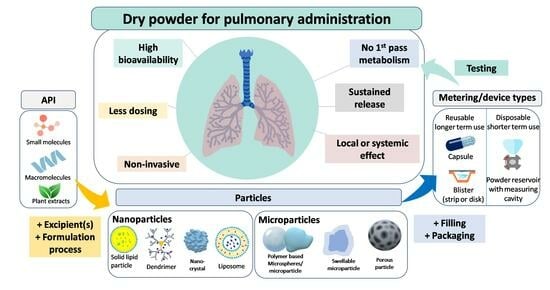
Inhalation Dosage Forms: A Focus on Dry Powder Inhalers and Their Advancements
Abstract
1. Introduction
2. Pulmonary Delivery: An Overview
2.1. Advantages of the Pulmonary Drug Delivery
2.2. Particle Size and Drug Deposition in the Lungs
- = probability of impaction
- θ = bending angle (change in the direction of the flow)
- Stk = Stokes number, defined as (Equation (2)):
- ρ = particle density
- = particle diameter
- υ = particle velocity
- µ = viscosity of fluid
- D = airway diameter
- = probability of sedimentation
- g = gravitational force
- C = Cunningham slip angle correction factor
- ρ = particle density
- = particle diameter
- L = length of the tube
- ø = inclination angle relative to gravity
- µ = viscosity of fluid
- R = radius of the airways
- υ = particle velocity
- = probability of diffusion
- K = Boltzmann’s constant
- T = absolute temperature
- C = Cunningham slip angle correction factor
- η = viscosity of gas
- = particle diameter
- R = airway diameter
3. Particle Engineering Techniques for DPI Formulations
3.1. Manufacturing Procedures for DPI Formulations
3.1.1. Milling
3.1.2. Spray Drying
3.1.3. Spray Freeze Drying (SFD)
3.1.4. Super Critical Fluid (SCF) Drying
3.1.5. Electrospinning
3.1.6. Thin Film Freezing (TFF)
3.2. Excipients
- Physico-chemical stability and compatibility with most low molecular weight drugs;
- Safe toxicological profile;
- Availability and affordability;
- Less hygroscopic than other sugars.
3.3. Types of Particles
3.4. Powder Processing in DPI Formulations
3.4.1. Powder Physico-Chemical Characterization in DPI Formulations
3.4.2. Formulation Characteristics of DPIs
3.5. DPI Formulations Testing
4. Design and Performance Considerations for Inhaler Devices
4.1. Performance Assessment of Inhalers
- R = resistance
- = pressure drop
- = flow rate
4.2. Patient Compliance and Device Optimization
4.3. Impact of Storage Conditions on DPI Capsules and Blisters
5. Inhalation Delivery Systems: Dry Powder Inhalers
5.1. Dry Powder Inhalers
5.1.1. Advantages of DPIs
5.1.2. Innovations in DPI Technology
5.1.3. Classifications of DPIs
5.1.4. Considerations in DPI Selection
6. Advancements in Inhaler Devices: Ideal Characteristics and Marketed Innovations
6.1. Ideal Characteristics of Inhaler Devices
6.2. Current Marketed Inhalers and Inhalation Therapy Innovations
6.3. Biocompatibility of DPIs
6.4. Significance of the Evolution of Inhaler Technology
6.4.1. Improved Efficiency
6.4.2. Improved Dispersion
6.4.3. Improved Patient Adherence
6.4.4. Alternative Dosage Forms
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, P.J. History of Aerosol Therapy: Liquid Nebulization to MDIs to DPIs. Respir. Care 2005, 50, 1139. [Google Scholar]
- Stein, S.W.; Thiel, C.G. The History of Therapeutic Aerosols: A Chronological Review. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Loo, C.-Y.; Traini, D.; Young, P.M. Inhalation of nanoparticle-based drug for lung cancer treatment: Advantages and challenges. Asian J. Pharm. Sci. 2015, 10, 481–489. [Google Scholar] [CrossRef]
- Ambrus, R.; Benke, E.; Farkas, Á.; Balásházy, I.; Szabó-Révész, P. Novel dry powder inhaler formulation containing antibiotic using combined technology to improve aerodynamic properties. Eur. J. Pharm. Sci. 2018, 123, 20–27. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; El-Baz, N.M.; Yacoub, M.H. Inhaled nano- and microparticles for drug delivery. Glob. Cardiol. Sci. Pract. 2015, 2015, 2. [Google Scholar] [CrossRef]
- Ashley, W.; Kai, M.B.; Hironori, S.; Simon, A.; Emmanuel, A.-Y.; Javaid, K.; Jørgen, V.; Helen, T. The environmental impact of inhaled therapy: Making informed treatment choices. Eur. Respir. J. 2022, 60, 2102106. [Google Scholar] [CrossRef]
- Buttini, F.; Quarta, E.; Allegrini, C.; Lavorini, F. Understanding the Importance of Capsules in Dry Powder Inhalers. Pharmaceutics 2021, 13, 1936. [Google Scholar] [CrossRef]
- de Boer, A.H.; Hagedoorn, P.; Hoppentocht, M.; Buttini, F.; Grasmeijer, F.; Frijlink, H.W. Dry powder inhalation: Past, present and future. Expert Opin. Drug Deliv. 2017, 14, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, S.I.; Sayed, M.A.; Mustafiz, M.I.; Kabir, M.J.; Aktar, J. Metered dose inhaler (MDI) versus dry powder inhaler (DPI): Patient’s compliance variation in asthma medication at rural Bangladesh perspective. J. Dent. Med. Sci. 2018, 17, 66–75. [Google Scholar]
- Clinical Trials. Available online: https://www.clinicaltrials.gov/ct2/home (accessed on 31 July 2023).
- Nelson, H.S. Inhalation devices, delivery systems, and patient technique. Ann. Allergy Asthma Immunol. 2016, 117, 606–612. [Google Scholar] [CrossRef]
- Rajendran, R.; Balan, R.; Ganesan, N.; Thiruvengadam, D. Recent modalities in drug delivery via inhalation therapy–An advanced treatment strategy for pulmonary Carcinoma. Int. J. Pharm. Pharm. Sci. 2015, 7, 8–21. [Google Scholar]
- Hickey, A.J. Back to the future: Inhaled drug products. J. Pharm. Sci. 2013, 102, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.; Zimmer, A.; Pardeike, J. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for pulmonary application: A review of the state of the art. Eur. J. Pharm. Biopharm. 2014, 86, 7–22. [Google Scholar] [CrossRef]
- Lexmond, A.; Forbes, B. Drug Delivery Devices for Inhaled Medicines. Handb. Exp. Pharmacol. 2017, 237, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Rangaraj, N.; Pailla, S.R.; Sampathi, S. Insight into pulmonary drug delivery: Mechanism of drug deposition to device characterization and regulatory requirements. Pulm. Pharmacol. Ther. 2019, 54, 1–21. [Google Scholar] [CrossRef]
- Darquenne, C. Deposition Mechanisms. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 181–185. [Google Scholar] [CrossRef]
- Darquenne, C. Aerosol deposition in health and disease. J. Aerosol Med. Pulm. Drug Deliv. 2012, 25, 140–147. [Google Scholar] [CrossRef]
- James, A.C.; Stahlhofen, W.; Rudolf, G.; Köbrich, R.; Briant, J.K.; Egan, M.J.; Nixon, W.; Birchall, A.G.; Annexe, D. deposition of inhaled particles. Ann. ICRP 1994, 24, 231–299. [Google Scholar] [CrossRef]
- Peng, T.; Lin, S.; Niu, B.; Wang, X.; Huang, Y.; Zhang, X.; Li, G.; Pan, X.; Wu, C. Influence of physical properties of carrier on the performance of dry powder inhalers. Acta Pharm. Sin. B 2016, 6, 308–318. [Google Scholar] [CrossRef]
- Yang, M.Y.; Chan, J.G.Y.; Chan, H.-K. Pulmonary drug delivery by powder aerosols. J. Control. Release 2014, 193, 228–240. [Google Scholar] [CrossRef]
- Kleinstreuer, C.; Zhang, Z.; Kim, C. Combined inertial and gravitational deposition of microparticles in small model airways of a human respiratory system. J. Aerosol Sci. 2007, 38, 1047–1061. [Google Scholar] [CrossRef]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef]
- Patton, J.S. Mechanisms of macromolecule absorption by the lungs. Adv. Drug Deliv. Rev. 1996, 19, 3–36. [Google Scholar] [CrossRef]
- Zanen, P.; Go, L.T.; Lammers, J.W. Optimal particle size for beta 2 agonist and anticholinergic aerosols in patients with severe airflow obstruction. Thorax 1996, 51, 977–980. [Google Scholar] [CrossRef]
- Cha, M.L.; Costa, L.R. Inhalation Therapy in Horses. Vet. Clin. N. Am. Equine Pract. 2017, 33, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Gokarn, Y.; Mitragotri, S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 2019, 18, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Hoppentocht, M.; Hagedoorn, P.; Frijlink, H.W.; de Boer, A.H. Technological and practical challenges of dry powder inhalers and formulations. Adv. Drug Deliv. Rev. 2014, 75, 18–31. [Google Scholar] [CrossRef]
- Shetty, N.; Cipolla, D.; Park, H.; Zhou, Q.T. Physical stability of dry powder inhaler formulations. Expert Opin. Drug Deliv. 2020, 17, 77–96. [Google Scholar] [CrossRef]
- Lau, M.; Young, P.M.; Traini, D. Co-milled API-lactose systems for inhalation therapy: Impact of magnesium stearate on physico-chemical stability and aerosolization performance. Drug Dev. Ind. Pharm. 2017, 43, 980–988. [Google Scholar] [CrossRef]
- Chougule, M.B.; Padhi, B.K.; Jinturkar, K.A.; Misra, A. Development of dry powder inhalers. Recent Pat. Drug Deliv. Formul. 2007, 1, 11–21. [Google Scholar] [CrossRef]
- Gradon, L.; Sosnowski, T.R. Formation of particles for dry powder inhalers. Adv. Powder Technol. 2014, 25, 43–55. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Chan, H.-K. Advancements in Particle Engineering for Inhalation Delivery of Small Molecules and Biotherapeutics. Pharm. Res. 2022, 39, 3047–3061. [Google Scholar] [CrossRef]
- Ruangchayajatuporn, J.; Amornsakchai, T.; Sinchaipanid, N.; Mitrevej, A. Compaction behavior and optimization of spray-dried lactose with various amorphous content. J. Drug Deliv. Sci. Technol. 2011, 21, 175–181. [Google Scholar] [CrossRef]
- Vromans, H.; Bolhuis, G.K.; Lerk, C.F.; van de Biggelaar, H.; Bosch, H. Studies on tableting properties of lactose. VII. The effect of variations in primary particle size and percentage of amorphous lactose in spray dried lactose products. Int. J. Pharm. 1987, 35, 29–37. [Google Scholar] [CrossRef]
- Haferkamp, L.; Haudenschild, L.; Spierings, A.; Wegener, K.; Riener, K.; Ziegelmeier, S.; Leichtfried, G.J. The Influence of Particle Shape, Powder Flowability, and Powder Layer Density on Part Density in Laser Powder Bed Fusion. Metals 2021, 11, 418. [Google Scholar] [CrossRef]
- Rassu, G.; Eissens, A.C.; Bolhuis, G.K. Tableting properties of an improved spray-dried lactose. J. Drug Deliv. Sci. Technol. 2006, 16, 455–459. [Google Scholar] [CrossRef]
- Vectura Group Ltd. Available online: https://www.vectura.com/services/formulation/dry-powder-formulation-development/ (accessed on 10 November 2023).
- D’Addio, S.M.; Chan, J.G.Y.; Kwok, P.C.L.; Benson, B.R.; Prud’homme, R.K.; Chan, H.-K. Aerosol Delivery of Nanoparticles in Uniform Mannitol Carriers Formulated by Ultrasonic Spray Freeze Drying. Pharm. Res. 2013, 30, 2891–2901. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, J.; Deng, S. Applications of Supercritical Anti-Solvent Process in Preparation of Solid Multicomponent Systems. Pharmaceutics 2021, 13, 475. [Google Scholar] [CrossRef]
- Nikolaou, M.; Krasia-Christoforou, T. Electrohydrodynamic methods for the development of pulmonary drug delivery systems. Eur. J. Pharm. Sci. 2018, 113, 29–40. [Google Scholar] [CrossRef]
- Mehta, P.P.; Pawar, V.S. Electrospun nanofiber scaffolds: Technology and applications. In Applications of Nanocomposite Materials in Drug Delivery; Inamuddin, Asiri, A.M., Mohammad, A., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 509–573. [Google Scholar]
- Hiwrale, A.; Bharati, S.; Pingale, P.; Rajput, A. Nanofibers: A current era in drug delivery system. Heliyon 2023, 9, e18917. [Google Scholar] [CrossRef]
- Li, X.; Thakkar, S.G.; Ruwona, T.B.; Williams, R.O., 3rd; Cui, Z. A method of lyophilizing vaccines containing aluminum salts into a dry powder without causing particle aggregation or decreasing the immunogenicity following reconstitution. J. Control. Release 2015, 204, 38–50. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Watts, A.B.; Peters, J.I.; Liu, S.; Batra, A.; Williams, R.O. In Vitro and In Vivo Performance of Dry Powder Inhalation Formulations: Comparison of Particles Prepared by Thin Film Freezing and Micronization. AAPS PharmSciTech 2014, 15, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, S.R.; Kole, E.B.; Kapare, H.S.; Chandankar, S.M.; Shinde, P.J.; Boisa, G.S.; Salgaonkar, S.S.; Giram, P.S.; More, M.P.; Kolimi, P.; et al. Progress on Thin Film Freezing Technology for Dry Powder Inhalation Formulations. Pharmaceutics 2022, 14, 2632. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, S.; Sahakijpijarn, S.; Moon, C.; Cui, Z.; Williams, R.O., III. The Development of Thin-Film Freezing and Its Application to Improve Delivery of Biologics as Dry Powder Aerosols. KONA Powder Part. J. 2022, 39, 176–192. [Google Scholar] [CrossRef]
- Praphawatvet, T.; Cui, Z.; Williams, R.O. Pharmaceutical dry powders of small molecules prepared by thin-film freezing and their applications–A focus on the physical and aerosol properties of the powders. Int. J. Pharm. 2022, 629, 122357. [Google Scholar] [CrossRef]
- TFF Pharmaceuticals Inc. Available online: https://tffpharma.com/ (accessed on 10 November 2023).
- Abdellah, A.; Noordin, M.I.; Wan Ismail, W.A. Importance and globalization status of good manufacturing practice (GMP) requirements for pharmaceutical excipients. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2015, 23, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Prime, D.; Atkins, P.J.; Slater, A.; Sumby, B. Review of dry powder inhalers. Adv. Drug Deliv. Rev. 1997, 26, 51–58. [Google Scholar] [CrossRef]
- Pilcer, G.; Wauthoz, N.; Amighi, K. Lactose characteristics and the generation of the aerosol. Adv. Drug Deliv. Rev. 2012, 64, 233–256. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Cleary, M.J. Developing an efficient and reliable dry powder inhaler for pulmonary drug delivery--a review for multidisciplinary researchers. Med. Eng. Phys. 2012, 34, 409–427. [Google Scholar] [CrossRef]
- Della Bella, A.; Salomi, E.; Buttini, F.; Bettini, R. The role of the solid state and physical properties of the carrier in adhesive mixtures for lung delivery. Expert Opin. Drug Deliv. 2018, 15, 665–674. [Google Scholar] [CrossRef]
- Telko, M.J.; Hickey, A.J. Dry Powder Inhaler Formulation. Respir. Care 2005, 50, 1209. [Google Scholar]
- Larramendi, C.H.; Marco, F.M.; Llombart, M.; de la Vega, A.; Chiner, E.; García-Abujeta, J.L.; Sempere, J.M. Allergenicity of casein containing chalk in milk allergic schoolchildren. Ann. Allergy Asthma Immunol. 2013, 110, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Barbi, E.; Gerarduzzi, T.; Longo, G.; Ventura, A. Fatal allergy as a possible consequence of long-term elimination diet. Allergy 2004, 59, 668–669. [Google Scholar] [CrossRef]
- Morikawa, M.; Kanemitsu, Y.; Tsukamoto, H.; Morikawa, A.; Tomioka, Y. A Case of Anaphylaxis in the Pediatric Patient with Milk Allergy Due to Traces of Milk Protein in the Lactose Used as an Excipient of Inavir Inhalation. Arerugi 2016, 65, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Shapiro, G.G.; Beyer, K.; Bardina, L.; Sampson, H.A. Contamination of dry powder inhalers for asthma with milk proteins containing lactose. J. Allergy Clin. Immunol. 2004, 113, 558–560. [Google Scholar] [CrossRef]
- Robles, J.; Motheral, L. Hypersensitivity reaction after inhalation of a lactose-containing dry powder inhaler. J. Pediatr. Pharmacol. Ther. 2014, 19, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Sa, A.; Oliveira, L.; Miyagi, K.; Mello, Y.; Cabral, E.; Carvalho, A.; Komaroff, F.; Gonçalves, R. Reaction due to milk proteins contaminating lactose added to inhaled corticosteroid. J. Allergy Clin. Immunol. 2011, 127, AB241. [Google Scholar] [CrossRef]
- Morisset, M.; Moneret-Vautrin, D.; Commun, N.; Schuller, A.; Kanny, G. Allergy to cow milk proteins contaminating lactose, common excipient of dry powder inhalers for asthma. J. Allergy Clin. Immunol. 2006, 117, S95. [Google Scholar] [CrossRef]
- Bar-On, O.; Levine, H.; Stafler, P.; Shmueli, E.; Jacobi, E.; Goldberg, O.; Steuer, G.; Prais, D.; Mei-Zahav, M. Lactose-Containing Dry-Powder Inhalers for Patients with Cow’s Milk Protein Allergy-The Conundrum; A National Survey of Pediatric Pulmonologists and Allergologists. J. Clin. Med. 2022, 11, 7346. [Google Scholar] [CrossRef]
- de Boer, A.H.; Hagedoorn, P.; Grasmeijer, F. Dry powder inhalation, part 2: The present and future. Expert Opin. Drug Deliv. 2022, 19, 1045–1059. [Google Scholar] [CrossRef]
- Kaialy, W.; Nokhodchi, A. Dry powder inhalers: Physicochemical and aerosolization properties of several size-fractions of a promising alterative carrier, freeze-dried mannitol. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2015, 68, 56–67. [Google Scholar] [CrossRef]
- Zhang, Q.; Ames, J.M.; Smith, R.D.; Baynes, J.W.; Metz, T.O. A Perspective on the Maillard Reaction and the Analysis of Protein Glycation by Mass Spectrometry: Probing the Pathogenesis of Chronic Disease. J. Proteome Res. 2009, 8, 754–769. [Google Scholar] [CrossRef]
- Zeng, X.M.; Martin, G.P.; Tee, S.-K.; Marriott, C. The role of fine particle lactose on the dispersion and deaggregation of salbutamol sulphate in an air stream in vitro. Int. J. Pharm. 1998, 176, 99–110. [Google Scholar] [CrossRef]
- Jones, M.D.; Price, R. The influence of fine excipient particles on the performance of carrier-based dry powder inhalation formulations. Pharm. Res. 2006, 23, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Young, P.M.; Edge, S.; Traini, D.; Jones, M.D.; Price, R.; El-Sabawi, D.; Urry, C.; Smith, C. The influence of dose on the performance of dry powder inhalation systems. Int. J. Pharm. 2005, 296, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Pai, R.V.; Jain, R.R.; Bannalikar, A.S.; Menon, M.D. Development and Evaluation of Chitosan Microparticles Based Dry Powder Inhalation Formulations of Rifampicin and Rifabutin. J. Aerosol Med. Pulm. Drug Deliv. 2016, 29, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.D.; Cavaco, J.S.; Guerreiro, F.; Lourenço, J.P.; Rosa da Costa, A.M.; Grenha, A. Inhalable Antitubercular Therapy Mediated by Locust Bean Gum Microparticles. Molecules 2016, 21, 702. [Google Scholar] [CrossRef]
- Devrim, B.; Bozkir, A.; Canefe, K. Preparation and evaluation of PLGA microparticles as carrier for the pulmonary delivery of rhIL-2 : I. Effects of some formulation parameters on microparticle characteristics. J. Microencapsul. 2011, 28, 582–594. [Google Scholar] [CrossRef]
- Yildiz, A.; John, E.; Özsoy, Y.; Araman, A.; Birchall, J.C.; Broadley, K.J.; Gumbleton, M. Inhaled extended-release microparticles of heparin elicit improved pulmonary pharmacodynamics against antigen-mediated airway hyper-reactivity and inflammation. J. Control. Release 2012, 162, 456–463. [Google Scholar] [CrossRef]
- Parumasivam, T.; Leung, S.S.; Quan, D.H.; Triccas, J.A.; Britton, W.J.; Chan, H.K. Rifapentine-loaded PLGA microparticles for tuberculosis inhaled therapy: Preparation and in vitro aerosol characterization. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2016, 88, 1–11. [Google Scholar] [CrossRef]
- Tavares, M.; Cabral, R.P.; Costa, C.; Martins, P.; Fernandes, A.R.; Casimiro, T.; Aguiar-Ricardo, A. Development of PLGA dry powder microparticles by supercritical CO2-assisted spray-drying for potential vaccine delivery to the lungs. J. Supercrit. Fluids 2017, 128, 235–243. [Google Scholar] [CrossRef]
- Doan, T.V.P.; Couet, W.; Olivier, J.C. Formulation and in vitro characterization of inhalable rifampicin-loaded PLGA microspheres for sustained lung delivery. Int. J. Pharm. 2011, 414, 112–117. [Google Scholar] [CrossRef]
- Jensen, D.K.; Jensen, L.B.; Koocheki, S.; Bengtson, L.; Cun, D.; Nielsen, H.M.; Foged, C. Design of an inhalable dry powder formulation of DOTAP-modified PLGA nanoparticles loaded with siRNA. J. Control. Release 2012, 157, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Dimer, F.A.; Ortiz, M.; Pohlmann, A.R.; Guterres, S.S. Inhalable resveratrol microparticles produced by vibrational atomization spray drying for treating pulmonary arterial hypertension. J. Drug Deliv. Sci. Technol. 2015, 29, 152–158. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Prestisya, I.; Suhariyono, G.; Miatmoko, A.; Rosita, N.; Rahmadi, M. Characterization of Dry Powder Inhaler Quercetin Solid Lipid Microparticle (SLM) as Lung Delivery System: Effect of Polymer Concentration. Egypt. J. Chem. 2022, 65, 281–289. [Google Scholar] [CrossRef]
- Scalia, S.; Haghi, M.; Losi, V.; Trotta, V.; Young, P.M.; Traini, D. Quercetin solid lipid microparticles: A flavonoid for inhalation lung delivery. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 49, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Aquino, R.; Del Gaudio, P.; Colombo, P.; Russo, P. Physical characteristics and aerosol performance of naringin dry powders for pulmonary delivery prepared by spray-drying. Eur. J. Pharm. Biopharm. 2009, 72, 206–213. [Google Scholar] [CrossRef]
- Prota, L.; Santoro, A.; Bifulco, M.; Aquino, R.P.; Mencherini, T.; Russo, P. Leucine enhances aerosol performance of naringin dry powder and its activity on cystic fibrosis airway epithelial cells. Int. J. Pharm. 2011, 412, 8–19. [Google Scholar] [CrossRef]
- Corcoran, T.; Venkataramanan, R.; Hoffman, R.; George, M.; Petrov, A.; Richards, T.; Zhang, S.; Choi, J.; Gao, Y.; Oakum, C. Systemic delivery of atropine sulfate by the microdose dry-powder inhaler. J. Aerosol Med. Pulm. Drug Deliv. 2013, 26, 46–55. [Google Scholar] [CrossRef]
- Kim, I.; Byeon, H.J.; Kim, T.H.; Lee, E.S.; Oh, K.T.; Shin, B.S.; Lee, K.C.; Youn, Y.S. Doxorubicin-loaded highly porous large PLGA microparticles as a sustained- release inhalation system for the treatment of metastatic lung cancer. Biomaterials 2012, 33, 5574–5583. [Google Scholar] [CrossRef]
- Dhanda, D.S.; Tyagi, P.; Mirvish, S.S.; Kompella, U.B. Supercritical fluid technology based large porous celecoxib-PLGA microparticles do not induce pulmonary fibrosis and sustain drug delivery and efficacy for several weeks following a single dose. J. Control. Release 2013, 168, 239–250. [Google Scholar] [CrossRef]
- Ungaro, F.; Giovino, C.; Coletta, C.; Sorrentino, R.; Miro, A.; Quaglia, F. Engineering gas-foamed large porous particles for efficient local delivery of macromolecules to the lung. Eur. J. Pharm. Sci. 2010, 41, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, S.; Lu, K.; Qiu, S.; Chen, X.D.; Wu, W.D. Spray freeze dried niclosamide nanocrystals embedded dry powder for high dose pulmonary delivery. Powder Technol. 2023, 415, 118168. [Google Scholar] [CrossRef] [PubMed]
- Alipour, S.; Montaseri, H.; Tafaghodi, M. Preparation and characterization of biodegradable paclitaxel loaded alginate microparticles for pulmonary delivery. Colloids Surf. B Biointerfaces 2010, 81, 521–529. [Google Scholar] [CrossRef]
- Möbus, K.; Siepmann, J.; Bodmeier, R. Zinc-alginate microparticles for controlled pulmonary delivery of proteins prepared by spray-drying. Eur. J. Pharm. Biopharm. 2012, 81, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Adi, H.; Young, P.M.; Chan, H.K.; Salama, R.; Traini, D. Controlled release antibiotics for dry powder lung delivery. Drug Dev. Ind. Pharm. 2010, 36, 119–126. [Google Scholar] [CrossRef]
- Abbas, Y.; Azzazy, H.M.; Tammam, S.; Lamprecht, A.; Ali, M.E.; Schmidt, A.; Sollazzo, S.; Mathur, S. Development of an inhalable, stimuli-responsive particulate system for delivery to deep lung tissue. Colloids Surf. B Biointerfaces 2016, 146, 19–30. [Google Scholar] [CrossRef]
- Secret, E.; Kelly, S.J.; Crannell, K.E.; Andrew, J.S. Enzyme-responsive hydrogel microparticles for pulmonary drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 10313–10321. [Google Scholar] [CrossRef]
- Patil-Gadhe, A.; Pokharkar, V. Single step spray drying method to develop proliposomes for inhalation: A systematic study based on quality by design approach. Pulm. Pharmacol. Ther. 2014, 27, 197–207. [Google Scholar] [CrossRef]
- Yu, S.; Yuan, H.; Chai, G.; Peng, K.; Zou, P.; Li, X.; Li, J.; Zhou, F.; Chan, H.K.; Zhou, Q.T. Optimization of inhalable liposomal powder formulations and evaluation of their in vitro drug delivery behavior in Calu-3 human lung epithelial cells. Int. J. Pharm. 2020, 586, 119570. [Google Scholar] [CrossRef]
- Cipolla, D.; Blanchard, J.; Gonda, I. Development of Liposomal Ciprofloxacin to Treat Lung Infections. Pharmaceutics 2016, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Chimote, G.; Banerjee, R. In vitro evaluation of inhalable isoniazid-loaded surfactant liposomes as an adjunct therapy in pulmonary tuberculosis. J. Biomed Mater. Res. B Appl. Biomater. 2010, 94, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chono, S.; Togami, K.; Itagaki, S. Aerosolized liposomes with dipalmitoyl phosphatidylcholine enhance pulmonary absorption of encapsulated insulin compared with co-administered insulin. Drug Dev. Ind. Pharm. 2017, 43, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, H.; Lu, X.; Jiang, L.; Xi, X.; Liu, J.; Zhu, J. Development and evaluation of a dry powder formulation of liposome-encapsulated oseltamivir phosphate for inhalation. Drug Deliv. 2015, 22, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Adel, I.M.; ElMeligy, M.F.; Abdelrahim, M.E.A.; Maged, A.; Abdelkhalek, A.A.; Abdelmoteleb, A.M.M.; Elkasabgy, N.A. Design and Characterization of Spray-Dried Proliposomes for the Pulmonary Delivery of Curcumin. Int. J. Nanomed. 2021, 16, 2667–2687. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.; Pandya, T.; Gandhi, R.; Patel, S.; Mashru, R.; Misra, A.; Tandel, H. Inhalable liposomal dry powder of gemcitabine-HCl: Formulation, in vitro characterization and in vivo studies. Int. J. Pharm. 2015, 496, 886–895. [Google Scholar] [CrossRef]
- Huang, W.H.; Yang, Z.J.; Wu, H.; Wong, Y.F.; Zhao, Z.Z.; Liu, L. Development of liposomal salbutamol sulfate dry powder inhaler formulation. Biol. Pharm. Bull 2010, 33, 512–517. [Google Scholar] [CrossRef][Green Version]
- Chen, X.; Huang, W.; Wong, B.C.; Yin, L.; Wong, Y.F.; Xu, M.; Yang, Z. Liposomes prolong the therapeutic effect of anti-asthmatic medication via pulmonary delivery. Int. J. Nanomed. 2012, 7, 1139–1148. [Google Scholar] [CrossRef]
- Changsan, N.; Chan, H.K.; Separovic, F.; Srichana, T. Physicochemical Characterization and Stability of Rifampicin Liposome Dry Powder Formulations for Inhalation. J. Pharm. Sci. 2009, 98, 628–639. [Google Scholar] [CrossRef]
- Chougule, M.; Padhi, B.; Misra, A. Nano-liposomal dry powder inhaler of tacrolimus: Preparation, characterization, and pulmonary pharmacokinetics. Int. J. Nanomed. 2007, 2, 675–688. [Google Scholar]
- Chougule, M.; Padhi, B.; Misra, A. Development of Spray Dried Liposomal Dry Powder Inhaler of Dapsone. AAPS PharmSciTech 2008, 9, 47–53. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Ge, Y.; Hu, Y.; Li, M.; Jin, Y. Inhalation treatment of primary lung cancer using liposomal curcumin dry powder inhalers. Acta Pharm. Sin. B 2018, 8, 440–448. [Google Scholar] [CrossRef]
- Ezzati NazhadDolatabadi, J.; Hamishehkar, H.; Valizadeh, H. Development of dry powder inhaler formulation loaded with alendronate solid lipid nanoparticles: Solid-state characterization and aerosol dispersion performance. Drug Dev. Ind. Pharm. 2015, 41, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Ghaffari, S.; Mirshojaei, S.F.; Jafarian, A.; Atyabi, F.; Kobarfard, F.; Azarmi, S. Biodistribution of amikacin solid lipid nanoparticles after pulmonary delivery. Biomed Res. Int. 2013, 2013, 136859. [Google Scholar] [CrossRef]
- Mussi, S.V.; Silva, R.C.; Oliveira, M.C.; Lucci, C.M.; Azevedo, R.B.; Ferreira, L.A. New approach to improve encapsulation and antitumor activity of doxorubicin loaded in solid lipid nanoparticles. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 48, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Shao, W.; Wang, Q.; Zhang, N. Solid lipid nanoparticles as insulin inhalation carriers for enhanced pulmonary delivery. J. Biomed. Nanotechnol. 2009, 5, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Maretti, E.; Rustichelli, C.; Romagnoli, M.; Balducci, A.G.; Buttini, F.; Sacchetti, F.; Leo, E.; Iannuccelli, V. Solid Lipid Nanoparticle assemblies (SLNas) for an anti-TB inhalation treatment-A Design of Experiments approach to investigate the influence of pre-freezing conditions on the powder respirability. Int. J. Pharm. 2016, 511, 669–679. [Google Scholar] [CrossRef]
- Emami, J.; Mohiti, H.; Hamishehkar, H.; Varshosaz, J. Formulation and optimization of solid lipid nanoparticle formulation for pulmonary delivery of budesonide using Taguchi and Box-Behnken design. Res. Pharm. Sci. 2015, 10, 17–33. [Google Scholar]
- Beck-Broichsitter, M.; Bohr, A.; Aragão-Santiago, L.; Klingl, A.; Kissel, T. Formulation and process considerations for the design of sildenafil-loaded polymeric microparticles by vibrational spray-drying. Pharm. Dev. Technol. 2017, 22, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Chaudhary, K.R.; Singh, A.; Singh, C. Inhalation potential of N-Acetylcysteine loaded PLGA nanoparticles for the management of tuberculosis: In vitro lung deposition and efficacy studies. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100084. [Google Scholar] [CrossRef]
- Oyarzun-Ampuero, F.A.; Brea, J.; Loza, M.I.; Torres, D.; Alonso, M.J. Chitosan-hyaluronic acid nanoparticles loaded with heparin for the treatment of asthma. Int. J. Pharm. 2009, 381, 122–129. [Google Scholar] [CrossRef]
- Mohtar, N.; Taylor, K.M.; Sheikh, K.; Somavarapu, S. Design and development of dry powder sulfobutylether-β-cyclodextrin complex for pulmonary delivery of fisetin. Eur. J. Pharm. Biopharm. 2017, 113, 1–10. [Google Scholar] [CrossRef]
- Pápay, Z.E.; Kósa, A.; Böddi, B.; Merchant, Z.; Saleem, I.Y.; Zariwala, M.G.; Klebovich, I.; Somavarapu, S.; Antal, I. Study on the Pulmonary Delivery System of Apigenin-Loaded Albumin Nanocarriers with Antioxidant Activity. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 274–288. [Google Scholar] [CrossRef]
- Taki, M.; Tagami, T.; Fukushige, K.; Ozeki, T. Fabrication of nanocomposite particles using a two-solution mixing-type spray nozzle for use in an inhaled curcumin formulation. Int. J. Pharm. 2016, 511, 104–110. [Google Scholar] [CrossRef]
- Mali, A.J.; Bothiraja, C.; Purohit, R.N.; Pawar, A.P. In Vitro and In Vivo Performance of Novel Spray Dried Andrographolide Loaded Scleroglucan Based Formulation for Dry Powder Inhaler. Curr. Drug Deliv. 2017, 14, 968–980. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.; Yu, J.; Wang, J.; Ju, J.; Dai, J. A dry powder inhalable formulation of salvianolic acids for the treatment of pulmonary fibrosis: Safety, lung deposition, and pharmacokinetic study. Drug Deliv. Transl. Res. 2021, 11, 1958–1968. [Google Scholar] [CrossRef]
- Sung, J.C.; Padilla, D.J.; Garcia-Contreras, L.; Verberkmoes, J.L.; Durbin, D.; Peloquin, C.A.; Elbert, K.J.; Hickey, A.J.; Edwards, D.A. Formulation and pharmacokinetics of self-assembled rifampicin nanoparticle systems for pulmonary delivery. Pharm. Res. 2009, 26, 1847–1855. [Google Scholar] [CrossRef]
- Cheow, W.S.; Ng, M.L.; Kho, K.; Hadinoto, K. Spray-freeze-drying production of thermally sensitive polymeric nanoparticle aggregates for inhaled drug delivery: Effect of freeze-drying adjuvants. Int. J. Pharm. 2011, 404, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Patil-Gadhe, A.; Kyadarkunte, A.; Patole, M.; Pokharkar, V. Montelukast-loaded nanostructured lipid carriers: Part II Pulmonary drug delivery and in vitro–in vivo aerosol performance. Eur. J. Pharm. Biopharm. 2014, 88, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Garg, T.; Rath, G.; Murthy, R.S.R.; Goyal, A.K. Development, optimization and evaluation of surfactant-based pulmonary nanolipid carrier system of paclitaxel for the management of drug resistance lung cancer using Box-Behnken design. Drug Deliv. 2016, 23, 1912–1925. [Google Scholar] [CrossRef] [PubMed]
- Plumley, C.; Gorman, E.M.; El-Gendy, N.; Bybee, C.R.; Munson, E.J.; Berkland, C. Nifedipine nanoparticle agglomeration as a dry powder aerosol formulation strategy. Int. J. Pharm. 2009, 369, 136–143. [Google Scholar] [CrossRef]
- Hu, L.; Kong, D.; Hu, Q.; Gao, N.; Pang, S. Evaluation of High-Performance Curcumin Nanocrystals for Pulmonary Drug Delivery Both In Vitro and In Vivo. Nanoscale Res. Lett. 2015, 10, 381. [Google Scholar] [CrossRef]
- Kurniawansyah, F.; Mammucari, R.; Foster, N.R. Inhalable curcumin formulations by supercritical technology. Powder Technol. 2015, 284, 289–298. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, H.; Jiang, K.; Gao, Y. Enhanced bioavailability after oral and pulmonary administration of baicalein nanocrystal. Int. J. Pharm. 2011, 420, 180–188. [Google Scholar] [CrossRef]
- Neumiller, J.J.; Campbell, R.K. Technosphere® Insulin. BioDrugs 2010, 24, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Geller, D.E.; Weers, J.; Heuerding, S. Development of an inhaled dry-powder formulation of tobramycin using PulmoSphere™ technology. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 175–182. [Google Scholar] [CrossRef]
- Singh, D.; Ravi, A.; Kane, K.; Schmalbach, T.; Hava, D.L. The pharmacokinetics, pharmacodynamics and tolerability of PUR0200, a novel tiotropium formulation, in chronic obstructive pulmonary disease. Br. J. Clin. Pharmacol. 2018, 84, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Rajabnezhad, S.; Casettari, L.; Lam, J.K.W.; Nomani, A.; Torkamani, M.R.; Palmieri, G.F.; Rajabnejad, M.R.; Darbandi, M.A. Pulmonary delivery of rifampicin microspheres using lower generation polyamidoamine dendrimers as a carrier. Powder Technol. 2016, 291, 366–374. [Google Scholar] [CrossRef]
- Shen, Q.; Jiang, Y.; Chen, J.; Wang, X.; Zheng, J. A Novel Curcumin-Based Drug Powder Inhalation Medicine for Chronic Obstructive Pulmonary Disease. Bioinorg. Chem. Appl. 2021, 2021, 8001787. [Google Scholar] [CrossRef]
- Agnoletti, M.; Bohr, A.; Thanki, K.; Wan, F.; Zeng, X.; Boetker, J.P.; Yang, M.; Foged, C. Inhalable siRNA-loaded nano-embedded microparticles engineered using microfluidics and spray drying. Eur. J. Pharm. Biopharm. 2017, 120, 9–21. [Google Scholar] [CrossRef]
- Zhong, Q. Co-Spray Dried Mannitol/Poly(amidoamine)-Doxorubicin Dry-Powder Inhaler Formulations for Lung Adenocarcinoma: Morphology, In Vitro Evaluation, and Aerodynamic Performance. AAPS PharmSciTech 2018, 19, 531–540. [Google Scholar] [CrossRef]
- Shahin, H.I.; Chablani, L. A comprehensive overview of dry powder inhalers for pulmonary drug delivery: Challenges, advances, optimization techniques, and applications. J. Drug Deliv. Sci. Technol. 2023, 84, 104553. [Google Scholar] [CrossRef]
- Dunber, C.A.; Hickey, A.J.; Holzner, P. Dispersion and characterization of pharmaceutical dry powder aerosols. KONA Powder Part. J. 1998, 16, 7–45. [Google Scholar] [CrossRef]
- Frijlink, H.W.; De Boer, A.H. Dry powder inhalers for pulmonary drug delivery. Expert Opin. Drug Deliv. 2004, 1, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Verma, R.; Garcia-Contreras, L. Inhalation drug delivery devices: Technology update. Med. Devices 2015, 8, 131–139. [Google Scholar] [CrossRef]
- Chan, J.G.Y.; Duke, C.C.; Ong, H.X.; Chan, J.C.Y.; Tyne, A.S.; Chan, H.-K.; Britton, W.J.; Young, P.M.; Traini, D. A Novel Inhalable Form of Rifapentine. J. Pharm. Sci. 2014, 103, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Claus, S.; Weiler, C.; Schiewe, J.; Friess, W. How can we bring high drug doses to the lung? Eur. J. Pharm. Biopharm. 2014, 86, 1–6. [Google Scholar] [CrossRef]
- Varun, N.; Ghoroi, C. Engineered inhalable micro-balloon shaped drug particles for carrier-free dry powder inhalation (DPI) application. Powder Technol. 2022, 408, 117705. [Google Scholar] [CrossRef]
- Lechanteur, A.; Evrard, B. Influence of Composition and Spray-Drying Process Parameters on Carrier-Free DPI Properties and Behaviors in the Lung: A review. Pharmaceutics 2020, 12, 55. [Google Scholar] [CrossRef]
- Wong, S.N.; Weng, J.; Ip, I.; Chen, R.; Lakerveld, R.; Telford, R.; Blagden, N.; Scowen, I.J.; Chow, S.F. Rational Development of a Carrier-Free Dry Powder Inhalation Formulation for Respiratory Viral Infections via Quality by Design: A Drug-Drug Cocrystal of Favipiravir and Theophylline. Pharmaceutics 2022, 14, 300. [Google Scholar] [CrossRef]
- Alhajj, N.; O’Reilly, N.J.; Cathcart, H. Designing enhanced spray dried particles for inhalation: A review of the impact of excipients and processing parameters on particle properties. Powder Technol. 2021, 384, 313–331. [Google Scholar] [CrossRef]
- Azari, F.; Ghanbarzadeh, S.; Safdari, R.; Yaqoubi, S.; Adibkia, K.; Hamishehkar, H. Development of a Carrier Free Dry Powder Inhalation Formulation of Ketotifen for Pulmonary Drug Delivery. Drug Res. 2020, 70, 26–32. [Google Scholar] [CrossRef]
- de Boer, A.H.; Chan, H.K.; Price, R. A critical view on lactose-based drug formulation and device studies for dry powder inhalation: Which are relevant and what interactions to expect? Adv. Drug Deliv. Rev. 2012, 64, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Guchardi, R.; Frei, M.; John, E.; Kaerger, J.S. Influence of fine lactose and magnesium stearate on low dose dry powder inhaler formulations. Int. J. Pharm. 2008, 348, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Lavorini, F.; Pistolesi, M.; Usmani, O.S. Recent advances in capsule-based dry powder inhaler technology. Multidiscip. Respir. Med. 2017, 12, 11. [Google Scholar] [CrossRef]
- Edwards, D. Applications of capsule dosing techniques for use in dry powder inhalers. Ther. Deliv. 2010, 1, 195–201. [Google Scholar] [CrossRef]
- Magramane, S.; Pápay, Z.; Turbucz, B.; Antal, I. Formulation and Characterization of Pulmonary Drug Delivery Systems. Acta Pharm. Hung. 2019, 89, 63–83. [Google Scholar] [CrossRef]
- Sibum, I.; Hagedoorn, P.; de Boer, A.H.; Frijlink, H.W.; Grasmeijer, F. Challenges for pulmonary delivery of high powder doses. Int. J. Pharm. 2018, 548, 325–336. [Google Scholar] [CrossRef]
- Smith, I.J.; Parry-Billings, M. The inhalers of the future? A review of dry powder devices on the market today. Pulm. Pharmacol. Ther. 2003, 16, 79–95. [Google Scholar] [CrossRef]
- Grasmeijer, F.; Grasmeijer, N.; Hagedoorn, P.; Frijlink, H.W.; Haaije de Boer, A. Recent advances in the fundamental understanding of adhesive mixtures for inhalation. Curr. Pharm. Des. 2015, 21, 5900–5914. [Google Scholar] [CrossRef]
- Gharse, S.; Fiegel, J. Large Porous Hollow Particles: Lightweight Champions of Pulmonary Drug Delivery. Curr. Pharm. Des. 2016, 22, 2463–2469. [Google Scholar] [CrossRef]
- Mohan, A.R.; Wang, Q.; Dhapare, S.; Bielski, E.; Kaviratna, A.; Han, L.; Boc, S.; Newman, B. Advancements in the Design and Development of Dry Powder Inhalers and Potential Implications for Generic Development. Pharmaceutics 2022, 14, 2495. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.A.; Hanes, J.; Caponetti, G.; Hrkach, J.; Ben-Jebria, A.; Eskew, M.L.; Mintzes, J.; Deaver, D.; Lotan, N.; Langer, R. Large porous particles for pulmonary drug delivery. Science 1997, 276, 1868–1871. [Google Scholar] [CrossRef] [PubMed]
- de Boer, A.H.; Hagedoorn, P.; Westerman, E.M.; Le Brun, P.P.H.; Heijerman, H.G.M.; Frijlink, H.W. Design and in vitro performance testing of multiple air classifier technology in a new disposable inhaler concept (Twincer®) for high powder doses. Eur. J. Pharm. Sci. 2006, 28, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Hossain, M.; Mamun, A.; Zaman, S.; Asaduzzaman, M.; Rashid, M. Pharmacopoeial Standards and Specifications for Pharmaceutical Aerosols: In-Process and Finished Products Quality Control Tests. Adv. Res. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Kulkarni, A.; Jagnade, V.; Hole, K. Effect of Formulation Excipients on Aerosolisation Performance of Budesonide. Indo Am. J. Pharm. Res. 2017, 7, 909–919. [Google Scholar]
- Agu, R.U.; Ugwoke, M.I. In vitro and in vivo testing methods for respiratory drug delivery. Expert Opin. Drug Deliv. 2011, 8, 57–69. [Google Scholar] [CrossRef]
- Ho, K.K.L.; Kellaway, I.W.; Tredree, R. Particle Size Analysis of Nebulised Aerosols Using Fraunhofer Laser Diffraction and Inertial Compaction Methods. J. Pharm. Pharmacol. 2011, 38. [Google Scholar] [CrossRef]
- Radivojev, S.; Zellnitz, S.; Paudel, A.; Frohlich, E. Searching for physiologically relevant in vitro dissolution techniques for orally inhaled drugs. Int. J. Pharm. 2019, 556, 45–56. [Google Scholar] [CrossRef]
- Eedara, B.B.; Tucker, I.G.; Das, S.C. In vitro dissolution testing of respirable size anti-tubercular drug particles using a small volume dissolution apparatus. Int. J. Pharm. 2019, 559, 235–244. [Google Scholar] [CrossRef]
- May, S.; Jensen, B.; Wolkenhauer, M.; Schneider, M.; Lehr, C.M. Dissolution techniques for in vitro testing of dry powders for inhalation. Pharm. Res. 2012, 29, 2157–2166. [Google Scholar] [CrossRef] [PubMed]
- Nokhodchi, A.; Chavan, S.; Ghafourian, T. In Vitro Dissolution and Permeability Testing of Inhalation Products: Challenges and Advances. Pharmaceutics 2023, 15, 983. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.J.; McConville, J.T. Development of a standardized dissolution test method for inhaled pharmaceutical formulations. Int. J. Pharm. 2009, 382, 15–22. [Google Scholar] [CrossRef]
- Davies, N.M.; Feddah, M.R. A novel method for assessing dissolution of aerosol inhaler products. Int. J. Pharm. 2003, 255, 175–187. [Google Scholar] [CrossRef]
- Salama, R.O.; Traini, D.; Chan, H.K.; Young, P.M. Preparation and characterisation of controlled release co-spray dried drug-polymer microparticles for inhalation 2: Evaluation of in vitro release profiling methodologies for controlled release respiratory aerosols. Eur. J. Pharm. Biopharm. 2008, 70, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.; Löbenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Moss, O.R. Simulants of Lung Interstitial Fluid; National Library of Medicine: Bethesda, MD, USA, 1979; Volume 36, pp. 447–448. [Google Scholar]
- Hassoun, M.; Royall, P.G.; Parry, M.; Harvey, R.D.; Forbes, B. Design and development of a biorelevant simulated human lung fluid. J. Drug Deliv. Sci. Technol. 2018, 47, 485–491. [Google Scholar] [CrossRef]
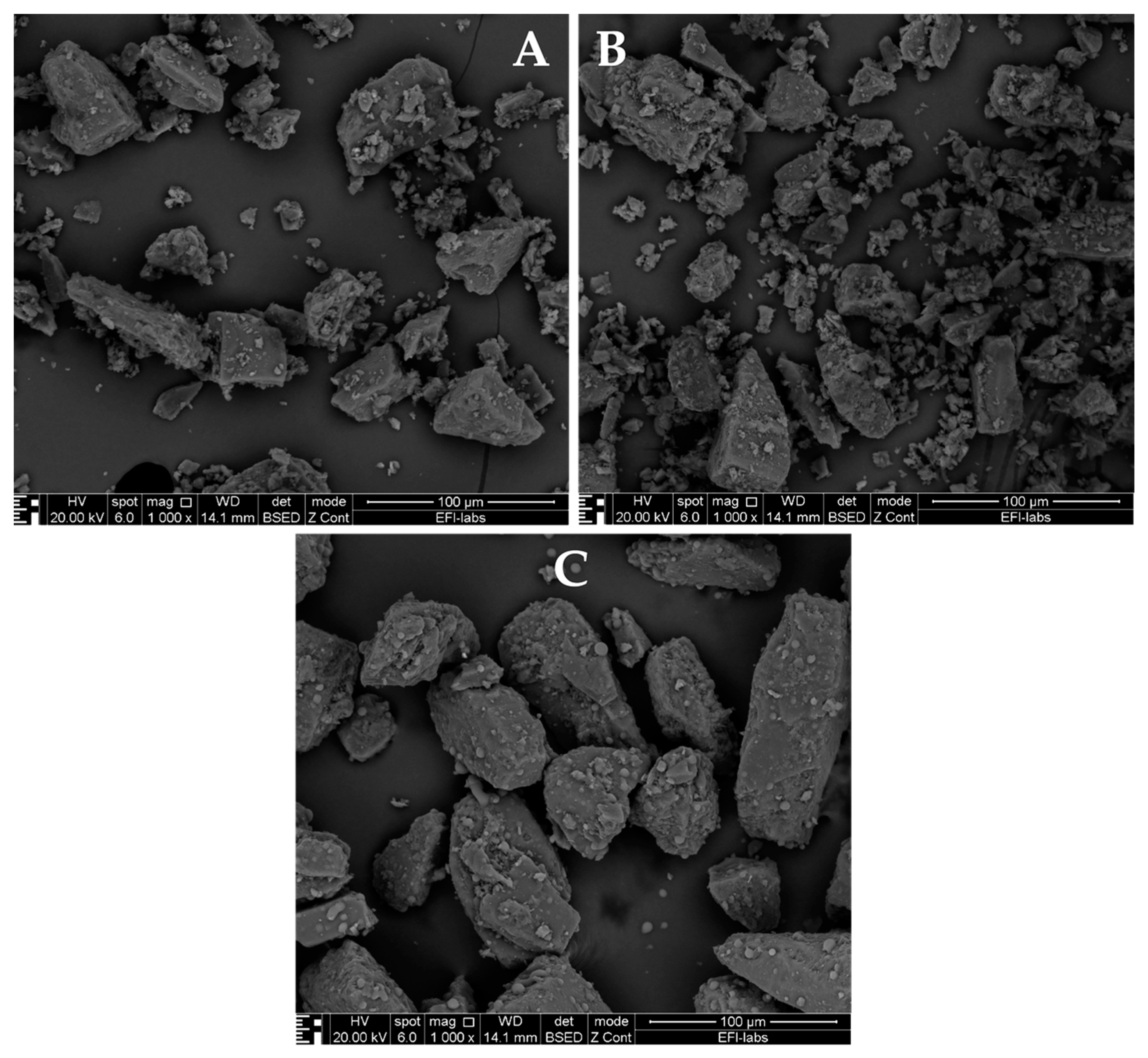
- Vernon-Parry, K.D. Scanning electron microscopy: An introduction. III-Vs Rev. 2000, 13, 40–44. [Google Scholar] [CrossRef]
- Scimeca, M.; Bischetti, S.; Lamsira, H.K.; Bonfiglio, R.; Bonanno, E. Energy Dispersive X-ray (EDX) microanalysis: A powerful tool in biomedical research and diagnosis. Eur. J. Histochem. 2018, 62, 2841. [Google Scholar] [CrossRef]
- Mahler, D.A. Peak Inspiratory Flow Rate as a Criterion for Dry Powder Inhaler Use in Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2017, 14, 1103–1107. [Google Scholar] [CrossRef]
- Dal Negro, R.W. Dry powder inhalers and the right things to remember: A concept review. Multidiscip. Respir. Med. 2015, 10, 13. [Google Scholar] [CrossRef]
- Janežič, A.; Locatelli, I.; Kos, M. Inhalation technique and asthma outcomes with different corticosteroid-containing inhaler devices. J. Asthma 2020, 57, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Kumar, L.; Vohra, V.; Sarin, R.; Jaiswal, A.; Puri, M.M.; Rathee, D.; Chakraborty, P. Evaluating the technique of using inhalation device in COPD and Bronchial Asthma patients. Respir. Med. 2014, 108, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Molimard, M.; Raherison, C.; Lignot, S.; Depont, F.; Abouelfath, A.; Moore, N. Assessment of Handling of Inhaler Devices in Real Life: An Observational Study in 3811 Patients in Primary Care. J. Aerosol Med. 2003, 16, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Richter, K. Successful use of DPI systems in asthmatic patients–key parameters. Respir. Med. 2004, 98 (Suppl. S2), S22–S27. [Google Scholar] [CrossRef] [PubMed]
- Benke, E.; Varga, P.; Szabó-Révész, P.; Ambrus, R. Stability and In Vitro Aerodynamic Studies of Inhalation Powders Containing Ciprofloxacin Hydrochloride Applying Different DPI Capsule Types. Pharmaceutics 2021, 13, 689. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Brunaugh, A.D.; Thakkar, R.; Lee, C.; Zhao, Q.J.; Kalafat, J.; Maniruzzaman, M.; Smyth, H.D.C. Comparison of HPMC Inhalation-Grade Capsules and Their Effect on Aerosol Performance Using Budesonide and Rifampicin DPI Formulations. AAPS PharmSciTech 2022, 23, 52. [Google Scholar] [CrossRef] [PubMed]
- Milind, K.; Biyani, A.K.; Fernando, D.; Ettore, C.; Erwin, P. Emerging Trends in Blister-Based Dry Powder Inhalers. Available online: https://pharma-trends.com/2021/01/14/emerging-trends-in-blister-based-dry-powder-inhalers/ (accessed on 3 August 2023).
- Pfizer. Exubera 1 mg Inhalation Powder Pre-Dispensed SmPC. Available online: https://www.ema.europa.eu/en/documents/product-information/exubera-epar-product-information_en.pdf (accessed on 3 August 2023).
- Chang, R.Y.K.; Chow, M.Y.T.; Khanal, D.; Chen, D.; Chan, H.-K. Dry powder pharmaceutical biologics for inhalation therapy. Adv. Drug Deliv. Rev. 2021, 172, 64–79. [Google Scholar] [CrossRef]
- Zhou, Q.T.; Tang, P.; Leung, S.S.; Chan, J.G.; Chan, H.K. Emerging inhalation aerosol devices and strategies: Where are we headed? Adv. Drug Deliv. Rev. 2014, 75, 3–17. [Google Scholar] [CrossRef]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part II: The role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 600–612. [Google Scholar] [CrossRef]
- Norderud Laerum, B.; Telg, G.; Stratelis, G. Need of education for dry powder inhaler storage and retention–-a patient-reported survey. Multidiscip. Respir. Med. 2016, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- 3M™ Drug Delivery Systems. Available online: https://news.3m.com/2016-04-19-3M-Unveils-Intelligent-Inhaler-Designed-to-Help-Control-Spiraling-Costs-of-Respiratory-Disease (accessed on 7 September 2023).
- Crompton, G.K. How to achieve good compliance with inhaled asthma therapy. Respir. Med. 2004, 98, S35–S40. [Google Scholar] [CrossRef]
- Kolewe, E.L.; Padhye, S.; Woodward, I.R.; Feng, Y.; Briddell, J.W.; Fromen, C.A. A Pediatric Upper Airway Library to Evaluate Interpatient Variability of In Silico Aerosol Deposition. AAPS PharmSciTech 2023, 24, 162. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, F.; Vanbever, R. Improving the efficacy of inhaled drugs for severe lung diseases: Emerging pulmonary delivery strategies. Adv. Drug Deliv. Rev. 2014, 75, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.S.; Pathare, S.R.; More, M.A.; Waykhinde, N.A.; Laddha, U.D.; Salunkhe, K.S.; Kshirsagar, S.J.; Patil, S.S.; Ramteke, K.H. Dry Powder Inhaler with the technical and practical obstacles, and forthcoming platform strategies. J. Control. Release 2023, 355, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.P. Dry powder inhalers: A concise summary of the electronic monitoring devices. Ther. Deliv. 2021, 12, 1–6. [Google Scholar] [CrossRef]
- Kumar, R.; Mehta, P.; Shankar, K.R.; Rajora, M.A.K.; Mishra, Y.K.; Mostafavi, E.; Kaushik, A. Nanotechnology-Assisted Metered-Dose Inhalers (MDIs) for High-Performance Pulmonary Drug Delivery Applications. Pharm. Res. 2022, 39, 2831–2855. [Google Scholar] [CrossRef]
- Electronic Medicines Compendium (EMC). Available online: https://www.medicines.org.uk/emc/ (accessed on 21 May 2023).
- European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en (accessed on 21 May 2023).
- RightBreathe. Available online: https://www.rightbreathe.com/ (accessed on 22 May 2023).
- DePietro, M.; Gilbert, I.; Millette, L.A.; Riebe, M. Inhalation device options for the management of chronic obstructive pulmonary disease. Postgrad. Med. 2018, 130, 83–97. [Google Scholar] [CrossRef]
- Digihaler. Available online: https://www.digihaler.com/inhalers (accessed on 13 November 2023).
- Propeller Health. Available online: https://propellerhealth.com/ (accessed on 13 November 2023).
- Adherium’s Hailie®. Available online: https://www.adherium.com/our-technology/ (accessed on 13 November 2023).
- Van Sickle, D.; Barrett, M.; Humblet, O.; Henderson, K.; Hogg, C. Randomized, controlled study of the impact of a mobile health tool on asthma SABA use, control and adherence. Eur. Respir. J. 2016, 48, PA1018. [Google Scholar] [CrossRef]
- Häußermann, S.; Arendsen, L.J.; Pritchard, J.N. Smart dry powder inhalers and intelligent adherence management. Adv. Drug Deliv. Rev. 2022, 191, 114580. [Google Scholar] [CrossRef]
- ISO 18562-1:2017; Biocompatibility Evaluation of Breathing Gas Pathways in Healthcare Applications—Part 1: Evaluation and Testing within a Risk Management Process. ISO (International Organization for Standardization): Geneva, Switzerland, 2017.
- Giannopoulou, E.; Efstratiadou, G.; Rozou, S.; Ismailos, G.; Theofanopoulos, K.; Panoelia, E.; Kalofonos, H.P.; Sivolapenko, G. In Vitro Cytotoxicity of Dry Powder Inhaler Medical Devices. Int. J. Res. Stud. Sci. Eng. Technol. 2016, 3, 7–14. [Google Scholar]
- Zillen, D.; Beugeling, M.; Hinrichs, W.L.J.; Frijlink, H.W.; Grasmeijer, F. Natural and bioinspired excipients for dry powder inhalation formulations. Curr. Opin. Colloid Interface Sci. 2021, 56, 101497. [Google Scholar] [CrossRef]
- Stoilova, S.; Fiore, W.; Trotta, V.; Mori, M. Performance and biocompatibility of a novel inhalable dry powder formulation based on hyaluronic acid intended to protect the respiratory tract mucosa. Int. J. Pharm. 2023, 638, 122889. [Google Scholar] [CrossRef] [PubMed]
- Lavorini, F.; Fontana, G.A.; Usmani, O.S. New inhaler devices-the good, the bad and the ugly. Respiration 2014, 88, 3–15. [Google Scholar] [CrossRef]
- Levy, M.L.; Dekhuijzen, P.N.R.; Barnes, P.J.; Broeders, M.; Corrigan, C.J.; Chawes, B.L.; Corbetta, L.; Dubus, J.C.; Hausen, T.; Lavorini, F.; et al. Inhaler technique: Facts and fantasies. A view from the Aerosol Drug Management Improvement Team (ADMIT). NPJ Prim. Care Respir. Med. 2016, 26, 16017. [Google Scholar] [CrossRef]
- Chan, J.G.Y.; Wong, J.; Zhou, Q.T.; Leung, S.S.Y.; Chan, H.-K. Advances in Device and Formulation Technologies for Pulmonary Drug Delivery. AAPS PharmSciTech 2014, 15, 882–897. [Google Scholar] [CrossRef]
- Berkenfeld, K.; Lamprecht, A.; McConville, J.T. Devices for Dry Powder Drug Delivery to the Lung. AAPS PharmSciTech 2015, 16, 479–490. [Google Scholar] [CrossRef]
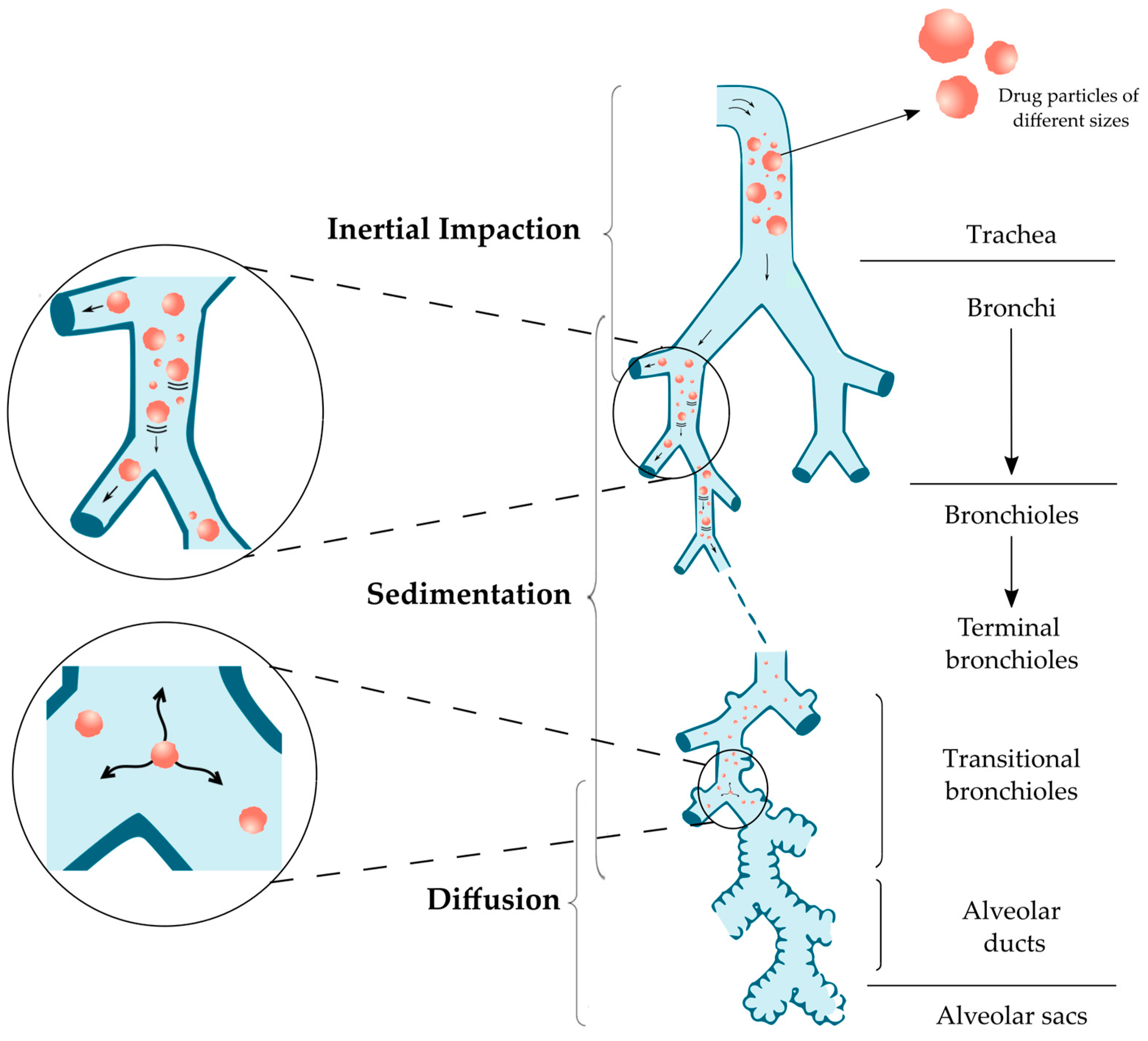

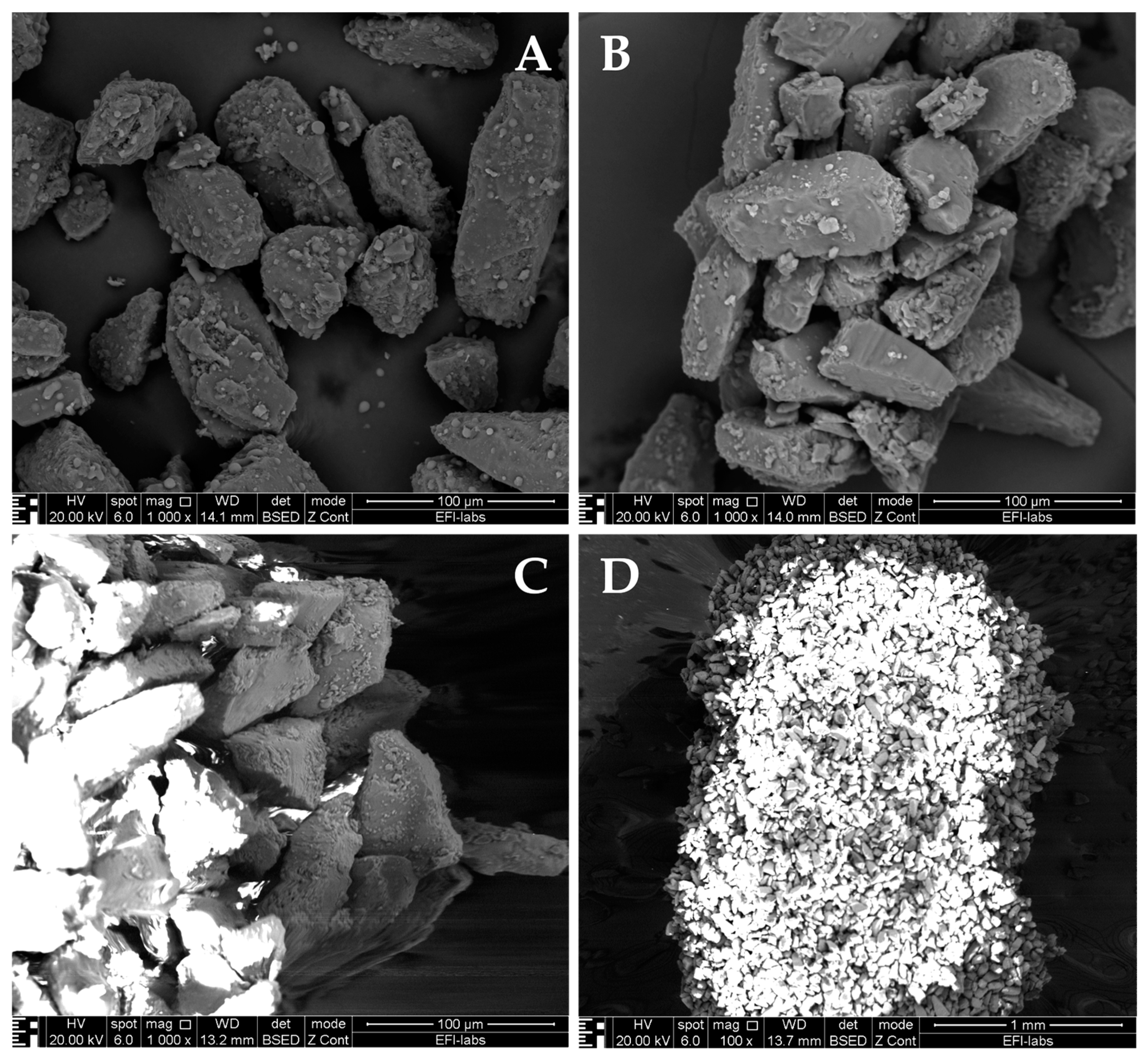


| Particle Size | Mechanism | Parts of Respiratory Tract |
|---|---|---|
| Above 5 μm | Inertial impaction | Oropharynx and conducting airways |
| 0.5–5 μm | Sedimentation | Bronchi, Bronchioles and Alveoli |
| 0.5–3 μm | Sedimentation and Diffusion | |
| Below 0.5 μm | Diffusion and Brownian motion | Alveolar region |
| Type of Particles | Characteristic | Active Pharmaceutical Ingredient | Method of Preparation | Size | Ref. |
|---|---|---|---|---|---|
| Polymeric microparticles (MPs) | Chitosan MPs | Rifabutin and Rifampicin | Spray drying | 1–5 μm | [70] |
| Locust bean gum (LBG) MPs | Isoniazid or Rifabutin | 1.15–1.67 μm | [71] | ||
| PLGA 1 MPs | Recombinant human interleukin-2 (rhIL-2) | Modified w/o/w double emulsion solvent extraction method | 4.02 μm | [72] | |
| Heparin | Spray drying | 2.55–3.86 µm | [73] | ||
| Rifapentine | ~2 µm | [74] | |||
| Bovine serum albumin (BSA) as a model vaccine | Supercritical CO2-assisted spray-drying (SASD) | 1.7–3.5 µm | [75] | ||
| Rifampicin-loaded microspheres | Solvent evaporation method with premix membrane homogenization | 0.64–4.1 µm | [76] | ||
| Modified PLGA | siRNA | Double emulsion solvent evaporation method | 207.7–261.1 nm | [77] | |
| PCL 2 MPs | Resveratrol | Vibrational atomization spray drying | 3.8 µm | [78] | |
| Microparticles | Solid Lipid Microparticles (SLMs) | Quercetin | o/w emulsification method | 5.72 µm | [79] |
| 2.90 µm | [80] | ||||
| None | Naringin | Spray drying | 3.29–3.92 µm | [81] | |
| Sprayed with amino acids | Spray drying | 2.75–3.42 µm | [82] | ||
| None | Atropine | Solid-phase extraction | 3.7 µm | [83] | |
| Porous particles | Large porous particles (LPPs) | Doxorubicin | w/o/w double emulsion method | 14.1 µm | [84] |
| Celecoxib | PLGA LPPs by supercritical fluid pressure-quench technology | 10.53 µm | [85] | ||
| PLGA-based gas-foamed LPPs | Rhodamine B isothiocyanate–dextran | Double emulsion solvent evaporation method | ~30 μm | [86] | |
| Porous particles | Nanocrystals embedded in microparticles | Niclosamide | Spray freeze drying | 0.18–4.29 µm | [87] |
| Swellable particles | Hydrogel microparticles | Paclitaxel | Emulsification/gelation method | <5 µm | [88] |
| BSA as a model protein | Spray drying | 3.6 µm | [89] | ||
| Swellable particles | Hydrogel microparticles | Ciprofloxacin and Doxycycline | Spray drying | ~2 μm | [90] |
| Chemotherapeutic drugs | ~6 μm | [91] | |||
| Matrix metalloproteinase (MMP) enzyme-responsive hydrogel | Modified polymerization method | 2.8–4.0 μm | [92] | ||
| Nanoparticles | Proliposomes | Rifapentine | Spray drying | 7.73 μm | [93] |
| Liposomes | Synergistic Ciprofloxacin and Colistin | Ultrasonic sprayfreeze drying | ~100 μm | [94] | |
| Ciprofloxacin | Membrane extrusion of multilamellar liposomes followed by remote loading of API | 3.6–4.0 µm | [95] | ||
| Isoniazid | Thin-film hydration method | 755 nm | [96] | ||
| Insulin | 100 nm | [97] | |||
| Oseltamivir phosphate | Spray drying | 3.5 µm | [98] | ||
| Curcumin | Nano-spray drying | 2.10 µm | [99] | ||
| Gemcitabine hydrochloride | Lyophilisation | 325 nm | [100] | ||
| Salbutamol sulfate | Vesicular phospholipid gel (VPG) technique | ~10 μm | [101] | ||
| 57 nm | [102] | ||||
| Rifampicin | Chloroform-film method, lyophilisation | 200–300 nm | [103] | ||
| Nanoparticles | Liposomes | Tacrolimus | Thin film evaporation, spray drying | 9.46–12.4 μm | [104] |
| Dapsone | 7.9–11.2 μm | [105] | |||
| Curcumin | Film method | 94.65 nm | [106] | ||
| Solid lipid nanoparticles (SLNs) 3 | Alendronate | Homogenization | <100 nm | [107] | |
| Amikacin | 164 nm | [108] | |||
| Solid lipid nanoparticles (SLNs) 3 | Doxorubicin | Homogenization | 94–113 nm (with d triethanolamine) 127–151 nm (with stearylamine) | [109] | |
| Insulin | w/o/w emulsion | 231.67 nm | [110] | ||
| Solid lipid nanoparticles (SLNs) 3 | Rifampicin | Melt emulsifying technique then freeze drying | 0.47–1.72 mm | [111] | |
| Budesonide | Emulsification-solvent diffusion method | 218.2 nm | [112] | ||
| Polymeric nanoparticles | Sildenafil | Vibrational spray drying | ~4–8 μm | [113] | |
| N-acetylcysteine | w/o/w double emulsion | 307.50 nm | [114] | ||
| Heparin | Ionotropic gelation technique | 162–217 nm | [115] | ||
| Fisetin | Spray drying | 1.5 µm | [116] | ||
| Protein-based nanoparticles | Apigenin | Spray drying | 376 nm | [117] | |
| Nanocomposite particles | Curcumin | Spray drying | 2.1 µm | [118] | |
| Andrographolide | 3.37 µm | [119] | |||
| Salvianolic acids | Freeze drying | <5 µm | [120] | ||
| Porous nanoparticle-aggregate particles | Rifampicin | Spray drying | 195 nm | [121] | |
| Levofloxacin | Spray freeze drying | 18 µm | [122] | ||
| Nanostructured lipid carrier (NLC) | Montelukast | Lyophilization | 184.6 nm | [123] | |
| Paclitaxel | Emulsification and ultrasonication method, spray drying | 283.4 nm | [124] | ||
| Nanoparticles | Nanoparticle agglomerates | Nifedipine | Solvent precipitation, controlled particle agglomeration, lyophilization | 470 nm | [125] |
| Nanocrystals | Curcumin | Spray drying | 924 nm | [126] | |
| Supercritical (ARISE) processing | 3–5 µm | [127] | |||
| Baicalein | Modified anti-solvent recrystallization then high pressure homogenization | Not specified | [128] | ||
| Microspheres | Technosphere® | Insulin | Precipitation, micoencapsulation | 2–5 µm | [129] |
| PulmoSphere™ | Tobramycin | Emulsion-based spray drying | 1–5 μm | [130] | |
| iSPERESE™ | Tiotropium bromide | iSPERSE dry powder delivery technology | ~3 μm 4 | [131] | |
| Polyamidoamine (PAMPAM) dendrimers | Rifampicin | Spray drying | ~6 μm | [132] | |
| Spherical particles | Curcumin | Spray drying | 1–5 μm | [133] | |
| Dendrimers | siRNA–dendrimer nanocomplexes | siRNA | Microfluidics, Spray-drying | Not specified | [134] |
| Doxorubicin–PAMAM dendrimer conjugate loaded with mannitol microparticles | Doxorubicin | Spray drying | 1 µm | [135] |
| Test | Description |
|---|---|
| Particle size determination | The determination is executed using a cascade impactor or using a light scattering decay method. The particle size is expressed in µm. |
| InVitro Aerodynamic Assessment | Evaluates the aerodynamic behavior of emitted particles, considering factors like the Mass Median Aerodynamic Diameter (MMAD). |
| Fine Particle Fraction (FPF) | Measures the fraction of fine particles (usually below 5 µm) that are emitted from the DPI, indicating their suitability for deep lung deposition. |
| Delivered dose | Ensures the delivered dose per actuation matches the intended dose and meets regulatory requirements. |
| Dose uniformity | Ensures uniformity of the dose by weighing the container before and after a specific number of actuations. The difference in weight per dose is calculated. |
| Content uniformity | Assesses the uniform distribution of the active pharmaceutical ingredient (API) within the DPI formulation. |
| Moisture content | Measures the moisture content using methods such as Karl-Fischer or gas chromatography. |
| Bulk density | Determines the bulk density of the DPI formulation using methods like pycnometry. |
| Tapped density | Measures the tapped density, which assesses the powder’s ability to pack and flow effectively. |
| Flowability | Evaluates the flow properties of the DPI formulation, which can affect device metering and aerosol dispersion. |
| Inhaler/Medicine Name | API 1 Dose Quantity and Name | Carrier Excipient | Company |
|---|---|---|---|
| Oxis Turbohaler 6 | 4.5 µg Formoterol fumarate dihydrate | Lactose monohydrate | AstraZeneca UK Ltd., London, UK. |
| Oxis Turbohaler 12 | 9 µg Formoterol fumarate dihydrate | Lactose monohydrate | AstraZeneca UK Ltd., London, UK. |
| Braltus 10 (with Zonda inhaler device) | 10 µg Tiotropium | Lactose monohydrate | Teva UK Ltd., Harlow, UK. |
| Spiriva 18 (with HandiHaler device) | 10 µg Tiotropium | Lactose monohydrate | Boehringer Ingelheim Ltd., Ingelheim, Germany. |
| Tiogiva 18 | 10 µg Tiotropium | Lactose monohydrate | Glenmark Pharmaceuticals Ltd., Mumbai, India. |
| Foradil 12 | 12 µg Formoterol fumarate dihydrate | Lactose monohydrate | Novartis Pharmaceuticals UK Ltd., London, UK. |
| Formoterol Easyhaler 12 | 12 µg Formoterol fumarate dihydrate | Lactose monohydrate | Orion Pharma UK Ltd., Reading, UK. |
| Acopair 18 (with NeumoHaler device) | 12 µg Tiotropium | Lactose anhydrous | Mylan, Hatfield, UK. |
| Flixotide Accuhaler 50 | 50 µg Fluticasone propionate | Lactose monohydrate | GlaxoSmithKline UK Ltd., London, UK. |
| Serevent Accuhaler 50 | 50 µg Salmeterol xinafoate | Lactose monohydrate | GlaxoSmithKline UK Ltd., London, UK. |
| Seebri Breezhaler 44 | 55 µg Glycopyrronium bromide | Lactose monohydrate | Novartis Pharmaceuticals UK Ltd., London, UK. |
| Incruse Ellipta 55 | 55 µg Umeclidinium | Lactose monohydrate | GlaxoSmithKline UK Ltd., London, UK. |
| Fobumix Easyhaler 80/4.5 | 84.5 µg Budesonide (80 µg) Formoterol fumarate dihydrate (4.5 µg) | Lactose monohydrate | Orion Pharma UK Ltd., Reading, UK. |
| Fostair NEXThaler 100/6 | 86.9 µg Beclometasone dipropionate anhydrous (81.9 µg) Formoterol fumarate dihydrate (5 µg) | Lactose monohydrate | Chiesi Ltd., Parma, Italy. |
| Symbicort Turbohaler 100/6 | 84.5 µg Budesonide (80 µg) Formoterol fumarate dihydrate (4.5 µg) | Lactose monohydrate | AstraZeneca UK Ltd., London, UK. |
| Anoro Ellipta 55/22 | 87 µg Umeclidinium bromide (65 µg) Vilanterol trifenatate (22 µg) | Lactose monohydrate | GlaxoSmithKline UK Ltd., London, UK. |
| Flixotide Accuhaler 100 | 100 µg Fluticasone propionate | Lactose monohydrate | GlaxoSmithKline UK Ltd., London, UK. |
| Pulmicort Turbohaler 100 | 100 µg Budesonide | None | AstraZeneca UK Ltd., London, UK. |
| Easyhaler Budesonide 100 | 100 µg Budesonide | Lactose monohydrate | Orion Pharma UK Ltd., Reading, UK. |
| Easyhaler Salbutamol 100 | 100 µg Salbutamol sulfate | Lactose monohydrate | Orion Pharma UK Ltd., Reading, UK. |
| Salbulin Novolizer 100 | 100 µg Salbutamol sulfate | Lactose monohydrate | Mylan, Hatfield, UK. |
| Trimbow NEXThaler 88/5/9 | 102 µg Beclometasone dipropionate (88 µg) Formoterol fumarate dihydrate (5 µg) Glycopyrronium (9 µg) | Lactose monohydrate | Chiesi Ltd., Parma, Italy. |
| Seffalair Spiromax 100/12.75 | 112.75 µg Fluticasone propionate (100 µg) Salmeterol xinafoate (12.75 µg) | Lactose monohydrate | Teva UK Ltd., Harlow, UK. |
| Relvar Ellipta 92/22 | 114 µg Fluticasone furoate (92 µg) Vilanterol trifenatate (22 µg) | Lactose monohydrate | GlaxoSmithKline UK Ltd., London, UK. |
| Onbrez Breezhaler 150 | 120 µg Indacaterol maleate | Lactose monohydrate | Novartis Pharmaceuticals UK Ltd., London, UK. |
| Seretide 100 Accuhaler | 139 µg Fluticasone propionate (92 µg) Salmeterol xinafoate (47 µg) | Lactose monohydrate | GlaxoSmithKline UK Ltd., London, UK. |
| Fixkoh Airmaster 50/100 | 139 µg Fluticasone propionate (92 µg) Salmeterol xinafoate (47 µg) | Lactose monohydrate | Genus Pharmaceuticals, Huddersfield, UK. |
| Ultibro Breezhaler 85/43 | 164 µg Indacaterol maleate (110 µg) Glycopyrronium bromide (54 µg) | Lactose monohydrate | Novartis Pharmaceuticals UK Ltd., London, UK. |
| Fobumix Easyhaler 160/4.5 | 164.5 µg Budesonide (160µg) Formoterol fumarate dihydrate (4.5 µg) | Lactose monohydrate | Orion Pharma UK Ltd., Reading, UK. |
| WockAir 160/4.5 | 164.5 µg Budesonide (160 µg) Formoterol fumarate dihydrate (4.5 µg) | Lactose monohydrate | Wockhardt UK Ltd., Wrexham, UK. |
| Symbicort Turbohaler 200/6 | 164.5 µg Budesonide (160 µg) Formoterol fumarate dihydrate (4.5 µg) | Lactose monohydrate | AstraZeneca UK Ltd., London, UK. |
| DuoResp Spiromax 160/4.5 | 164.5 µg Budesonide (160 µg) Formoterol fumarate dihydrate (4.5 µg) | Lactose monohydrate | Teva UK Ltd., Harlow, UK. |
| Fostair NEXThaler 200/6 | 164.8 µg Beclometasone dipropionate anhydrous (158.8 µg) Formoterol fumarate dihydrate (6 µg) | Lactose monohydrate | Chiesi Ltd., Parma, Italy. |
| Trelegy Ellipta 92/55/22 | 179 µg Fluticasone furoate (92 µg) Umeclidinium bromide (65 µg) Vilanterol trifenatate (22 µg) | Lactose monohydrate | GlaxoSmithKline UK Ltd., London, UK. |
| Easyhaler Beclometasone 200 | 180 µg Beclometasone dipropionate | Lactose monohydrate | Orion Pharma UK Ltd., Reading, UK. |
| Atectura Breezhaler 125/62.5 | 187.5 µg Indacaterol acetate (125 µg) Mometasone furoate (62.5 µg) | Lactose monohydrate | Novartis Pharmaceuticals UK Ltd., London, UK. |
| Pulmicort Turbohaler 200 | 200 µg Budesonide | None | AstraZeneca UK Ltd., London, UK. |
| Easyhaler Budesonide 200 | 200 µg Budesonide | Lactose monohydrate | Orion Pharma UK Ltd., Reading, UK. |
| Easyhaler Salbutamol 200 | 200 µg Salbutamol sulfate | Lactose monohydrate | Orion Pharma UK Ltd., Reading, UK. |
| Budelin Novolizer | 200 µg Budesonide | Lactose monohydrate | Mylan, Hatfield, UK. |
| Asmanex Twisthaler 200 | 200 µg Mometasone furoate | Lactose anhydrous | Organon Pharma UK Ltd., London, UK. |
| Ventolin Accuhaler 200 | 200 µg Salbutamol sulfate | Lactose monohydrate | GlaxoSmithKline UK Ltd., London, UK. |
| Relvar Ellipta 184/22 | 206 µg Fluticasone furoate (184 µg) Vilanterol trifenatate (22 µg) | Lactose monohydrate | GlaxoSmithKline UK Ltd., London, UK. |
| Seffalair Spiromax 202/12.75 | 214.75 µg Fluticasone propionate (202 µg) Salmeterol xinafoate (12.75 µg) | Lactose monohydrate | Teva UK Ltd., Harlow, UK. |
| Onbrez Breezhaler 300 | 240 µg Indacaterol maleate | Lactose monohydrate | Novartis Pharmaceuticals UK Ltd., London, UK. |
| Flixotide Accuhaler 250 | 250 µg Fluticasone propionate | Lactose monohydrate | GlaxoSmithKline UK Ltd., London, UK. |
| Atectura Breezhaler 125/127.5 | 252.5 µg Indacaterol acetate (125 µg) Mometasone furoate (127.5 µg) | Lactose monohydrate | Novartis Pharmaceuticals UK Ltd., London, UK. |
| Fixkoh Airmaster 50/250 | 274 µg Fluticasone propionate (229 µg) Salmeterol xinafoate (45 µg) | Lactose monohydrate | Genus Pharmaceuticals, Huddersfield, UK. |
| Seretide 250 Accuhaler | 278 µg Fluticasone propionate (231 µg) Salmeterol xinafoate (47 µg) | Lactose monohydrate | GlaxoSmithKline UK Ltd., London, UK. |
| Sereflo Ciphaler 50/250 | 278 µg Fluticasone propionate (231 µg) Salmeterol xinafoate (47 µg) | Lactose monohydrate | Cipla EU Ltd., Addlestone, UK. |
| Fusacomb Easyhaler 50/250 | 286 µg Fluticasone propionate (238 µg) Salmeterol xinafoate (48 µg) | Lactose monohydrate | Orion Pharma UK Ltd., Reading, UK. |
| Enerzair Breezhaler | 308 µg Indacaterol acetate (114 µg) Glycopyrronium bromide (58 µg) Mometasone furoate (136 µg) | Lactose monohydrate | Novartis Pharmaceuticals UK Ltd., London, UK. |
| Fobumix Easyhaler 320/9 | 329 µg Budesonide (320 µg) Formoterol fumarate dihydrate (9 µg) | Lactose monohydrate | Orion Pharma UK Ltd., Reading, UK. |
| WockAir 320/9 | 329 µg Budesonide (320 µg) Formoterol fumarate dihydrate (9 µg) | Lactose monohydrate | Wockhardt UK Ltd., Wrexham, UK. |
| Symbicort Turbohaler 400/12 | 329 µg Budesonide (320 µg) Formoterol fumarate dihydrate (9 µg) | Lactose monohydrate | AstraZeneca UK Ltd., London, UK. |
| DuoResp Spiromax 320/9 | 329 µg Budesonide (320 µg) Formoterol fumarate dihydrate (9 µg) | Lactose monohydrate | Teva UK Ltd., Harlow, UK. |
| Atectura Breezhaler 125/260 | 385 µg Indacaterol acetate (125 µg) Mometasone furoate (260 µg) | Lactose monohydrate | Novartis Pharmaceuticals UK Ltd., London, UK. |
| Pulmicort Turbohaler 400 | 400 µg Budesonide | None | AstraZeneca UK Ltd., London, UK. |
| Easyhaler Budesonide 400 | 400 µg Budesonide | Lactose monohydrate | Orion Pharma UK Ltd., Reading, UK. |
| Asmanex Twisthaler 400 | 400 µg Mometasone furoate | Lactose anhydrous | Organon Pharma UK Ltd., London, UK. |
| Bricanyl Turbohaler 0.5mg | 400 µg Terbutaline sulfate | Lactose monohydrate | AstraZeneca UK Ltd., London, UK. |
| Duaklir Genuair 340/12 | 407.8 µg Aclidinium bromide (396 µg) Formoterol fumarate dihydrate (11.8 µg) | Lactose monohydrate | Zentiva, Prague, Czech Republic. |
| Fixkoh Airmaster 50/500 | 475 µg Fluticasone propionate (432 µg) Salmeterol xinafoate (43 µg) | Lactose monohydrate | Genus Pharmaceuticals, Huddersfield, UK. |
| Flixotide Accuhaler 500 | 500 µg Fluticasone propionate | Lactose monohydrate | GlaxoSmithKline UK Ltd., London, UK. |
| Seretide 500 Accuhaler | 507 µg Fluticasone propionate (460 µg) Salmeterol xinafoate (47 µg) | Lactose monohydrate | GlaxoSmithKline UK Ltd., London, UK. |
| Sereflo Ciphaler 50/500 | 507 µg Fluticasone propionate (460 µg) Salmeterol xinafoate (47 µg) | Lactose monohydrate | Cipla EU Ltd., Addlestone, UK. |
| Stalpex 500/50 | 507 µg Fluticasone propionate (460 µg) Salmeterol xinafoate (47 µg) | Lactose monohydrate | Glenmark Pharmaceuticals Ltd., Mumbai, India. |
| AirFluSal Forspiro 50/500 | 510 µg Fluticasone propionate (465 µg) Salmeterol xinafoate (45 µg) | Lactose monohydrate | Sandoz Ltd., Basel, Switzerland. |
| Fusacomb Easyhaler 50/500 | 544 µg Fluticasone propionate (496 µg) Salmeterol xinafoate (48 µg) | Lactose monohydrate | Orion Pharma UK Ltd., Reading, UK. |
| Afrezza | 4, 8, or 12 units—Insulin | Fumaryl diketopiperazine (FDKP) | MannKind Corporation, Westlake Village, CA, USA. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magramane, S.; Vlahović, K.; Gordon, P.; Kállai-Szabó, N.; Zelkó, R.; Antal, I.; Farkas, D. Inhalation Dosage Forms: A Focus on Dry Powder Inhalers and Their Advancements. Pharmaceuticals 2023, 16, 1658. https://doi.org/10.3390/ph16121658
Magramane S, Vlahović K, Gordon P, Kállai-Szabó N, Zelkó R, Antal I, Farkas D. Inhalation Dosage Forms: A Focus on Dry Powder Inhalers and Their Advancements. Pharmaceuticals. 2023; 16(12):1658. https://doi.org/10.3390/ph16121658
Chicago/Turabian StyleMagramane, Sabrina, Kristina Vlahović, Péter Gordon, Nikolett Kállai-Szabó, Romána Zelkó, István Antal, and Dóra Farkas. 2023. "Inhalation Dosage Forms: A Focus on Dry Powder Inhalers and Their Advancements" Pharmaceuticals 16, no. 12: 1658. https://doi.org/10.3390/ph16121658
APA StyleMagramane, S., Vlahović, K., Gordon, P., Kállai-Szabó, N., Zelkó, R., Antal, I., & Farkas, D. (2023). Inhalation Dosage Forms: A Focus on Dry Powder Inhalers and Their Advancements. Pharmaceuticals, 16(12), 1658. https://doi.org/10.3390/ph16121658