Development and Preclinical Evaluation of [68Ga]BMSH as a New Potent Positron Emission Tomography Tracer for Imaging Programmed Death-Ligand 1 Expression
Abstract
:1. Introduction
2. Results
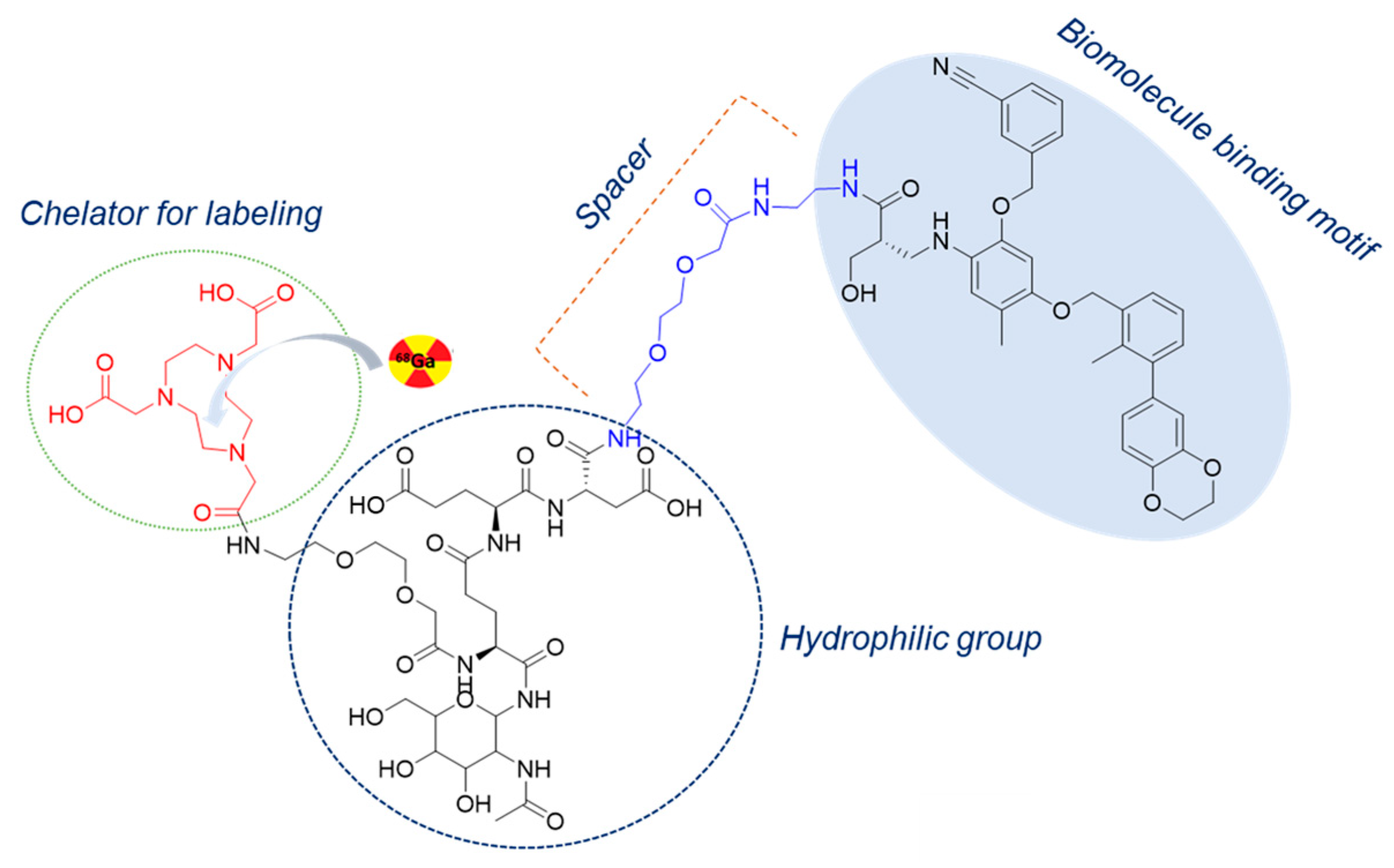
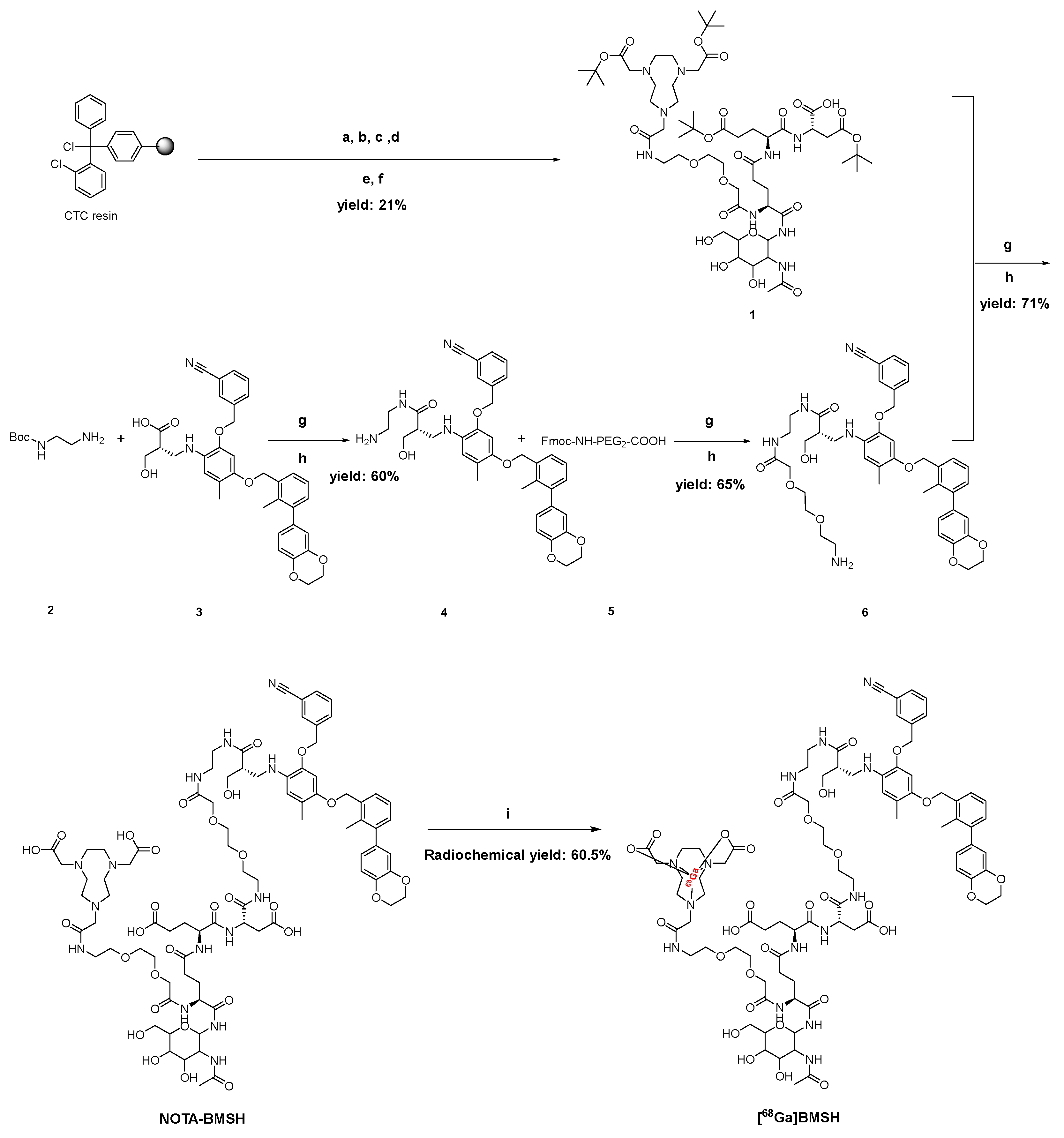
2.1. Chemical and Radiochemical Properties
2.2. Characterization of [68Ga]BMSH
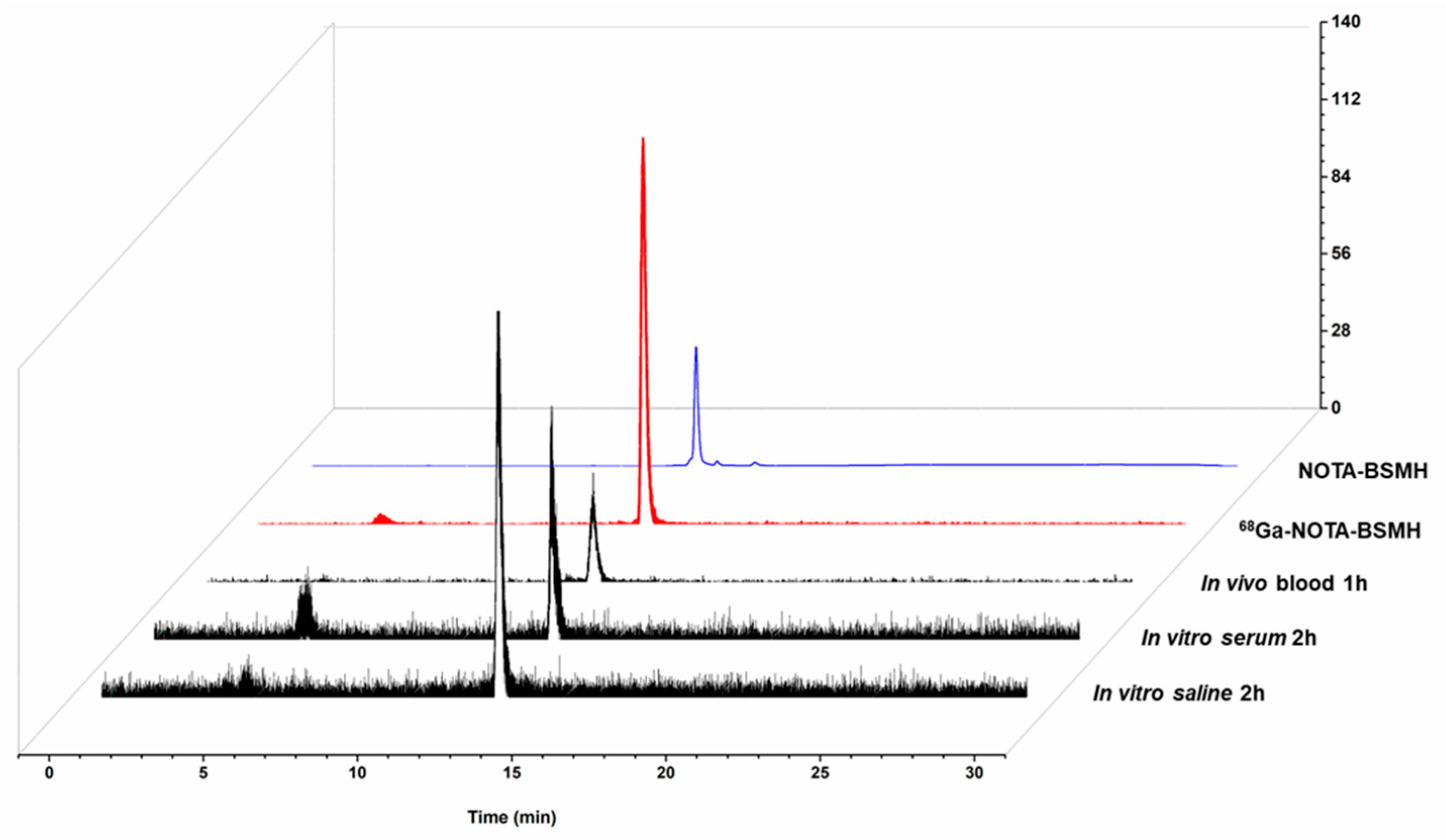
2.3. In Vitro Evaluation of [68Ga]BMSH
2.4. Biodistribution of [68Ga]BMSH
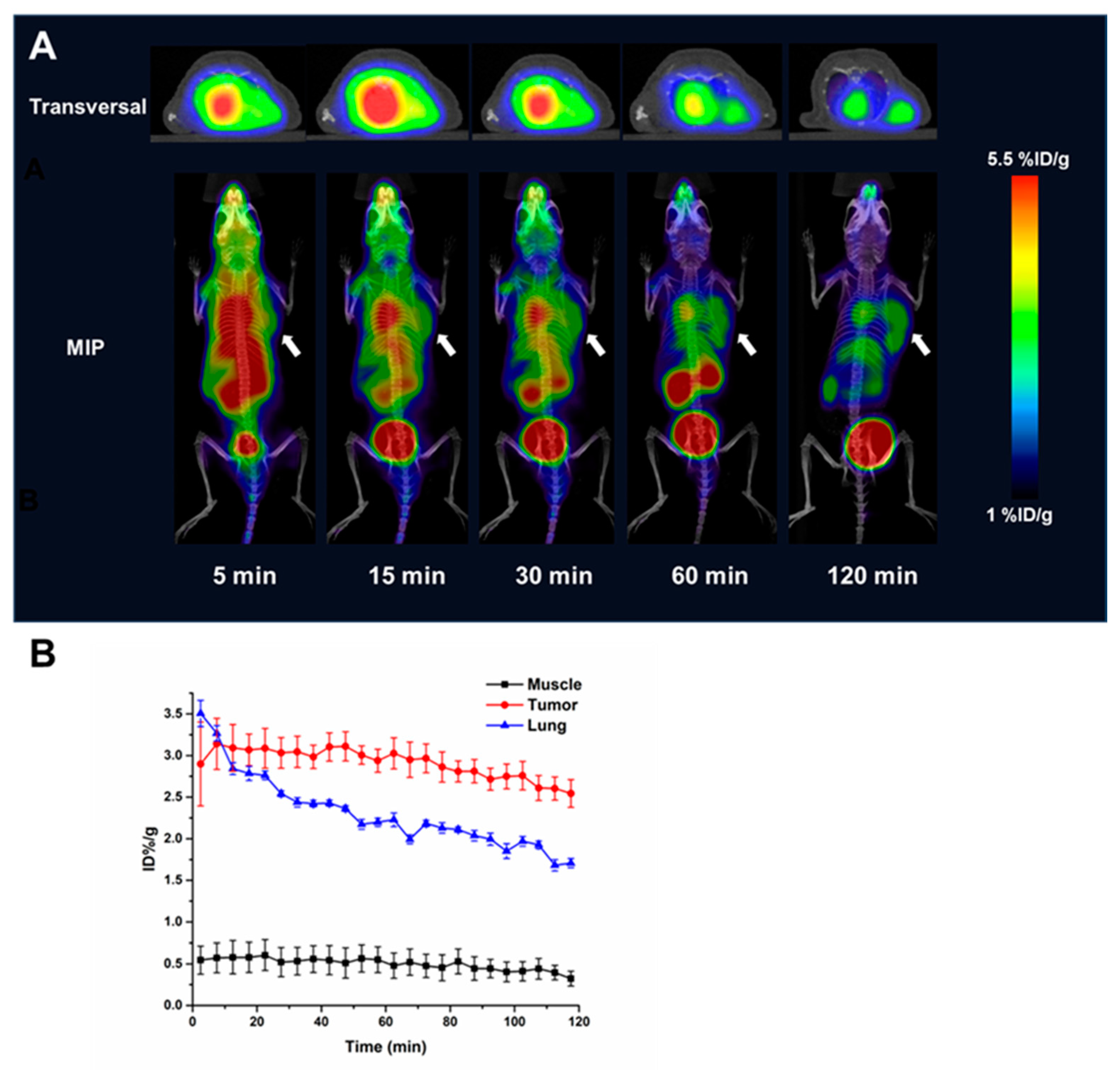
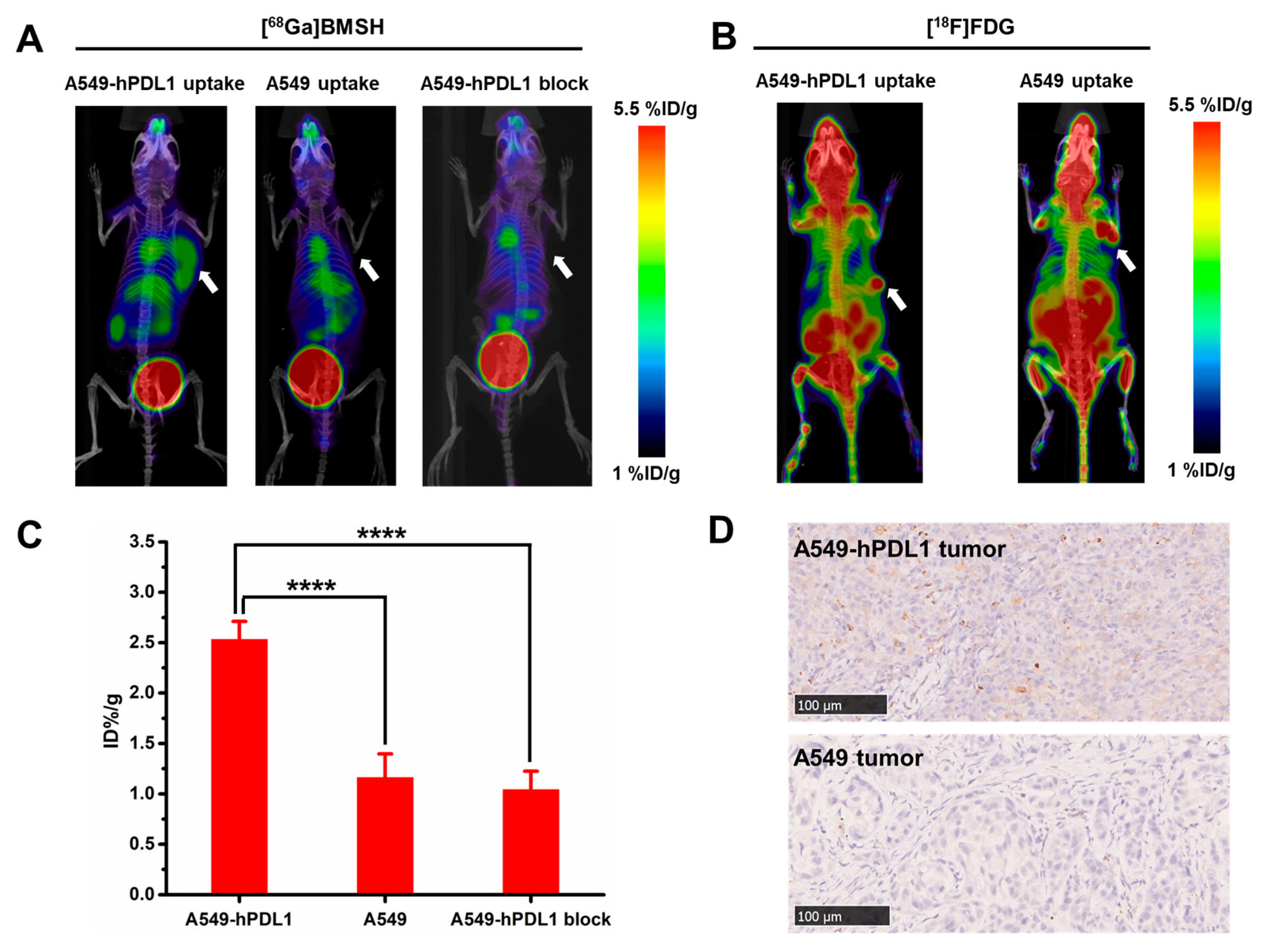
2.5. Micro-PET Imaging and Immunohistochemical Staining
2.6. Internal Dose Assessment
3. Discussion
4. Materials and Methods
4.1. Reagents and Instruments
4.2. General Procedure for the Synthesis of Resin-Bound Compound 1
4.3. General Procedure for the Synthesis of Compound 6
4.4. Synthesis of NOTA-BMSH
4.5. Radiochemical Synthesis of [68Ga]BMSH
4.6. Partition Coefficient
4.7. In Vitro Serum Stability and In Vivo Stability
4.8. Cell Lines and Tumor Models
4.9. In Vitro Cell Study
4.10. Tissue Biodistribution Studies
4.11. Micro-PET Scanner Imaging
4.12. Immunohistochemical Staining
4.13. Internal Radiation Dose of [68Ga]BMSH
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Muro, K.; Chung, H.C.; Shankaran, V.; Geva, R.; Catenacci, D.; Gupta, S.; Eder, J.P.; Golan, T.; Le, D.T.; Burtness, B. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): A multicentre, open–label, phase 1b trial. Lancet Oncol. 2016, 17, 717–726. [Google Scholar] [CrossRef]
- Kang, Y.K.; Boku, N.; Satoh, T.; Ryu, M.H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.S.; Muro, K.; Kang, W.K. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO–4538–12, ATTRACTION–2): A randomised, double–blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef]
- Moehler, M.; Högner, A.; Wagner, A.D.; Obermannova, R.; Alsina, M.; Thuss-Patience, P.; van Laarhoven, H.; Smyth, E. Recent progress and current challenges of immunotherapy in advanced/metastatic esophagogastric adenocarcinoma. Eur. J. Cancer 2022, 176, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Botticelli, A.; Visconti, I.C.; Angeletti, D.; Fiore, M.; Marchetti, P.; Lambiase, A.; de Vincentiis, M.; Greco, A. Immunotherapy in the treatment of metastatic melanoma: Current knowledge and future directions. J. Immunol. Res. 2020, 2020, 925638. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Remon, J.; Hellmann, M.D. First-line immunotherapy for non–small-cell lung cancer. J. Clin. Oncol. 2022, 40, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Labriola, M.K.; Batich, K.A.; Zhu, J.; McNamara, M.A.; Harrison, M.R.; Armstrong, A.J.; George, D.J.; Zhang, T. Immunotherapy is changing first-line treatment of metastatic renal-cell carcinoma. Clin. Genitourin. Cancer 2019, 17, e513–e521. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; Weil, R.; Dovey, Z.; Davis, A.; Tewari, A.K. The tumor microenvironment and immunotherapy in prostate and bladder cancer. Urol. Clin. N. Am. 2020, 47, e17–e54. [Google Scholar] [CrossRef] [PubMed]
- Homet Moreno, B.; Ribas, A. Anti–programmed cell death protein–1/ligand–1 therapy in different cancers. Br. J. Cancer 2015, 112, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Sunshine, J.; Taube, J.M. Pd-1/pd–l1 inhibitors. Curr. Opin. Pharmacol. 2015, 23, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Fan, R.; Shi, N.; Ma, W. The efficacy and safety of anti–PD–1/PD–L1 antibodies for treatment of advanced or refractory cancers: A meta–analysis. Oncotarget 2016, 7, 73068–73079. [Google Scholar] [CrossRef]
- Gandini, S.; Massi, D.; Mandalà, M. PD–L1 expression in cancer patients receiving anti PD–1/PD–L1 antibodies: A systematic review and meta-analysis. Crit. Rev. Oncol./Hematol. 2016, 100, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti–PD-1 therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef] [PubMed]
- Brody, R.; Zhang, Y.; Ballas, M.; Siddiqui, M.K.; Gupta, P.; Barker, C.; Midha, A.; Walker, J. PD–L1 expression in advanced NSCLC: Insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer 2017, 112, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.; Long–Mira, E.; Bence, C.; Butori, C.; Lassalle, S.; Bouhlel, L.; Fazzalari, L.; Zahaf, K.; Lalvée, S.; Washetine, K. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: A potential issue for anti-PD–L1 therapeutic strategies. Ann. Oncol. 2016, 27, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, Z.; Jiang, T.; Hou, L.; Wu, F.; Gao, G.; He, Y.; Zhao, J.; Li, X.; Zhao, C. Heterogeneity of PD–L1 expression among the different histological components and metastatic lymph nodes in patients with resected lung adenosquamous carcinoma. Clin. Lung Cancer 2018, 19, e421–e430. [Google Scholar] [CrossRef]
- Alauddin, M.M.; Khawli, L.A. Advances in Immuno-PET for the Detection of Cancer and Assessment of Response to Therapy. Curr. Med. Chem. 2021, 28, 647–672. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Cléry, P.-F.; Faivre–Chauvet, A.; Bourgeois, M.; Guérard, F.; Haddad, F.; Barbet, J.; Chérel, M.; Kraeber–Bodéré, F.; Carlier, T. Immuno-PET for clinical theranostic approaches. Int. J. Mol. Sci. 2016, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Rosenkrans, Z.T.; Liu, J.; Huang, G.; Luo, Q.Y.; Cai, W. ImmunoPET: Concept, design, and applications. Chem. Rev. 2020, 120, 3787–3851. [Google Scholar] [CrossRef]
- Smit, J.; Borm, F.J.; Niemeijer, A.L.N.; Huisman, M.C.; Hoekstra, O.S.; Boellaard, R.; Oprea–Lager, D.E.; Vugts, D.J.; van Dongen, G.A.; Thunnissen, E. PD–L1 PET/CT Imaging with Radiolabeled Durvalumab in Patients with Advanced–Stage Non–Small Cell Lung Cancer. J. Nucl. Med. 2022, 63, 686–693. [Google Scholar] [CrossRef]
- Lesniak, W.G.; Chatterjee, S.; Gabrielson, M.; Lisok, A.; Wharram, B.; Pomper, M.G.; Nimmagadda, S. PD-L1 detection in tumors using [64Cu] atezolizumab with PET. Bioconjugate Chem. 2016, 27, 2103–2110. [Google Scholar] [CrossRef]
- Jagoda, E.M.; Vasalatiy, O.; Basuli, F.; Opina, A.C.L.; Williams, M.R.; Wong, K.; Lane, K.C.; Adler, S.; Ton, A.T.; Szajek, L.P. Immuno-PET imaging of the programmed cell death–1 ligand (PD-L1) using a zirconium–89 labeled therapeutic antibody, avelumab. Mol. Imaging 2019, 18, 1536012119829986. [Google Scholar] [CrossRef] [PubMed]
- Christensen, C.; Kristensen, L.K.; Alfsen, M.Z.; Nielsen, C.H.; Kjaer, A. Quantitative PET imaging of PD-L1 expression in xenograft and syngeneic tumour models using a site–specifically labelled PD–L1 antibody. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jiang, L.; Li, K.; Li, H.; Lv, G.; Lin, J.; Qiu, L. Immuno–PET imaging of 68 Ga-labeled nanobody Nb109 for dynamic monitoring the PD–L1 expression in cancers. Cancer Immunol. Immunother. 2021, 70, 1721–1733. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lesniak, W.G.; Miller, M.S.; Lisok, A.; Sikorska, E.; Wharram, B.; Kumar, D.; Gabrielson, M.; Pomper, M.G.; Gabelli, S.B. Rapid PD–L1 detection in tumors with PET using a highly specific peptide. Biochem. Biophys. Res. Commun. 2017, 483, 258–263. [Google Scholar] [CrossRef] [PubMed]
- De Silva, R.A.; Kumar, D.; Lisok, A.; Chatterjee, S.; Wharram, B.; Venkateswara Rao, K.; Mease, R.; Dannals, R.F.; Pomper, M.G.; Nimmagadda, S. Peptide-based 68Ga–PET radiotracer for imaging PD-L1 expression in cancer. Mol. Pharm. 2018, 15, 3946–3952. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, W.G.; Mease, R.C.; Chatterjee, S.; Kumar, D.; Lisok, A.; Wharram, B.; Kalagadda, V.R.; Emens, L.A.; Pomper, M.G.; Nimmagadda, S. Development of [18F] FPy–WL12 as a PD-L1 specific PET imaging peptide. Mol. Imaging 2019, 18, 1536012119852189. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jiang, J.; Yang, X.; Liu, T.; Ding, J.; Nimmagadda, S.; Pomper, M.G.; Zhu, H.; Zhao, J.; Yang, Z. First–in–Humans evaluation of a PD–L1–binding peptide PET radiotracer in non–small cell lung cancer patients. J. Nucl. Med. 2022, 63, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Guzik, K.; Zak, K.M.; Grudnik, P.; Magiera, K.; Musielak, B.; Torner, R.; Skalniak, L.; Domling, A.; Dubin, G.; Holak, T.A. Small-molecule inhibitors of the programmed cell death–1/programmed death-ligand 1 (PD–1/PD–L1) interaction via transiently induced protein states and dimerization of PD–L1. J. Med. Chem. 2017, 60, 5857–5867. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, L.; Liang, B.; Zhu, S.; Lv, G.; Qiu, L.; Lin, J. Design, Synthesis, and Biological Evaluation of a Small-Molecule PET Agent for Imaging PD-L1 Expression. Pharmaceuticals 2023, 16, 213. [Google Scholar] [CrossRef]
- Krutzek, F.; Donat, C.K.; Ullrich, M.; Zarschler, K.; Ludik, M.-C.; Feldmann, A.; Loureiro, L.R.; Kopka, K.; Stadlbauer, S. Design and Biological Evaluation of Small-Molecule PET-Tracers for Imaging of Programmed Death Ligand 1. Cancers 2023, 15, 2638. [Google Scholar] [CrossRef]
- Huisman, M.C.; Niemeijer, A.L.N.; Windhorst, A.D.; Schuit, R.C.; Leung, D.; Hayes, W.; Poot, A.; Bahce, I.; Radonic, T.; Oprea-Lager, D.E. Quantification of PD-L1 expression with 18F–BMS–986192 PET/CT in patients with advanced-stage non–small cell lung cancer. J. Nucl. Med. 2020, 61, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, X.; Chen, B.; Xiang, S.; Rao, W.; Zhang, Z.; Liu, H.; Fang, J.; Yin, X.; Deng, P. Preclinical and first-in-human evaluation of 18F–labeled D-peptide antagonist for PD-L1 status imaging with PET. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4312–4324. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jiang, L.; Li, S.-C.; He, Q.-J.; Yang, B.; Cao, J. Small molecule inhibitors targeting the PD-1/PD-L1 signaling pathway. Acta Pharmacol. Sin. 2021, 42, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lu, X.; Luo, G.; Xiang, H. Progress in PD–1/PD–L1 pathway inhibitors: From biomacromolecules to small molecules. Eur. J. Med. Chem. 2020, 186, 111876. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Hsu, J.M.; Yang, W.H.; Hung, M.C. Mechanisms regulating PD-L1 expression in cancers and associated opportunities for novel small–molecule therapeutics. Nat. Rev. Clin. Oncol. 2022, 19, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cai, S.; Cheng, Y.; Zhang, W.; Wang, M.; Sun, H.; Guo, B.; Li, Z.; Xiao, Y.; Jiang, S. Discovery of small-molecule inhibitors of the PD-1/PD-L1 axis that promote PD-L1 internalization and degradation. J. Med. Chem. 2022, 65, 3879–3893. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, S.; Huizinga, H.P.S.; Butera, R.; Kouchi, A.; Guzik, K.; Magiera–Mularz, K.; Holak, T.A.; Domling, A. A patent review on PD-1/PD-L1 antagonists: Small molecules, peptides, and macrocycles (2015–2018). Expert Opin. Ther. Pat. 2018, 28, 665–678. [Google Scholar] [CrossRef]
- Kawashita, S.; Aoyagi, K.; Yamanaka, H.; Hantani, R.; Naruoka, S.; Tanimoto, A.; Hori, Y.; Toyonaga, Y.; Fukushima, K.; Miyazaki, S.; et al. Symmetry–based ligand design and evaluation of small molecule inhibitors of programmed cell death-1/programmed death-ligand 1 interaction. Bioorg. Med. Chem. Lett. 2019, 29, 2464–2467. [Google Scholar] [CrossRef]
- Schottelius, M.; Wester, H.J.; Reubi, J.C.; Senekowitsch–Schmidtke, R.; Schwaiger, M. Improvement of pharmacokinetics of radioiodinated Tyr(3)-octreotide by conjugation with carbohydrates. Bioconjugate Chem. 2002, 13, 1021–1030. [Google Scholar] [CrossRef]
- Penchala, S.C.; Miller, M.R.; Pal, A.; Dong, J.; Madadi, N.R.; Xie, J.; Joo, H.; Tsai, J.; Batoon, P.; Samoshin, V.; et al. A biomimetic approach for enhancing the in vivo half-life of peptides. Nat. Chem. Biol. 2015, 11, 793–798. [Google Scholar] [CrossRef]
- Huang, J.; Fu, L.; Hu, K.; Huang, S.; Han, Y.; Lin, R.; Xu, W.; Tang, G.; Huang, Y. Automatic Production and Preliminary PET Imaging of a New Imaging Agent [(18)F]AlF–FAPT. Front. Oncol. 2021, 11, 802676. [Google Scholar] [CrossRef]

| Organ | 30 min | 60 min | 120 min |
|---|---|---|---|
| Bone | 1.60 ± 0.33 | 1.38 ± 0.20 | 0.88 ± 0.19 |
| Muscle | 1.39 ± 0.13 | 1.09 ± 0.12 | 0.73 ± 0.27 |
| Lung | 8.38 ± 0.54 | 7.16 ± 1.06 | 3.01 ± 0.53 |
| Brain | 0.32 ± 0.03 | 0.25 ± 0.02 | 0.19 ± 0.02 |
| Heart | 3.70 ± 0.08 | 3.10 ± 0.47 | 2.00 ± 0.33 |
| Liver | 3.73 ± 0.25 | 3.76 ± 0.22 | 3.08 ± 0.35 |
| Kidney | 6.42 ± 1.34 | 6.12 ± 0.23 | 3.94± 0.21 |
| Spleen | 2.37 ± 0.18 | 1.84 ± 0.05 | 1.31 ± 0.28 |
| Gall bladder | 1.97 ± 0.96 | 1.10 ± 0.18 | 1.33 ± 0.43 |
| Stomach | 2.86 ± 0.83 | 2.30 ± 0.51 | 1.26 ± 0.10 |
| Intestine | 1.71 ± 0.03 | 1.66 ± 0.33 | 1.71 ± 0.36 |
| Blood | 16.21 ±3.02 | 11.49 ± 1.67 | 7.80 ± 1.14 |
| A549-hPDL1 tumor | 4.61 ± 0.16 | 4.40 ± 0.36 | 4.22 ± 0.65 |
| A549 tumor | - | - | 2.23 ± 0.41 |
| A549-hPDL1 T/M | 3.49 ± 0.21 | 4.32 ± 0.84 | 5.78 ± 0.60 |
| A549-hPDL1 T/L | 0.58 ± 0.05 | 0.65 ± 0.07 | 1.26 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Li, C.; Li, Z.; Wang, Q.; Huang, S.; Liu, Q.; Liang, Y. Development and Preclinical Evaluation of [68Ga]BMSH as a New Potent Positron Emission Tomography Tracer for Imaging Programmed Death-Ligand 1 Expression. Pharmaceuticals 2023, 16, 1487. https://doi.org/10.3390/ph16101487
Huang Y, Li C, Li Z, Wang Q, Huang S, Liu Q, Liang Y. Development and Preclinical Evaluation of [68Ga]BMSH as a New Potent Positron Emission Tomography Tracer for Imaging Programmed Death-Ligand 1 Expression. Pharmaceuticals. 2023; 16(10):1487. https://doi.org/10.3390/ph16101487
Chicago/Turabian StyleHuang, Yong, Chengze Li, Zhongjing Li, Qiong Wang, Size Huang, Qi Liu, and Ying Liang. 2023. "Development and Preclinical Evaluation of [68Ga]BMSH as a New Potent Positron Emission Tomography Tracer for Imaging Programmed Death-Ligand 1 Expression" Pharmaceuticals 16, no. 10: 1487. https://doi.org/10.3390/ph16101487
APA StyleHuang, Y., Li, C., Li, Z., Wang, Q., Huang, S., Liu, Q., & Liang, Y. (2023). Development and Preclinical Evaluation of [68Ga]BMSH as a New Potent Positron Emission Tomography Tracer for Imaging Programmed Death-Ligand 1 Expression. Pharmaceuticals, 16(10), 1487. https://doi.org/10.3390/ph16101487







