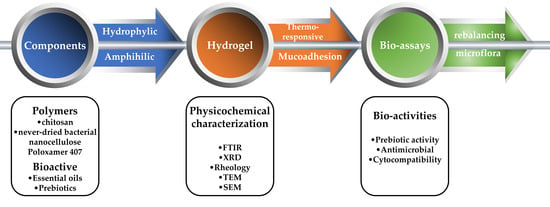
Bioactive-Loaded Hydrogels Based on Bacterial Nanocellulose, Chitosan, and Poloxamer for Rebalancing Vaginal Microbiota
Abstract
:1. Introduction
2. Results
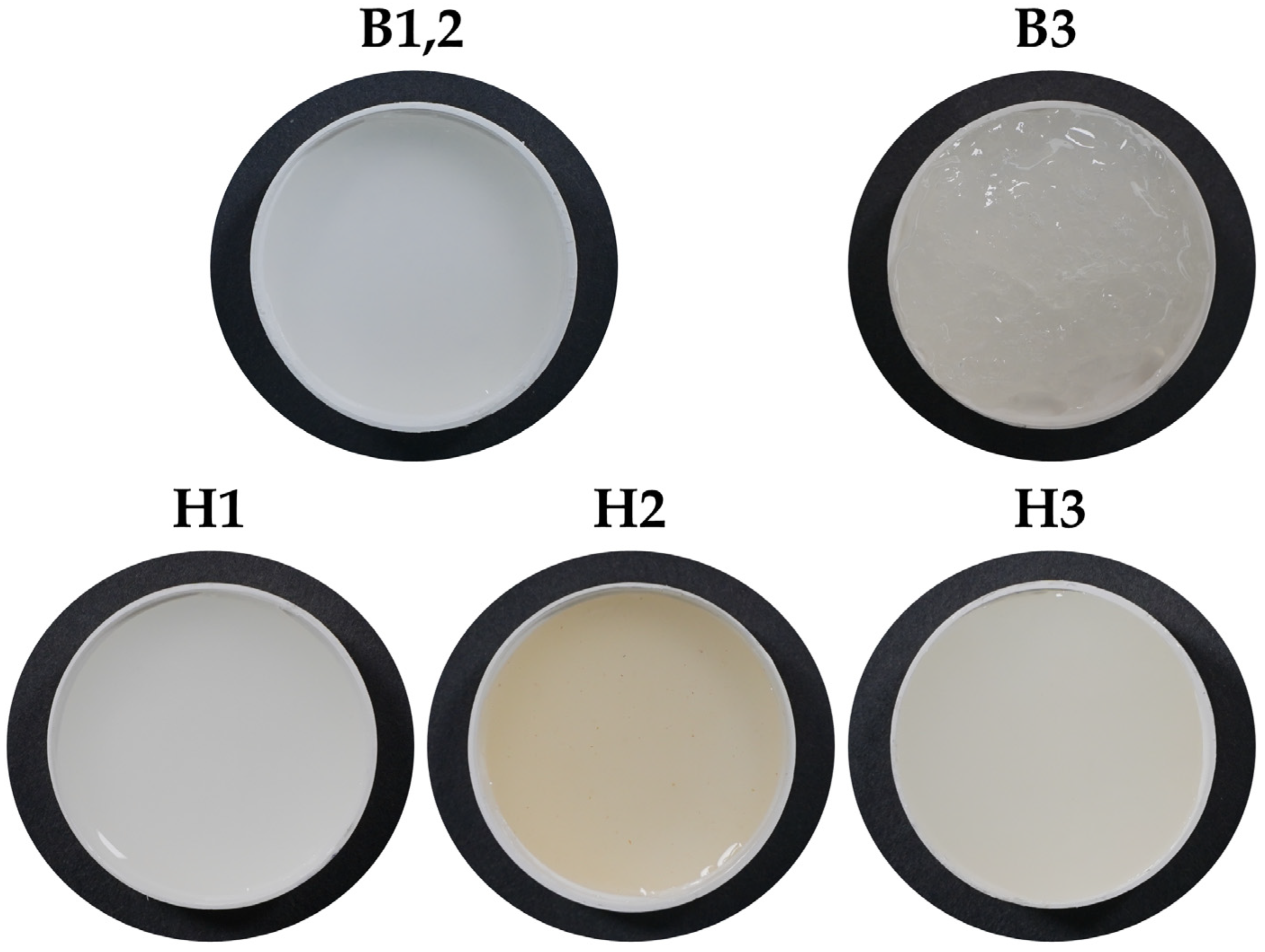
2.1. Structure and Physical-Chemical Properties of the Hydrogels
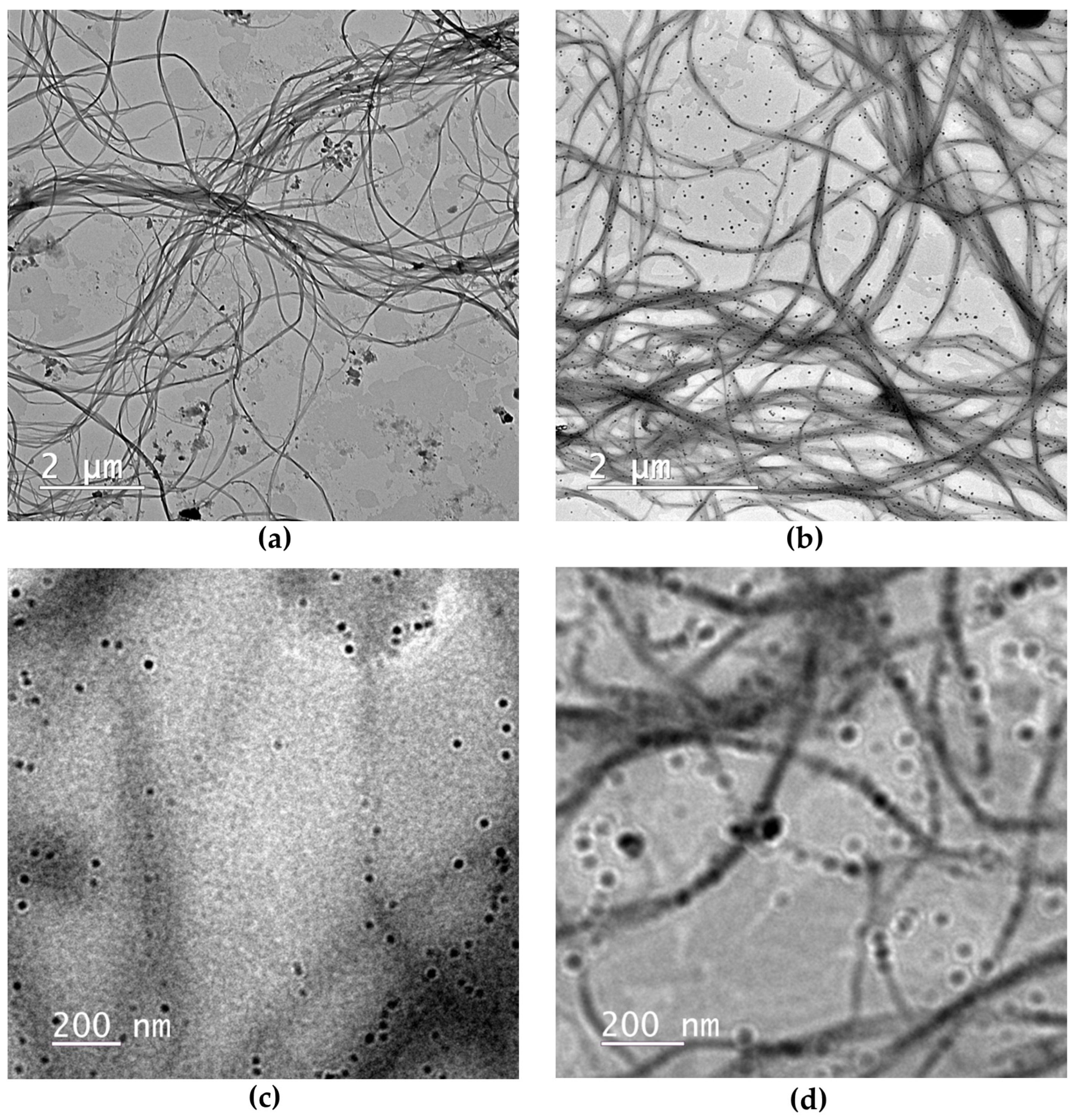
2.1.1. Microscopic Structure of Hydrogels via TEM
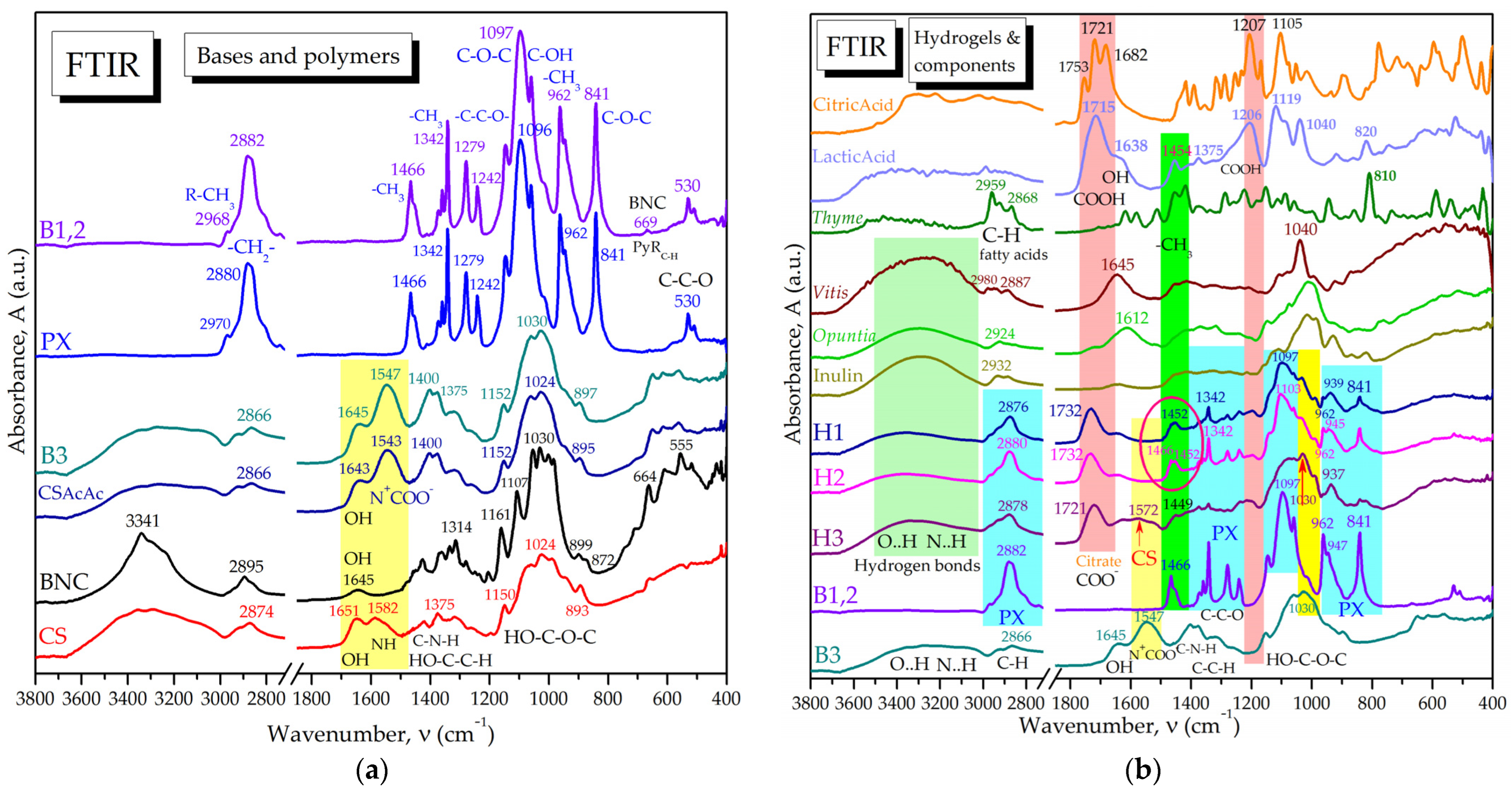
2.1.2. Molecular Interactions in Hydrogels by FTIR Spectroscopy
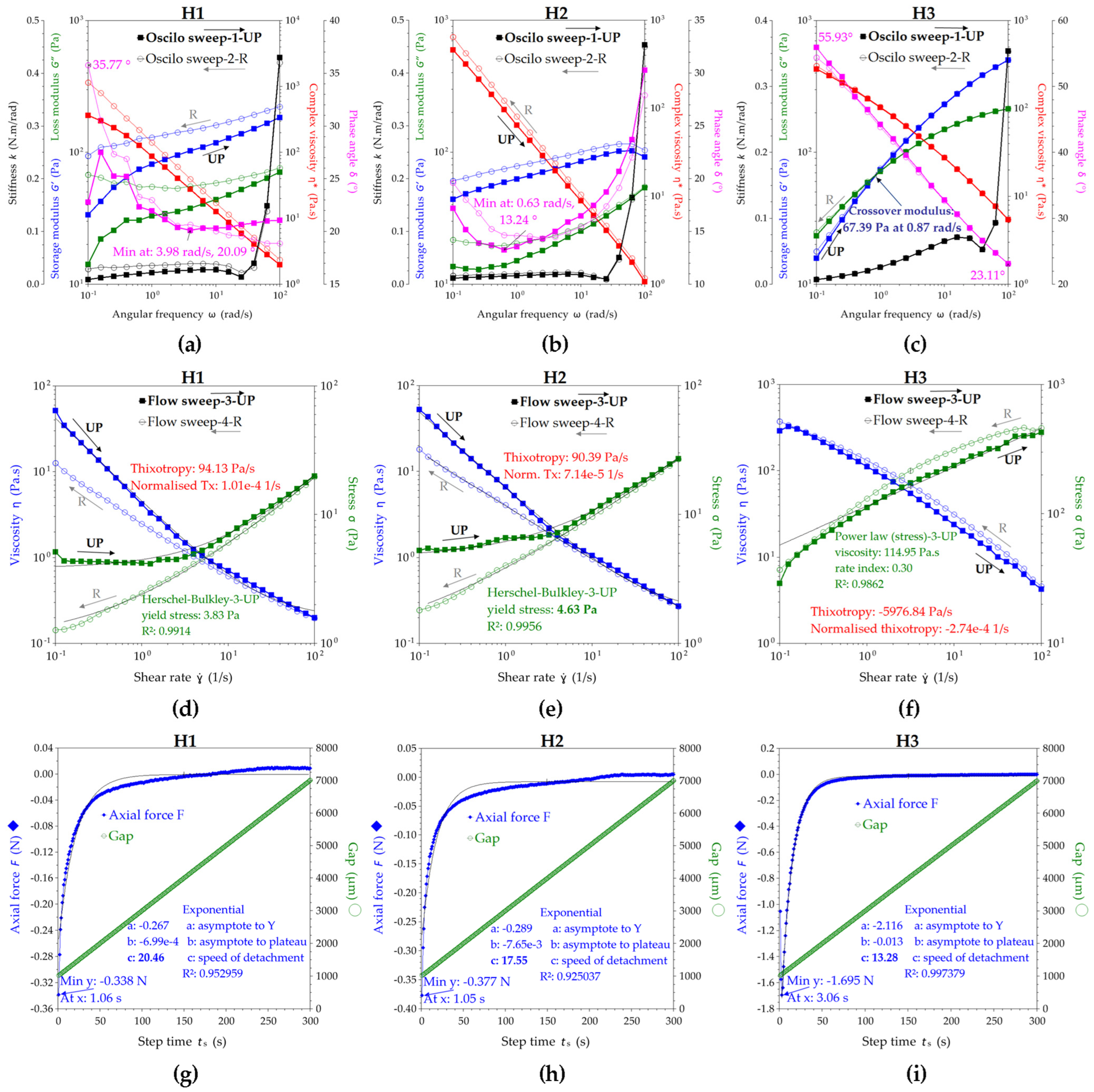
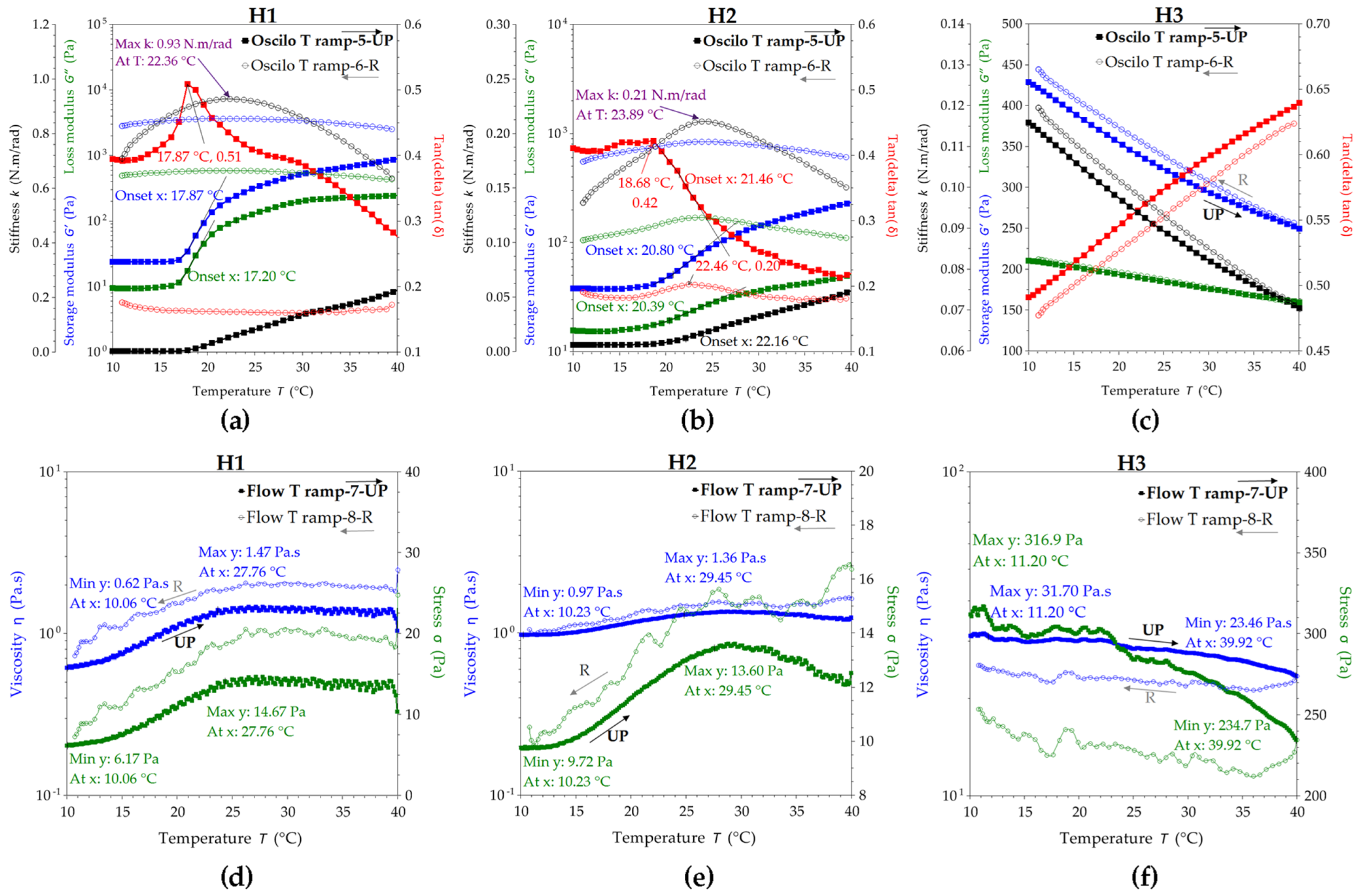
2.1.3. Rheological Properties of the Hydrogels
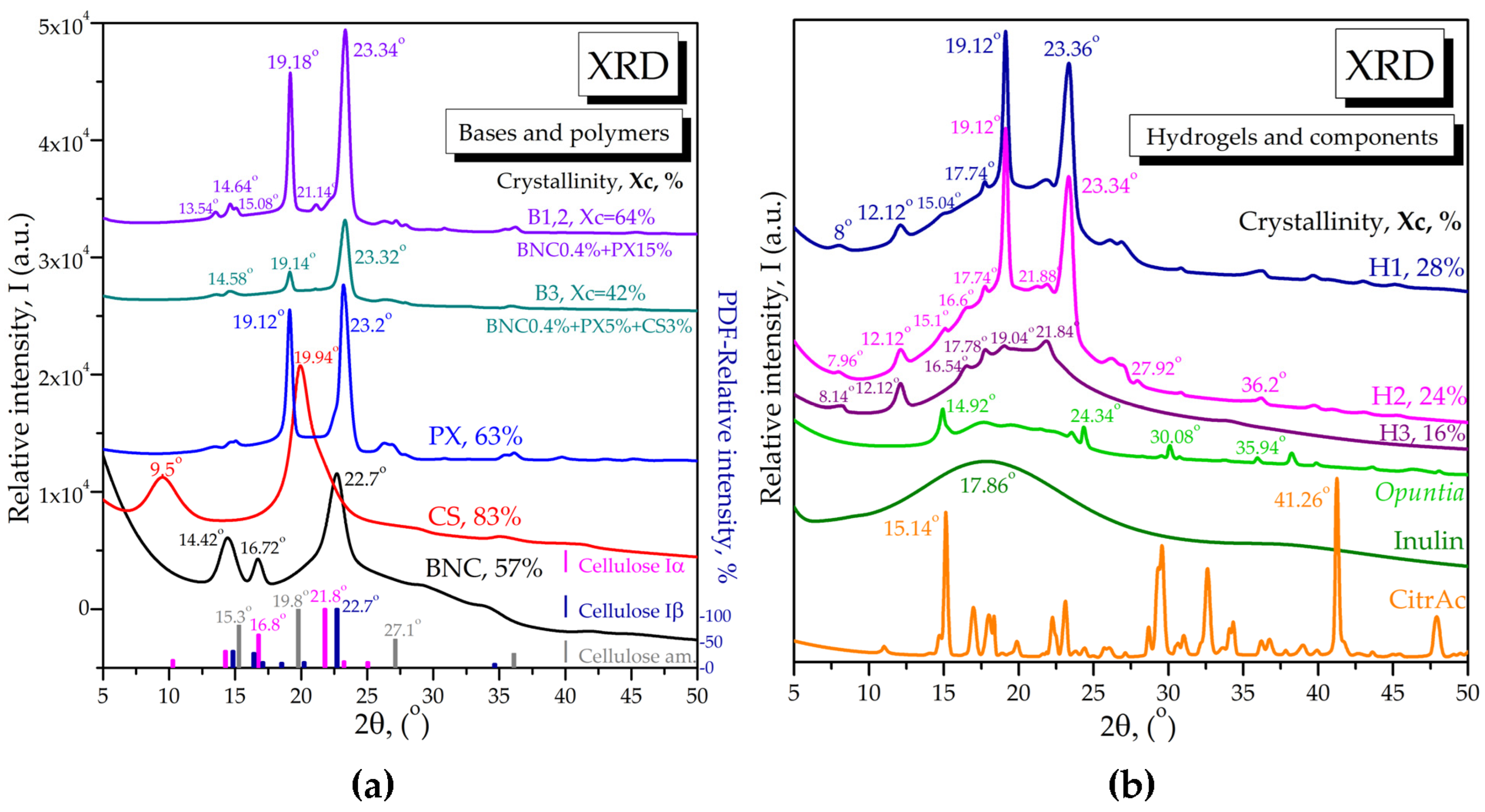
2.1.4. X-ray Diffraction of Bases and Loaded Hydrogels
2.2. Hydrogel Interaction with Mucin
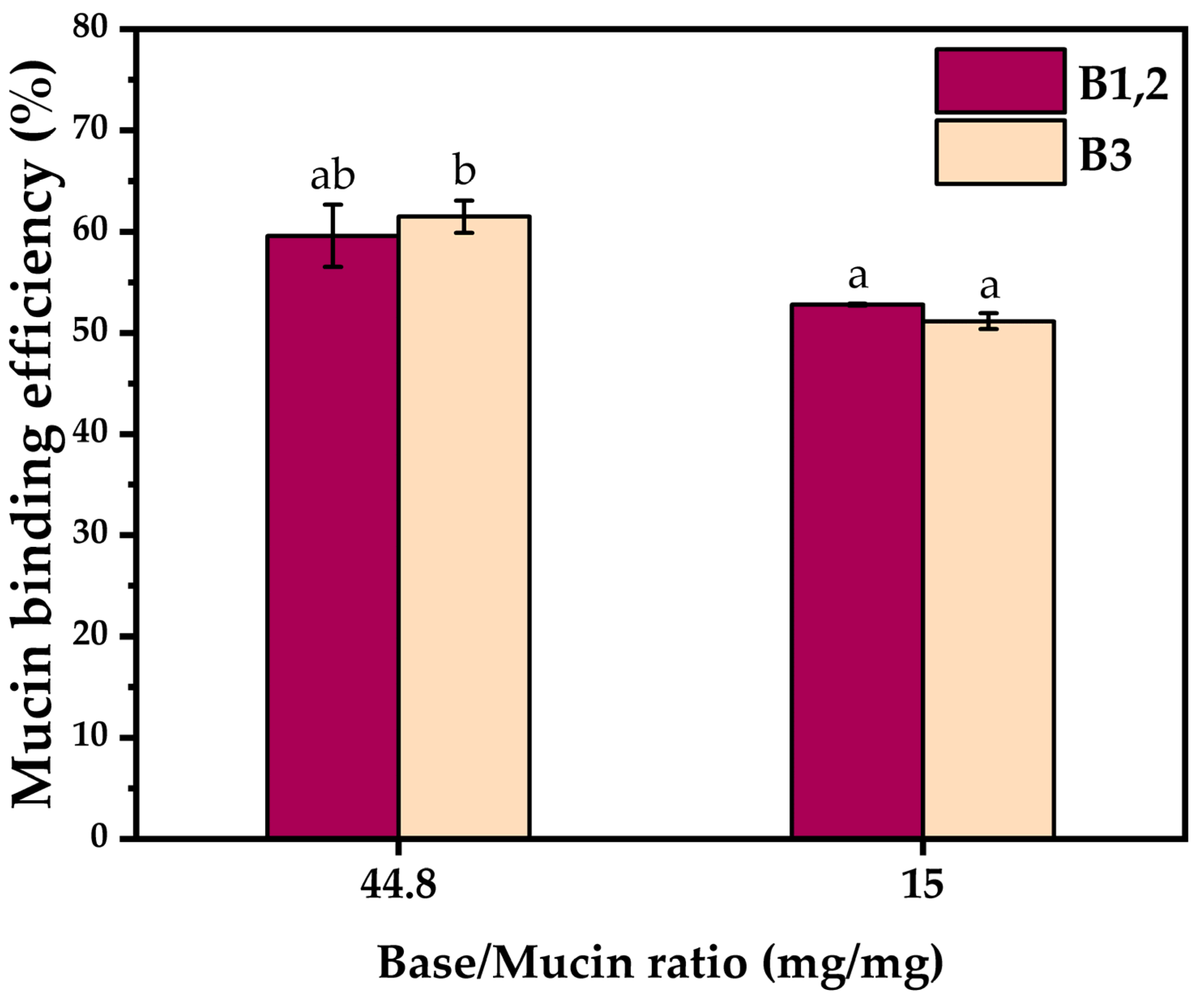
2.2.1. Quantitative Evaluation of Hydrogel–Mucin Interaction
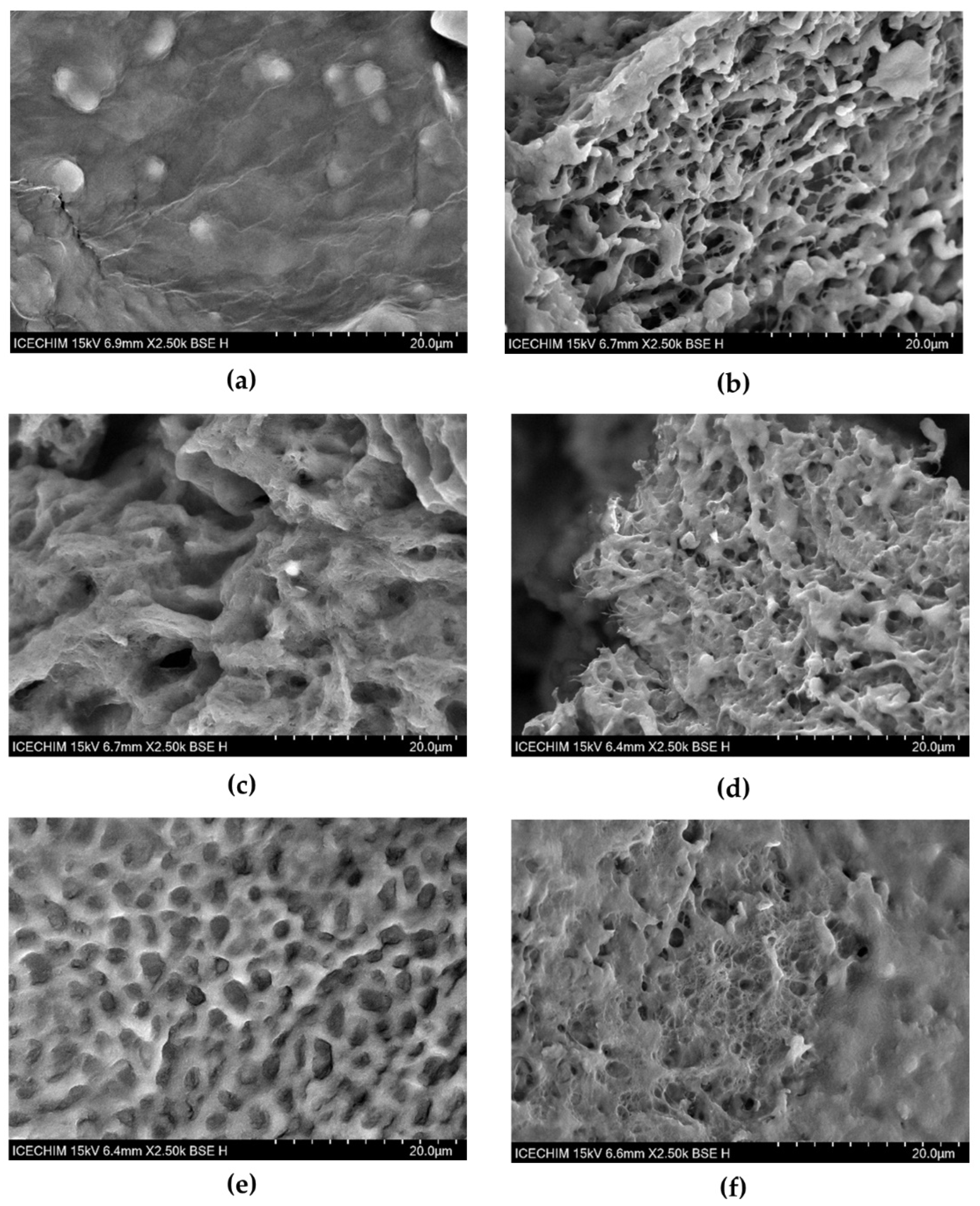
2.2.2. SEM Microscopic Structure of Hydrogel Interaction with Mucin
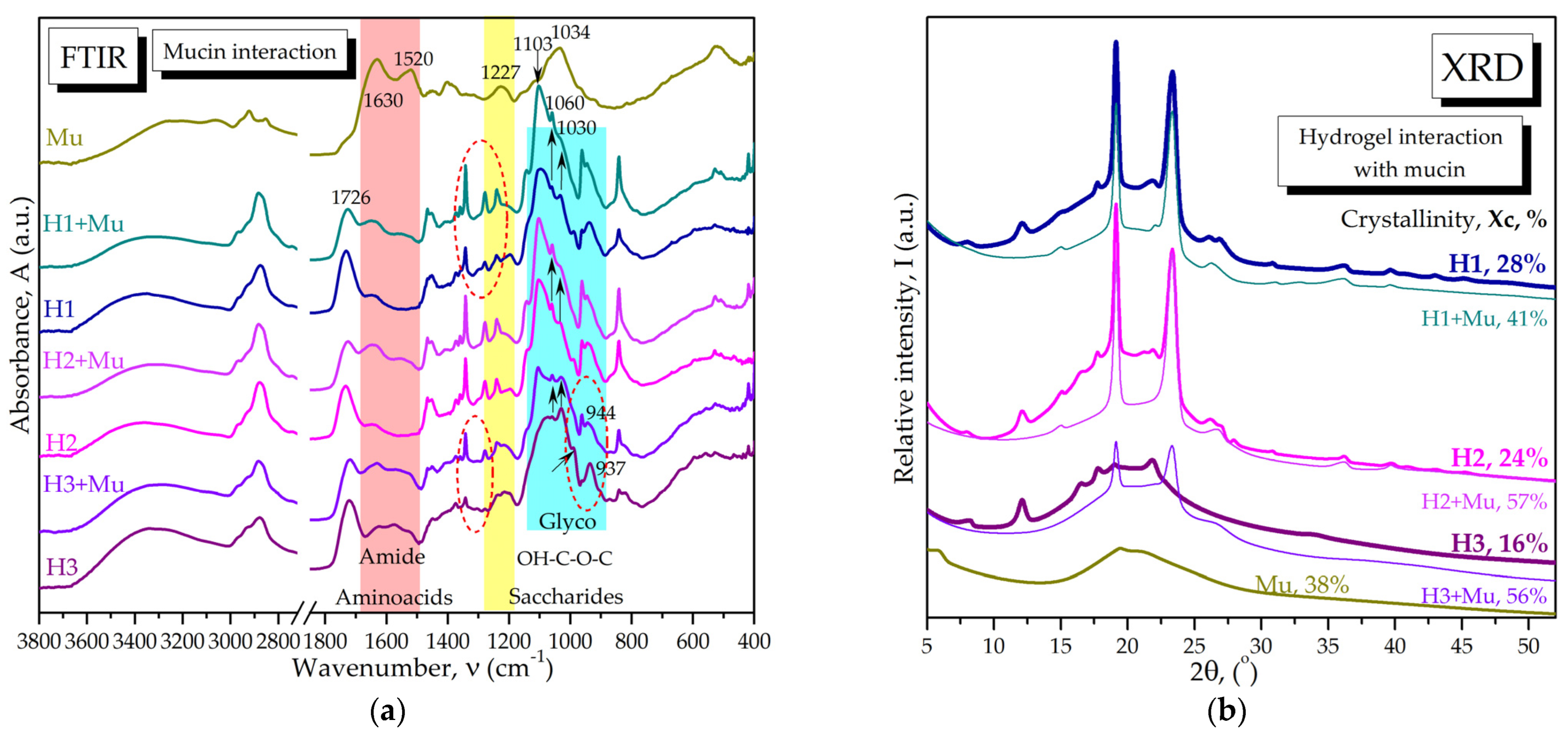
2.2.3. Molecular Interactions of Hydrogels with Mucin via FTIR Spectroscopy and XRD
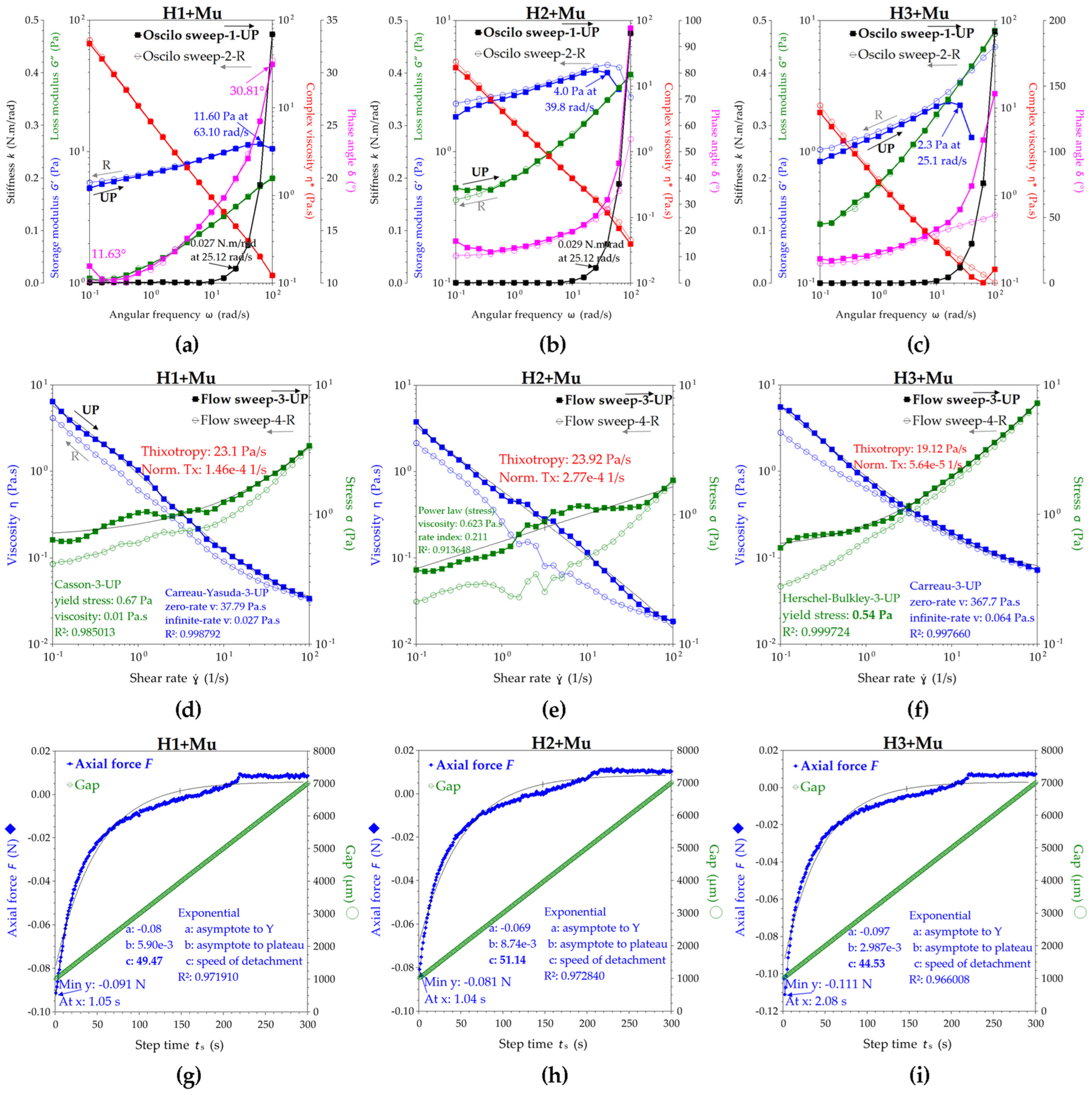
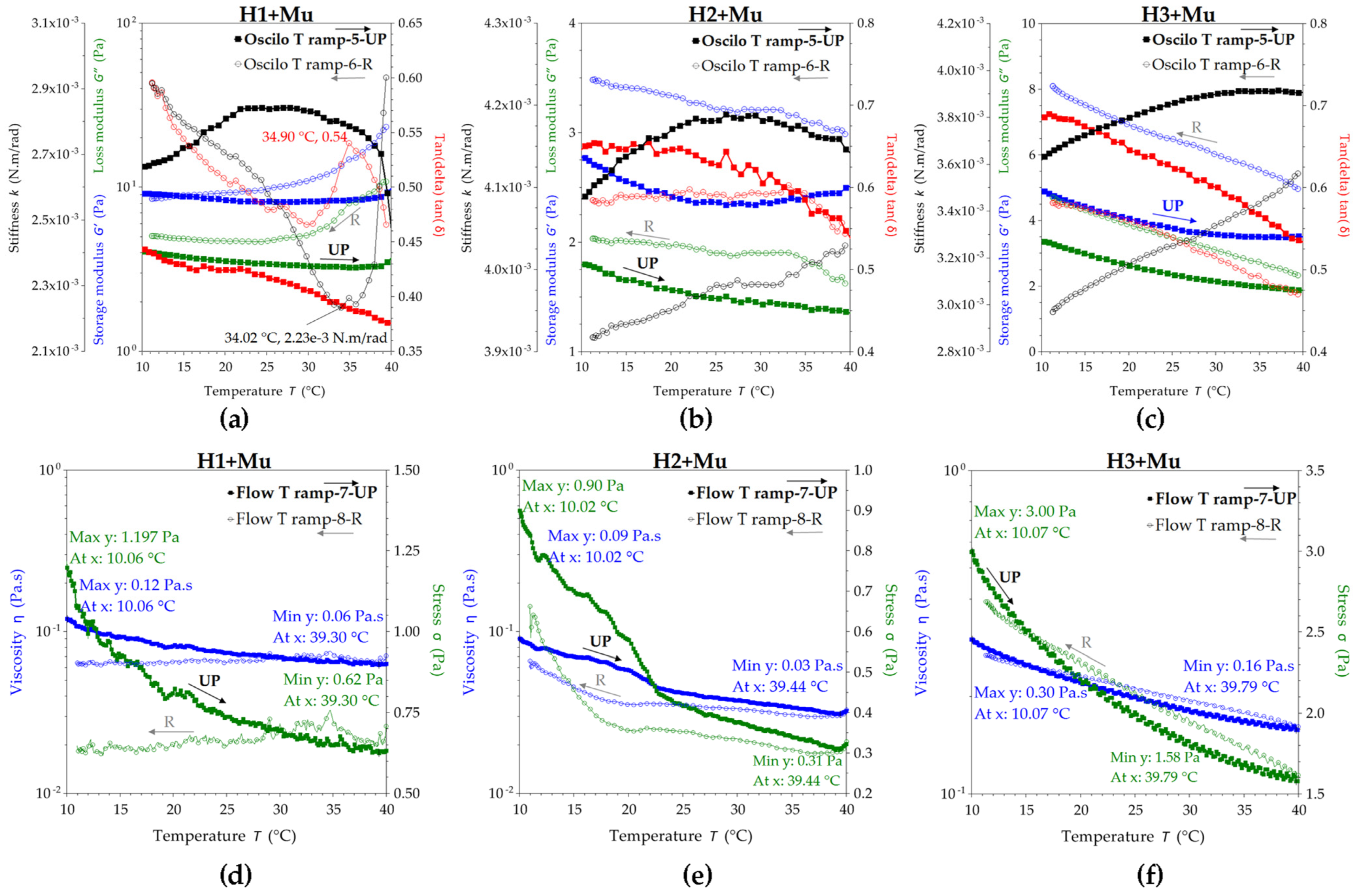
2.2.4. Rheological Studies of Hydrogel–Mucin Complex
2.3. Biocompatibility and Bioactivity of Hydrogels
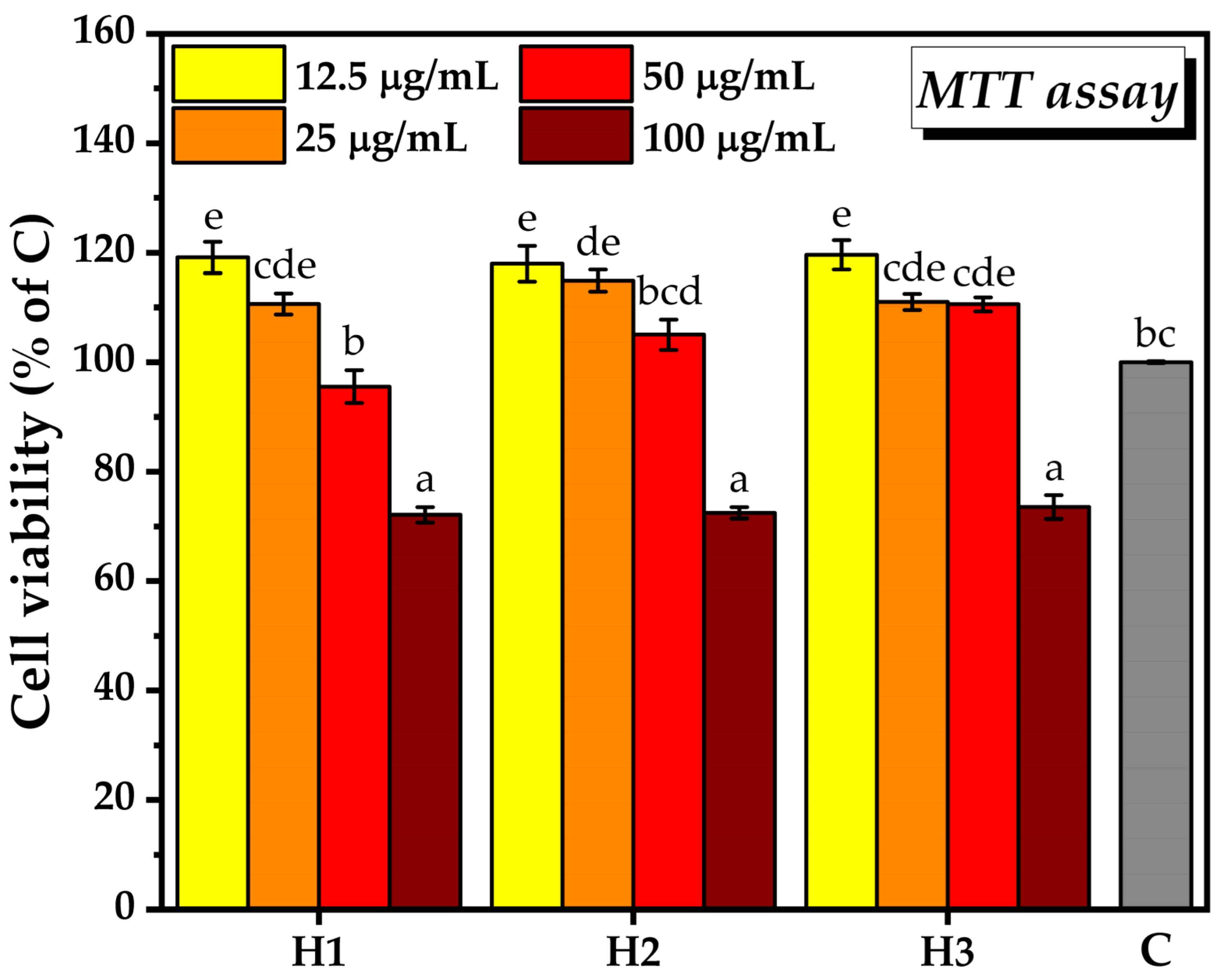
2.3.1. Biocompatibility Assay
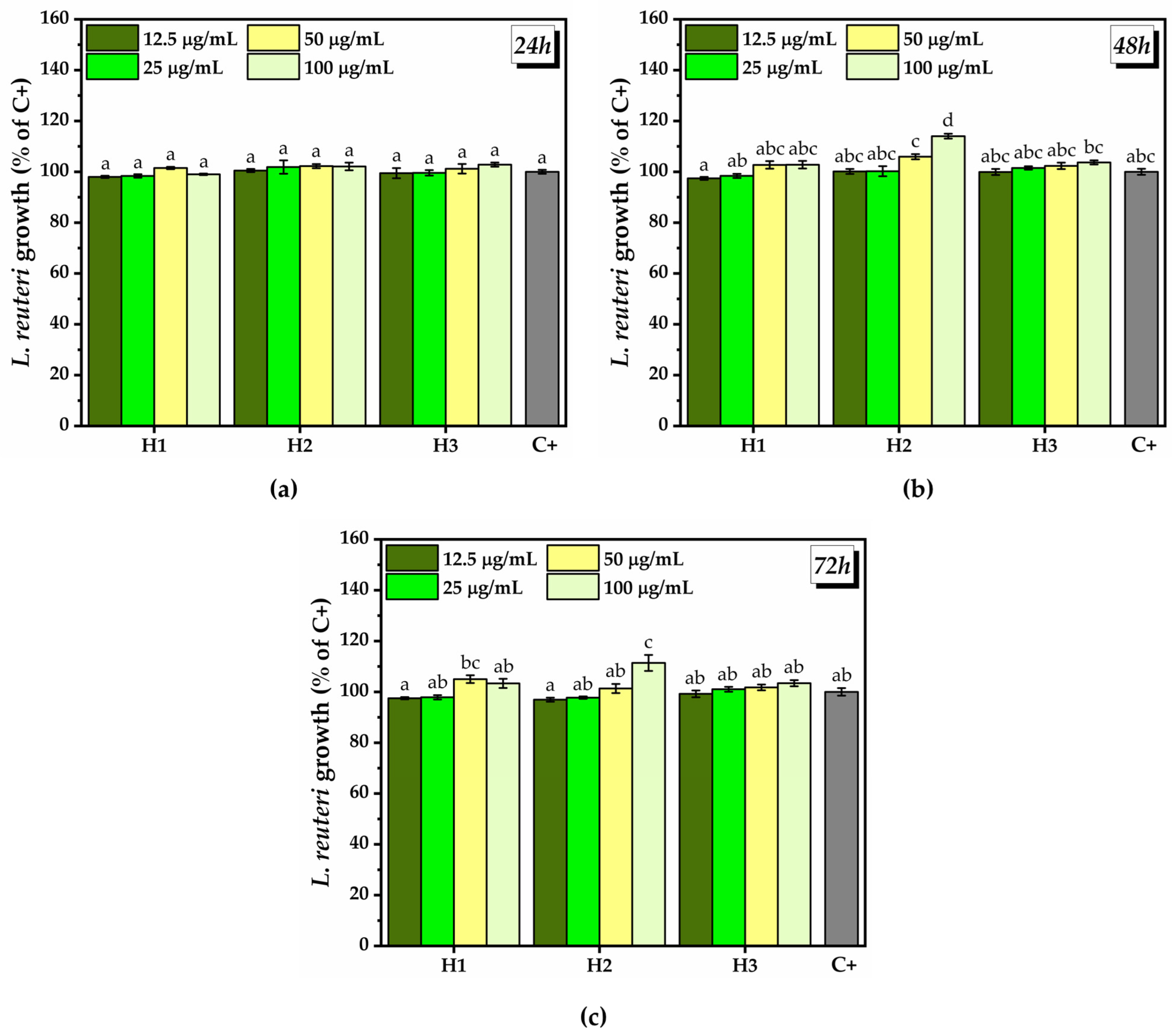
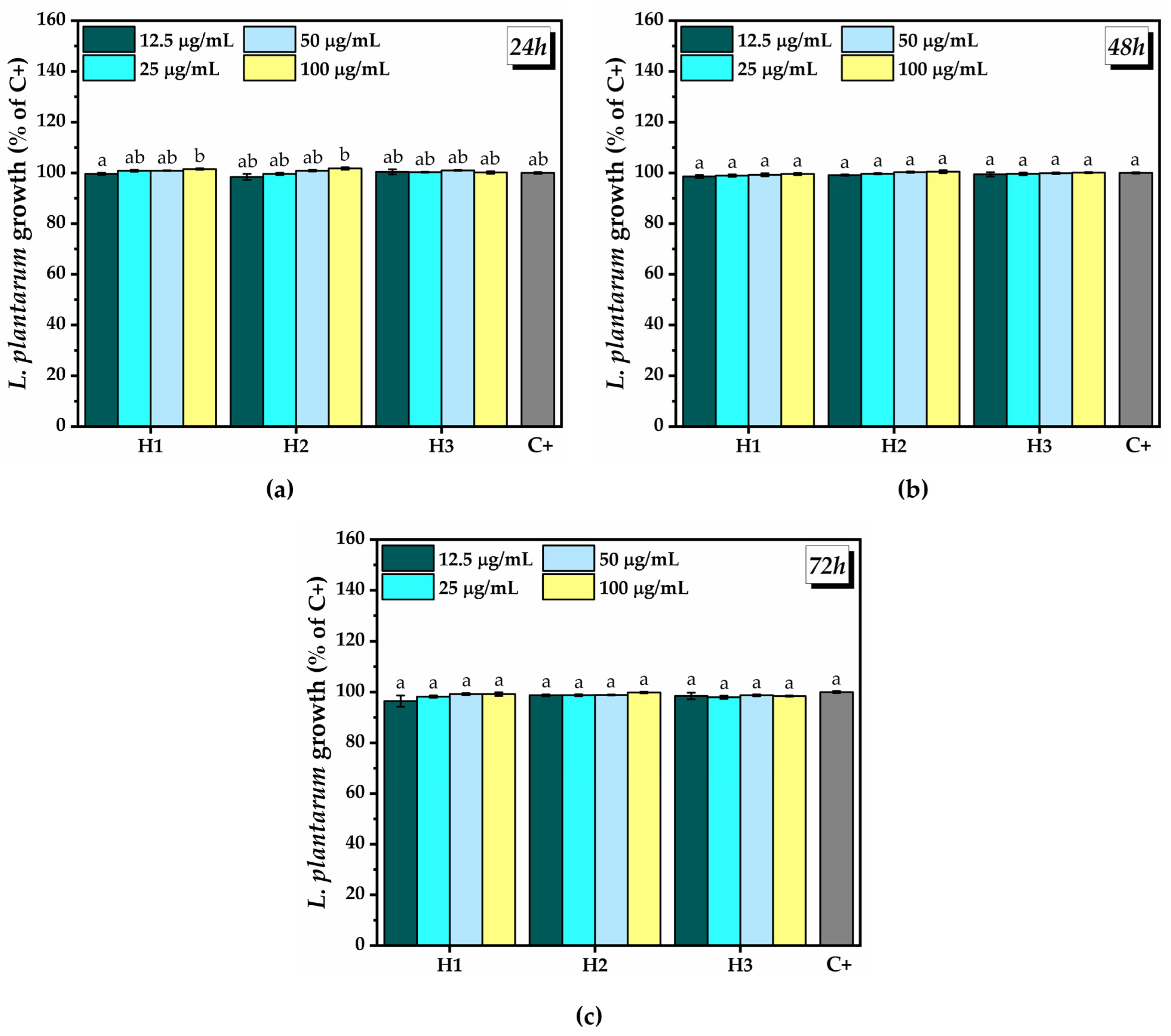
2.3.2. Prebiotic Activity of the Hydrogels
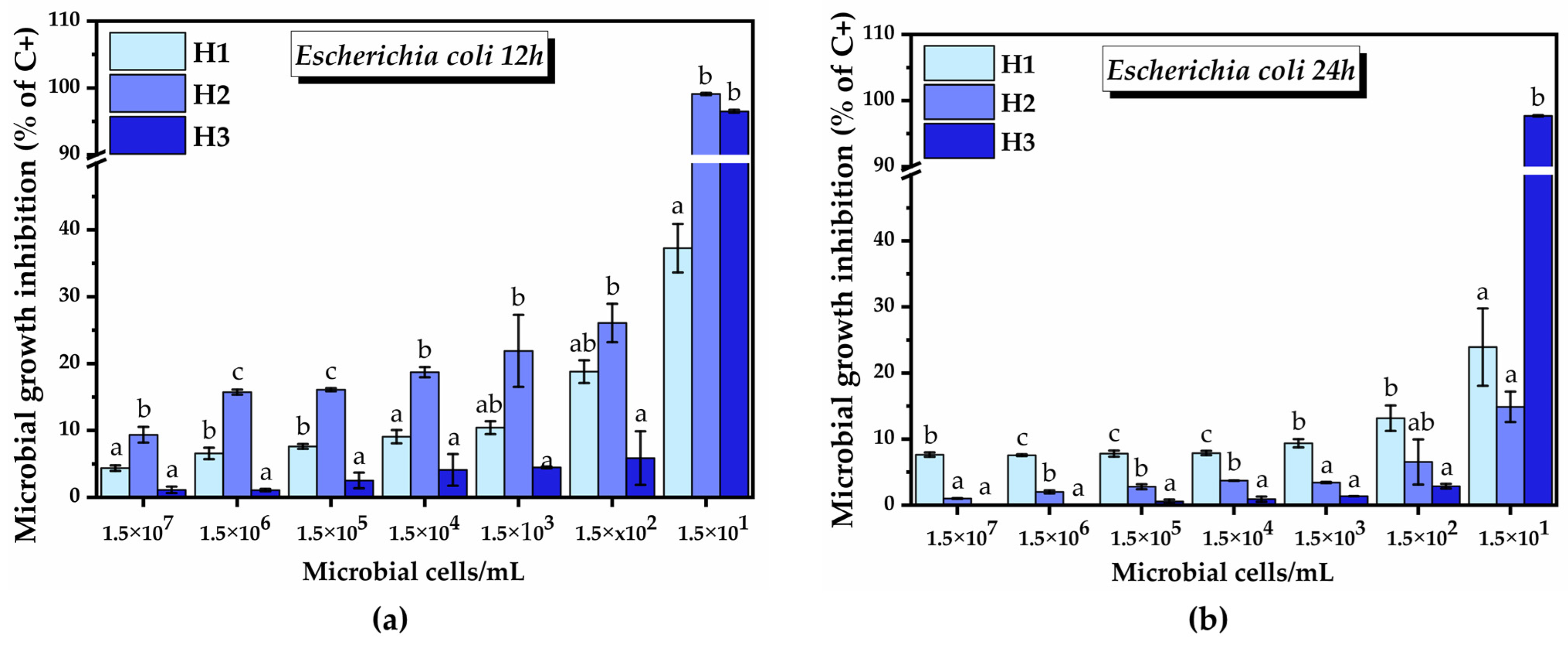
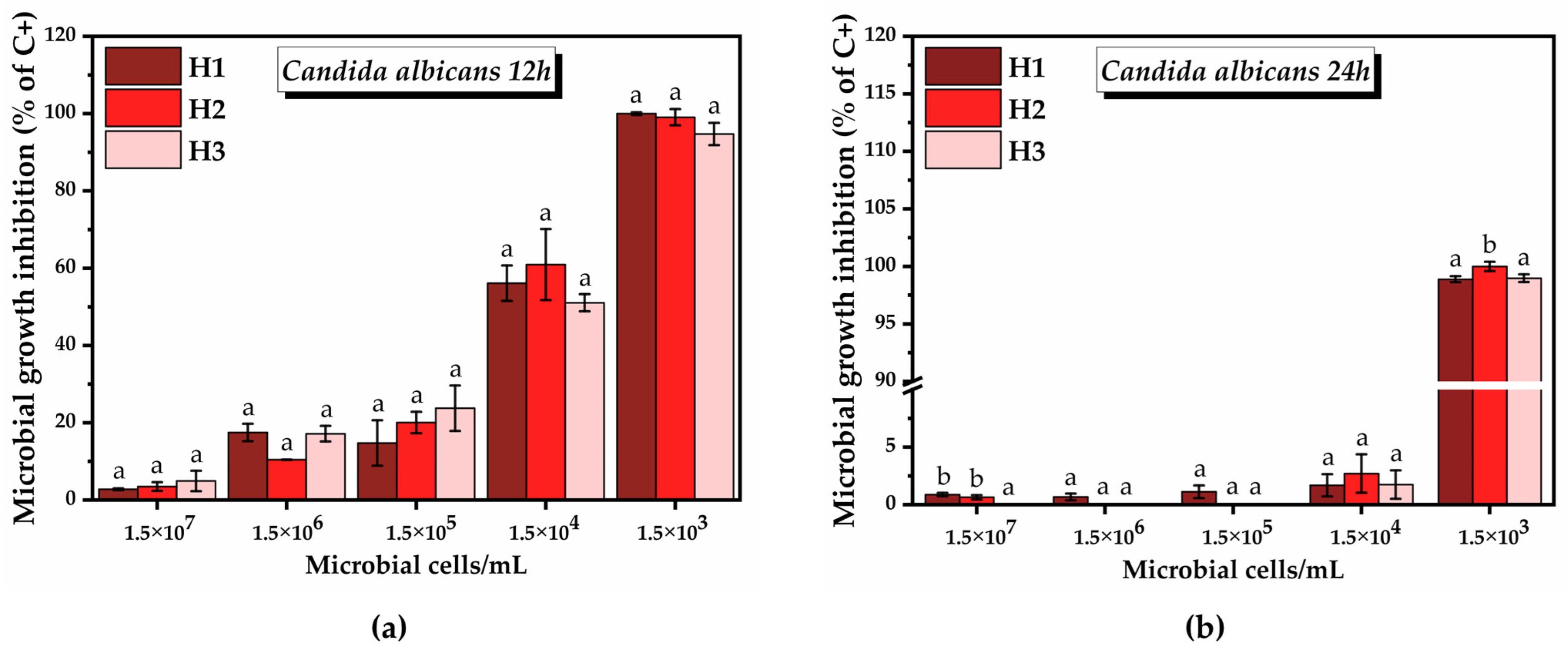
2.3.3. Antimicrobial Activity of the Hydrogels
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Hydrogels
4.3. Hydrogel Ultrastructural Characterization
4.4. FTIR Characterization
4.5. X-ray Diffraction
4.6. Viscosity and Mucoadhesion Determination by Rheology
4.7. Quantitative Mucoadhesion Assay of the Hydrogel Systems
4.8. Cell Viability Assay
4.9. Probiotic Growth Assay
4.10. Effects on Pathogenic Bacteria
4.10.1. Antimicrobial Activity
4.10.2. Antibiofilm Activity
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gosecka, M.; Gosecki, M. Antimicrobial Polymer-Based Hydrogels for the Intravaginal Therapies—Engineering Considerations. Pharmaceutics 2021, 13, 1393. [Google Scholar] [CrossRef]
- Martins dos Santos, A.; Carvalho, S.; Hugo, V.; Carvalho, G.; Gremiao, M.; Chorilli, M. Recent advances in hydrogels as strategy for drug delivery intended to vaginal infections. Int. J. Pharm. 2020, 590, 119867. [Google Scholar] [CrossRef]
- Ballell-Hosa, L.; González-Mira, E.; Santana, H.; Morla-Folch, J.; Moreno-Masip, M.; Martínez-Prieto, Y.; Revuelta, A.; Di Mauro, P.P.; Veciana, J.; Sala, S.; et al. DELOS Nanovesicles-Based Hydrogels: An Advanced Formulation for Topical Use. Pharmaceutics 2022, 14, 199. [Google Scholar] [CrossRef]
- Zamboulis, A.; Michailidou, G.; Koumentakou, I.; Bikiaris, D.N. Polysaccharide 3D Printing for Drug Delivery Applications. Pharmaceutics 2022, 14, 145. [Google Scholar] [CrossRef]
- Hasan, N.; Lee, J.; Ahn, H.-J.; Hwang, W.R.; Bahar, M.A.; Habibie, H.; Amir, M.N.; Lallo, S.; Son, H.-J.; Yoo, J.-W. Nitric Oxide-Releasing Bacterial Cellulose/Chitosan Crosslinked Hydrogels for the Treatment of Polymicrobial Wound Infections. Pharmaceutics 2022, 14, 22. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Kumar, S.; Khan, M.S.; Kumar, P.; Singh, I. Preparation and Evaluation of Chitosan/PVA Based Hydrogel Films Loaded with Honey for Wound Healing Application. Gels 2022, 8, 111. [Google Scholar] [CrossRef]
- Deng, W.T.; Yan, Y.; Zhuang, P.P.; Liu, X.X.; Tian, K.; Huang, W.F.; Li, C. Synthesis of nanocapsules blended polymeric hydrogel loaded with bupivacaine drug delivery system for local anesthetics and pain management. Drug Deliv. 2022, 29, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest Advances in Cryogel Technology for Biomedical Applications. Adv. Therap. 2019, 2, 45. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.Y.; Lee, D.S. Advances in biodegradable and injectable hydrogels for biomedical applications. J. Control. Release 2021, 330, 151–160. [Google Scholar] [CrossRef] [PubMed]
- David; Guerrero, M.; Pariguana, M.; Marican, A.; Durán-Lara, E.F. Hydrogel-Based Microneedle as a Drug Delivery System. Pharmaceutics 2023, 15, 2444. [Google Scholar] [CrossRef]
- Chiesa, I.; Ceccarini, M.R.; Bon, S.B.; Codini, M.; Beccari, T.; Valentini, L.; De Maria, C. 4D Printing Shape-Morphing Hybrid Biomaterials for Advanced Bioengineering Applications. Materials 2023, 16, 6661. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.M.; Muniz, B.V.; Castro, S.R.; de Araujo, J.S.; de Souza Amorim, K.; Ribeiro, L.N.M.; Ferreira, L.E.; de Araújo, D.R.; de Paula, E.; Franz-Montan, M. Mucoadhesive, Thermoreversible Hydrogel, Containing Tetracaine-Loaded Nanostructured Lipid Carriers for Topical, Intranasal Needle-Free Anesthesia. Pharmaceutics 2021, 13, 1760. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gonzalez, N.; Bozal-de Febrer, N.; Calpena-Campmany, A.C.; Nardi-Ricart, A.; Rodriguez-Lagunas, M.J.; Morales-Molina, J.A.; Soriano-Ruiz, J.L.; Fernandez-Campos, F.; Clares-Naveros, B. New Formulations Loading Caspofungin for Topical Therapy of Vulvovaginal Candidiasis. Gels 2021, 7, 259. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, E.; Czajkowska-Kosnik, A.; Winnicka, K. Comparison of Rheological, Drug Release, and Mucoadhesive Characteristics upon Storage between Hydrogels with Unmodified or Beta-Glycerophosphate-Crosslinked Chitosan. Int. J. Polym. Sci. 2018, 2018, 3592843. [Google Scholar] [CrossRef]
- Mehta, P.; Sharma, M.; Devi, M. Hydrogels: An overview of its classifications, properties, and applications. J. Mech. Behav. Biomed. Mater. 2023, 147, 106145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Wang, Z.F.; Sun, Q.Q.; Li, Q.; Li, S.H.; Li, X.M. Dynamic Hydrogels with Viscoelasticity and Tunable Stiffness for the Regulation of Cell Behavior and Fate. Materials 2023, 16, 5161. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, G.; Wang, J.; Zhang, R.; Zhong, S.; Wang, J.; Cui, X. Injectable chitosan-based self-healing supramolecular hydrogels with temperature and pH dual-responsivenesses. Int. J. Biol. Macromol. 2023, 227, 1038–1047. [Google Scholar] [CrossRef]
- Zubik, K.; Singhsa, P.; Wang, Y.A.; Manuspiya, H.; Narain, R. Thermo-Responsive Poly(N-Isopropylacrylamide)-Cellulose Nanocrystals Hybrid Hydrogels for Wound Dressing. Polymers 2017, 9, 119. [Google Scholar] [CrossRef]
- Dumanli, A.G. Nanocellulose and its Composites for Biomedical Applications. Curr. Med. Chem. 2017, 24, 512–528. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xue, Y.; Zhu, Z.; Hu, Y.; Zeng, Q.; Wu, Y.; Wang, Y.; Shen, C.; Jiang, C.; Liu, L.; et al. Quantitative Structure-Activity Relationship of Enhancers of Licochalcone A and Glabridin Release and Permeation Enhancement from Carbomer Hydrogel. Pharmaceutics 2022, 14, 262. [Google Scholar] [CrossRef]
- Batista, J.V.D.C.; Matos, A.P.S.; Oliveria, A.P.; Ricci Júnior, E.; Freitas, Z.M.; Oliveira, C.A.; Toma, H.K.; Capella, M.A.M.; Rocha, L.M.; Weissenstein, U.; et al. Thermoresponsive Hydrogel Containing Viscum album Extract for Topic and Transdermal Use: Development, Stability and Cytotoxicity Activity. Pharmaceutics 2022, 14, 37. [Google Scholar] [CrossRef]
- Khade, S.M.; Behera, B.; Sagiri, S.S.; Singh, V.K.; Thirugnanam, A.; Pal, K.; Ray, S.S.; Pradhan, D.K.; Bhattacharya, M.K. Gelatin-PEG based metronidazole-loaded vaginal delivery systems: Preparation, characterization and in vitro antimicrobial efficiency. Iran. Polym. J. 2014, 23, 171–184. [Google Scholar] [CrossRef]
- Wu, S.Y.; Xiao, R.J.; Wu, Y.; Xu, L.J. Advances in tissue engineering of gellan gum-based hydrogels. Carbohydr. Polym. 2023, 324, 121484. [Google Scholar] [CrossRef]
- Hosseinzadeh, F.; Tabesh, H.; Farzaneh, F. Nano drug delivery platform based on thermosensitive PEG-PCL hydrogel encapsulated in silver-bearing micelles and its antifungal activity investigation against vaginal candidiasis. Front. Mater. 2023, 10, 1210542. [Google Scholar] [CrossRef]
- Dima, S.-O.; Panaitescu, D.-M.; Orban, C.; Ghiurea, M.; Doncea, S.-M.; Fierascu, R.C.; Nistor, C.L.; Alexandrescu, E.; Nicolae, C.-A.; Trica, B.; et al. Bacterial Nanocellulose from Side-Streams of Kombucha Beverages Production: Preparation and Physical-Chemical Properties. Polymers 2017, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.W.; Xia, Z.P.; Pan, J.J.; Wang, N.; Gao, H.C.; Ren, J.L.; Xia, X.K. Bacterial Cellulose Applied in Wound Dressing Materials: Production and Functional Modification—A Review. Macromol. Biosci. 2023, 14, e2300333. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.C.; Meng, Q.S.; Hu, J.G. Bacterial Nanocellulose Hydrogel: A Promising Alternative Material for the Fabrication of Engineered Vascular Grafts. Polymers 2023, 15, 3812. [Google Scholar] [CrossRef]
- Muller, A.; Ni, Z.X.; Hessler, N.; Wesarg, F.; Muller, F.A.; Kralisch, D.; Fischer, D. The biopolymer bacterial nanocellulose as drug delivery system: Investigation of drug loading and release using the model protein albumin. J. Pharm. Sci. 2013, 102, 579–592. [Google Scholar] [CrossRef]
- Fonseca, D.F.S.; Carvalho, J.P.F.; Bastos, V.; Oliveira, H.; Moreirinha, C.; Almeida, A.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Antibacterial Multi-Layered Nanocellulose-Based Patches Loaded with Dexpanthenol for Wound Healing Applications. Nanomaterials 2020, 10, 2469. [Google Scholar] [CrossRef]
- Napavichayanun, S.; Yamdech, R.; Aramwit, P. The safety and efficacy of bacterial nanocellulose wound dressing incorporating sericin and polyhexamethylene biguanide: In vitro, in vivo and clinical studies. Arch. Dermatol. Res. 2016, 308, 123–132. [Google Scholar] [CrossRef]
- Pahwa, R.; Ahuja, M. Nanocellulose-gellan cross-linked scaffolds for vaginal delivery of fluconazole. Int. J. Biol. Macromol. 2023, 229, 668–683. [Google Scholar] [CrossRef]
- Lengowski, E.C.; Franco, T.S.; Viana, L.C.; Bonfatti, E.A., Jr.; de Muñiz, G.I.B. Micro and nanoengineered structures and compounds: Nanocellulose. Cellulose 2023, 30, 10595–10632. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Lv, H.F.; Lu, C.T.; Chen, L.J.; Lin, M.; Zhang, M.; Jiang, X.; Shen, X.T.; Jin, R.R.; Cai, J.; et al. Evaluation of a Novel Thermosensitive Heparin-Poloxamer Hydrogel for Improving Vascular Anastomosis Quality and Safety in a Rabbit Model. PLoS ONE 2013, 8, e73178. [Google Scholar] [CrossRef]
- Ganji, F.; Abdekhodaie, M.J.; Ramazani, A. Gelation time and degradation rate of chitosan-based injectable hydrogel. J. Sol-Gel Sci. Technol. 2007, 42, 47–53. [Google Scholar] [CrossRef]
- Ruel-Gariepy, E.; Leroux, J.C. In situ-forming hydrogels—Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef]
- Kim, D.; Kim, S.; Jo, S.; Woo, J.; Noh, I. Physicochemical properties of chitosan-poly(ethylene oxide) hydrogel modified through linoleic acid. Macromol. Res. 2011, 19, 396–402. [Google Scholar] [CrossRef]
- Tan, H.P.; Chu, C.R.; Payne, K.A.; Marra, K.G. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.R.; Oshiro-Junior, J.A.; Rodero, C.F.; Boni, F.I.; Araujo, V.H.S.; Bauab, T.M.; Nicholas, D.; Callan, J.F.; Chorilli, M. Enhancing Antifungal Treatment of Candida albicans with Hypericin-Loaded Nanostructured Lipid Carriers in Hydrogels: Characterization, In Vitro, and In Vivo Photodynamic Evaluation. Pharmaceuticals 2023, 16, 1094. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.N.; Li, D.; Zou, X.N.; Wei, H.G.; Zhong, C.; Chu, L.Q. Preparation and antibacterial activity of injectable methylcellulose/chitosan double network hydrogel. Cellulose 2023, 30, 10357–10372. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef]
- Dai, T.; Tanaka, M.; Huang, Y.-Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti. Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, J.; Li, P.; Ning, N. The Effect of Chitosan in Wound Healing: A Systematic Review. Adv. Ski. Wound Care 2021, 34, 262–266. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- Valipour, F.; Rahimabadi, E.Z.; Rostamzad, H. Preparation and characterization of wound healing hydrogel based on fish skin collagen and chitosan cross-linked by dialdehyde starch. Int. J. Biol. Macromol. 2023, 253, 126704. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The Prebiotic Potential of Inulin-Type Fructans: A Systematic Review. Adv. Nutr. 2022, 13, 492–529. [Google Scholar] [CrossRef] [PubMed]
- Balthazar, C.F.; Guimarães, J.F.; Coutinho, N.M.; Pimentel, T.C.; Ranadheera, C.S.; Santillo, A.; Albenzio, M.; Cruz, A.G.; Sant’Ana, A.S. The future of functional food: Emerging technologies application on prebiotics, probiotics and postbiotics. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2560–2586. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.; Oomah, B.D.; Oliveira, W.P.; Burgos-Díaz, C.; Rubilar, M.; Shene, C. Probiotics and prebiotics potential for the care of skin, female urogenital tract, and respiratory tract. Folia Microbiol. 2020, 65, 245–264. [Google Scholar] [CrossRef]
- Boskovic, M.; Zdravkovic, N.; Ivanovic, J.; Janjic, J.; Djordjevic, J.; Starcevic, M.; Baltic, M.Z. Antimicrobial Activity of Thyme (Tymus vulgaris) and Oregano (Origanum vulgare) Essential Oils against Some Food-Borne Microorganisms. Procedia Food Sci. 2015, 5, 18–21. [Google Scholar] [CrossRef]
- Cai, C.; Ma, R.; Duan, M.; Lu, D. Preparation and antimicrobial activity of thyme essential oil microcapsules prepared with gum arabic. RSC Adv. 2019, 9, 19740–19747. [Google Scholar] [CrossRef]
- Bogavac, M.; Karaman, M.; Janjušević, L.; Sudji, J.; Radovanović, B.; Novaković, Z.; Simeunović, J.; Božin, B. Alternative treatment of vaginal infections—In vitro antimicrobial and toxic effects of Coriandrum sativum L. and Thymus vulgaris L. essential oils. J. Appl. Microbiol. 2015, 119, 697–710. [Google Scholar] [CrossRef]
- Tomás, M.; Sousa, L.G.V.; Oliveira, A.S.; Gomes, C.P.; Palmeira-de-Oliveira, A.; Cavaleiro, C.; Salgueiro, L.; Cerca, N.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Vaginal Sheets with Thymbra capitata Essential Oil for the Treatment of Bacterial Vaginosis: Design, Characterization and In Vitro Evaluation of Efficacy and Safety. Gels 2023, 9, 293. [Google Scholar] [CrossRef]
- Hasan, B.; Khudair, A.; Alkalby, J. Effect of Vaginal Candidiasis and Treatments With Oils of Thyme and Oregano Compared With Nystatin on Blood Parameters of Mature Female Rabbits. Indian J. Appl. Res. 2015, 5, 35–38. [Google Scholar]
- Gadelha, L.; Valadas, L.; Fiallos, N.; Luque Peralta, S.; Mendonça, K.; Lotif, M.; Bezerra, G.; Aguiar, M.; Diógenes, É.; Martins Rodrigues Neto, E.; et al. Evaluation of the Antifungal Effect Vitis vinifera Extract on Candida albicans. J. Young Pharm. 2018, 10, 164–168. [Google Scholar] [CrossRef]
- Simonetti, G.A.-O.; Santamaria, A.R.; D’Auria, F.D.; Mulinacci, N.A.-O.; Innocenti, M.; Cecchini, F.A.-O.; Pericolini, E.A.-O.; Gabrielli, E.; Panella, S.; Antonacci, D.; et al. Evaluation of Anti-Candida activity of Vitis vinifera L. seed extracts obtained from wine and table cultivars. BioMed Res. Int. 2014, 2014, 127021. [Google Scholar] [CrossRef] [PubMed]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Lambros, M.; Tran, T.H.; Fei, Q.; Nicolaou, M. Citric Acid: A Multifunctional Pharmaceutical Excipient. Pharmaceutics 2022, 14, 972. [Google Scholar] [CrossRef] [PubMed]
- Nangare, S.; Vispute, Y.; Tade, R.; Dugam, S.; Patil, P. Pharmaceutical applications of citric acid. Future J. Pharm. Sci. 2021, 7, 54. [Google Scholar] [CrossRef]
- Giraldo-Silva, L.; Ferreira, B.; Rosa, E.; Dias, A.C.P. Opuntia ficus-indica Fruit: A Systematic Review of Its Phytochemicals and Pharmacological Activities. Plants 2023, 12, 543. [Google Scholar] [CrossRef]
- Guedes, B.N.; Fathi, F.; Silva, A.M.; Santini, A.; Oliveira, M.B.P.P.; Souto, E.B. Biopharmaceutical applications of Opuntia ficus-indica: Bibliometric map, bioactivities and extraction techniques. Eur. Food Res. Technol. 2023, 249, 2457–2469. [Google Scholar] [CrossRef]
- Sánchez, E.; Rivas Morales, C.; Castillo, S.; Leos-Rivas, C.; García-Becerra, L.; Ortiz Martínez, D.M. Antibacterial and Antibiofilm Activity of Methanolic Plant Extracts against Nosocomial Microorganisms. Evid.-Based Complement. Altern. Med. 2016, 2016, 1572697. [Google Scholar] [CrossRef]
- Smeriglio, A.; Bonasera, S.; Germanò, M.; D’Angelo, V.; Barreca, D.; Denaro, M.; Monforte, M.; Galati, E.; Trombetta, D. Opuntia ficus-indica (L.) Mill. fruit as source of betalains with antioxidant, cytoprotective, and anti-angiogenic properties. Phytother. Res. 2019, 33, 1526–1537. [Google Scholar] [CrossRef]
- Lehr, C.M.; Bouwstra, J.A.; Schacht, E.H.; Junginger, H.E. In vitro Evaluation of Mucoadhesive Properties of Chitosan and Some Other Natural Polymers. Int. J. Pharm. 1992, 78, 43–48. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. Hydrogels as mucoadhesive and bioadhesive materials: A review. Biomaterials 1996, 17, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Parodi, B.; Caviglioli, G.; Cafaggi, S.; Bignardi, G.; Milani, M.; Prini, M. Development, characterization and preliminary clinical evaluation of mucoadhesive vaginal gels containing chlorhexidine digluconate. J. Drug Deliv. Sci. Technol. 2004, 14, 489–494. [Google Scholar] [CrossRef]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why is chitosan mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar] [CrossRef]
- Avlani, D.; Kumar, A.; Shivakumar, H.N. Development of Dispersible Vaginal Tablets of Tenofovir Loaded Mucoadhesive Chitosan Microparticles for Anti-HIV Pre-Exposure Prophylaxis. Mol. Pharm. 2023, 20, 5006–5018. [Google Scholar] [CrossRef] [PubMed]
- Abou-Taleb, H.A.; Fathalla, Z.; Naguib, D.M.; Al Fatease, A.; Abdelkader, H. Chitosan/Solid-Lipid Nanoparticles Hybrid Gels for Vaginal Delivery of Estradiol for Management of Vaginal Menopausal Symptoms. Pharmaceuticals 2023, 16, 1284. [Google Scholar] [CrossRef]
- Hickey, R.J.; Zhou, X.; Pierson, J.D.; Ravel, J.; Forney, L.J. Understanding vaginal microbiome complexity from an ecological perspective. Transl. Res. 2012, 160, 267–282. [Google Scholar] [CrossRef]
- Spiegel, C.A. Bacterial vaginosis. Clin. Microbiol. Rev. 1991, 4, 485–502. [Google Scholar] [CrossRef]
- Vanic, Z.; Skalko-Basnet, N. Hydrogels for Vaginal Drug Delivery. In Functional Hydrogels in Drug Delivery: Key Features and Future Perspectives; Spizzirri, U.G., Cirillo, G., Eds.; Crc Press-Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 259–302. [Google Scholar]
- Khan, A.; Wang, B.; Ni, Y. Chitosan-Nanocellulose Composites for Regenerative Medicine Applications. Curr. Med. Chem. 2020, 27, 4584–4592. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, Y.; Dewaldt, M.; Moritz, S.; Nitzsche, R.; Kralisch, D.; Fischer, D. Controlled extended octenidine release from a bacterial nanocellulose/Poloxamer hybrid system. Eur. J. Pharm. Biopharm. 2017, 112, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Pei, L.; Zhang, W.; Shu, G.; Lin, J.; Li, H.; Xu, F.; Tang, H.; Peng, G.; Zhao, L.; et al. Chitosan-poloxamer-based thermosensitive hydrogels containing zinc gluconate/recombinant human epidermal growth factor benefit for antibacterial and wound healing. Mater. Sci. Eng. C 2021, 130, 112450. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Lien, C.-C.; Yeh, H.-J.; Yu, C.-M.; Hsu, S.-h. Bacterial cellulose and bacterial cellulose–chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef]
- Wu, Q.; Li, L. Thermal sensitive Poloxamer/Chitosan hydrogel for drug delivery in vagina. Mater. Res. Express 2020, 7, 105401. [Google Scholar] [CrossRef]
- Timur, S.S.; Şahin, A.; Aytekin, E.; Öztürk, N.; Polat, K.H.; Tezel, N.; Gürsoy, R.N.; Çalış, S. Design and in vitro evaluation of tenofovir-loaded vaginal gels for the prevention of HIV infections. Pharm. Dev. Technol. 2018, 23, 301–310. [Google Scholar] [CrossRef]
- Argenta, D.F.; Bernardo, B.d.C.; Chamorro, A.F.; Matos, P.R.; Caon, T. Thermosensitive hydrogels for vaginal delivery of secnidazole as an approach to overcome the systemic side-effects of oral preparations. Eur. J. Pharm. Sci. 2021, 159, 105722. [Google Scholar] [CrossRef]
- Carvalho, G.C.; Araujo, V.H.S.; Fonseca-Santos, B.; de Araújo, J.T.C.; de Souza, M.P.C.; Duarte, J.L.; Chorilli, M. Highlights in poloxamer-based drug delivery systems as strategy at local application for vaginal infections. Int. J. Pharm. 2021, 602, 120635. [Google Scholar] [CrossRef]
- Rimpy; Ahuja, M. Fluconazole-loaded TEOS-modified nanocellulose 3D scaffolds—Fabrication, characterization and its application as vaginal drug delivery system. J. Drug Deliv. Sci. Technol. 2022, 75, 103646. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhai, D.D.; He, Y. Graphene oxide/polyacrylamide/carboxymethyl cellulose sodium nanocomposite hydrogel with enhanced mechanical strength: Preparation, characterization and the swelling behavior. RSC Adv. 2014, 4, 44600–44609. [Google Scholar] [CrossRef]
- Lee, S.; Choi, J.; Youn, J.; Lee, Y.; Kim, W.; Choe, S.; Song, J.; Reis, R.L.; Khang, G. Development and Evaluation of Gellan Gum/Silk Fibroin/Chondroitin Sulfate Ternary Injectable Hydrogel for Cartilage Tissue Engineering. Biomolecules 2021, 11, 1184. [Google Scholar] [CrossRef]
- Jin, Z.Y.; Zhou, H.W.; Lai, J.L.; Jin, X.L.; Liu, H.B.; Wu, P.; Chen, W.X.; Ma, A.J. Self-Recoverable, Stretchable, and Sensitive Wearable Sensors Based on Ternary Semi-interpenetrating Ionic Hydrogels. Acs Appl. Polym. Mater. 2021, 3, 2732–2741. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Espinosa-Andrews, H.; Velasquillo-Martinez, C.; Garcia-Carvajal, Z.Y. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Gokila, S.; Gomathi, T.; Vijayalakshmi, K.; Alsharani, F.A.; Anil, S.; Sudha, P.N. Development of 3D scaffolds using nanochitosan/silk-fibroin/hyaluronic acid biomaterials for tissue engineering applications. Int. J. Biol. Macromol. 2018, 120, 876–885. [Google Scholar] [CrossRef]
- Tang, R.X.; Meng, Q.Y.; Wang, Z.S.; Lu, C.J.; Zhang, M.H.; Li, C.C.; Li, Y.Y.; Shen, X.P.; Sun, Q.F. Multifunctional Ternary Hybrid Hydrogel Sensor Prepared via the Synergistic Stabilization Effect. Acs Appl. Mater. Interfaces 2021, 13, 57725–57734. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qin, H.L.; Zhang, T.; Cong, H.P.; Yu, S.H. Highly Tough Bioinspired Ternary Hydrogels Synergistically Reinforced by Graphene/Xonotlite Network. Small 2018, 14, 1800673. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, J.X.; Li, P.; Fan, Y.B. Ternary hydrogels with tunable mechanical and self-healing properties based on the synergistic effects of multiple dynamic bonds. J. Mater. Chem. B 2020, 8, 4660–4671. [Google Scholar] [CrossRef]
- Costa, E.D.; Pereira, M.M.; Mansur, H.S. Properties and biocompatibility of chitosan films modified by blending with PVA and chemically crosslinked. J. Mater. Sci.-Mater. Med. 2009, 20, 553–561. [Google Scholar] [CrossRef]
- Molina, R.; Jovancic, P.; Vilchez, S.; Tzanov, T.; Solans, C. In situ chitosan gelation initiated by atmospheric plasma treatment. Carbohydr. Polym. 2014, 103, 472–479. [Google Scholar] [CrossRef]
- Cabana, A.; AitKadi, A.; Juhasz, J. Study of the gelation process of polyethylene oxide(a) polypropylene oxide(b) polyethylene oxide(a) copolymer (Poloxamer 407) aqueous solutions. J. Colloid Interface Sci. 1997, 190, 307–312. [Google Scholar] [CrossRef]
- Van Ngo, H.; Park, C.; Tran, T.T.D.; Nguyen, V.; Lee, B.J. Mechanistic understanding of salt-induced drug encapsulation in nanosuspension via acid-base neutralization as a nanonization platform technology to enhance dissolution rate of pH-dependent poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2020, 154, 8–17. [Google Scholar] [CrossRef]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2017, 52, 456–506. [Google Scholar] [CrossRef]
- Yildirim, O.E.; Basaran, O.A. Deformation and breakup of stretching bridges of Newtonian and sheer-thinning liquids: Comparison of one- and two-dimensional models. Chem. Eng. Sci. 2001, 56, 211–233. [Google Scholar] [CrossRef]
- Perrin, C.L.; Tardy, P.M.J.; Sorbie, K.S.; Crawshaw, J.C. Experimental and modeling study of Newtonian and non-Newtonian fluid flow in pore network micromodels. J. Colloid Interface Sci. 2006, 295, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, M.; Hoshahili, A.R.T.; Ramaswamy, H.S. Rheological properties of selected hydrocolloids as a function of concentration and temperature. Food Res. Int. 2001, 34, 695–703. [Google Scholar] [CrossRef]
- Coussot, P. Yield stress fluid flows: A review of experimental data. J. Non-Newton. Fluid Mech. 2014, 211, 31–49. [Google Scholar] [CrossRef]
- Chen, T. Rheology: Basic Theory and Applications Training. Available online: https://www.tainstruments.com/wp-content/uploads/2020-Rheology-Online-Training-2.pdf (accessed on 27 September 2023).
- El-Badry, M.; Hassan, M.A.; Ibrahim, M.A.; Elsaghir, H. Performance of Poloxamer 407 as hydrophilic carrier on the binary mixtures with nimesulide. Farmacia 2013, 61, 1137–1150. [Google Scholar]
- Han, Y.F.; Duan, Q.; Li, Y.Q.; Li, Y.H.; Tian, J. Preparation and Characterization of Chitosan-Based Nanoparticles as Protein Delivery System. Adv. Polym. Technol. 2018, 37, 1214–1220. [Google Scholar] [CrossRef]
- Owen, D.H.; Peters, J.J.; Katz, D.F. Rheological properties of contraceptive gels. Contraception 2000, 62, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Shibeshi, S.S.; Collins, W.E. The rheology of blood flow in a branched arterial system. Appl. Rheol. 2005, 15, 398–405. [Google Scholar] [CrossRef]
- Wan, Y.C.; Qiu, W.L.; Zhu, H.; Zhang, Q.; Zhu, S.P. Engineering cohesion and adhesion through dynamic bonds for advanced adhesive materials. Can. J. Chem. Eng. 2023, 101, 4941–4954. [Google Scholar] [CrossRef]
- Yang, J.W.; Bai, R.B.; Chen, B.H.; Suo, Z.G. Hydrogel Adhesion: A Supramolecular Synergy of Chemistry, Topology, and Mechanics. Adv. Funct. Mater. 2020, 30, 27. [Google Scholar] [CrossRef]
- Mateescu, M.; Baixe, S.; Garnier, T.; Jierry, L.; Ball, V.; Haikel, Y.; Metz-Boutigue, M.H.; Nardin, M.; Schaaf, P.; Etienne, O.; et al. Antibacterial Peptide-Based Gel for Prevention of Medical Implanted-Device Infection. PLoS ONE 2015, 10, e0145143. [Google Scholar] [CrossRef]
- Sutherland, A.; Tester, R.; Al-Ghazzewi, F.; McCulloch, E.; Connolly, M. Glucomannan hydrolysate (GMH) inhibition of Candida albicans growth in the presence of Lactobacillus and Lactococcus species. Microb. Ecol. Health Dis. 2008, 20, 127–134. [Google Scholar] [CrossRef]
- García-Gamboa, R.; Domínguez-Simi, M.Á.; Gradilla-Hernández, M.S.; Bravo-Madrigal, J.; Moya, A.; González-Avila, M. Antimicrobial and Antibiofilm Effect of Inulin-Type Fructans, Used in Synbiotic Combination with Lactobacillus spp. Against Candida albicans. Plant Foods Hum. Nutr. 2022, 77, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, K.; Kunicka-Styczyńska, A.; Maroszyńska, M. Selected Essential Oils as Antifungal Agents Against Antibiotic-Resistant Candida spp.: In Vitro Study on Clinical and Food-Borne Isolates. Microb. Drug Resist. 2016, 23, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Romejko, K.; Markowska, M.; Niemczyk, S. The Review of Current Knowledge on Neutrophil Gelatinase-Associated Lipocalin (NGAL). Int. J. Mol. Sci. 2023, 24, 10470. [Google Scholar] [CrossRef]
- Virzì, G.M.; Clementi, A.; de Cal, M.; Cruz, D.N.; Ronco, C. Genomics and Biological Activity of Neutrophil Gelatinase-Associated Lipocalin in Several Clinical Settings. Blood Purif. 2013, 35, 139–143. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef]
- Mashatan, N.; Heidari, R.; Altafi, M.; Amir, A.; Ommati, M.M.; Hashemzaei, M. Probiotics in vaginal health. Pathog. Dis. 2023, 81, ftad012. [Google Scholar] [CrossRef]
- Herbein, G.; Wendling, D. Histone deacetylases in viral infections. Clin. Epigenetics 2010, 1, 13–24. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Bui, Q.A.; Huynh, P.D.; Nguyen, Q.H.; Tran, N.Q.; Viet, N.T.; Nguyen, D.T. Curcumin and Paclitaxel Co-Loaded Heparin and Poloxamer P403 Hybrid Nanocarrier for Improved Synergistic Efficacy in Breast Cancer. Curr. Drug Deliv. 2022, 19, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Pozharani, L.B.; Baloglu, E.; Suer, K.; Guler, E.; Burgaz, V.; Kunter, I. Development and optimization of in-situ gels for vaginal delivery of metronidazole and curcumin via box-behnken design: In vitro characterization and anti-trichomonas activity. J. Drug Deliv. Sci. Technol. 2023, 86, 104739. [Google Scholar] [CrossRef]
- Zahir-Jouzdani, F.; Wolf, J.D.; Atyabi, F.; Bernkop-Schnurch, A. In situ gelling and mucoadhesive polymers: Why do they need each other? Expert Opin. Drug Deliv. 2018, 15, 1007–1019. [Google Scholar] [CrossRef]
- Jalalvandi, E.; Shavandi, A. In situ-forming and pH-responsive hydrogel based on chitosan for vaginal delivery of therapeutic agents. J. Mater. Sci.-Mater. Med. 2018, 29, 158. [Google Scholar] [CrossRef]
- Balakrishnan, S.A.-O.; Yamang, H.A.-O.; Lorenz, M.C.; Chew, S.A.-O.X.; Than, L.A.-O. Role of Vaginal Mucosa, Host Immunity and Microbiota in Vulvovaginal Candidiasis. Pathogens 2022, 11, 618. [Google Scholar] [CrossRef]
- Vagios, S.; Mitchell, C.M. Mutual Preservation: A Review of Interactions Between Cervicovaginal Mucus and Microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 676114. [Google Scholar] [CrossRef]
- Prasitsilp, M.; Jenwithisuk, R.; Kongsuwan, K.; Damrongchai, N.; Watts, P. Cellular responses to chitosan in vitro: The importance of deacetylation. J. Mater. Sci. Mater. Med. 2000, 11, 773–778. [Google Scholar] [CrossRef]
- Xi Loh, E.Y.; Fauzi, M.B.; Ng, M.H.; Ng, P.Y.; Ng, S.F.; Ariffin, H.; Mohd Amin, M.C.I. Cellular and Molecular Interaction of Human Dermal Fibroblasts with Bacterial Nanocellulose Composite Hydrogel for Tissue Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 39532–39543. [Google Scholar] [CrossRef] [PubMed]
- Alessandro Di, C.; Beniamino, P.; Maria, A.; Julio Cesar, M.-M.; Tommaso, I. Mechanisms and therapeutic effectiveness of lactobacilli. J. Clin. Pathol. 2016, 69, 187. [Google Scholar] [CrossRef]
- Liu, P.; Lu, Y.; Li, R.; Chen, X. Use of probiotic lactobacilli in the treatment of vaginal infections: In vitro and in vivo investigations. Front. Cell. Infect. Microbiol. 2023, 13, 1153894. [Google Scholar] [CrossRef] [PubMed]
- Rampersaud, R.; Randis, T.M.; Ratner, A.J. Microbiota of the upper and lower genital tract. Semin. Fetal Neonatal Med. 2012, 17, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Willems, H.M.E.; Ahmed, S.S.; Liu, J.; Xu, Z.; Peters, B.M. Vulvovaginal Candidiasis: A Current Understanding and Burning Questions. J. Fungi 2020, 6, 27. [Google Scholar] [CrossRef]
- Halstead, F.D.; Rauf, M.; Moiemen, N.S.; Bamford, A.; Wearn, C.M.; Fraise, A.P.; Lund, P.A.; Oppenheim, B.A.; Webber, M.A. The Antibacterial Activity of Acetic Acid against Biofilm-Producing Pathogens of Relevance to Burns Patients. PLoS ONE 2015, 10, e0136190. [Google Scholar] [CrossRef] [PubMed]
- Mantle, M.; Allen, A. A Colorimetric Assay for Glycoproteins Based on the Periodic Acid/Schiff Stain. Biochem. Soc. Trans. 1978, 6, 607–609. [Google Scholar] [CrossRef]
- Hejjaji, E.; Smith, A.; Morris, G. Evaluation of the mucoadhesive properties of chitosan nanoparticles prepared using different chitosan to tripolyphosphate (CS:TPP) ratios. Int. J. Biol. Macromol. 2018, 120, 1610–1617. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival—Application to proliferation and cyto-toxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Gingasu, D.; Mindru, I.; Patron, L.; Calderon-Moreno, J.; Mocioiu, O.C.; Preda, S.; Stanica, N.; Nita, S.; Dobre, N.; Popa, M.; et al. Green Synthesis Methods of CoFe2O4 and Ag-CoFe2O4 Nanoparticles Using Hibiscus Extracts and Their Antimicrobial Potential. J. Nanomater. 2016, 2016, 2106756. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Kacurakova, M.; Mathlouthi, M. FTIR and laser-Raman spectra of oligosaccharides in water: Characterization of the glycosidic bond. Carbohydr. Res. 1996, 284, 145–157. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2004. [Google Scholar]
- Balaban, A.T.; Banciu, M.; Pogany, I.I. Aplicatii ale Metodelor Fizice in Chimica Organica-RO (Applications of Physical Methods in Organic Chemistry); Editura Stiintifica si Enciclopedica: Bucuresti, Romania, 1983; p. 288. [Google Scholar]
- Domszy, J.G.; Roberts, G.A.F. Evaluation of infrared spectroscopic techniques for analysing chitosan. Die Makromol. Chem. 1985, 186, 1671–1677. [Google Scholar] [CrossRef]
- Baxter, A.; Dillon, M.; Taylor, K.D.; Roberts, G.A. Improved method for i.r. determination of the degree of N-acetylation of chitosan. Int. J. Biol. Macromol. 1992, 14, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Peh, K.K.; Ch’ng, H.S. Reporting degree of deacetylation values of chitosan: The influence of analytical methods. J. Pharm. Pharm. Sci. 2002, 5, 205–212. [Google Scholar]
- Dash, M.; Chiellini, F.; Fernandez, E.G.; Piras, A.M.; Chiellini, E. Statistical approach to the spectroscopic determination of the deacetylation degree of chitins and chitosans. Carbohydr. Polym. 2011, 86, 65–71. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Munir, A. Bioinspired sodium alginate based thermosensitive hydrogel membranes for accelerated wound healing. Int. J. Biol. Macromol. 2020, 155, 751–765. [Google Scholar] [CrossRef]
- Fonseca-Garcia, A.; Caicedo, C.; Jimenez-Regalado, E.J.; Morales, G.; Aguirre-Loredo, R.Y. Effects of Poloxamer Content and Storage Time of Biodegradable Starch-Chitosan Films on Its Thermal, Structural, Mechanical, and Morphological Properties. Polymers 2021, 13, 2341. [Google Scholar] [CrossRef]
- Bashir, M.; Syed, H.K.; Asghar, S.; Irfan, M.; Almalki, W.H.; Menshawi, S.A.; Khan, I.U.; Shah, P.A.; Khalid, I.; Ahmad, J.; et al. Effect of Hydrophilic Polymers on Complexation Efficiency of Cyclodextrins in Enhancing Solubility and Release of Diflunisal. Polymers 2020, 12, 1564. [Google Scholar] [CrossRef]
- Kacurakova, M.; Smith, A.C.; Gidley, M.J.; Wilson, R.H. Molecular interactions in bacterial cellulose composites studied by 1D FT-IR and dynamic 2D FT-IR spectroscopy. Carbohydr. Res. 2002, 337, 1145–1153. [Google Scholar] [CrossRef]
- Nikonenko, N.A.; Buslov, D.K.; Sushko, N.I.; Zhbankov, R.G. Investigation of stretching vibrations of glycosidic linkages in disaccharides and polysaccarides with use of IR spectra deconvolution. Biopolymers 2000, 57, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Kruer-Zerhusen, N.; Cantero-Tubilla, B.; Wilson, D.B. Characterization of cellulose crystallinity after enzymatic treatment using Fourier transform infrared spectroscopy (FTIR). Cellulose 2018, 25, 37–48. [Google Scholar] [CrossRef]
| Average Diameter of the Inhibition Zone (cm) ± Standard Error (SE) | |||||||
|---|---|---|---|---|---|---|---|
| Strain | Dose | H1 | C1 | H2 | C2 | H3 | C3 |
| E. coli | 25 µL hydrogel | 1.13 ± 0.08 c | 0 | 1.01 ± 0.004 b | 0 | 0.74 ± 0.008 a | 0.19 ± 0.08 |
| 100 µL hydrogel | 1.62 ± 0.01 a | 0 | 1.61± 0.004 a | 0 | 1.74 ± 0.006 b | 1.26 ± 0.03 | |
| 25 µL of 2:1 diluted hydrogel (v/v) | 1.27 ± 0.01 b | - | 1.21 ± 0.02 b | - | 0.81 ± 0.05 a | - | |
| C. albicans | 25 µL hydrogel | 0.28 ± 0.01 a | 0 | 0.31 ± 0.01 a | 0 | 1.01 ± 0.01 b | 0 |
| 100 µL hydrogel | 1.27 ± 0.01 a | 0 | 1.15 ± 0.02 b | 0 | 1.4 ± 0.04 c | 0 | |
| 25 µL of 2:1 diluted hydrogel (v/v) | 0 a | - | 0 a | - | 0.65 ± 0.01 b | - | |
| Inhibition of Bacterial Biofilm (% of C+) | |||
|---|---|---|---|
| H1 | H2 | H3 | |
| 1.5 × 107 bacterial cells | 60.75 ± 1.08 | 100 | 23.90 ± 3.80 |
| 1.5 × 106 bacterial cells | 60.40 ± 3.08 | 100 | 26.90 ± 1.66 |
| 1.5 × 105 bacterial cells | 100 | 100 | 26.36 ± 1.60 |
| 1.5 × 104 bacterial cells | 100 | 100 | 32.30 ± 0.44 |
| 1.5 × 103 bacterial cells | 100 | 100 | 37.75 ± 2.40 |
| Compounds | B1,2 | H1 | H2 | B3 | H3 |
|---|---|---|---|---|---|
| PX (w/v) | 15% | 15% | 15% | 5% | 5% |
| BNC (w/v) | 0.4% | 0.4% | 0.4% | 0.4% | 0.4% |
| CS (w/v) | - | - | - | 3% | 3% |
| Inulin (w/v) | - | 3% | 3% | - | 3% |
| Thyme essential oil (v/v) | - | 0.5% | 0.5% | - | 0.5% |
| Hydro-glycero-alcoholic extract of Vitis vinifera (v/v) | - | 0.5% | 0.5% | - | 0.5% |
| Opuntia ficus-indica powder (w/v) | - | - | 0.1% | - | - |
| Lactic acid (v/v) | - | 6% | 3% | - | 3% |
| Citric acid (w/v) | - | 3% | 3% | - | 3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraru, A.; Dima, Ș.-O.; Tritean, N.; Oprița, E.-I.; Prelipcean, A.-M.; Trică, B.; Oancea, A.; Moraru, I.; Constantinescu-Aruxandei, D.; Oancea, F. Bioactive-Loaded Hydrogels Based on Bacterial Nanocellulose, Chitosan, and Poloxamer for Rebalancing Vaginal Microbiota. Pharmaceuticals 2023, 16, 1671. https://doi.org/10.3390/ph16121671
Moraru A, Dima Ș-O, Tritean N, Oprița E-I, Prelipcean A-M, Trică B, Oancea A, Moraru I, Constantinescu-Aruxandei D, Oancea F. Bioactive-Loaded Hydrogels Based on Bacterial Nanocellulose, Chitosan, and Poloxamer for Rebalancing Vaginal Microbiota. Pharmaceuticals. 2023; 16(12):1671. https://doi.org/10.3390/ph16121671
Chicago/Turabian StyleMoraru, Angela, Ștefan-Ovidiu Dima, Naomi Tritean, Elena-Iulia Oprița, Ana-Maria Prelipcean, Bogdan Trică, Anca Oancea, Ionuț Moraru, Diana Constantinescu-Aruxandei, and Florin Oancea. 2023. "Bioactive-Loaded Hydrogels Based on Bacterial Nanocellulose, Chitosan, and Poloxamer for Rebalancing Vaginal Microbiota" Pharmaceuticals 16, no. 12: 1671. https://doi.org/10.3390/ph16121671
APA StyleMoraru, A., Dima, Ș.-O., Tritean, N., Oprița, E.-I., Prelipcean, A.-M., Trică, B., Oancea, A., Moraru, I., Constantinescu-Aruxandei, D., & Oancea, F. (2023). Bioactive-Loaded Hydrogels Based on Bacterial Nanocellulose, Chitosan, and Poloxamer for Rebalancing Vaginal Microbiota. Pharmaceuticals, 16(12), 1671. https://doi.org/10.3390/ph16121671