Uridine Diphosphate Glucose (UDP-G) Activates Oxidative Stress and Respiratory Burst in Isolated Neutrophils
Abstract
:1. Introduction
2. Results
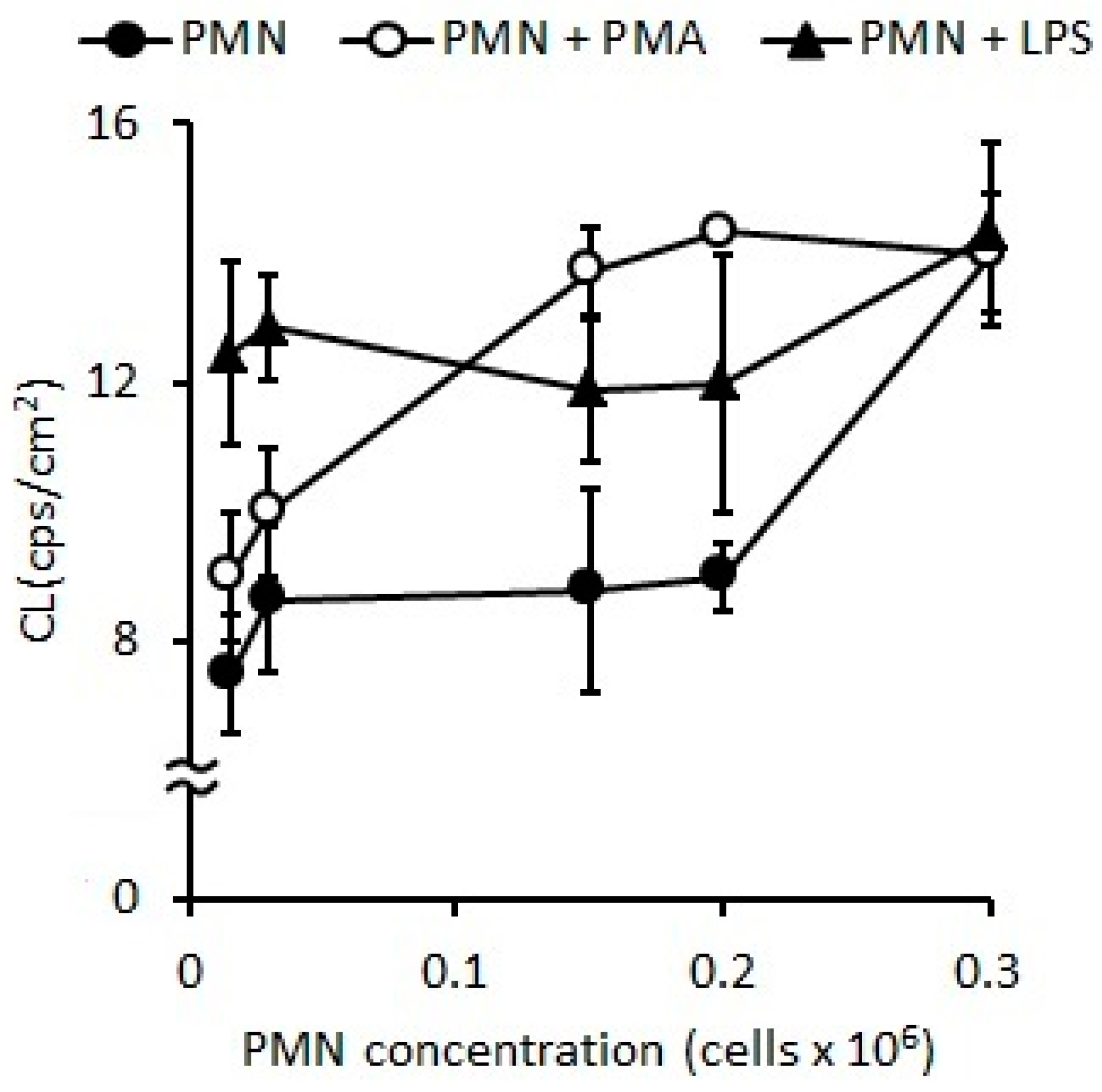
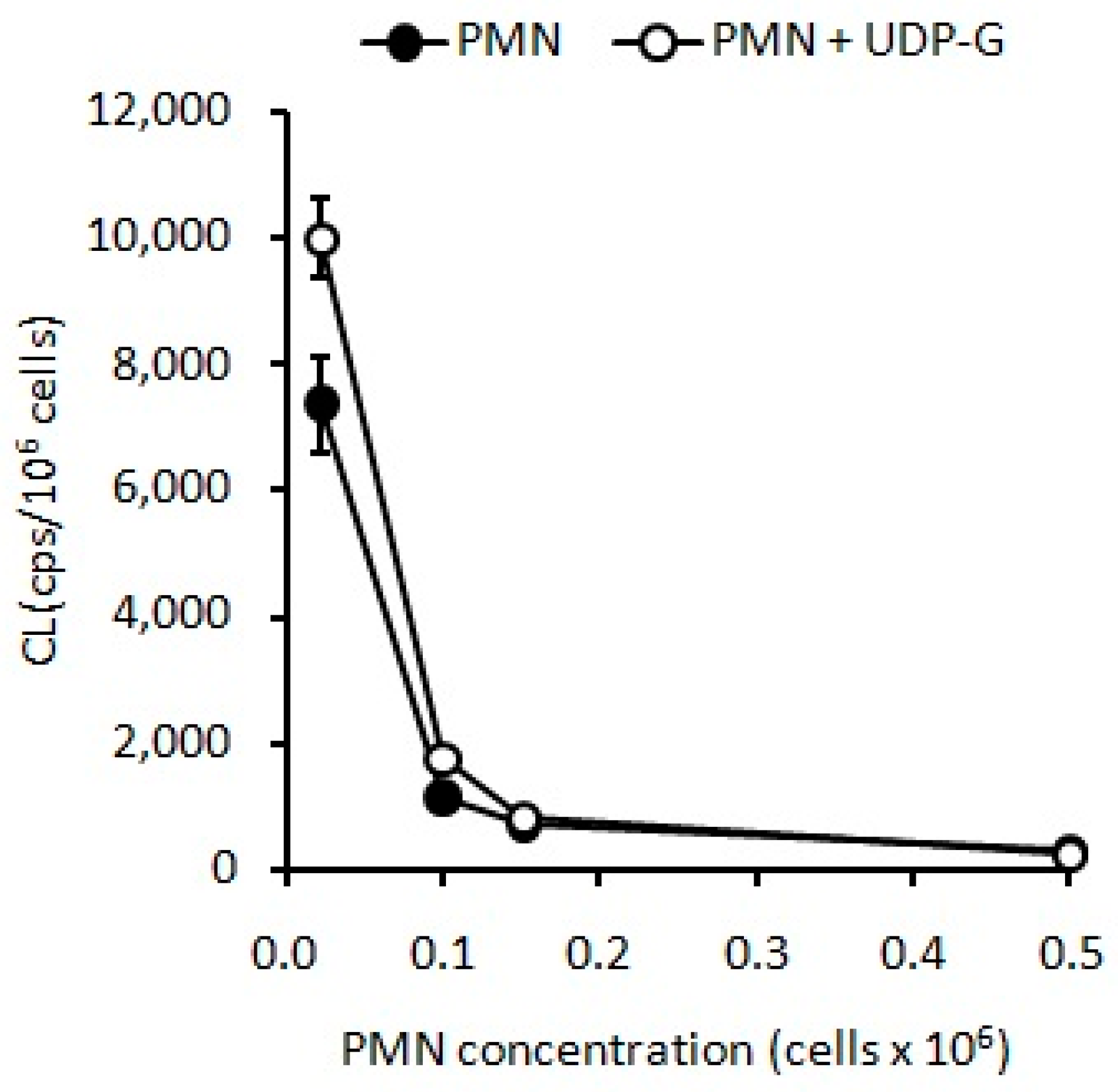
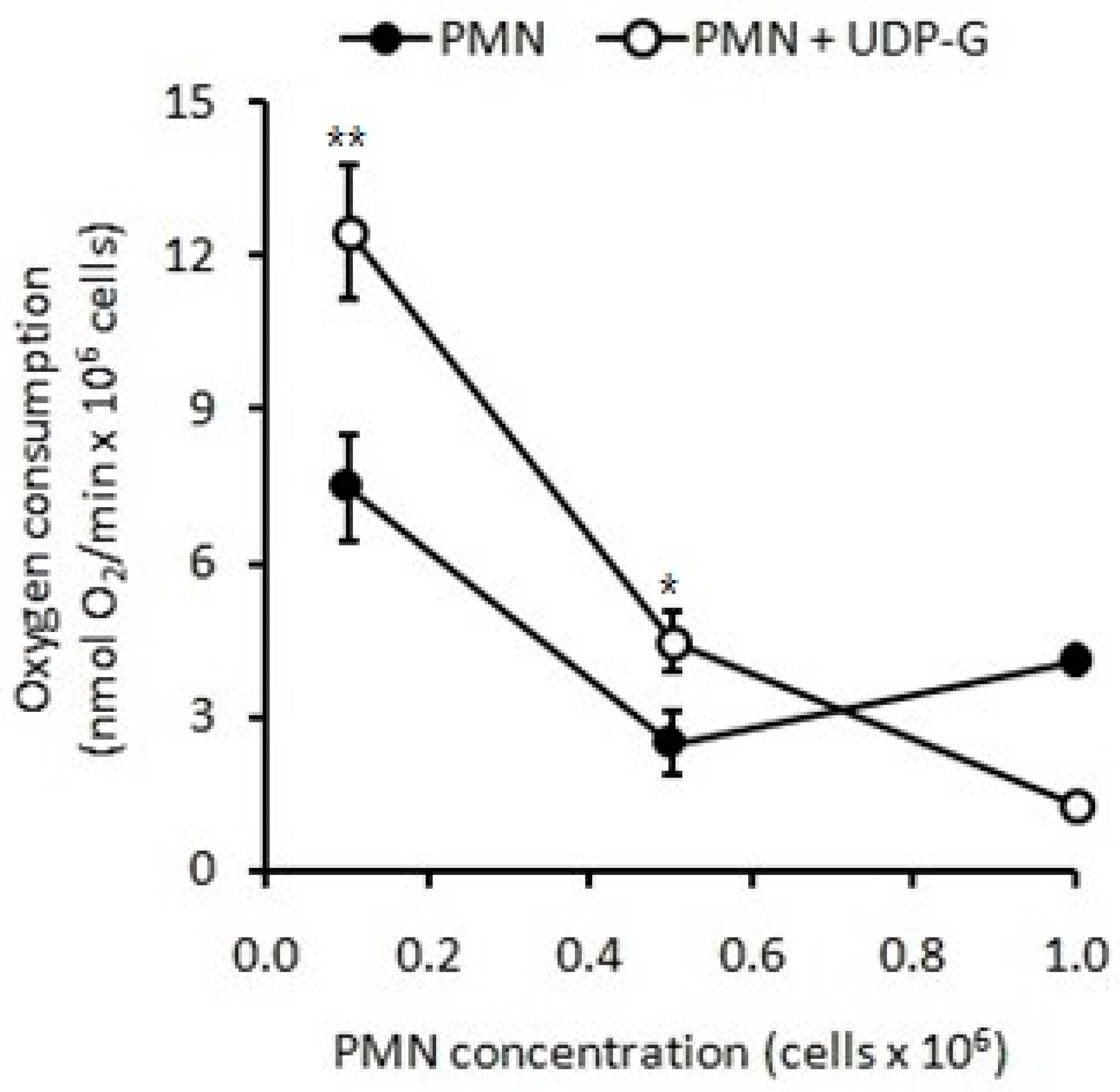
2.1. Standardization of Optimal PMN Concentration
2.1.1. Oxidative Stress Measured as Spontaneous Chemiluminescence
2.1.2. Respiratory Burst Measured as Oxygen Consumption
2.2. PMN Activation Effect of Agonists: Stimulation Index
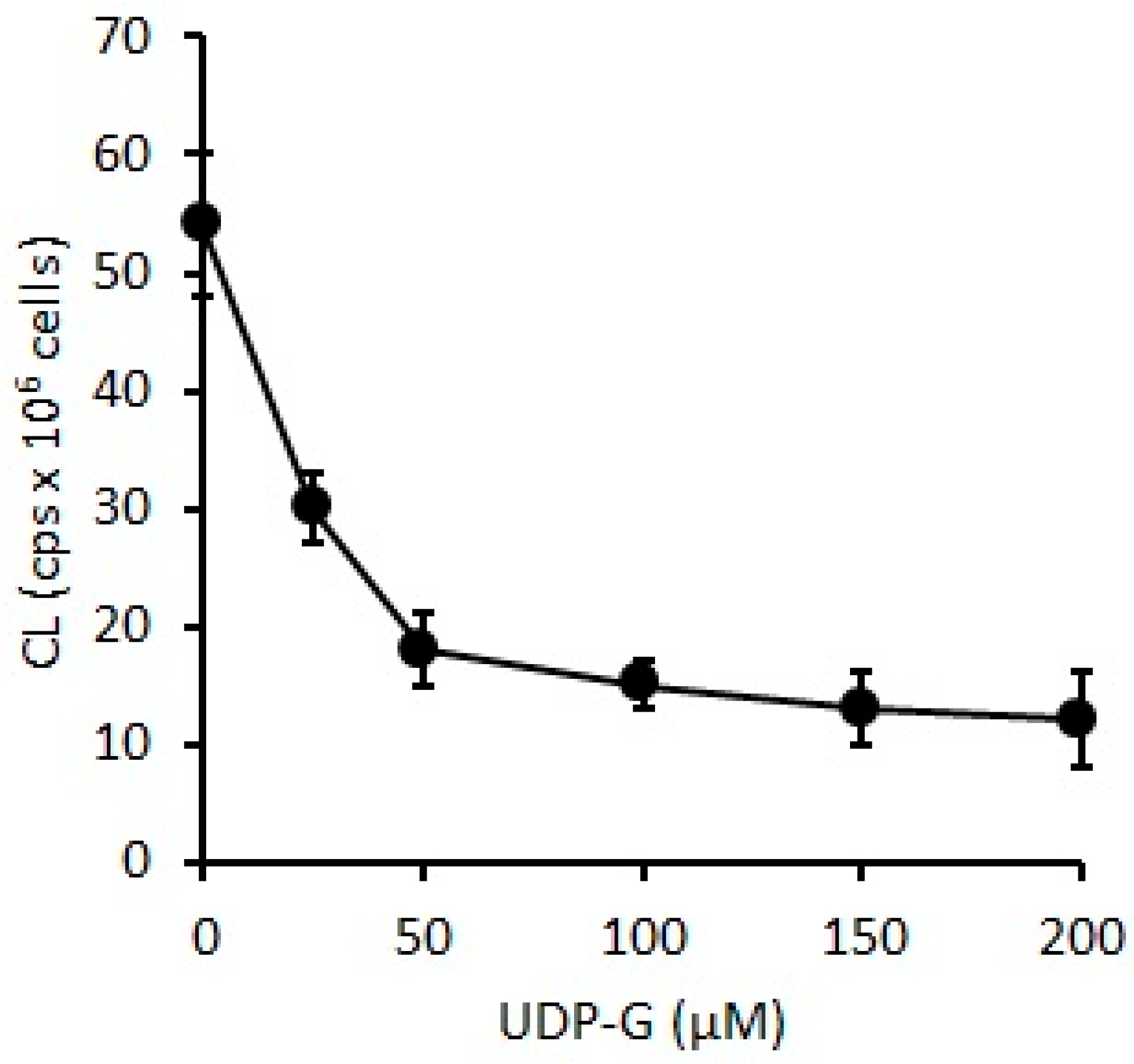
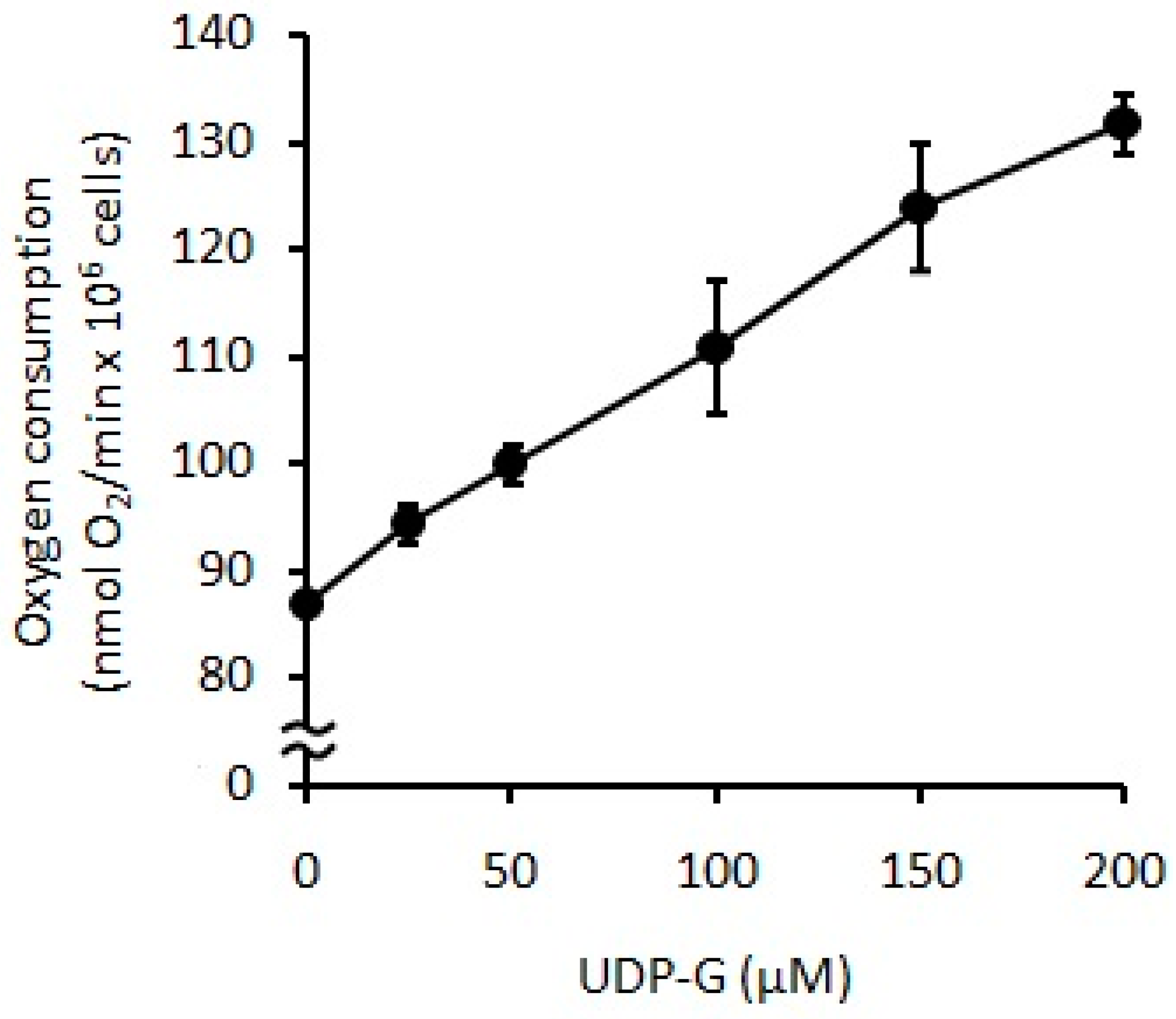
2.3. Effect of UDP-G on PMN Function: OS and RB
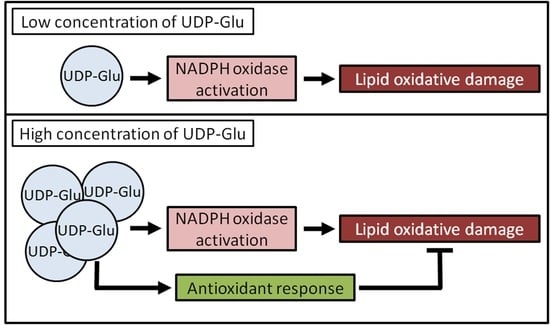
3. Discussion
4. Materials and Methods
4.1. Isolation of Human PMNs and Cell Viability
4.2. Stimuli and Activation of PMNs
4.3. Standardization of Optimal PMN Concentration for OS and RB Measurement
4.4. Biochemical Determinations of OS and RB
4.4.1. PMNs Spontaneous CL
4.4.2. PMNs Oxygen Consumption
4.5. Activation Effect of Agonists: Stimulation Index
4.6. Effect of UDP-G on PMN Function
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CL | Chemiluminescence |
| EDTA | Ethylenediaminetetraacetic acid |
| fMLP | N-formyl-methionyl- leucyl-phenylalanine |
| LPS | Lipopolysaccharide |
| OS | Oxidative stress |
| PMA | Phorbol 12-myristate 13-acetate |
| PMN | Polymorphonuclear neutrophil |
| CTX | Chemotaxis |
| RB | Respiratory burst |
| SI | Stimulation index |
| UDP-G | Uridine diphosphate glucose |
References
- Burnstock, G.; Campbell, G.; Satchell, D.; Smythe, A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by nonadrenergic inhibitory nerves in the gut. Br. J. Pharmacol. 1970, 40, 668–688. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic signalling. Br. J. Pharmacol. 2006, 147, S172–S181. [Google Scholar] [CrossRef] [PubMed]
- Lazarowski, E.R. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012, 8, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Abbracchio, M.P.; Burnstock, G.; Boeynaems, J.M.; Barnard, E.A.; Boyer, J.L.; Kennedy, C.; Knight, G.E.; Fumagalli, M.; Gachet, C.; Jacobson, K.A.; et al. International Union of Pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 2006, 58, 281–341. [Google Scholar] [CrossRef] [PubMed]
- Lazarowski, E.R.; Harden, T.K. UDP-sugars as extracellular signaling molecules: Cellular and physiologic consequences of P2Y14 receptor activation. Mol. Pharmacol. 2015, 88, 151–160. [Google Scholar] [CrossRef]
- Muller, T.; Bayer, H.; Myrtek, D.; Ferrari, D.; Sorichter, S.; Ziegenhagen, M.W.; Zissel, G.; Virchow, J.C., Jr.; Luttmann, W.; Norgauer, J.; et al. The P2Y14 receptor of airway epithelial cells: Coupling to intracellular Ca2+ and IL-8 secretion. Am. J. Respir. Cell Mol. Biol. 2005, 33, 601–609. [Google Scholar] [CrossRef]
- Moore, D.J.; Murdock, P.R.; Watson, J.M.; Faull, R.L.; Waldvogel, H.J.; Szekeres, P.G.; Wilson, S.; Freeman, K.B.; Emson, P.C. GPR105, a novel Gi/o-coupled UDP-glucose receptor expressed on brain glia and peripheral immune cells, is regulated by immunologic challenge: Possible role in neuroimmune function. Brain Res. Mol. Brain Res. 2003, 118, 10–23. [Google Scholar] [CrossRef]
- Lee, B.C.; Cheng, T.; Adams, G.B.; Attar, E.C.; Miura, N.; Lee, S.B.; Saito, Y.; Olszak, I.; Dombkowski, D.; Olson, D.P.; et al. P2Y-like receptor, GPR105 (P2Y14), identifies and mediates chemotaxis of bone-marrow hematopoietic stem cells. Genes Dev. 2003, 17, 1592–1604. [Google Scholar] [CrossRef]
- Sesma, J.I.; Weitzer, C.D.; Livraghi-Butrico, A.; Dang, H.; Donaldson, S.; Alexis, N.; Jacobson, K.A.; Harden, K.; Lazarowski, E.R. UDP-glucose promotes neutrophil recruitment in the lung. Purinergic Signal. 2016, 12, 627–635. [Google Scholar] [CrossRef]
- Barrett, M.O.; Sesma, J.I.; Ball, C.B.; Jayasekara, P.S.; Jacobson, K.A.; Lazarowski, E.R.; Harden, T.K. A selective high-affinity antagonist of the P2Y14 receptor inhibits UDP-glucose-stimulated chemotaxis of human neutrophils. Mol. Pharmacol. 2013, 84, 41–49. [Google Scholar] [CrossRef]
- Sesma, J.I.; Kreda, S.M.; Steinckwich-Besancon, N.; Dang, H.; García-Mata, R.; Harden, T.K.; Lazarowski, E.R. The UDP-sugar-sensing P2Y(14) receptor promotes Rho-mediated signaling and chemotaxis in human neutrophils. Am. J. Physiol. Cell Physiol. 2012, 303, C490–C498. [Google Scholar] [CrossRef]
- Kreda, S.; Okada, S.F.; van Heusden, C.A.; O’Neal, W.; Gabriel, S.; Abdullah, L.; Davis, C.W.; Boucher, R.C.; Lazarowski, E.R. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J. Physiol. 2007, 584, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.F.; Zhang, L.; Kreda, S.M.; Abdullah, L.H.; Davis, C.W.; Pickles, R.J.; Lazarowski, E.R.; Boucher, R.C. Coupled nucleotide and mucin hypersecretion from goblet-cell metaplastic human airway epithelium. Am. J. Respir. Cell Mol. Biol. 2011, 45, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Borda, N.; Carbia, C.; Rabossi, G.; Arcavi, M.; García Gonçalves, L.; Ameneiros, M.; Martínez, R.; Lazarowski, A.; Merelli, A. Activación de polimorfonucleares neutrófilos por UDP-Glucosa extracelular. Revista Bioquímica y Patología Clínica 2015, 79, 10–17. Available online: http://www.revistabypc.org.ar/index.php/bypc/article/view/166 (accessed on 9 July 2023).
- Geerdink, R.J.; Pillay, J.; Meyaard, L.; Bont, L. Neutrophils in respiratory syncytial virus infection: A target for asthma prevention. J. Allergy Clin. Immunol. 2015, 136, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Borregaard, N.; Sorensen, O.E.; Theilgaard-Monch, K. Neutrophil granules: A library of innate immunity proteins. Trends Immunol 2007, 28, 340–345. [Google Scholar] [CrossRef]
- Abramson, J.S.; Mills, E.L.; Giebink, G.S.; Quie, P.G. Depression of monocyte and polymorphonuclear leukocyte oxidative metabolism and bactericidal capacity by influenza A virus. Infect. Immun. 1982, 35, 350–355. [Google Scholar] [CrossRef]
- Boveris, A.; Repetto, M.; Bustamante, J.; Boveris, A.D.; Valdez, L.B. The Concept of Oxidative Stress in Pathology. In Free Radical Pathophysiology; Alvarez, S., Evelson, P., Boveris, A., Eds.; Transword Research Network: Kerala, India, 2008; pp. 1–17. [Google Scholar]
- Repetto, M.; Semprine, J.; Boveris, A. Lipid Peroxidation: Chemical Mechanisms, Biological Implications and Analytical Determinations. In Lipid Peroxidation; Catala, A., Ed.; Intech: Rijeka, Croatia, 2012; pp. 3–30. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Nagata, N.; Iwata-Yoshikawa, N.; Taguchi, F. Studies of Severe Acute Respiratory Syndrome Coronavirus Pathology in Human Cases and Animal Models. Vet. Pathol. 2010, 47, 881–892. [Google Scholar] [CrossRef]
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front. Cell Infect. Microbiol. 2017, 7, 373. [Google Scholar] [CrossRef]
- Tian, S.; Xiong, Y.; Liu, H.; Niu, L.; Guo, J.; Liao, M.; Xiao, X.-Y. Pathological study of the 2019 novel coronavirus disease (COVID-19) through post-mortem core biopsies. Mod Pathol. 2020, 33, 1007–1014. [Google Scholar] [CrossRef]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp Med. 2020, 217, e20200652. [Google Scholar] [CrossRef] [PubMed]
- Harden, T.K.; Sesma, J.I.; Fricks, I.P.; Lazarowski, E.R. Signalling and pharmacological properties of the P2Y14 receptor. Acta Physiol. (Oxf.) 2010, 199, 149–160. [Google Scholar] [CrossRef]
- Lazarowski, E.R.; Boucher, R.C. Purinergic receptors in airway epithelia. Curr. Opin. Pharmacol. 2009, 9, 262–267. [Google Scholar] [CrossRef]
- Van Acker, H.; Coenye, T. The role of reactive oxygen species in antibiotic-mediated killing of bacteria. Trends Microbiol. 2017, 25, 456–466. [Google Scholar] [CrossRef]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, W. Infarction of the heart. Am. J. Physiol. 1899, 2, 243–265. [Google Scholar] [CrossRef]
- Sadiku, P.; Walmsley, S.R. Hypoxia and the regulation of myeloid cell metabolic imprinting: Consequences for the inflammatory response. EMBO Rep. 2019, 20, e47388. [Google Scholar] [CrossRef]
- Clark, R.A.; Nausees, W.M. Isolation and Functional Analysis of Neutrophils. In Current Protocols in Immunology; Coligan, J.E., Krusibeek, A.M., Margulies, D.H., Shevach, E.M., Strober, W., Eds.; John Wiley & Sons: Oxford, UK, 1991; pp. 7.23.1–7.23.3. [Google Scholar] [CrossRef]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef]
- Carreras, M.C.; Pargament, G.A.; Catz, S.D.; Poderoso, J.J.; Boveris, A. Kinetics of nitric oxide and hydrogen peroxide production and formation of peroxynitrite during the respiratory burst of human neutrophils. FEBS Lett. 1994, 341, 65–68. [Google Scholar] [CrossRef]
- Valdez, L.; Boveris, A. Nitric oxide and superoxide radical production by human mononuclear leukocytes. Antioxid. and Redox Signal. 2001, 3, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Lintzmaier Petiz, L.; Glaser, T.; Scharfstein, J.; Ratajczak, M.Z.; Ulrich, H. P2Y14 Receptor as a Target for Neutrophilia Attenuation in Severe COVID-19 Cases: From Hematopoietic Stem Cell Recruitment and Chemotaxis to Thrombo-inflammation. Stem Cell Rev. Rep. 2021, 17, 241–252. [Google Scholar] [CrossRef] [PubMed]


| PMN + Agonist | Oxygen Consumption (nmol O2/min/106 PMN) |
|---|---|
| Control (PBS) | 1.8 ± 1.2 |
| LPS | 2.1 ± 0.8 *** |
| PMA | 2.8 ± 0.6 * |
| PMN + Agonist | Oxygen Consumption (nmol O2/min/106 PMN) | |
|---|---|---|
| 0.1 × 106 PMN/ mL | 0.5 × 106 PMN/ mL | |
| Control (PBS) | 7.2 ± 1.3 | 1.8 ± 1.2 |
| LPS | 7.5 ± 2.0 | 2.1 ± 0.7 |
| PMA | 10 ± 2 *** | 2.5 ± 1.5 * |
| UDP-G | 13 ± 1.5 *** | 4.0 ± 1.0 ** |
| PMN + Agonist | Oxygen Consumption (nmol O2/min/106 PMN) |
|---|---|
| 0.02 × 106 PMN/ mL | |
| Control (PBS) | 55 ± 10 |
| LPS | 60 ± 5 |
| PMA | 52 ± 1 |
| UDP-G | 60 ± 5 |
| LPS + UDP-G | 69 ± 4 * |
| Agonist | CL-SI | RB-SI |
|---|---|---|
| None | 1.0 | 1.0 |
| LPS | 1.7 | 1.2 |
| PMA | 2.2 | 1.4 |
| UDP-G | 1.6 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lairion, F.; Carbia, C.; Chiesa, I.M.; Saporito-Magriña, C.; Borda, N.; Lazarowski, A.; Repetto, M.G. Uridine Diphosphate Glucose (UDP-G) Activates Oxidative Stress and Respiratory Burst in Isolated Neutrophils. Pharmaceuticals 2023, 16, 1501. https://doi.org/10.3390/ph16101501
Lairion F, Carbia C, Chiesa IM, Saporito-Magriña C, Borda N, Lazarowski A, Repetto MG. Uridine Diphosphate Glucose (UDP-G) Activates Oxidative Stress and Respiratory Burst in Isolated Neutrophils. Pharmaceuticals. 2023; 16(10):1501. https://doi.org/10.3390/ph16101501
Chicago/Turabian StyleLairion, Fabiana, Claudio Carbia, Iris Maribel Chiesa, Christian Saporito-Magriña, Natalia Borda, Alberto Lazarowski, and Marisa Gabriela Repetto. 2023. "Uridine Diphosphate Glucose (UDP-G) Activates Oxidative Stress and Respiratory Burst in Isolated Neutrophils" Pharmaceuticals 16, no. 10: 1501. https://doi.org/10.3390/ph16101501
APA StyleLairion, F., Carbia, C., Chiesa, I. M., Saporito-Magriña, C., Borda, N., Lazarowski, A., & Repetto, M. G. (2023). Uridine Diphosphate Glucose (UDP-G) Activates Oxidative Stress and Respiratory Burst in Isolated Neutrophils. Pharmaceuticals, 16(10), 1501. https://doi.org/10.3390/ph16101501