Statistically Optimized Polymeric Buccal Films of Eletriptan Hydrobromide and Itopride Hydrochloride: An In Vivo Pharmacokinetic Study
Abstract
1. Introduction
Advantages of Buccal Films
- Due to first-pass avoidance, the presence of high blood supply, and administration, oral mucosal ease is a striking route for the administration of medications.
- As buccal films remain intact for a longer period, they exhibit a higher release and availability of drugs compared to other routes, such as suspensions or solutions.
- The release pattern in films depends on their mode of use or formulation design. These can be used for both local and systemic effects. They can be prepared in one or more layers as well as in a modified release format.
- They increase patient compliance due to their small size, flexibility, and increased absorption time [43].
2. Results
2.1. Characterization of EHBR–ITHC Buccal Films
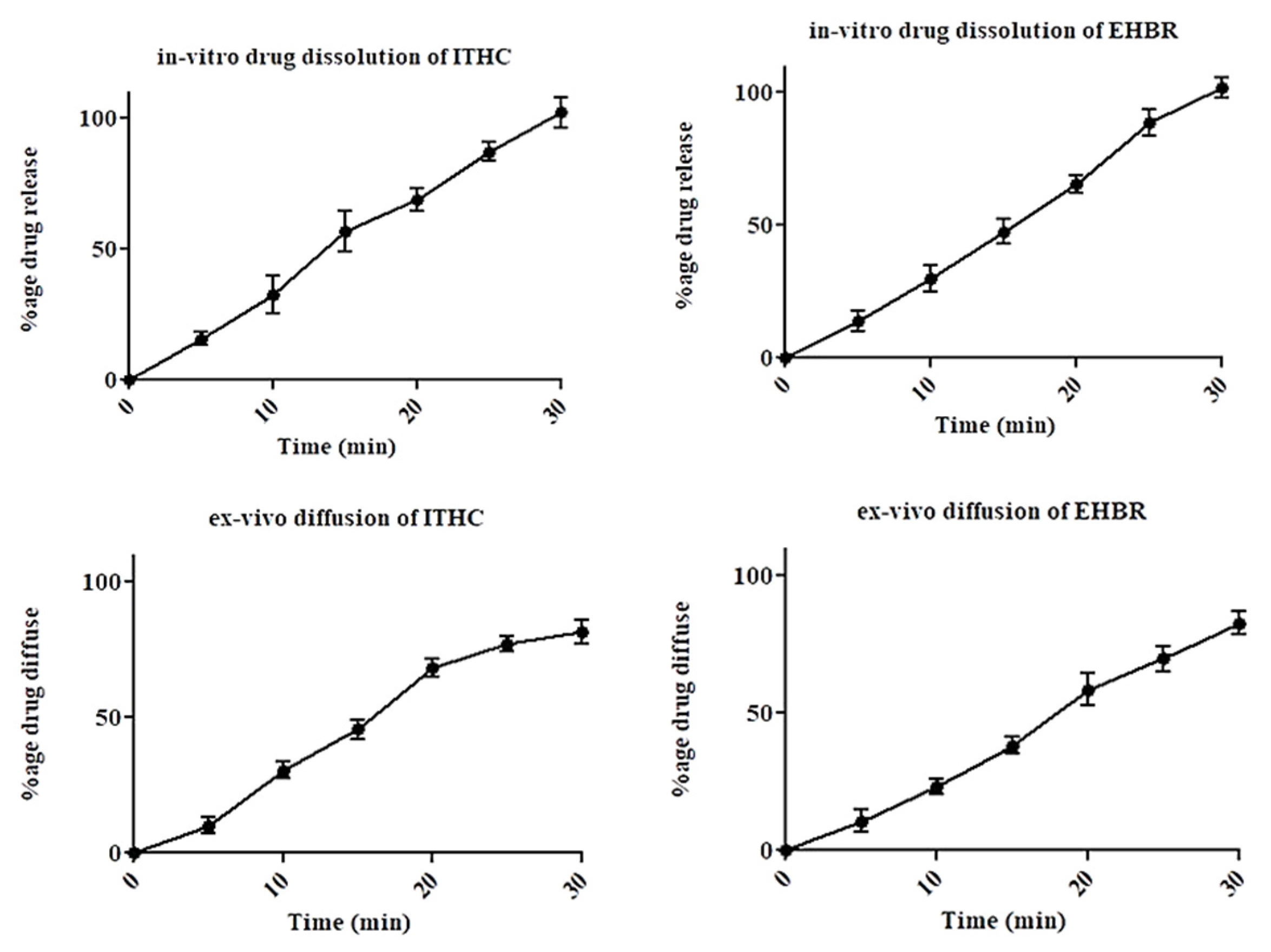
2.2. In Vitro Dissolution and Ex Vivo Permeation Studies
2.3. Scanning Electron Microscopy (SEM) of EHBR–ITHC Buccal Films
2.4. X-ray Diffractometer (XRD) of EHBR–ITHC Buccal Films
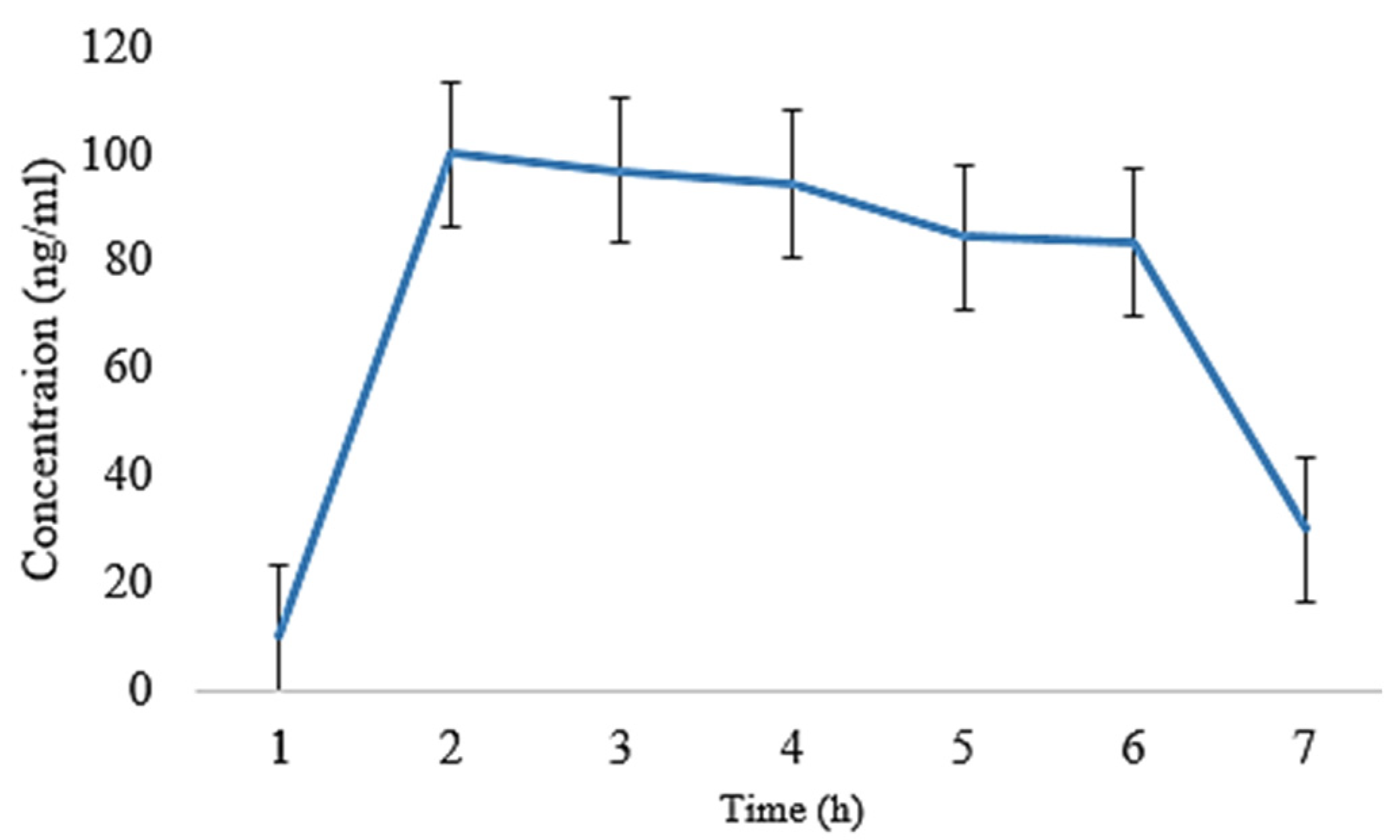
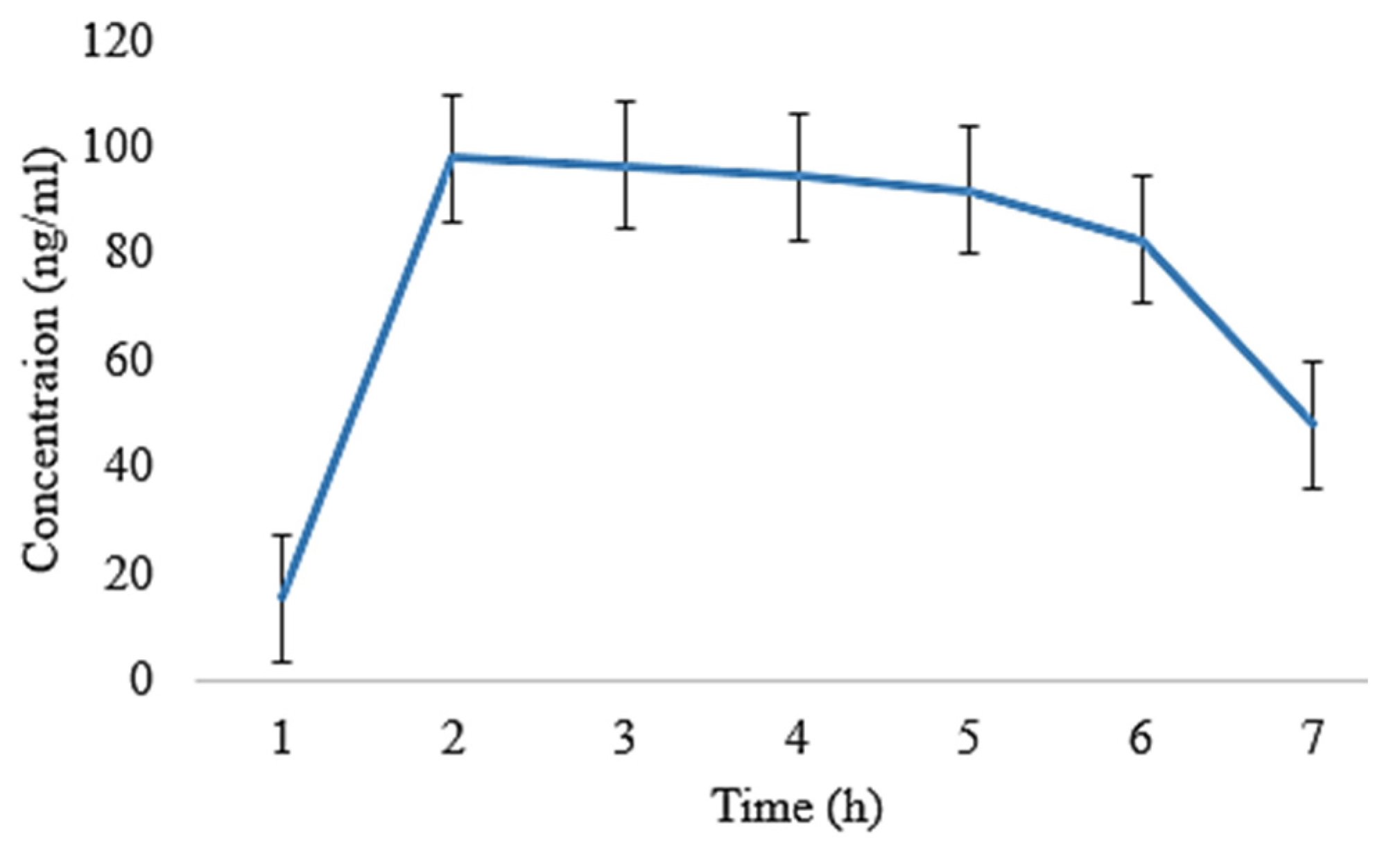
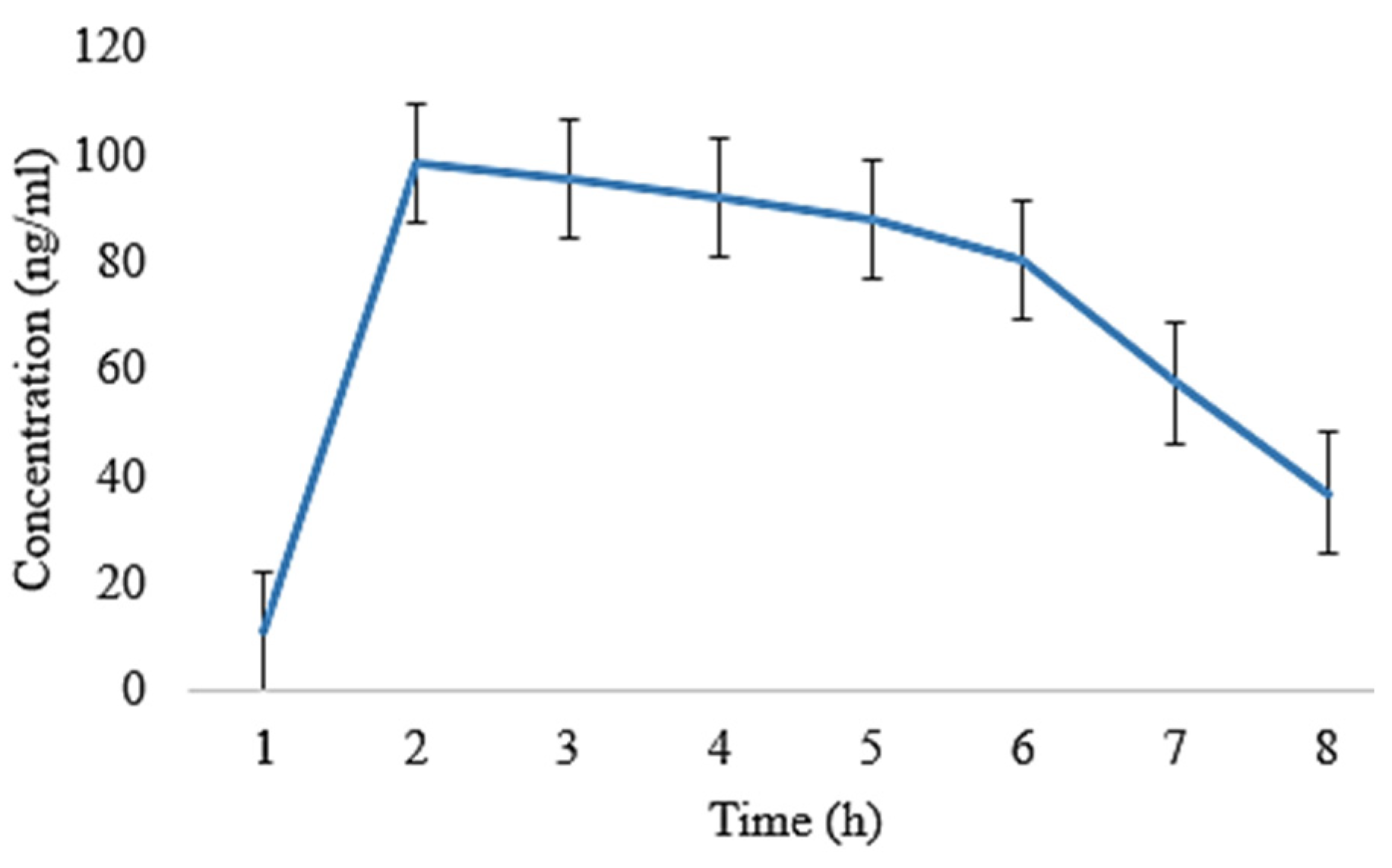
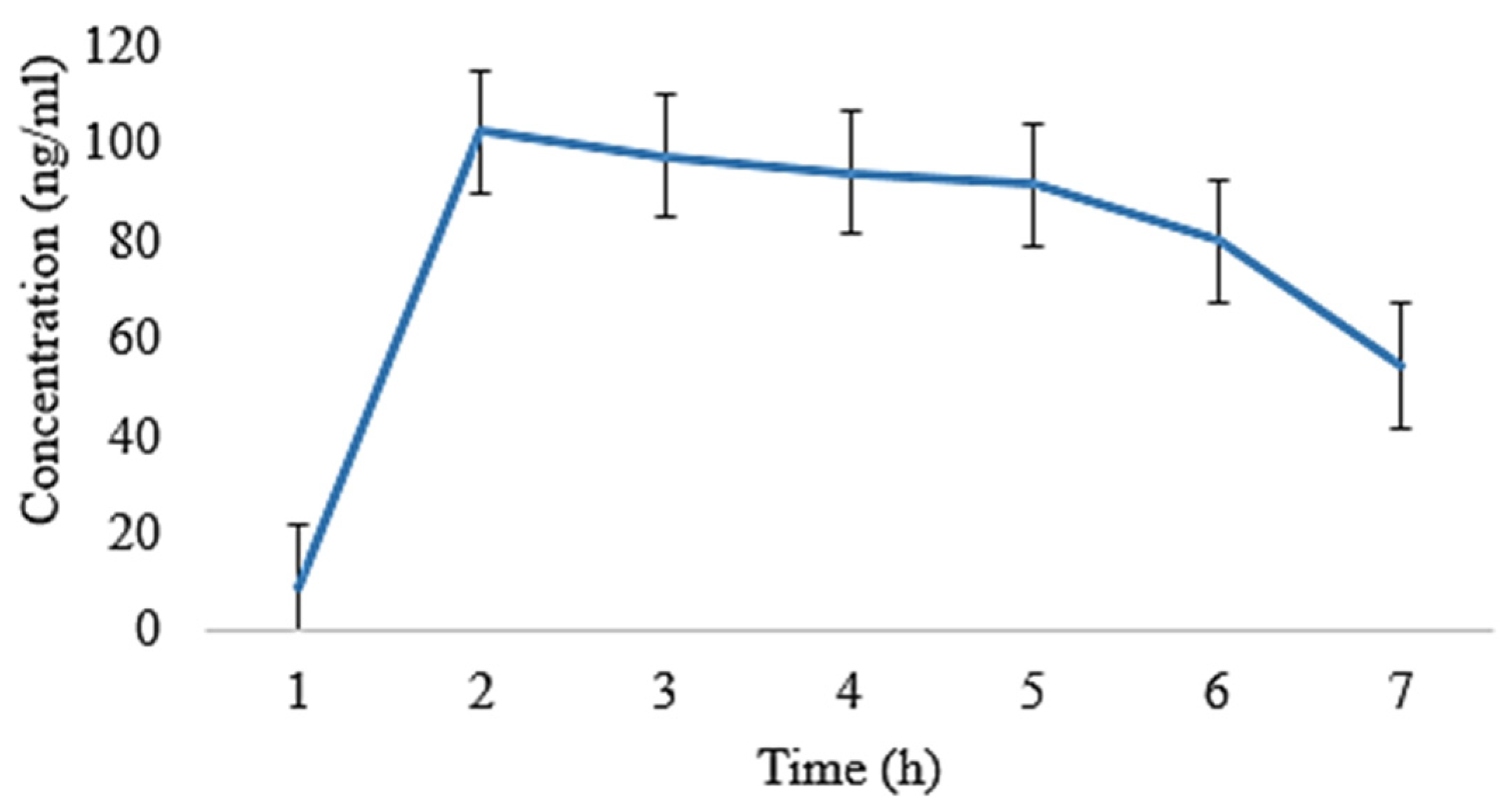
2.5. In Vivo Pharmacokinetic Analysis
2.6. Statistical Analysis
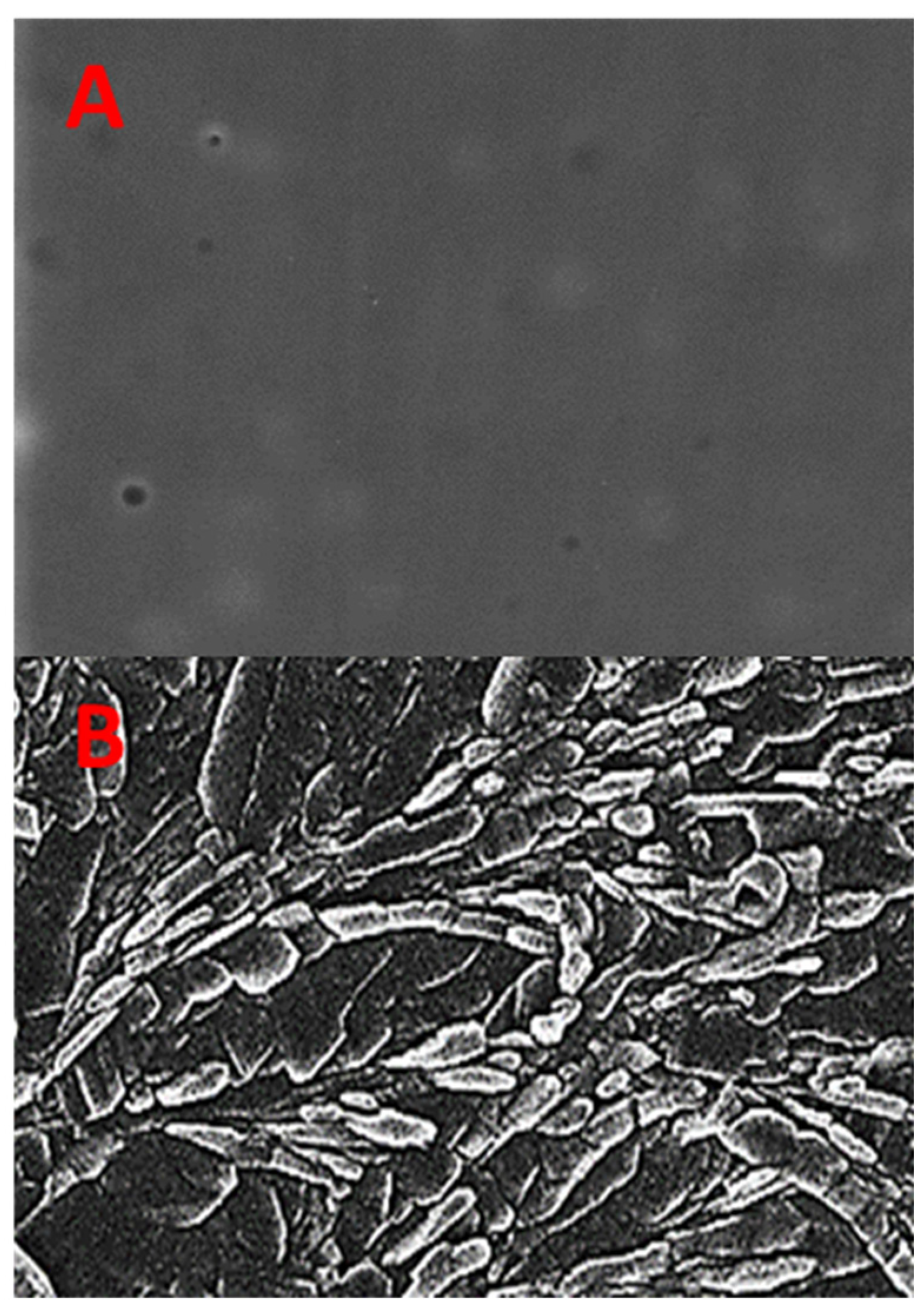
2.7. Histopathological Evaluation of Formulated Films
Kinetic Modeling
3. Discussion
4. Materials and Methods
4.1. Preparation of EHBR and ITHC Loaded Buccal Films
4.2. Evaluation of Buccal Films
Disintegration Time (DT) and Total Dissolving Time (TDT)
4.3. Surface pH
4.4. Moisture Content (MC)
4.5. Thickness
4.6. Folding Endurance (FE)
4.7. Content Uniformity
4.8. Scanning Electron Microscopy (SEM)
4.9. X-ray Diffraction (XRD)
4.10. In Vitro Dissolution Studies
4.11. Ex Vivo Permeation Studies
4.12. In Vivo Pharmacokinetic Study
Chromatographic Condition
4.13. Statistical Analysis
4.14. Histopathological Evaluation of Formulated Films
4.15. Kinetic Modeling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goel, A.N.; Long, J.L. Chapter 2—The Oral Cavity. In Dysphagia Evaluation and Management in Otolaryngology; Chhetri, D.K., Dewan, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 5–12. [Google Scholar]
- Arya, A.; Chandra, A.; Sharma, V.; Pathak, K. Fast dissolving oral films: An innovative drug delivery system and dosage form. Int. J. ChemTech Res. 2010, 2, 576–583. [Google Scholar]
- Jagtap, V.D. Buccal Film—A Review on Novel Drug Delivery System. Int. J. Res. Rev. 2020, 7, 17–28. [Google Scholar]
- Huanbutta, K.; Sangnim, T. Bioadhesive Films for Drug Delivery Systems. In Bioadhesives in Drug Delivery; Scrivener Publishing: Beverly, MA, USA, 2020; pp. 99–122. [Google Scholar]
- Parikh, T.; Gupta, S.S.; Meena, A.K.; Vitez, I.; Mahajan, N.; Serajuddin, A.T. Application of film-casting technique to investigate drug–polymer miscibility in solid dispersion and hot-melt extrudate. J. Pharm. Sci. 2015, 104, 2142–2152. [Google Scholar] [CrossRef] [PubMed]
- Arora, L.; Chakraborty, T. A review on new generation orodispersible films and its novel approaches. Indo Am. J. Pharm. Res. 2017, 7, 7451–7470. [Google Scholar]
- Borsook, D.; Maleki, N.; Burstein, R. Chapter 42—Migraine. In Neurobiology of Brain Disorders; Zigmond, M.J., Rowland, L.P., Coyle, J.T., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 693–708. [Google Scholar]
- Muehlberger, T. What Is Migraine? In Migraine Surgery: A Clinical Guide to Theory and Practice; Springer International Publishing: Cham, Switzerland, 2018; pp. 7–30. [Google Scholar]
- World Health Organisation. Atlas of Headache Disorders and Resources in the World 2011; World Health Organisation: Geneva, Switzerland, 2011. [Google Scholar]
- Ruthirago, D.; Julayanont, P.; Kim, J. Chapter 7.2—Translational Correlation: Migraine. In Conn’s Translational Neuroscience; Conn, P.M., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 159–165. [Google Scholar]
- Cortelli, P.; Allais, G.; Benedetto, C. Overview of triptans in the treatment of acute migraine. Eur. Neurol. Rev. 2017, 12, 71–77. [Google Scholar] [CrossRef]
- Kothapuvari, P.K.; Rawat, S.; Bhikshapathi, D. Estimation of Eletriptan Hydrobromide in Oral Film Dosage Form by. Int. J. Pharm. Sci. Drug Res. 2015, 7, 484–488. [Google Scholar]
- Capi, M.; Curto, M.; Lionetto, L.; de Andrés, F.; Gentile, G.; Negro, A.; Martelletti, P. Eletriptan in the management of acute migraine: An update on the evidence for efficacy, safety, and consistent response. Ther. Adv. Neurol. Disord. 2016, 9, 414–423. [Google Scholar] [CrossRef]
- Leroux, E.; Buchanan, A.; Lombard, L.; Loo, L.S.; Bridge, D.; Rousseau, B.; Hopwood, N.; Matthews, B.R.; Reuter, U. Evaluation of Patients with Insufficient Efficacy and/or Tolerability to Triptans for the Acute Treatment of Migraine: A Systematic Literature Review. Adv. Ther. 2020, 37, 4765–4796. [Google Scholar] [CrossRef]
- Esim, O.; Savaser, A.; Ozkan, C.K.; Oztuna, A.; Goksel, B.A.; Ozler, M.; Tas, C.; Ozkan, Y. Nose to brain delivery of eletriptan hydrobromide nanoparticles: Preparation, in vitro/in vivo evaluation and effect on trigeminal activation. J. Drug Deliv. Sci. Technol. 2020, 59, 101919. [Google Scholar] [CrossRef]
- Volans, G. Absorption of effervescent aspirin during migraine. Br. Med. J. 1974, 4, 265–268. [Google Scholar] [CrossRef]
- Cutler, N.; Hussey, E.; Sramek, J.; Clements, B.; Paulsgrove, L.; Busch, M.; Donn, K. Oral sumatriptan in pharmacokinetics in the migrainous state. Cephalalgia 1991, 11, 222–223. [Google Scholar] [CrossRef]
- Thomsen, L.; Dixon, R.; Lassen, L.; Giboens, M.; Langemark, M.; Bendtsen, L.; Daugaard, D.; Olesen, J. 311C90 (Zolmitriptan), a novel centrally and peripheral acting oral 5-hydroxytryptamine-1D agonist: A comparison of its absorption during a migraine attack and in a migraine-free period. Cephalalgia 1996, 16, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Shelke, S.; Shahi, S.; Tayade, P.; Joshi, A. Development and Validation of UV Spectrophotometric Method of Eletriptan Hydrobromide in Bulk and Pharmaceutical Formulation. Inven. Rapid Pharm. Anal. Qual. Assur. 2015, 2015, 1–4. [Google Scholar]
- Grujich, N.N.; Gawel, M.J. Eletriptan. Expert Opin. Investig. Drugs 2001, 10, 1869–1874. [Google Scholar] [CrossRef]
- Spandana, B.; Shashidher, B.; Dinesh, S.; Nagaraj, B. Eletriptan hydrobromide Orodispersible tablets: Design, Development and In vitro characterization. Res. J. Pharm. Technol. 2020, 13, 5339–5344. [Google Scholar]
- Milton, K.A.; Scott, N.R.; Allen, M.J.; Abel, S.; Jenkins, V.C.; James, G.; Rance, D.J.; Eve, M.D. Pharmacokinetics, Pharmacodynamics, and Safety of the 5-HT1B/1D Agonist Eletriptan following Intravenous and Oral Administration. J. Clin. Pharmacol. 2002, 42, 528–539. [Google Scholar] [CrossRef]
- Sandrini, G.; Perrotta, A.; Tassorelli, C.; Nappi, G. Eletriptan. Expert Opin. Drug Metab. Toxicol. 2009, 5, 1587–1598. [Google Scholar] [CrossRef]
- Aurora, S.K.; Papapetropoulos, S.; Kori, S.H.; Kedar, A.; Abell, T.L. Gastric stasis in migraineurs: Etiology, characteristics, and clinical and therapeutic implications. Cephalalgia 2013, 33, 408–415. [Google Scholar] [CrossRef]
- Masaoka, T.; Tack, J. Gastroparesis: Current concepts and management. Gut Liver 2009, 3, 166. [Google Scholar] [CrossRef][Green Version]
- Parkman, H.P. Migraine and gastroparesis from a gastroenterologist’s perspective. Headache J. Head Face Pain 2013, 53, 4–10. [Google Scholar] [CrossRef]
- van Hemert, S.; Breedveld, A.C.; Rovers, J.M.; Vermeiden, J.P.; Witteman, B.J.; Smits, M.G.; de Roos, N.M. Migraine associated with gastrointestinal disorders: Review of the literature and clinical implications. Front. Neurol. 2014, 5, 241. [Google Scholar] [CrossRef]
- Cámara-Lemarroy, C.R.; Rodriguez-Gutierrez, R.; Monreal-Robles, R.; Marfil-Rivera, A. Gastrointestinal disorders associated with migraine: A comprehensive review. World J. Gastroenterol. 2016, 22, 8149. [Google Scholar] [CrossRef]
- Ratnaparkhi, M.P. Formulation and development of floating drug delivery of Itopride Hcl. J. Drug Deliv. Ther. 2013, 3, 222–228. [Google Scholar] [CrossRef][Green Version]
- Ranjan, A.; Chandra, A.; Kumar, D. The comparative effects of Itopride and Levosulpiride orally used in patients suffering from Non-ulcer dyspepsia. Int. J. Basic Clin. Pharmacol. 2019, 8, 1915. [Google Scholar] [CrossRef]
- Nasiri, M.I.; Yousuf, R.I.; Shoaib, M.H.; Siddiqui, F.; Qazi, F.; Ahmed, K.; Anwer, S.; Zaheer, K. Comparative pharmacokinetic evaluation of extended release itopride HCl pellets with once daily tablet formulation in healthy human subjects: A two treatment, four period crossover study in fasted and fed condition. Drug Dev. Ind. Pharm. 2019, 45, 415–422. [Google Scholar] [CrossRef]
- Felice, C.S.; Srinivasulu, K.; Kumar, V.P.; Saradhi, S.V. Visible Spectrophotometric Determination of Itopride Hydrochloride in Pharmaceutical Formulations. Int. J. Chem. Sci. 2008, 6, 832–838. [Google Scholar]
- Gupta, K.; Joshi, R.; Chawla, R.; Wadodkar, S. UV spectrophotometric method for theestimation of itopride hydrochloride in pharmaceutical formulation. J. Chem. 2010, 7, S49–S54. [Google Scholar] [CrossRef]
- Bansal, S.; Beg, S.; Asthana, A.; Garg, B.; Asthana, G.S.; Kapil, R.; Singh, B. QbD-enabled systematic development of gastroretentive multiple-unit microballoons of itopride hydrochloride. Drug Deliv. 2016, 23, 437–451. [Google Scholar] [CrossRef]
- Maboos, M.; Yousuf, R.I.; Shoaib, M.H.; Nasiri, I.; Hussain, T.; Ahmed, H.F.; Iffat, W. Effect of lipid and cellulose based matrix former on the release of highly soluble drug from extruded/spheronized, sintered and compacted pellets. Lipids Health Dis. 2018, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-J.; Cho, W.; Cha, K.-H.; Park, J.; Kim, M.-S.; Kim, J.-S.; Hwang, S.-J. Pharmacokinetic and bioequivalence study of itopride HCl in healthy volunteers. Arzneimittelforschung 2010, 60, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kapoor, V.; Kapoor, B. Drug Review: Itopride. A Novel Prokinetic Agent. JK Sci. 2004, 6, 106–108. [Google Scholar]
- Sawant, P.; Das, H.; Desai, N.; Kalokhe, S.; Patil, S. Comparative evaluation of the efficacy and tolerability of itopride hydrochloride and domperidone in patients with non-ulcer dyspepsia. JAPI 2004, 52, 626–628. [Google Scholar] [PubMed]
- Kim, Y.S.; Kim, T.H.; Choi, C.S.; Shon, Y.W.; Kim, S.W.; Seo, G.S.; Nah, Y.H.; Choi, M.G.; Choi, S.C. Effect of itopride, a new prokinetic, in patients with mild GERD: A pilot study. World J. Gastroenterol. WJG 2005, 11, 4210. [Google Scholar] [CrossRef]
- Khan, K.; Arain, M.I.; Asghar, M.A.; Rehman, A.A.; Ghoto, M.A.; Dayo, A.; Imtiaz, M.S.; Rana, M.H.; Asghar, M.A. Analysis of treatment cost and persistence among migraineurs: A two-year retrospective cohort study in Pakistan. PLoS ONE 2021, 16, e0248761. [Google Scholar] [CrossRef] [PubMed]
- Aurora, S.K.; Shrewsbury, S.B.; Ray, S.; Hindiyeh, N.; Nguyen, L. A link between gastrointestinal disorders and migraine: Insights into the gut–brain connection. Headache J. Head Face Pain 2021, 61, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Seher, S.; Ali, M.K.; Ilyas, M.; Raza, M.; Hussain, F. Scintigraphic Evaluation of Gastric Emptying in Diabetic Patients with Suspected Gastroparesis. Pak. J. Physiol. 2016, 12, 30–32. [Google Scholar]
- Montenegro-Nicolini, M.; Morales, J.O. Overview and future potential of buccal mucoadhesive films as drug delivery systems for biologics. AAPS PharmSciTech 2017, 18, 3–14. [Google Scholar] [CrossRef]
- Naik, D.S.; Ramadevi, K.; Bhagawan, D. Method develop and validation of eletriptan hydrobromide pharmaceutical dosage form by RP-HPLC. Int. J. Eng. Res. Appl. 2013, 3, 1–5. [Google Scholar]
- Siddique, W.; Sarfraz, R.M.; Zaman, M.; Khan, R.; Gul, M.; Asghar, F.; Malik, T.; Saif, A.; Shamim, Q.-U.-A.; Salawi, A. Method development and validation for simultaneous determination of Eletriptan hydrobromide and Itopride hydrochloride from fast dissolving buccal films by using RP-HPLC. Acta Chromatogr. 2022, 35, 310–318. [Google Scholar] [CrossRef]
- Elbary, A.A.; Ali, A.A.; Aboud, H.M. Enhanced dissolution of meloxicam from orodispersible tablets prepared by different methods. Bull. Fac. Pharm. Cairo Univ. 2012, 50, 89–97. [Google Scholar] [CrossRef]
- Prabhu, P.; Malli, R.; Koland, M.; Vijaynarayana, K.; D’Souza, U.; Harish, N.; Shastry, C.; Charyulu, R. Formulation and evaluation of fast dissolving films of levocitirizine di hydrochloride. Int. J. Pharm. Investig. 2011, 1, 99. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, M.; Dubey, A.; Narayan, R.; Prabhu, P.; Priya, S. Formulation and evaluation of buccal film of Ivabradine hydrochloride for the treatment of stable angina pectoris. Int. J. Pharm. Investig. 2013, 3, 47. [Google Scholar] [PubMed]
- Rao, N.; Munde, M. Formulation and in-vitro evaluation of mucoadhesive buccal films of zolmitriptan. J. Pharm. Res. 2011, 4, 2682–2685. [Google Scholar]
- Lv, Q.; Shen, C.; Li, X.; Shen, B.; Yu, C.; Xu, P.; Xu, H.; Han, J.; Yuan, H. Mucoadhesive buccal films containing phospholipid-bile salts-mixed micelles as an effective carrier for Cucurbitacin B delivery. Drug Deliv. 2015, 22, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Pedada, R.B.; Vanka, E.; Babu, A.S.; Desu, P.K.; Bharathi, P.R.; Rao, P.V. Enhancement of Solubility: An Over View. PharmaTutor 2013, 1, 60–74. [Google Scholar]
- Hanif, M.; Zaman, M. Thiolation of arabinoxylan and its application in the fabrication of controlled release mucoadhesive oral films. DARU J. Pharm. Sci. 2017, 25, 6. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Boddu, S.H.; Gorain, B.; Sreeharsha, N.; Shah, J. An updated overview of the emerging role of patch and film-based buccal delivery systems. Pharmaceutics 2021, 13, 1206. [Google Scholar] [CrossRef]
- Salehi, S.; Boddohi, S. New formulation and approach for mucoadhesive buccal film of rizatriptan benzoate. Prog. Biomater. 2017, 6, 175–187. [Google Scholar] [CrossRef]
- Ali, M.; Vijendar, C.; Kumar, S.; Krishnaveni, J. Formulation and evaluation of fast dissolving oral films of diazepam. J. Pharmacovigil. 2016, 4, 210. [Google Scholar]
- Dangre, P.V.; Phad, R.D.; Surana, S.J.; Chalikwar, S.S. Quality by design (QbD) assisted fabrication of fast dissolving buccal film for clonidine hydrochloride: Exploring the quality attributes. Adv. Polym. Technol. 2019, 2019, 3682402. [Google Scholar] [CrossRef]
- Zaman, M.; Hanif, M.; Sultana, K. Synthesis of thiolated arabinoxylan and its application as sustained release mucoadhesive film former. Biomed. Mater. 2018, 13, 025019. [Google Scholar] [CrossRef] [PubMed]
- Prieto, C.; Calvo, L. Performance of the biocompatible surfactant Tween 80, for the formation of microemulsions suitable for new pharmaceutical processing. J. Appl. Chem. 2013, 2013, 930356. [Google Scholar] [CrossRef]
- Buya, A.B.; Beloqui, A.; Memvanga, P.B.; Préat, V. Self-Nano-Emulsifying Drug-Delivery Systems: From the Development to the Current Applications and Challenges in Oral Drug Delivery. Pharmaceutics 2020, 12, 1194. [Google Scholar] [CrossRef]
- Akhtar, N.; Rehman, M.; Khan, H.; Rasool, F.; Saeed, T.; Murtaz, G. Penetration enhancing effect of polysorbate 20 and 80 on the in vitro percutaneous absorption of lascorbic acid. Trop. J. Pharm. Res. 2011, 10, 281–288. [Google Scholar] [CrossRef]
- Pawar Harshal, A.; Jadhav Surekha, M.; Jadhav Pravin, T.; Geevarghese, R. Development and Evaluation of Mucoadhesive Patch Using a Natural Polysaccharide Isolated from Cordia dichotoma Fruit. J. Mol. Pharm. Org. Process Res. 2014, 2, 120. [Google Scholar]
- Luciano, B.M. 5—Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. [Google Scholar]
- Mane, P.; Bushetti, S.; Keshavshetti, G. Development and In vitro evaluation of mucoadhesive buccal films of nebivolol. Indian J. Pharm. Sci. 2014, 76, 166. [Google Scholar]
- Kumar, S.; Singh, A.K.; Prajapati, S.; Singh, V.K. Formulation and evaluation of once daily sustained release matrix tablets of aceclofenac using natural gums. J. Drug Deliv. Ther. 2012, 2, 16–24. [Google Scholar] [CrossRef]
- Anod, H.V.; Gupta, N.V.; Gowda, D.; Manohar, M. Preparation and evaluation of simvastatin transdermal film. Int. J. Appl. Pharm. 2018, 10, 235–238. [Google Scholar] [CrossRef][Green Version]
- El-Maghraby, G.M.; Abdelzaher, M.M. Formulation and evaluation of simvastatin buccal film. J. Appl. Pharm. Sci. 2015, 5, 70–77. [Google Scholar] [CrossRef]
- Bîrsan, M.; Apostu, M.; Todoran, N.; Antonoaea, P.; Rusu, A.; Ciurba, A. Development of dermal films containing miconazole nitrate. Molecules 2018, 23, 1640. [Google Scholar] [CrossRef] [PubMed]
- Siddique, W.; Zaman, M.; Sarfraz, R.M.; Butt, M.H.; Rehman, A.U.; Fassih, N.; Albadrani, G.M.; Bayram, R.; Alfaifi, M.Y.; Abdel-Daim, M.M. The Development of Eletriptan Hydrobromide Immediate Release Buccal Films Using Central Composite Rotatable Design: An In Vivo and In Vitro Approach. Polymers 2022, 14, 3981. [Google Scholar] [CrossRef] [PubMed]
- Speer, I.; Lenhart, V.; Preis, M.; Breitkreutz, J. Prolonged release from orodispersible films by incorporation of diclofenac-loaded micropellets. Int. J. Pharm. 2019, 554, 149–160. [Google Scholar] [CrossRef]
- Hanif, M.; Zaman, M.; Chaurasiya, V. Polymers used in buccal film: A review. Des. Monomers Polym. 2015, 18, 105–111. [Google Scholar] [CrossRef]
- Suksaeree, J.; Chaichawawut, B.; Srichan, M.; Tanaboonsuthi, N.; Monton, C.; Maneewattanapinyo, P.; Pichayakorn, W. Applying design of experiments (DoE) on the properties of buccal film for nicotine delivery. e-Polymers 2021, 21, 566–574. [Google Scholar] [CrossRef]
- Salawi, A. An insight into preparatory methods and characterization of orodispersible film—A review. Pharmaceuticals 2022, 15, 844. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Zaman, M.; Raja, M.A.G.; Mahmood, A.; Amjad, M.W. Rosuvastatin, Perindopril and Ezetimibe loaded instant release buccal films: Development and in vitro characterization. J. Appl. Biomed. 2020, 18, 115–125. [Google Scholar] [CrossRef]
- Talekar, S.D.; Haware, R.V.; Dave, R.H. Evaluation of self-nanoemulsifying drug delivery systems using multivariate methods to optimize permeability of captopril oral films. Eur. J. Pharm. Sci. 2019, 130, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Hanif, M.; Shaheryar, Z.A. Development of Tizanidine HCl-Meloxicam loaded mucoadhesive buccal films: In-vitro and in-vivo evaluation. PLoS ONE 2018, 13, e0194410. [Google Scholar] [CrossRef]
- Li, X.-Q.; Ye, Z.-M.; Wang, J.-B.; Fan, C.-R.; Pan, A.-W.; Li, C.; Zhang, R.-B. Mucoadhesive buccal films of tramadol for effective pain management. Rev. Bras. Anestesiol. 2017, 67, 231–237. [Google Scholar] [CrossRef]
- Chauhan, I.; Yasir, M.; Verma, M. Oral delivery of zolmitriptan loaded fast disintegrating film: Formulation development, statistical optimization, in-vitro and in-vivo evaluation. J. Appl. Pharm. Sci. Res. 2019, 2, 13–22. [Google Scholar] [CrossRef]
- Hadi, M.A.; Rao, N.G.R.; Rao, A.S. Pharmacokinetic parameters determination and in vitro–in vivo correlation of ileocolonic-targeted pH-responsive coated mini-tablets of naproxen. Sci. Pharm. 2015, 83, 645–658. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Michael, J.; Bessa de Sousa, D.; Conway, J.; Gonzalez-Labrada, E.; Obeid, R.; Tevini, J.; Felder, T.; Hutter-Paier, B.; Zerbe, H.; Paiement, N. Improved bioavailability of montelukast through a novel oral mucoadhesive film in humans and mice. Pharmaceutics 2020, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Boddohi, S. Design and optimization of kollicoat® IR based mucoadhesive buccal film for co-delivery of rizatriptan benzoate and propranolol hydrochloride. Mater. Sci. Eng. C 2019, 97, 230–244. [Google Scholar] [CrossRef] [PubMed]
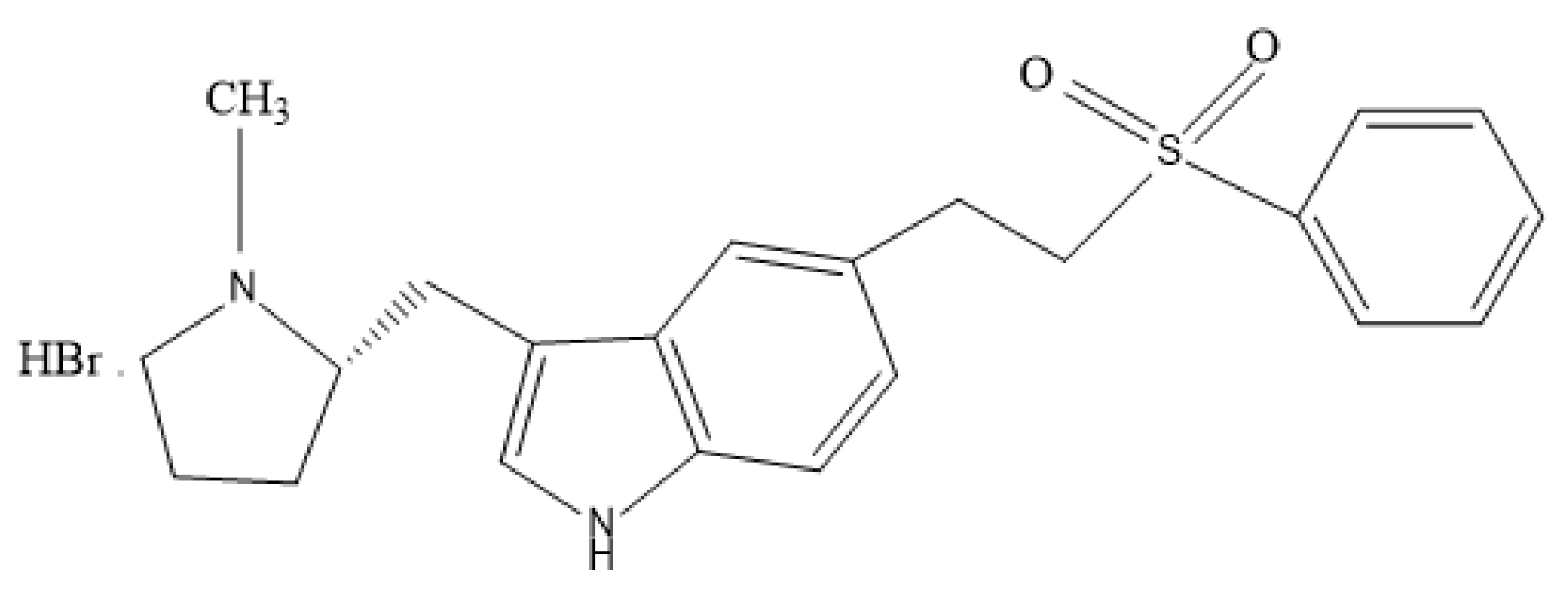
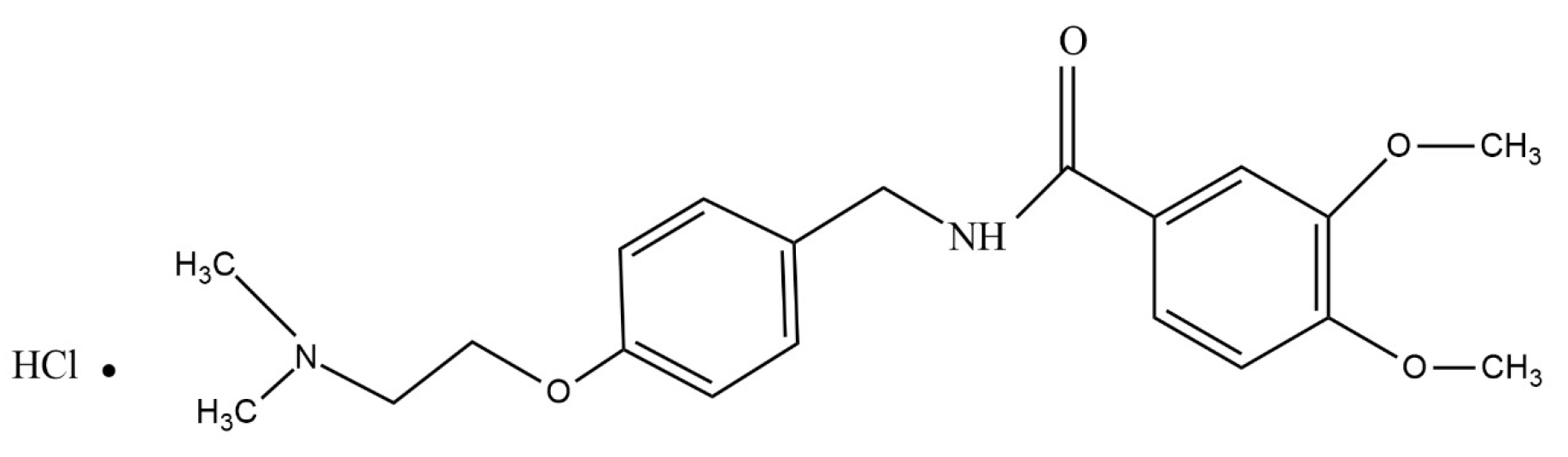


| pH | Thickness (mm) | DT (s) | TDT (s) | Weight Variation (mg) | MC (%) | FE | Content Uniformity | |
|---|---|---|---|---|---|---|---|---|
| EHBR (%) | ITHC (%) | |||||||
| 6.5 ± 0.02 | 0.23 ± 0.1 | 13 ± 1 | 42.6 ± 0.75 | 0.035 ± 0.05 | 45 ± 0.78 | 249 ± 1 | 101 ± 0.1 | 97 ± 0.3 |
| Parameters | ITHC–EHBR Buccal Films | ITHC–EHBR Solution | ||
|---|---|---|---|---|
| ITHC | EHBR | ITHC | EHBR | |
| t1/2 (h) | 4.86 ± 0.02 | 4.70 ± 0.11 | 5.01 ± 0.008 | 5.21 ± 0.009 |
| tmax (h) | 1 ± 0.01 | 1 ± 0.05 | 1 ± 0.02 | 1 ± 0.03 |
| Cmax (ng/mL) | 130 ± 0.15 | 119 ± 0.14 | 96 ± 0.11 | 90.5 ± 0.07 |
| Clast_obs/Cmax | 0.32 ± 0.01 | 0.43 ± 0.05 | 0.460 ± 0.01 | 0.46 ± 0.03 |
| AUC0–t (ng/mL·h) | 693 ± 1.01 | 650 ± 1.20 | 648.5 ± 0.29 | 639.5 ± 0.55 |
| AUC0–inf_obs (ng/mL·h) | 987.76 ± 1.06 | 881.76 ± 1.19 | 993.03 ± 1.21 | 984.03 ± 1.11 |
| AUC0–t/0–inf_obs | 0.701 ± 0.11 | 0.737 ± 0.14 | 0.65 ± 0.10 | 0.649 ± 0.13 |
| AUMC0–inf_obs (ng/mL·h2) | 6966.73 ± 1.12 | 5772.72 ± 1.08 | 7749.29 ± 1.23 | 7747.79 ± 1.11 |
| MRT0–inf_obs (h) | 7.05 ± 0.16 | 6.30 ± 0.35 | 7.80 ± 0.16 | 7.87 ± 0.13 |
| Formulation No | Zero-Order | First-Order | Highuci Model | Hixson–Crowell Model | Korsmeyer–Peppas Model | Value of n | Best-Fit Model |
|---|---|---|---|---|---|---|---|
| 1 | 0.5169 | 0.9646 | 0.9723 | 0.9157 | 0.9733 | 0.503 | Korsmeyer–Peppas model |
| 2 | 0.5067 | 0.9616 | 0.9590 | 0.9101 | 0.9690 | 0.500 | Korsmeyer–Peppas model |
| 3 | 0.5605 | 0.9748 | 0.9670 | 0.9870 | 0.9680 | 0.519 | Hixson–Crowell model |
| 4 | 0.5597 | 0.8696 | 0.9475 | 0.8148 | 0.9485 | 0.520 | Korsmeyer–Peppas model |
| 5 | 0.5605 | 0.9748 | 0.9670 | 0.9870 | 0.9680 | 0.519 | Hixson–Crowell model |
| 6 | 0.9498 | 0.9527 | 0.8907 | 0.9787 | 0.9878 | 0.783 | Korsmeyer–Peppas model |
| 7 | 0.6912 | 0.9064 | 0.9555 | 0.8533 | 0.9659 | 0.568 | Korsmeyer–Peppas model |
| 8 | 0.9478 | 0.9608 | 0.8819 | 0.9720 | 0.9813 | 0.792 | Korsmeyer–Peppas model |
| 9 | 0.2271 | 0.5515 | 0.8997 | 0.3480 | 0.9664 | 0.383 | Korsmeyer–Peppas model |
| 10 | 0.1243 | 0.9728 | 0.9035 | 0.9556 | 0.9539 | 0.395 | First-order |
| 11 | 0.1638 | 0.6226 | 0.8813 | 0.4270 | 0.9378 | 0.389 | Korsmeyer–Peppas model |
| 12 | 0.7101 | 0.8939 | 0.9566 | 0.8459 | 0.9694 | 0.576 | Korsmeyer–Peppas model |
| 13 | 0.9183 | 0.9509 | 0.9044 | 0.9711 | 0.9799 | 0.736 | Hixson–Crowell model |
| 14 | 0.8471 | 0.9666 | 0.9504 | 0.9416 | 0.9890 | 0.648 | Korsmeyer–Peppas model |
| 15 | 0.5605 | 0.9748 | 0.9670 | 0.9870 | 0.9680 | 0.519 | Hixson–Crowell model |
| 16 | 0.1121 | 0.9942 | 0.9161 | 0.9806 | 0.9378 | 0.425 | Hixson–Crowell model |
| 17 | 0.7591 | 0.9240 | 0.9637 | 0.8825 | 0.9826 | 0.595 | Korsmeyer–Peppas model |
| Formulation No | Zero-Order | First-Order | Highuci Model | Hixson–Crowell Model | Korsmeyer–Peppas Model | Value of n | Best-Fit Model |
|---|---|---|---|---|---|---|---|
| 1 | 0.9540 | 0.8877 | 0.7325 | 0.9186 | 0.9585 | 1.101 | Korsmeyer–Peppas model |
| 2 | 0.8067 | 0.8844 | 0.9222 | 0.9650 | 0.9872 | 0.641 | Korsmeyer–Peppas model |
| 3 | 0.7041 | 0.9825 | 0.9479 | 0.9601 | 0.9602 | 0.575 | First-order |
| 4 | 0.9011 | 0.9613 | 0.8512 | 0.9650 | 0.9424 | 0.774 | Hixson–Crowell model |
| 5 | 0.7041 | 0.9825 | 0.9479 | 0.9601 | 0.9602 | 0.575 | First-order |
| 6 | 0.7462 | 0.9655 | 0.9302 | 0.9808 | 0.9513 | 0.603 | Hixson–Crowell model |
| 7 | 0.9583 | 0.8873 | 0.7327 | 0.9192 | 0.9638 | 1.114 | Korsmeyer–Peppas model |
| 8 | 0.8635 | 0.9610 | 0.9162 | 0.9706 | 0.9882 | 0.682 | Korsmeyer–Peppas model |
| 9 | 0.0773 | 0.9324 | 0.8326 | 0.8553 | 0.9738 | 0.400 | Korsmeyer–Peppas model |
| 10 | 0.7004 | 0.9549 | 0.9049 | 0.9201 | 0.9214 | 0.591 | First-order |
| 11 | 0.7652 | 0.9462 | 0.8641 | 0.9401 | 0.8999 | 0.648 | First-order |
| 12 | 0.9217 | 0.9685 | 0.8588 | 0.9738 | 0.9574 | 0.789 | First-order |
| 13 | 0.9766 | 0.8681 | 0.7623 | 0.9145 | 0.9787 | 1.067 | Korsmeyer–Peppas model |
| 14 | 0.9799 | 0.9730 | 0.8133 | 0.9810 | 0.9814 | 0.948 | Korsmeyer–Peppas model |
| 15 | 0.7041 | 0.9825 | 0.9479 | 0.9601 | 0.9602 | 0.575 | First-order |
| 16 | 0.9941 | 0.9752 | 0.7975 | 0.9844 | 0.9943 | 1.022 | Korsmeyer–Peppas model |
| 17 | 0.9844 | 0.9430 | 0.8018 | 0.9677 | 0.9846 | 0.981 | Korsmeyer–Peppas model |
| Trials | Polymer (mg) | Plasticizer (%) | Surfactant (%) |
|---|---|---|---|
| 1 | 500.00 | 87.5 | 28.4 |
| 2 | 500.00 | 66.5 | 20 |
| 3 | 500.00 | 87.5 | 20 |
| 4 | 500.00 | 87.5 | 11.6 |
| 5 | 500.00 | 87.5 | 20 |
| 6 | 350.00 | 70 | 10.5 |
| 7 | 350.00 | 52.5 | 10.5 |
| 8 | 500.00 | 108.5 | 20 |
| 9 | 650.00 | 97.5 | 32.5 |
| 10 | 650.00 | 130 | 32.5 |
| 11 | 350.00 | 70 | 17.5 |
| 12 | 650.00 | 97.5 | 19.5 |
| 13 | 650.00 | 130 | 19.5 |
| 14 | 752.27 | 131.7 | 30.1 |
| 15 | 500.00 | 87.5 | 20 |
| 16 | 247.73 | 43.4 | 9.9 |
| 17 | 350.00 | 52.5 | 17.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safhi, A.Y.; Siddique, W.; Zaman, M.; Sarfraz, R.M.; Shafeeq Ur Rahman, M.; Mahmood, A.; Salawi, A.; Sabei, F.Y.; Alsalhi, A.; Zoghebi, K. Statistically Optimized Polymeric Buccal Films of Eletriptan Hydrobromide and Itopride Hydrochloride: An In Vivo Pharmacokinetic Study. Pharmaceuticals 2023, 16, 1551. https://doi.org/10.3390/ph16111551
Safhi AY, Siddique W, Zaman M, Sarfraz RM, Shafeeq Ur Rahman M, Mahmood A, Salawi A, Sabei FY, Alsalhi A, Zoghebi K. Statistically Optimized Polymeric Buccal Films of Eletriptan Hydrobromide and Itopride Hydrochloride: An In Vivo Pharmacokinetic Study. Pharmaceuticals. 2023; 16(11):1551. https://doi.org/10.3390/ph16111551
Chicago/Turabian StyleSafhi, Awaji Y., Waqar Siddique, Muhammad Zaman, Rai Muhammad Sarfraz, Muhammad Shafeeq Ur Rahman, Asif Mahmood, Ahmad Salawi, Fahad Y. Sabei, Abdullah Alsalhi, and Khalid Zoghebi. 2023. "Statistically Optimized Polymeric Buccal Films of Eletriptan Hydrobromide and Itopride Hydrochloride: An In Vivo Pharmacokinetic Study" Pharmaceuticals 16, no. 11: 1551. https://doi.org/10.3390/ph16111551
APA StyleSafhi, A. Y., Siddique, W., Zaman, M., Sarfraz, R. M., Shafeeq Ur Rahman, M., Mahmood, A., Salawi, A., Sabei, F. Y., Alsalhi, A., & Zoghebi, K. (2023). Statistically Optimized Polymeric Buccal Films of Eletriptan Hydrobromide and Itopride Hydrochloride: An In Vivo Pharmacokinetic Study. Pharmaceuticals, 16(11), 1551. https://doi.org/10.3390/ph16111551








