The Risk of Age-Related Macular Degeneration Is Reduced in Type 2 Diabetes Patients Who Use Metformin
Abstract
1. Introduction
2. Results
3. Materials and Methods
- Analysis restricted to patients enrolled during 1999–2002;
- Analysis restricted to patients enrolled during 2003–2005;
- Including only patients aged 50–64 years;
- Including only patients aged 65–79 years;
- Including only male patients;
- Including only female patients;
- Excluding patients with a diagnosis of anemia (ICD-9-CM 280–285) and/or nutritional deficiency (ICD-9-CM 260–269);
- Patients with diabetic retinopathy (ICD-9-CM 362.0X);
- Patients without diabetic retinopathy (ICD-9-CM 362.0X);
- Outcome defined as nonexudative AMD (ICD-9-CM 362.50 and 362.51);
- Outcome defined as exudative AMD (ICD-9-CM 362.52);
- All covariates defined at censor.
4. Discussion
4.1. Main Findings
4.2. Potential Mechanisms
4.3. Clinical Implications
4.4. Limitations
4.5. Strengths
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, U.; Wong, T.Y.; Fletcher, A.; Piault, E.; Evans, C.; Zlateva, G.; Buggage, R.; Pleil, A.; Mitchell, P. Clinical risk factors for age-related macular degeneration: A systematic review and meta-analysis. BMC Ophthalmol. 2010, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Qiao, L.; Du, M.; Qu, C.; Wan, L.; Li, J.; Huang, L. Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis. 2021, 9, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- He, L. Metformin and systemic metabolism. Trends Pharm. Sci. 2020, 41, 868–881. [Google Scholar] [CrossRef]
- Maniar, K.; Moideen, A.; Mittal, A.; Patil, A.; Chakrabarti, A.; Banerjee, D. A story of metformin-butyrate synergism to control various pathological conditions as a consequence of gut microbiome modification: Genesis of a wonder drug? Pharm. Res. 2017, 117, 103–128. [Google Scholar] [CrossRef]
- Chen, F.; Moat, J.; McFeely, D.; Clarkson, G.; Hands-Portman, I.J.; Furner-Pardoe, J.P.; Harrison, F.; Dowson, C.G.; Sadler, P.J. Biguanide iridium(III) complexes with potent antimicrobial activity. J. Med. Chem. 2018, 61, 7330–7344. [Google Scholar] [CrossRef]
- Nath, M.; Bhattacharjee, K.; Choudhury, Y. Pleiotropic effects of anti-diabetic drugs: A comprehensive review. Eur. J. Pharmacol. 2020, 884, 173349. [Google Scholar] [CrossRef]
- Ugwueze, C.V.; Ogamba, O.J.; Young, E.E.; Onyenekwe, B.M.; Ezeokpo, B.C. Metformin: A possible option in cancer chemotherapy. Anal. Cell. Pathol. 2020, 2020, 7180923. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin may reduce breast cancer risk in Taiwanese women with type 2 diabetes. Breast Cancer Res. Treat. 2014, 145, 785–790. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin may reduce bladder cancer risk in Taiwanese patients with type 2 diabetes. Acta Diabetol. 2014, 51, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Use of metformin and risk of kidney cancer in patients with type 2 diabetes. Eur. J. Cancer 2016, 52, 19–25. [Google Scholar] [CrossRef]
- Tseng, C.H. The effect of metformin on male reproductive function and prostate: An updated review. World J. Mens Health 2022, 40, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin may reduce oral cancer risk in patients with type 2 diabetes. Oncotarget 2016, 7, 2000–2008. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin is associated with a lower risk of colorectal cancer in Taiwanese patients with type 2 diabetes: A retrospective cohort analysis. Diabetes Metab. 2017, 43, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin decreases risk of tuberculosis infection in type 2 diabetes patients. J. Clin. Med. 2018, 7, 264. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin reduces the risk of diverticula of intestine in Taiwanese patients with type 2 diabetes mellitus. Front. Pharmacol. 2021, 12, 739141. [Google Scholar] [CrossRef]
- El-Arabey, A.A.; Abdalla, M.; Eltayb, W.A. Metformin: Ongoing journey with superdrug revolution. Adv. Pharm. Bull. 2019, 9, 1–4. [Google Scholar] [CrossRef]
- Chen, L.; Pawlikowski, B.; Schlessinger, A.; More, S.S.; Stryke, D.; Johns, S.J.; Portman, M.A.; Chen, E.; Ferrin, T.E.; Sali, A.; et al. Role of organic cation transporter 3 (SLC22A3) and its missense variants in the pharmacologic action of metformin. Pharm. Genomics 2010, 20, 687–699. [Google Scholar] [CrossRef]
- Massry, M.E.; Alaeddine, L.M.; Ali, L.; Saad, C.; Eid, A.A. Metformin: A growing journey from glycemic control to the treatment of Alzheimer’s disease and depression. Curr. Med. Chem. 2021, 28, 2328–2345. [Google Scholar] [CrossRef]
- Xu, L.; Kong, L.; Wang, J.; Ash, J.D. Stimulation of AMPK prevents degeneration of photoreceptors and the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA. 2018, 115, 10475–10480. [Google Scholar] [CrossRef]
- Brown, E.E.; Ball, J.D.; Chen, Z.; Khurshid, G.S.; Prosperi, M.; Ash, J.D. The common antidiabetic drug metformin reduces odds of developing age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jeon, H.L.; Park, S.J.; Shin, J.Y. Effect of statins, metformin, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers on age-related macular degeneration. Yonsei Med. J. 2019, 60, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Shen, Y.C.; Lai, Y.J.; Wang, C.Y.; Lin, K.H.; Feng, S.C.; Liang, C.Y.; Wei, L.C.; Chou, P. Association between metformin and a lower risk of age-related macular degeneration in patients with type 2 diabetes. J. Ophthalmol. 2019, 2019, 1649156. [Google Scholar] [CrossRef]
- Stewart, J.M.; Lamy, R.; Wu, F.; Keenan, J.D. Relationship between oral metformin use and age-related macular degeneration. Ophthalmol. Retina 2020, 4, 1118–1119. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, A.L.; Ham, S.A.; Colby, K.A.; Skondra, D. Association of metformin use with age-related macular degeneration: A case-control study. JAMA Ophthalmol. 2021, 139, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Eton, E.A.; Wubben, T.J.; Besirli, C.G.; Hua, P.; McGeehan, B.; VanderBeek, B.L. Association of metformin and development of dry age-related macular degeneration in a U.S. insurance claims database. Eur. J. Ophthalmol. 2022, 32, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Su, C.C.; Shao, S.C.; Sung, S.F.; Lin, S.J.; Kao Yang, Y.H.; Lai, E.C. Taiwan’s National Health Insurance Research Database: Past and future. Clin. Epidemiol. 2019, 11, 349–358. [Google Scholar] [CrossRef]
- Chang, L. A Study of Validation on Comorbidity Derived from Claims Data. Master’s Thesis, National Yang-Ming University, Hsinchu, Taiwan, 2004. Available online: https://etd.lib.nctu.edu.tw/cgi-bin/gs32/ymgsweb.cgi/ccd=9x8X4y/record?r1=1&h1=0 (accessed on 21 May 2020).
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin and endometrial cancer risk in Chinese women with type 2 diabetes mellitus in Taiwan. Gynecol. Oncol. 2015, 138, 147–153. [Google Scholar] [CrossRef]
- Abeysekera, W.W.M.; Sooriyarachchi, M.R. Use of Schoenfeld’s global test to test the proportional hazards assumption in the Cox proportional hazards model: An application to a clinical study. J. Natn. Sci. Found. Sri Lanka 2009, 37, 41–51. [Google Scholar] [CrossRef]
- Brown, E.E.; Lewin, A.S.; Ash, J.D. Mitochondria: Potential targets for protection in age-related macular degeneration. Adv. Exp. Med. Biol. 2018, 1074, 11–17. [Google Scholar] [PubMed]
- Zhao, X.; Liu, L.; Jiang, Y.; Silva, M.; Zhen, X.; Zheng, W. Protective effect of metformin against hydrogen peroxide-induced oxidative damage in human retinal pigment epithelial (RPE) cells by enhancing autophagy via activating the AMPK pathway. Oxidative Med. Cell. Longev. 2020, 2020, 2524174. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Zhang, C.; Liu, D.; Wu, J.; Tian, H.; Lu, L.; Xu, G.T.; Liu, F.; Zhang, J. Metformin protects ARPE-19 cells from glyoxal-induced oxidative stress. Oxidative Med. Cell. Longev. 2020, 2020, 1740943. [Google Scholar]
- Nita, M.; Grzybowski, A. Interplay between reactive oxygen species and autophagy in the course of age-related macular degeneration. EXCLI J. 2020, 19, 1353–1371. [Google Scholar] [PubMed]
- Rowan, S.; Taylor, A. The role of microbiota in retinal disease. Adv. Exp. Med. Biol. 2018, 1074, 429–435. [Google Scholar]
- Cheng, Q.; Saaddine, J.B.; Klein, R.; Rothenberg, R.; Chou, C.F.; Il’yasova, D. Early age-related macular degeneration with cardiovascular and renal comorbidities: An analysis of the National Health and Nutrition Examination Survey, 2005–2008. Ophthalmic Epidemiol. 2017, 24, 413–419. [Google Scholar] [CrossRef]
- Rastogi, N.; Smith, R.T. Association of age-related macular degeneration and reticular macular disease with cardiovascular disease. Surv. Ophthal-Mol. 2016, 61, 422–433. [Google Scholar] [CrossRef]
- Farkas, M.H.; DeAngelis, M.M. Age-related macular degeneration: From epigenetics to therapeutic implications. Adv. Exp. Med. Biol. 2021, 1256, 221–235. [Google Scholar]
- Mauschitz, M.M.; Finger, R.P. Age-related macular degeneration and cardiovascular diseases: Revisiting the common soil theory. Asia Pac. J. Oph-Thalmol. 2022, 11, 94–99. [Google Scholar] [CrossRef]
- Cheung, C.M.; Wong, T.Y. Is age-related macular degeneration a manifestation of systemic disease? New prospects for early intervention and treatment . J. Intern. Med. 2014, 276, 140–153. [Google Scholar] [PubMed]
- Wang, J.; Xue, Y.; Thapa, S.; Wang, L.; Tang, J.; Ji, K. Relation between age-related macular degeneration and cardiovascular events and mortality: A systematic review and meta-analysis. Biomed Res. Int. 2016, 2016, 8212063. [Google Scholar] [CrossRef] [PubMed]
- Keilhauer, C.N.; Fritsche, L.G.; Guthoff, R.; Haubitz, I.; Weber, B.H. Age-related macular degeneration and coronary heart disease: Evaluation of genetic and environmental associations. Eur. J. Med. Genet. 2013, 56, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Yeung, L.; Sun, C.C.; Huang, C.C.; Chen, K.S.; Lu, Y.H. Age-related macular degeneration in chronic kidney disease: A meta-analysis of observational studies. Am. J. Nephrol. 2018, 48, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Leisy, H.B.; Ahmad, M.; Marmor, M.; Smith, R.T. Association between decreased renal function and reticular macular disease in age-related macular degeneration. Ophthalmol. Retina 2017, 1, 42–48. [Google Scholar] [CrossRef]
- Chen, C.Y.; Dai, C.S.; Lee, C.C.; Shyu, Y.C.; Huang, T.S.; Yeung, L.; Sun, C.C.; Yang, H.Y.; Wu, I.W. Association between macular degeneration and mild to moderate chronic kidney disease: A nationwide population-based study. Medicine 2017, 96, e6405. [Google Scholar] [CrossRef]
- Karesvuo, P.; Gursoy, U.K.; Pussinen, P.J.; Suominen, A.L.; Huumonen, S.; Vesti, E.; Könönen, E. Alveolar bone loss associated with age-related macular degeneration in males. J. Periodontol. 2013, 84, 58–67. [Google Scholar] [CrossRef]
- Pockpa, Z.A.D.; Struillou, X.; Kone, D.; Mobio, G.S.; Soueidan, A.; Badran, Z. Periodontal diseases and age-related macular degeneration: Is there a link? A Review. Perm. J. 2019, 23, 18–260. [Google Scholar] [CrossRef]
- Lindner, M.; Arefnia, B.; Ivastinovic, D.; Sourij, H.; Lindner, E.; Wimmer, G. Association of periodontitis and diabetic macular edema in various stages of diabetic retinopathy. Clin. Oral Investig. 2022, 26, 505–512. [Google Scholar] [CrossRef]
- Di Spirito, F.; La Rocca, M.; De Bernardo, M.; Rosa, N.; Sbordone, C.; Sbordone, L. Possible association of periodontal disease and macular degeneration: A case-control study. Dent. J. 2020, 9, 1. [Google Scholar] [CrossRef]
- Lv, X.; Li, W.; Fang, Z.; Xue, X.; Pan, C. Periodontal disease and age-related macular degeneration: A meta-analysis of 112,240 participants. Biomed Res. Int. 2020, 2020, 4753645. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Sculean, A.; Romanos, G.E. Association between age-related macular degeneration and periodontal and peri-implant diseases: A systematic review. Acta Ophthalmol. 2021, 99, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.C.; Chen, S.J.; Huang, C.C.; Yuan, M.K.; Leu, H.B. Age-related macular degeneration and risk of degenerative dementia among the elderly in Taiwan: A population-based cohort study. Ophthalmology 2015, 122, 2327–2335. [Google Scholar] [CrossRef]
- Wen, L.Y.; Wan, L.; Lai, J.N.; Chen, C.S.; Chen, J.J.; Wu, M.Y.; Hu, K.C.; Chiu, L.T.; Tien, P.T.; Lin, H.J. Increased risk of Alzheimer’s disease among patients with age-related macular degeneration: A nationwide population-based study. PLoS ONE 2021, 16, e0250440. [Google Scholar] [CrossRef]
- Liao, C.; Xu, J.; Chen, Y.; Ip, N.Y. Retinal dysfunction in Alzheimer’s disease and implications for biomarkers. Biomolecules 2021, 11, 1215. [Google Scholar] [CrossRef] [PubMed]
- Asahi, M.G.; Avaylon, J.; Wallsh, J.; Gallemore, R.P. Emerging biological therapies for the treatment of age-related macular degeneration. Expert Opin. Emerg. Drugs 2021, 26, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.V.; Khanna, S.; Parvar, S.P.; Shaw, L.T.; Dao, D.; Hariprasad, S.M.; Skondra, D. Metformin and retinal diseases in preclinical and clinical studies: Insights and review of literature. Exp. Biol. Med. 2022, 247, 317–329. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, M.; Zhu, Y.; Li, X.; Liu, J. Association of metformin treatment with enhanced effect of anti-VEGF agents in diabetic macular edema patients. Acta Diabetol. 2022, 59, 553–559. [Google Scholar] [CrossRef]
- Foster, T.; Ionescu, C.; Walker, D.; Jones, M.; Wagle, S.; Kovacevic, B.; Brown, D.; Mikov, M.; Mooranian, A.; Al-Salami, H. Chemotherapy-induced hearing loss: The applications of bio-nanotechnologies and bile acid-based delivery matrices. Ther. Deliv. 2021, 12, 723–737. [Google Scholar] [CrossRef]
- Chester, J.; Johnston, E.; Walker, D.; Jones, M.; Ionescu, C.M.; Wagle, S.R.; Kovacevic, B.; Brown, D.; Mikov, M.; Mooranian, A.; et al. A review on recent advancement on age-related hearing loss: The applications of nanotechnology, drug pharmacology, and biotechnology. Pharmaceutics 2021, 13, 1041. [Google Scholar] [CrossRef]
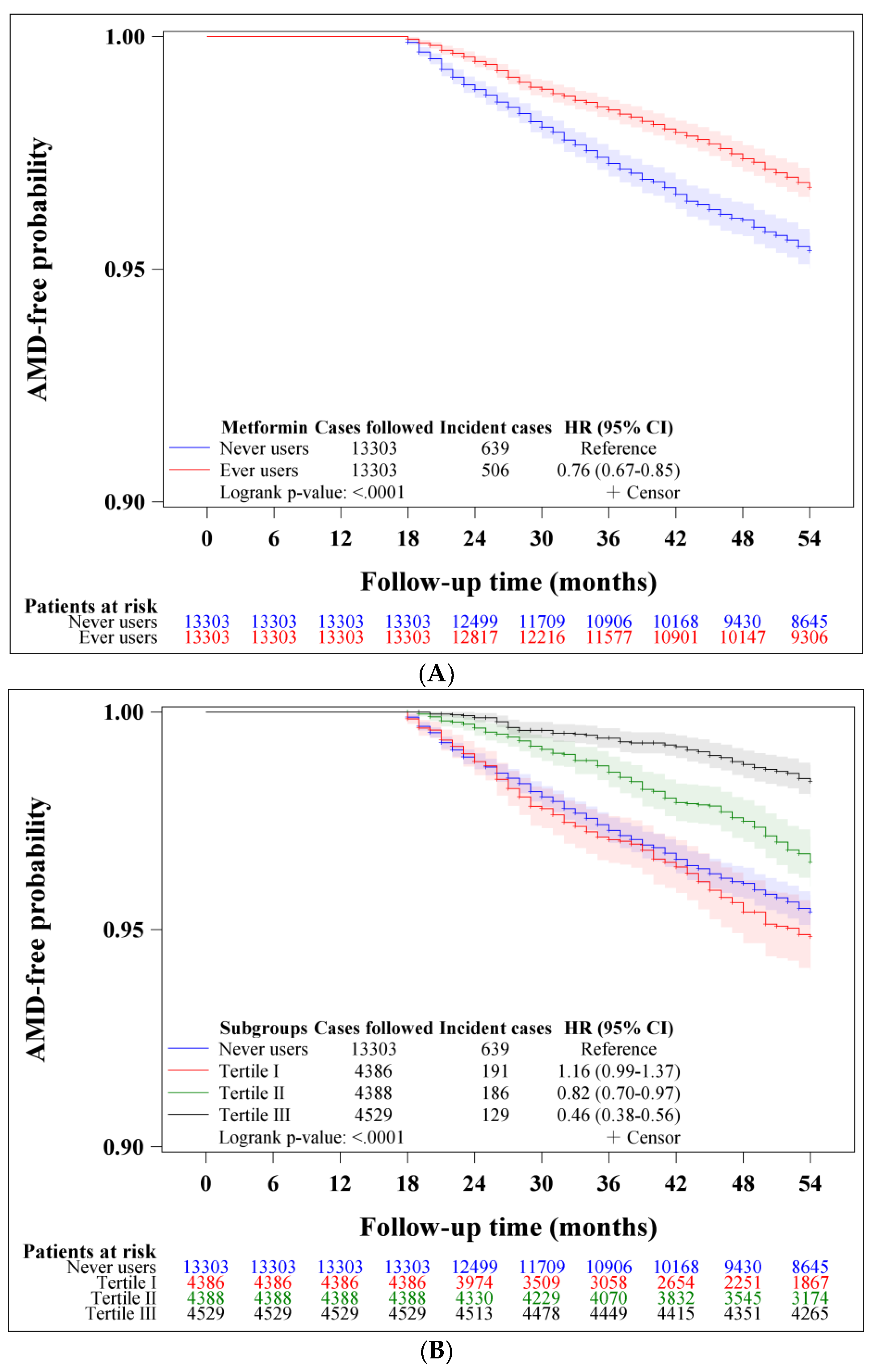
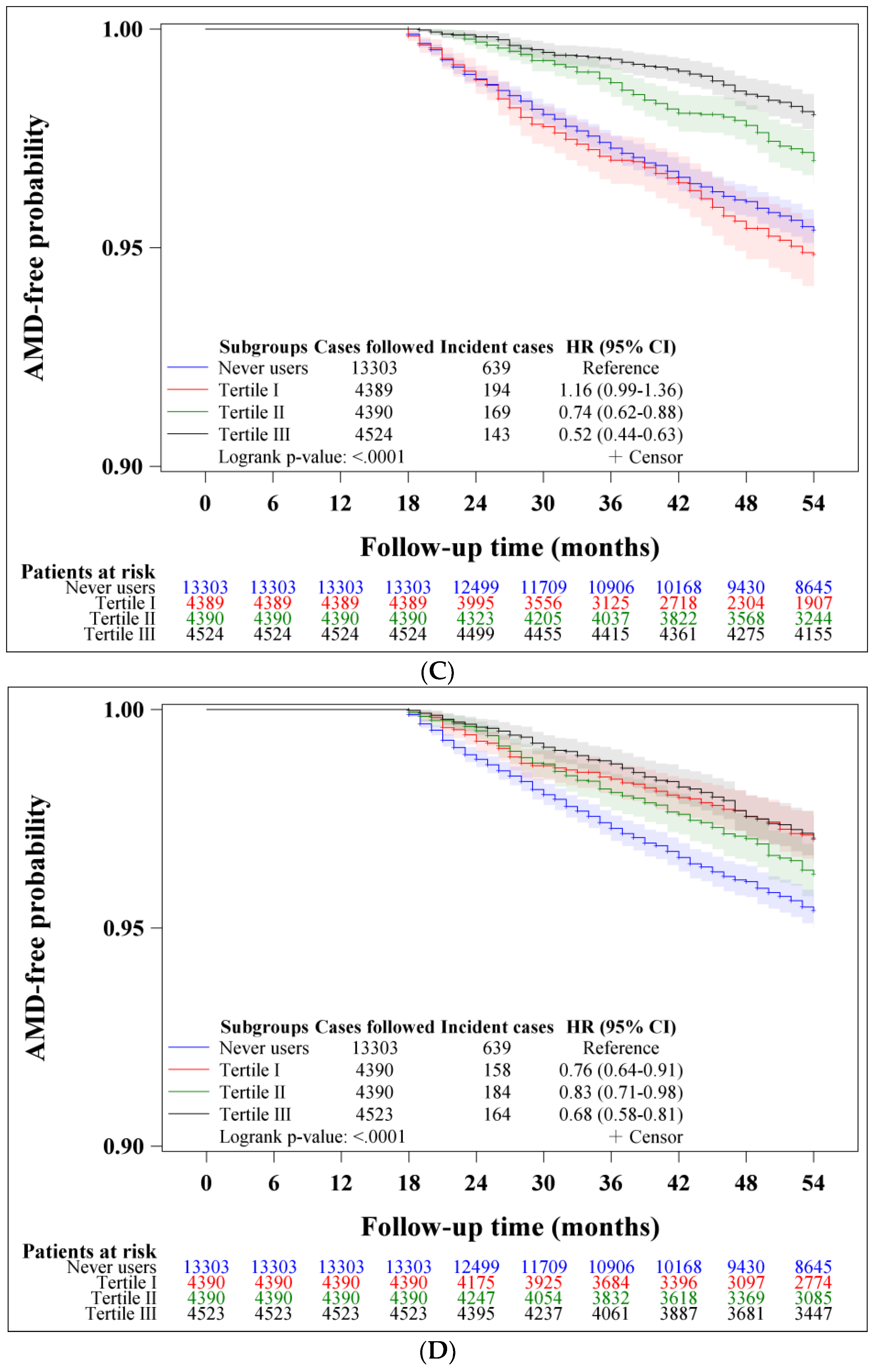
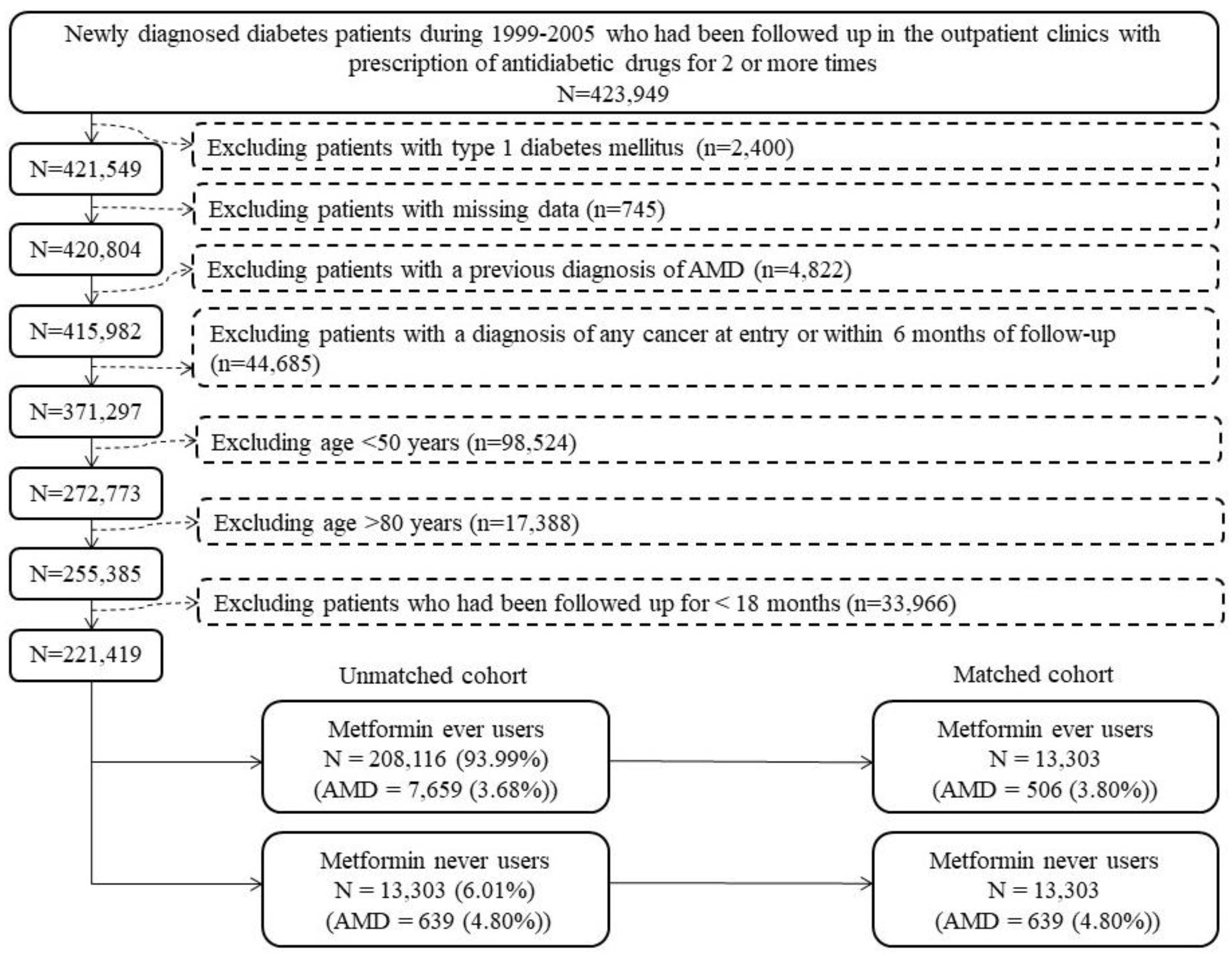
| Metformin Use | Incident Case Number | Cases Followed | Person-Years | Incidence Rate (Per 100,000 Person-Years) | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|---|---|---|---|
| Never users | 639 | 13,303 | 62,855.47 | 1016.62 | 1.000 | ||
| Ever users | 506 | 13,303 | 64,978.49 | 778.72 | 0.756 | (0.673–0.850) | <0.0001 |
| Tertiles of cumulative duration of metformin therapy (months) | |||||||
| Never users | 639 | 13,303 | 62,855.47 | 1016.62 | 1.000 | ||
| <31.8 | 191 | 4386 | 17,487.30 | 1092.22 | 1.131 | (0.961–1.330) | 0.1381 |
| 31.8–63.9 | 186 | 4388 | 21,823.66 | 852.29 | 0.821 | (0.697–0.967) | 0.0181 |
| >63.9 | 129 | 4529 | 25,667.53 | 502.58 | 0.464 | (0.384–0.561) | <0.0001 |
| Tertiles of cumulative dose of metformin therapy (grams) | |||||||
| Never users | 639 | 13,303 | 62,855.47 | 1016.62 | 1.000 | ||
| <947.1 | 194 | 4389 | 17,679.78 | 1097.30 | 1.131 | (0.962–1.329) | 0.1352 |
| 947.1–2193.5 | 169 | 4390 | 21,997.90 | 768.26 | 0.739 | (0.624–0.876) | 0.0005 |
| >2193.5 | 143 | 4524 | 25,300.82 | 565.20 | 0.525 | (0.438–0.629) | <0.0001 |
| Tertiles of defined daily dose of metformin therapy per day | |||||||
| Never users | 639 | 13,303 | 62,855.47 | 1016.62 | 1.000 | ||
| <0.49 | 158 | 4390 | 20,483.93 | 771.34 | 0.761 | (0.640–0.906) | 0.0021 |
| 0.49–0.64 | 184 | 4390 | 21,484.15 | 856.45 | 0.832 | (0.706–0.980) | 0.0274 |
| >0.64 | 164 | 4523 | 23,010.41 | 712.72 | 0.684 | (0.576–0.812) | <0.0001 |
| Model/Metformin Use | Incident Case Number | Cases Followed | Person-Years | Incidence Rate (Per 100,000 Person-Years) | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|---|---|---|---|
| 1. Analysis restricted to patients enrolled from 1999 to 2002 | |||||||
| Never users | 279 | 5979 | 27,844.59 | 1001.99 | 1.000 | ||
| Ever users | 331 | 7984 | 39,501.77 | 837.94 | 0.815 | (0.695–0.956) | 0.0118 |
| 2. Analysis restricted to patients enrolled from 2003 to 2005 | |||||||
| Never users | 360 | 7324 | 35,010.89 | 1028.25 | 1.000 | ||
| Ever users | 175 | 5319 | 25,476.73 | 686.90 | 0.668 | (0.558–0.801) | <0.0001 |
| 3. Including only patients aged 50–64 years | |||||||
| Never users | 246 | 6534 | 31,625.48 | 777.85 | 1.000 | ||
| Ever users | 205 | 6512 | 32,740.69 | 626.13 | 0.794 | (0.660–0.956) | 0.0148 |
| 4. Including patients aged 65–79 years | |||||||
| Never users | 393 | 6769 | 31,229.99 | 1258.41 | 1.000 | ||
| Ever users | 301 | 6791 | 32,237.80 | 933.69 | 0.733 | (0.631–0.851) | <0.0001 |
| 5. Including only male patients | |||||||
| Never users | 330 | 7109 | 33,515.89 | 984.61 | 1.000 | ||
| Ever users | 274 | 7212 | 35,057.37 | 781.58 | 0.784 | (0.668–0.920) | 0.0029 |
| 6. Including only female patients | |||||||
| Never users | 309 | 6194 | 29,339.59 | 1053.18 | 1.000 | ||
| Ever users | 232 | 6091 | 29,921.12 | 775.37 | 0.727 | (0.613–0.862) | 0.0002 |
| 7. Excluding patients with a diagnosis of anemia and/or nutritional deficiencies | |||||||
| Never users | 472 | 9591 | 46,129.75 | 1023.20 | 1.000 | ||
| Ever users | 376 | 10,088 | 49,569.05 | 758.54 | 0.736 | (0.643–0.843) | <0.0001 |
| 8. Patients with diabetic retinopathy | |||||||
| Never users | 56 | 810 | 3862.97 | 1449.66 | 1.000 | ||
| Ever users | 43 | 773 | 3768.50 | 1141.04 | 0.780 | (0.524–1.160) | 0.2199 |
| 9. Patients without diabetic retinopathy | |||||||
| Never users | 583 | 12,493 | 58,992.50 | 988.26 | 1.000 | ||
| Ever users | 463 | 12,530 | 61,209.99 | 756.41 | 0.755 | (0.668–0.853) | <0.0001 |
| 10. Outcome defined as nonexudative age-related macular degeneration | |||||||
| Never users | 495 | 13,303 | 63,198.84 | 783.24 | 1.000 | ||
| Ever users | 361 | 13,303 | 65,244.76 | 553.30 | 0.697 | (0.609–0.799) | <0.0001 |
| 11. Outcome defined as exudative age-related macular degeneration | |||||||
| Never users | 23 | 13,303 | 64,237.81 | 35.80 | 1.000 | ||
| Ever users | 22 | 13,303 | 65,868.88 | 33.40 | 0.924 | (0.515–1.658) | 0.7919 |
| 12. All covariates defined at censor | |||||||
| Never users | 639 | 13,303 | 62,855.47 | 1016.62 | 1.000 | ||
| Ever users | 506 | 13,303 | 64,978.49 | 778.72 | 0.756 | (0.673–0.850) | <0.0001 |
| Variables | Never Users | Ever Users | Standardized Difference | ||
|---|---|---|---|---|---|
| (n = 13,303) | (n = 13,303) | ||||
| n | % | n | % | ||
| Basic data | |||||
| Age (years) * | 65.05 | 8.48 | 65.04 | 8.22 | 0.29 |
| Sex (male) | 7109 | 53.44 | 7212 | 54.21 | 1.47 |
| Occupation | |||||
| I | 4786 | 35.98 | 4749 | 35.70 | |
| II | 2321 | 17.45 | 2369 | 17.81 | 0.90 |
| III | 3345 | 25.14 | 3360 | 25.26 | 0.26 |
| IV | 2851 | 21.43 | 2825 | 21.24 | −0.36 |
| Living region | |||||
| Taipei | 4450 | 33.45 | 4501 | 33.83 | |
| Northern | 1407 | 10.58 | 1370 | 10.30 | −1.07 |
| Central | 2285 | 17.18 | 2372 | 17.83 | 1.66 |
| Southern | 2350 | 17.67 | 2294 | 17.24 | −1.11 |
| Kao-Ping and Eastern | 2811 | 21.13 | 2766 | 20.79 | −0.65 |
| Major comorbidities commonly seen in diabetes patients | |||||
| Obesity | 235 | 1.77 | 244 | 1.83 | 0.51 |
| Hypertension | 10,694 | 80.39 | 10,739 | 80.73 | 1.02 |
| Dyslipidemia | 8168 | 61.40 | 8069 | 60.66 | −1.33 |
| Complications related to diabetes | |||||
| Diabetic polyneuropathy | 1373 | 10.32 | 1367 | 10.28 | −0.33 |
| Eye diseases | 1091 | 8.20 | 995 | 7.48 | −2.96 |
| Nephropathy | 3521 | 26.47 | 3556 | 26.73 | 0.16 |
| Ischemic heart disease | 5923 | 44.52 | 5963 | 44.82 | 0.59 |
| Stroke | 3981 | 29.93 | 4014 | 30.17 | 0.58 |
| Peripheral arterial disease | 2267 | 17.04 | 2289 | 17.21 | 0.31 |
| Hypoglycemia | 216 | 1.62 | 188 | 1.41 | −1.89 |
| Antidiabetic drugs | |||||
| Sulfonylurea | 10,000 | 75.17 | 10,328 | 77.64 | 6.11 |
| Acarbose | 1461 | 10.98 | 1393 | 10.47 | −2.93 |
| Meglitinide | 1106 | 8.31 | 1045 | 7.86 | −1.69 |
| Rosiglitazone | 418 | 3.14 | 438 | 3.29 | 0.50 |
| Pioglitazone | 329 | 2.47 | 341 | 2.56 | 0.25 |
| Insulin | 776 | 5.83 | 659 | 4.95 | −5.23 |
| Drugs commonly used by diabetes patients or drugs that may affect the outcome | |||||
| Statins | 5494 | 41.30 | 5526 | 41.54 | 0.64 |
| Fibrates | 3665 | 27.55 | 3643 | 27.38 | −0.20 |
| Calcium channel blockers | 8315 | 62.50 | 8386 | 63.04 | 1.23 |
| Angiotensin converting enzyme inhibitors/angiotensin receptor blockers | 8723 | 65.57 | 8657 | 65.08 | −0.95 |
| Aspirin | 7070 | 53.15 | 7074 | 53.18 | 0.23 |
| Non-steroidal anti-inflammatory drugs ** | 5166 | 38.83 | 5163 | 38.81 | 0.00 |
| Selective serotonin re-uptake inhibitors | 1020 | 7.67 | 916 | 6.89 | −3.03 |
| Opioid analgesics | 2100 | 15.79 | 2101 | 15.79 | −0.12 |
| Immunosuppressants ** | 687 | 5.16 | 656 | 4.93 | −1.22 |
| Common comorbidities that may affect the exposure/outcome | |||||
| Chronic obstructive pulmonary disease | 5923 | 44.52 | 5842 | 43.91 | −1.09 |
| Tobacco abuse | 176 | 1.32 | 164 | 1.23 | −0.77 |
| Alcohol-related diagnoses | 578 | 4.34 | 534 | 4.01 | −2.03 |
| Head injury | 140 | 1.05 | 162 | 1.22 | 1.32 |
| Dementia | 871 | 6.55 | 848 | 6.37 | −0.67 |
| Parkinson’s disease | 383 | 2.88 | 407 | 3.06 | 0.96 |
| Heart failure | 2390 | 17.97 | 2323 | 17.46 | −1.50 |
| Valvular heart disease | 1372 | 10.31 | 1308 | 9.83 | −1.65 |
| Gingival and periodontal diseases | 9972 | 74.96 | 10,028 | 75.38 | 1.02 |
| Pneumonia | 1446 | 10.87 | 1415 | 10.64 | −1.02 |
| Osteoporosis | 2915 | 21.91 | 2936 | 22.07 | 0.48 |
| Arthropathies and related disorders | 9591 | 72.10 | 9656 | 72.59 | 1.30 |
| Psoriasis and similar disorders | 274 | 2.06 | 307 | 2.31 | 1.72 |
| Dorsopathies | 9344 | 70.24 | 9348 | 70.27 | 0.12 |
| Liver cirrhosis | 600 | 4.51 | 559 | 4.20 | −1.86 |
| Hepatitis B virus infection | 180 | 1.35 | 126 | 0.95 | −4.24 |
| Hepatitis C virus infection | 567 | 4.26 | 541 | 4.07 | −1.16 |
| Other chronic non-alcoholic liver diseases | 971 | 7.30 | 965 | 7.25 | 0.06 |
| Organ transplantation | 65 | 0.49 | 46 | 0.35 | −2.63 |
| Human immunodeficiency virus infection | 6 | 0.05 | 4 | 0.03 | −0.76 |
| Helicobacter pylori infection | 70 | 0.53 | 76 | 0.57 | 0.73 |
| Peptic ulcer site unspecified | 5067 | 38.09 | 4997 | 37.56 | −1.04 |
| Appendicitis | 198 | 1.49 | 204 | 1.53 | 0.37 |
| Irritable bowel syndrome | 1728 | 12.99 | 1637 | 12.31 | −2.12 |
| Noninfective enteritis and colitis | 5990 | 45.03 | 5919 | 44.49 | −1.02 |
| Abscess of anal/rectal regions | 143 | 1.07 | 142 | 1.07 | −0.24 |
| Anal fissure/fistula | 265 | 1.99 | 262 | 1.97 | −0.09 |
| Episodic mood disorders | 642 | 4.83 | 565 | 4.25 | −2.79 |
| Depressive disorder | 362 | 2.72 | 383 | 2.88 | 0.97 |
| Suicidal attempt | 4 | 0.03 | 3 | 0.02 | −0.43 |
| Insomnia | 3126 | 23.50 | 3099 | 23.30 | −0.45 |
| Drug dependence | 56 | 0.42 | 55 | 0.41 | 0.00 |
| Diseases of the ear and mastoid process | 5842 | 43.91 | 5901 | 44.36 | 1.00 |
| Hearing loss | 855 | 6.43 | 903 | 6.79 | 1.64 |
| Inflammatory diseases of the central nervous system (encephalitis and meningitis) | 149 | 1.12 | 156 | 1.17 | 0.42 |
| Tuberculosis | 443 | 3.33 | 436 | 3.28 | −0.38 |
| Malaria | 4 | 0.03 | 1 | 0.01 | −2.36 |
| Some parasitic diseases | 900 | 6.77 | 834 | 6.27 | −1.98 |
| Epilepsy and recurrent seizures | 316 | 2.38 | 270 | 2.03 | −2.55 |
| Disorders of fluid electrolyte and acid-base balance | 1218 | 9.16 | 1150 | 8.64 | −2.15 |
| Cancer during follow-up | 1485 | 11.16 | 1463 | 11.00 | −0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, C.-H. The Risk of Age-Related Macular Degeneration Is Reduced in Type 2 Diabetes Patients Who Use Metformin. Pharmaceuticals 2023, 16, 224. https://doi.org/10.3390/ph16020224
Tseng C-H. The Risk of Age-Related Macular Degeneration Is Reduced in Type 2 Diabetes Patients Who Use Metformin. Pharmaceuticals. 2023; 16(2):224. https://doi.org/10.3390/ph16020224
Chicago/Turabian StyleTseng, Chin-Hsiao. 2023. "The Risk of Age-Related Macular Degeneration Is Reduced in Type 2 Diabetes Patients Who Use Metformin" Pharmaceuticals 16, no. 2: 224. https://doi.org/10.3390/ph16020224
APA StyleTseng, C.-H. (2023). The Risk of Age-Related Macular Degeneration Is Reduced in Type 2 Diabetes Patients Who Use Metformin. Pharmaceuticals, 16(2), 224. https://doi.org/10.3390/ph16020224





