Carborane-Based ABCG2-Inhibitors Sensitize ABC-(Over)Expressing Cancer Cell Lines for Doxorubicin and Cisplatin
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Reagents and Cells
4.2. Establishment of the Doxorubicin-Resistant MCF-7 (MCF-7 Doxo) Cell Line
4.3. Gene Expression Analysis
4.4. Cell Viability
4.5. Isobologram Analysis
4.6. JC-1 Staining
4.7. Statistical Analysis
4.8. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takeshima, H.; Ushijima, T. Accumulation of genetic and epigenetic alterations in normal cells and cancer risk. NPJ Precis. Oncol. 2019, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Robey, R.W.; Ambudkar, S.V. New mechanisms of multidrug resistance: An introduction to the Cancer Drug Resistance special collection. Cancer Drug Resist. 2023, 6, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005, 5, 30. [Google Scholar] [CrossRef][Green Version]
- Dieck, C.L.; Ferrando, A. Genetics and mechanisms of NT5C2-driven chemotherapy resistance in relapsed ALL. Blood 2019, 133, 2263–2268. [Google Scholar] [CrossRef]
- Rivera, G.; Wakelee, H.A. Resistance to Therapy. Cancer Treat. Res. 2016, 170, 183–202. [Google Scholar] [CrossRef] [PubMed]
- McMellen, A.; Woodruff, E.R.; Corr, B.R.; Bitler, B.G.; Moroney, M.R. Wnt Signaling in Gynecologic Malignancies. Int. J. Mol. Sci. 2020, 21, 4272. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Li, Y.; Xia, J.; Li, D.; Li, H.; Ren, M.; Liao, Y.; Yu, S.; Chen, Y.; et al. Cell apoptosis, autophagy and necroptosis in osteosarcoma treatment. Oncotarget 2016, 7, 44763–44778. [Google Scholar] [CrossRef]
- Shi, W.-J.; Gao, J.-B. Molecular mechanisms of chemoresistance in gastric cancer. World J. Gastrointest. Oncol. 2016, 8, 673–681. [Google Scholar] [CrossRef]
- Narasaki, F.; Matsuo, I.; Ikuno, N.; Fukuda, M.; Soda, H.; Oka, M. Multidrug resistance-associated protein (MRP) gene expression in human lung cancer. Anticancer Res. 1996, 16, 2079–2082. [Google Scholar]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Kanwal, R.; Shukla, S.; Walker, E.; Gupta, S. Acquisition of tumorigenic potential and therapeutic resistance in CD133+ subpopulation of prostate cancer cells exhibiting stem-cell like characteristics. Cancer Lett. 2018, 430, 25–33. [Google Scholar] [CrossRef]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef]
- Malik, B.; Nie, D. Cancer stem cells and resistance to chemo and radio therapy. Front. Biosci. 2012, E4, 2142–2149. [Google Scholar] [CrossRef]
- Matei, D.; Nephew, K.P. Epigenetic Attire in Ovarian Cancer: The Emperor’s New Clothes. Cancer Res. 2020, 80, 3775–3785. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Ling, V. The molecular basis of multidrug resistance in cancer: The early years of P-glycoprotein research. FEBS Lett. 2006, 580, 998–1009. [Google Scholar] [CrossRef]
- Dassa, E.; Bouige, P. The ABC of ABCS: A phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 2001, 152, 211–229. [Google Scholar] [CrossRef]
- Stefková, J.; Poledne, R.; Hubácek, J.A. ATP-binding cassette (ABC) transporters in human metabolism and diseases. Physiol. Res. 2004, 53, 235–243. [Google Scholar] [CrossRef]
- Lye, P.; Bloise, E.; Imperio, G.E.; Chitayat, D.; Matthews, S.G. Functional Expression of Multidrug-Resistance (MDR) Transporters in Developing Human Fetal Brain Endothelial Cells. Cells 2022, 11, 2259. [Google Scholar] [CrossRef]
- Hira, D.; Terada, T. BCRP/ABCG2 and high-alert medications: Biochemical, pharmacokinetic, pharmacogenetic, and clinical implications. Biochem. Pharm. 2018, 147, 201–210. [Google Scholar] [CrossRef]
- Steinbach, D.; Sell, W.; Voigt, A.; Hermann, J.; Zintl, F.; Sauerbrey, A. BCRP gene expression is associated with a poor response to remission induction therapy in childhood acute myeloid leukemia. Leukemia 2002, 16, 1443–1447. [Google Scholar] [CrossRef]
- Suvannasankha, A.; Minderman, H.; O’Loughlin, K.L.; Nakanishi, T.; Greco, W.R.; Ross, D.D.; Baer, M.R. Breast cancer resistance protein (BCRP/MXR/ABCG2) in acute myeloid leukemia: Discordance between expression and function. Leukemia 2004, 18, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Uggla, B.; Ståhl, E.; Wågsäter, D.; Paul, C.; Karlsson, M.G.; Sirsjö, A.; Tidefelt, U. BCRP mRNA expression v. clinical outcome in 40 adult AML patients. Leuk. Res. 2005, 29, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Benderra, Z.; Faussat, A.-M.; Sayada, L.; Perrot, J.-Y.; Chaoui, D.; Marie, J.-P.; Legrand, O. Breast cancer resistance protein and P-glycoprotein in 149 adult acute myeloid leukemias. Clin. Cancer Res. 2004, 10, 7896–7902. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.L.; Cassinelli, G.; Pennati, M.; Zuco, V.; Gatti, L. Overcoming ABC transporter-mediated multidrug resistance: The dual role of tyrosine kinase inhibitors as multitargeting agents. Eur. J. Med. Chem. 2017, 142, 271–289. [Google Scholar] [CrossRef]
- Gounder, M.K.; Nazar, A.S.; Saleem, A.; Pungaliya, P.; Kulkarni, D.; Versace, R.; Rubin, E.H. Effects of drug efflux proteins and topoisomerase I mutations on the camptothecin analogue gimatecan. Investig. New Drugs 2008, 26, 205–213. [Google Scholar] [CrossRef]
- Sabet, Z.; Vagiannis, D.; Budagaga, Y.; Zhang, Y.; Novotná, E.; Hanke, I.; Rozkoš, T.; Hofman, J. Talazoparib Does Not Interact with ABCB1 Transporter or Cytochrome P450s, but Modulates Multidrug Resistance Mediated by ABCC1 and ABCG2: An in Vitro and Ex Vivo Study. Int. J. Mol. Sci. 2022, 23, 14338. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; van Loevezijn, A.; Lakhai, J.M.; van der Valk, M.; van Tellingen, O.; Reid, G.; Schellens, J.H.M.; Koomen, G.-J.; Schinkel, A.H. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol. Cancer Ther. 2002, 1, 417–425. [Google Scholar]
- Tamaki, A.; Ierano, C.; Szakacs, G.; Robey, R.W.; Bates, S.E. The controversial role of ABC transporters in clinical oncology. Essays Biochem. 2011, 50, 209–232. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, N.; Sharma, S. ATP binding cassette transporters and cancer: Revisiting their controversial role. Pharmacogenomics 2021, 22, 1211–1235. [Google Scholar] [CrossRef]
- Kuhnert, L.; Kuhnert, R.; Sárosi, M.B.; Lakoma, C.; Scholz, B.K.; Lönnecke, P.; Hey-Hawkins, E.; Honscha, W.; Scholz, B.K. Enhanced reversal of ABCG2-mediated drug resistance by replacing a phenyl ring in baicalein with a meta-carborane. Mol. Oncol. 2023. [Google Scholar] [CrossRef]
- Stockmann, P.; Kuhnert, L.; Zörner, L.; Honscha, W.; Hey-Hawkins, E. 2-Carboranylquinazoline: The Path to an ABCG2 Inhibitor. ChemMedChem 2023, 18, e202300094. [Google Scholar] [CrossRef]
- Stockmann, P.; Kuhnert, L.; Leinung, W.; Lakoma, C.; Scholz, B.; Paskas, S.; Mijatović, S.; Maksimović-Ivanić, D.; Honscha, W.; Hey-Hawkins, E. The More the Better-Investigation of Polymethoxylated N-Carboranyl Quinazolines as Novel Hybrid Breast Cancer Resistance Protein Inhibitors. Pharmaceutics 2023, 15, 241. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, P.; Kuhnert, L.; Krajnović, T.; Mijatović, S.; Maksimović-Ivanić, D.; Honscha, W.; Hey-Hawkins, E. Carboranes, the better phenyl ring?—A comparative study on the reversal of ABCG2-mediated drug resistance of carboranylquinazolines and their organic isoters. ChemMedChem 2023. accepted. [Google Scholar]
- Stockmann, P.; Gozzi, M.; Kuhnert, R.; Sárosi, M.-B.; Hey-Hawkins, E. New keys for old locks: Carborane-containing drugs as platforms for mechanism-based therapies. Chem. Soc. Rev. 2019, 48, 3497–3512. [Google Scholar] [CrossRef] [PubMed]
- AbuHammad, S.; Zihlif, M. Gene expression alterations in doxorubicin resistant MCF7 breast cancer cell line. Genomics 2013, 101, 213–220. [Google Scholar] [CrossRef]
- Taylor, N.M.I.; Manolaridis, I.; Jackson, S.M.; Kowal, J.; Stahlberg, H.; Locher, K.P. Structure of the human multidrug transporter ABCG2. Nature 2017, 546, 504–509. [Google Scholar] [CrossRef]
- Chen, Y.; Du, F.; Tang, L.; Xu, J.; Zhao, Y.; Wu, X.; Li, M.; Shen, J.; Wen, Q.; Cho, C.H.; et al. Carboranes as unique pharmacophores in antitumor medicinal chemistry. Mol. Ther. Oncolytics 2022, 24, 400–416. [Google Scholar] [CrossRef]
- Hey-Hawkins, E. (Ed.) Boron-Based Compounds: Potential and Emerging Applications in Medicine; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; ISBN 9781119275596. [Google Scholar]
- Scholz, M.; Hey-Hawkins, E. Carbaboranes as pharmacophores: Properties, synthesis, and application strategies. Chem. Rev. 2011, 111, 7035–7062. [Google Scholar] [CrossRef]
- Das, B.C.; Nandwana, N.K.; Das, S.; Nandwana, V.; Shareef, M.A.; Das, Y.; Saito, M.; Weiss, L.M.; Almaguel, F.; Hosmane, N.S.; et al. Boron Chemicals in Drug Discovery and Development: Synthesis and Medicinal Perspective. Molecules 2022, 27, 2615. [Google Scholar] [CrossRef]
- Leśnikowski, Z.J. Challenges and Opportunities for the Application of Boron Clusters in Drug Design. J. Med. Chem. 2016, 59, 7738–7758. [Google Scholar] [CrossRef]
- Calcagno, A.M.; Fostel, J.M.; To, K.K.W.; Salcido, C.D.; Martin, S.E.; Chewning, K.J.; Wu, C.-P.; Varticovski, L.; Bates, S.E.; Caplen, N.J.; et al. Single-step doxorubicin-selected cancer cells overexpress the ABCG2 drug transporter through epigenetic changes. Br. J. Cancer 2008, 98, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Shivhare, S.; Das, A. Cell density modulates chemoresistance in breast cancer cells through differential expression of ABC transporters. Mol. Biol. Rep. 2023, 50, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Xiang, D.; Wang, T.; Zhang, Y.; Pham, C.V.; Zhou, S.; Jiang, G.; Hou, Y.; Zhu, Y.; Han, Y.; et al. The inhibition of ABCB1/MDR1 or ABCG2/BCRP enables doxorubicin to eliminate liver cancer stem cells. Sci. Rep. 2021, 11, 10791. [Google Scholar] [CrossRef] [PubMed]
- Omori, M.; Noro, R.; Seike, M.; Matsuda, K.; Hirao, M.; Fukuizumi, A.; Takano, N.; Miyanaga, A.; Gemma, A. Inhibitors of ABCB1 and ABCG2 overcame resistance to topoisomerase inhibitors in small cell lung cancer. Thorac. Cancer 2022, 13, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Sarkadi, B.; Homolya, L.; Hegedűs, T. The ABCG2/BCRP transporter and its variants—From structure to pathology. FEBS Lett. 2020, 594, 4012–4034. [Google Scholar] [CrossRef]
- Orlando, B.J.; Liao, M. ABCG2 transports anticancer drugs via a closed-to-open switch. Nat. Commun. 2020, 11, 2264. [Google Scholar] [CrossRef]
- Gose, T.; Aitken, H.M.; Wang, Y.; Lynch, J.; Rampersaud, E.; Fukuda, Y.; Wills, M.; Baril, S.A.; Ford, R.C.; Shelat, A.; et al. The net electrostatic potential and hydration of ABCG2 affect substrate transport. Nat. Commun. 2023, 14, 5035. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Haber, M.; Henderson, M.J.; Norris, M.D. ABC transporters in cancer: More than just drug efflux pumps. Nat. Rev. Cancer 2010, 10, 147–156. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Gao, S.; Wu, K.; Bai, F.; Zhang, Q.; Wang, H.; Ye, Q.; Xu, F.; Sun, H.; et al. Human drug efflux transporter ABCC5 confers acquired resistance to pemetrexed in breast cancer. Cancer Cell Int. 2021, 21, 136. [Google Scholar] [CrossRef]
- Lewis, I.J.; Weeden, S.; Machin, D.; Stark, D.; Craft, A.W. Received dose and dose-intensity of chemotherapy and outcome in nonmetastatic extremity osteosarcoma. European Osteosarcoma Intergroup. J. Clin. Oncol. 2000, 18, 4028–4037. [Google Scholar] [CrossRef]
- Milowsky, M.I.; Nanus, D.M.; Maluf, F.C.; Mironov, S.; Shi, W.; Iasonos, A.; Riches, J.; Regazzi, A.; Bajorin, D.F. Final results of sequential doxorubicin plus gemcitabine and ifosfamide, paclitaxel, and cisplatin chemotherapy in patients with metastatic or locally advanced transitional cell carcinoma of the urothelium. J. Clin. Oncol. 2009, 27, 4062–4067. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Theriault, R.L.; Hortobagyi, G.N.; Kau, S.W.; Holmes, F.A.; Hug, V.; Fraschini, G.; Jabboury, K.; Buzdar, A.U. Sequential multiagent chemotherapy incorporating cisplatin, doxorubicin, and cyclophosphamide in the treatment of metastatic breast cancer. Cancer 1988, 62, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Marinello, P.C.; Panis, C.; Silva, T.N.X.; Binato, R.; Abdelhay, E.; Rodrigues, J.A.; Mencalha, A.L.; Lopes, N.M.D.; Luiz, R.C.; Cecchini, R.; et al. Metformin prevention of doxorubicin resistance in MCF-7 and MDA-MB-231 involves oxidative stress generation and modulation of cell adaptation genes. Sci. Rep. 2019, 9, 5864. [Google Scholar] [CrossRef] [PubMed]
- Vesel, M.; Rapp, J.; Feller, D.; Kiss, E.; Jaromi, L.; Meggyes, M.; Miskei, G.; Duga, B.; Smuk, G.; Laszlo, T.; et al. ABCB1 and ABCG2 drug transporters are differentially expressed in non-small cell lung cancers (NSCLC) and expression is modified by cisplatin treatment via altered Wnt signaling. Respir. Res. 2017, 18, 52. [Google Scholar] [CrossRef]
- Mojic, M.; Mijatovic, S.; Maksimovic-Ivanic, D.; Dinic, S.; Grdovic, N.; Miljkovic, D.; Stosic-Grujicic, S.; Tumino, S.; Fagone, P.; Mangano, K.; et al. Saquinavir-NO-targeted S6 protein mediates sensitivity of androgen-dependent prostate cancer cells to TRAIL. Cell Cycle 2012, 11, 1174–1182. [Google Scholar] [CrossRef]
- Tallarida, R.J. An overview of drug combination analysis with isobolograms. J. Pharmacol. Exp. Ther. 2006, 319, 1–7. [Google Scholar] [CrossRef]
- Wolosin, J.M.; Zamudio, A.; Wang, Z. Application of JC1 for non-toxic isolation of cells with MDR transporter activity by flow cytometry. PLoS ONE 2017, 12, e0174905. [Google Scholar] [CrossRef]
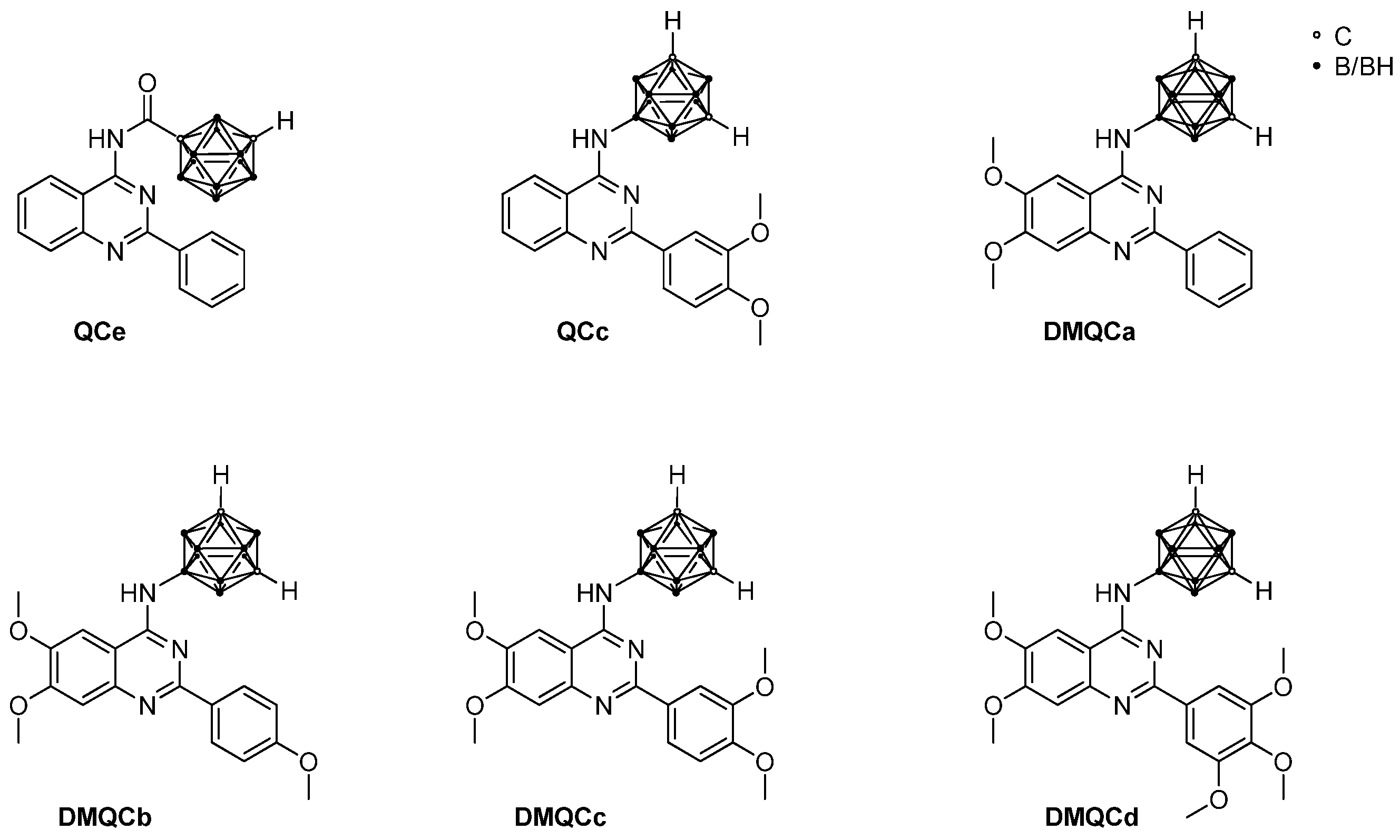
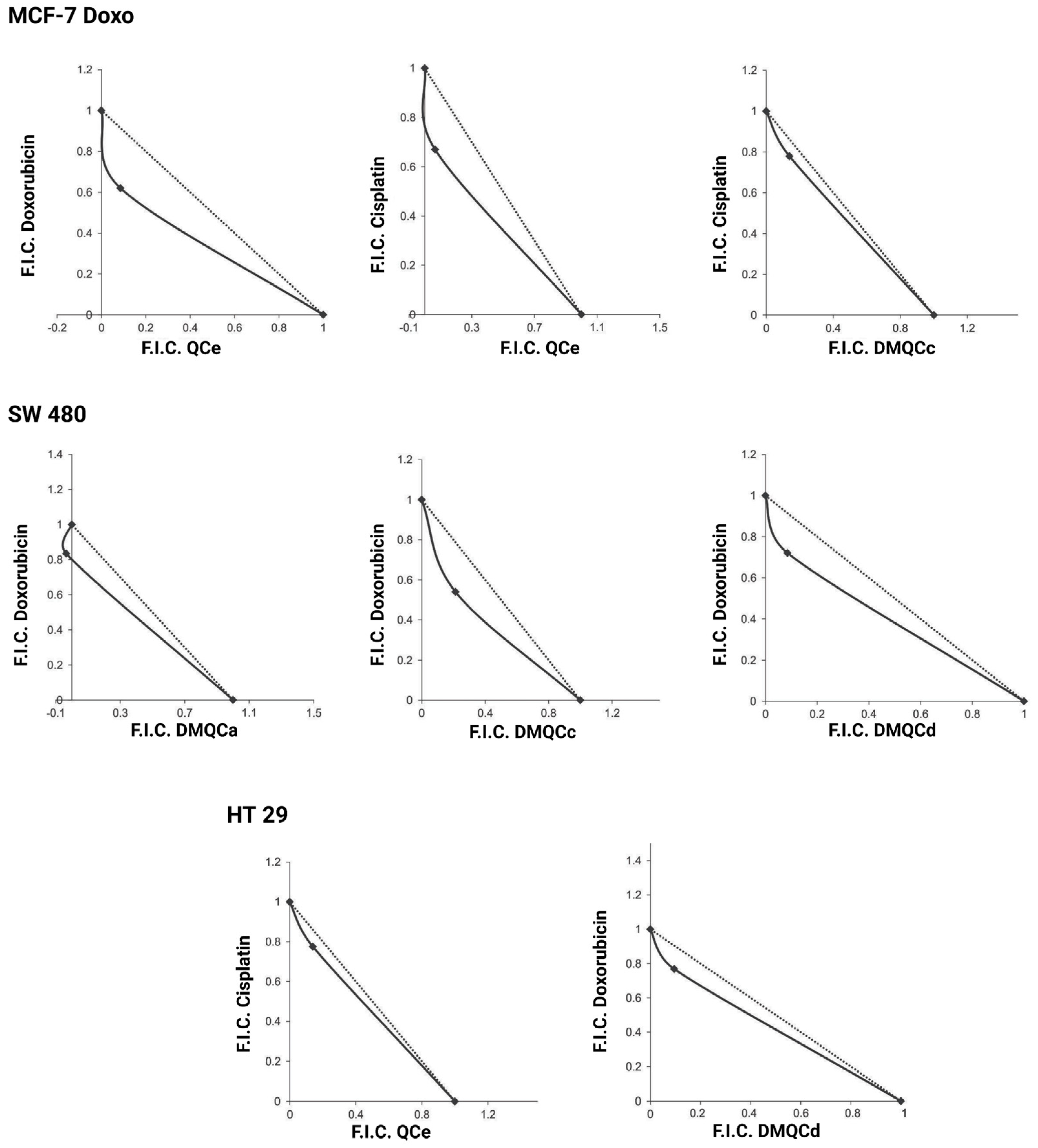
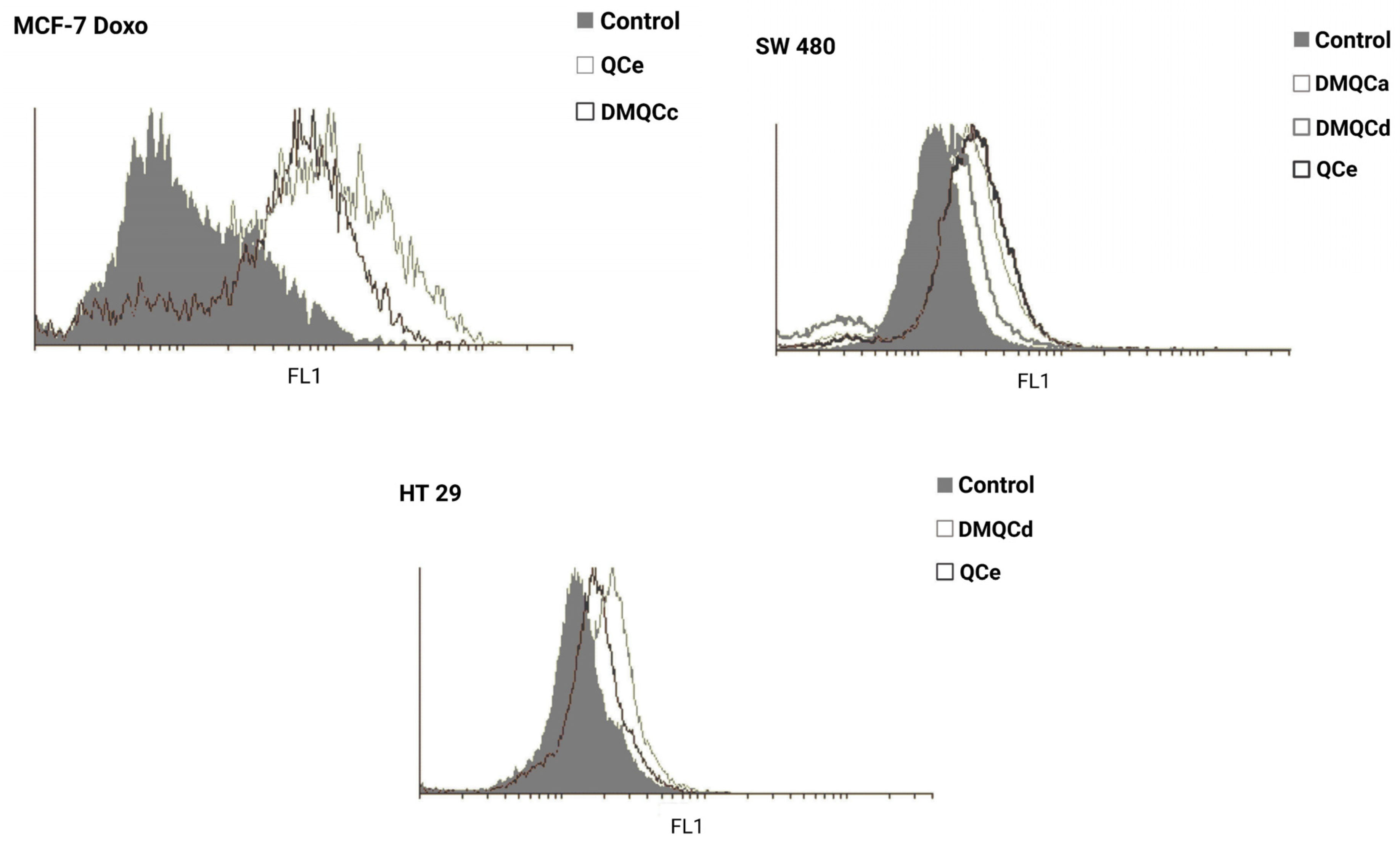
| Compound | MCF-7 | MCF-7 Doxo | SW480 | HT29 | ||||
|---|---|---|---|---|---|---|---|---|
| Doxo | CisPt | Doxo | CisPt | Doxo | CisPt | Doxo | CisPt | |
| QCc | Ant | Ant | Ant | Ant | Ant | Ant | Ant | Ant |
| QCe | Ant | Ant | Syn | Syn | Ant | Ant | Ant | Syn |
| DMQCa | Ant | Ant | Ant | Ant | Syn | Ant | Ant | Ant |
| DMQCb | Ant | Ant | Ant | Ant | Ant | Ant | Ant | Ant |
| DMQCc | Ant | Ant | Ant | Syn | Syn | Ant | Ant | Ant |
| DMQCd | Ant | Ant | Ant | Ant | Syn | Ant | Syn | Ant |
| Cell Line | Therapeutic | Inhibitor | IC50 (Therapeutic) [µM] | IC50 (Therapeutic + Inhibitor) [µM] |
|---|---|---|---|---|
| MCF-7 Doxo | Doxorubicin | QCe | 1.15 ± 0.20 | 0.80 ± 0.23 |
| Cisplatin | QCe | 25.10 ± 0.06 | 12.05 ± 1.30 | |
| Cisplatin | DMQCc | 26.65 ± 0.43 | 19.65 ± 0.03 | |
| SW480 | Doxorubicin | DMQCa | 1.25 ± 0.20 | 0.95 ± 0.14 |
| Doxorubicin | DMQCc | 0.60 ± 0.00 | 0.45 ± 0.03 | |
| Doxorubicin | DMQCd | 0.90 ± 0.10 | 0.45 ± 0.08 | |
| HT29 | Cisplatin | QCe | 25.05 ± 2.05 | 19.50 ± 1.16 |
| Doxorubicin | DMQCd | 1.00 ± 0.05 | 0.45 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paskas, S.; Stockmann, P.; Mijatović, S.; Kuhnert, L.; Honscha, W.; Hey-Hawkins, E.; Maksimović-Ivanić, D. Carborane-Based ABCG2-Inhibitors Sensitize ABC-(Over)Expressing Cancer Cell Lines for Doxorubicin and Cisplatin. Pharmaceuticals 2023, 16, 1582. https://doi.org/10.3390/ph16111582
Paskas S, Stockmann P, Mijatović S, Kuhnert L, Honscha W, Hey-Hawkins E, Maksimović-Ivanić D. Carborane-Based ABCG2-Inhibitors Sensitize ABC-(Over)Expressing Cancer Cell Lines for Doxorubicin and Cisplatin. Pharmaceuticals. 2023; 16(11):1582. https://doi.org/10.3390/ph16111582
Chicago/Turabian StylePaskas, Svetlana, Philipp Stockmann, Sanja Mijatović, Lydia Kuhnert, Walther Honscha, Evamarie Hey-Hawkins, and Danijela Maksimović-Ivanić. 2023. "Carborane-Based ABCG2-Inhibitors Sensitize ABC-(Over)Expressing Cancer Cell Lines for Doxorubicin and Cisplatin" Pharmaceuticals 16, no. 11: 1582. https://doi.org/10.3390/ph16111582
APA StylePaskas, S., Stockmann, P., Mijatović, S., Kuhnert, L., Honscha, W., Hey-Hawkins, E., & Maksimović-Ivanić, D. (2023). Carborane-Based ABCG2-Inhibitors Sensitize ABC-(Over)Expressing Cancer Cell Lines for Doxorubicin and Cisplatin. Pharmaceuticals, 16(11), 1582. https://doi.org/10.3390/ph16111582








