The Effects of Hydrogen-Rich Water on Blood Lipid Profiles in Clinical Populations: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Results
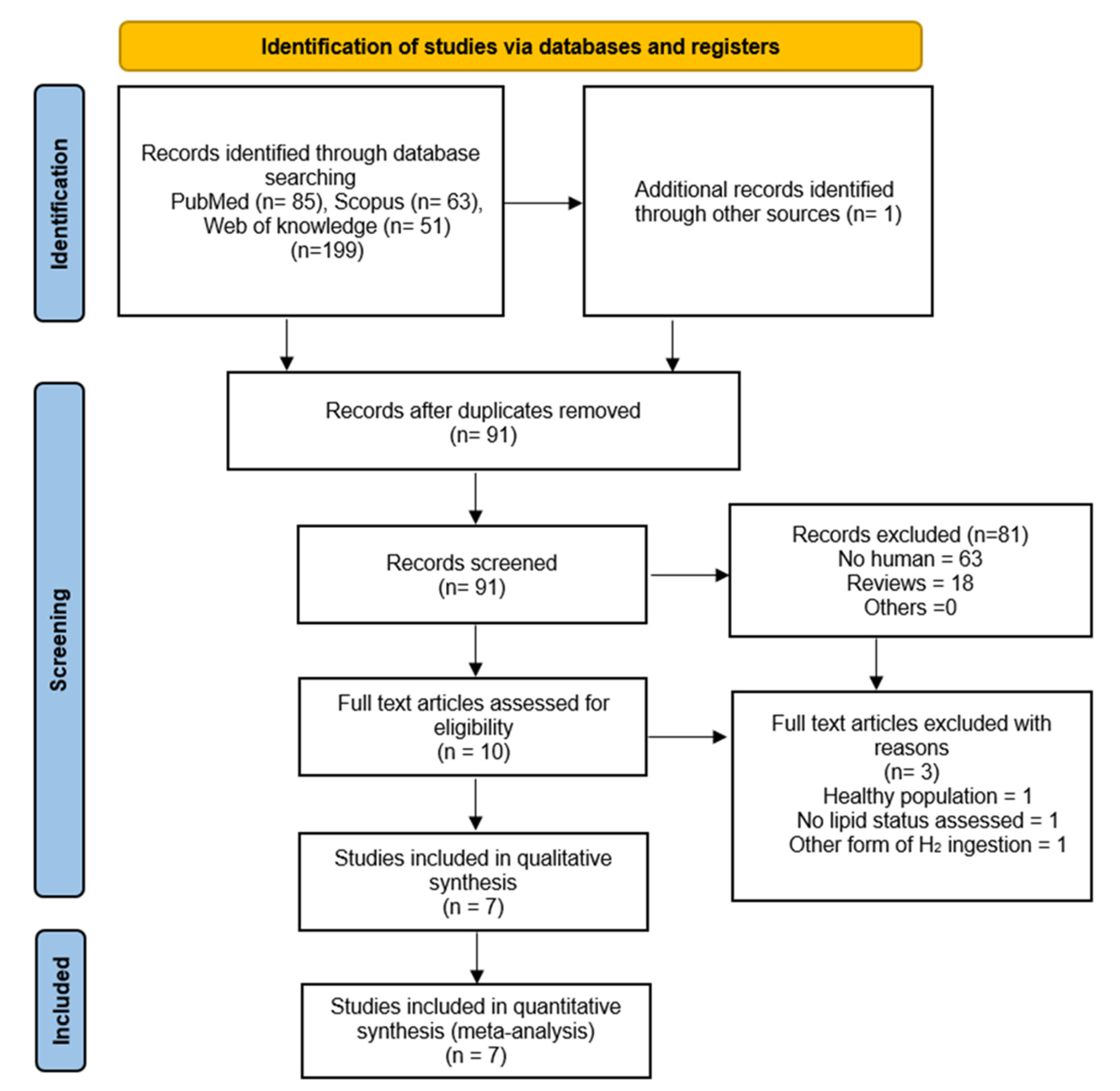
2.1. Literature Search
2.2. Level of the Quality of the Studies
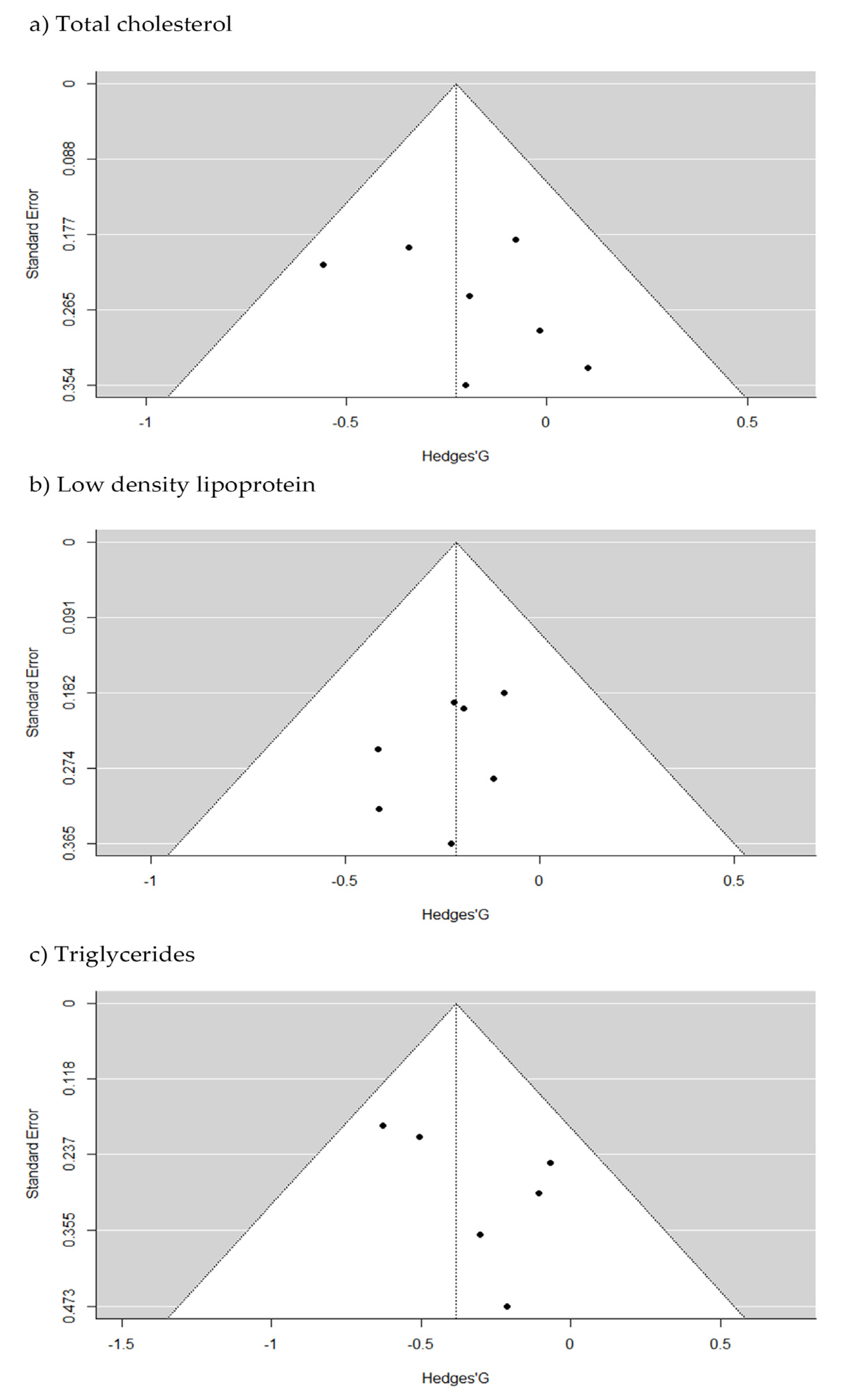
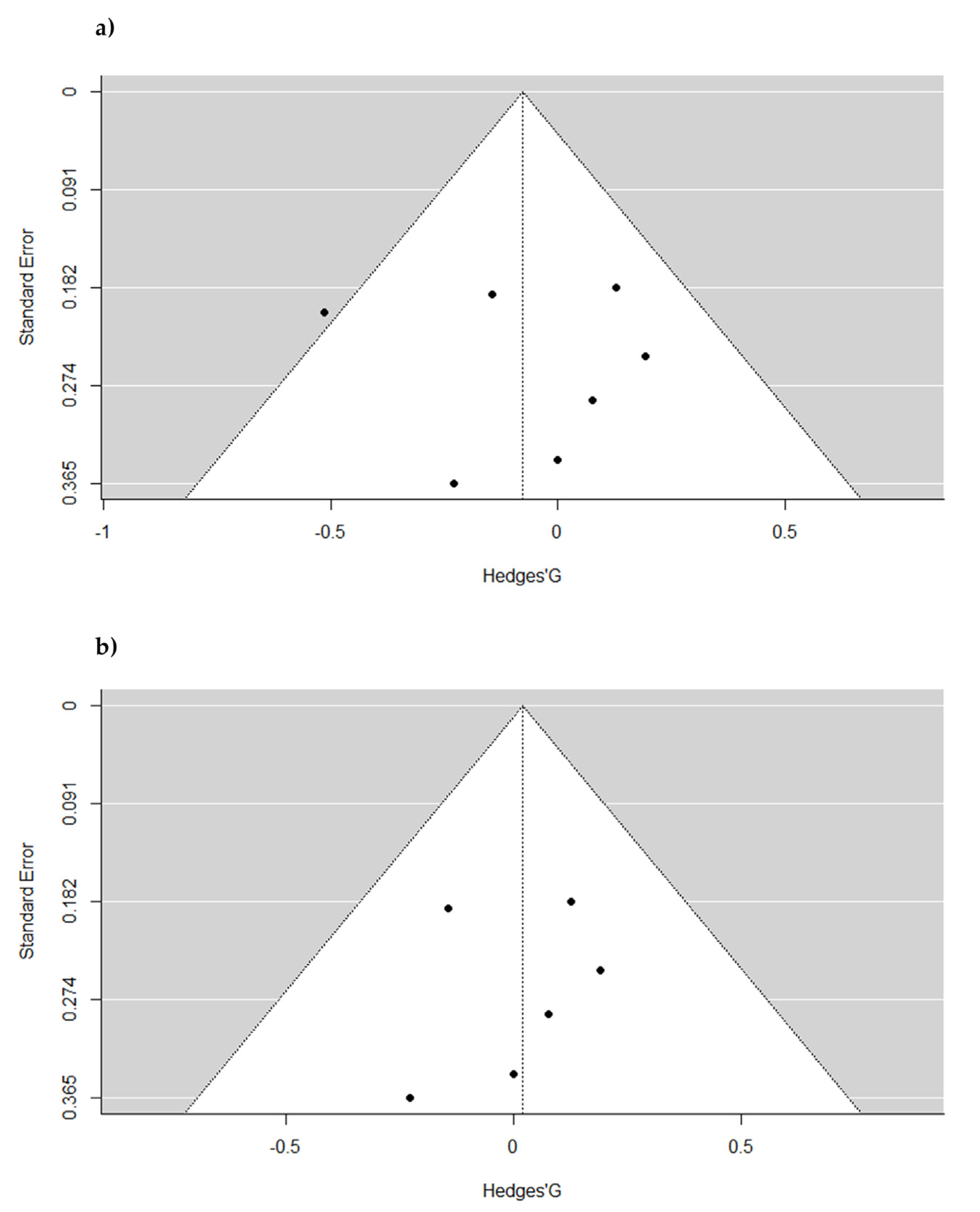
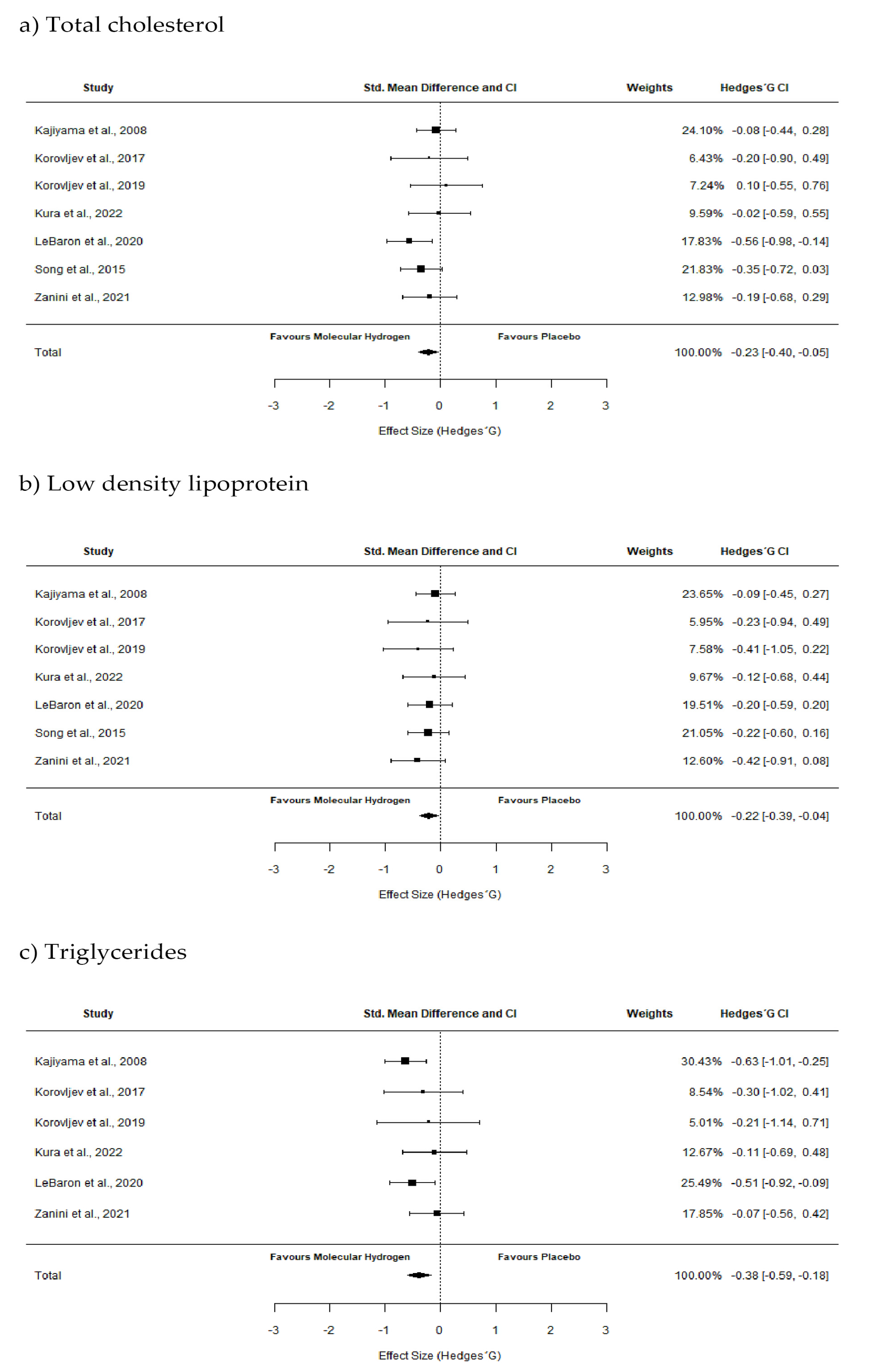
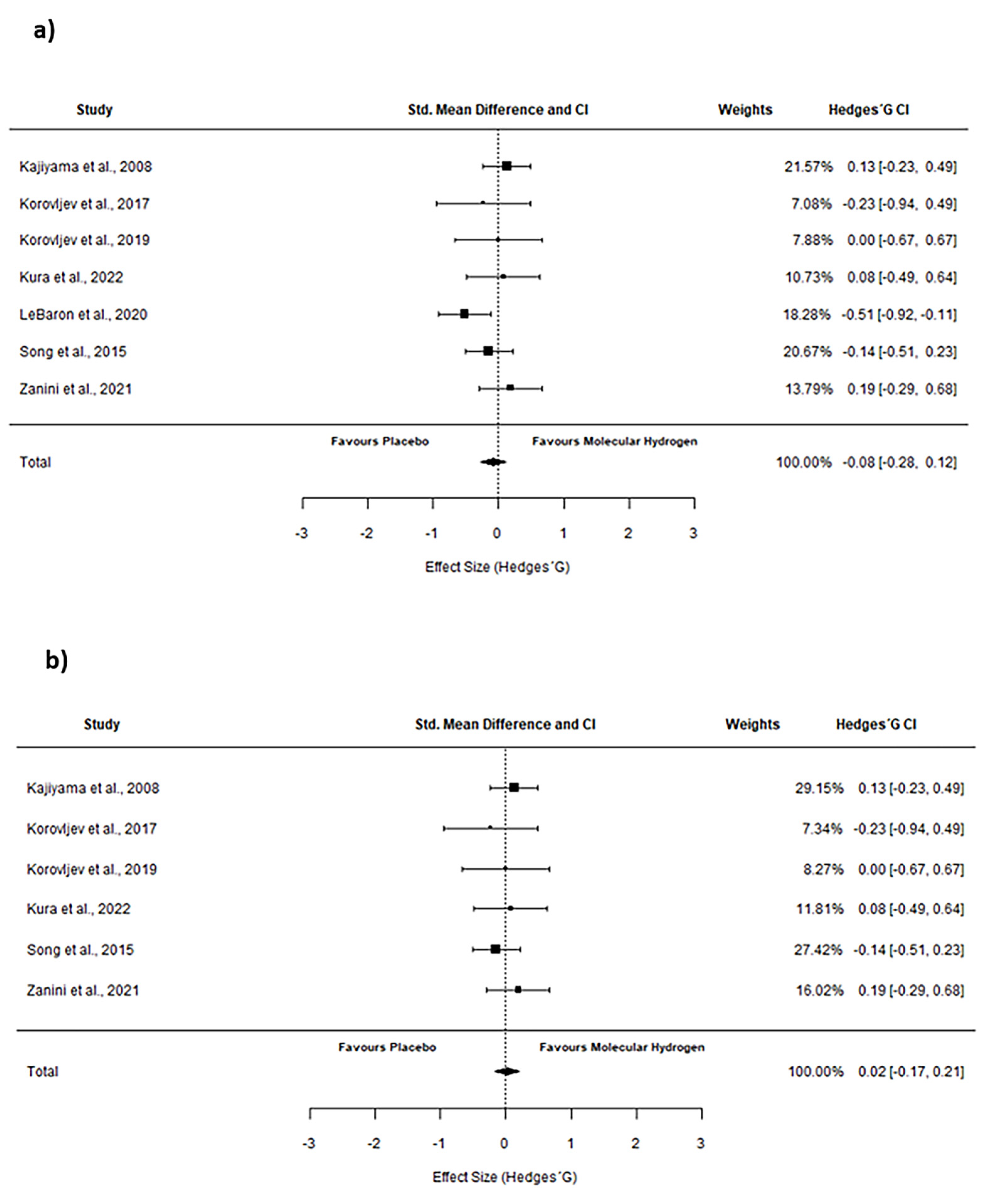
2.3. Pooled Effect Estimate
3. Discussion
4. Materials and Methods
4.1. Inclusion and Exclusion Criteria
4.2. Text Screening
4.3. Data Extraction and Study Coding
4.4. Quality Assessment of Included Studies
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dole, M.; Wilson, F.R.; Fife, W.P. Hyperbaric hydrogen therapy: A possible treatment for cancer. Science 1975, 190, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Molecular hydrogen: An inert gas turns clinically effective. Ann. Med. 2015, 47, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Targeting molecular hydrogen to mitochondria: Barriers and gateways. Pharmacol. Res 2015, 94, 51–53. [Google Scholar] [CrossRef]
- Ishibashi, T. Therapeutic efficacy of molecular hydrogen: A new mechanistic insight. Curr. Pharm. Des. 2019, 25, 946–955. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Kura, B.; Kalocayova, B.; Tribulova, N.; Slezak, J. A new approach for the prevention and treatment of cardiovascular disorders. Molecular hydrogen significantly reduces the effects of oxidative stress. Molecules 2019, 24, 2076. [Google Scholar] [CrossRef]
- Ohta, S. Recent progress toward hydrogen medicine: Potential of molecular hydrogen for preventive and therapeutic applications. Curr. Pharm. Des. 2011, 17, 2241–2252. [Google Scholar] [CrossRef]
- Ostojic, S.M. Serum alkalinization and hydrogen-rich water I healthy men. Mayo Clin. Proc. 2012, 87, 501–502. [Google Scholar] [CrossRef]
- Kamimura, N.; Ichimiya, H.; Iuchi, K.; Ohta, S. Molecular hydrogen stimulates the gene expression of transcriptional coactivator PGC-1α to enhance fatty acid metabolism. Aging Mech. Dis. 2016, 2, 16008. [Google Scholar] [CrossRef]
- Masuda, H.; Sato, A.; Miyata, K.; Shizuno, T.; Oyamada, A.; Ishiwata, K.; Nakagawa, Y.; Asahara, T. Drinking molecular hydrogen water is beneficial to cardiovascular function in diet-induced obesity mice. Biology 2021, 10, 364. [Google Scholar] [CrossRef]
- Ostojic, S.M. Hydrogen-rich water as a dietary activator of brown adipose tissue and UCP1? Ann. Nutr. Metab. 2022, 78, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Korovljev, D.; Trivic, T.; Drid, P.; Ostojic, S.M. Molecular hydrogen affects body composition, metabolic profiles, and mitochondrial function in middle-aged overweight women. Ir. J. Med. Sci. 2018, 187, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Shiiya, T.; Nakazato, M.; Mizuta, M.; Date, Y.; Mondal, M.S.; Tanaka, M.; Nozoe, S.-I.; Hosoda, H.; Kangawa, K.; Matsukura, S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J. Clin. Endocrinol. Metab. 2002, 87, 240–244. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart disease and stroke statistics—2018 update: A report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Klag, M.J.; Ford, D.E.; Mead, L.A.; He, J.; Whelton, P.K.; Liang, K.Y.; Levine, D.M. Serum cholesterol in young men and subsequent cardiovascular disease. N. Engl. J. Med. 1993, 328, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Gowshall, M.; Taylor-Robinson, S.D. The increasing prevalence of non-communicable diseases in low-middle income countries: The view from Malawi. Int. J. Gen. Med. 2018, 11, 255–264. [Google Scholar] [CrossRef]
- Kura, B.; Szantova, M.; LeBaron, T.W.; Mojto, V.; Barancik, M.; Szeiffova Bacova, B.; Kalocayova, B.; Sykora, M.; Okruhlicova, L.; Tribulova, N.; et al. Biological Effects of Hydrogen Water on Subjects with NAFLD: A Randomized, Placebo-Controlled Trial. Antioxidants 2022, 11, 1935. [Google Scholar] [CrossRef]
- Korovljev, D.; Stajer, V.; Ostojic, J.; LeBaron, T.W.; Ostojic, S.M. Hydrogen-rich water reduces liver fat accumulation and improves liver enzyme profiles in patients with non-alcoholic fatty liver disease: A randomized controlled pilot trial. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 688–693. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Singh, R.B.; Fatima, G.; Kartikey, K.; Sharma, J.P.; Ostojic, S.M.; Gvozdjakova, A.; Kura, B.; Noda, M.; Mojto, V.; et al. The effects of 24-week, high-concentration hydrogen-rich water on body composition, blood lipid profiles and inflammation biomarkers in men and women with metabolic syndrome: A randomized controlled trial. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 889–896. [Google Scholar] [CrossRef]
- Zanini, D.; Todorovic, N.; Korovljev, D.; Stajer, V.; Ostojic, J.; Purac, J.; Kojic, D.; Vukasinovic, E.; Djordjievski, S.; Sopic, M.; et al. The effects of 6-month hydrogen-rich water intake on molecular and phenotypic biomarkers of aging in older adults aged 70 years and over: A randomized controlled pilot trial. Exp. Gerontol. 2021, 155, 111574. [Google Scholar] [CrossRef]
- Song, G.; Lin, Q.; Zhao, H.; Liu, M.; Ye, F.; Sun, Y.; Yu, Y.; Guo, S.; Jiao, P.; Wu, Y.; et al. Hydrogen activates ATP-binding cassette transporter A1-dependent efflux ex vivo and improves high-density lipoprotein function in patients with hypercholesterolemia: A double-blinded, randomized, and placebo-controlled trial. J. Clin. Endocrinol. Metab. 2015, 100, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Kitawaki, J.; Imai, S.; Nakano, K.; Ohta, M.; et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Trexler, E.T.; Persky, A.M.; Ryan, E.D.; Schwartz, T.A.; Stoner, L.; Smith-Ryan, A.E. Acute effects of citrulline supplementation on high-intensity strength and power performance: A systematic review and meta-analysis. Sports Med. 2019, 49, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Ichihara, G.; Katsumata, Y.; Moriyama, H.; Kitakata, H.; Hirai, A.; Momoi, M.; Ko, S.; Shinya, Y.; Kinouchi, K.; Kobayashi, E.; et al. Pharmacokinetics of hydrogen after ingesting a hydrogen-rich solution: A study in pigs. Heliyon 2021, 7, e08359. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.N.; Clark, J.P.; Anderson, R.M. Mitochondrial regulator PGC-1a—Modulating the modulator. Curr. Opin. Endocr. Metab. Res. 2019, 5, 37–44. [Google Scholar] [CrossRef]
- Kamimura, N.; Nishimaki, K.; Ohsawa, I.; Ohta, S. Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in db/db mice. Obesity 2011, 19, 1396–1403. [Google Scholar] [CrossRef]
- Yan, J.; Nie, Y.; Cao, J.; Luo, M.; Yan, M.; Chen, Z.; He, B. The roles and pharmacological effects of FGF21 in preventing aging-associated metabolic diseases. Front. Cardiovasc. Med. 2021, 8, 655575. [Google Scholar] [CrossRef]
- Ostojic, S.M. Does H2 alter mitochondrial bioenergetics via GHS-R1α activation? Theranostics 2017, 7, 1330–1332. [Google Scholar] [CrossRef]
- Martins, A.D.; Sá, R.; Monteiro, M.P.; Barros, A.; Sousa, M.; Carvalho, R.A.; Silva, B.M.; Oliveira, P.F.; Alves, M.G. Ghrelin acts as energy status sensor of male reproduction by modulating Sertoli cells glycolytic metabolism and mitochondrial bioenergetics. Mol. Cell. Endocrinol. 2016, 434, 199–209. [Google Scholar] [CrossRef]
- Blüher, M. Adipose tissue dysfunction in obesity. Exp. Clin. Endocrinol. Diabetes 2009, 117, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Paulis, M.G.; Hassan, O.A.; Abbass, M.F.; Mohammad, M.A.A.H. Structural and lipid peroxidation effects of lead on rat hippocampus and its attenuation by hydrogen rich water. J. Chem. Neuroanat. 2018, 91, 55–62. [Google Scholar] [CrossRef]
- Sun, X.; Ohta, S.; Nakao, A. (Eds.) Hydrogen Molecular Biology and Medicine; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- LeLorier, J.; Gregoire, G.; Benhaddad, A.; Lapierre, J.; Derderian, F. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N. Engl. J. Med. 1997, 337, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Peacock, R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: Audit of primary sources. BMJ 2005, 331, 1064–1065. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: New York, NY, USA, 2021. [Google Scholar]
- Cooper, H.; Hedges, L.V.; Valentine, J.C. The handbook of Research Synthesis and Meta-Analysis; Russell Sage Foundation: New York, NY, USA, 2019. [Google Scholar]
- De Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef]
- Cohen, J. Quantitative methods in psychology: A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Hedges, L.V. Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
| Study | Population | Intervention | Variables Analyzed | Outcomes |
|---|---|---|---|---|
| Kajiyama et al., 2008 [22] | 36 subjects with T2DM (58.6 ± 4.7 y) | Study type: Randomized, double-blind, placebo-controlled crossover study Supplementation protocol: 900 mL/d of HRW (H2 concentration = 12 ppm) or placebo water Duration: 8 weeks | TC LDL sdLDL HDL TG | ↔ ↔ ↓ ↑ ↔ |
| Song et al., 2015 [21] | 68 subjects (35–60 years old) with hypercholesterolemia | Study type: Randomized, double-blind, placebo-controlled study Supplementation protocol: 0.9 L/d (0.3 L 3 times/d) of HRW (H2 concentration ≈ 0.6 ppm) or placebo Duration: 12 weeks | TC LDL HDL | ↓ ↓ ↔ |
| Korovljev et al., 2018 [12] | 10 middle-aged overweight women (56.4 ± 12.6 y) | Study type: Randomized, double-blind, placebo-controlled crossover study Supplementation protocol: blend of hydrogen generating minerals (46 mg of calcium and 40 mg of magnesium) (H2 concentration ≈ 6 ppm) or placebo (cellulose) dissolved in water Duration: 4 weeks | TC LDL HDL TG | ↔ ↔ ↔ ↓ |
| Korovljev et al., 2019 [18] | 12 subjects with NAFLD (56.2 ± 10 y) | Study type: Randomized, double-blind, placebo-controlled study Supplementation protocol: 4 doses daily of 250 mL HRW (H2 concentratio = 4.76 ppm) or placebo water Duration: 4 weeks | TC LDL HDL TG | ↔ ↓ ↔ ↔ |
| LeBaron et al., 2020 [19] | 60 subjects with metabolic syndrome(43.4 ± 9.2 y) | Study type: Randomized, double-blind, placebo-controlled study Supplementation protocol: 3 doses daily of 250 mL HRW (H2 concentration > 5.5 ppm) or placebo water Duration: 24 weeks | TC LDL VLDL HDL TG | ↓ ↓ ↓ ↔ ↓ |
| Zanini et al., 2021 [20] | 40 elderly man and women (76 ± 5.6 y) | Study type: Randomized, double-blind, placebo-controlled study Supplementation protocol: 500 mL/d of HRW (H2 concentratio = 15 ppm) or Placebo water Duration: 24 weeks | TC LDL HDL TG | ↔ ↓ ↔ ↔ |
| Kura et al., 2022 [17] | 30 subjects with NAFLD (53.2 ± 9.1 y) | Study type: Randomized, double-blind, placebo-controlled study Supplementation protocol: 3 doses daily of 330 mL HRW (H2 concentratio > 4 ppm) or placebo water Duration: 8 weeks | TC LDL HDL TG | ↔ ↔ ↔ ↔ |
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kajiyama et al., 2008 | Yes | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Song et al., 2015 | Yes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Korovljev et al., 2018 | Yes | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Korovljev et al., 2019 | Yes | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| LeBaron et al., 2020 | Yes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Zanini et al., 2021 | Yes | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Kura et al., 2022 | Yes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorovic, N.; Fernández-Landa, J.; Santibañez, A.; Kura, B.; Stajer, V.; Korovljev, D.; Ostojic, S.M. The Effects of Hydrogen-Rich Water on Blood Lipid Profiles in Clinical Populations: A Systematic Review and Meta-Analysis. Pharmaceuticals 2023, 16, 142. https://doi.org/10.3390/ph16020142
Todorovic N, Fernández-Landa J, Santibañez A, Kura B, Stajer V, Korovljev D, Ostojic SM. The Effects of Hydrogen-Rich Water on Blood Lipid Profiles in Clinical Populations: A Systematic Review and Meta-Analysis. Pharmaceuticals. 2023; 16(2):142. https://doi.org/10.3390/ph16020142
Chicago/Turabian StyleTodorovic, Nikola, Julen Fernández-Landa, Asier Santibañez, Branislav Kura, Valdemar Stajer, Darinka Korovljev, and Sergej M. Ostojic. 2023. "The Effects of Hydrogen-Rich Water on Blood Lipid Profiles in Clinical Populations: A Systematic Review and Meta-Analysis" Pharmaceuticals 16, no. 2: 142. https://doi.org/10.3390/ph16020142
APA StyleTodorovic, N., Fernández-Landa, J., Santibañez, A., Kura, B., Stajer, V., Korovljev, D., & Ostojic, S. M. (2023). The Effects of Hydrogen-Rich Water on Blood Lipid Profiles in Clinical Populations: A Systematic Review and Meta-Analysis. Pharmaceuticals, 16(2), 142. https://doi.org/10.3390/ph16020142








