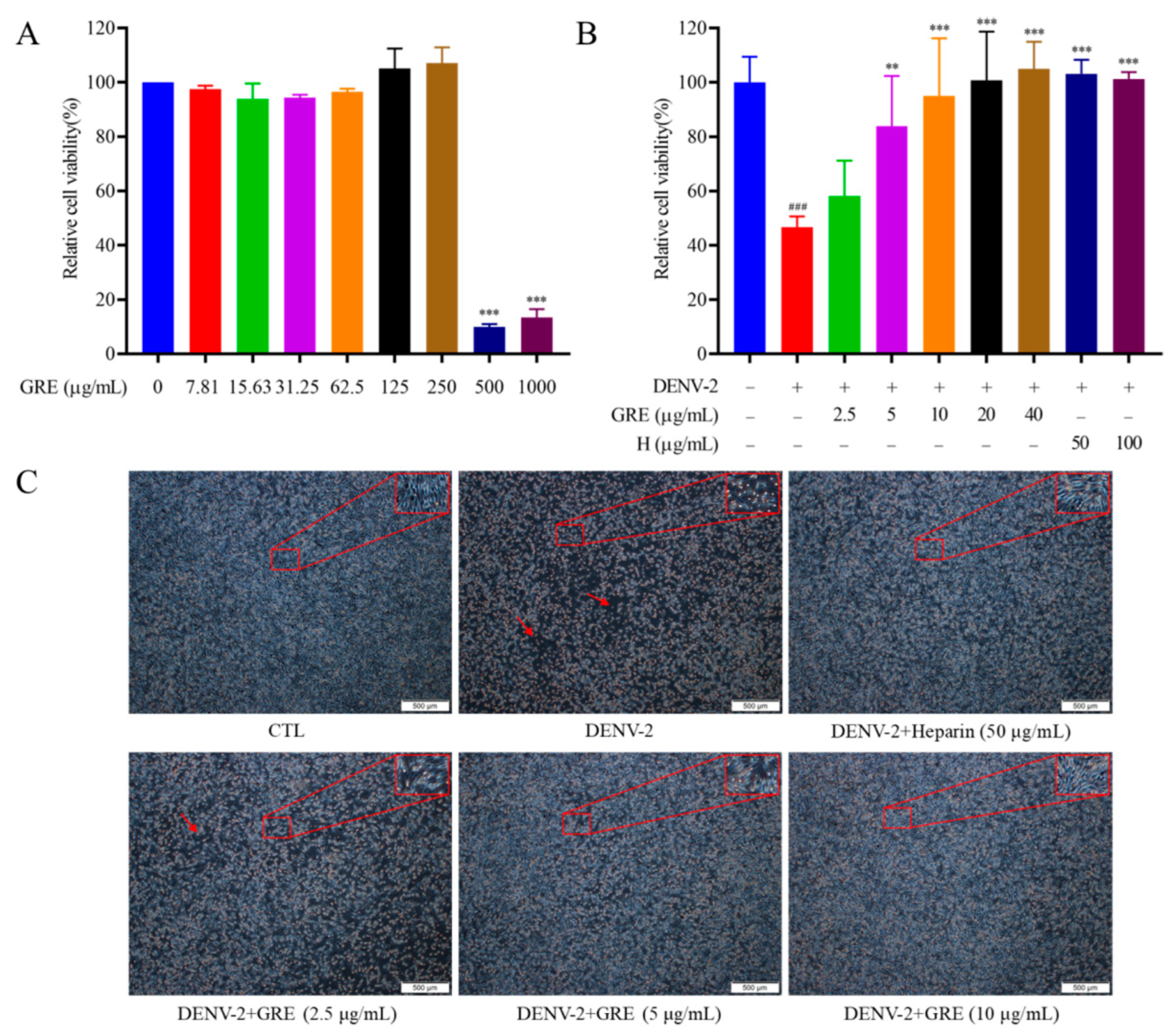
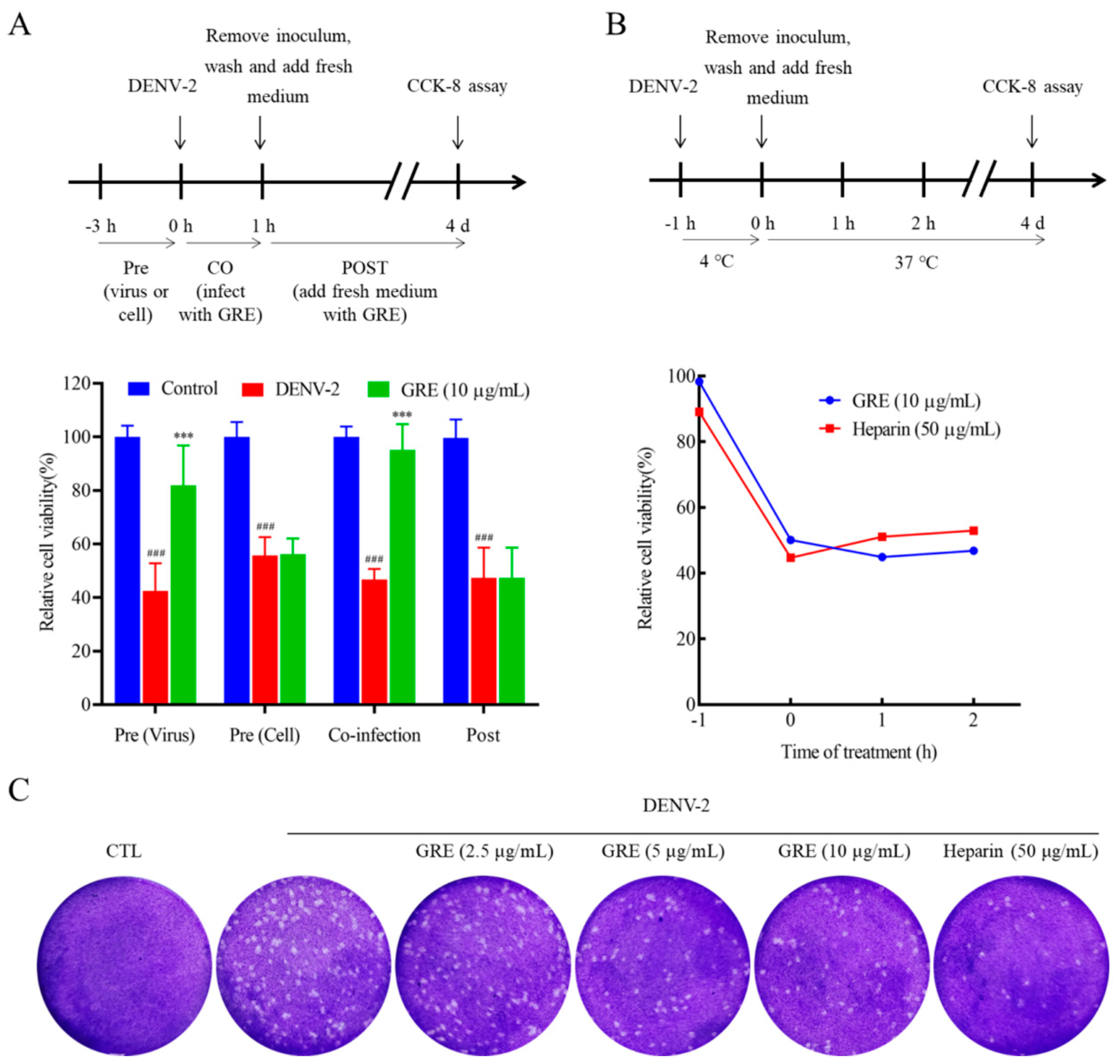
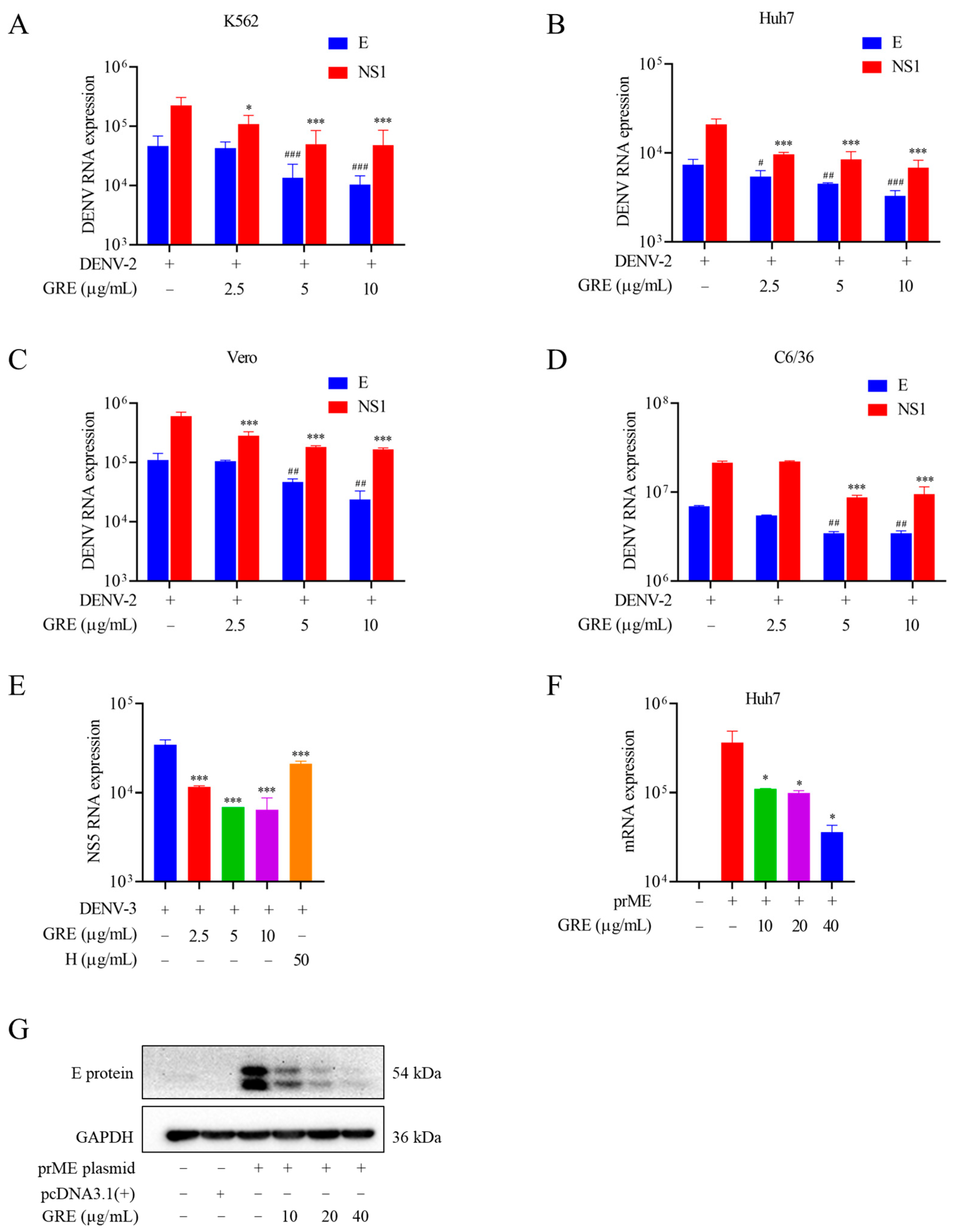
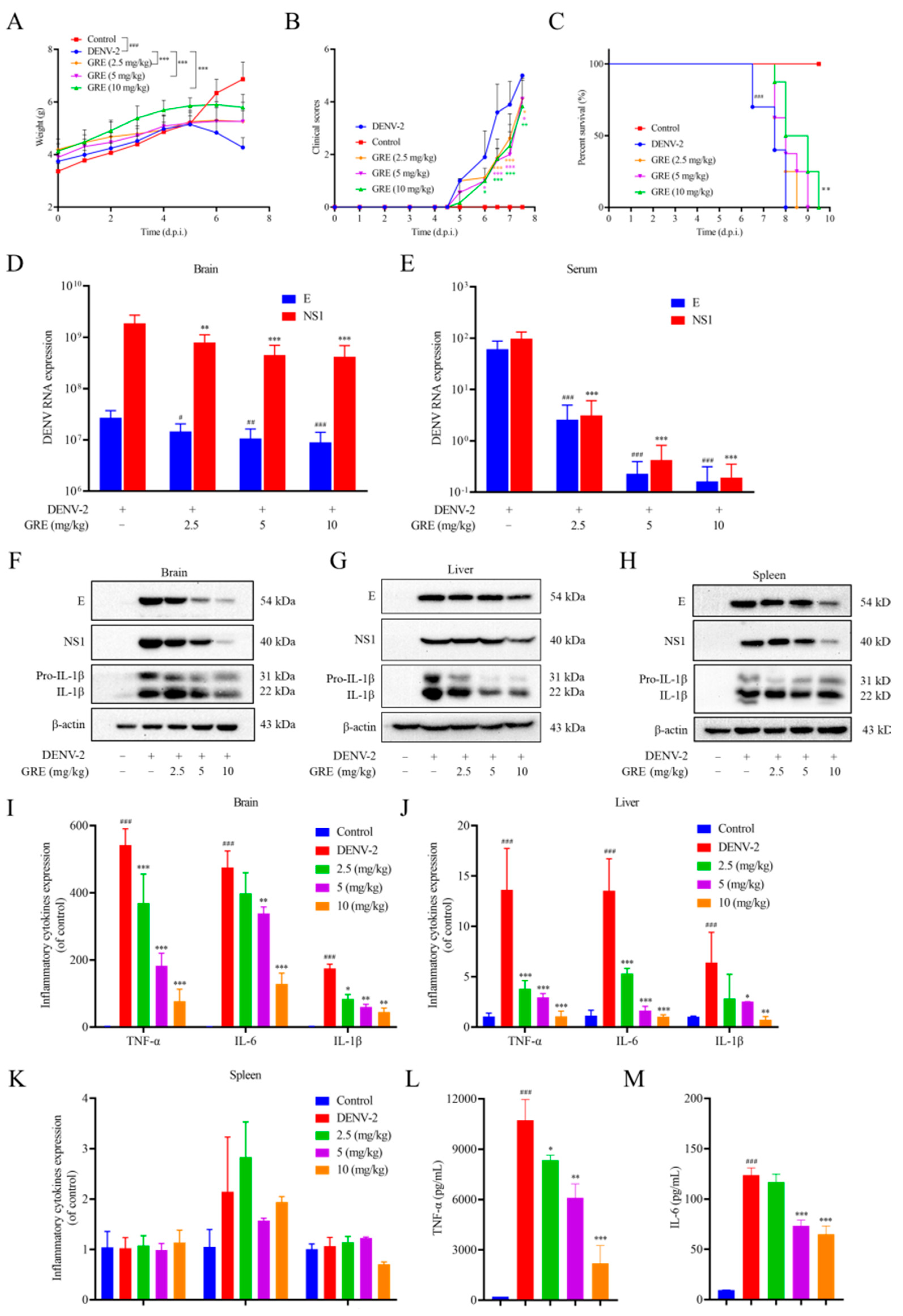
In the present study, we found that GRE, the n-butanol extract of Glycyrrhizae Radix et Rhizome, exerted significant antiviral activities against DENV-2 infection. Then, with the aim of clarifying the anti-DENV mechanism of GRE, we conducted time of addition and temperature-shift assays to determine the stage of the virus replication cycle at which GRE had antiviral effects. Our data showed that GRE exerted inhibitory activities during the adsorption process in the DENV replication cycle, which was similar to heparin. Additionally, when DENV-2 was pre-infected with GRE for 3 h prior to infection, the infectivity of the virus was severely impaired. Altogether, these results suggested that GRE showed significant inhibitory activities against virus attachment. It was likely that GRE directly targeted the virus itself. In addition, GRE exerted inhibitory activities against several cell types relevant to DENV infection and reduced the viral infectivity of DENV-3.
The E protein is responsible for the attachment of a virus to the host cell membrane, and ED III serves as the binding site for cellular receptors prior to its internalization into host endosomes [
23]. To determine the effect of GRE on the E protein, Huh7 cells were transfected with pcDNA3.1(+)-prME plasmid. Consistent with our deduction, GRE significantly inhibited E protein expression in transfected cells. The following SPR assays indicated that several constituents of the GRE could bind to the III domain of the E protein. It is possible that GRE interacted with the III domain of the E protein, induced a conformational change, and finally blocked the normal function of the E protein. However, how GRE impacted ED III needs to be further explored.
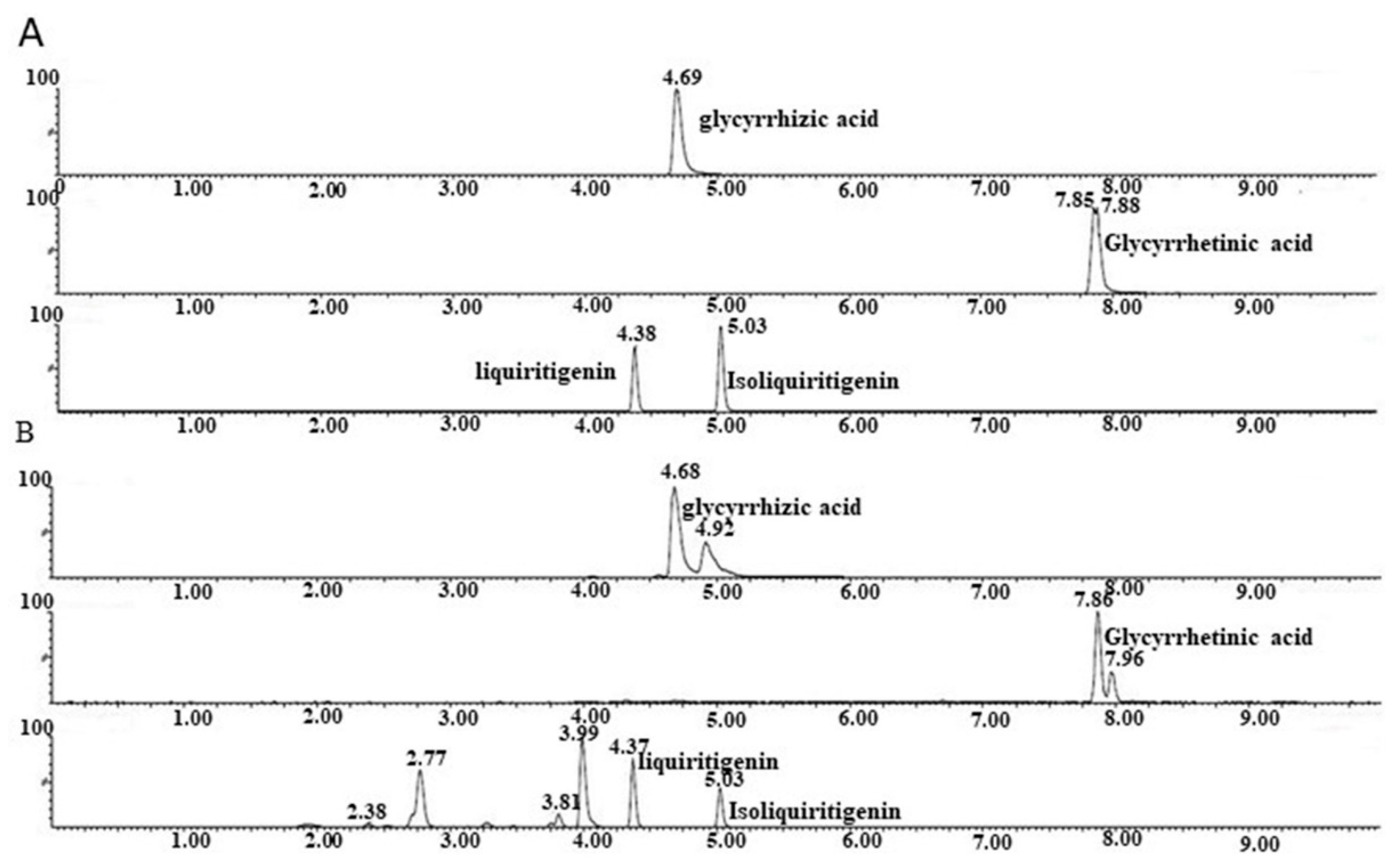
Glycyrrhizic acid, Glycyrrhetnic acid, liquiritigenin, and isoliquiritigenin were preliminarily investigated to evaluate their antiviral activities. Unfortunately, these compounds did not show significant anti-DENV effects (
Figure S3). This may indicate that additional active molecules or a combination of them might be responsible for the robust anti-DENV effects of GRE and further highlight the value of GRE as an excellent starting source for discovering a novel DAA.
Seven-day-old ICR suckling mice were used to examine the protective effects of GRE against DENV in vivo, which was a successfully established DENV infection model in previous studies. Our results showed that GRE evidently decreased weight change, improved disease symptoms, and prolonged the survival duration of DENV-infected mice. Furthermore, we found that GRE significantly alleviated viremia and decreased the viral load in the brain, liver, and spleen tissues of DENV-infected mice. Studies have shown that pro-inflammatory cytokines play an important role in the pathological processes in DENV-infected mice [
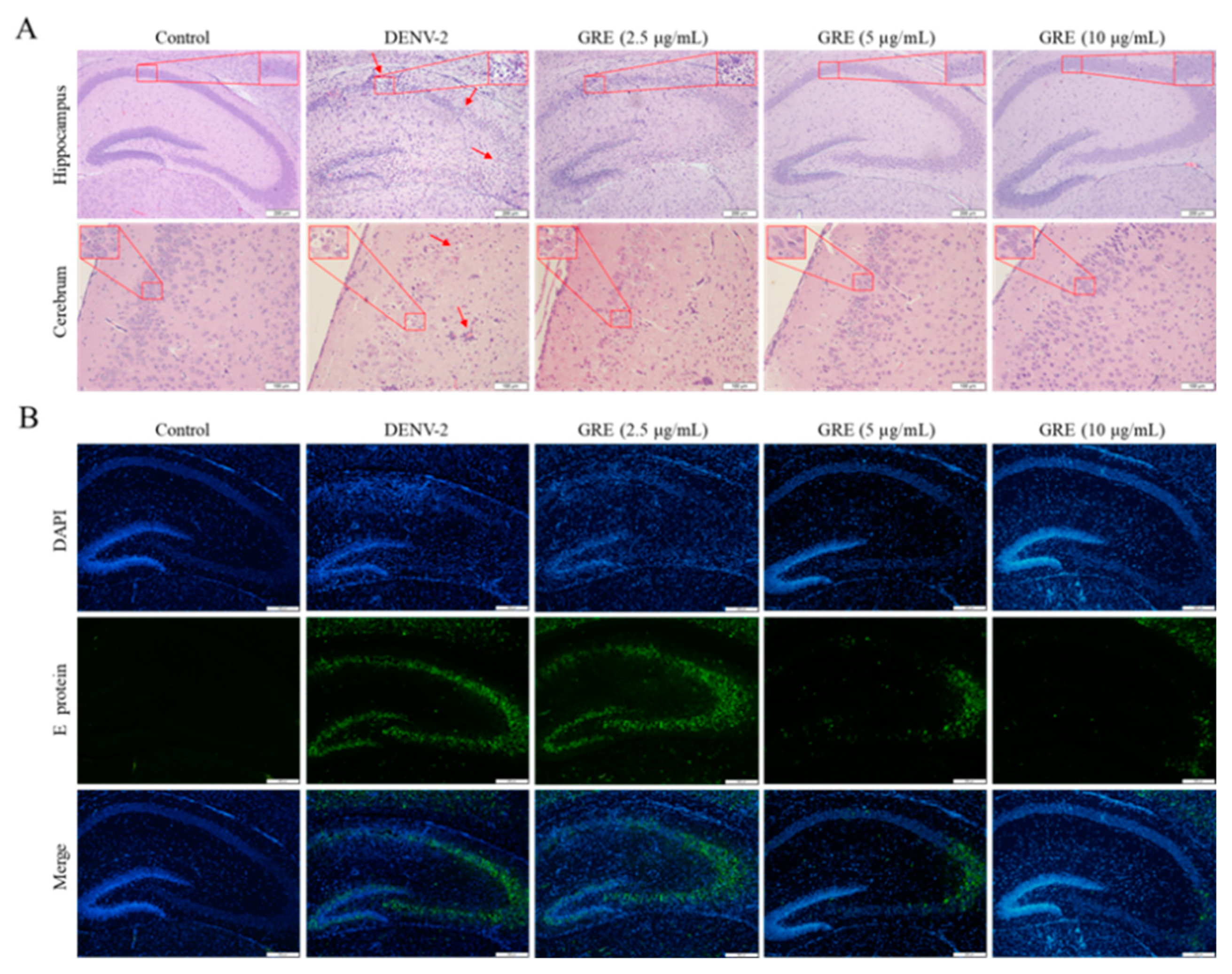
24]. In our mice model, we found that GRE significantly decreased the expression levels of TNF-α, IL-6, and IL-1β in the brain tissue compared with the model group, as well as in liver tissue. However, we did not observe a significant difference in the spleen tissue. Moreover, only pathological damage in the brain was observed among these tissues, which was probably due to the low levels of viral load and pro-inflammatory cytokines in the liver and spleen tissues that did not cause organic lesions. Although ICR suckling mice have been demonstrated to be susceptible to DENV infection, an obvious limitation of this model is that paralysis is not a relevant phenotype in human disease [
25]. Therefore, immunocompromised mice should be further used to test the antiviral effects of GRE in vivo.
5. Materials and Methods
5.1. Reagents
Glycyrrhizae Radix et Rhizoma was purchased from Kangmei Pharmaceutical Co. Ltd. (Guangzhou, China) and authenticated by Professor Zhi Chao (Southern Medical University) as the dried root and rhizome of Glycyrrhiza uralensis Fisch. Enzyme-linked immunosorbent assay (ELISA) kits for tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were obtained from Cusabio (Wuhan, China). PrimeScriptTM RT Master Mix and TB GreenTM Premix Ex TaqTM II were from Takara (Shiga, Japan). Bestar R qPCR Master Mix was obtained from DBI Bioscience (Shanghai, China). Fetal bovine serum (FBS) was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Antibodies against dengue virus E protein and NS1 protein were purchased from GeneTex (San Antonio, TX, USA) and Arigo Biolaboratories (Taiwan, China), respectively. The antibody against β-actin was provided by Santa Cruz (Santa Cruz, CA, USA). Anti-mouse IgG HRP-linked antibody and Anti-rabbit IgG HRP-linked antibody were obtained from Cell Signaling Technology (Danvers, MA, USA). Alexa Fluor 488-conjugated anti-Rabbit IgG antibody, Alexa Fluor 555-conjugated anti-Mouse IgG antibody, and Lipofectamine® 2000 transfection reagent were purchased from Invitrogen (Grand Island, NE, USA). 4′, 6-diamidino-2-phenylindole (DAPI) was obtained from Bioss (Beijing, China). RPMI-1640, DMEM, penicillin, streptomycin, the BCA protein assay kit, and the enhanced chemiluminescence (ECL) kit were purchased from Thermo Fisher Scientific. Methylcellulose, crystal violet, and other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). ED III protein was provided by China Peptides Co., Ltd.
5.2. Cells and Viruses
The mosquito larva C6/36 cells and baby hamster kidney cell line BHK-21 cells were obtained from the American Type Culture Collection (Rockville, MD, USA). African green monkey kidney cell line Vero cells, human hepatocellular carcinoma Huh7 cells, and human myelogenous leukemia K562 cells were obtained from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). BHK-21, Huh7, and K562 cells were cultured in RPMI-1640 supplemented with 10% FBS (v/v). Vero cells were maintained in DMEM containing 10% FBS (v/v). C6/36 cells were grown in RPMI-1640 supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 28 °C. All cells, except for C6/36, were cultured in an incubator with 5% CO2 at 37 °C.
DENV-2 New Guinea C derivative strain was generously provided by Professor Xingang Yao (Southern Medical University). DENV-3 was obtained from the BSL-3 Laboratory, Guangdong Provincial Key Laboratory of Tropical Disease Research (Guangdong, China). All virus stocks were propagated in C6/36 cells and the cell supernatants were harvested, clarified, and stored at −80 °C. The DENV titer in the harvested supernatants was determined by the TCID50 assay.
5.3. Preparation of Extracts
Glycyrrhizae Radix et Rhizoma (150 g) were successively soaked in 90%, 70%, and 50% ethanol for 2 h with a material-to-solvent ratio of 1:10 and then extracted by ultrasonic extraction for 30 min. The merged supernatant was evaporated by a rotary evaporator, the residue of which was dissolved in water. Subsequently, the solution was extracted by petroleum ether (60–90 °C), ethyl acetate, and n-butanol, respectively. Finally, the concentrated extract was lyophilized into powder and stored in desiccators. The freeze-dried powder was dissolved in DMSO at a concentration of 500 mg/mL and stored at −20 °C.
5.4. Component Analysis of GRE
Qualitative analysis of the components of the GRE was conducted using HPLC. The Vanquish high-performance liquid chromatography instrument (Thermo Fisher Scientific) was used and the separation was carried out using an analytical column (Hypersil Gold 100 × 2.1 mm, 1.9 μm, USA) with a column temperature of 35 °C at a flow rate of 0.3 mL/min. Water containing 0.1% formic acid (A) and acetonitrile (B) were prepared as mobile phases. A gradient program was used as follows: 0–7 min, 95% A; 7–19 min, 55% A; 19–21 min, 5% A. The mass spectrometric experiment was operated via electron spray ionization in the negative- and positive-ion modes of an Orbitrap Fusion Tribrid mass spectrometer (Thermo Fisher Scientific). The operating parameters were as follows: positive ion, 3500 V; negative ion, 3000 V; sheath gas, 40 mL/min; aux gas, 10 mL/min; sweep gas, 1 mL/min; ion transfer tube temperature, 350 °C; vaporizer temperature, 300 °C. The mass data were recorded within the range of 100 to 1000 m/z. Finally, the compounds in the GRE were identified by comparing their retention times and MS as well as MS/MS data with those of the standards or reported data.
Quantitative analyses of glycyrrhizic acid, glycyrrhetinic acid, liquiritigenin, and isoliquiritigenin in GRE were analyzed via ultra-performance liquid chromatography/tandem mass spectrometry (UHPLC-MS/MS). Standard products of glycyrrhizic acid, glycyrrhetinic acid, liquiritigenin, and isoliquiritigenin were purchased from Biopurify. The purity of the four standards was 95–99%. The separation was carried out using an Agilent ZORBAX Eclipse Plus C18 (2.1 mm × 150 mm, 1.8 μm, USA). The column temperature was set at 35 °C and the flow rate was 0.3 mL/min. Water solutions containing 10 mM ammonium formate and 0.1% formic acid (A) or methanol (B) were prepared as mobile phases. The elution gradient was 70% A in the first 5 min; 2% A for 5–7 min; and 70% A for 7–10 min. The typical ion source parameters were: capillary voltage = –2500 V, cone voltage = 30 V, desolvation temperature = 550 °C, desolvation gas flow = 1000 L/Hr, collision gas flow = 0.15 mL/min, and nebulizer gas flow = 7 Bar. Finally, quantitation was employed following the calibration curve method using the reference standards.
5.5. Cell Viability Assay
BHK-21 cells were seeded in a 96-well culture plate at a density of 8 × 103 cells per well and then exposed to different concentrations of GRE. After 96 h of incubation at 37 °C, the cells were cultured in 100 µL of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide (MTT) solution (0.5 mg/mL) for another 4 h. Then, the MTT solution was removed and 100 μL of DMSO was added to solubilize the formazan crystals. Finally, the absorbance was measured using a microplate reader (Thermo Fisher Scientific) at 570 nm.
5.6. Lactate Dehydrogenase (LDH) Release Assay and CCK-8 Assay
BHK-21 cells were seeded in 96-well plates and incubated for 24 h. For the viral adsorption and entry experiment, the cells were infected with 100 μL of DENV-2 (200 plaque-forming units/mL, PFU/mL) in the presence or absence of GRE at 37 °C for 1 h. After rinsing twice with PBS to remove the unbound virus, the cells were grown in RPMI-1640 medium with 2% FBS. For the viral intracellular replication experiment, the cells were infected with dengue virus for 1 h at 37 °C and then treated with different compounds at the indicated concentrations. After 4 d of incubation, the LDH and CCK-8 assays were performed according to the manufacturer’s protocol to evaluate the antiviral activities of the extracts.
5.7. Time of Addition and Temperature-Shift Assay
Experiments were carried out in BHK-21 cells using DENV-2 (200 PFU/mL) in the presence of GRE (10 μg/mL) or heparin (50 μg/mL) at the times noted in the schematic. For the “Pre” conditions, either cells or the virus were pre-incubated in 100 μL medium containing the compound for 3 h at 37 °C (“Pre cells” and “Pre virus”). For “Co-infection” conditions, GRE was present during the 1 h incubation of the viral inoculum with cells. For “Post”, the cells were infected with the virus for 1 h prior to adding the compound. For the temperature-shift assay, the cells were incubated with DENV-2 (200 PFU/mL) at 4 °C for 1 h. Next, after washing with cold PBS 3 times, the cells were shifted to a 37 °C incubator and incubated for 4 d. During the time allowed for viral adsorption and infection, 100 μL of RPMI-1640 containing GRE or heparin was added to the cells at appropriate time points (−1, 0, 1, and 2 h). The inhibitory activities of GRE against the absorption of DENV-2 were measured by the CCK-8 assay.
5.8. Cytopathic Effect (CPE)
BHK-21 cells were grown in a 6-well plate at 37 °C overnight. After 1 h of infection with GRE or heparin at 37 °C, the cells were washed twice with PBS and cultured in RPMI-1640 supplemented with 2% FBS at 37 °C for another 4 d. The CPE was visualized under an IX 53 light microscope (Olympus, Tokyo, Japan).
5.9. Plaque Assay
BHK-21 cells cultured in 6-well microplates (1 × 105 cells per well) were firstly infected with DENV-2 at 4 °C in the presence of different concentrations of GRE (2.5, 5, and 10 μg/mL) or Heparin (50 μg/mL). After 2 d of incubation, the supernatants containing progeny virus were collected to infect new BHK-21 cells for 1 h at 37 °C and then the medium was replaced with RPMI-1640 supplemented with 2% FBS and 1.2% methylcellulose for another 5 days. Finally, the cells were fixed in 4% paraformaldehyde (PFA) and stained with 2% crystal violet for 15 min, and the plaques formed were visualized.
5.10. Western Blot Analysis
The samples were homogenized in lysis buffer (50 mM Tris, pH 7.5, 150 mM sodium chloride, 1% Triton X-100, 1 mM EDTA, 1 mM PMSF, 1 mM sodium orthovanadate, 1 mM dithiothreitol, and 1 mM phosphatase inhibitor) on ice for 15 min and then the homogenates were centrifuged at 15,000× g for 15 min at 4 °C. The supernatants were collected and stored at −20 °C. The protein concentration of the lysates was quantified using a BCA kit. Subsequently, an equal number of proteins was separated by 10% SDS–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad, Hercules, USA). After blocking in Tris-buffered saline Tween-20 (TBS-T) containing 5% (w/v) skimmed milk for 1 h, the membranes were incubated with primary antibodies against E protein (1:3000), NS1 protein (1:1000), IL-1β (1:1000), GAPDH (1:5000), and β-actin (1:5000) at 4 °C overnight, respectively. Next, the membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (1:1000) at 4 °C for 2 h. Finally, the protein bands were detected using ECL reagent and visualized by a FluorChem E™ system (ProteinSimple, San Francisco, CA, USA).
5.11. Immunofluorescence
Cells at 2 days post-infection were rinsed with PBS thrice, fixed with 4% (v/v) paraformaldehyde for 15 min, and then permeabilized with 0.2% (v/v) TritonX-100 in PBS for 15 min. Subsequently, the cells were blocked with 5% (w/v) skimmed milk followed by incubation with E protein (1:500) or NS1 protein (1:400) antibody at 4 °C overnight. For slices, the tissue sections were firstly deparaffinized, rehydrated, and washed in PBS thrice. Epitope retrieval was performed in the boiling citrate buffer (pH = 6.0) for 10 min. Subsequently, brain slices were blocked with 5% bovine serum albumin (BSA) for 1 h and incubated with E protein (1:500) antibody at 4 °C overnight. Then, the cells and slices were incubated with AlexaFluor488-conjugated anti-Rabbit IgG antibody (1:500) for 1 h and stained with DAPI for 5 min under darkness at room temperature, respectively. Finally, fluorescent imaging was conducted using a confocal microscope (LSM800, CarlZeiss, Oberkochen, Germany) or an IX 53 light microscope.
5.12. Quantitative Real-Time PCR (qRT-PCR) Analysis
Total RNAs were extracted from the cells or tissues using RNAiso Plus (Takara) following the manufacturer’s instructions, and reverse transcription was immediately performed using a PrimeScript
TM RT reagent Kit with gDNA Eraser (Takara). Then, qRT-PCR was carried out on a LightCycler
® 96 real-time PCR instrument (Roche, Switzerland) with Bestar R qPCR Master Mix (for the absolute expression levels of virus genes) or TB green
TM Premix Ex Taq
TM II (for the relative expression levels of cytokine genes). The expression ratio of inflammatory cytokines was calculated by the 2
−ΔΔCt method normalized to the expression level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). All of the TaqMan probes and primers used in this study are listed in
Table S1.
5.13. Plasmids and Transfection
Plasmid pcDNA3.1(+)-prME was obtained from Guangzhou Youming Biotechnology Co. Ltd. (Guangzhou, China). DENV prME proteins were cloned into pcDNA3.1(+) vector using specific primers (GenBank accession number AF038403, nucleotides: 367 bp–2421 bp). The empty vector pcDNA3.1(+) served as a negative control.
Huh7 cells were seeded in either 12-well microplates or 6-well microplates. For the 12-well microplates, 0.5 µg pcDNA3.1(+)-prME plasmid and GRE were mixed with 2.5 µL Lipofectamine 2000 in 500 µL Opti-MEM (Thermo Fisher Scientific). For the 6-well microplates, 1 µg of pcDNA3.1(+)-prME plasmid and GRE were mixed with 5 µL of Lipofectamine 2000 in 1000 µL Opti-MEM. After 20 min of incubation at room temperature, cells were transfected with the plasmid mixture for 4 h to express prME. Finally, the levels of the E protein in the transfected cells were detected by qRT-PCR and Western blotting.
5.14. DENV-2 Infection in ICR Suckling Mice
ICR-strain suckling mice were obtained from SPF Biotechnology Co., Ltd. (Beijing, China). The housing and experimental use of the mice were carried out in a biosafety level 2 facility at the Animal Experimental Center (Permit number: 20220526002).
To investigate the therapeutic effect of GRE on mice, an established DENV-2-infected suckling mice model was used. Seven-day-old ICR suckling mice were inoculated intracerebrally with 400 PFU/mL and intraperitoneally with 4 × 105 PFU/mL DENV-2. For the treatment with drugs, GRE (2.5, 5, and 10 mg/kg) or the vehicle control were administered intragastrically at 0, 1, 3, 5, and 7 days post-infection (d.p.i). The mortality and clinical scores were monitored every day for 10 days. At 6 d.p.i, the brain, liver, kidneys, spleen, large intestine, small intestine, and serum of mice were collected and analyzed by qRT-PCR, Western blotting, ELISA, immunofluorescence, and histological analyses to monitor DENV replication. The clinical illness was scored as follows: 0, healthy; 1, reduced mobility; 2, limbic seizure; 3, limbic weakness; 4, limbic paralysis; and 5, death.
5.15. ELISA
The TNF-α, IL-6, and IL-1β levels in the homogenate were determined using commercially available ELISA kits according to the manufacturers’ protocols. Briefly, the samples were added to a 96-well ELISA plate and then reacted with relevant primary antibodies and HRP-conjugated secondary antibodies. Eventually, the absorbance value at 450 nm was measured.
5.16. Histopathological Analysis
The paraffin-embedded slices (4 μm) were stained with hematoxylin and eosin (H&E) to observe and image the pathological changes as described previously.
5.17. Statistical Analysis
All data were presented as the means ± standard deviations (SDs) from three independent experiments. Analysis of variance (ANOVA) was used to assess differences between multiple groups in GraphPad Prism 8.0 (San Diego, CA, USA). p < 0.05 was considered statistically significant.