Plausible Protective Role of Encephalartos villosus Extract in Acetic-Acid-Induced Ulcerative Colitis in Rats
Abstract
:1. Introduction
2. Results
2.1. HPLC Analysis
2.2. Colon Weight
2.3. Colon Length
2.4. Colon Weight/Length Ratio
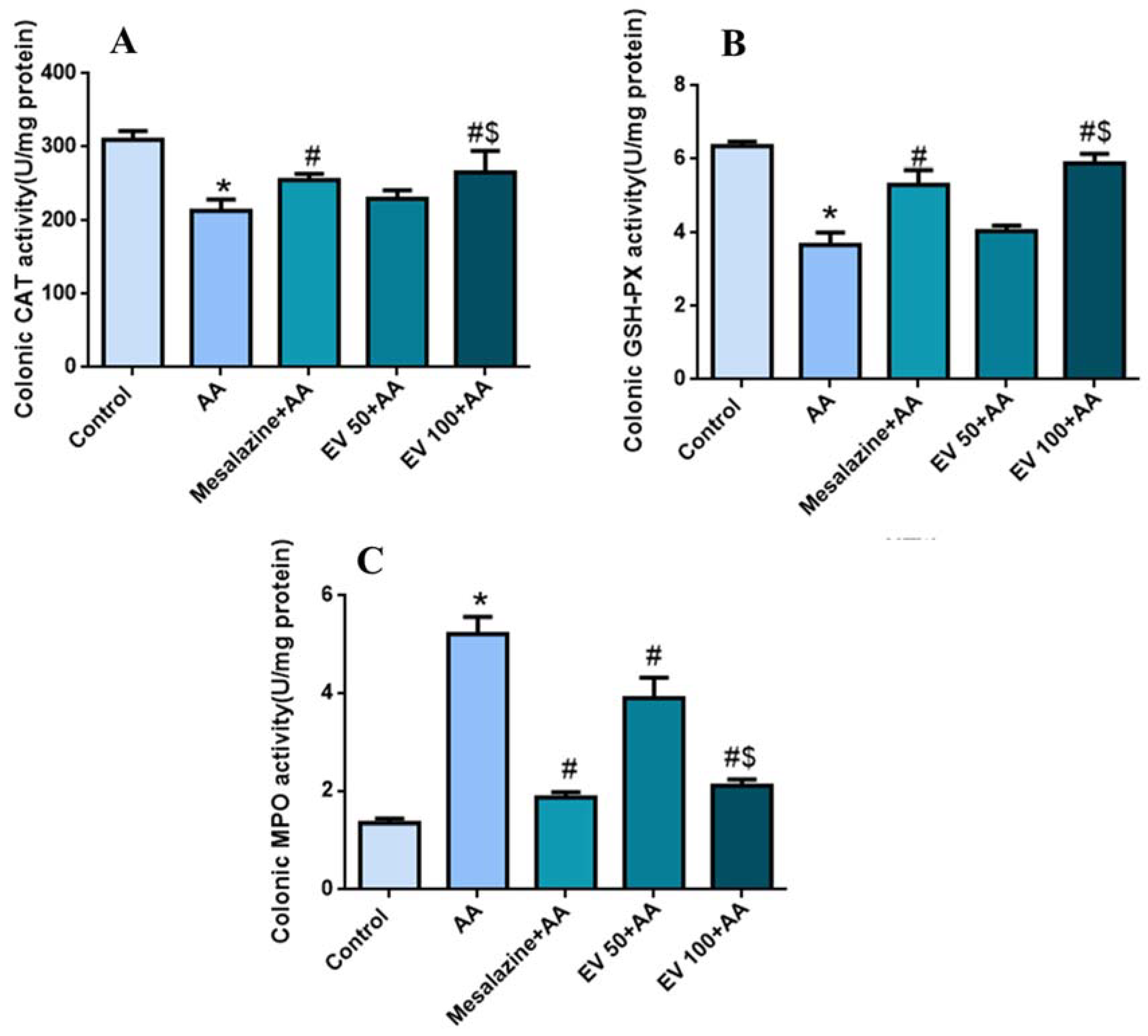
2.5. Colon Catalase (CAT) Activity
2.6. Colon Glutathione Peroxidase (GSH-PX) Activity
2.7. Colon Myeloperoxidase (MPO) Activity
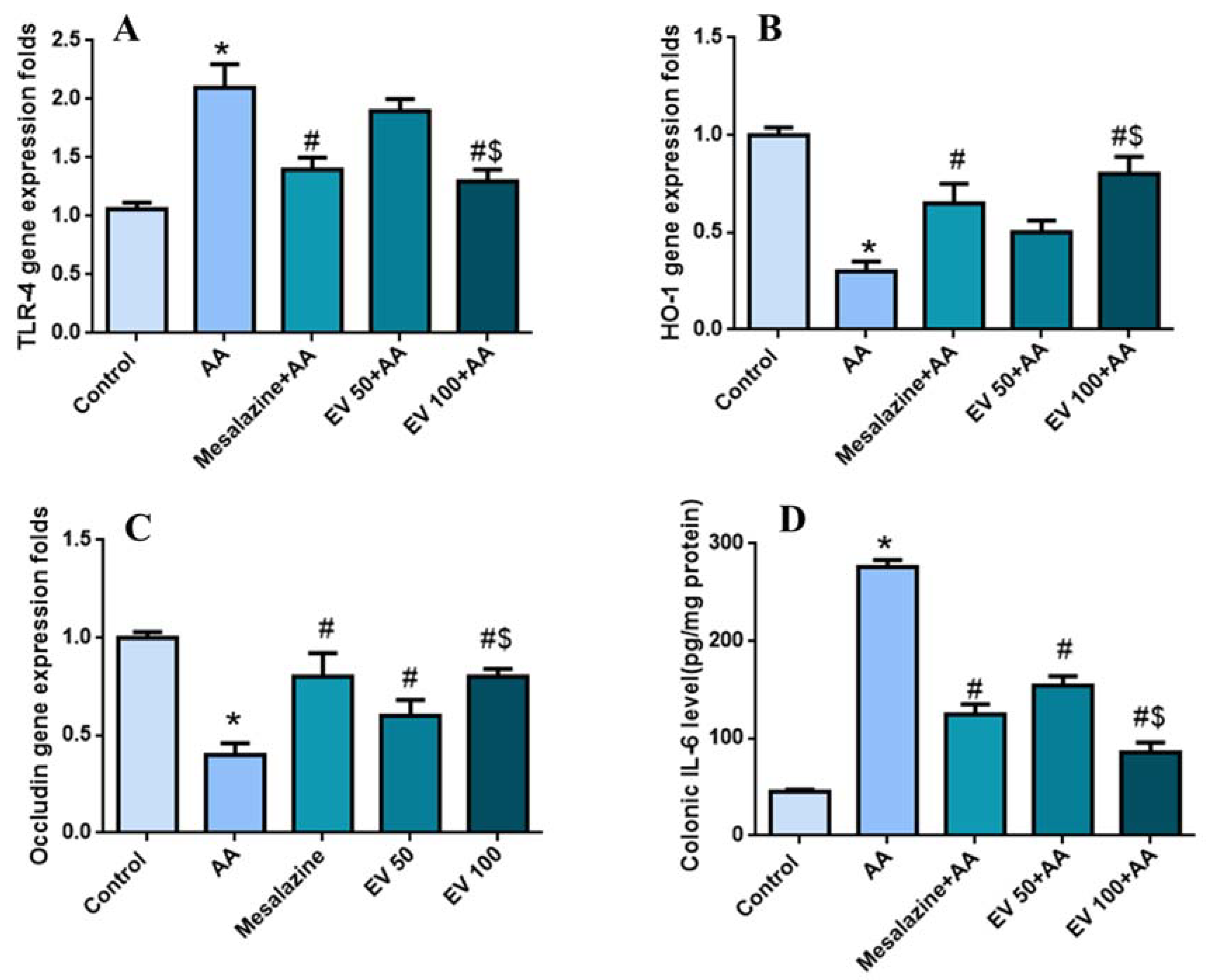
2.8. Colon Gene Expression of TLR-4, Hemoxygenase (HO-1), and Occludin
2.9. Colon Interleukin-6 (IL-6) Level
2.10. Histopathological Examination
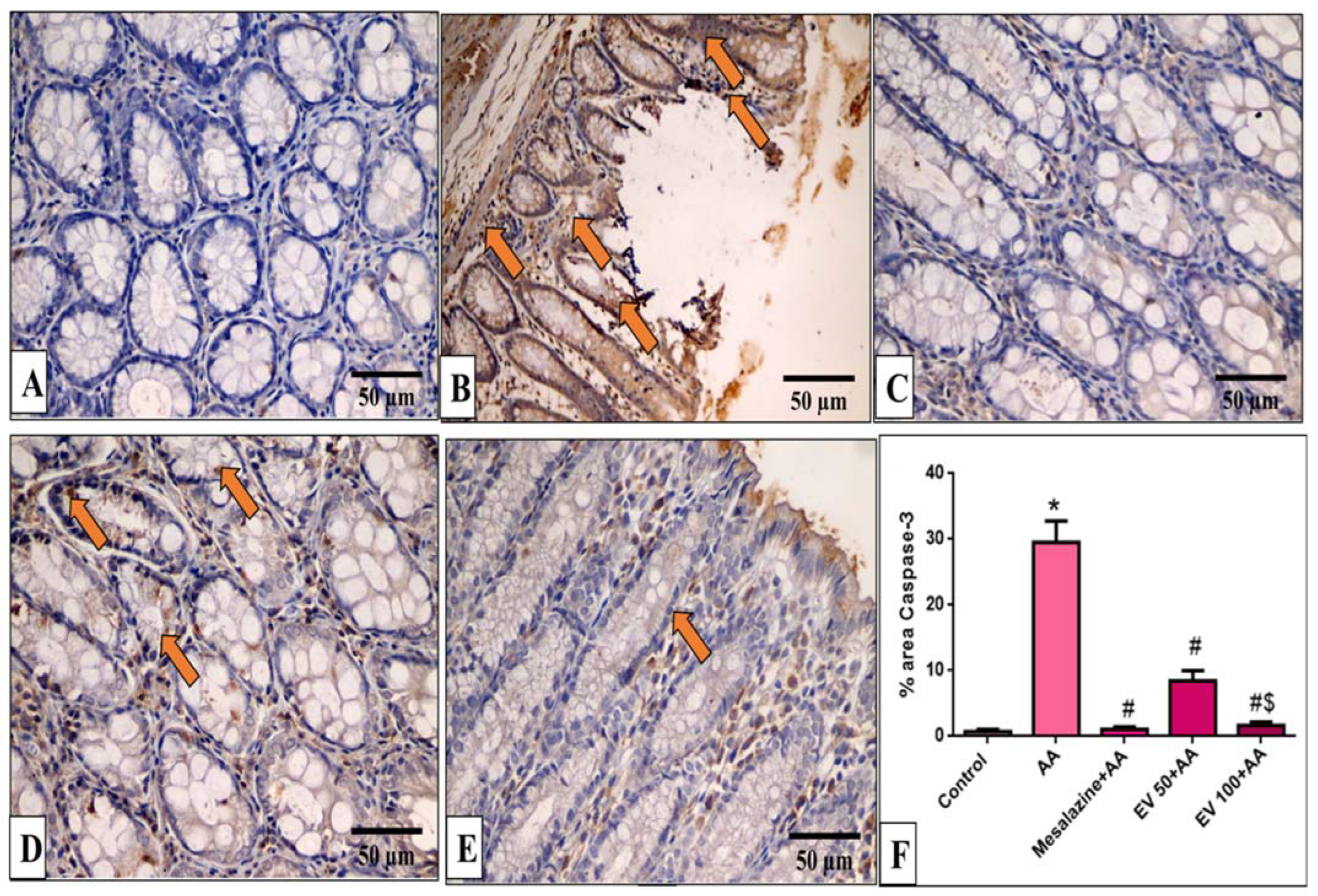
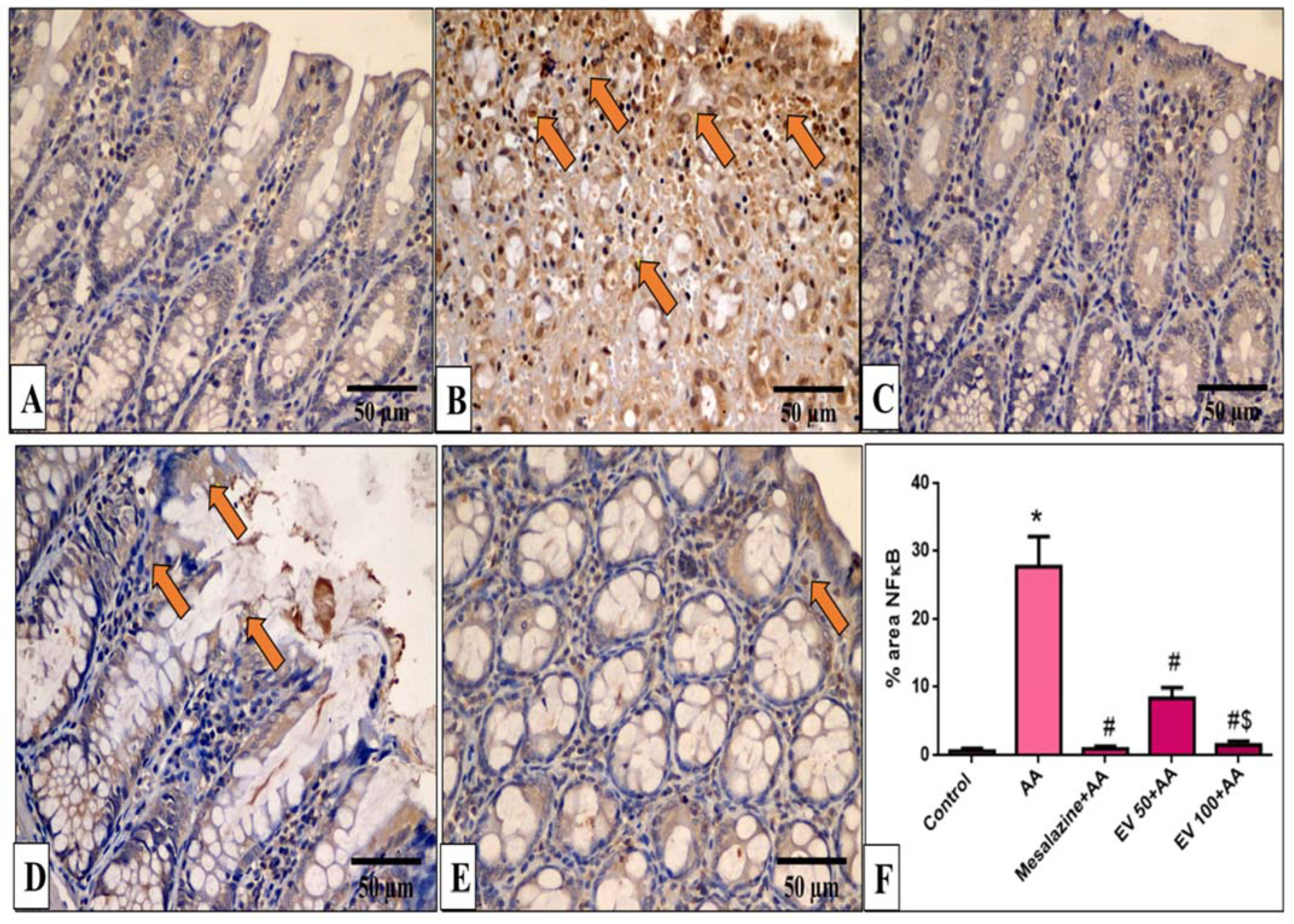
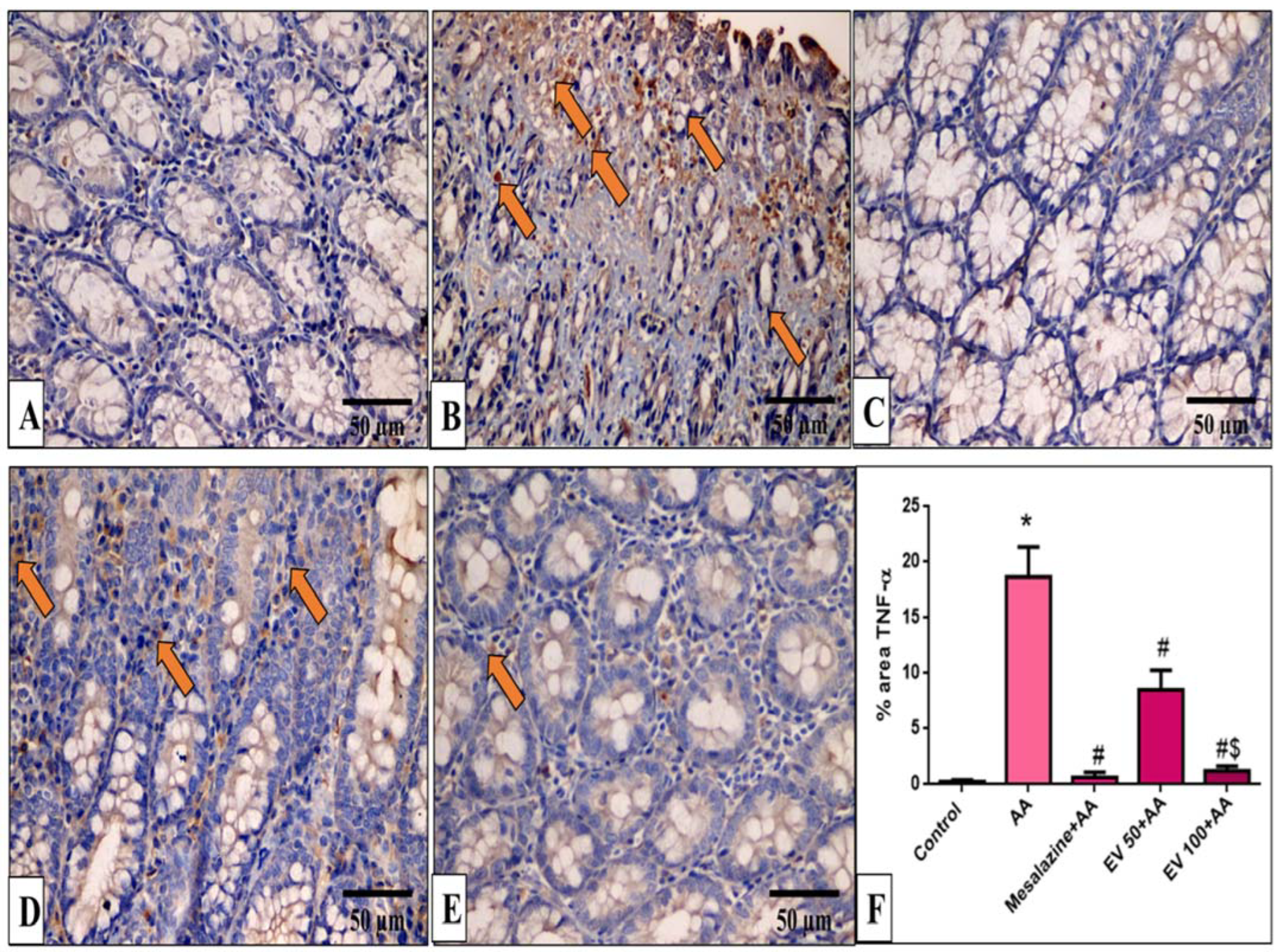
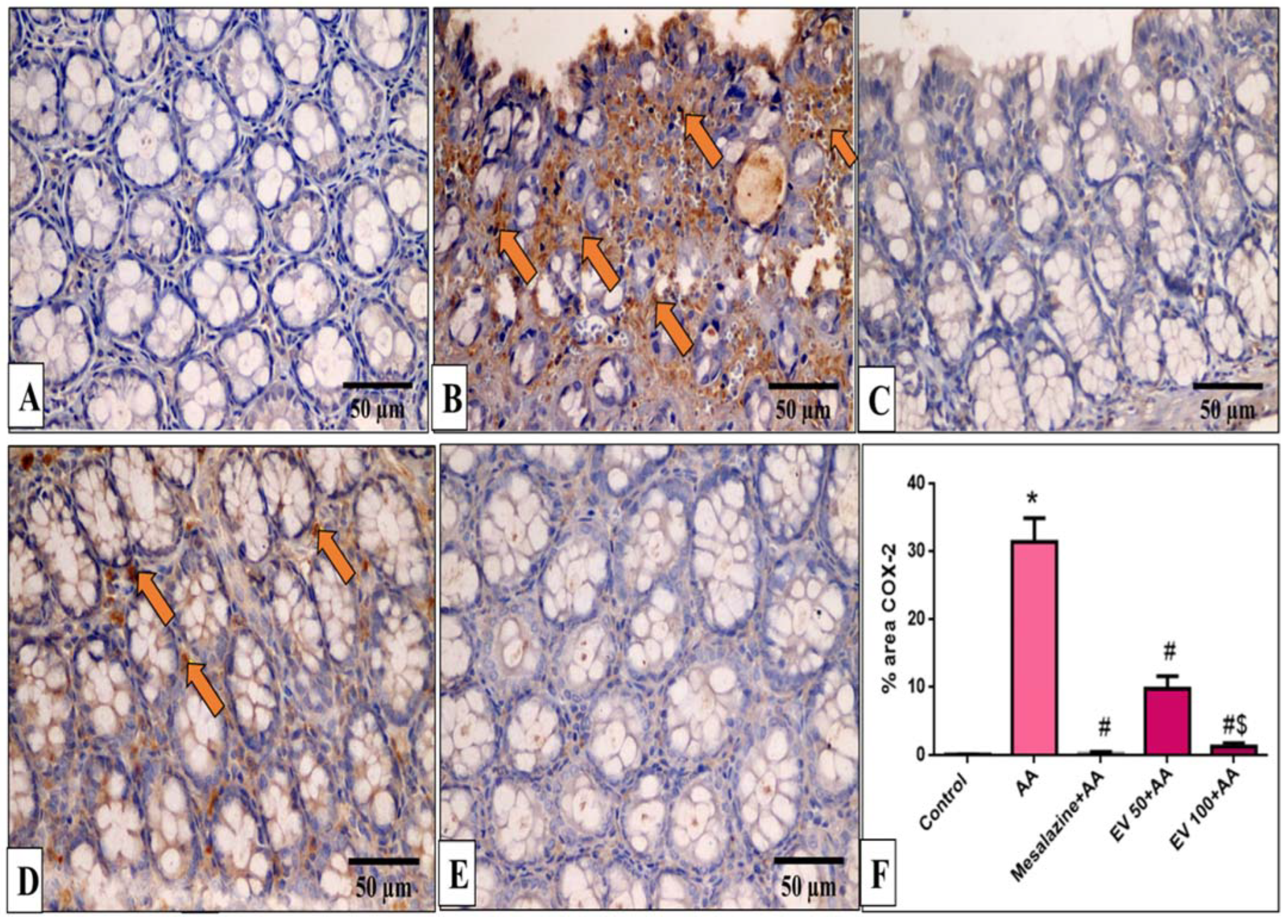
2.11. Immunohistochemical Features of Caspase-3, NF-kB, TNF-α, and COX-2
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Plants, Drugs, and Chemicals
4.2.1. Encephalartos villosus Extract Preparation
4.2.2. High-Performance Liquid Chromatography (HPLC) Analysis
4.3. Experimental Procedure
4.3.1. Induction of UC
4.3.2. Design
4.3.3. Tissue Collection
4.4. Colorimetric Determination of Colon Catalase and Glutathione Peroxidase
4.5. Colorimetric Determination of the Colon Myeloperoxidase Activity
4.6. ELISA for Interlukin-6 Levels
4.7. Quantitative Real-Time (qRT-PCR) for TLR-4, Heme Oxygenase-1, and Occludin
4.8. Histopathology of Colon Sections
4.9. Immunohistochemical Detection of Caspase-3, NF-kB, TNF-α, and COX-2
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molodecky, N.A.; Soon, S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Pallone, F.; MacDonald, T.T. Emerging immunological targets in inflammatory bowel disease. Curr. Opin. Pharmacol. 2011, 11, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef] [PubMed]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like receptors activation, signaling, and targeting: An overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Ke, X.; Zhou, F.; Gao, Y.; Xie, B.; Hu, G.; Fang, W.; Peng, J.; Chen, Y.; Sferra, T.J. Qing Hua Chang Yin exerts therapeutic effects against ulcerative colitis through the inhibition of the TLR4/NF-κB pathway. Int. J. Mol. Med. 2013, 32, 926–930. [Google Scholar] [CrossRef]
- Lin, S.; Yin, Q.; Zhong, Q.; Lv, F.-L.; Zhou, Y.; Li, J.-Q.; Wang, J.-Z.; Su, B.-Y.; Yang, Q.-W. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J. Neuroinflamm. 2012, 9, 46. [Google Scholar] [CrossRef]
- Ahmed, S.; Dewan, M.Z.; Xu, R. Nuclear factor-kappaB in inflammatory bowel disease and colorectal cancer. Am. J. Dig. Dis 2014, 1, 84–96. [Google Scholar]
- Laurindo, L.F.; Santos, A.R.d.O.d.; Carvalho, A.C.A.d.; Bechara, M.D.; Guiguer, E.L.; Goulart, R.d.A.; Vargas Sinatora, R.; Araújo, A.C.; Barbalho, S.M. Phytochemicals and Regulation of NF-kB in Inflammatory Bowel Diseases: An Overview of In Vitro and In Vivo Effects. Metabolites 2023, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Le Loupp, A.-G.; Bach-Ngohou, K.; Bourreille, A.; Boudin, H.; Rolli-Derkinderen, M.; Denis, M.G.; Neunlist, M.; Masson, D. Activation of the prostaglandin D2 metabolic pathway in Crohn’s disease: Involvement of the enteric nervous system. BMC Gastroenterol. 2015, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; DuBois, R.N. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 2010, 29, 781–788. [Google Scholar] [CrossRef]
- Piechota-Polanczyk, A.; Fichna, J. The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive oxygen species: Drivers of physiological and pathological processes. J. Inflamm. Res. 2020, 10, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Goel, S.; Ghatule, R.; Singh, A.; Nath, G.; Goel, R. Curative effect of Terminalia chebula extract on acetic acid-induced experimental colitis: Role of antioxidants, free radicals and acute inflammatory marker. Inflammopharmacology 2013, 21, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wei, W.; Liang, S.; Fang, H.; Cao, J. Esculentoside A could attenuate apoptosis and inflammation in TNBS-induced ulcerative colitis via inhibiting the nuclear translocation of NF-κB. Ann. Transl. Med. 2022, 10, 771. [Google Scholar] [CrossRef]
- Raish, M.; Shahid, M.; Bin Jardan, Y.A.; Ansari, M.A.; Alkharfy, K.M.; Ahad, A.; Abdelrahman, I.A.; Ahmad, A.; Al-Jenoobi, F.I. Gastroprotective effect of sinapic acid on ethanol-induced gastric ulcers in rats: Involvement of Nrf2/HO-1 and NF-κB signaling and antiapoptotic role. Front. Pharmacol. 2021, 12, 622815. [Google Scholar] [CrossRef] [PubMed]
- Celiberto, L.S.; Graef, F.A.; Healey, G.R.; Bosman, E.S.; Jacobson, K.; Sly, L.M.; Vallance, B.A. Inflammatory bowel disease and immunonutrition: Novel therapeutic approaches through modulation of diet and the gut microbiome. Immunology 2018, 155, 36–52. [Google Scholar] [CrossRef]
- Temraz, A. Novel illudalane sesquiterpenes from Encephalartos villosus Lehm. antimicrobial activity. Nat. Prod. Res. 2016, 30, 2791–2797. [Google Scholar] [CrossRef]
- Aleisa, A.M.; Al-Rejaie, S.S.; Abuohashish, H.M.; Ola, M.S.; Parmar, M.Y.; Ahmed, M.M. Pretreatment of Gymnema sylvestre revealed the protection against acetic acid-induced ulcerative colitis in rats. BMC Complement. Altern. Med. 2014, 14, 49. [Google Scholar] [CrossRef]
- Chen, M.L.; Sundrud, M.S. Cytokine networks and T-cell subsets in inflammatory bowel diseases. Inflamm. Bowel Dis. 2016, 22, 1157–1167. [Google Scholar] [CrossRef]
- Vishwakarma, N.; Ganeshpurkar, A.; Pandey, V.; Dubey, N.; Bansal, D. Mesalazine–probiotics beads for acetic acid experimental colitis: Formulation and characterization of a promising new therapeutic strategy for ulcerative colitis. Drug Deliv. 2015, 22, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.J.; Elekhnawy, E.; Negm, W.A.; Mahgoub, S.; Hussein, I.A. Encephalartos villosus Lem. Displays a strong in vivo and in vitro antifungal potential against Candida glabrata clinical isolates. J. Fungi 2022, 8, 521. [Google Scholar] [CrossRef] [PubMed]
- Dejban, P.; Sahraei, M.; Chamanara, M.; Dehpour, A.; Rashidian, A. Anti-inflammatory effect of amitriptyline in a rat model of acetic acid-induced colitis: The involvement of the TLR4/NF-kB signaling pathway. Fundam. Clin. Pharmacol. 2021, 35, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Hasan Khudhair, D.; Al-Gareeb, A.I.; Al-Kuraishy, H.M.; El-Kadem, A.H.; Elekhnawy, E.; Negm, W.A.; Saber, S.; Cavalu, S.; Tirla, A.; Alotaibi, S.S. Combination of vitamin C and curcumin safeguards against methotrexate-induced acute liver injury in mice by synergistic antioxidant effects. Front. Med. 2022, 9, 866343. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhan, L.; Liao, H.; Chen, L.; Lv, X. Curcumin improves TNBS-induced colitis in rats by inhibiting IL-27 expression via the TLR4/NF-κB signaling pathway. Planta Medica 2013, 29, 102–109. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, B.; Lee, H.-S.; Bae, E.-A.; Lee, H.; Ahn, Y.-T.; Lim, K.-S.; Huh, C.-S.; Kim, D.-H. Lactobacillus suntoryeus inhibits pro-inflammatory cytokine expression and TLR-4-linked NF-κB activation in experimental colitis. Int. J. Color. Dis. 2009, 24, 231–237. [Google Scholar] [CrossRef]
- Momtaz, S.; Navabakhsh, M.; Bakouee, N.; Dehnamaki, M.; Rahimifard, M.; Baeeri, M.; Abdollahi, A.; Abdollahi, M.; Farzaei, M.H.; Abdolghaffari, A.H. Cinnamaldehyde targets TLR-4 and inflammatory mediators in acetic-acid induced ulcerative colitis model. Biologia 2021, 76, 1817–1827. [Google Scholar] [CrossRef]
- Almukainzi, M.; A El-Masry, T.; A Negm, W.; Elekhnawy, E.; Saleh, A.; E Sayed, A.; A Khattab, M.; H Abdelkader, D. Gentiopicroside PLGA nanospheres: Fabrication, in vitro characterization, antimicrobial action, and in vivo effect for enhancing wound healing in diabetic rats. Int. J. Nanomed. 2022, 22, 1203–1225. [Google Scholar] [CrossRef]
- Qu, Y.; Li, X.; Xu, F.; Zhao, S.; Wu, X.; Wang, Y.; Xie, J. Kaempferol alleviates murine experimental colitis by restoring gut microbiota and inhibiting the LPS-TLR4-NF-κB axis. Front. Immunol. 2021, 12, 679897. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.; Silva, A.M. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Chaen, Y.; Yamamoto, Y.; Suzuki, T. Naringenin promotes recovery from colonic damage through suppression of epithelial tumor necrosis factor–α production and induction of M2-type macrophages in colitic mice. Nutr. Res. 2019, 64, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Wu, X.; Guo, H.; Pan, X.; Huang, R.; Wang, G.; Liu, J. Naringin exhibited therapeutic effects against DSS-induced mice ulcerative colitis in intestinal barrier–dependent manner. Molecules 2021, 26, 6604. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Zhang, J.; Sun, A.; Zhang, E.; Ding, L.; Mukherjee, S.; Wei, X.; Chou, G.; Wang, Z.-T.; Mani, S. Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κB signalling. Br. J. Nutr. 2013, 110, 599–608. [Google Scholar] [CrossRef]
- Romier, B.; Schneider, Y.-J.; Larondelle, Y.; During, A. Dietary polyphenols can modulate the intestinal inflammatory response. Nutr. Rev. 2009, 67, 363–378. [Google Scholar] [CrossRef]
- Shi, L.; Dai, Y.; Jia, B.; Han, Y.; Guo, Y.; Xie, T.; Liu, J.; Tan, X.; Ding, P.; Li, J. The inhibitory effects of Qingchang Wenzhong granule on the interactive network of inflammation, oxidative stress, and apoptosis in rats with dextran sulfate sodium-induced colitis. J. Cell. Biochem. 2019, 120, 9979–9991. [Google Scholar] [CrossRef]
- Negm, W.A.; El-Kadem, A.H.; Elekhnawy, E.; Attallah, N.G.; Al-Hamoud, G.A.; El-Masry, T.A.; Zayed, A. Wound-Healing Potential of Rhoifolin-Rich Fraction Isolated from Sanguisorba officinalis Roots Supported by Enhancing Re-Epithelization, Angiogenesis, Anti-Inflammatory, and Antimicrobial Effects. Pharmaceuticals 2022, 15, 178. [Google Scholar] [CrossRef] [PubMed]
- Attallah, N.G.; El-Sherbeni, S.A.; El-Kadem, A.H.; Elekhnawy, E.; El-Masry, T.A.; Elmongy, E.I.; Altwaijry, N.; Negm, W.A. elucidation of the metabolite profile of Yucca gigantea and assessment of its cytotoxic, antimicrobial, and anti-inflammatory activities. Molecules 2022, 27, 1329. [Google Scholar] [CrossRef]
- Ramadan, A.; Afifi, N.; Yassin, N.Z.; Abdel-Rahman, R.F.; Abd El-Rahman, S.S.; Fayed, H.M. Mesalazine, an osteopontin inhibitor: The potential prophylactic and remedial roles in induced liver fibrosis in rats. Chem.-Biol. Interact. 2018, 289, 109–118. [Google Scholar] [CrossRef]
- Attallah, N.G.; El-Kadem, A.H.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Elmongy, E.I.; Altwaijry, N.; Alanazi, A.S.; Al-Hamoud, G.A.; Ragab, A.E. Promising Antiviral Activity of Agrimonia pilosa Phytochemicals against Severe Acute Respiratory Syndrome Coronavirus 2 Supported with In Vivo Mice Study. Pharmaceuticals 2021, 14, 1313. [Google Scholar] [CrossRef]
- Arab, H.H.; Al-Shorbagy, M.Y.; Abdallah, D.M.; Nassar, N.N. Telmisartan attenuates colon inflammation, oxidative perturbations and apoptosis in a rat model of experimental inflammatory bowel disease. PLoS ONE 2014, 9, e97193. [Google Scholar] [CrossRef]
- Hussein, S.Z.; Mohd Yusoff, K.; Makpol, S.; Mohd Yusof, Y.A. Gelam honey inhibits the production of proinflammatory, mediators NO, PGE2, TNF-α, and IL-6 in carrageenan-induced acute paw edema in rats. Evid. Based Complement. Altern. Med. 2012, 2012, 109636. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Grover, J.; Jachak, S.M. Coumarins as privileged scaffold for anti-inflammatory drug development. RSC Adv. 2015, 5, 38892–38905. [Google Scholar] [CrossRef]
- Raish, M.; Ahmad, A.; Jardan, Y.A.B.; Shahid, M.; Alkharfy, K.M.; Ahad, A.; Ansari, M.A.; Abdelrahman, I.A.; Al-Jenoobi, F.I. Sinapic acid ameliorates cardiac dysfunction and cardiomyopathy by modulating NF-κB and Nrf2/HO-1 signaling pathways in streptozocin induced diabetic rats. Biomed. Pharmacother. 2022, 145, 112412. [Google Scholar] [CrossRef]
- Ali, A.A.; Abd Al Haleem, E.N.; Khaleel, S.A.-H.; Sallam, A.S. Protective effect of cardamonin against acetic acid-induced ulcerative colitis in rats. Pharmacol. Rep. 2017, 69, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Luissint, A.-C.; Parkos, C.A.; Nusrat, A. Inflammation and the intestinal barrier: Leukocyte–epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 2016, 151, 616–632. [Google Scholar] [CrossRef] [PubMed]
- Almero, J.M. Influence of Toll-like receptor 2 and interleukin 10 on the intestinal epithelial barrier and their roles in inflammatory bowel disease. Inmunología 2011, 30, 8–16. [Google Scholar] [CrossRef]
- Albert-Garay, J.S.; Riesgo-Escovar, J.R.; Salceda, R. High glucose concentrations induce oxidative stress by inhibiting Nrf2 expression in rat Müller retinal cells in vitro. Sci. Rep. 2022, 12, 1261. [Google Scholar] [CrossRef]
- Wu, M.-L.; Ho, Y.-C.; Lin, C.-Y.; Yet, S.-F. Heme oxygenase-1 in inflammation and cardiovascular disease. Am. J. Cardiovasc. Dis. 2011, 1, 150. [Google Scholar] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A.; Zhang, M.; Yin, F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharmacol. 2012, 3, 119. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Yeh, C.-H.; Yang, M.-L.; Kuan, Y.-H. Luteolin suppresses inflammatory mediator expression by blocking the Akt/NFκB pathway in acute lung injury induced by lipopolysaccharide in mice. Evid. Based Complement. Altern. Med. 2012, 2012, 383608. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.-H.; Weng, J.-R.; Tsao, L.-T.; Yen, M.-H.; Wang, J.-P.; Lin, C.-N. Anti-inflammatory flavonoids and pterocarpanoid from Crotalaria pallida and C. assamica. Bioorg. Med. Chem. Lett. 2004, 14, 1011–1014. [Google Scholar] [CrossRef]
- Buchmann, D.; Schultze, N.; Borchardt, J.; Böttcher, I.; Schaufler, K.; Guenther, S. Synergistic antimicrobial activities of epigallocatechin gallate, myricetin, daidzein, gallic acid, epicatechin, 3-hydroxy-6-methoxyflavone and genistein combined with antibiotics against ESKAPE pathogens. J. Appl. Microbiol. 2022, 132, 949–963. [Google Scholar] [CrossRef]
- El-Akabawy, G.; El-Sherif, N.M. Zeaxanthin exerts protective effects on acetic acid-induced colitis in rats via modulation of pro-inflammatory cytokines and oxidative stress. Biomed. Pharmacother. 2019, 111, 841–851. [Google Scholar] [CrossRef]
- Attallah, N.G.; Kabbash, A.; Negm, W.A.; Elekhnawy, E.; Binsuwaidan, R.; Al-Fakhrany, O.M.; Shaldam, M.A.; Moglad, E.; Tarek, M.; Samir, N. Protective Potential of Saussurea costus (Falc.) Lipsch. Roots against Cyclophosphamide-Induced Pulmonary Injury in Rats and Its In Vitro Antiviral Effect. Pharmaceuticals 2023, 16, 318. [Google Scholar] [CrossRef]
- Bezerra, G.B.; de Souza, L.d.M.; Dos Santos, A.S.; de Almeida, G.K.M.; Souza, M.T.S.; Santos, S.L.; Camargo, E.A.; dos Santos Lima, B.; de Souza Araújo, A.A.; Cardoso, J.C. Hydroalcoholic extract of Brazilian red propolis exerts protective effects on acetic acid-induced ulcerative colitis in a rodent model. Biomed. Pharmacother. 2017, 85, 687–696. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Cao, X.-N.; Wang, G.-H.; Hu, J.-B.; Wang, J. Efficacy of topical versus oral 5-aminosalicylate for treatment of 2, 4, 6-trinitrobenzene sulfonic acid-induced ulcerative colitis in rats. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 59–65. [Google Scholar] [CrossRef]
- Abdin, A.A. Targeting sphingosine kinase 1 (SphK1) and apoptosis by colon-specific delivery formula of resveratrol in treatment of experimental ulcerative colitis in rats. Eur. J. Pharmacol. 2013, 718, 145–153. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Jeon, H.; Bae, C.H.; Lee, Y.; Kim, H.; Kim, S. Rumex japonicus Houtt. alleviates dextran sulfate sodium-induced colitis by protecting tight junctions in mice. Integr. Med. Res. 2020, 9, 100398. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, B.; El-Masry, T.A.; Elekhnawy, E.; El-Kadem, A.H.; Saleh, A.; Negm, W.A.; Abdelkader, D.H. Aqueous core epigallocatechin gallate PLGA nanocapsules: Characterization, antibacterial activity against uropathogens, and in vivo reno-protective effect in cisplatin induced nephrotoxicity. Drug Deliv. 2022, 29, 1848–1862. [Google Scholar] [CrossRef] [PubMed]
- Elekhnawy, E.; Negm, W.A. The potential application of probiotics for the prevention and treatment of COVID-19. Egypt. J. Med. Hum. Genet. 2022, 23, 36. [Google Scholar] [CrossRef]
- Sivakumar, M.; Yoithapprabhunath, T.R.; Nirmal, R.M.; Veeravarmal, V.; Dineshshankar, J.; Amsaveni, R. Immunohistochemical analysis of Nuclear Factor-kappa B (NF-κB) between follicular and plexiform ameloblastomas: A pilot study. J. Oral Maxillofac. Pathol. JOMFP 2020, 24, 466. [Google Scholar]
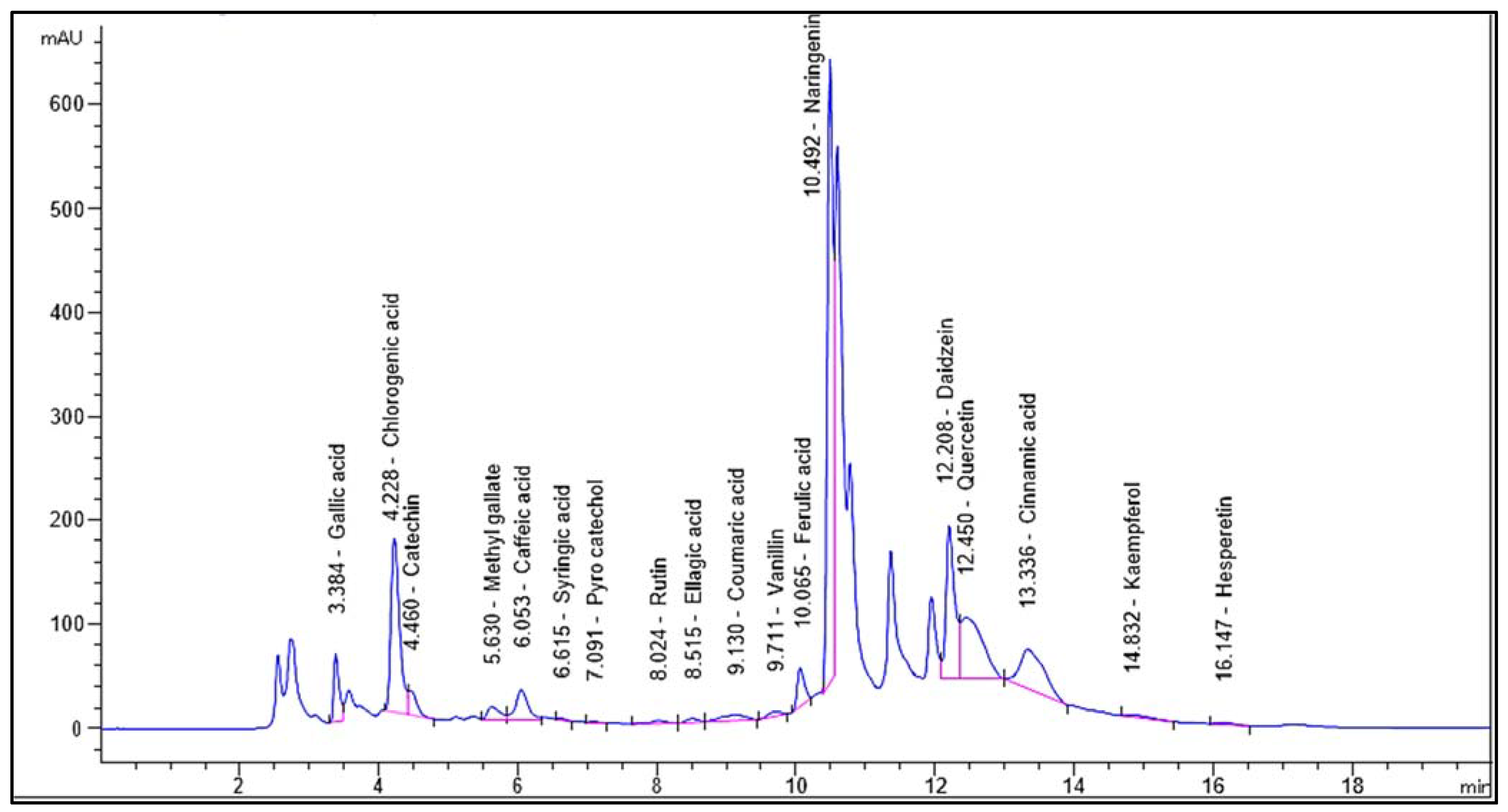

| Identified Compound | RT (Min) | Area % | Area [mAU*s] | Conc. (µg/g) |
|---|---|---|---|---|
| Gallic acid | 3.384 | 3.4543 | 345.99289 | 1008.68 |
| Chlorogenic acid | 4.228 | 14.5071 | 1453.08276 | 7595.27 |
| Catechin | 4.460 | 1.8801 | 188.31728 | 1883.93 |
| Methyl-gallate | 5.630 | 1.4192 | 142.15103 | 333.51 |
| Caffeic acid | 6.053 | 3.3520 | 335.75253 | 926.40 |
| Syringic acid | 6.615 | 0.0768 | 7.68790 | 22.68 |
| Pyrocatechol | 7.091 | 0.0551 | 5.51670 | 28.18 |
| Rutin | 8.024 | 0.3438 | 34.43813 | 91.14 |
| Ellagic acid | 8.515 | 0.4069 | 40.75592 | 508.97 |
| Coumaric acid | 9.130 | 1.3329 | 133.50352 | 157.58 |
| Vanillin | 9.711 | 0.5434 | 54.42558 | 74.84 |
| Ferulic acid | 10.065 | 2.5549 | 255.90369 | 580.48 |
| Naringenin | 10.492 | 34.7159 | 3477.27783 | 11,476.89 |
| Daidzein | 12.208 | 13.0087 | 1302.99866 | 3239.40 |
| Quercetin | 12.450 | 12.3932 | 1241.34583 | 5485.34 |
| Cinnamic acid | 13.336 | 9.1824 | 919.74677 | 702.02 |
| Apigenin | 14.496 | ND | ND | ND |
| Kaempferol | 14.832 | 0.4681 | 46.88348 | 179.35 |
| Hesperetin | 16.147 | 0.3054 | 30.59113 | 64.45 |
| Colon Weight (gm) | Colon Length (cm) | Colon Weight/Length Ratio (gm/cm) | |
|---|---|---|---|
| Control | 1.136 ± 0.092 | 12.9 ± 0.42 | 8.8 ± 0.57 |
| AA | 1.418 ± 0.143 * | 10.1 ± 0.74 * | 14.14 ± 2.27 * |
| Mesalazine + AA | 1.064 ± 0.1 # | 12 ± 0.8 # | 8.89 ± 1.03 # |
| EV 50 + AA | 1.23 ± 0.08 | 10.6 ± 0.65 | 11.61 ± 0.93 # |
| EV 100 + AA | 1.122 ± 0.098 # | 12.58 ± 0.94 #$ | 8.98 ± 0.6 #$ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, A.S.; Alanazi, M.M.; Elekhnawy, E.; Attallah, N.G.M.; Negm, W.A.; El-Kadem, A.H. Plausible Protective Role of Encephalartos villosus Extract in Acetic-Acid-Induced Ulcerative Colitis in Rats. Pharmaceuticals 2023, 16, 1431. https://doi.org/10.3390/ph16101431
Alanazi AS, Alanazi MM, Elekhnawy E, Attallah NGM, Negm WA, El-Kadem AH. Plausible Protective Role of Encephalartos villosus Extract in Acetic-Acid-Induced Ulcerative Colitis in Rats. Pharmaceuticals. 2023; 16(10):1431. https://doi.org/10.3390/ph16101431
Chicago/Turabian StyleAlanazi, Ashwag S., Mohammed M. Alanazi, Engy Elekhnawy, Nashwah G. M. Attallah, Walaa A. Negm, and Aya H. El-Kadem. 2023. "Plausible Protective Role of Encephalartos villosus Extract in Acetic-Acid-Induced Ulcerative Colitis in Rats" Pharmaceuticals 16, no. 10: 1431. https://doi.org/10.3390/ph16101431
APA StyleAlanazi, A. S., Alanazi, M. M., Elekhnawy, E., Attallah, N. G. M., Negm, W. A., & El-Kadem, A. H. (2023). Plausible Protective Role of Encephalartos villosus Extract in Acetic-Acid-Induced Ulcerative Colitis in Rats. Pharmaceuticals, 16(10), 1431. https://doi.org/10.3390/ph16101431










