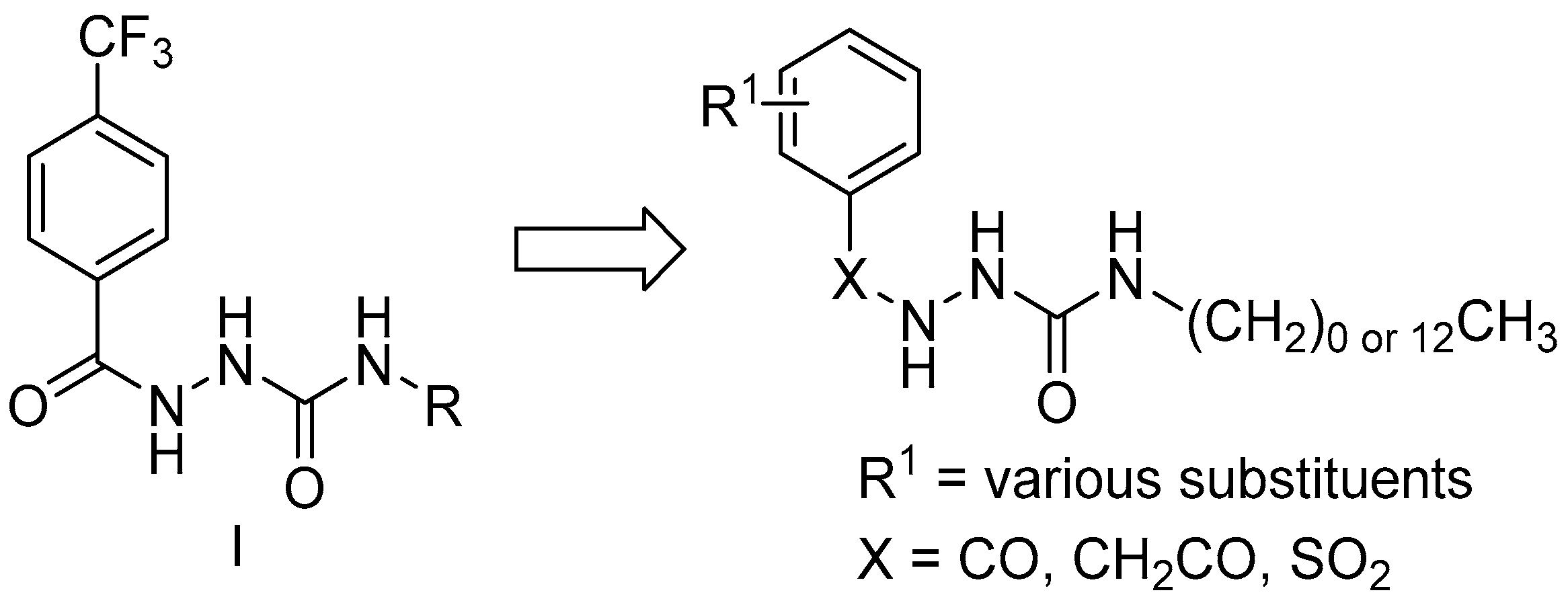
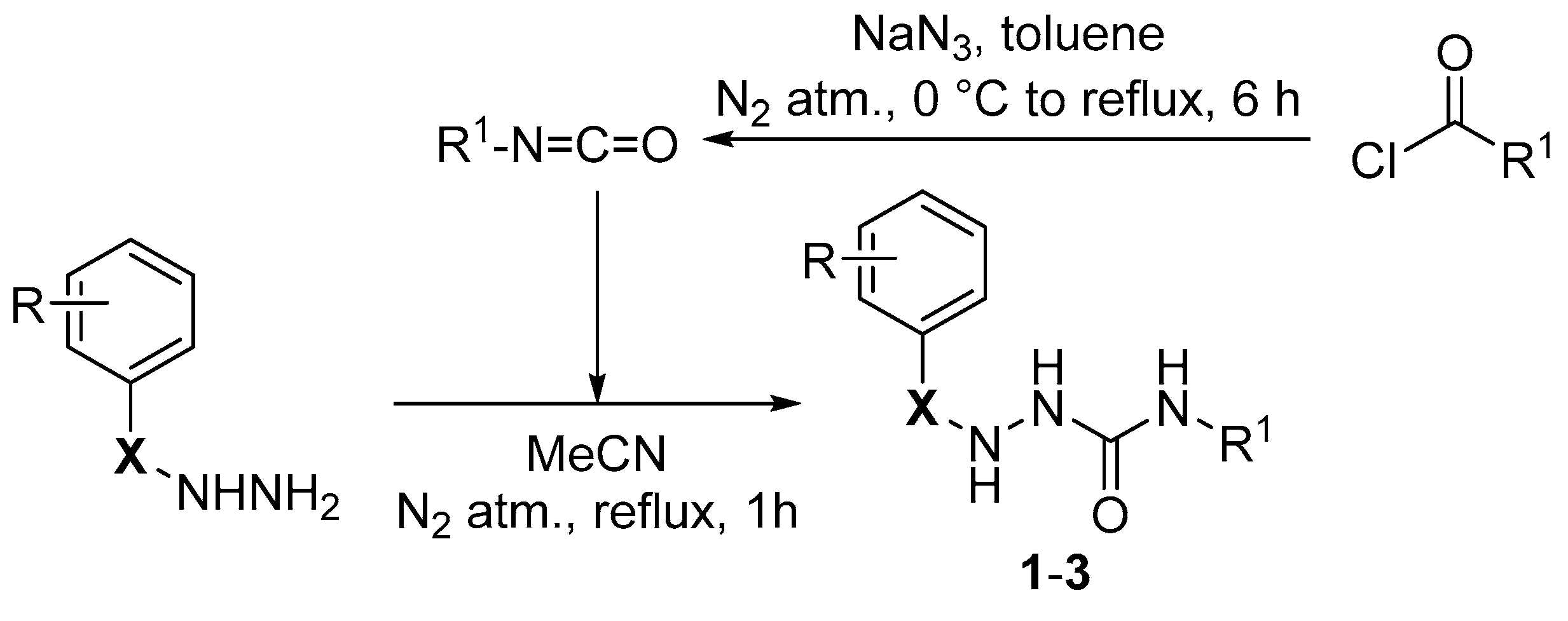
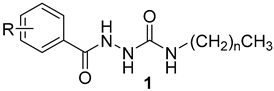
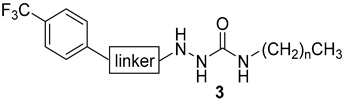
Synthesis of Hydrazine-1-carboxamides
Method A
Substituted benzohydrazide (1.0 mmol) was dissolved in acetonitrile (5 mL) and mixed with N,N-diisopropylethylamine (DIPEA; 2.0 mmol, 348 µL) followed by N-succinimidyl N-methylcarbamate (1.5 mmol; 258.2 mg). The reaction mixture was stirred at room temperature for 24 h, the formed precipitate was filtered off, it was washed with cold water and diethyl ether, and it was crystallized from ethyl acetate.
Method B
Triphosgene (0.4 mol, 118.7 mg) was dissolved in anhydrous dichloromethane (DCM; 5 mL) under a nitrogen atmosphere. Then, tridecylamine (1.01 mmol; 201.4 mg) dissolved in anhydrous DCM (5 mL) was added dropwise. After 30 min of stirring at room temperature, triethylamine (2.1 mmol, 293 µL) was added. After an additional 30 min, substituted benzohydrazide (1.0 mmol) was added. The reaction mixture was stirred for 10 h at room temperature and was then evaporated to dryness, treated with water (10 mL) and extracted with ethyl acetate (3 × 15 mL). The combined organic phase was dried over anhydrous sodium sulfate, filtered off and evaporated to dryness to give the final product, which was crystallized from ethyl acetate.
Method C
Both alkyl isocyanates were prepared via the Curtius rearrangement from commercially available acyl chlorides according to the known method [
10]. The crude isocyanate was used immediately for a subsequent reaction.
Then, 1 mmol of the appropriate benzohydrazide (benzenesulfonyl hydrazide) was dissolved/suspended in 10 mL of anhydrous acetonitrile and was heated to a boil under a nitrogen atmosphere, and then a 1.1 equivalent (1.1 mmol, as a solution in toluene) of alkyl isocyanate was added in one portion to the vigorously stirred reaction mixture. The reaction mixture was heated under reflux for one hour. The solvent was evaporated under reduced pressure. The crude product was purified via column chromatography on silica gel using gradient elution (pure DCM → mixture of DCM to CH3OH; 93:7 V/V). The reaction progress was monitored via TLC (DCM + CH3OH; 93:7 V/V).
N-Methyl-2-[4-(trifluoromethoxy)benzoyl]hydrazine-1-carboxamide 1a. White solid. Yield 91% (method C), mp 237–239 °C. 1H NMR (600 MHz, DMSO-D6) δ 10.18 (1H, s, NH), 8.00–7.96 (2H, m, H2, H6), 7.88 (1H, s, NH), 7.46–7.43 (2H, m, H3, H5), 6.42 (1H, q, J = 4.5 Hz, NH-CH3), 2.54 (3H, d, J = 4.6 Hz, NH-CH3). 13C NMR (151 MHz, DMSO-D6) δ 165.75, 159.36, 151.08, 132.46, 130.50, 121.14, 120.91 (q, J = 255.0 Hz), 26.75. IR (ATR): 636, 760, 851, 912, 930, 988, 1016, 1064, 1165, 1211, 1252, 1344, 1424, 1502, 1544, 1584, 1609, 1644, 2890, 3074, 3274 cm−1. Elemental analysis for C10H10F3N3O3 (277.20); calculated: C, 43.33; H, 3.64; N, 15.16, found: C, 43.44; H, 3.77; N, 15.01. Rf: 0.46.
N-Tridecyl-2-[4-(trifluoromethoxy)benzoyl]hydrazine-1-carboxamide 1b. White needle-like solid. Yield 90% (method C), mp 200–201 °C. 1H NMR (600 MHz, DMSO-D6) δ 9.98 (1H, s, NH), 7.97 (2H, d, J = 8.3 Hz, H2, H6), 7.63 (1H, s, NH), 7.39 (2H, d, J = 8.3 Hz, H3, H5), 6.24 (1H, t, J = 5.9 Hz, NH-CH2), 3.01 (2H, q, J = 6.6 Hz, NH-CH2), 1.38 (2H, p, J = 6.9 Hz, C2H2), 1.24–1.21 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.83 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 165.68, 158.66, 151.17, 132.65, 130.38, 121.43, 120.91 (q, J = 255.0 Hz), 31.75, 30.32, 29.50, 29.49, 29.48, 29.47, 29.46, 29.44, 29.29, 29.12, 26.84, 22.49, 14.25. IR (ATR): 627, 722, 862, 899, 922, 1015, 1059, 1168, 1204, 1266, 1339, 1470, 1490, 1549, 1591, 1640, 1668, 2853, 2921, 3278 cm−1. Elemental analysis for C22H34F3N3O3 (445.53); calculated: C, 59.31; H, 7.69; N, 9.43, found: C, 59.45; H, 7.61; N, 9.50. Rf: 0.46.
2-(4-Fluorobenzoyl)-N-methylhydrazine-1-carboxamide 1c. White solid. Yield 33% (method A), mp 221–223 °C. 1H NMR (500 MHz, DMSO-D6) δ 10.13 (1H, s, NH), 7.99–7.93 (2H, m, H2, H6), 7.86 (1H, s, NH), 7.34–7.27 (2H, m, H3, H5), 6.43 (1H, q, J = 4.9 Hz, NH-CH3), 2.57 (3H, d, J = 4.6 Hz, NH-CH3). 13C NMR (126 MHz, DMSO-D6) δ 165.56, 164.29 (d, J = 249.0 Hz), 159.08, 130.46 (d, J = 9.1 Hz), 129.46 (d, J = 2.9 Hz), 115.42 (d, J = 21.8 Hz), 26.42. IR (ATR): 3309, 3070, 1665, 1644, 1603, 1581, 1542, 1494, 1341, 1299, 1254, 1230, 1163, 1100, 1010, 912, 848, 927, 813, 758, 650, 629 cm−1. Elemental analysis for C9H10FN3O2 (211.20); calculated: C, 51.18; H, 4.77; N, 19.90, found: C, 51.24; H, 4.70; N, 20.01.
2-(4-Fluorobenzoyl)-N-tridecylhydrazine-1-carboxamide 1d. White solid. Yield 25% (method B), mp 196–198 °C. 1H NMR (600 MHz, DMSO-D6) δ 9.95 (1H, d, J = 2.0 Hz, NH), 7.94–7.90 (2H, m, H2, H6), 7.62 (1H, s, NH), 7.26–7.21 (2H, m, H3, H5), 6.26 (1H, t, J = 5.9 Hz, NH-CH2), 3.00 (2H, q, J = 6.6 Hz, NH-CH2), 1.38 (2H, p, J = 7.0 Hz, C2H2), 1.29–1.21 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.83 (3H, t, J = 7.0 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 165.85, 164.67 (d, J = 249.1 Hz), 158.76, 130.72 (d, J = 9.1 Hz), 130.01, 115.66 (d, J = 21.9 Hz), 39.87, 31.77, 30.35, 29.52, 29.50, 29.48, 29.31, 29.15, 26.84, 22.52, 14.31. IR (ATR): 3303, 3073, 2922, 2850, 1660, 1638, 1605, 1568, 1501, 1465, 1339, 1300, 1279, 1258, 1232, 1164, 1068, 914, 849, 771, 755, 725, 642, 627 cm−1. Elemental analysis for C21H34FN3O2 (379.52); calculated: C, 66.46; H, 9.03; N, 11.07, found: C, 66.41; H, 9.10; N, 11.05.
2-(4-Chlorobenzoyl)-N-methylhydrazine-1-carboxamide 1e. White solid. Yield 59% (method A), mp 227–229 °C. 1H NMR (500 MHz, DMSO-D6) δ 10.18 (1H, s, NH), 7.92–7.88 (3H, m, NH, H2, H6), 7.57–7.54 (2H, m, H3, H5), 6.44 (1H, q, J = 4.7 Hz, NH-CH3), 2.57 (3H, d, J = 4.6 Hz, NH-CH3). 13C NMR (126 MHz, DMSO-D6) δ 165.61, 159.02, 136.64, 131.74, 129.70, 128.56, 26.42. IR (ATR): 3318, 3253, 3063, 1663, 1642, 1597, 1574, 1543, 1487, 1424, 1340, 1252, 1095, 1010, 910, 895, 845, 759, 727, 700, 663, 651, 625, 608 cm−1. Elemental analysis for C9H10ClN3O2 (227.65); calculated: C, 47.49; H, 4.43; N, 18.46, found: C, 47.54; H, 4.40; N, 18.51.
2-(4-Chlorobenzoyl)-N-tridecylhydrazine-1-carboxamide 1f. White solid. Yield 26% (method B), mp 195–197 °C. 1H NMR (500 MHz, DMSO-D6) δ 10.16 (1H, s, NH), 7.91–7.87 (2H, m, H2, H6), 7.80 (1H, s, NH), 7.56–7.53 (2H, m, H3, H5), 6.49 (1H, t, J = 6.0 Hz, NH-CH2), 2.99 (2H, q, J = 6.5 Hz, NH-CH2), 1.36 (2H, p, J = 6.9 Hz, C2H2), 1.23–1.15 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.84 (3H, t, J = 6.8 Hz, CH3). 13C NMR (126 MHz, DMSO-D6) δ 165.54, 158.45, 136.64, 131.75, 129.67, 128.57, 39.41, 31.49, 30.04, 29.26, 29.21, 29.12, 29.03, 28.91, 27.13, 26.50, 22.29, 14.14. IR (ATR): 3309, 2952, 2919, 2850, 1663, 1637, 1599, 1580, 1540, 1486, 1469, 1338, 1279, 1261, 1092, 1012, 907, 844, 751, 720, 667, 636, 622, 606 cm−1. Elemental analysis for C21H34ClN3O2 (395.97); calculated: C, 63.70; H, 8.66; N, 10.61, found: C, 63.74; H, 8.50; N, 10.51.
2-(4-Bromobenzoyl)-N-methylhydrazine-1-carboxamide 1g. White solid. Yield 40% (method A), mp 225–227 °C. 1H NMR (500 MHz, DMSO-D6) δ 10.18 (1H, s, NH), 7.89 (1H, s, NH), 7.84–7.81 (2H, m, H2, H6), 7.71–7.68 (2H, m, H3, H5), 6.44 (1H, q, J = 5.0 Hz, NH-CH3), 2.57 (3H, d, J = 4.5 Hz, NH-CH3). 13C NMR (126 MHz, DMSO-D6) δ 165.74, 159.00, 132.11, 131.50, 129.88, 125.58, 26.43. IR (ATR): 3287, 1683, 1665, 1637, 1589, 1558, 1536, 1482, 1419, 1342, 1315, 1277, 1254, 1176, 1108, 1072, 1008, 898, 847, 824, 759, 715 cm−1. Elemental analysis for C9H10BrN3O2 (272.10); calculated: C, 39.73; H, 3.70; N, 15.44, found: C, 39.74; H, 3.65; N, 15.51.
2-(4-Bromobenzoyl)-N-tridecylhydrazine-1-carboxamide 1h. White solid. Yield 34% (method B), mp 144–146 °C. 1H NMR (500 MHz, DMSO-D6) δ 10.16 (1H, s, NH), 7.86–7.81 (3H, m, NH, H2, H6), 7.72–7.68 (2H, m, H3, H5), 6.49 (1H, t, J = 5.0 Hz, NH-CH2), 3.06 (2H, q, J = 6.5 Hz, NH-CH2), 1.36 (2H, p, J = 6.8 Hz, C2H2), 1.24–1.19 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.84 (3H, t, J = 6.8 Hz, CH3). 13C NMR (126 MHz, DMSO-D6) δ 165.59, 158.38, 132.48, 131.47, 129.82, 127.34, 38.92, 31.46, 29.23, 29.19, 29.10, 29.00, 28.88, 28.69, 27.12, 26.48, 25.98, 22.26, 14.11. IR (ATR): 2954, 2919, 2849, 1663, 1637, 1595, 1521, 1496, 1468, 1398, 1377, 1340, 1263, 1147, 1121, 1074, 1010, 981, 943, 897, 841, 754, 721, 646, 632, 625, 612 cm−1. Elemental analysis for C21H34BrN3O2 (440.43); calculated: C, 57.27; H, 7.78; N, 9.54, found: C, 57.24; H, 7.75; N, 9.59.
2-(4-Iodobenzoyl)-N-methylhydrazine-1-carboxamide 1i. White solid. Yield 24% (method A), mp 238–240 °C. 1H NMR (500 MHz, DMSO-D6) δ 10.16 (1H, s, NH), 7.89–7.83 (3H, m, NH, H2, H6), 7.70–7.64 (2H, m, H3, H5), 6.43 (1H, s, NH-CH3), 2.57 (3H, s, NH-CH3). 13C NMR (126 MHz, DMSO-D6) δ 165.99, 158.99, 137.35, 132.41, 129.69, 99.51, 26.43. IR (ATR): 3286, 1664, 1638, 1586, 1558, 1538, 1480, 1418, 1343, 1310, 1276, 1253, 1173, 1107, 1064, 1005, 898, 845, 825, 754, 706, 651, 633 cm−1. Elemental analysis for C9H10IN3O2 (319.10); calculated: C, 33.88; H, 3.16; N, 13.17, found: C, 33.79; H, 3.15; N, 13.10.
2-(4-Iodobenzoyl)-N-tridecylhydrazine-1-carboxamide 1j. White solid. Yield 24% (method B), mp 190–193 °C. 1H NMR (600 MHz, DMSO-D6) δ 10.12 (1H, s, NH), 7.90–7.83 (3H, m, NH, H2, H6), 7.52 (2H, d, J = 8.3 Hz, H3, H5), 6.47 (1H, t, J = 5.0 Hz, NH-CH2), 2.90 (2H, q, J = 6.6 Hz, NH-CH2), 1.31 (2H, p, J = 6.8 Hz, C2H2), 1.25–1.19 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.81 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 157.18, 154.85, 138.64, 127.46, 124.00, 99.23, 40.76, 31.83, 29.95, 29.59, 29.57, 29.55, 29.52, 29.27, 29.25, 29.07, 26.76, 22.63, 14.46. IR (ATR): 3340, 2956, 2919, 2850, 1691, 1613, 1583, 1536, 1479, 1463, 1398, 1351, 1332, 1284, 1263, 1247, 1198, 1144, 1076, 1033, 1006, 967, 938, 823, 720, 668, 639, 627, 612 cm−1. Elemental analysis for C21H34IN3O2 (487.43); calculated: C, 51.75; H, 7.03; N, 8.62, found: C, 51.84; H, 7.05; N, 8.59.
N-Methyl-2-(4-nitrobenzoyl)hydrazine-1-carboxamide 1k. Yellowish solid. Yield 39% (method A), mp 252–254 °C. 1H NMR (500 MHz, DMSO-D6) δ 10.44 (1H, s, NH), 8.33 (2H, d, J = 8.7 Hz, H3, H5), 8.15–8.00 (3H, m, NH, H2, H6), 6.52 (1H, s, NH-CH3), 2.59 (3H, s, NH-CH3). 13C NMR (126 MHz, DMSO-D6) δ 165.11, 158.88, 149.47, 138.73, 129.33, 123.67, 26.45. IR (ATR): 3283, 3070, 1670, 1635, 1604, 1582, 1547, 1519, 1490, 1419, 1341, 1318, 1258, 1190, 1171, 1107, 1014, 989, 904, 875, 850, 716, 680, 644, 609 cm−1. Elemental analysis for C9H10N4O4 (238.20); calculated: C, 45.38; H, 4.23; N, 23.52, found: C, 45.49; H, 4.15; N, 23.60.
2-(4-Nitrobenzoyl)-N-tridecylhydrazine-1-carboxamide 1l. Yellowish solid. Yield 30% (method B), mp 252–252 °C. 1H NMR (500 MHz, DMSO-D6) δ 10.45 (1H, s, NH), 8.31 (2H, d, J = 8.8 Hz, H3, H5), 8.16–8.05 (3H, m, NH, H2, H6), 6.48 (1H, t, J = 5.0 Hz, NH-CH2), 2.71 (2H, q, J = 6.6 Hz, NH-CH2), 1.53 (2H, p, J = 6.8 Hz, C2H2), 1.24–1.19 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.83 (3H, t, J = 7.0 Hz, CH3). 13C NMR (126 MHz, DMSO-D6) δ 164.33, 159.12, 150.04, 136.25, 129.64, 124.02, 38.90, 31.52, 29.29, 29.26, 29.24, 29.16, 29.07, 28.94, 28.77, 27.14, 26.08, 22.32, 14.18. IR (ATR): 3268, 2954, 2929, 2850, 1673, 1605, 1589, 1530, 1481, 1344, 1326, 1286, 1191, 1173, 1147, 1118, 1039, 1009, 882, 845, 717, 648, 624, 606 cm−1. Elemental analysis for C21H34N4O4 (406.53); calculated: C, 62.05; H, 8.43; N, 13.78, found: C, 62.04; H, 8.35; N, 15.69.
2-(4-Cyanobenzoyl)-N-methylhydrazine-1-carboxamide 1m. White solid. Yield 94% (method C), mp 209–210 °C (decomp.). 1H NMR (600 MHz, DMSO-D6) δ 10.31 (1H, s, NH), 8.01–7.98 (2H, m, H2, H6), 7.95–7.92 (3H, m, NH, H3, H5), 6.45 (1H, q, J = 4.4 Hz, NH-CH3), 2.54 (3H, d, J = 4.5 Hz, NH-CH3). 13C NMR (151 MHz, DMSO-D6) δ 165.62, 159.22, 137.38, 132.93, 128.97, 118.83, 114.52, 26.77. IR (ATR): 612, 760, 774, 859, 905, 991, 1018, 1172, 1259, 1312, 1338, 1424, 1496, 1541, 1586, 1641, 1669, 2230, 3297 cm−1. Elemental analysis for C10H10N4O2 (218.22); calculated: C, 55.04; H, 4.62; N, 25.68, found: C, 55.18; H, 4.74; N, 25.59. Rf: 0.27.
2-(4-Cyanobenzoyl)-N-tridecylhydrazine-1-carboxamide 1n. White solid. Yield 99% (method C), mp 206–208°C. 1H NMR (600 MHz, DMSO-D6) δ 10.22 (1H, s, NH), 7.99 (2H, d, J = 8.4 Hz, H2, H6), 7.92 (2H, d, J = 8.1 Hz, H3, H5), 7.76 (1H, s, NH), 6.37 (1H, t, J = 5.8 Hz, NH-CH2), 2.99 (2H, q, J = 6.6 Hz, NH-CH2), 1.36 (2H, p, J = 6.9 Hz, C2H2), 1.21–1.12 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.82 (3H, t, J = 7.0 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 165.51, 158.57, 137.50, 132.86, 128.92, 118.74, 114.54, 31.79, 30.34, 29.56, 29.55, 29.54, 29.52, 29.51, 29.50, 29.32, 29.18, 26.83, 22.56, 14.38. IR (ATR): 626, 650, 724, 757, 855, 912, 1016, 1060, 1245, 1258, 1339, 1465, 1489, 1544, 1586, 1638, 1667, 2851, 2922, 3271, 3307 cm−1. Elemental analysis for C22H34N4O2 (386.54); calculated: C, 68.36; H, 8.87; N, 14.49, found: C, 68.42; H, 8.99; N, 14.40. Rf: 0.45.
N-Methyl-2-(4-methylbenzoyl)hydrazine-1-carboxamide 1o. White solid. Yield 36% (method A), mp 193–195 °C. 1H NMR (500 MHz, DMSO-D6) δ 10.01 (1H, s, NH), 7.83–7.87 (3H, m, NH, H2, H6), 7.27 (2H, d, J = 8.1 Hz, H3, H5), 6.38 (1H, q, J = 5.7 Hz, NH-CH3), 2.57 (3H, d, J = 4.6 Hz, NH-CH3), 2.35 (3H, s, Ph-CH3). 13C NMR (126 MHz, DMSO-D6) δ 166.49, 159.17, 144.27, 130.16, 128.96, 127.77, 26.44, 21.18. IR (ATR): 3310, 3254, 3063, 1666, 1644, 1613, 1575, 1541, 1505, 1420, 1337, 1255, 1191, 1126, 1108, 1016, 910, 840, 750, 701, 646, 634, 629 cm−1. Elemental analysis for C10H13N3O2 (207.23); calculated: C, 57.96; H, 6.32; N, 20.28, found: C, 57.99; H, 6.35; N, 20.30.
2-(4-Methylbenzoyl)-N-tridecylhydrazine-1-carboxamide 1p. White solid. Yield 22% (method B), mp 159–161 °C. 1H NMR (500 MHz, DMSO-D6) δ 10.51 (1H, s, NH), 7.87–7.83 (3H, m, NH, H2, H6), 7.28–7.24 (2H, m, H3, H5), 6.48 (1H, t, J = 5.0 Hz, NH-CH2), 3.04 (2H, q, J = 6.6 Hz, NH-CH2), 2.36 (3H, s, Ph-CH3), 1.36 (2H, p, J = 6.8 Hz, C2H2), 1.26–1.19 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.84 (3H, t, J = 7.0 Hz, CH3). 13C NMR (126 MHz, DMSO-D6) δ 166.35, 158.53, 141.66, 130.15, 128.93, 127.71, 39.10, 31.47, 30.03, 29.28, 29.24, 29.20, 29.17, 29.01, 28.88, 26.48, 22.26, 21.15, 14.11. IR (ATR): 3293, 2917, 2850, 1666, 1643, 1614, 1586, 1542, 1504, 1468, 1377, 1339, 1262, 1190, 1124, 1063, 1017, 912, 838, 746, 721, 655, 641, 625 cm−1. Elemental analysis for C22H37N3O2 (375.56); calculated: C, 70.36; H, 9.93; N, 11.19, found: C, 70.44; H, 10.00; N, 11.15.
2-[(4-(tert-Butyl)benzoyl]-N-methylhydrazine-1-carboxamide 1q. White solid. Yield 25% (method A), mp 180–182 °C. 1H NMR (500 MHz, DMSO-D6) δ 10.02 (1H, s, NH), 7.85–7.81 (3H, m, NH, H2, H6), 7.50–7.47 (2H, m, H3, H5), 6.37 (1H, q, J = 4.7 Hz, NH-CH3), 2.57 (3H, d, J = 4.6 Hz, NH-CH3), 1.29 (9H, s, C-CH3). 13C NMR (126 MHz, DMSO-D6) δ 166.45, 159.19, 154.68, 130.20, 127.64, 125.22, 34.86, 31.13, 26.46. IR (ATR): 3353, 3221, 2963, 2906, 2870, 1682, 1655, 1611, 1573, 1519, 1463, 1408, 1366, 1297, 1286, 1196, 1163, 1113, 1079, 1019, 926, 890, 854, 839, 765, 705, 658, 623, 607 cm−1. Elemental analysis for C13H19N3O2 (249.31); calculated: C, 62.63; H, 7.68; N, 16.85, found: C, 62.59; H, 7.65; N, 16.90.
2-[(4-(tert-Butyl)benzoyl]-N-tridecylhydrazine-1-carboxamide 1r. White solid. Yield 39% (method B), mp 103–105 °C. 1H NMR (500 MHz, DMSO-D6) δ 9.99 (1H, s, NH), 7.80–7.77 (2H, m, H2, H6), 7.75 (1H, s, NH), 7.48–7.45 (2H, m, H3, H5), 6.44 (1H, t, J = 5.8 Hz, NH-CH2), 2.98 (2H, q, J = 6.6 Hz, NH-CH2), 1.34 (2H, p, J = 6.6 Hz, C2H2), 1.28–1.19 (29H, m, C-CH3, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.82 (3H, t, J = 6.9 Hz, CH3). 13C NMR (126 MHz, DMSO-D6) δ 166.99, 159.05, 155.17, 130.32, 127.90, 125.59, 39.96, 31.76, 31.38, 29.51, 29.48, 29.46, 29.39, 29.27, 29.16, 28.98, 27.41, 26.73, 26.25, 22.57, 14.43. IR (ATR): 3356, 3258, 2955, 2920, 2851, 1702, 1628, 1610, 1544, 1500, 1468, 1364, 1333, 1268, 1258, 1240, 1123, 1016, 978, 895, 852, 788, 761, 721, 663, 652, 635, 616 cm−1. Elemental analysis for C25H43N3O2 (417.64); calculated: C, 71.90; H, 10.38; N, 10.06, found: C, 72.01; H, 10.40; N, 10.13.
N-Methyl-2-(4-phenylbenzoyl)hydrazine-1-carboxamide 1s. White solid. Yield 20% (method A), mp 172–174 °C. 1H NMR (600 MHz, DMSO-D6) δ 10.12 (1H, s, NH), 7.96 (2H, d, J = 8.2 Hz, H2, H6), 7.85 (1H, s, NH), 7.75 (2H, d, J = 8.3 Hz, H3, H5), 7.70 (2H, d, J = 7.7 Hz, H2’, H6’), 7.46 (2H, t, J = 7.5 Hz, H3’, H5’), 7.37 (1H, t, J = 7.3 Hz, H4’), 6.40 (1H, q, J = 5.7 Hz, NH-CH3), 2.55 (3H, d, J = 4.6 Hz, NH-CH3). 13C NMR (151 MHz, DMSO-D6) δ 166.61, 159.49, 143.66, 139.65, 132.14, 129.59, 128.82, 128.66, 127.42, 126.99, 26.81. IR (ATR): 3411, 3209, 1706, 1680, 1650, 1637, 1609, 1561, 1485, 1413, 1329, 1240, 1207, 1130, 1105, 1006, 901, 857, 780, 744, 690, 661, 636, 609 cm−1. Elemental analysis for C15H15N3O2 (269.30); calculated: C, 66.90; H, 5.61; N, 15.60, found: C, 66.96; H, 5.55; N, 15.70.
2-(4-Phenylbenzoyl)-N-tridecylhydrazine-1-carboxamide 1t. White solid. Yield 84% (method B), mp 249–251 °C. 1H NMR (600 MHz, DMSO-D6) δ 10.63 (1H, s, NH), 8.02 (2H, d, J = 8.2 Hz, H2, H6), 7.97 (1H, s, NH), 7.81 (2H, d, J = 8.2 Hz, H3, H5), 7.72 (2H, d, J = 7.8 Hz, H2’, H6’), 7.47 (2H, t, J = 7.7 Hz, H3’, H5’), 7.39 (1H, t, J = 7.5 Hz, H4’), 6.46 (1H, t, J = 5.0 Hz, NH-CH2), 2.69 (2H, q, J = 6.3 Hz, NH-CH2), 1.40 (2H, p, J = 6.8 Hz, C2H2), 1.24–1.17 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.81 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 166.06, 158.32, 144.77, 139.36, 129.74, 129.64, 129.02, 128.92, 127.51, 127.40, 39.24, 31.82, 29.59, 29.56, 29.54, 29.46, 29.36, 29.24, 29.07, 27.46, 26.39, 22.62, 14.48. IR (ATR): 2955, 2920, 2850, 1673, 1610, 1575, 1558, 1520, 1484, 1468, 1449, 1401, 1303, 1238, 1163, 1146, 1078, 1036, 1006, 852, 779, 742, 722, 698, 648, 631, 614 cm−1. Elemental analysis for C27H39N3O2 (437.63); calculated: C, 74.10; H, 8.98; N, 9.60, found: C, 74.17; H, 9.05; N, 9.55.
2-(4-Methoxybenzoyl)-N-methylhydrazine-1-carboxamide 1u. White solid. Yield 34% (method A), mp 171–173 °C. 1H NMR (500 MHz, DMSO-D6) δ 9.95 (1H, s, NH), 7.88–7.85 (2H, m, H2, H6), 7.78 (1H, s, NH), 7.02–6.99 (2H, m, H3, H5), 6.37 (1H, q, J = 5.0 Hz, NH-CH3), 3.81 (3H, s, OCH3), 2.57 (3H, d, J = 4.6 Hz, NH-CH3). 13C NMR (126 MHz, DMSO-D6) δ 166.10, 162.05, 159.24, 129.63, 125.12, 113.67, 55.55, 26.43. IR (ATR): 3347, 3323, 3004, 2944, 1685, 1634, 1610, 1574, 1524, 1496, 1467, 1420, 1324, 1260, 1173, 1104, 1027, 980, 902, 840, 797, 767, 738, 700, 652, 624 cm−1. Elemental analysis for C10H13N3O3 (223.23); calculated: C, 53.81; H, 5.87; N, 18.82, found: C, 53.89; H, 5.85; N, 18.90.
2-(4-Methoxybenzoyl)-N-tridecylhydrazine-1-carboxamide 1v. White solid. Yield 27% (method B), mp 149–151 °C. 1H NMR (500 MHz, DMSO-D6) δ 9.96 (1H, s, NH), 7.86 (2H, d, J = 8.9 Hz, H2, H6), 7.76 (1H, s, NH), 6.99 (2H, d, J = 8.9 Hz, H3, H5), 6.51 (1H, t, J = 5.0 Hz, NH-CH2), 3.80 (3H, s, OCH3), 2.98 (2H, q, J = 6.6 Hz, NH-CH2), 1.35 (2H, p, J = 6.8 Hz, C2H2), 1.25–1.19 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.84 (3H, t, J = 6.9 Hz, CH3). 13C NMR (126 MHz, DMSO-D6) δ 165.99, 162.02, 158.67, 129.60, 125.15, 113.65, 55.55, 45.41, 39.96, 31.47, 30.05, 29.24, 29.20, 29.18, 29.02, 28.88, 26.51, 22.27, 14.13. IR (ATR): 3291, 1954, 2918, 2851, 1645, 1609, 1583, 1541, 1504, 1647, 1376, 1340, 1309, 1255, 1180, 1038, 912, 842, 763, 722, 656, 640, 625 cm−1. Elemental analysis for C22H37N3O3 (391.56); calculated: C, 67.49; H, 9.53; N, 10.73, found: C, 67.53; H, 9.46; N, 10.65.
N-Methyl-2-(4-phenoxybenzoyl)hydrazine-1-carboxamide 1w. White solid. Yield 88% (method C), mp 176–177 °C. 1H NMR (600 MHz, DMSO-D6) δ 10.01 (1H, s, NH), 7.89–7.87 (2H, m, H2, H6), 7.80 (1H, s, NH), 7.44–7.37 (2H, m, H3’, H5’), 7.21–7.15 (1H, m, H4’), 7.07–7.03 (2H, m, H3, H5), 7.02–6.98 (2H, m, H2’, H6’), 6.36 (1H, q, J = 4.5 Hz, NH-CH3), 2.54 (3H, d, J = 4.5 Hz, NH-CH3). 13C NMR (151 MHz, DMSO-D6) δ 166.20, 160.33, 159.50, 156.11, 130.81, 130.36, 127.99, 124.90, 120.07, 117.87, 26.78. IR (ATR): 618, 658, 692, 717, 760, 873, 899, 980, 1078, 1102, 1170, 1238, 1319, 1410, 1487, 1527, 1566, 1642, 1663, 2837, 3016, 3262 cm−1. Elemental analysis for C15H15N3O3 (285.30); calculated: C, 63.15; H, 5.30; N, 14.73, found: C, 63.24; H, 5.20; N, 14.84. Rf: 0.36.
2-(4-Phenoxybenzoyl)-N-tridecylhydrazine-1-carboxamide 1x. White flakes. Yield 86% (method C), mp 127–129 °C. 1H NMR (600 MHz, DMSO-D6) δ 10.00 (1H, s, NH), 7.88 (2H, d, J = 8.5 Hz, H2, H6), 7.71 (1H, s, NH), 7.41 (2H, t, J = 7.8 Hz, H3’, H5’), 7.18 (1H, t, J = 7.4 Hz, H4’), 7.05 (2H, d, J = 7.9 Hz, H3, H5), 7.00 (2H, d, J = 8.6 Hz, H2’, H6’), 6.39 (1H, t, J = 5.9 Hz, NH-CH2), 2.97 (2H, q, J = 6.6 Hz, NH-CH2), 1.34 (2H, p, J = 7.1 Hz, C2H2), 1.22–1.18 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.81 (3H, t, J = 6.8 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 166.12, 160.32, 158.91, 156.10, 130.80, 130.32, 127.98, 124.89, 120.07, 117.86, 31.83, 30.41, 29.61, 29.59, 29.58, 29.57, 29.56, 29.55, 29.38, 29.25, 26.85, 22.63, 14.48. IR (ATR): 629, 692, 721, 749, 793, 848, 872, 900, 1072, 1168, 1200, 1245, 1257, 1309, 1469, 1491, 1548, 1573, 1589, 1647, 2849, 2920, 2954, 3286 cm−1. Elemental analysis for C27H39N3O3 (453.63); calculated: C, 71.49; H, 8.67; N, 9.26, found: C, 71.57; H, 8.60; N, 9.12. Rf: 0.48.
2-[4-(Dimethylamino)benzoyl]-N-methylhydrazine-1-carboxamide 1y. White solid. Yield 31% (method A), mp 201–203 °C. 1H NMR (500 MHz, DMSO-D6) δ 9.74 (1H, s, NH), 7.78–7.73 (2H, m, H2, H6), 7.68 (1H, s, NH), 6.72–6.68 (2H, m, H3, H5), 6.32 (1H, q, J = 4.6 Hz, NH-CH3), 2.96 (6H, s, N-CH3), 2.56 (3H, d, J = 4.6 Hz, NH-CH3). 13C NMR (126 MHz, DMSO-D6) δ 166.57, 159.47, 152.57, 129.16, 119.38, 110.88, 39.86, 26.45. IR (ATR): 3365, 3273, 2945, 1677, 1659, 1602, 1537, 1511, 1443, 1402, 1377, 1335, 1289, 1239, 1201, 1169, 1130, 1070, 950, 917, 886, 831, 770, 703, 650 cm−1. Elemental analysis for C11H16N4O2 (236.28); calculated: C, 55.92; H, 6.83; N, 23.71, found: C, 55.98; H, 6.75; N, 23.80.
2-[4-(Dimethylamino)benzoyl]-N-tridecylhydrazine-1-carboxamide 1z. White solid. Yield 83% (method B), mp 148–150 °C. 1H NMR (500 MHz, DMSO-D6) δ 9.74 (1H, s, NH), 7.77–7.73 (2H, m, H2, H6), 7.62 (1H, s, NH), 6.70 (2H, d, J = 9.0 Hz, H3, H5), 6.37 (1H, t, J = 5.0 Hz, NH-CH2), 2.98 (2H, q, J = 6.5 Hz, NH-CH2), 2.96 (6H, s, N-CH3), 1.36 (2H, p, J = 6.9 Hz, C2H2), 1.26–1.20 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.84 (3H, t, J = 6.9 Hz, CH3). 13C NMR (126 MHz, DMSO-D6) δ 166.43, 158.85, 152.50, 129.10, 118.26, 110.89, 39.80, 38.94, 31.50, 30.09, 29.28, 29.24, 29.14, 29.05, 28.93, 27.15, 26.52, 22.30, 14.15. IR (ATR): 3367, 3280, 2950, 2918, 2849, 1685, 1636, 1609, 1543, 1516, 1469, 1445, 1375, 1340, 1329, 1261, 1240, 1212, 1174, 1071, 952, 902, 835, 766, 722, 656, 609 cm−1. Elemental analysis for C23H40N4O2 (404.60); calculated: C, 68.28; H, 9.97; N, 13.85, found: C, 68.34; H, 10.03; N, 13.78.
2-(4-Hydroxybenzoyl)-N-methylhydrazine-1-carboxamide 1aa. White solid. Yield 30% (method A), mp 234–235 °C. 1H NMR (500 MHz, DMSO-D6) δ 10.04 (1H, s, OH), 9.85 (1H, s, NH), 7.78–7.72 (3H, m, NH, H2, H6), 6.82–7.78 (2H, m, H3, H5), 6.35 (1H, q, J = 4.7 Hz, NH-CH3), 2.56 (3H, d, J = 4.6 Hz, NH-CH3). 13C NMR (126 MHz, DMSO-D6) δ 166.32, 160.70, 159.33, 129.76, 123.56, 114.99, 26.44. IR (ATR): 3407, 3331, 3183, 1687, 1635, 1607, 1587, 1542, 1512, 1493, 1462, 1442, 1412, 1310, 1284, 1259, 1226, 1169, 1117, 1102, 976, 903, 844, 757, 688, 657, 634, 610 cm−1. Elemental analysis for C9H11N3O3 (209.21); calculated: C, 51.67; H, 5.30; N, 20.09, found: C, 51.64; H, 5.27; N, 20.01.
2-(4-Hydroxybenzoyl)-N-tridecylhydrazine-1-carboxamide 1bb. White solid. Yield 25% (method B), 146–148 °C. 1H NMR (500 MHz, DMSO-D6) δ 10.43 (1H, s, OH), 9.83 (1H, s, NH), 7.99 (1H, s, NH), 7.82–7.79 (2H, m, H2, H6), 6.90–7.87 (2H, m, H3, H5), 6.42 (1H, t, J = 5.9 Hz, NH-CH2), 3.04 (2H, q, J = 6.4 Hz, NH-CH2), 1.37 (2H, p, J = 6.9 Hz, C2H2), 1.26–1.20 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.84 (3H, t, J = 6.9 Hz, CH3). 13C NMR (126 MHz, DMSO-D6) δ 166.25, 160.78, 158.77, 129.70, 123.50, 115.01, 38.93, 31.53, 30.11, 29.31, 29.26, 29.08, 28.96, 28.78, 27.16, 26.55, 26.07, 22.33, 14.19. IR (ATR): 3392, 3197, 2955, 2919, 2850, 1660, 1639, 1613, 1582, 1501, 1468, 1378, 1327, 1291, 1270, 1246, 1172, 1074, 904, 847, 767, 721, 659, 652, 633 cm−1. Elemental analysis for C21H35N3O3 (377.53); calculated: C, 66.81; H, 9.34; N, 11.13, found: C, 66.75; H, 9.41; N, 11.02.
N-Methyl-2-(4-sulfamoylbenzoyl)hydrazine-1-carboxamide 1cc. White solid. Yield 72% (method C), mp 233–234 °C. 1H NMR (600 MHz, DMSO-D6) δ 10.24 (1H, s, NH), 8.00 (2H, d, J = 8.2 Hz, H2, H6), 7.91 (1H, s, NH), 7.87 (2H, d, J = 8.3 Hz, H3, H5), 7.46 (2H, s, SO2NH2), 6.43 (2H, q, J = 4.6 Hz, NH-CH3), 2.54 (3H, d, J = 4.6 Hz, NH-CH3). 13C NMR (151 MHz, DMSO-D6) δ 165.99, 159.28, 147.16, 136.27, 128.82, 126.08, 26.79. IR (ATR): 904, 1015, 1074, 1098, 1120, 1162, 1187, 1291, 1314, 1416, 1473, 1541, 1567, 1647, 3100, 3241, 3360 cm−1. Elemental analysis for C9H12N4O4S (272.28); calculated: C, 39.70; H, 4.44; N, 20.58, found: C, 39.80; H, 4.44; N, 20.62. Rf: 0.12.
2-(4-Sulfamoylbenzoyl)-N-tridecylhydrazine-1-carboxamide 1dd. White solid. Yield 99% (method C), mp 116–118 °C. 1H NMR (600 MHz, DMSO-D6) δ 10.01 (1H, s, NH), 7.98 (2H, d, J = 8.2 Hz, H2, H6), 7.88 (2H, d, J = 8.2 Hz, H3, H5), 7.67 (1H, s, NH), 7.28 (2H, s, SO2NH2), 6.25 (1H, t, J = 5.9 Hz, NH-CH2), 3.01 (2H, q, J = 6.6 Hz, NH-CH2), 1.38 (2H, p, J = 6.9 Hz, C2H2), 1.25–1.21 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.83 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 165.86, 158.58, 147.30, 136.46, 128.62, 126.10, 31.75, 30.34, 29.50, 29.49, 29.48, 29.47, 29.46, 29.45, 29.29, 29.12, 26.84, 22.50, 14.28. IR (ATR): 676, 717, 748, 767, 853, 860, 901, 1022, 1093, 1163, 1257, 1289, 1326, 1343, 1377, 1472, 1478, 1519, 1572, 1647, 1667, 2850, 2918, 2954, 3288 cm−1. Elemental analysis for C21H36N4O4S (440.60); calculated: C, 57.25; H, 8.24; N, 12.72, found: C, 57.33; H, 8.20; N, 12.80. Rf: 0.17.
2-Benzoyl-N-methylhydrazine-1-carboxamide 1ee. White solid. Yield 28% (method A), mp 165–168 °C. 1H NMR (500 MHz, DMSO-D6) δ 10.10 (1H, s, NH), 7.91–7.86 (3H, m, NH, H2, H6), 7.57–7.53 (1H, m, H4), 7.49–7.45 (2H, m, H3, H5), 6.42 (2H, q, J = 4.7 Hz, NH-CH3), 2.58 (3H, d, J = 4.6 Hz, NH-CH3). 13C NMR (126 MHz, DMSO-D6) δ 166.58, 159.14, 132.96, 131.82, 128.46, 127.77, 26.45. IR (ATR): 3310, 3280, 3056, 1648, 1603, 1581, 1536, 1490, 1419, 1344, 1330, 1255, 1188, 1109, 1074, 1025, 982, 911, 804, 748, 684, 636, 616 cm−1. Elemental analysis for C9H11N3O2 (193.21); calculated: C, 55.95; H, 5.74; N, 21.75, found: C, 56.00; H, 5.84; N, 21.69.
2-Benzoyl-N-tridecylhydrazine-1-carboxamide 1ff. White solid. Yield 34% (method B), mp 129–131 °C. 1H NMR (500 MHz, DMSO-D6) δ 10.11 (1H, s, NH), 7.83 (1H, s, NH), 7.65–7.61 (1H, m, H4), 7.56–7.51 (2H, m, H2, H6), 7.48–7.44 (2H, m, H3, H5), 6.53 (1H, t, J = 6.0 Hz, NH-CH2), 3.03 (2H, q, J = 6.5 Hz, NH-CH2), 1.36 (2H, p, J = 6.9 Hz, C2H2), 1.25–1.20 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.84 (3H, t, J = 6.9 Hz, CH3). 13C NMR (126 MHz, DMSO-D6) δ 165.96, 158.55, 132.96, 131.78, 128.88, 127.96, 45.43, 31.50, 30.08, 29.27, 29.23, 29.14, 29.05, 28.92, 26.53, 26.04, 22.30, 14.16, 8.58. IR (ATR): 3300, 2948, 2918, 2849, 1668, 1645, 1604, 1582, 1540, 1500, 1469, 1343, 1330, 1260, 1189, 1124, 1069, 1017, 982, 912, 806, 747, 684, 638, 618 cm−1. Elemental analysis for C21H35N3O2 (361.53); calculated: C, 69.77; H, 9.76; N, 11.62, found: C, 69.84; H, 9.83; N, 11.69.
N-Methyl-2-[3-(trifluoromethyl)benzoyl]hydrazine-1-carboxamide 2a. White solid. Yield 21% (method A), mp 202–204 °C. 1H NMR (500 MHz, DMSO-D6) δ 10.38 (1H, s, NH), 8.23 (1H, t, J = 1.9 Hz, H2), 8.19–8.16 (1H, m, H6), 7.96 (1H, s, NH), 7.93–7.90 (1H, m, H4), 7.73 (1H, t, J = 7.8 Hz, H5), 6.51 (1H, q, J = 4.5 Hz, NH-CH3), 2.58 (3H, d, J = 4.6 Hz, NH-CH3). 13C NMR (126 MHz, DMSO-D6) δ 164.99, 158.92, 134.16, 131.82, 129.76, 129.26 (q, J = 32.0 Hz), 128.19 (q, J = 3.8 Hz), 124.41 (q, J = 3.9 Hz), 124.18 (q, J = 272.3 Hz), 26.40. IR (ATR): 3235, 3049, 1655, 1617, 1581, 1548, 1488, 1421, 1348, 1326, 1312, 1288, 1259, 1220, 1163, 1116, 1075, 983, 923, 819, 800, 762, 698, 667, 649, 615 cm−1. Elemental analysis for C10H10F3N3O2 (261.20); calculated: C, 45.98; H, 3.86; N, 16.09, found: C, 46.07; H, 3.90; N, 16.08.
N-Tridecyl-2-[3-(trifluoromethyl)benzoyl]hydrazine-1-carboxamide 2b. White solid. Yield 95% (method C), mp 140–141 °C. 1H NMR (600 MHz, DMSO-D6) δ 10.16 (1H, s, NH), 8.18 (1H, s, NH), 8.16–8.12 (1H, m, H2), 7.88–7.84 (1H, m, H6), 7.71–7.66 (2H, m, H4, H5), 6.29 (1H, t, J = 6.1 Hz, NH-CH2), 3.01 (2H, q, J = 6.6 Hz, NH-CH2), 1.38 (2H, p, J = 6.9 Hz, C2H2), 1.25–1.21 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.83 (3H, t, J = 6.7 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 165.49, 158.62, 134.55, 132.05, 130.04, 129.73 (q, J = 31.8 Hz), 128.15 (q, J = 3.9 Hz), 124.55 (q, J = 272.3 Hz), 124.73 (q, J = 4.7 Hz), 31.75, 30.33, 29.49, 29.48, 29.47, 29.46, 29.45, 29.44, 29.29, 29.11, 26.83, 22.49, 14.25. IR (ATR): 663, 725, 817, 922, 1073, 1124, 1170, 1244, 1258, 1287, 1311, 1326, 1468, 1558, 1568, 1650, 1668, 1688, 2851, 2921, 3278 cm−1. Elemental analysis for C22H34F3N3O2 (429.53); calculated: C, 61.52; H, 7.98; N, 9.78, found: C, 61.67; H, 8.09; N, 9.88. Rf: 0.48.
N-Methyl-2-[2-(trifluoromethyl)benzoyl]hydrazine-1-carboxamide 2c. White solid. Yield 21% (method A), mp 196–198 °C. 1H NMR (500 MHz, DMSO-D6) δ 10.38 (1H, s, NH), 8.23 (1H, s, NH), 8.17 (1H, d, J = 7.9 Hz, H6), 7.96–7.91 (2H, m, H3, H4), 7.74 (1H, t, J = 7.8 Hz, H5), 6.51 (1H, q, J = 4.6 Hz, NH-CH3), 2.58 (3H, d, J = 4.5 Hz, NH-CH3). 13C NMR (126 MHz, DMSO-D6) δ 165.24, 158.96, 133.92, 131.87, 129.89, 129.29 (q, J = 32.2 Hz), 128.35 (q, J = 3.8 Hz), 124.46 (q, J = 4.0 Hz), 124.15 (q, J = 272.2 Hz), 26.40. IR (ATR): 3369, 3253, 3050, 1654, 1617, 1582, 1548, 1421, 1348, 1326, 1313, 1259, 1220, 1163, 1116, 1075, 986, 923, 819, 801, 762, 698, 667, 649, 611 cm−1. Elemental analysis for C10H10F3N3O2 (261.20); calculated: C, 45.98; H, 3.86; N, 16.09, found: C, 46.02; H, 3.93; N, 16.12.
N-Tridecyl-2-[2-(trifluoromethyl)benzoyl]hydrazine-1-carboxamide 2d. White solid. Yield 92% (method C), mp 134–136 °C. 1H NMR (600 MHz, DMSO-D6) δ 9.82 (1H, s, NH), 7.78–7.73 (2H, m, H3, H6), 7.72–7.68 (1H, m, H4), 7.66–7.61 (2H, m, NH, H5), 5.98 (1H, t, J = 5.8 Hz, NH-CH2), 3.03 (2H, q, J = 6.6 Hz, NH-CH2), 1.40 (2H, p, J = 7.0 Hz, C2H2), 1.26–1.23 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.83 (3H, t, J = 6.8 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 167.28, 158.31, 134.99, 132.72, 130.68, 129.74, 127.15 (q, J = 31.8 Hz), 126.77 (q, J = 4.2 Hz), 124.19 (q, J = 273.7 Hz), 31.75, 30.31, 29.50, 29.49, 29.48, 29.47, 29.46, 29.45, 29.28, 29.12, 26.82, 22.49, 14.26. IR (ATR): 665, 727, 765, 900, 1036, 1056, 1110, 1120, 1140, 1188, 1240, 1256, 1271, 1315, 1378, 1448, 1463, 1538, 1588, 1602, 1613, 1660, 2849, 2924, 2954, 3180 cm−1. Elemental analysis for C22H34F3N3O2 (429.53); calculated: C, 61.52; H, 7.98; N, 9.78, found: C, 61.49; H, 8.10; N, 9.84. Rf: 0.38.
N-Methyl-2-(3-nitrobenzoyl)hydrazine-1-carboxamide 2e. Pale yellow solid. Yield 33% (method A), mp 220–222 °C. 1H NMR (600 MHz, DMSO-D6) δ 10.44 (1H, s, NH), 8.68 (1H, t, J = 2.0 Hz, H2), 8.38 (1H, dd, J = 8.2, 2.4 Hz, H4), 8.29–8.26 (1H, m, H6), 7.96 (1H, s, NH), 7.77 (1H, t, J = 8.0 Hz, H5), 6.50 (1H, s, NH-CH3), 2.55 (3H, d, J = 4.5 Hz, NH-CH3). 13C NMR (151 MHz, DMSO-D6) δ 165.02, 159.23, 148.23, 134.87, 134.52, 130.68, 126.75, 122.95, 26.76. IR (ATR): 3305, 3099, 2949, 1668, 1644, 1587, 1542, 1519, 1476, 1437, 1416, 1354, 1340, 1257, 1228, 1161, 1130, 1086, 985, 935, 827, 777, 726, 673, 652, 644, 606 cm−1. Elemental analysis for C9H10N4O4 (238.20); calculated: C, 45.38; H, 4.23; N, 23.52, found: C, 45.41; H, 4.30; N, 23.46.
2-(3-Nitrobenzoyl)-N-tridecylhydrazine-1-carboxamide 2f. Pale yellow solid. Yield 64% (method C), mp 146–147 °C. 1H NMR (600 MHz, DMSO-D6) δ 10.40 (1H, s, NH), 8.67 (1H, t, J = 2.0 Hz, H2), 8.36 (1H, dd, J = 8.1, 2.3 Hz, H4), 8.27 (1H, dd, J = 8.00, 1.4 Hz, H6), 7.82 (1H, s, NH), 7.76 (1H, t, J = 8.0 Hz, H5), 6.46 (1H, t, J = 5.9 Hz, NH-CH2), 2.99 (2H, q, J = 6.6 Hz, NH-CH2), 1.36 (2H, p, J = 7.0 Hz, C2H2), 1.24–1.18 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.82 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 164.84, 158.60, 148.32, 135.01, 134.42, 130.64, 126.63, 122.88, 31.80, 30.38, 29.57, 29.56, 29.55, 29.54, 29.52, 29.50, 29.34, 29.20, 26.84, 22.58, 14.41. IR (ATR): 629, 720, 820, 924, 1084, 1238, 1256, 1346, 1467, 1482, 1526, 1539, 1583, 1566, 1613, 2850, 2925, 2955, 3223, 3351 cm−1. Elemental analysis for C21H34N4O4 (406.53); calculated: C, 62.05; H, 8.43; N, 13.78, found: C, 62.14; H, 8.31; N, 13.84. Rf: 0.48.
N-Methyl-2-(2-nitrobenzoyl)hydrazine-1-carboxamide 2g. Pale yellow solid. Yield 17% (method A), mp 200–202 °C. 1H NMR (600 MHz, DMSO-D6) δ 10.50 (1H, s, NH), 8.09 (1H, d, J = 8.1 Hz, H3), 8.03 (1H, s, NH), 7.80 (1H, t, J = 7.5 Hz, H5), 7.71–7.64 (2H, m, H4, H6), 6.15 (1H, q, J = 4.6 Hz, NH-CH3), 2.53 (3H, d, J = 4.5 Hz, NH-CH3). 13C NMR (151 MHz, DMSO-D6) δ 164.92, 159.03, 143.84, 135.50, 133.28, 131.12, 129.80, 124.97, 26.78. IR (ATR): 3355, 3103, 2954, 1671, 1644, 1585, 1540, 1524, 1477, 1356, 1255, 1232, 1061, 901, 935, 827, 770, 729, 671, 649 cm−1. Elemental analysis for C9H10N4O4 (238.20); calculated: C, 45.38; H, 4.23; N, 23.52, found: C, 45.42; H, 4.17; N, 23.56.
2-(2-Nitrobenzoyl)-N-tridecylhydrazine-1-carboxamide 2h. Pale yellow solid. Yield 21% (method B), mp 148–150 °C. 1H NMR (600 MHz, DMSO-D6) δ 10.21 (1H, s, NH), 8.03 (1H, d, J = 8.1 Hz, H3), 8.00 (1H, s, NH), 7.80 (1H, t, J = 7.5 Hz, H5), 7.73–7.67 (2H, m, H4, H6), 6.15 (1H, t, J = 5.8 Hz, NH-CH2), 3.00 (2H, q, J = 6.6 Hz, NH-CH2), 1.36 (2H, p, J = 6.8 Hz, C2H2), 1.24–1.18 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.81 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 165.84, 158.28, 147.70, 134.18, 131.86, 131.00, 130.19, 124.75, 39.61, 31.83, 30.33, 29.60, 26.59, 29.56, 29.55, 29.36, 29.30, 29.25, 26.82, 22.63, 14.48. IR (ATR): 3356, 3179, 2955, 2924, 2489, 1608, 1585, 1539, 1524, 1478, 1460, 1352, 1255, 1238, 1064, 901, 859, 786, 757, 728, 698, 666 cm−1. Elemental analysis for C21H34N4O4 (406.53); calculated: C, 62.05; H, 8.43; N, 13.78, found: C, 62.10; H, 8.41; N, 13.75.
2-(3-Bromobenzoyl)-N-methylhydrazine-1-carboxamide 2i. White solid. Yield 65% (method A), mp 190–192 °C. 1H NMR (600 MHz, DMSO-D6) δ 10.18 (1H, s, NH), 8.04 (1H, t, J = 1.9 Hz, H2), 7.88–7.81 (2H, m, NH, H6), 7.74–7.71 (1H, m, H4), 7.42 (1H, t, J = 7.9 Hz, H5), 6.44 (1H, s, NH-CH3), 2.54 (3H, d, J = 4.5 Hz, NH-CH3). 13C NMR (151 MHz, DMSO-D6) δ 165.58, 159.31, 135.55, 134.87, 131.10, 130.86, 127.23, 122.12, 26.76. IR (ATR): 3289, 1670, 1646, 1588, 1532, 1489, 1470, 1416, 1337, 1261, 1224, 1072, 995, 920, 808, 752, 724, 678, 644, 631, 616 cm−1. Elemental analysis for C9H10BrN3O2 (277.10); calculated: C, 39.73; H, 3.70; N, 15.44, found: C, 39.74; H, 3.76; N, 15.51.
2-(3-Bromobenzoyl)-N-tridecylhydrazine-1-carboxamide 2j. White solid. Yield 43% (method B), mp 145–147 °C. 1H NMR (600 MHz, DMSO-D6) δ 10.16 (1H, s, NH), 8.03 (1H, s, H2), 7.83 (1H, d, J = 7.8 Hz, H6), 7.78 (1H, s, NH), 7.72 (1H, d, J = 8.0 Hz, H4), 7.41 (1H, t, J = 7.9 Hz, H5), 6.47 (1H, t, J = 5.9 Hz, NH-CH2), 2.96 (2H, q, J = 6.6 Hz, NH-CH2), 1.34 (2H, p, J = 6.8 Hz, C2H2), 1.26–1.21 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.81 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 165.45, 158.74, 135.54, 134.86, 131.10, 130.83, 127.18, 122.13, 39.73, 31.84, 30.40, 29.63, 29.61, 29.56, 29.54, 29.48, 29.38, 29.26, 26.84, 22.64, 14.48. IR (ATR): 3322, 3038, 2921, 2850, 1660, 1650, 1588, 1566, 1468, 1336, 1257, 1244, 1070, 997, 924, 901, 805, 724, 683, 659, 644, 632, 613 cm−1. Elemental analysis for C21H34BrN3O2 (440.43); calculated: C, 57.27; H, 7.78; N, 9.54, found: C, 57.24; H, 7.74; N, 9.59.
2-(2-Bromobenzoyl)-N-methylhydrazine-1-carboxamide 2k. White solid. Yield 20% (method A), mp 180–182 °C. 1H NMR (600 MHz, DMSO-D6) δ 9.93 (1H, d, J = 2.1 Hz, NH), 8.01 (1H, d, J = 2.1 Hz, NH), 7.63 (1H, dd, J = 8.0, 1.8 Hz, H6), 7.52 (1H, dd, J = 7.8, 1.8 Hz, H3), 7.42 (1H, td, J = 7.8, 1.8 Hz, H4), 7.36 (1H, td, J = 7.8, 1.7 Hz, H5), 6.14 (1H, q, J = 4.6 Hz, NH-CH3), 2.58 (3H, d, J = 4.6 Hz, NH-CH3). 13C NMR (151 MHz, DMSO-D6) δ 167.64, 158.96, 137.33, 133.41, 132.00, 130.13, 127.99, 119.93, 26.84. IR (ATR): 3385, 3284, 3188, 3002, 1710, 1645, 1591, 1568, 1542, 1472, 1429, 1412, 1356, 1327, 1251, 1147, 1123, 1029, 972, 901, 772, 747, 724, 701, 663, 646, 628 cm−1. Elemental analysis for C9H10BrN3O2 (277.10); calculated: C, 39.73; H, 3.70; N, 15.44, found: C, 39.70; H, 3.72; N, 15.39.
2-(2-Bromobenzoyl)-N-tridecylhydrazine-1-carboxamide 2l. White solid. Yield 67% (method B), mp 120–121 °C. 1H NMR (500 MHz, DMSO-D6) δ 9.96 (1H, d, J = 2.1 Hz, NH), 7.96 (1H, d, J = 2.1 Hz, NH), 7.67 (1H, d, J = 8.0, H6), 7.51 (1H, dd, J = 7.6, 1.8 Hz, H3), 7.46 (1H, td, J = 7.8, 1.8 Hz, H4), 7.39 (1H, td, J = 7.8, 1.9 Hz, H5), 6.20 (1H, t, J = 5.8 Hz, NH-CH2), 3.03 (2H, q, J = 6.6 Hz, NH-CH2), 1.42–1.36 (2H, m, C2H2), 1.28–1.20 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.85 (3H, t, J = 6.7 Hz, CH3). 13C NMR (126 MHz, DMSO-D6) δ 167.17, 157.97, 136.97, 133.01, 131.61, 129.67, 127.64, 119.51, 45.78, 31.46, 30.01, 29.26, 29.24, 29.21, 29.19, 29.00, 28.88, 26.48, 26.33, 22.26, 14.12. IR (ATR): 3354, 3201, 2954, 2924, 2849, 1607, 1591, 1573, 1591, 1541, 1478, 1467, 1456, 1257, 1237, 1223, 1027, 984, 899, 777, 756, 741, 724, 654, 636 cm−1. Elemental analysis for C21H34BrN3O2 (440.43); calculated: C, 57.27; H, 7.78; N, 9.54, found: C, 57.20; H, 7.80; N, 9.49.
N-Methyl-2-{2-[4-(trifluoromethyl)phenyl]acetyl}hydrazine-1-carboxamide 3a. White solid. Yield 50% (method A), mp 209–210 °C. 1H NMR (600 MHz, DMSO-D6) δ 9.73 (1H, d, J = 2.0 Hz, NH), 7.76 (1H, d, J = 2.0 Hz, NH), 7.63 (2H, d, J = 8.1 Hz, H3, H5), 7.47 (2H, d, J = 7.9 Hz, H2, H6), 6.27 (1H, q, J = 4.9 Hz, NH-CH3), 3.52 (2H, s, CH2), 2.52 (3H, d, J = 4.5 Hz, NH-CH3). 13C NMR (151 MHz, DMSO-D6) δ 169.92, 159.12, 141.18, 130.56, 127.78 (q, J = 31.9 Hz), 125.54 (q, J = 3.9 Hz), 124.93 (q, J = 273.6 Hz), 42.49, 26.72. IR (ATR): 3310, 3269, 3072, 3033, 1655, 1644, 1583, 1544, 1509, 1425, 1325, 1314, 1260, 1168, 1116, 1070, 1015, 986, 914, 858, 777, 765, 743, 693, 644, 629 cm−1. Elemental analysis for C11H12F3N3O2 (275.23); calculated: C, 48.00; H, 4.39; N, 15.27, found: C, 47.99; H, 4.31; N, 15.33.
N-Tridecyl-2-{2-[4-(trifluoromethyl)phenyl]acetyl}hydrazine-1-carboxamide 3b. White solid. Yield 25% (method B), mp 154–156 °C. 1H NMR (500 MHz, DMSO-D6) δ 9.77 (1H, s, NH), 7.71 (1H, s, NH), 7.65 (2H, d, J = 7.9 Hz, H3, H5), 7.51 (2H, d, J = 7.9 Hz, H2, H6), 6.29 (1H, t, J = 5.8 Hz, NH-CH2), 3.55 (2H, s, CH2), 2.97 (2H, q, J = 6.6 Hz, NH-CH2), 1.34 (2H, p, J = 7.4 Hz, C2H2), 1.26–1.20 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.84 (3H, t, J = 6.8 Hz, CH3). 13C NMR (126 MHz, DMSO-D6) δ 169.42, 158.13, 140.86, 130.15, 127.42 (q, J = 31.8 Hz), 125.14 (q, J = 4.0 Hz), 124.56 (q, J = 271.9 Hz), 40.03, 37.48, 31.47, 30.00, 29.24, 29.22, 29.20, 29.01, 28.89, 26.47, 22.27, 14.10. IR (ATR): 3311, 2950, 2918, 2849, 1667, 1639, 1569, 1558, 1508, 1489, 1469, 1417, 1326, 1243, 1165, 1119, 1068, 1020, 981, 873, 827, 723, 652, 644, 635, 626 cm−1. Elemental analysis for C23H36F3N3O2 (443.56); calculated: C, 62.28; H, 8.18; N, 9.47, found: C, 62.29; H, 8.10; N, 9.55.
N-Methyl-2-{[4-(trifluoromethyl)phenyl]sulfonyl}hydrazine-1-carboxamide 3c. White solid. Yield 64% (method C), mp 209–210 °C (decomp.). 1H NMR (600 MHz, DMSO-D6) δ 9.69 (1H, s, NH), 8.04 (1H, s, NH), 7.99–7.91 (4H, m, H2, H3, H5, H6), 6.36 (1H, q, J = 4.6 Hz, NH-CH3), 2.47 (3H, d, J = 4.6 Hz, NH-CH3). 13C NMR (151 MHz, DMSO-D6) δ 158.61, 142.87, 133.15 (q, J = 32.1 Hz), 129.32, 126.71 (q, J = 4.4 Hz), 124.11 (q, J = 272.9 Hz), 26.74. IR (ATR): 626, 707, 762, 793, 845, 964, 1016, 1062, 1095, 1110, 1135, 1172, 1192, 1302, 1319, 1358, 1405, 1418, 1489, 1564, 1648, 3113, 3417 cm−1. Elemental analysis for C9H10F3N3O3S (297.25); calculated: C, 36.37; H, 3.39; N, 14.14, found: C, 36.49; H, 3.30; N, 14.13. Rf: 0.43.
N-Tridecyl-2-{[4-(trifluoromethyl)phenyl]sulfonyl}hydrazine-1-carboxamide 3d. White solid. Yield 96% (method C), mp 157–158 °C. 1H NMR (600 MHz, DMSO-D6) δ 9.67 (1H, s, NH), 8.02 (1H, s, NH), 7.97 (2H, d, J = 8.3 Hz, H3, H5), 7.92 (2H, d, J = 8.3 Hz, H2, H6), 6.28 (1H, t, J = 6.0 Hz, NH-CH2), 2.85 (2H, q, J = 6.8 Hz, NH-CH2), 1.22–1.13 (22H, m, C2H2, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.81 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 157.75, 142.92, 133.13 (q, J = 32.1 Hz), 129.36, 126.61 (q, J = 4.5 Hz), 124.11 (q, J = 272.9 Hz), 31.83, 30.16, 29.60, 29.59, 29.58, 29.57, 29.56, 29.55, 29.29, 29.24, 26.70, 22.62, 14.45. IR (ATR): 635, 717, 744, 790, 842, 892, 1018, 1065, 1094, 1111, 1142, 1161, 1172, 1316, 1330, 1406, 1471, 1489, 1549, 1636, 1655, 2850, 2917, 2955, 3342 cm−1. Elemental analysis for C21H34F3N3O3S (465.58); calculated: C, 54.18; H, 7.36; N, 9.03, found: C, 54.29; H, 7.47; N, 8.92. Rf: 0.56.