Cichorium intybus L. Hairy Roots as a Platform for Antimicrobial Activity
Abstract
1. Introduction
2. Results
2.1. VL-70 Hairy Roots
2.2. K1793 Hairy Roots
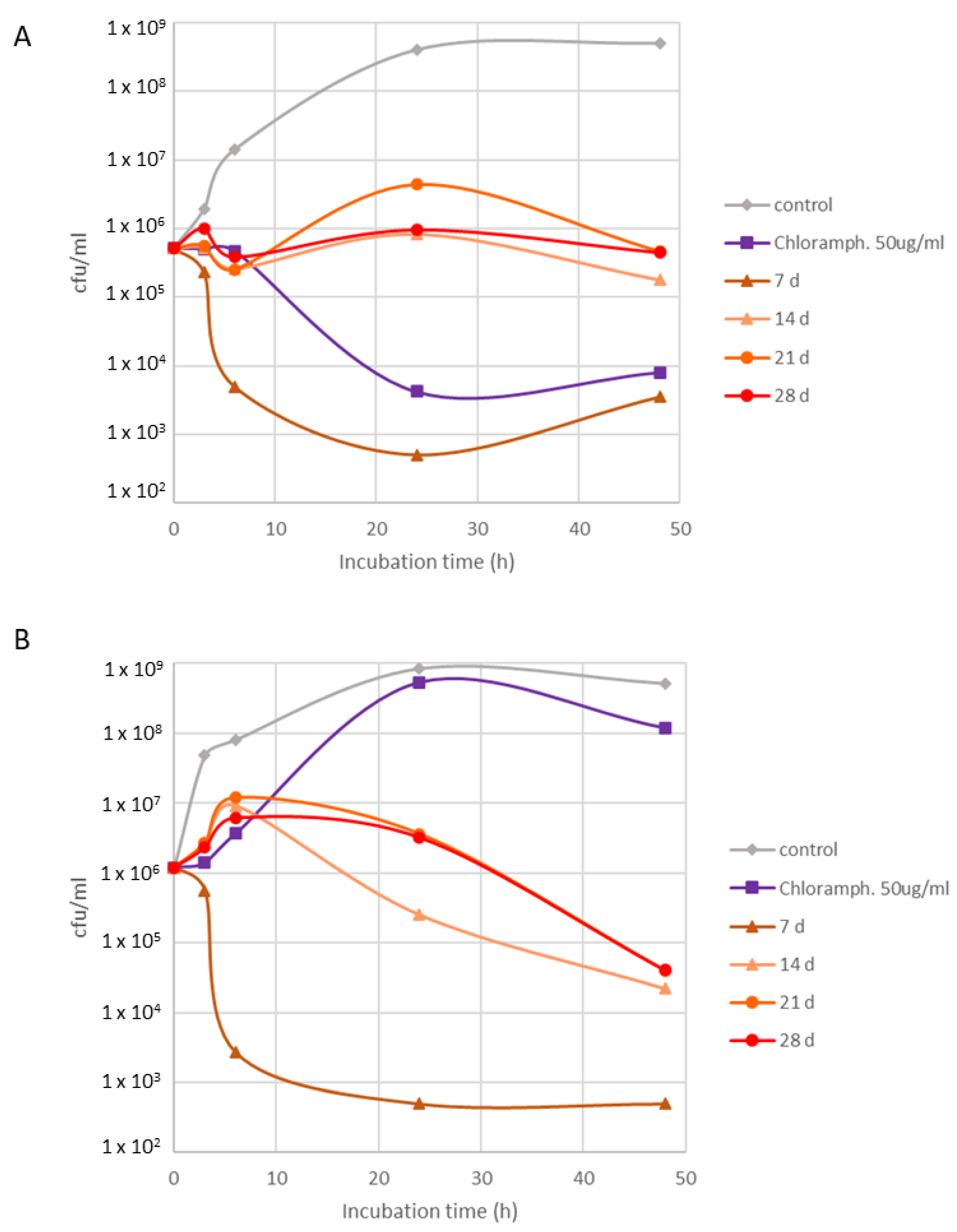
2.3. Bioactivity Assessment
3. Discussion
4. Materials and Methods
4.1. Hairy Root Induction and Maintenance and Chicory Taproots
4.2. Metabolite Analysis
4.3. Antimicrobial Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Arkel, J.; Vergauwen, R.; Sévenier, R.; Hakkert, J.C.; van Laere, A.; Bouwmeester, H.J.; Koops, A.J.; van der Meer, I.M. Sink Filling, Inulin Metabolizing Enzymes and Carbohydrate Status in Field Grown Chicory (Cichorium Intybus L.). J. Plant Physiol. 2012, 169, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Sessa, R.A.; Bennett, M.H.; Lewis, M.J.; Mansfield, J.W.; Beale, M.H. Metabolite Profiling of Sesquiterpene Lactones from Lactuca Species: Major Latexcomponents Are Novel Oxalate and Sulfate Conjugates of Lactucin and Its Derivatives. J. Biol. Chem. 2000, 275, 26877–26884. [Google Scholar] [CrossRef] [PubMed]
- van Beek, T.A.; Maas, P.; King, B.M.; Leclercq, E.; Voragen, A.G.J.; de Groot, A. Bitter Sesquiterpene Lactones from Chicory Roots. J. Agric. Food Chem. 1990, 38, 1035–1038. [Google Scholar] [CrossRef]
- Malarz, J.; Stojakowska, A.; Kisiel, W. Sesquiterpene Lactones in a Hairy Root Culture of Cichorium Intybus. Z. Für Nat. C 2002, 57, 994–997. [Google Scholar] [CrossRef] [PubMed]
- van Arkel, J.; Twarogowska, A.; Cornelis, Y.; de Marez, T.; Engel, J.; Maenhout, P.; de Vos, R.C.H.; Beekwilder, J.; van Droogenbroeck, B.; Cankar, K. Effect of Root Storage and Forcing on the Carbohydrate and Secondary Metabolite Composition of Belgian Endive (Cichorium Intybus L. Var. Foliosum). ACS Food Sci. Technol. 2022, 2, 1546–1557. [Google Scholar] [CrossRef]
- Häkkinen, S.T.; Soković, M.; Nohynek, L.; Ćirić, A.; Ivanov, M.; Stojković, D.; Tsitko, I.; Matos, M.; Baixinho, J.P.; Ivasiv, V.; et al. Chicory Extracts and Sesquiterpene Lactones Show Potent Activity against Bacterial and Fungal Pathogens. Pharmaceuticals 2021, 14, 941. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Ludwig-Müller, J.; Bley, T. Hairy Root Culture: Copying Nature in New Bioprocesses Copying Nature: Transformation with Agrobacterium Rhizogenes. ©CAB Int. Med. Plant Biotechnol. 2010, 156-175, 156–175. [Google Scholar]
- Sevón, N.; Oksman-Caldentey, K.M. Agrobacterium Rhizogenes-Mediated Transformation: Root Cultures as a Source of Alkaloids. Planta Med.. 2002, 68, 859–868. [Google Scholar] [CrossRef]
- Häkkinen, S.T.; Oksman-Caldentey, K.-M. Progress and Prospects of Hairy Root Research. In Hairy Roots: An Effective Tool of Plant Biotechnology; Srivastava, V., Mehrotra, S., Mishra, S., Eds.; Springer Singapore: Singapore, 2018; pp. 3–19. ISBN 978-981-13-2562-5. [Google Scholar]
- Gutierrez-Valdes, N.; Häkkinen, S.T.; Lemasson, C.; Guillet, M.; Oksman-Caldentey, K.-M.; Ritala, A.; Cardon, F. Hairy Root Cultures—A Versatile Tool With Multiple Applications. Front. Plant Sci. 2020, 11, 33. [Google Scholar] [CrossRef]
- Ritala, A.; Dong, L.; Imseng, N.; Seppänen-Laakso, T.; Vasilev, N.; van der Krol, S.; Rischer, H.; Maaheimo, H.; Virkki, A.; Brändli, J.; et al. Evaluation of Tobacco (Nicotiana Tabacum L. Cv. Petit Havana SR1) Hairy Roots for the Production of Geraniol, the First Committed Step in Terpenoid Indole Alkaloid Pathway. J. Biotechnol. 2014, 176, 20–28. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Eibl, R.; Zhong, J.-J. Hosting the Plant Cells in Vitro: Recent Trends in Bioreactors. Appl. Microbiol. Biotechnol. 2013, 97, 3787–3800. [Google Scholar] [CrossRef]
- Matos, M.S.; Anastácio, J.D.; Allwood, J.W.; Carregosa, D.; Marques, D.; Sungurtas, J.; McDougall, G.J.; Menezes, R.; Matias, A.A.; Stewart, D.; et al. Assessing the Intestinal Permeability and Anti-Inflammatory Potential of Sesquiterpene Lactones from Chicory. Nutrients 2020, 12, 3547. [Google Scholar] [CrossRef]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.A.; Darwiche, N. What Made Sesquiterpene Lactones Reach Cancer Clinical Trials? Drug Discov. Today 2010, 15, 668–678. [Google Scholar] [CrossRef]
- Häkkinen, S.T.; Cankar, K.; Nohynek, L.; Suomalainen, M.; van Arkel, J.; Siika-Aho, M.; Twarogowska, A.; van Droogenbroeck, B.; Oksman-Caldentey, K.-M. Enzyme-Treated Chicory for Cosmetics: Application Assessment and Techno-Economic Analysis. AMB Express 2022, 12, 152. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Suvanto, J.; Salminen, J.-P.; Seppänen-Laakso, T.; Tähtiharju, J.; Honkapää, K.; Oksman-Caldentey, K.-M. Natural Antimicrobials from Cloudberry (Rubus Chamaemorus) Seeds by Sanding and Hydrothermal Extraction. ACS Food Sci. Technol. 2021, 1, 917–927. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Xu, Y.; Zhang, B.; Xia, X. Antimicrobial Effect and Mode of Action of Chlorogenic Acid on Staphylococcus Aureus. Eur. Food Res. Technol. 2014, 238, 589–596. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Cankar, K.; Bundock, P.; Sevenier, R.; Häkkinen, S.T.; Hakkert, J.C.; Beekwilder, J.; Meer, I.M.; Both, M.; Bosch, D. Inactivation of the Germacrene A Synthase Genes by CRISPR/Cas9 Eliminates the Biosynthesis of Sesquiterpene Lactones in Cichorium Intybus L. Plant Biotechnol. J. 2021. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Morgan, A.J.; Cox, P.N.; Turner, D.A.; Peel, E.; Davey, M.R.; Gartland, K.M.A.; Mulligan, B.J. Transformation of Tomato Using an Ri Plasmid Vector. Plant Sci. 1987, 49, 37–49. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Murashige, T.; Thorpe, T.A.; Vasil, I.K. Plant Tissue Culture Media. In Vitro 1976, 12, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.T.; Moyano, E.; Cusidó, R.M.; Oksman-Caldentey, K.-M. Exploring the Metabolic Stability of Engineered Hairy Roots after 16 Years Maintenance. Front. Plant Sci. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- de Vos, R.C.H.; Moco, S.; Lommen, A.; Keurentjes, J.J.B.; Bino, R.J.; Hall, R.D. Untargeted Large-Scale Plant Metabolomics Using Liquid Chromatography Coupled to Mass Spectrometry. Nat. Protoc. 2007, 2, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, L.J.; Alakomi, H.L.; Kähkönen, M.P.; Heinonen, M.; Helander, I.M.; Oksman-Caldentey, K.M.; Puupponen-Pimiä, R.H. Berry Phenolics: Antimicrobial Properties and Mechanisms of Action against Severe Human Pathogens. Nutr. Cancer 2006, 54, 18–32. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Juvonen, R.; Kössö, T.; Truchado, P.; Westerlund-Wikström, B.; Leppänen, T.; Moilanen, E.; Oksman-Caldentey, K.-M. Fermentation and Dry Fractionation Increase Bioactivity of Cloudberry (Rubus Chamaemorus). Food Chem. 2016, 197, 950–958. [Google Scholar] [CrossRef]
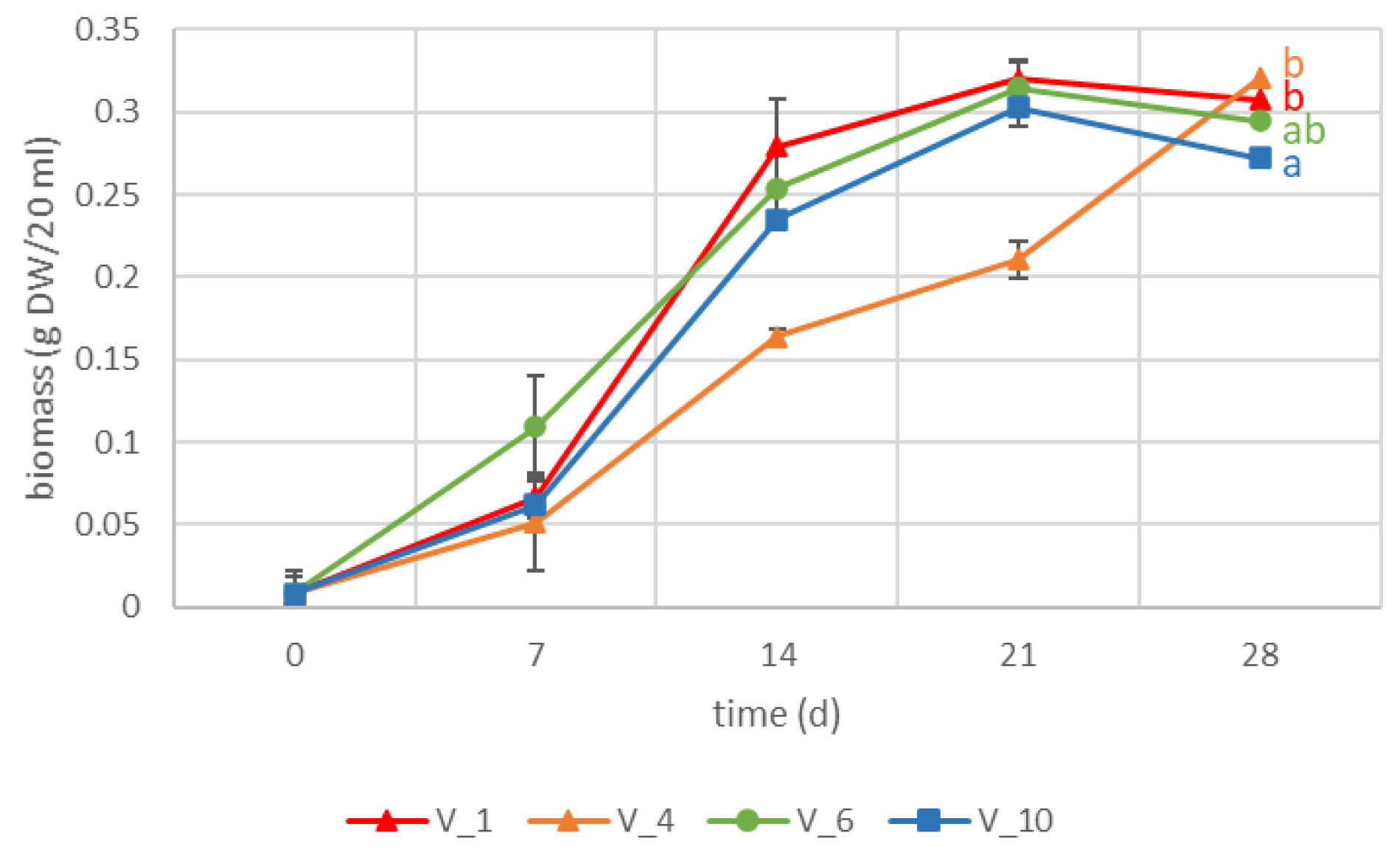
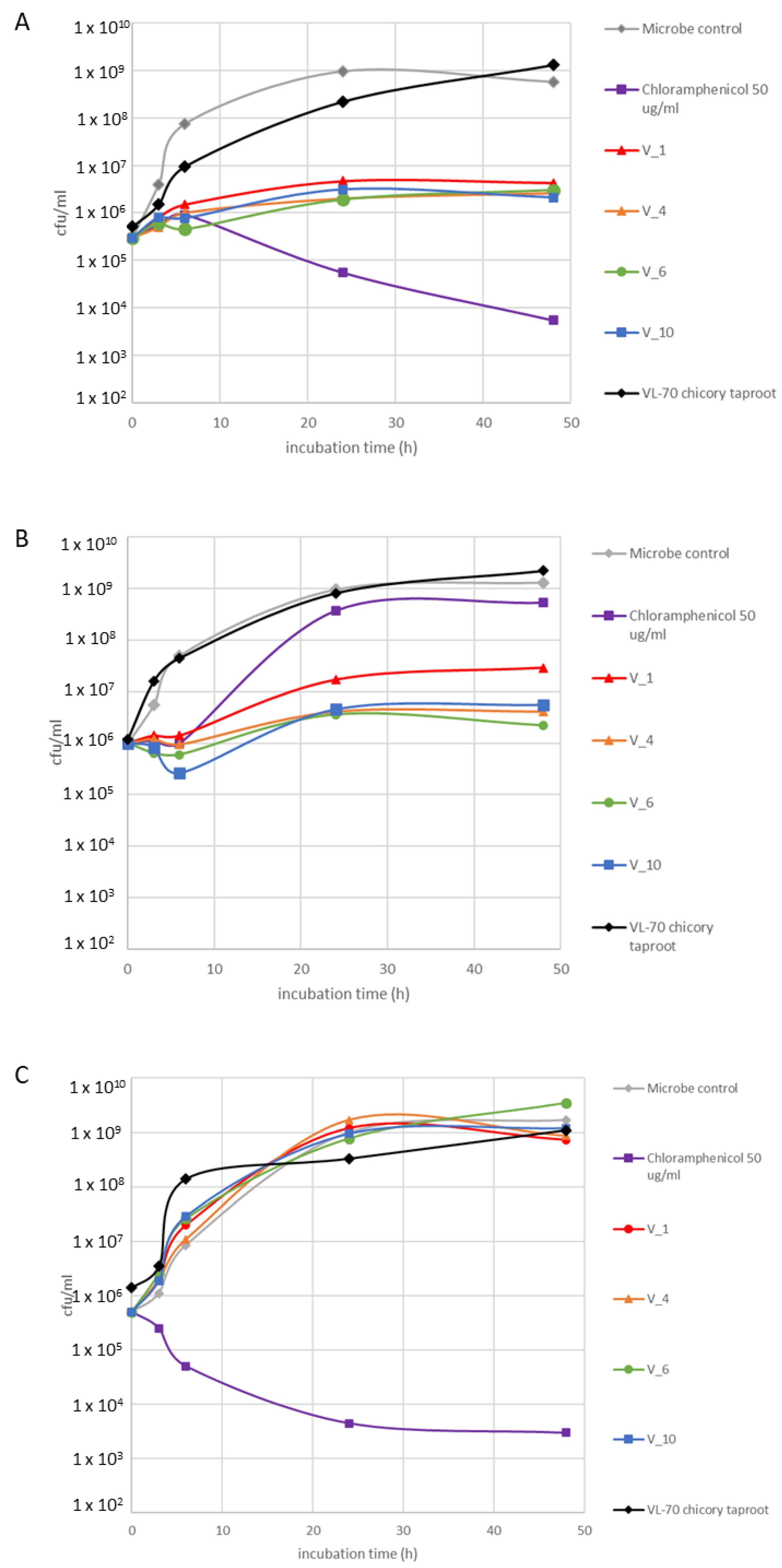
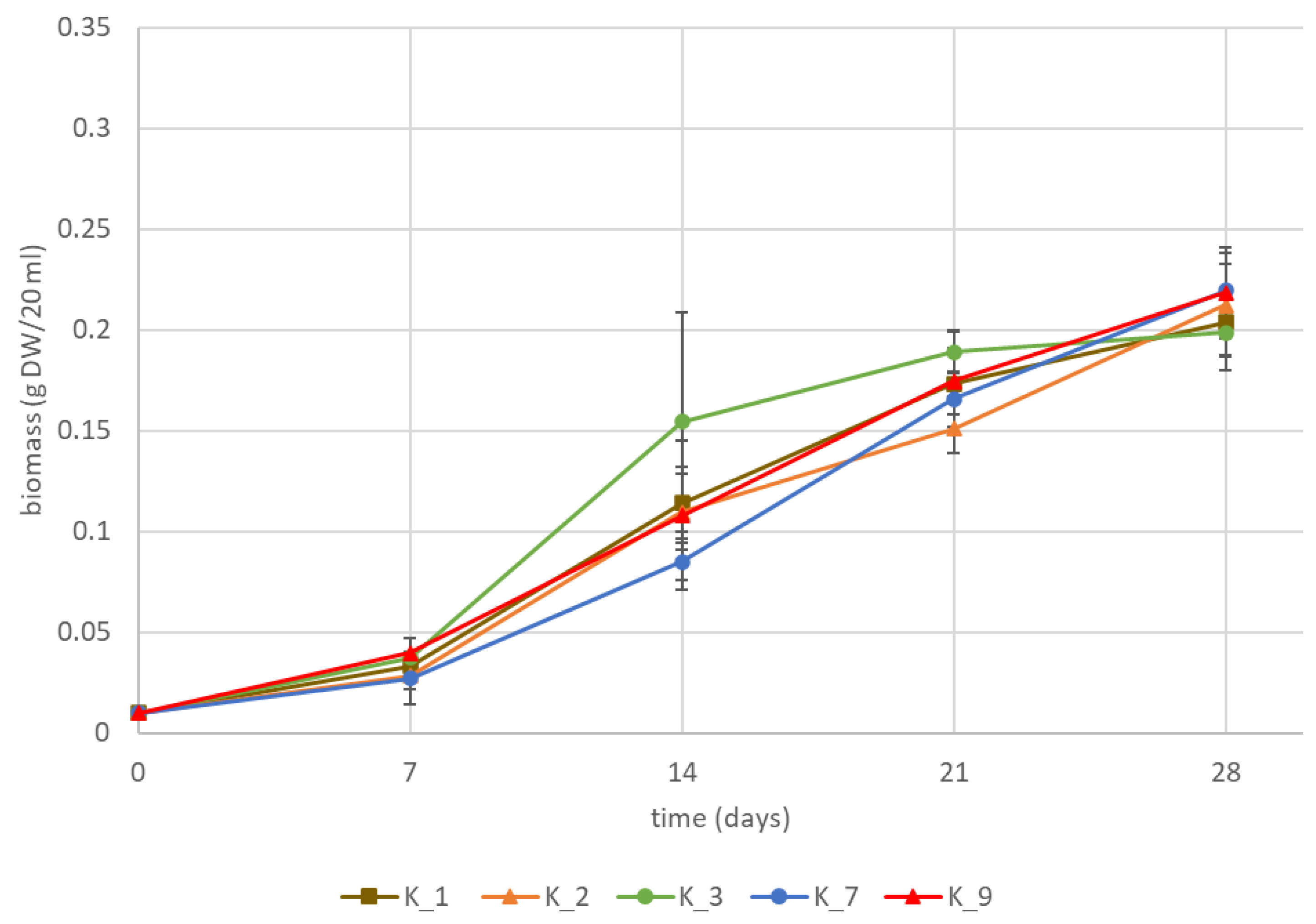
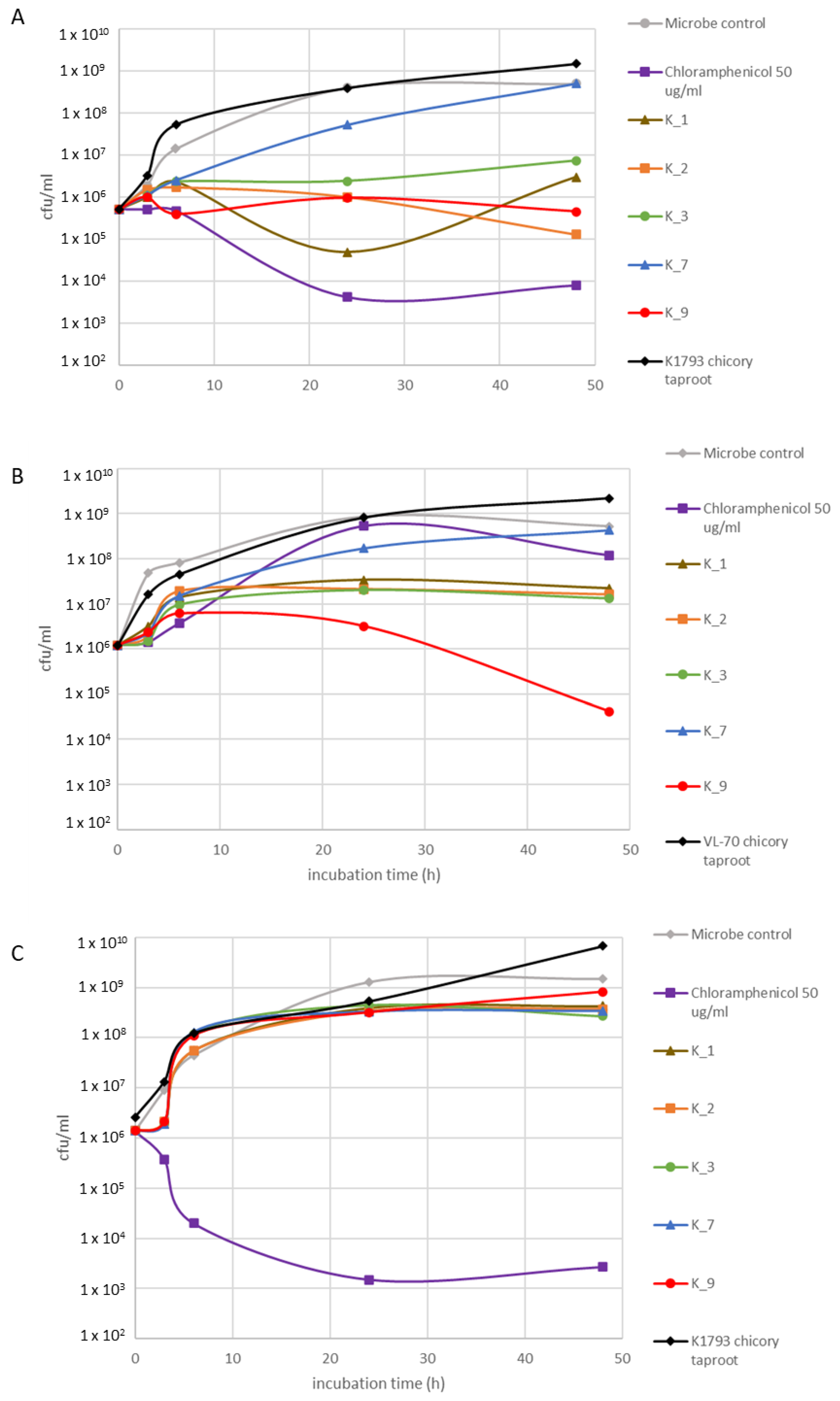
| Hairy Root Line | Chlorogenic Acid (mg/g DW) | Isochlorogenic Acid A (mg/g DW) | Chicoric Acid (mg/g DW) | Lactucin (mg/g DW) | 8-Deoxylactucin (AR) | Lactucopicrin (mg/g DW) | Lactucin 15-Oxalate (AR) | 8-Deoxylactucin 15-Oxalate (AR) | Lactucopicrin 15-Oxalate (AR) |
|---|---|---|---|---|---|---|---|---|---|
| V_1 | 5.95 ± 0.71 ab | 17.61 ± 2.12 ab | 0.38 ± 0.03 a | 0.04 ± 0.02 ab | 12.41 ± 2.73 ab | 0.090 ± 0.045 a | 8.72 ± 1.75 bc | 17.28 ± 5.00 cd | 43.30 ± 8.89 c |
| V_3 | 7.58 ± 1.73 ab | 20.11 ± 4.42 ab | 0.80 ± 0.20 ab | 0.02 ± 0.01 ab | 19.53 ± 2.24 bc | 0.089 ± 0.028 a | 3.75 ± 0.35 a | 11.48 ± 1.30 bc | 32.26 ± 5.73 bc |
| V_4 | 5.18 ± 1.10 a | 14.24 ± 1.70 a | 0.38 ± 0.05 a | 0.04 ± 0.00 b | 11.18 ± 0.62 ab | 0.060 ± 0.010 a | 11.47 ± 1.08 c | 20.18 ± 0.89 d | 34.73 ± 0.81 bc |
| V_6 | 9.25 ± 1.83 abc | 21.09 ± 4.01 ab | 0.83 ± 0.23 ab | 0.02 ± 0.01 ab | 22.14 ± 5.27 c | 0.084 ± 0.030 a | 1.77 ± 0.78 a | 3.81 ± 1.27 ab | 20.86 ± 5.98 ab |
| V_8 | 6.69 ± 1.48 ab | 19.86 ± 1.86 ab | 0.39 ± 0.07 a | 0.03 ± 0.01 ab | 10.04 ± 1.01 a | 0.073 ± 0.014 a | 8.93 ± 2.38 bc | 20.32 ± 3.86 d | 43.84 ± 4.41 c |
| V_10 | 13.61 ± 1.95 c | 28.77 ± 5.54 b | 1.04 ± 0.14 b | 0.01 ± 0.00 a | 9.78 ± 0.49 a | 0.029 ± 0.009 a | 2.75 ± 0.69 a | 2.05 ± 0.29 a | 14.11 ± 1.23 a |
| V_15 | 10.61 ± 0.38 bc | 25.96 ± 0.86 b | 0.55 ± 0.06 a | 0.02 ± 0.00 ab | 14.60 ± 0.75 abc | 0.063 ± 0.012 a | 5.86 ± 0.13 ab | 11.75 ± 0.51 bcd | 43.18 ± 3.22 c |
| Hairy Root Line | Chlorogenic Acid (mg/g DW) | Isochlorogenic Acid A (mg/g DW) | Chicoric Acid (mg/g DW) | Lactucin (mg/g DW) | 8-Deoxylactucin (AR) | Lactucopicrin (mg/g DW) | Lactucin 15-Oxalate (AR) | 8-Deoxylactucin 15-Oxalate (AR) | Lactucopicrin 15-Oxalate (AR) |
|---|---|---|---|---|---|---|---|---|---|
| K_1 | 2.31 ± 0.08 b | 6.04 ± 0.05 b | 0.71 ± 0.04 b | 0.01 ± 0.00 a | 2.69 ± 0.74 a | 0.008 ± 0.001 a | 7.71 ± 1.00 a | 4.62 ± 0.51 a | 17.91 ± 1.64 a |
| K_2 | 2.09 ± 0.24 ab | 5.88 ± 0.23 b | 0.51 ± 0.11 a | 0.01 ± 0.00 a | 1.53 ± 0.12 a | 0.011 ± 0.002 a | 5.97 ± 0.36 a | 3.49 ± 0.15 a | 16.38 ± 0.70 a |
| K_3 | 2.29 ± 0.12 ab | 6.24 ± 0.14 ab | 0.66 ± 0.04 ab | 0.01 ± 0.00 a | 1.92 ± 0.15 a | 0.012 ± 0.000 ab | 8.75 ± 1.93 a | 3.88 ± 0.07 a | 18.23 ± 0.72 ab |
| K_7 | 2.27 ± 0.05 ab | 5.89 ± 0.08 ab | 0.97 ± 0.04 c | 0.02 ± 0.00 c | 6.22 ± 0.95 b | 0.016 ± 0.001 bc | 27.11 ± 1.22 b | 7.68 ± 0.74 b | 22.85 ± 0.25 b |
| K_9 | 1.87 ± 0.08 a | 5.50 ± 0.05 a | 0.51 ± 0.04 a | 0.01 ± 0.00 b | 2.29 ± 0.38 a | 0.018 ± 0.002 c | 7.74 ± 0.36 a | 4.40 ± 0.50 a | 16.70 ± 2.54 a |
| Plant Line | Chlorogenic Acid (mg/g DW) | Isochlorogenic Acid A (mg/g DW) | Chicoric Acid (mg/g DW) | Lactucin (mg/g DW) | 8-Deoxylactucin (AR) | Lactucopicrin (mg/g DW) | Lactucin 15-Oxalate (AR) | 8-Deoxylactucin 15-Oxalate (AR) | Lactucopicrin 15-Oxalate (AR) |
|---|---|---|---|---|---|---|---|---|---|
| VL-70 | 0.89 ± 0.09 a | 1.05 ± 0.23 a | 0.45 ± 0.08 b | 0.02 ± 0.00 a | 11.57 ± 4.46 a | 0.013 ± 0.003 a | 0.75 ± 0.25 a | 7.17 ± 0.93 a | 18.8 ± 7.61 a |
| K1793 | 0.58 ± 0.04 a | 17.24 ± 4.63 b | 0.05 ± 0.00 a | 0.20 ± 0.00 b | 38.49 ± 4.65 b | 0.199 ± 0.015 b | 157.48 ± 10.38 b | 83.65 ± 10 b | 197 ± 5.92 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Häkkinen, S.T.; Cankar, K.; Nohynek, L.; van Arkel, J.; Laurel, M.; Oksman-Caldentey, K.-M.; Van Droogenbroeck, B. Cichorium intybus L. Hairy Roots as a Platform for Antimicrobial Activity. Pharmaceuticals 2023, 16, 140. https://doi.org/10.3390/ph16020140
Häkkinen ST, Cankar K, Nohynek L, van Arkel J, Laurel M, Oksman-Caldentey K-M, Van Droogenbroeck B. Cichorium intybus L. Hairy Roots as a Platform for Antimicrobial Activity. Pharmaceuticals. 2023; 16(2):140. https://doi.org/10.3390/ph16020140
Chicago/Turabian StyleHäkkinen, Suvi T., Katarina Cankar, Liisa Nohynek, Jeroen van Arkel, Markus Laurel, Kirsi-Marja Oksman-Caldentey, and Bart Van Droogenbroeck. 2023. "Cichorium intybus L. Hairy Roots as a Platform for Antimicrobial Activity" Pharmaceuticals 16, no. 2: 140. https://doi.org/10.3390/ph16020140
APA StyleHäkkinen, S. T., Cankar, K., Nohynek, L., van Arkel, J., Laurel, M., Oksman-Caldentey, K.-M., & Van Droogenbroeck, B. (2023). Cichorium intybus L. Hairy Roots as a Platform for Antimicrobial Activity. Pharmaceuticals, 16(2), 140. https://doi.org/10.3390/ph16020140








