Pharmacological Evaluation of Signals of Disproportionality Reporting Related to Adverse Reactions to Antiepileptic Cannabidiol in VigiBase
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Reports
2.2. Disproportionality Analysis
2.3. Characteristics of Insomnia Reports
3. Discussion
4. Material and Methods
4.1. Case of Interest Definition
4.2. Exposure Definition
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBD | cannabidiol |
| CB1 | cannabinoid receptors 1 |
| CB2 | cannabinoid receptor 2 |
| CI | confidence interval |
| ENT-1 | equilibrative nucleoside transporter type 1 |
| FAAH | fatty-acid amide hydrolase 1 |
| GPR55 | G protein-coupled receptor 55 |
| HLT | Higher-Level Terms |
| 8-OH-DPAT | 8-hydroxy-2-(di-n-propilamino)tetralin |
| IC | information component |
| ICSRs | individual case safety report |
| MedDRA | Medical Dictionary for Regulatory Activities |
| PTs | Preferred Terms |
| READUS-PV | Reporting of A Disproportionality analysis for drUg Safety signal detection using spontaneously reported adverse events in Pharmacovigilance |
| ROR | Reporting odds ratio |
| SSRIs | selective serotonin reuptake inhibitors |
| THC | delta-9-tetrahydrocannabinol |
| TRPV | transient receptor potential vanilloid |
| UMC | Uppsala Monitoring Centre |
References
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar] [CrossRef]
- Rosenberg, E.C.; Tsien, R.W.; Whalley, B.J.; Devinsky, O. Cannabinoids and Epilepsy. Neurotherapeutics 2015, 12, 747–768. [Google Scholar] [CrossRef] [PubMed]
- Galaj, E.; Xi, Z.X. Possible Receptor Mechanisms Underlying Cannabidiol Effects on Addictive-like Behaviors in Experimental Animals. Int. J. Mol. Sci. 2020, 22, 134. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, D.L.; Devi, L.A. Diversity of molecular targets and signaling pathways for CBD. Pharmacol. Res. Perspect. 2020, 8, e00682. [Google Scholar] [CrossRef]
- Freeman, T.P.; Craft, S.; Wilson, J.; Stylianou, S.; ElSohly, M.; Di Forti, M.; Lynskey, M.T. Changes in delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: Systematic review and meta-analysis. Addiction 2021, 116, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Puchalski, K.; Shokoohinia, Y.; Zolfaghari, B.; Asgary, S. Differentiating Cannabis Products: Drugs, Food, and Supplements. Front. Pharmacol. 2022, 13, 906038. [Google Scholar] [CrossRef] [PubMed]
- Costa, B. On the pharmacological properties of Delta9-tetrahydrocannabinol (THC). Chem. Biodivers. 2007, 4, 1664–1677. [Google Scholar] [CrossRef]
- Calapai, F.; Cardia, L.; Sorbara, E.E.; Navarra, M.; Gangemi, S.; Calapai, G.; Mannucci, C. Cannabinoids, Blood-Brain Barrier, and Brain Disposition. Pharmaceutics 2020, 12, 265. [Google Scholar] [CrossRef]
- Britch, S.C.; Babalonis, S.; Walsh, S.L. Cannabidiol: Pharmacology and therapeutic targets. Psychopharmacology 2021, 238, 9–28. [Google Scholar] [CrossRef]
- Mannucci, C.; Navarra, M.; Calapai, F.; Spagnolo, E.V.; Busardò, F.P.; Cas, R.D.; Ippolito, F.M.; Calapai, G. Neurological Aspects of Medical Use of Cannabidiol. CNS Neurol. Disord. Drug Targets 2017, 16, 541–553. [Google Scholar] [CrossRef]
- Calapai, G.; Mannucci, C.; Chinou, I.; Cardia, L.; Calapai, F.; Sorbara, E.E.; Firenzuoli, B.; Ricca, V.; Gensini, G.F.; Firenzuoli, F. Preclinical and Clinical Evidence Supporting Use of Cannabidiol in Psychiatry. Evid. Based Complement. Altern. Med. 2019, 2019, 2509129. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- Granja, A.G.; Carrillo-Salinas, F.; Pagani, A.; Gómez-Cañas, M.; Negri, R.; Navarrete, C.; Mecha, M.; Mestre, L.; Fiebich, B.L.; Cantarero, I.; et al. A cannabigerol quinone alleviates neuroinflammation in a chronic model of multiple sclerosis. J. Neuroimmune Pharmacol. 2012, 7, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Schiano Moriello, A.; Imperatore, R.; Cristino, L.; Starowicz, K.; Di Marzo, V. A re-evaluation of 9-HODE activity at TRPV1 channels in comparison with anandamide: Enantioselectivity and effects at other TRP channels and in sensory neurons. Br. J. Pharmacol. 2012, 167, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, P.; Silveirinha, V.; dos Santos-Rodrigues, A.; Venance, L.; Ledent, C.; Takahashi, R.N.; Cunha, R.A.; Köfalvi, A. Cannabinoids inhibit the synaptic uptake of adenosine and dopamine in the rat and mouse striatum. Eur. J. Pharmacol. 2011, 655, 38–45. [Google Scholar] [CrossRef]
- Castillo, A.; Tolón, M.R.; Fernández-Ruiz, J.; Romero, J.; Martinez-Orgado, J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol. Dis. 2010, 37, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Kathmann, M.; Flau, K.; Redmer, A.; Tränkle, C.; Schlicker, E. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2006, 372, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, Y.; Gozal, D.; Carney, P. Channelopathy of Dravet Syndrome and Potential Neuroprotective Effects of Cannabidiol. J. Cent. Nerv. Syst. Dis. 2021, 13, 11795735211048045. [Google Scholar] [CrossRef]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.; Traynor, J.R. The [35S]GTPgammaS binding assay: Approaches and applications in pharmacology. Life Sci. 2003, 74, 489–508. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Clinical endocannabinoid deficiency (CECD): Can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? Neuro Endocrinol. Lett. 2008, 29, 192–200. [Google Scholar] [PubMed]
- Müller, C.P.; Homberg, J.R. The role of serotonin in drug use and addiction. Behav. Brain Res. 2015, 277, 146–192. [Google Scholar] [CrossRef] [PubMed]
- Vaseghi, S.; Arjmandi-Rad, S.; Nasehi, M.; Zarrindast, M.R. Cannabinoids and sleep-wake cycle: The potential role of serotonin. Behav. Brain Res. 2021, 412, 113440. [Google Scholar] [CrossRef]
- Resstel, L.B.; Tavares, R.F.; Lisboa, S.F.; Joca, S.R.; Corrêa, F.M.; Guimarães, F.S. 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 2009, 156, 181–188. [Google Scholar] [CrossRef]
- Summary of the Risk Management Plan. Available online: https://www.ema.europa.eu/en/documents/rmp-summary/epidyolex-epar-risk-management-plan-summary_en.pdf (accessed on 15 July 2023).
- Ammendolia, I.; Mannucci, C.; Cardia, L.; Calapai, G.; Gangemi, S.; Esposito, E.; Calapai, F. Pharmacovigilance on cannabidiol as an antiepileptic agent. Front. Pharmacol. 2023, 14, 1091978. [Google Scholar] [CrossRef]
- Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/epidyolex-epar-product-information_en.pdf (accessed on 4 August 2023).
- Hauben, M.; Zhou, X. Quantitative methods in pharmacovigilance: Focus on signal detection. Drug Saf. 2003, 26, 159–186. [Google Scholar] [CrossRef]
- Palapra, H.; Viswam, S.K.; Kalaiselvan, V.; Undela, K. SGLT2 inhibitors associated pancreatitis: Signal identification through disproportionality analysis of spontaneous reports and review of case reports. Int. J. Clin. Pharm. 2022, 44, 1425–1433. [Google Scholar] [CrossRef]
- Blake, M.J.; Trinder, J.A.; Allen, N.B. Mechanisms underlying the association between insomnia, anxiety, and depression in adolescence: Implications for behavioral sleep interventions. Clin. Psychol. Rev. 2018, 63, 25–40. [Google Scholar] [CrossRef]
- Suraev, A.S.; Marshall, N.S.; Vandrey, R.; McCartney, D.; Benson, M.J.; McGregor, I.S.; Grunstein, R.R.; Hoyos, C.M. Cannabinoid therapies in the management of sleep disorders: A systematic review of preclinical and clinical studies. Sleep. Med. Rev. 2020, 53, 101339. [Google Scholar] [CrossRef] [PubMed]
- Linares, I.M.P.; Guimaraes, F.S.; Eckeli, A.; Crippa, A.C.S.; Zuardi, A.W.; Souza, J.D.S.; Hallak, J.E.; Crippa, J.A.S. No Acute Effects of Cannabidiol on the Sleep-Wake Cycle of Healthy Subjects: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study. Front. Pharmacol. 2018, 9, 315. [Google Scholar] [CrossRef] [PubMed]
- Geffrey, A.L.; Pollack, S.F.; Bruno, P.L.; Thiele, E.A. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 2015, 56, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Gidal, B.; Blakey, G.; Tayo, B.; Morrison, G. A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects. CNS Drugs 2018, 32, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Rodríguez, E. The role of the CB1 receptor in the regulation of sleep. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008, 32, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Jouvet, M. Sleep and serotonin: An unfinished story. Neuropsychopharmacology 1999, 21 (Suppl. S2), 24S–27S. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blier, P.; Piñeyro, G.; el Mansari, M.; Bergeron, R.; de Montigny, C. Role of somatodendritic 5-HT autoreceptors in modulating 5-HT neurotransmission. Ann. N. Y. Acad. Sci. 1998, 861, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.R.; Mukhin, Y.V.; Gettys, T.W.; Garnovskaya, M.N. The recombinant 5-HT1A receptor: G protein coupling and signalling pathways. Br. J. Pharmacol. 1999, 127, 1751–1764. [Google Scholar] [CrossRef]
- Pompeiano, M.; Palacios, J.M.; Mengod, G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: Correlation with receptor binding. J. Neurosci. 1992, 12, 440–453. [Google Scholar] [CrossRef]
- Berumen, L.C.; Rodríguez, A.; Miledi, R.; García-Alcocer, G. Serotonin receptors in hippocampus. Sci. World J. 2012, 2012, 823493. [Google Scholar] [CrossRef]
- Brown, J.W.; Sirlin, E.A.; Benoit, A.M.; Hoffman, J.M.; Darnall, R.A. Activation of 5-HT1A receptors in medullary raphé disrupts sleep and decreases shivering during cooling in the conscious piglet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R884–R894. [Google Scholar] [CrossRef][Green Version]
- Toth, M. 5-HT1A receptor knockout mouse as a genetic model of anxiety. Eur. J. Pharmacol. 2003, 463, 177–184. [Google Scholar] [CrossRef]
- Artigas, F. Developments in the field of antidepressants, where do we go now? Eur. Neuropsychopharmacol. 2015, 25, 657–670. [Google Scholar] [CrossRef]
- Meneses, A.; Hong, E. Mechanism of action of 8-OH-DPAT on learning and memory. Pharmacol. Biochem. Behav. 1994, 49, 1083–1086. [Google Scholar] [CrossRef]
- Bjorvatn, B.; Fagerland, S.; Eid, T.; Ursin, R. Sleep/waking effects of a selective 5-HT1A receptor agonist given systemically as well as perfused in the dorsal raphe nucleus in rats. Brain Res. 1997, 770, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Fiske, E.; Portas, C.M.; Grønli, J.; Sørensen, E.; Bjorvatn, B.; Bjørkum, A.A.; Ursin, R. Increased extracellular 5-HT but no change in sleep after perfusion of a 5-HT1A antagonist into the dorsal raphe nucleus of rats. Acta Physiol. 2008, 193, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ukaegbu, O.; Smith, J.; Hall, D.; Frain, T.; Abbasian, C. Staff awareness of the use of cannabidiol (CBD): A trust-wide survey study in the UK. J. Cannabis Res. 2021, 3, 51. [Google Scholar] [CrossRef]
- Chrétien, B.; Nguyen, S.; Dolladille, C.; Morice, P.M.; Heraudeau, M.; Loilier, M.; Fedrizzi, S.; Bourgine, J.; Cesbron, A.; Alexandre, J.; et al. Association between road traffic accidents and drugs belonging to the antiseizure medications class: A pharmacovigilance analysis in VigiBase. Br. J. Clin. Pharmacol. 2023, 89, 222–231. [Google Scholar] [CrossRef]
- Lindquist, M. Use of triage strategies in the WHO signal-detection process. Drug Saf. 2007, 30, 635–637. [Google Scholar] [CrossRef] [PubMed]
- MedDRA Hierarchy|MedDRA. Available online: https://www.meddra.org/how-to-use/basics/hierarchy (accessed on 4 August 2023).
- Bate, A.; Evans, S.J. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol. Drug Saf. 2009, 18, 427–436. [Google Scholar] [CrossRef]
- Faillie, J.L. Case-non-case studies: Principle, methods, bias and interpretation. Therapie 2019, 74, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Trillenberg, P.; Sprenger, A.; Machner, B. Sensitivity and specificity in signal detection with the reporting odds ratio and the information component. Pharmacoepidemiol. Drug Saf. 2023, 32, 910–917. [Google Scholar] [CrossRef] [PubMed]
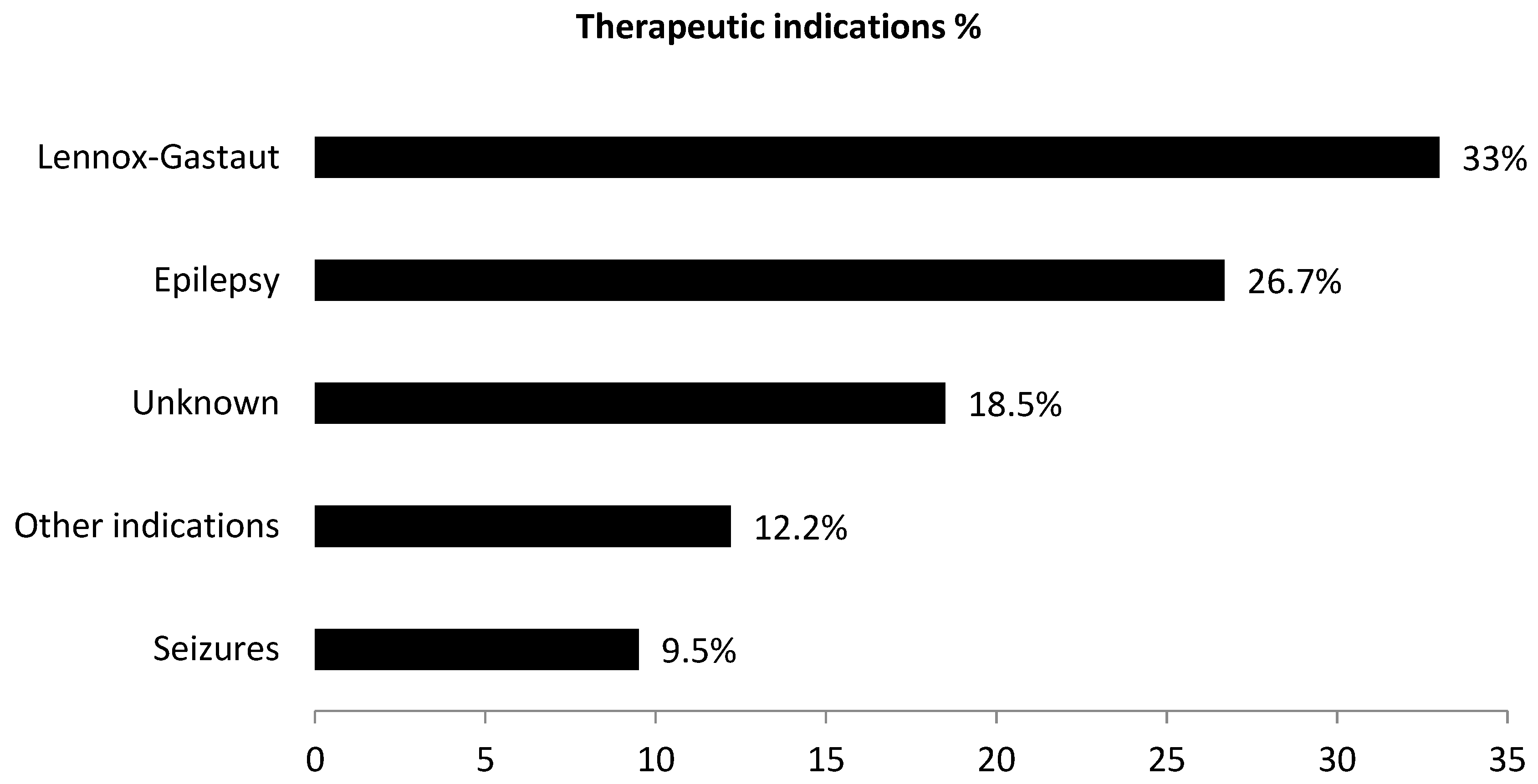
| Main Characteristics | N° Cases (820) | % |
|---|---|---|
| Patient Age | ||
| 28 days–23 months | 1 | 0.1% |
| 2–11 years | 23 | 2.8% |
| 12–17 years | 23 | 2.8% |
| 18–44 years | 57 | 7.0% |
| 45–64 years | 18 | 2.2% |
| 65–74 years | 6 | 0.7% |
| ≥75 years | 6 | 0.7% |
| Unknown | 685 | 83.5% |
| 28 days–23 months | 1 | 0.1% |
| Sex | ||
| Male | 149 | 18.2% |
| Female | 147 | 17.9% |
| Unknown | 524 | 63.9% |
| Countries | ||
| USA | 754 | 92.0% |
| UK | 18 | 2.2% |
| France | 12 | 1.5% |
| Germany | 8 | 1.0% |
| other countries | 28 | 3.4% |
| Reporter qualification | ||
| Physician | 154 | 18.8% |
| Pharmacist | 22 | 2.7% |
| Other Health Professional | 179 | 21.8% |
| Consumer/Non Health Professional | 463 | 56.5% |
| Unknown | 8 | 1.0% |
| Drug/Product | ||
| Epidiolex | 753 | 91.8% |
| Unknown (Cannabidiol) | 47 | 5.7% |
| CBD oil | 15 | 1.8% |
| Convupidiol (Argentina) | 3 | 0.4% |
| Xannadiol (Uruguay) | 2 | 0.2% |
| Indication | ||
| Epilepsy NOS | 200 | 24.4% |
| Lennox Gastaut Syndrome | 315 | 38.4% |
| Seizures | 40 | 4.9% |
| Partial seizure | 41 | 5% |
| Idiopatic epilepsy | 34 | 4.1% |
| Tuberous Sclerosis Complex | 11 | 1.3% |
| Product use for unknown indication | 79 | 9.6% |
| Other indications | 100 | 12.2% |
| Serious/non serious adverse reactions | ||
| Yes | 233 | 28.4% |
| No | 587 | 71.6% |
| Adverse Reaction | N. of Cases | ROR (95% CI) | IC (IC025) |
|---|---|---|---|
| Weight decreased | 456 | 5.19° (4.54–5.70) | 2.4 * (2.2) |
| Hypophagia | 30 | 3.68° (3.22–5.27) | 1.8 * (1.3) |
| Insomnia | 221 | 1.60° (1.40–1.83) | 0.7 * (0.5) |
| Dizziness | 118 | 0.23 (0.20–0.27) | −2.1 (−2.4) |
| Palpitations | 16 | 0.12 (0.10–0.19) | −3.1 (−3.9) |
| Main Characteristics | N° Cases (221) | % |
|---|---|---|
| Patient Age | ||
| 2–11 years | 8 | 3.6% |
| 12–17 years | 5 | 2.3% |
| 18–44 years | 4 | 1.8% |
| 45–64 years | 4 | 1.8% |
| 65–74 years | 2 | 0.9% |
| ≥75 years | 2 | 0.9% |
| Unknown | 196 | 88.7% |
| Sex | ||
| Male | 28 | 12.7% |
| Female | 25 | 11.3% |
| Unknown | 168 | 76.0% |
| Drug/Product | ||
| Epidiolex | 206 | 93.2% |
| Unknown Cannabidiol | 11 | 5.0% |
| CBD oil | 2 | 0.9% |
| Convupidiol (Argentina) | 2 | 0.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calapai, F.; Mannucci, C.; McQuain, L.; Salvo, F. Pharmacological Evaluation of Signals of Disproportionality Reporting Related to Adverse Reactions to Antiepileptic Cannabidiol in VigiBase. Pharmaceuticals 2023, 16, 1420. https://doi.org/10.3390/ph16101420
Calapai F, Mannucci C, McQuain L, Salvo F. Pharmacological Evaluation of Signals of Disproportionality Reporting Related to Adverse Reactions to Antiepileptic Cannabidiol in VigiBase. Pharmaceuticals. 2023; 16(10):1420. https://doi.org/10.3390/ph16101420
Chicago/Turabian StyleCalapai, Fabrizio, Carmen Mannucci, Liana McQuain, and Francesco Salvo. 2023. "Pharmacological Evaluation of Signals of Disproportionality Reporting Related to Adverse Reactions to Antiepileptic Cannabidiol in VigiBase" Pharmaceuticals 16, no. 10: 1420. https://doi.org/10.3390/ph16101420
APA StyleCalapai, F., Mannucci, C., McQuain, L., & Salvo, F. (2023). Pharmacological Evaluation of Signals of Disproportionality Reporting Related to Adverse Reactions to Antiepileptic Cannabidiol in VigiBase. Pharmaceuticals, 16(10), 1420. https://doi.org/10.3390/ph16101420







