Synergistic Effect of Plant Compounds in Combination with Conventional Antimicrobials against Biofilm of Staphylococcus aureus, Pseudomonas aeruginosa, and Candida spp.
Abstract
:1. Introduction
2. Biofilm of Bacteria and Yeasts
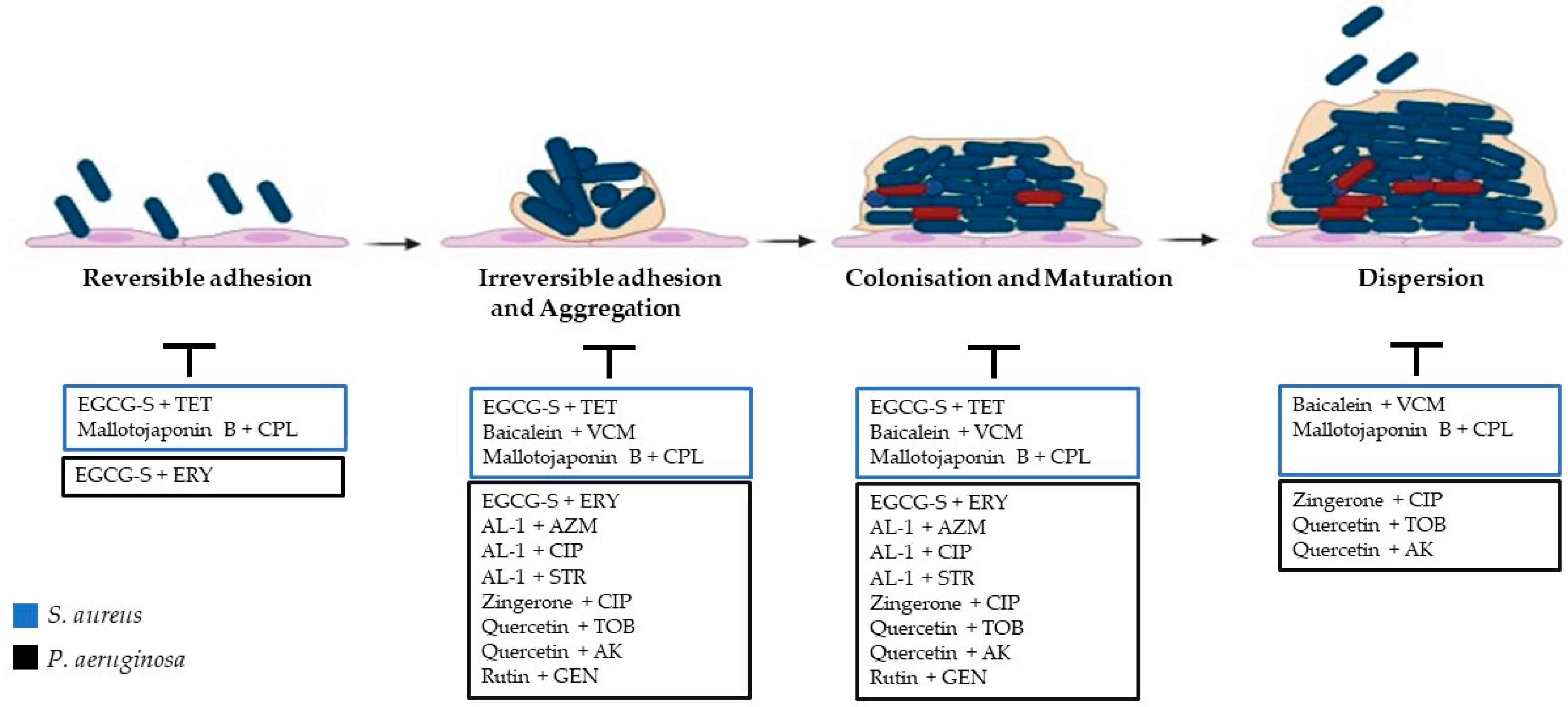
2.1. Steps of Biofilm Formation
2.1.1. Adhesion
2.1.2. Irreversible Adhesion and Aggregation
2.1.3. Colonisation and Maturation
2.1.4. Dispersion
3. Bacterial and Fungal Resistance
3.1. Bacterial Biofilm Resistance
3.2. Candida Biofilm Resistance
4. Antimicrobial Activity of Plant Compounds
5. Synergy between Antibacterial Chemotherapeutics and Natural Compounds against S. aureus
6. Synergy between Antibacterial Chemotherapeutics and Natural Compounds against P. aeruginosa
7. Synergy between Antifungal Chemotherapeutics and Natural Compounds against Candida spp.
8. Synergy between Antimicrobial Chemotherapeutics and Natural Compounds against Polymicrobial Biofilms
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berlanga, M.; Gomez-Perez, L.; Guerrero, R. Biofilm formation and antibiotic susceptibility in dispersed cells versus planktonic cells from clinical, industry and environmental origins. Antonie Van Leeuwenhoek 2017, 110, 1691–1704. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert. Rev. Anti Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Ghods, S.; Rehm, B.H. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef]
- Rokas, A. Evolution of the human pathogenic lifestyle in fungi. Nat. Microbiol. 2022, 7, 607–619. [Google Scholar] [CrossRef]
- Maurice, N.M.; Bedi, B.; Sadikot, R.T. Pseudomonas aeruginosa Biofilms: Host Response and Clinical Implications in Lung Infections. Am. J. Respir. Cell Mol. Biol. 2018, 58, 428–439. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Epifano, F.; Fiorito, S.; Alvarez-Suarez, J.M. Phytochemical Analysis and Biological Investigation of Nepeta juncea Benth. Different Extracts. Plants 2020, 9, 646. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Mohanta, Y.K.; Pohl, P.; Jaradat, N.; Aboul-Soud, M.A.M.; Zengin, G. Variation of phytochemical constituents, antioxidant, antibacterial, antifungal, and anti-inflammatory properties of Grantia aucheri (Boiss.) at different growth stages. Microb. Pathog. 2022, 172, 105805. [Google Scholar] [CrossRef]
- Zhang, J.; Miao, Y.; Zhang, Q.; Sun, Y.; Wu, L.; Peng, Y. Mechanism of stable sewage nitrogen removal in a partial nitrification-anammox biofilm system at low temperatures: Microbial community and EPS analysis. Bioresour. Technol. 2020, 297, 122459. [Google Scholar] [CrossRef]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Magana, M.; Sereti, C.; Ioannidis, A.; Mitchell, C.A.; Ball, A.R.; Magiorkinis, E.; Chatzipanagiotou, S.; Hamblin, M.R.; Hadjifrangiskou, M.; Tegos, G.P. Options and Limitations in Clinical Investigation of Bacterial Biofilms. Clin. Microbiol. Rev. 2018, 31, e00084-16. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, Y.Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef]
- Tobudic, S.; Kratzer, C.; Lassnigg, A.; Presterl, E. Antifungal susceptibility of Candida albicans in biofilms. Mycoses 2012, 55, 199–204. [Google Scholar] [CrossRef]
- Taff, H.T.; Mitchell, K.F.; Edward, J.A.; Andes, D.R. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013, 8, 1325–1337. [Google Scholar] [CrossRef]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa Biofilm: A Review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef]
- Kamali, E.; Jamali, A.; Ardebili, A.; Ezadi, F.; Mohebbi, A. Evaluation of antimicrobial resistance, biofilm forming potential, and the presence of biofilm-related genes among clinical isolates of Pseudomonas aeruginosa. BMC Res. Notes 2020, 13, 27. [Google Scholar] [CrossRef]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef]
- Galie, S.; Garcia-Gutierrez, C.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.; Vlamakis, H.; Kolter, R. Biofilms. Cold Spring Harb. Perspect. Biol. 2010, 2, a000398. [Google Scholar] [CrossRef] [PubMed]
- Dunne, W.M., Jr. Bacterial adhesion: Seen any good biofilms lately? Clin. Microbiol. Rev. 2002, 15, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv. Colloid. Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bos, R.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry of initial microbial adhesive interactions--its mechanisms and methods for study. FEMS Microbiol. Rev. 1999, 23, 179–230. [Google Scholar] [CrossRef]
- Berne, C.; Ducret, A.; Hardy, G.G.; Brun, Y.V. Adhesins Involved in Attachment to Abiotic Surfaces by Gram-Negative Bacteria. Microbiol. Spectr. 2015, 3, 4. [Google Scholar] [CrossRef]
- Sionov, R.V.; Steinberg, D. Targeting the Holy Triangle of Quorum Sensing, Biofilm Formation, and Antibiotic Resistance in Pathogenic Bacteria. Microorganisms 2022, 10, 1239. [Google Scholar] [CrossRef]
- Jiang, Q.; Jin, Z.; Sun, B. MgrA Negatively Regulates Biofilm Formation and Detachment by Repressing the Expression of psm Operons in Staphylococcus aureus. Appl. Environ. Microbiol. 2018, 84, e01008-18. [Google Scholar] [CrossRef]
- Laventie, B.J.; Sangermani, M.; Estermann, F.; Manfredi, P.; Planes, R.; Hug, I.; Jaeger, T.; Meunier, E.; Broz, P.; Jenal, U. A Surface-Induced Asymmetric Program Promotes Tissue Colonization by Pseudomonas aeruginosa. Cell Host Microbe 2019, 25, 140–152.e146. [Google Scholar] [CrossRef]
- Comolli, J.C.; Waite, L.L.; Mostov, K.E.; Engel, J.N. Pili binding to asialo-GM1 on epithelial cells can mediate cytotoxicity or bacterial internalization by Pseudomonas aeruginosa. Infect. Immun. 1999, 67, 3207–3214. [Google Scholar] [CrossRef]
- Dueholm, M.S.; Sondergaard, M.T.; Nilsson, M.; Christiansen, G.; Stensballe, A.; Overgaard, M.T.; Givskov, M.; Tolker-Nielsen, T.; Otzen, D.E.; Nielsen, P.H. Expression of Fap amyloids in Pseudomonas aeruginosa, P. fluorescens, and P. putida results in aggregation and increased biofilm formation. Microbiologyopen 2013, 2, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- McCall, A.D.; Pathirana, R.U.; Prabhakar, A.; Cullen, P.J.; Edgerton, M. Candida albicans biofilm development is governed by cooperative attachment and adhesion maintenance proteins. NPJ Biofilms Microbiomes 2019, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Mourer, T.; El Ghalid, M.; Pehau-Arnaudet, G.; Kauffmann, B.; Loquet, A.; Brule, S.; Cabral, V.; d’Enfert, C.; Bachellier-Bassi, S. The Pga59 cell wall protein is an amyloid forming protein involved in adhesion and biofilm establishment in the pathogenic yeast Candida albicans. NPJ Biofilms Microbiomes 2023, 9, 6. [Google Scholar] [CrossRef]
- Abraham, W.R. Going beyond the Control of Quorum-Sensing to Combat Biofilm Infections. Antibiotics 2016, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Tian, X. Quorum sensing and bacterial social interactions in biofilms. Sensors 2012, 12, 2519–2538. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef]
- Sperandio, V.; Torres, A.G.; Giron, J.A.; Kaper, J.B. Quorum sensing is a global regulatory mechanism in enterohemorrhagic Escherichia coli O157:H7. J. Bacteriol. 2001, 183, 5187–5197. [Google Scholar] [CrossRef]
- Sun, J.; Daniel, R.; Wagner-Dobler, I.; Zeng, A.P. Is autoinducer-2 a universal signal for interspecies communication: A comparative genomic and phylogenetic analysis of the synthesis and signal transduction pathways. BMC Evol. Biol. 2004, 4, 36. [Google Scholar] [CrossRef]
- Zawrotniak, M.; Wojtalik, K.; Rapala-Kozik, M. Farnesol, a Quorum-Sensing Molecule of Candida albicans Triggers the Release of Neutrophil Extracellular Traps. Cells 2019, 8, 1611. [Google Scholar] [CrossRef]
- Myszka, K.; Czaczyk, K. Characterization of adhesive exopolysaccharide (EPS) produced by Pseudomonas aeruginosa under starvation conditions. Curr. Microbiol. 2009, 58, 541–546. [Google Scholar] [CrossRef]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3, 3. [Google Scholar] [CrossRef]
- Jayathilake, P.G.; Jana, S.; Rushton, S.; Swailes, D.; Bridgens, B.; Curtis, T.; Chen, J. Extracellular Polymeric Substance Production and Aggregated Bacteria Colonization Influence the Competition of Microbes in Biofilms. Front. Microbiol. 2017, 8, 1865. [Google Scholar] [CrossRef]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Toyofuku, M.; Inaba, T.; Kiyokawa, T.; Obana, N.; Yawata, Y.; Nomura, N. Environmental factors that shape biofilm formation. Biosci. Biotechnol. Biochem. 2016, 80, 7–12. [Google Scholar] [CrossRef]
- Dogsa, I.; Kriechbaum, M.; Stopar, D.; Laggner, P. Structure of bacterial extracellular polymeric substances at different pH values as determined by SAXS. Biophys. J. 2005, 89, 2711–2720. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Ghafoor, A.; Hay, I.D.; Rehm, B.H. Role of exopolysaccharides in Pseudomonas aeruginosa biofilm formation and architecture. Appl. Environ. Microbiol. 2011, 77, 5238–5246. [Google Scholar] [CrossRef]
- Friedman, L.; Kolter, R. Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J. Bacteriol. 2004, 186, 4457–4465. [Google Scholar] [CrossRef]
- Jackson, K.D.; Starkey, M.; Kremer, S.; Parsek, M.R.; Wozniak, D.J. Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J. Bacteriol. 2004, 186, 4466–4475. [Google Scholar] [CrossRef] [PubMed]
- Overhage, J.; Schemionek, M.; Webb, J.S.; Rehm, B.H. Expression of the psl operon in Pseudomonas aeruginosa PAO1 biofilms: PslA performs an essential function in biofilm formation. Appl. Environ. Microbiol. 2005, 71, 4407–4413. [Google Scholar] [CrossRef]
- Campisano, A.; Schroeder, C.; Schemionek, M.; Overhage, J.; Rehm, B.H. PslD is a secreted protein required for biofilm formation by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2006, 72, 3066–3068. [Google Scholar] [CrossRef] [PubMed]
- Reichhardt, C.; Jacobs, H.M.; Matwichuk, M.; Wong, C.; Wozniak, D.J.; Parsek, M.R. The Versatile Pseudomonas aeruginosa Biofilm Matrix Protein CdrA Promotes Aggregation through Different Extracellular Exopolysaccharide Interactions. J. Bacteriol. 2020, 202, e00216-20. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.F.; Zarnowski, R.; Andes, D.R. Fungal Super Glue: The Biofilm Matrix and Its Composition, Assembly, and Functions. PLoS Pathog. 2016, 12, e1005828. [Google Scholar] [CrossRef] [PubMed]
- Visini, R.; Jin, X.; Bergmann, M.; Michaud, G.; Pertici, F.; Fu, O.; Pukin, A.; Branson, T.R.; Thies-Weesie, D.M.; Kemmink, J.; et al. Structural Insight into Multivalent Galactoside Binding to Pseudomonas aeruginosa Lectin LecA. ACS Chem. Biol. 2015, 10, 2455–2462. [Google Scholar] [CrossRef]
- Passos da Silva, D.; Matwichuk, M.L.; Townsend, D.O.; Reichhardt, C.; Lamba, D.; Wozniak, D.J.; Parsek, M.R. The Pseudomonas aeruginosa lectin LecB binds to the exopolysaccharide Psl and stabilizes the biofilm matrix. Nat. Commun. 2019, 10, 2183. [Google Scholar] [CrossRef]
- Secchi, E.; Savorana, G.; Vitale, A.; Eberl, L.; Stocker, R.; Rusconi, R. The structural role of bacterial eDNA in the formation of biofilm streamers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113723119. [Google Scholar] [CrossRef]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef]
- Flemming, H.C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The “house of biofilm cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef]
- Powell, L.C.; Pritchard, M.F.; Ferguson, E.L.; Powell, K.A.; Patel, S.U.; Rye, P.D.; Sakellakou, S.M.; Buurma, N.J.; Brilliant, C.D.; Copping, J.M.; et al. Targeted disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate oligosaccharides. NPJ Biofilms Microbiomes 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.K.; Dreifus, J.E.; Reichhardt, C.; Storek, K.M.; Secor, P.R.; Wozniak, D.J.; Hisert, K.B.; Parsek, M.R. Pseudomonas aeruginosa aggregates in cystic fibrosis sputum produce exopolysaccharides that likely impede current therapies. Cell Rep. 2021, 34, 108782. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Liu, H.; Zhang, L.; Guo, Y.; Yu, S.; Wozniak, D.J.; Ma, L.Z. The exopolysaccharide Psl-eDNA interaction enables the formation of a biofilm skeleton in Pseudomonas aeruginosa. Environ. Microbiol. Rep. 2015, 7, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, J.; Oh, S. Machine learning reveals the complex ecological interplay of microbiome in a full-scale membrane bioreactor wastewater treatment plant. Environ. Res. 2023, 222, 115366. [Google Scholar] [CrossRef] [PubMed]
- Vopalenska, I.; St’ovicek, V.; Janderova, B.; Vachova, L.; Palkova, Z. Role of distinct dimorphic transitions in territory colonizing and formation of yeast colony architecture. Environ. Microbiol. 2010, 12, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Uppuluri, P.; Thomas, D.P.; Cleary, I.A.; Henriques, M.; Lopez-Ribot, J.L.; Oliveira, R. Presence of extracellular DNA in the Candida albicans biofilm matrix and its contribution to biofilms. Mycopathologia 2010, 169, 323–331. [Google Scholar] [CrossRef]
- Kean, R.; Delaney, C.; Sherry, L.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R.; Williams, C.; Ramage, G. Transcriptome Assembly and Profiling of Candida auris Reveals Novel Insights into Biofilm-Mediated Resistance. mSphere 2018, 3, e00334-18. [Google Scholar] [CrossRef]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef]
- Schilcher, K.; Horswill, A.R. Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies. Microbiol. Mol. Biol. Rev. 2020, 84, e00026-19. [Google Scholar] [CrossRef]
- Desai, J.V.; Mitchell, A.P. Candida albicans Biofilm Development and Its Genetic Control. Microbiol. Spectr. 2015, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Langenheder, S.; Jurgens, K. Dispersal Modifies the Diversity and Composition of Active Bacterial Communities in Response to a Salinity Disturbance. Front. Microbiol. 2018, 9, 2188. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, P.; Chaturvedi, A.K.; Srinivasan, A.; Banerjee, M.; Ramasubramaniam, A.K.; Kohler, J.R.; Kadosh, D.; Lopez-Ribot, J.L. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 2010, 6, e1000828. [Google Scholar] [CrossRef]
- Pokhrel, S.; Boonmee, N.; Tulyaprawat, O.; Pharkjaksu, S.; Thaipisutikul, I.; Chairatana, P.; Ngamskulrungroj, P.; Mitrpant, C. Assessment of Biofilm Formation by Candida albicans Strains Isolated from Hemocultures and Their Role in Pathogenesis in the Zebrafish Model. J. Fungi 2022, 8, 1014. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Garcia-Betancur, J.C.; Lopez, D. Cell Heterogeneity in Staphylococcal Communities. J. Mol. Biol. 2019, 431, 4699–4711. [Google Scholar] [CrossRef]
- Aguila-Arcos, S.; Alvarez-Rodriguez, I.; Garaiyurrebaso, O.; Garbisu, C.; Grohmann, E.; Alkorta, I. Biofilm-Forming Clinical Staphylococcus Isolates Harbor Horizontal Transfer and Antibiotic Resistance Genes. Front. Microbiol. 2017, 8, 2018. [Google Scholar] [CrossRef]
- Fernandez-Billon, M.; Llambias-Cabot, A.E.; Jordana-Lluch, E.; Oliver, A.; Macia, M.D. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa biofilms. Biofilm 2023, 5, 100129. [Google Scholar] [CrossRef]
- Tseng, B.S.; Zhang, W.; Harrison, J.J.; Quach, T.P.; Song, J.L.; Penterman, J.; Singh, P.K.; Chopp, D.L.; Packman, A.I.; Parsek, M.R. The extracellular matrix protects Pseudomonas aeruginosa biofilms by limiting the penetration of tobramycin. Environ. Microbiol. 2013, 15, 2865–2878. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, H.; Charron-Mazenod, L.; Lewenza, S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008, 4, e1000213. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Nobile, C.J. Antifungal drug-resistance mechanisms in Candida biofilms. Curr. Opin. Microbiol. 2023, 71, 102237. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.F.; Zarnowski, R.; Andes, D.R. The Extracellular Matrix of Fungal Biofilms. Adv. Exp. Med. Biol. 2016, 931, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Saville, S.P.; Wickes, B.L.; Lopez-Ribot, J.L. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 2002, 68, 5459–5463. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Soto, S.M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 2013, 4, 223–229. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Chandra, J.; Kuhn, D.M.; Ghannoum, M.A. Mechanism of fluconazole resistance in Candida albicans biofilms: Phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 2003, 71, 4333–4340. [Google Scholar] [CrossRef]
- Bizerra, F.C.; Nakamura, C.V.; de Poersch, C.; Estivalet Svidzinski, T.I.; Borsato Quesada, R.M.; Goldenberg, S.; Krieger, M.A.; Yamada-Ogatta, S.F. Characteristics of biofilm formation by Candida tropicalis and antifungal resistance. FEMS Yeast Res. 2008, 8, 442–450. [Google Scholar] [CrossRef]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal biofilm resistance. Int. J. Microbiol. 2012, 2012, 528521. [Google Scholar] [CrossRef]
- Finkel, J.S.; Mitchell, A.P. Genetic control of Candida albicans biofilm development. Nat. Rev. Microbiol. 2011, 9, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.E.; Lepak, A.J.; Marchillo, K.; Andes, D.R. Time course global gene expression analysis of an in vivo Candida biofilm. J. Infect. Dis. 2009, 200, 307–313. [Google Scholar] [CrossRef] [PubMed]
- LaFleur, M.D.; Kumamoto, C.A.; Lewis, K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 2006, 50, 3839–3846. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhaheri, R.S.; Douglas, L.J. Absence of amphotericin B-tolerant persister cells in biofilms of some Candida species. Antimicrob. Agents Chemother. 2008, 52, 1884–1887. [Google Scholar] [CrossRef]
- Perumal, P.; Mekala, S.; Chaffin, W.L. Role for cell density in antifungal drug resistance in Candida albicans biofilms. Antimicrob. Agents Chemother. 2007, 51, 2454–2463. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Jin, L.J.; Samaranayake, Y.H.; Samaranayake, L.P. Cell density and cell aging as factors modulating antifungal resistance of Candida albicans biofilms. Antimicrob. Agents Chemother. 2008, 52, 3259–3266. [Google Scholar] [CrossRef]
- Alem, M.A.; Oteef, M.D.; Flowers, T.H.; Douglas, L.J. Production of tyrosol by Candida albicans biofilms and its role in quorum sensing and biofilm development. Eukaryot. Cell 2006, 5, 1770–1779. [Google Scholar] [CrossRef]
- Hornby, J.M.; Jensen, E.C.; Lisec, A.D.; Tasto, J.J.; Jahnke, B.; Shoemaker, R.; Dussault, P.; Nickerson, K.W. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 2001, 67, 2982–2992. [Google Scholar] [CrossRef]
- Jabra-Rizk, M.A.; Shirtliff, M.; James, C.; Meiller, T. Effect of farnesol on Candida dubliniensis biofilm formation and fluconazole resistance. FEMS Yeast Res. 2006, 6, 1063–1073. [Google Scholar] [CrossRef]
- Weber, K.; Schulz, B.; Ruhnke, M. The quorum-sensing molecule E,E-farnesol—Its variable secretion and its impact on the growth and metabolism of Candida species. Yeast 2010, 27, 727–739. [Google Scholar] [CrossRef]
- Uppuluri, P.; Nett, J.; Heitman, J.; Andes, D. Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob. Agents Chemother. 2008, 52, 1127–1132. [Google Scholar] [CrossRef]
- Robbins, N.; Uppuluri, P.; Nett, J.; Rajendran, R.; Ramage, G.; Lopez-Ribot, J.L.; Andes, D.; Cowen, L.E. Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS Pathog. 2011, 7, e1002257. [Google Scholar] [CrossRef]
- Borges, A.; Abreu, A.C.; Dias, C.; Saavedra, M.J.; Borges, F.; Simoes, M. New Perspectives on the Use of Phytochemicals as an Emergent Strategy to Control Bacterial Infections Including Biofilms. Molecules 2016, 21, 877. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Chemical diversity and defence metabolism: How plants cope with pathogens and ozone pollution. Int. J. Mol. Sci. 2009, 10, 3371–3399. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Rausher, M.D. Co-evolution and plant resistance to natural enemies. Nature 2001, 411, 857–864. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Furstenberg-Hagg, J.; Zagrobelny, M.; Bak, S. Plant defense against insect herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef]
- Gonzalez-Lamothe, R.; Mitchell, G.; Gattuso, M.; Diarra, M.S.; Malouin, F.; Bouarab, K. Plant antimicrobial agents and their effects on plant and human pathogens. Int. J. Mol. Sci. 2009, 10, 3400–3419. [Google Scholar] [CrossRef]
- Dehghan, H.; Sarrafi, Y.; Salehi, P. Antioxidant and antidiabetic activities of 11 herbal plants from Hyrcania region, Iran. J. Food Drug Anal. 2016, 24, 179–188. [Google Scholar] [CrossRef]
- Lahiri, D.; Dash, S.; Dutta, R.; Nag, M. Elucidating the effect of anti-biofilm activity of bioactive compounds extracted from plants. J. Biosci. 2019, 44, 52. [Google Scholar] [CrossRef]
- Mahizan, N.A.; Yang, S.K.; Moo, C.L.; Song, A.A.; Chong, C.M.; Chong, C.W.; Abushelaibi, A.; Lim, S.E.; Lai, K.S. Terpene Derivatives as a Potential Agent against Antimicrobial Resistance (AMR) Pathogens. Molecules 2019, 24, 2631. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Bano, N.; Ahmad, T.; Sharangi, A.B.; Upadhyay, T.K.; Alraey, Y.; Alabdallah, N.M.; Rauf, M.A.; Saeed, M. Synergistic Role of Plant Extracts and Essential Oils against Multidrug Resistance and Gram-Negative Bacterial Strains Producing Extended-Spectrum beta-Lactamases. Antibiotics 2022, 11, 855. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Chamkhi, I.; Balahbib, A.; Rebezov, M.; Shariati, M.A.; Wilairatana, P.; Mubarak, M.S.; Benali, T.; El Omari, N. Mechanisms, Anti-Quorum-Sensing Actions, and Clinical Trials of Medicinal Plant Bioactive Compounds against Bacteria: A Comprehensive Review. Molecules 2022, 27, 1484. [Google Scholar] [CrossRef] [PubMed]
- Lillicrap, A.; Macken, A.; Wennberg, A.C.; Grung, M.; Rundberget, J.T.; Fredriksen, L.; Scheie, A.A.; Benneche, T.; d’Auriac, M.A. Environmental fate and effects of novel quorum sensing inhibitors that can control biofilm formation. Chemosphere 2016, 164, 52–58. [Google Scholar] [CrossRef]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef]
- Anju, V.T.; Busi, S.; Ranganathan, S.; Ampasala, D.R.; Kumar, S.; Suchiang, K.; Kumavath, R.; Dyavaiah, M. Sesamin and sesamolin rescues Caenorhabditis elegans from Pseudomonas aeruginosa infection through the attenuation of quorum sensing regulated virulence factors. Microb. Pathog. 2021, 155, 104912. [Google Scholar] [CrossRef]
- Kumar, S.N.; Aravind, S.R.; Sreelekha, T.T.; Jacob, J.; Kumar, B.S. Asarones from Acorus calamus in combination with azoles and amphotericin B: A novel synergistic combination to compete against human pathogenic Candida species in vitro. Appl. Biochem. Biotechnol. 2015, 175, 3683–3695. [Google Scholar] [CrossRef]
- Lu, M.; Li, T.; Wan, J.; Li, X.; Yuan, L.; Sun, S. Antifungal effects of phytocompounds on Candida species alone and in combination with fluconazole. Int. J. Antimicrob. Agents 2017, 49, 125–136. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Doke, S.K.; Raut, J.S.; Dhawale, S.; Karuppayil, S.M. Sensitization of Candida albicans biofilms to fluconazole by terpenoids of plant origin. J. Gen. Appl. Microbiol. 2014, 60, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Merghni, A.; Noumi, E.; Hadded, O.; Dridi, N.; Panwar, H.; Ceylan, O.; Mastouri, M.; Snoussi, M. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1,8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb. Pathog. 2018, 118, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Lakhundi, S.; Zhang, K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Meza, M.E.; Galarde-Lopez, M.; Carrillo-Quiroz, B.; Alpuche-Aranda, C.M. Antimicrobial resistance: One Health approach. Vet. World 2022, 15, 743–749. [Google Scholar] [CrossRef]
- Shinde, S.; Lee, L.H.; Chu, T. Inhibition of Biofilm Formation by the Synergistic Action of EGCG-S and Antibiotics. Antibiotics 2021, 10, 102. [Google Scholar] [CrossRef]
- Hengge, R. Targeting Bacterial Biofilms by the Green Tea Polyphenol EGCG. Molecules 2019, 24, 2403. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, P.; Chen, H.; Wang, Y.; Li, C.; Wang, Q. Baicalein Inhibits the Staphylococcus aureus Biofilm and the LuxS/AI-2 System in vitro. Infect. Drug Resist. 2023, 16, 2861–2882. [Google Scholar] [CrossRef]
- Nguena-Dongue, B.N.; Tchamgoue, J.; Ngandjui Tchangoue, Y.A.; Lunga, P.K.; Toghueo, K.R.M.; Zeuko, O.M.; Melogmo, Y.K.D.; Tchouankeu, J.C.; Kouam, S.F.; Fekam, B.F. Potentiation effect of mallotojaponin B on chloramphenicol and mode of action of combinations against Methicillin-resistant Staphylococcus aureus. PLoS ONE 2023, 18, e0282008. [Google Scholar] [CrossRef]
- Kumar, L.; Chhibber, S.; Harjai, K. Zingerone inhibit biofilm formation and improve antibiofilm efficacy of ciprofloxacin against Pseudomonas aeruginosa PAO1. Fitoterapia 2013, 90, 73–78. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, X.; Bian, J.; Pei, G.; Dai, H.; Polyak, S.W.; Song, F.; Ma, L.; Wang, Y.; Zhang, L. Synergistic effect of 14-alpha-lipoyl andrographolide and various antibiotics on the formation of biofilms and production of exopolysaccharide and pyocyanin by Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2011, 55, 3015–3017. [Google Scholar] [CrossRef]
- Deepika, M.S.; Thangam, R.; Sakthidhasan, P.; Arun, S.; Sivasubramanian, S.; Thirumurugan, R. Combined effect of a natural flavonoid rutin from Citrus sinensis and conventional antibiotic gentamicin on Pseudomonas aeruginosa biofilm formation. Food Control 2018, 90, 282–294. [Google Scholar] [CrossRef]
- Vipin, C.; Saptami, K.; Fida, F.; Mujeeburahiman, M.; Rao, S.S.; Athmika; Arun, A.B.; Rekha, P.D. Potential synergistic activity of quercetin with antibiotics against multidrug-resistant clinical strains of Pseudomonas aeruginosa. PLoS ONE 2020, 15, e0241304. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Tarar, S.M.; Gul, I.; Nawaz, U.; Arshad, M. Challenges of antibiotic resistance biofilms and potential combating strategies: A review. 3 Biotech. 2021, 11, 169. [Google Scholar] [CrossRef]
- Diaz-Puertas, R.; Alvarez-Martinez, F.J.; Falco, A.; Barrajon-Catalan, E.; Mallavia, R. Phytochemical-Based Nanomaterials against Antibiotic-Resistant Bacteria: An Updated Review. Polymers 2023, 15, 1392. [Google Scholar] [CrossRef] [PubMed]
- Najarzadeh, Z.; Mohammad-Beigi, H.; Nedergaard Pedersen, J.; Christiansen, G.; Sonderby, T.V.; Shojaosadati, S.A.; Morshedi, D.; Stromgaard, K.; Meisl, G.; Sutherland, D.; et al. Plant Polyphenols Inhibit Functional Amyloid and Biofilm Formation in Pseudomonas Strains by Directing Monomers to Off-Pathway Oligomers. Biomolecules 2019, 9, 659. [Google Scholar] [CrossRef]
- Sudano Roccaro, A.; Blanco, A.R.; Giuliano, F.; Rusciano, D.; Enea, V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob. Agents Chemother. 2004, 48, 1968–1973. [Google Scholar] [CrossRef]
- Bhat, S.D.; Ashok, B.K.; Acharya, R.N.; Ravishankar, B. Anticonvulsant activity of raw and classically processed Vacha (Acorus calamus Linn.) rhizomes. Ayu 2012, 33, 119–122. [Google Scholar] [CrossRef]
- Aqil, F.; Zahin, M.; Ahmad, I. Antimutagenic activity of methanolic extracts of four ayurvedic medicinal plants. Indian. J. Exp. Biol. 2008, 46, 668–672. [Google Scholar]
- Su, L.Y.; Ni, G.H.; Liao, Y.C.; Su, L.Q.; Li, J.; Li, J.S.; Rao, G.X.; Wang, R.R. Antifungal Activity and Potential Mechanism of 6,7, 4’-O-Triacetylscutellarein Combined With Fluconazole Against Drug-Resistant C. albicans. Front. Microbiol. 2021, 12, 692693. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, K.Y.; Ryu, S.Y.; Yoon, Y.; Hahm, D.H.; Kang, S.A.; Cho, S.H.; Lim, J.S.; Moon, E.Y.; Yoon, S.R.; et al. Asarone inhibits adipogenesis and stimulates lipolysis in 3T3-L1 adipocytes. Cell Mol. Biol. 2010, 56 (Suppl. S1), OL1215-1222. [Google Scholar]
- Geng, Y.; Li, C.; Liu, J.; Xing, G.; Zhou, L.; Dong, M.; Li, X.; Niu, Y. Beta-asarone improves cognitive function by suppressing neuronal apoptosis in the beta-amyloid hippocampus injection rats. Biol. Pharm. Bull. 2010, 33, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.B.; Karuppayil, S.M. beta-Asarone, an active principle of Acorus calamus rhizome, inhibits morphogenesis, biofilm formation and ergosterol biosynthesis in Candida albicans. Phytomedicine 2013, 20, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Han, Y. Synergic anticandidal effect of epigallocatechin-O-gallate combined with amphotericin B in a murine model of disseminated candidiasis and its anticandidal mechanism. Biol. Pharm. Bull. 2007, 30, 1693–1696. [Google Scholar] [CrossRef]
- Hirasawa, M.; Takada, K. Multiple effects of green tea catechin on the antifungal activity of antimycotics against Candida albicans. J. Antimicrob. Chemother. 2004, 53, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhai, L.; Arendrup, M.C. In vitro activity of 23 tea extractions and epigallocatechin gallate against Candida species. Med. Mycol. 2015, 53, 194–198. [Google Scholar] [CrossRef]
- Sitheeque, M.A.; Panagoda, G.J.; Yau, J.; Amarakoon, A.M.; Udagama, U.R.; Samaranayake, L.P. Antifungal activity of black tea polyphenols (catechins and theaflavins) against Candida species. Chemotherapy 2009, 55, 189–196. [Google Scholar] [CrossRef]
- Navarro-Martinez, M.D.; Garcia-Canovas, F.; Rodriguez-Lopez, J.N. Tea polyphenol epigallocatechin-3-gallate inhibits ergosterol synthesis by disturbing folic acid metabolism in Candida albicans. J. Antimicrob. Chemother. 2006, 57, 1083–1092. [Google Scholar] [CrossRef]
- Ning, Y.; Ling, J.; Wu, C.D. Synergistic effects of tea catechin epigallocatechin gallate and antimycotics against oral Candida species. Arch. Oral. Biol. 2015, 60, 1565–1570. [Google Scholar] [CrossRef]
- Behbehani, J.M.; Irshad, M.; Shreaz, S.; Karched, M. Synergistic effects of tea polyphenol epigallocatechin 3-O-gallate and azole drugs against oral Candida isolates. J. Mycol. Med. 2019, 29, 158–167. [Google Scholar] [CrossRef]
- Evensen, N.A.; Braun, P.C. The effects of tea polyphenols on Candida albicans: Inhibition of biofilm formation and proteasome inactivation. Can. J. Microbiol. 2009, 55, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morato, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Perez-Mana, C.; Khymenets, O.; Fito, M.; Farre, M.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro Rde, A.; Teixeira, C.E.; Brilhante, R.S.; Castelo-Branco, D.S.; Alencar, L.P.; de Oliveira, J.S.; Monteiro, A.J.; Bandeira, T.J.; Sidrim, J.J.; Moreira, J.L.; et al. Exogenous tyrosol inhibits planktonic cells and biofilms of Candida species and enhances their susceptibility to antifungals. FEMS Yeast Res. 2015, 15, fov012. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, D.R.; Feresin, L.P.; Arias, L.S.; Barao, V.A.; Barbosa, D.B.; Delbem, A.C. Effect of tyrosol on adhesion of Candida albicans and Candida glabrata to acrylic surfaces. Med. Mycol. 2015, 53, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, Z.; Yin, H.; Hu, J.; Xue, Y.; Zhang, G.; Zheng, X.; Chen, W.; Hu, X. Synergistic Antibiofilm Effects of Pseudolaric Acid A Combined with Fluconazole against Candida albicans via Inhibition of Adhesion and Yeast-To-Hypha Transition. Microbiol. Spectr. 2022, 10, e0147821. [Google Scholar] [CrossRef]
- Li, Z.; Yin, H.; Chen, W.; Jiang, C.; Hu, J.; Xue, Y.; Yao, D.; Peng, Y.; Hu, X. Synergistic Effect of Pseudolaric Acid B with Fluconazole Against Resistant Isolates and Biofilm of Candida tropicalis. Infect. Drug Resist. 2020, 13, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, B.; Chen, W.; Hu, J.; Xue, Y.; Yin, H.; Hu, X.; Liu, W. Pseudolaric Acid A: A Promising Antifungal Agent Against Prevalent Non-albicans Candida Species. Infect. Drug Resist. 2023, 16, 5953–5964. [Google Scholar] [CrossRef]
- Li, D.D.; Chai, D.; Huang, X.W.; Guan, S.X.; Du, J.; Zhang, H.Y.; Sun, Y.; Jiang, Y.Y. Potent In Vitro Synergism of Fluconazole and Osthole against Fluconazole-Resistant Candida albicans. Antimicrob. Agents Chemother. 2017, 61, e00436-17. [Google Scholar] [CrossRef]
- de Almeida, I.; Alviano, D.S.; Vieira, D.P.; Alves, P.B.; Blank, A.F.; Lopes, A.H.; Alviano, C.S.; Rosa Mdo, S. Antigiardial activity of Ocimum basilicum essential oil. Parasitol. Res. 2007, 101, 443–452. [Google Scholar] [CrossRef]
- Zore, G.B.; Thakre, A.D.; Jadhav, S.; Karuppayil, S.M. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine 2011, 18, 1181–1190. [Google Scholar] [CrossRef]
- Kotan, R.; Kordali, S.; Cakir, A. Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Z. Naturforsch C J. Biosci. 2007, 62, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Zore, G.B.; Thakre, A.D.; Rathod, V.; Karuppayil, S.M. Evaluation of anti-Candida potential of geranium oil constituents against clinical isolates of Candida albicans differentially sensitive to fluconazole: Inhibition of growth, dimorphism and sensitization. Mycoses 2011, 54, e99–e109. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y.; Hwang, S.T.; Sethi, G.; Fan, L.; Arfuso, F.; Ahn, K.S. Potential Anti-Inflammatory and Anti-Cancer Properties of Farnesol. Molecules 2018, 23, 2827. [Google Scholar] [CrossRef] [PubMed]
- Katragkou, A.; McCarthy, M.; Alexander, E.L.; Antachopoulos, C.; Meletiadis, J.; Jabra-Rizk, M.A.; Petraitis, V.; Roilides, E.; Walsh, T.J. In vitro interactions between farnesol and fluconazole, amphotericin B or micafungin against Candida albicans biofilms. J. Antimicrob. Chemother. 2015, 70, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Nagy, F.; Vitalis, E.; Jakab, A.; Borman, A.M.; Forgacs, L.; Toth, Z.; Majoros, L.; Kovacs, R. In vitro and in vivo Effect of Exogenous Farnesol Exposure Against Candida auris. Front. Microbiol. 2020, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, H.; Wang, D.; Zhang, M.; Sun, S.; Zhao, Y. The synergistic antifungal effects of gypenosides combined with fluconazole against resistant Candida albicans via inhibiting the drug efflux and biofilm formation. Biomed. Pharmacother. 2020, 130, 110580. [Google Scholar] [CrossRef]
- Zhao, J.; Ming, Y.; Wan, Q.; Ye, S.; Xie, S.; Zhu, Y.; Wang, Y.; Zhong, Z.; Li, L.; Ye, Q. Gypenoside attenuates hepatic ischemia/reperfusion injury in mice via anti-oxidative and anti-apoptotic bioactivities. Exp. Ther. Med. 2014, 7, 1388–1392. [Google Scholar] [CrossRef]
- Yu, H.; Guan, Q.; Guo, L.; Zhang, H.; Pang, X.; Cheng, Y.; Zhang, X.; Sun, Y. Gypenosides alleviate myocardial ischemia-reperfusion injury via attenuation of oxidative stress and preservation of mitochondrial function in rat heart. Cell Stress. Chaperones 2016, 21, 429–437. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, C.; Zhao, X.; Wang, D.; Liu, Y.; Sun, S. Antifungal activity and potential mechanism of Asiatic acid alone and in combination with fluconazole against Candida albicans. Biomed. Pharmacother. 2021, 139, 111568. [Google Scholar] [CrossRef]
- Agagunduz, D.; Sahin, T.O.; Yilmaz, B.; Ekenci, K.D.; Duyar Ozer, S.; Capasso, R. Cruciferous Vegetables and Their Bioactive Metabolites: From Prevention to Novel Therapies of Colorectal Cancer. Evid. Based Complement. Alternat Med. 2022, 2022, 1534083. [Google Scholar] [CrossRef]
- Davaatseren, M.; Hwang, J.T.; Park, J.H.; Kim, M.S.; Wang, S.; Sung, M.J. Allyl isothiocyanate ameliorates angiogenesis and inflammation in dextran sulfate sodium-induced acute colitis. PLoS ONE 2014, 9, e102975. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.S.; Lee, S.Y.; Dougherty, R.H.; Kang, D.H. Antimicrobial effects of mustard flour and acetic acid against Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella enterica serovar Typhimurium. Appl. Environ. Microbiol. 2003, 69, 2959–2963. [Google Scholar] [CrossRef]
- Nowicki, D.; Rodzik, O.; Herman-Antosiewicz, A.; Szalewska-Palasz, A. Isothiocyanates as effective agents against enterohemorrhagic Escherichia coli: Insight to the mode of action. Sci. Rep. 2016, 6, 22263. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Abreu, A.C.; Ferreira, C.; Saavedra, M.J.; Simoes, L.C.; Simoes, M. Antibacterial activity and mode of action of selected glucosinolate hydrolysis products against bacterial pathogens. J. Food Sci. Technol. 2015, 52, 4737–4748. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.C.; Borges, A.; Simoes, L.C.; Saavedra, M.J.; Simoes, M. Antibacterial activity of phenyl isothiocyanate on Escherichia coli and Staphylococcus aureus. Med. Chem. 2013, 9, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Bansode, B.S.; Jadhav, A.K.; Karuppayil, S.M. Activity of Allyl Isothiocyanate and Its Synergy with Fluconazole against Candida albicans Biofilms. J. Microbiol. Biotechnol. 2017, 27, 685–693. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, W.; Huang, X.; Hao, L.; Li, Y.; Sun, S. Antifungal Activity and Potential Mechanism of N-Butylphthalide Alone and in Combination with Fluconazole against Candida albicans. Front. Microbiol. 2019, 10, 1461. [Google Scholar] [CrossRef]
- Vollekova, A.; Kost’alova, D.; Kettmann, V.; Toth, J. Antifungal activity of Mahonia aquifolium extract and its major protoberberine alkaloids. Phytother. Res. 2003, 17, 834–837. [Google Scholar] [CrossRef]
- Ishikawa, S.; Tamaki, M.; Ogawa, Y.; Kaneki, K.; Zhang, M.; Sunagawa, M.; Hisamitsu, T. Inductive Effect of Palmatine on Apoptosis in RAW 264.7 Cells. Evid. Based Complement. Alternat Med. 2016, 2016, 7262054. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Q.; Zhang, N.; Xue, C.; Leung, A.W.; Zhang, H.; Xu, C.; Tang, Q.J. Photodynamic action of palmatine hydrochloride on colon adenocarcinoma HT-29 cells. Photodiagnosis Photodyn. Ther. 2016, 15, 53–58. [Google Scholar] [CrossRef]
- Wang, T.; Shao, J.; Da, W.; Li, Q.; Shi, G.; Wu, D.; Wang, C. Strong Synergism of Palmatine and Fluconazole/Itraconazole against Planktonic and Biofilm Cells of Candida Species and Efflux-Associated Antifungal Mechanism. Front. Microbiol. 2018, 9, 2892. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, H.; Tasneem, S.; Farooq, S.; Sami, A.; Rahman, A.U.; Choudhary, M.I. Harmaline and its Derivatives Against the Infectious Multi-Drug Resistant Escherichia coli. Med. Chem. 2017, 13, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Gao, Y.; Hao, L. Synergistic Effects and Mechanisms of Combined Treatment With Harmine Hydrochloride and Azoles for Resistant Candida albicans. Front. Microbiol. 2019, 10, 2295. [Google Scholar] [CrossRef] [PubMed]
- Schmeller, T.; Latz-Bruning, B.; Wink, M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores. Phytochemistry 1997, 44, 257–266. [Google Scholar] [CrossRef]
- Quan, H.; Cao, Y.Y.; Xu, Z.; Zhao, J.X.; Gao, P.H.; Qin, X.F.; Jiang, Y.Y. Potent in vitro synergism of fluconazole and berberine chloride against clinical isolates of Candida albicans resistant to fluconazole. Antimicrob. Agents Chemother. 2006, 50, 1096–1099. [Google Scholar] [CrossRef]
- Wei, G.X.; Xu, X.; Wu, C.D. In vitro synergism between berberine and miconazole against planktonic and biofilm Candida cultures. Arch. Oral. Biol. 2011, 56, 565–572. [Google Scholar] [CrossRef]
- Jafri, H.; Ahmad, I. Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis. J. Mycol. Med. 2020, 30, 100911. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, S.; Zhang, S. Enhanced in vitro antimicrobial activity of amphotericin B with berberine against dual-species biofilms of Candida albicans and Staphylococcus aureus. J. Appl. Microbiol. 2021, 130, 1154–1172. [Google Scholar] [CrossRef]
- Tan, Y.; Leonhard, M.; Moser, D.; Ma, S.; Schneider-Stickler, B. Antibiofilm efficacy of curcumin in combination with 2-aminobenzimidazole against single- and mixed-species biofilms of Candida albicans and Staphylococcus aureus. Colloids Surf. B Biointerfaces 2019, 174, 28–34. [Google Scholar] [CrossRef]

| Plant Compounds (1) | Drugs (2) | Strains | Preformed Biofilm | Biofilm Formation | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | 1 (µg/mL) | 2 (µg/mL) | Inhibition (%) | FICI 1 | 1 (µg/mL) | 2 (µg/mL) | Inhibition (%) | 1 FICI | |||
| Epigallocatechin-3- gallate-stearate | Tetracycline | ATCC 14990 CRM-6538 | 200 | 15 | 94 | [125] | |||||
| Baicalein | Vancomycin | 17546 (t037) | 32 | 4 | [127] | ||||||
| Mallotojaponin B | Chloramphe- nicol | ATCC 33591 | 1.56 | 3.9 | 73.97 | 1.56 | 3.9 | 50 | 0.393 | [128] | |
| P. aeruginosa | 1 (µg/mL) | 2 (µg/mL) | Inhibition (%) | FICI 1 | 1 (µg/mL) | 2 (µg/mL) | Inhibition (%) | FICI | |||
| Zingerone | Ciprofloxacin | PAO-1 | 10,000 | 0.06 | 10,000 | 0.06 | [129] | ||||
| 14-alpha-lipoyl andrographolide | Azithromycin | PAO-1 | 269.4 | 8 | [130] | ||||||
| 14-alpha-lipoyl andrographolide | Gentamicin | PAO-1 | 269.4 | 1 | [130] | ||||||
| 14-alpha-lipoyl andrographolide | Ciprofloxacin | PAO-1 | 269.4 | 0.75 | [130] | ||||||
| 14-alpha-lipoyl andrographolide | Streptomycin | PAO-1 | 269.4 | 8 | [130] | ||||||
| Rutin | Gentamicin | MTCC 2488 | 200 | 2.5 | 85 | 0.50 | [131] | ||||
| Quercetin | Amikacin | YU-V10, YU-V11, YU-V15, YU-V28, and PAO-1 | 125 | 0.5 to 128 | >90 | 125 | 0.5 to 128 | >90 | 0.25 | [132] | |
| Quercetin | Tobramycin | YU-V10, YU-V11, YU-V15, YU-V28 PAO-1 | 125 | 0.5 to 128 | >90 | 125 | 0.5 to 128 | >90 | 0.50 | [132] | |
| Epigallocatechin-3-gallate-stearate | Erythromycin | ATCC CRM-9027 | 100 | 15 | 95 | [125] | |||||
| Plant Compounds (1) | Drugs (2) | Strains | Preformed Biofilm | Biofilm Formation | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C. albicans | 1 (µg/mL) | 2 (µg/mL) | inhibition (%) | FICI 2 | 1 (µg/mL) | 2 (µg/mL) | inhibition (%) | FICI 2 | |||
| α-Asarones from Acorus calamusin rhizomes | Amphotericin B | MTCC 277 | 8 | 0.03 | 36 | [118] | |||||
| α-Asarones from Acorus calamusin rhizomes | Fluconazole | MTCC 277 | 8 | 0.06 | 40 | [118] | |||||
| α-Asarones from Acorus calamusin rhizomes | Clotrimazole | MTCC 277 | 16 | 0.12 | 39 | [118] | |||||
| β-Asarones from Acorus calamusin rhizomes | Amphotericin B | MTCC 277 | 8 | 0.06 | 21 | [118] | |||||
| β-Asarones from Acorus calamusin rhizomes | Fluconazole | MTCC 277 | 2 | 0.03 | 16 | [118] | |||||
| β-Asarones from Acorus calamusin rhizomes | Clotrimazole | MTCC 277 | 4 | 0.06 | 23 | [118] | |||||
| Pseudolaric Acid A | Fluconazole | ATCC 90028 | 4 | 0.5 | 58 | 4 | 0.5 | 49 | [155] | ||
| Allyl isothiocyanate | Fluconazole | ATCC 90028 | 250 | 16 | 50 | 0.265 | 125 | 4 | 50 | 0.257 | [176] |
| Carvacrol | Fluconazole | ATCC 90028 | 500 | 32 | 0.516 | 62 | 32 | 0.311 | [121] | ||
| Eugenol | Fluconazole | ATCC 90028 | 1000 | 2 | 1 | 125 | 2 | 0.25 | [121] | ||
| Thymol | Fluconazole | ATCC 90028 | 2000 | 2 | 1.001 | 1000 | 2 | 1.003 | [121] | ||
| osthole, coumarin derivative | Fluconazole | SC5314 | 8 | 8 | 90 | [158] | |||||
| Berberine | Miconazole | SC5314 | 250,000 | 7800 | 90 | 0.25 | 16,000 | 800 | 91 | [186] | |
| Palmatine | Fluconazole | SC5314 | 64 | 256 | 80 | 0.2813 | [181] | ||||
| Palmatine | Itraconazole | SC5314 | 64 | 128 | 80 | 0.127 | [181] | ||||
| Farnesol | Fluconazole | SC5314 | 75 | 64 | 50 | [165] | |||||
| Farnesol | Voriconazole | SC5314 | 4.69 | 1 | 50 | [165] | |||||
| Farnesol | Itraconazole | SC5314 | 4.69 | 0.5 | 50 | [165] | |||||
| Farnesol | Posaconazole | SC5314 | 4.69 | 0.25 | 50 | [165] | |||||
| Farnesol | Isoconazole | SC5314 | 4.69 | 0.5 | 50 | [165] | |||||
| Epigallocatechin gallate | Miconazole | SC5314 | 1500 | 400 | 90 | 0.31 | [149] | ||||
| Epigallocatechin gallate | Fluconazole | SC5314 | 3000 | 400 | 90 | 0.19 | [149] | ||||
| Epigallocatechin gallate | Amphotericin B | SC5314 | 3000 | 1.56 | 90 | 0.37 | [149] | ||||
| Farnesol | Fluconazole | SC5314 | 33.356 | 64 | 0.5 | [164] | |||||
| Farnesol | Amphotericin B | SC5314 | 3.313 | 1 | 0.79 | [164] | |||||
| Farnesol | Micafungin | SC5314 | 66.711 | 0.25 | 0.49 | [164] | |||||
| Tyrosol | Amphotericin B | Clinical isolate | 25.9 | 52.5 | 36.5 | 25.9 | 52.5 | 69 | [153] | ||
| Tyrosol | Fluconazole | Clinical isolate | 25.9 | 3490 | 0 | 25.9 | 3490 | 55 | [153] | ||
| Tyrosol | Itraconazole | Clinical isolate | 25.9 | 1020 | 0 | 25.9 | 1020 | 70 | [153] | ||
| Allyl isothiocyanate | Fluconazole | GMC 03 | 250 | 32 | 50 | 0.312 | 62 | 4 | 50 | 0.132 | [176] |
| N-butylphthalide | Fluconazole | Clinical isolate 04 | 64 | 0.5 | 80 | >0.5 | [177] | ||||
| N-butylphthalide | Fluconazole | Clinical isolate 04 | 64 | 0.5 | 80 | >0.5 | [177] | ||||
| N-butylphthalide | Fluconazole | Clinical isolate 04 | 64 | 4 | 80 | >0.5 | [177] | ||||
| Asiatic Acid | Fluconazole | Clinical isolate 04 | 32 | 0.25 | 80 | 0.504 | [169] | ||||
| Asiatic Acid | Fluconazole | Clinical isolate 08 | 32 | 0.25 | 80 | 0.504 | [169] | ||||
| N-butylphthalide | Fluconazole | Clinical isolate 10 | 32 | 0.5 | 80 | 0.25 | [177] | ||||
| N-butylphthalide | Fluconazole | Clinical isolate 10 | 32 | 4 | 80 | 0.26 | [177] | ||||
| N-butylphthalide | Fluconazole | Clinical isolate 10 | 64 | 8 | 80 | 0.27 | [177] | ||||
| Harmine hydrochloride | Fluconazole | Clinical isolate 10 | >1024 | >128 | 50 | 2 | 16 | 0.25 | 50 | 0.018 | [183] |
| Harmine hydrochloride | Itraconazole | Clinical isolate 10 | >1024 | >64 | 50 | 2 | 64 | 0.125 | 50 | 0.064 | [183] |
| Harmine hydrochloride | Voriconazole | Clinical isolate 10 | >1024 | >64 | 50 | 2 | 64 | 0.125 | 50 | 0.064 | [183] |
| Gypenosides | Fluconazole | Clinical isolate 10 | 32 | 0.5 | 80 | 0.254 | [166] | ||||
| Asiatic Acid | Fluconazole | Clinical isolate 10 | 32 | 0.25 | 80 | 0.504 | [169] | ||||
| N-butylphthalide | Fluconazole | Clinical isolate 16 | 32 | 0.5 | 80 | 0.25 | [177] | ||||
| N-butylphthalide | Fluconazole | Clinical isolate 16 | 32 | 2 | 80 | 0.25 | [177] | ||||
| N-butylphthalide | Fluconazole | Clinical isolate 16 | 64 | 4 | 80 | 0.26 | [177] | ||||
| Harmine hydrochloride | Fluconazole | Clinical isolate 16 | >1024 | >128 | 50 | 2 | 16 | 0.25 | 50 | 0.018 | [183] |
| Harmine hydrochloride | Itraconazole | Clinical isolate 16 | >1024 | >64 | 50 | 2 | 64 | 0.25 | 50 | 0.066 | [183] |
| Harmine hydrochloride | Voriconazole | Clinical isolate 16 | >1024 | >64 | 50 | 2 | 64 | 0.125 | 50 | 0.064 | [183] |
| Gypenosides | Fluconazole | Clinical isolate 16 | 32 | 1 | 80 | [166] | |||||
| Asiatic Acid | Fluconazole | Clinical isolate 16 | 32 | 0.25 | 80 | 0.504 | [169] | ||||
| Palmatine | Fluconazole | Clinical isolate 73044 | 64 | 256 | 80 | 0.375 | [181] | ||||
| Palmatine | Itraconazole | Clinical isolate 73044 | 16 | 256 | 80 | 1 | [181] | ||||
| Palmatine | Fluconazole | Clinical isolate Z2003 | 32 | 256 | 80 | 0.281 | [181] | ||||
| Palmatine | Itraconazole | Clinical isolate Z2003 | 32 | 128 | 80 | 0.094 | [181] | ||||
| Palmatine | Fluconazole | Clinical isolate Z1402 | 32 | 256 | 80 | 0.156 | [181] | ||||
| Palmatine | Itraconazole | Clinical isolate Z1402 | 64 | 256 | 80 | 0.129 | [181] | ||||
| Palmatine | Fluconazole | Clinical isolate Z1407 | 64 | 64 | 80 | 0.156 | [181] | ||||
| Palmatine | Itraconazole | Clinical isolate Z1407 | 32 | 32 | 80 | 0.281 | [181] | ||||
| Palmatine | Fluconazole | Clinical isolates Z826 | 64 | 256 | 80 | 0.312 | [181] | ||||
| Palmatine | Itraconazole | Clinical isolates Z826 | 64 | 128 | 80 | 0.187 | [181] | ||||
| Palmatine | Fluconazole | Clinical isolate Z1309 | 32 | 128 | 80 | 0.312 | [181] | ||||
| Palmatine | Itraconazole | Clinical isolate Z1309 | 32 | 64 | 80 | 0.266 | [181] | ||||
| Epigallocatechin 3-O-gallate | Fluconazole | CDC 27974 | 62.5 | 32 | 80 | 0.563 | [150] | ||||
| Epigallocatechin 3-O-gallate | Ketoconazole | CDC 27974 | 31.25 | 32 | 80 | 0.313 | [150] | ||||
| Epigallocatechin 3-O-gallate | Fluconazole | CDC 28304 | 31.25 | 32 | 80 | 0.375 | [150] | ||||
| Epigallocatechin 3-O-gallate | Ketoconazole | CDC 28304 | 31.25 | 16 | 80 | 0.313 | [150] | ||||
| Epigallocatechin 3-O-gallate | Fluconazole | ATCC 24433 | 31.25 | 16 | 80 | 0.531 | [150] | ||||
| Epigallocatechin 3-O-gallate | Ketoconazole | ATCC 24433 | 31.25 | 8 | 80 | 0.281 | [150] | ||||
| Epigallocatechin gallate | Miconazole | ATCC 10231 | 375 | 50 | 90 | 0.16 | [149] | ||||
| Epigallocatechin gallate | Fluconazole | ATCC 10231 | 375 | 800 | 90 | 0.28 | [149] | ||||
| Epigallocatechin gallate | Amphotericin B | ATCC 10231 | 750 | 0.13 | 90 | 0.19 | [149] | ||||
| Plant compounds | Drugs | C. parapsilosis | 1 (µg/mL) | 2 (µg/mL) | inhibition (%) | FICI 2 | 1 (µg/mL) | 2 (µg/mL) | inhibition (%) | FICI 2 | |
| Palmatine | Fluconazole | ATCC 22019 | 32 | 0.0625 | 80 | 0.281 | [181] | ||||
| Palmatine | Itraconazole | ATCC 22019 | 8 | 128 | 80 | 0.156 | [181] | ||||
| Epigallocatechin gallate | Miconazole | ATCC 22019 | 6000 | 800 | 90 | 0.5 | [149] | ||||
| Epigallocatechin gallate | Fluconazole | ATCC 22019 | 1500 | 1600 | 90 | 0.56 | [149] | ||||
| Epigallocatechin gallate | Amphotericin B | ATCC 22019 | 375 | 1.56 | 90 | 0.27 | [149] | ||||
| C. tropicalis | 1 (µg/mL) | 2 (µg/mL) | inhibition (%) | FICI 2 | 1 (µg/mL) | 2 (µg/mL) | inhibition (%) | FICI 2 | |||
| α-Asarones from Acorus calamusin rhizomes | Amphotericin B |
MTCC 184 | 4 | 0.25 | 39 | [118] | |||||
| α-Asarones from Acorus calamusin rhizomes | Fluconazole | MTCC 184 | 16 | 0.12 | 50 | [118] | |||||
| α-Asarones from Acorus calamusin rhizomes | Clotrimazole | MTCC 184 | 8 | 0.25 | 41 | [118] | |||||
| β-Asarones from Acorus calamusin rhizomes | Amphotericin B | MTCC 184 | 1 | 0.03 | 21 | [118] | |||||
| β-Asarones from Acorus calamusin rhizomes | Fluconazole | MTCC 184 | 2 | 0.03 | 16 | [118] | |||||
| β-Asarones from Acorus calamusin rhizomes | Clotrimazole | MTCC 184 | 2 | 0.06 | 23 | [118] | |||||
| Tyrosol | Amphotericin B | Clinical isolate | 311 | 90 | 36,4 | 311 | 90 | 63 | [153] | ||
| Tyrosol | Fluconazole | Clinical isolate | 311 | 4720 | 0 | 311 | 4720 | 58 | [153] | ||
| Tyrosol | Itraconazole | Clinical isolate | 311 | 1045 | 0 | 311 | 1045 | 61 | [153] | ||
| Palmatine | Fluconazole | Clinical isolate GDM 2.147 | 8 | 256 | 80 | 0.125 | [181] | ||||
| Palmatine | Itraconazole | Clinical isolate GDM 2.147 | 16 | 32 | 80 | 0.062 | [181] | ||||
| Epigallocatechin gallate | Miconazole | ATCC 13803 | 1500 | 800 | 90 | 0.31 | [149] | ||||
| Epigallocatechin gallate | Fluconazole | ATCC 13803 | 1500 | 400 | 90 | 0.19 | [149] | ||||
| Epigallocatechin gallate | Amphotericin B | ATCC 13803 | 3000 | 0.19 | 90 | 0.19 | [149] | ||||
| Pseudolaric Acid B | Fluconazole | ATCC 750 | 64 | 2 | 50 | 2 | 16 | 80.36 | [156] | ||
| C. krusei | 1 (µg/mL) | 2 (µg/mL) | inhibition (%) | FICI 2 | 1 (µg/mL) | 2 (µg/mL) | inhibition (%) | FICI 2 | |||
| Palmatine | Fluconazole | ATCC 1182 | 8 | 128 | 80 | 0.281 | [181] | ||||
| Palmatine | Itraconazole | ATCC 1182 | 128 | 4 | 80 | 0.312 | [181] | ||||
| Epigallocatechin gallate | Miconazole | ATCC 14243 | 375 | 0.20 | 90 | 0.14 | [149] | ||||
| Epigallocatechin gallate | Fluconazole | ATCC 14243 | 1500 | 39.06 | 90 | 0.75 | [149] | ||||
| Epigallocatechin gallate | Amphotericin B | ATCC 14243 | 187.5 | 0.16 | 90 | 0.31 | [149] | ||||
| C. glabrata | 1 (µg/mL) | 2 (µg/mL) | inhibition (%) | FICI 2 | 1 (µg/mL) | 2 (µg/mL) | inhibition (%) | FICI 2 | |||
| Palmatine | Fluconazole | ATCC 2340 | 256 | 128 | 80 | 0.312 | [181] | ||||
| Palmatine | Itraconazole | ATCC 2340 | 512 | 512 | 80 | 0.187 | [181] | ||||
| Epigallocatechin 3-O-gallate | Fluconazole | CDC 27845 | 15.63 | 16 | 80 | 0.375 | [150] | ||||
| Epigallocatechin 3-O-gallate | Ketoconazole | CDC 27845 | 15.63 | 8 | 80 | 0.375 | [150] | ||||
| Epigallocatechin 3-O-gallate | Fluconazole | CDC 28398 | 15.63 | 16 | 80 | 0.313 | [150] | ||||
| Epigallocatechin 3-O-gallate | Ketoconazole | CDC 28398 | 15.63 | 16 | 80 | 0.313 | [150] | ||||
| Epigallocatechin 3-O-gallate | Fluconazole | ATCC 15126 | 15.63 | 8 | 80 | 0.281 | [150] | ||||
| Epigallocatechin 3-O-gallate | Ketoconazole | ATCC 15126 | 15.63 | 8 | 80 | 0.281 | [150] | ||||
| Epigallocatechin gallate | Miconazole | ATCC 66032 | 1500 | 200 | 90 | 0.31 | [149] | ||||
| Epigallocatechin gallate | Fluconazole | ATCC 66032 | 6000 | 400 | 90 | 0.5 | [149] | ||||
| Epigallocatechin gallate | Amphotericin B | ATCC 66032 | 1500 | 0.63 | 90 | 0.31 | [149] | ||||
| C. auris | 1 (µg/mL) | 2 (µg/mL) | inhibition (%) | FICI 2 | 1 (µg/mL) | 2 (µg/mL) | inhibition (%) | FICI 2 | |||
| Farnesol | Fluconazole | Clinical isolate 10 | 75 | 64 | 50 | [165] | |||||
| Farnesol | Itraconazole | Clinical isolate 10 | 4.69 | 0.5 | 50 | [165] | |||||
| Farnesol | Posaconazole | Clinical isolate 10 | 2.34 | 0.25 | 50 | [165] | |||||
| Farnesol | Isoconazole | Clinical isolate 10 | 9.375 | 0.125 | 50 | [165] | |||||
| Farnesol | Fluconazole | Clinical isolate 12 | 75 | 64 | 50 | [165] | |||||
| Farnesol | Voriconazole | Clinical isolate 12 | 4.69 | 0.5 | 50 | [165] | |||||
| Farnesol | Itraconazole | Clinical isolate 12 | 9.375 | 0.5 | 50 | [165] | |||||
| Farnesol | Posaconazole | Clinical isolate 12 | 2.34 | 0.25 | 50 | [165] | |||||
| Farnesol | Isoconazole | Clinical isolate 12 | 18.75 | 0.125 | 50 | [165] | |||||
| Farnesol | Fluconazole | Clinical isolate 27 | 75 | 64 | 50 | [165] | |||||
| Farnesol | Voriconazole | Clinical isolate 27 | 9.375 | 0.5 | 50 | [165] | |||||
| Farnesol | Itraconazole | Clinical isolate 27 | 9.375 | 0.5 | 50 | [165] | |||||
| Farnesol | Posaconazole | Clinical isolate 27 | 2.34 | 0.25 | 50 | [165] | |||||
| Farnesol | Isoconazole | Clinical isolate 27 | 9.38 | 0.125 | 50 | [165] | |||||
| C. guilliermondi | 1 (µg/mL) | 2 (µg/mL) | inhibition (%) | FICI 2 | 1 (µg/mL) | 2 (µg/mL) | inhibition (%) | FICI 2 | |||
| Palmatine | Fluconazole | ATCC 6260 | 32 | 256 | 80 | 0.156 | [181] | ||||
| Palmatine | Itraconazole | ATCC 6260 | 2 | 64 | 80 | 0.094 | [181] | ||||
| C. dubliniensis | 1 (µg/mL) | 2 (µg/mL) | inhibition (%) | FICI 2 | 1 (µg/mL) | 2 (µg/mL) | inhibition (%) | FICI 2 | |||
| Epigallocatechin 3-O-gallate | Fluconazole | CDC 27963 | 15.63 | 16 | 80 | 0.313 | [150] | ||||
| Epigallocatechin 3-O-gallate | Ketoconazole | CDC 27963 | 15.63 | 16 | 80 | 0.313 | [150] | ||||
| Epigallocatechin 3-O-gallate | Fluconazole | CDC 28551 | 15.63 | 16 | 80 | 0.375 | [150] | ||||
| Epigallocatechin 3-O-gallate | Ketoconazole | CDC 28551 | 15.63 | 8 | 80 | 0.375 | [150] | ||||
| C. kefir | 1 (µg/mL) | 2 (µg/mL) | inhibition (%) | FICI 2 | 1 (µg/mL) | 2 (µg/mL) | inhibition (%) | FICI 2 | |||
| Epigallocatechin gallate | Miconazole | ATCC 46764 | 750 | 100 | 90 | 0.63 | [149] | ||||
| Epigallocatechin gallate | Fluconazole | ATCC 46764 | 750 | 200 | 90 | 0.63 | [149] | ||||
| Plant Compounds (1) | Drugs (2) | Strains | Preformed Biofilm | Biofilm Formation | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (µg/mL) | 2 (µg/mL) | Inhibition (%) | FICI | 1 (µg/mL) | 2 (µg/mL) | Inhibition (%) | FICI 3 | ||||
| Thymol | Fluconazole | C. albicans J-01 | 3.25 | 32 | 80 | 0.250 | [187] | ||||
| S. aureus MTCC3160 | |||||||||||
| Eugenol | Fluconazole | C. albicans J-01 | 12.5 | 512 | 80 | 0.531 | [187] | ||||
| S. aureus MTCC3160 | |||||||||||
| Thymol | Vancomycin | C. albicans J-01 | 3.25 | 128 | 80 | 0.125 | [187] | ||||
| S. aureus MTCC3160 | |||||||||||
| Eugenol | Vancomycin | C. albicans J-01 | 12.5 | 512 | 80 | 0.281 | [187] | ||||
| S. aureus MTCC3160 | |||||||||||
| Thymol | Fluconazole | C. albicans J-12 | 3.12 | 256 | 80 | 0.187 | [187] | ||||
| S. aureus MTCC3160 | |||||||||||
| Eugenol | Fluconazole | C. albicans J-12 | 12.5 | 1024 | 80 | 0.562 | [187] | ||||
| S. aureus MTCC3160 | |||||||||||
| Thymol | Vancomycin | C. albicans J-12 | 3.12 | 128 | 80 | 0.125 | [187] | ||||
| S. aureus MTCC3160 | |||||||||||
| Eugenol | Vancomycin | C. albicans J-12 | 50 | 256 | 80 | 0.375 | [187] | ||||
| S. aureus MTCC3160 | |||||||||||
| Berberine | Amphotericin B | C. albicans SC5314 | 128 | 4 | 60 | [188] | |||||
| S. aureus HNS0029 | |||||||||||
| Berberine | Amphotericin B | C. albicans SC5314 | 128 | 4 | 69 | [188] | |||||
| S. aureus ATCC 25923 | |||||||||||
| Curcumin | 2-aminobenzimidazole | C. albicans DAY185 | 200 | 200 | 73.3 | 100 | 100 | 97.6 | [189] | ||
| S. aureus ATCC 6538 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonincontro, G.; Scuderi, S.A.; Marino, A.; Simonetti, G. Synergistic Effect of Plant Compounds in Combination with Conventional Antimicrobials against Biofilm of Staphylococcus aureus, Pseudomonas aeruginosa, and Candida spp. Pharmaceuticals 2023, 16, 1531. https://doi.org/10.3390/ph16111531
Bonincontro G, Scuderi SA, Marino A, Simonetti G. Synergistic Effect of Plant Compounds in Combination with Conventional Antimicrobials against Biofilm of Staphylococcus aureus, Pseudomonas aeruginosa, and Candida spp. Pharmaceuticals. 2023; 16(11):1531. https://doi.org/10.3390/ph16111531
Chicago/Turabian StyleBonincontro, Graziana, Sarah Adriana Scuderi, Andreana Marino, and Giovanna Simonetti. 2023. "Synergistic Effect of Plant Compounds in Combination with Conventional Antimicrobials against Biofilm of Staphylococcus aureus, Pseudomonas aeruginosa, and Candida spp." Pharmaceuticals 16, no. 11: 1531. https://doi.org/10.3390/ph16111531
APA StyleBonincontro, G., Scuderi, S. A., Marino, A., & Simonetti, G. (2023). Synergistic Effect of Plant Compounds in Combination with Conventional Antimicrobials against Biofilm of Staphylococcus aureus, Pseudomonas aeruginosa, and Candida spp. Pharmaceuticals, 16(11), 1531. https://doi.org/10.3390/ph16111531







