Strategies to Enhance the Solubility and Bioavailability of Tocotrienols Using Self-Emulsifying Drug Delivery System
Abstract
:1. Introduction
2. Pharmacokinetics of Tocotrienol
2.1. Absorption of Tocotrienol
2.2. Distribution of Tocotrienol
2.3. Metabolism of Tocotrienol
2.4. Excretion of Tocotrienol
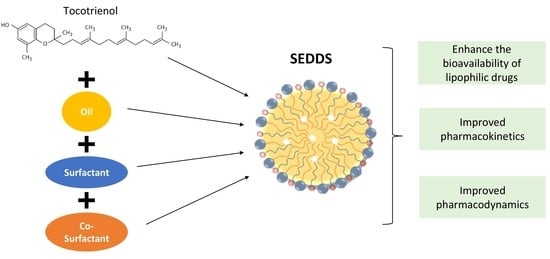
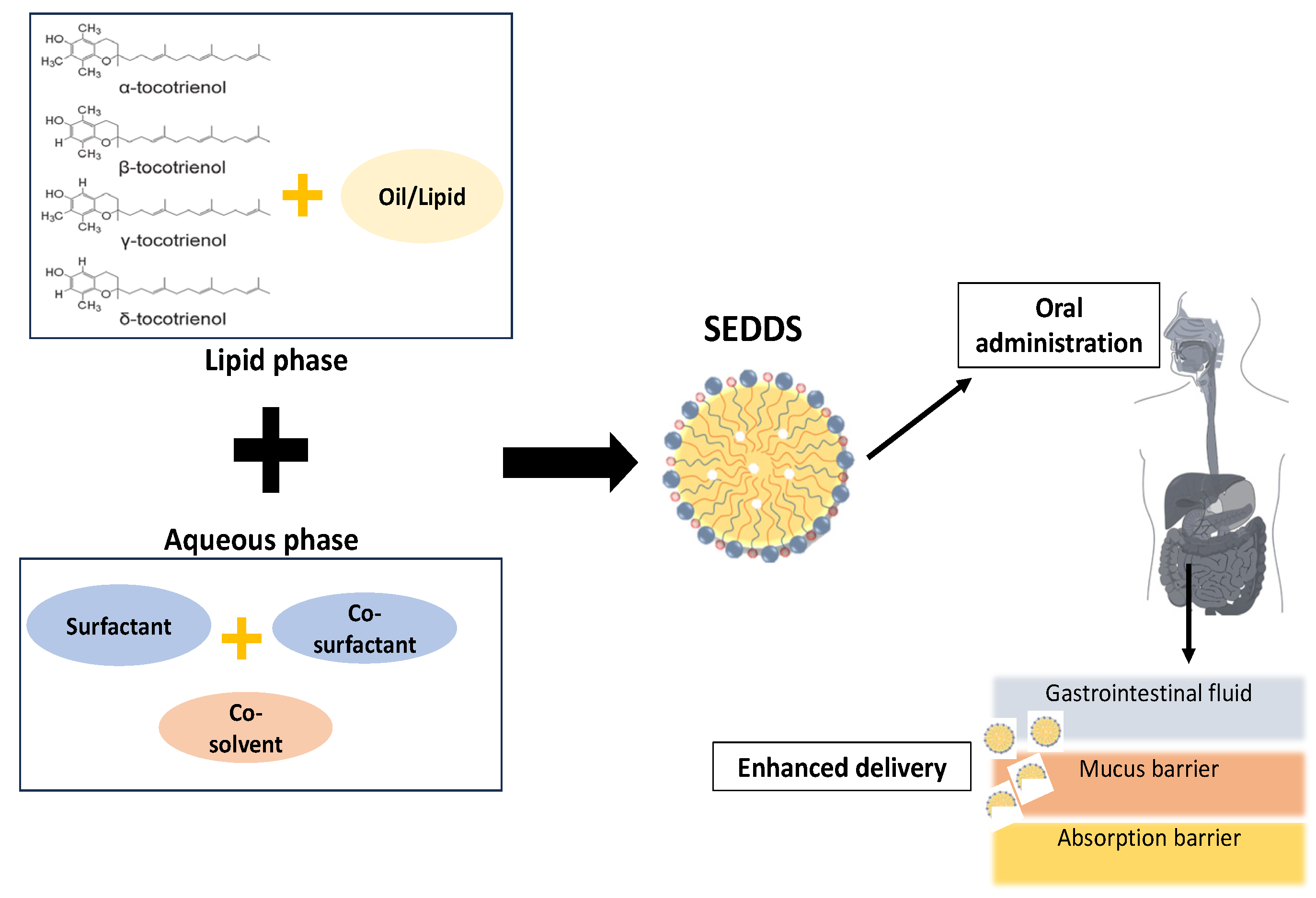
3. Lipid-Based System
3.1. Components of SEDDSs
3.1.1. Lipids (Natural/Synthetic)
3.1.2. Surfactants (Hydrophilic/Hydrophobic)
3.1.3. Co-Surfactants
3.1.4. Co-Solvents
3.2. Process of SEDDSs
4. Oral Bioavailability of Tocotrienol with SEDDSs
| In Vitro/In Vivo/ Human Study | Treatment | Formulation of SEDDSs | Mode/Treatment Duration | Findings | Reference |
|---|---|---|---|---|---|
| Male Sprague–Dawley rats (n = 18, weight 250–350 g) | δ-T3 and γ-T3 formulated with SEDDS Doses: 0.5, 2.5, and 25 mg/kg | Primary surfactant: Cremophor EL (40.7% w/w) Co-surfactant: Labrasol (40.7% w/w) Secondary oil: Captex 355 (7.2% w/w) Co-solvent: ethanol (11.4% w/w) | Oral gavage— 45 min (blood samples were collected at 1, 2, 3, 4, 6, 8, 10, and 12 h) | ↑ Oral bioavailability— (i) δ-T3 at 0.5 and 2.5 mg/kg doses 0.05 (ii) γ-T3 at 2.5 mg/kg ↑ Passive permeability of δ-T3 and γ-T3 (threefold) | [28] |
| Caco2 cells | Doses: (i) 1–25 μM for δ-T3 (ii) 0.1–2.5 μM for γ-T3 | Incubate—45 min | ↑ Cellular uptake at high and low concentrations of δ-T3 ↑ Cellular uptake at 0.1–2.5 μM of γ-T3 | ||
| Male Sprague–Dawley rats (n = 25, weight 250–400 g) | γ-T3 formulated with SEDDS Doses: 1, 2.5, 10, 25 and 50 mg/kg | Primary surfactant: Cremophor EL (40.7% w/w) Co-surfactant: labrasol (40.7% w/w) Secondary oil: captex 355 (7.2% w/w) Co-solvent: ethanol (11.4% w/w) | Oral gavage— 45 min (blood samples were collected at 1, 2, 3, 4, 6, 8, 10, and 12 h) | ↑ Oral bioavailability: 10, 25, and 50 mg/kg (twofold) | [102] |
| Caco2 cells | Doses: 0, 5, 10, 15, 20, 25, 30, 35, 40, 45, and 50 μM | Incubate—45 min | ↑ Cellular uptake | ||
| Osteoporotic female Sprague–Dawley rats (n = 36, weight 200–250 g) | Annatto-T3 formulated with SEDDS Dose: 60 mg/kg | Primary surfactant: Cremophor EL (40.7% w/w) Co-surfactant: Labrasol (40.7% w/w) Secondary oil: Captex 355 (7.2% w/w) Co-solvent: ethanol (11.4% w/w) | Oral gavage— 2 months treatment | ↑ Plasma annatto concentration ↑ Bone parameters (cortical bone thickness, preserved bone calcium content, bone biomechanical strength, and antioxidant enzyme activities) | [103] |
| Healthy adult male volunteers (n = 6, aged 26–41 years old and body weight 55–75 kg) | Tocomin® 50% (21.6: γ-, 6.4: δ-, 10.7 α-tocotrienol and 10.9% α-tocopherol) formulated with SEF Dose: 200 mg mixed tocotrienols at 9:00 a.m. after a 12 h fast with 240 mL of water | SES-A- Surfactants: i. Tween 80 (12.5 mg) ii. Labrasol (87.5 mg) Soybean oil: 351.3 mg SES-B- Surfactants: i.Tween 80 (367.5 mg) ii. Labrasol (52.5 mg) Soybean oil: 31.3 mg | Orally— Blood samples were collected at 0 (before dosing), 1–8, 10, 12, 14, 18, and 24 h after supplemented | Both SES-A and B- ↑ plasma levels and faster rate of drug absorption | [104] |
| Young healthy Caucasian Women (n = 8, aged 23.5 ± 2.2 years old and body weight 58 ± 7.5 kg) | ToCOVID Suprabio® by Carotech Inc, New Jersey contains: 77 mg α-T3 96 mg δ-T3 3 mg γ-T3 62 mg α-TCP 96 mg γ-TCP Dose: 400 mg (one time) with a fat-loaded meal | Tocomin® 50: 200 mg Soya Oil: 305.4 mg Labrasol®: 50 mg Cremophor EL: 50 mg | Orally— Blood samples were collected at 2, 4, 6, and 8 h after supplemented. | Tocotrienols were detected in the blood plasma, and all lipoprotein subfractions studied postprandially | [105] |
| Healthy participants (n = 16, age 21–40 years old) | ToCOVID Suprabio® b.i.d. by Carotech Inc, New Jersey contains: 61.52 mg α-T3 112.8 mg γ-T3 25.68 mg δ-T3 Dose: 400 mg (twice 200 mg per day) | Tocomin® 50: 200 mg Soya Oil: 305.4 mg Labrasol®: 50 mg Cremophor EL: 50 mg | Orally— Blood samples were collected at 0, 6, and 12 weeks. | ↑ Tissue concentrations in blood, skin, adipose, brain, cardiac muscle, and liver ↓ Model for end-stage liver disease score in 50% | [107] |
| Healthy adult male volunteers (n = 8, age 22 ± 47 years and body weight 50 ± 79 kg) | ToCOVID Suprabio® by Hovid Pte. Ltd. Malaysia contains: 21.8 mg α-T3 41.6 mg γ-T3 10.7 mg δ-T3 34.8 int. units d-α-TCP Dose: 300 mg after fasting for a minimum of 12 h overnight and standard meals were given at 4 and 10 h after administration | Tocomin® 50: 200 mg Soya Oil: 305.4 mg Labrasol®: 50 mg Cremophor EL: 50 mg | Orally— Blood samples were collected at 1, 2, 3, 4, 5, 6, 7, 8, 10, 14, 18, and 24 h after supplemented | ↑ The onset and extent of absorption for all T3 isomers by more than two folds between fed and fasted. ↔ Peak plasma concentration of T3 isomers between fed and fasted Elimination half-life of T3 were 4.5- to 8.7-fold shorter than α-TCP ↔ For elimination half time between fed and fasted | [25] |
| Healthy male subjects (n = 36, aged < 40 years old) | ToCOVID Suprabio® by Hovid Sdn Bhd, Malaysia contains: 23.54% α-T3 43.16% γ-T3 9.83% δ-T3 23.5% α-TCP Doses: 50 mg, 100 mg and 200 mg | Tocomin® 50: 200 mg Soya Oil: 305.4 mg Labrasol®: 50 mg Cremophor EL: 50 mg | Orally— Blood samples were collected after 2 months supplemented | ↑ Plasma δ, α, and γ- T3 concentrations Linear dose–concentration relationship for all the isomers ↔ On BP and serum TC and LDL-C | [106] |
| Hypercholesterolemic but otherwise healthy subjects (n = 32 (20 males and 12 females), aged between 31 and 53 years old) | ToCOVID Suprabio® by Hovid Sdn Bhd, Malaysia One capsule contains 50 mg of mixed T3: 30.8% α-T3 56.4% γ-T3 12.8% δ-T3 22.9 IU α-TCP Dose: 300 mg daily = 3x after breakfast and 3x after dinner | Tocomin® 50: 200 mg Soya Oil: 305.4 mg Labrasol®: 50 mg Cremophor EL: 50 mg | Orally— Blood samples were collected twice at 2 weeks for 24 weeks | ↑ Serum T3 concentration relative to the TCP level T3 ↑ 22-fold compared to baseline T3 ↓ TC and LDL at 4 months until 6 months compared with baseline | [108] |
| Untreated hypercholesterolaemic adults (n = 87, aged > 35 years old) | ToCOVID Suprabio® by Hovid Sdn Bhd, Malaysia contains: 61.5 mg α-T3 112.8 mg γ-T3 25.7 mg δ-T3 61.1 mg α-TCP Dose: 400 mg (twice 200 mg per day) | Tocomin® 50: 200 mg Soya Oil: 305.4 mg Labrasol®: 50 mg Cremophor EL: 50 mg | Orally— Blood samples were collected after an overnight fast of 1 year treatment period | Normalisation of the hepatic echogenic response in NAFLD | [109] |
| Healthy women (n = 108, aged 18–25 years old) | ToCOVID Suprabio® by Hovid Sdn Bhd, Malaysia contains: 61.52 mg α-T3 112.8 mg γ-T3 25.68 mg δ-T3 91.60 IU α-TCP Dose: 400 mg (twice 200 mg per day) | Tocomin® 50: 200 mg Soya Oil: 305.4 mg Labrasol®: 50 mg Cremophor EL: 50 mg | Orally— Blood samples were collected at 0, 28, and 56 days after supplemented | ↑ Total vitamin E level in the plasma ↑ Interferon-γ and IL-4 and anti-TT IgG ↓ IL-6 level | [110] |
5. Limitation
6. Future Perspective
7. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sundram, K.; Sambanthamurthi, R.; Tan, Y.-A. Palm fruit chemistry and nutrition. Asia Pac. J. Clin. Nutr. 2003, 12, 355–362. [Google Scholar]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols in health and disease: The other half of the natural vitamin E family. Mol. Asp. Med. 2007, 28, 692–728. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.H.; Choo, Y.M.; Ma, A.N.; Chuah, C.H.; Hashim, M.A. Separation of vitamin E (tocopherol, tocotrienol, and tocomonoenol) in palm oil. Lipids 2004, 39, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Shammugasamy, B.; Ramakrishnan, Y.; Manan, F.; Muhammad, K. Rapid Reversed-Phase Chromatographic Method for Determination of Eight Vitamin E Isomers and γ-Oryzanols in Rice Bran and Rice Bran Oil. Food Anal. Methods 2015, 8, 649–655. [Google Scholar] [CrossRef]
- Moreau, R.A.; Wayns, K.E.; Flores, R.A.; Hicks, K.B. Tocopherols and Tocotrienols in Barley Oil Prepared from Germ and Other Fractions from Scarification and Sieving of Hulless Barley. Cereal Chem. 2007, 84, 587–592. [Google Scholar] [CrossRef]
- Kumar, G.S.; Krishna, A.G. Studies on the nutraceuticals composition of wheat derived oils wheat bran oil and wheat germ oil. J. Food Sci. Technol. 2015, 52, 1145–1151. [Google Scholar] [CrossRef]
- Tan, B.; Brzuskiewicz, L. Separation of tocopherol and tocotrienol isomers using normal- and reverse-phase liquid chromatography. Anal. Biochem. 1989, 180, 368–373. [Google Scholar] [CrossRef]
- Wong, S.K.; Kamisah, Y.; Mohamed, N.; Muhammad, N.; Masbah, N.; Fahami, N.A.M.; Mohamed, I.N.; Shuid, A.N.; Saad, Q.M.; Abdullah, A. Potential Role of Tocotrienols on Non-Communicable Diseases: A Review of Current Evidence. Nutrients 2020, 12, 259. [Google Scholar] [CrossRef]
- Nazrun, A.; Khairunnur, A.; Norliza, M.; Norazlina, M. Effects of palm tocotrienols on oxidative stress and bone strength in ovariectomised rats. Med. Health 2008, 3, 247–255. [Google Scholar]
- Radhakrishnan, A.; Tudawe, D.; Chakravarthi, S.; Chiew, G.S.; Haleagrahara, N. Effect of γ-tocotrienol in counteracting oxidative stress and joint damage in collagen-induced arthritis in rats. Exp. Ther. Med. 2014, 7, 1408–1414. [Google Scholar] [CrossRef]
- Devi, R.R.; Arumughan, C. Antiradical efficacy of phytochemical extracts from defatted rice bran. Food Chem. Toxicol. 2007, 45, 2014–2021. [Google Scholar] [CrossRef] [PubMed]
- Norazlina, M.; Lee, P.L.; Lukman, H.I.; Nazrun, A.S.; Ima-Nirwana, S. Effects of vitamin E supplementation on bone metabolism in nicotine-treated rats. Singap. Med. J. 2007, 48, 195–199. [Google Scholar]
- Ahmad, N.S.; Khalid, B.A.; Luke, D.A.; Nirwana, S. Tocotrienol offers better protection than tocopherol from free radical-induced damage of rat bone. Clin. Exp. Pharmacol. Physiol. 2005, 32, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Reis, J.C.; Qureshi, N.; Papasian, C.J.; Morrison, D.C.; Schaefer, D.M. δ-Tocotrienol and quercetin reduce serum levels of nitric oxide and lipid parameters in female chickens. Lipids Health Dis. 2011, 10, 39. [Google Scholar] [CrossRef]
- Shah, M.K.; Khatri, P.; Vora, N.; Patel, N.K.; Jain, S.; Lin, S. Lipid nanocarriers: Preparation, characterization and absorption mechanism and applications to improve oral bioavailability of poorly water-soluble drugs. Biomed. Appl. Nanoparticles 2019, 117–147. [Google Scholar] [CrossRef]
- Gao, P.; Morozowich, W. Development of supersaturatable self-emulsifying drug delivery system formulations for improving the oral absorption of poorly soluble drugs. Expert Opin. Drug Deliv. 2006, 3, 97–110. [Google Scholar] [CrossRef]
- Abuasal, B.S.; Qosa, H.; Sylvester, P.W.; Kaddoumi, A. Comparison of the intestinal absorption and bioavailability of γ-tocotrienol and α-tocopherol: In vitro, in situ and in vivo studies. Biopharm. Drug Dispos. 2012, 33, 246–256. [Google Scholar] [CrossRef]
- Lim, Y.; Traber, M.G. Alpha-Tocopherol Transfer Protein (α-TTP): Insights from Alpha-Tocopherol Transfer Protein Knockout Mice. Nutr. Res. Pr. 2007, 1, 247–253. [Google Scholar] [CrossRef]
- Boyd, B.J.; Bergström, C.A.; Vinarov, Z.; Kuentz, M.; Brouwers, J.; Augustijns, P.; Brandl, M.; Bernkop-Schnürch, A.; Shrestha, N.; Préat, V. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur. J. Phar. Sci. 2019, 137, 104967. [Google Scholar] [CrossRef]
- Yap, S.P.; Yuen, K.H.; Lim, A.B. Influence of route of administration on the absorption and disposition of alpha-, gamma- and delta-tocotrienols in rats. J. Pharm. Pharmacol. 2003, 55, 53–58. [Google Scholar] [CrossRef]
- Gursoy, R.N.; Benita, S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharmacothe.r 2004, 58, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Yuen, K.-H.; Ng, B.-H.; Wong, J.-W. 22 Absorption and Disposition of Tocotrienols, Tocotrienols: Vitamin E Beyond Tocopherols; CRC Press: Taylor & Francis Group, Boca Raton, FL, USA, 2008; p. 297. [Google Scholar]
- Qureshi, A.; Khan, D.; Saleem, S.; Silswal, N.; Trias, A.; Tan, B. Pharmacokinetics and bioavailability of annatto δ-tocotrienol in healthy fed subjects. J. Clin. Exp. Cardiol. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Khan, D.A.A.; Silswal, N.; Saleem, S.; Qureshi, N. Evaluation of Pharmacokinetics, and Bioavailability of Higher Doses of Tocotrienols in Healthy Fed Humans. J. Clin. Exp. Cardiol. 2016, 7, 434. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.P.; Yuen, K.H.; Wong, J.W. Pharmacokinetics and bioavailability of alpha-, gamma- and delta-tocotrienols under different food status. J. Pharm. Pharmacol. 2001, 53, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Iyanagi, T. Molecular Mechanism of Phase I and Phase II Drug-Metabolizing Enzymes: Implications for Detoxification. Int. Rev. Cytol. 2007, 260, 35–112. [Google Scholar] [CrossRef]
- Cao, X.; Gibbs, S.T.; Fang, L.; Miller, H.A.; Landowski, C.P.; Shin, H.-C.; Lennernas, H.; Zhong, Y.; Amidon, G.L.; Yu, L.X.; et al. Why is it Challenging to Predict Intestinal Drug Absorption and Oral Bioavailability in Human Using Rat Model. Pharm. Res. 2006, 23, 1675–1686. [Google Scholar] [CrossRef]
- Alqahtani, S.; Alayoubi, A.; Nazzal, S.; Sylvester, P.W.; Kaddoumi, A. Nonlinear Absorption Kinetics of Self-Emulsifying Drug Delivery Systems (SEDDS) Containing Tocotrienols as Lipophilic Molecules: In Vivo and In Vitro Studies. AAPS J. 2013, 15, 684–695. [Google Scholar] [CrossRef]
- Abuasal, B.S.; Lucas, C.; Peyton, B.; Alayoubi, A.; Nazzal, S.; Sylvester, P.W.; Kaddoumi, A. Enhancement of Intestinal Permeability Utilizing Solid Lipid Nanoparticles Increases γ-Tocotrienol Oral Bioavailability. Lipids 2012, 47, 461–469. [Google Scholar] [CrossRef]
- Sylvester, P.W.; Shah, S.J. Mechanisms mediating the antiproliferative and apoptotic effects of vitamin E in mammary cancer cells. Front. Biosci. 2005, 10, 699–709. [Google Scholar] [CrossRef]
- Fu, J.-Y.; Che, H.-L.; Tan, D.M.-Y.; Teng, K.-T. Bioavailability of tocotrienols: Evidence in human studies. Nutr. Metab. 2014, 11, 5. [Google Scholar] [CrossRef]
- Fairus, S.; Nor, R.M.; Cheng, H.M.; Sundram, K. Alpha-tocotrienol is the most abundant tocotrienol isomer circulated in plasma and lipoproteins after postprandial tocotrienol-rich vitamin E supplementation. Nutr. J. 2012, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Gee, P.T. Vitamin E - essential knowledge for supplementation. Lipid Technol. 2011, 23, 79–82. [Google Scholar] [CrossRef]
- Abuasal, B.; Sylvester, P.W.; Kaddoumi, A. Intestinal Absorption of γ-Tocotrienol Is Mediated by Niemann-Pick C1-Like 1: In Situ Rat Intestinal Perfusion Studies. Drug Metab. Dispos. 2010, 38, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Narushima, K.; Takada, T.; Yamanashi, Y.; Suzuki, H. Niemann-Pick C1-Like 1 Mediates α-Tocopherol Transport. Mol. Pharmacol. 2008, 74, 42–49. [Google Scholar] [CrossRef]
- Hathcock, J.N.; Azzi, A.; Blumberg, J.; Bray, T.; Dickinson, A.; Frei, B.; Jialal, I.; Johnston, C.S.; Kelly, F.J.; Kraemer, K. Vitamins E and C are safe across a broad range of intakes. Am. J. Clin. Nutr. 2005, 81, 736–745. [Google Scholar] [CrossRef]
- Ikeda, S.; Toyoshima, K.; Yamashita, K. Dietary Sesame Seeds Elevate α- and γ-Tocotrienol Concentrations in Skin and Adipose Tissue of Rats Fed the Tocotrienol-Rich Fraction Extracted from Palm Oil. J. Nutr. 2001, 131, 2892–2897. [Google Scholar] [CrossRef]
- Kawakami, Y.; Tsuzuki, T.; Nakagawa, K.; Miyazawa, T. Distribution of Tocotrienols in Rats Fed a Rice Bran Tocotrienol Concentrate. Biosci. Biotechnol. Biochem. 2007, 71, 464–471. [Google Scholar] [CrossRef]
- Deng, L.; Peng, Y.; Wu, Y.; Yang, M.; Ding, Y.; Chen, Q.; Fu, Q. Tissue distribution of emulsified γ-tocotrienol and its long-term biological effects after subcutaneous administration. Lipids Health Dis. 2014, 13, 66. [Google Scholar] [CrossRef]
- Shibata, A.; Nakagawa, K.; Shirakawa, H.; Kobayashi, T.; Kawakami, Y.; Takashima, R.; Ohashi, A.; Sato, S.; Ohsaki, Y.; Kimura, F. Physiological Effects and Tissue Distribution from Large Doses of Tocotrienol in Rats. Biosci. Biotechnol. Biochem. 2012, 76, 1805–1808. [Google Scholar] [CrossRef]
- Ikeda, S.; Tohyama, T.; Yoshimura, H.; Hamamura, K.; Abe, K.; Yamashita, K. Dietary α-tocopherol decreases α-tocotrienol but not γ-tocotrienol concentration in rats. J. Nutr. 2003, 133, 428–434. [Google Scholar] [CrossRef]
- Uchida, T.; Abe, C.; Nomura, S.; Ichikawa, T.; Ikeda, S. Tissue distribution of α- and γ-tocotrienol and γ-tocopherol in rats and interference with their accumulation by α-tocopherol. Lipids 2012, 47, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Okabe, M.; Oji, M.; Ikeda, I.; Tachibana, H.; Yamada, K. Tocotrienol Levels in Various Tissues of Sprague-Dawley Rats after Intragastric Administration of Tocotrienols. Biosci. Biotechnol. Biochem. 2002, 66, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Ikeda, S.; Iizuka, Y.; Ikeda, I. Effect of sesaminol on plasma and tissue α-tocopherol and α-tocotrienol concentrations in rats fed a vitamin E concentrate rich in tocotrienols. Lipids 2002, 37, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Birringer, M.; Pfluger, P.; Kluth, D.; Landes, N.; Brigelius-Flohé, R. Identities and Differences in the Metabolism of Tocotrienols and Tocopherols in HepG2 Cells. J. Nutr. 2002, 132, 3113–3118. [Google Scholar] [CrossRef]
- Parker, R.S.; Sontag, T.J.; Swanson, J.E.; McCormick, C.C. Discovery, characterization, and significance of the cytochrome P450 omega-hydroxylase pathway of vitamin E catabolism. Ann. N. Y. Acad. Sci. 2004, 1031, 13–21. [Google Scholar] [CrossRef]
- Sontag, T.J.; Parker, R.S. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J. Biol. Chem. 2002, 277, 25290–25296. [Google Scholar] [CrossRef]
- Qing, J.; Helene, F.; Wood, K.V.; Xinmin, Y. Identification and quantitation of novel vitamin E metabolites, sulfated long-chain carboxychromanols, in human A549 cells and in rats. J. Lipid Res. 2007, 48, 1221–1230. [Google Scholar] [CrossRef]
- Freiser, H.; Jiang, Q. γ-Tocotrienol and γ-tocopherol are primarily metabolized to conjugated 2-(β-carboxyethyl)-6-hydroxy-2, 7, 8-trimethylchroman and sulfated long-chain carboxychromanols in rats. J. Nutr. 2009, 139, 884–889. [Google Scholar] [CrossRef]
- Traber, M.G. Mechanisms for the prevention of vitamin E excess. J. Lipid Res. 2013, 54, 2295–2306. [Google Scholar] [CrossRef]
- Lebold, K.M.; Ang, A.; Traber, M.G.; Arab, L. Urinary α-carboxyethyl hydroxychroman can be used as a predictor of α-tocopherol adequacy, as demonstrated in the Energetics Study. Am. J. Clin. Nutr. 2012, 96, 801–809. [Google Scholar] [CrossRef]
- Lodge, J.K.; Ridlington, J.; Leonard, S.; Vaule, H.; Traber, M.G. Alpha- and gamma-tocotrienols are metabolized to carboxyethyl-hydroxychroman derivatives and excreted in human urine. Lipids 2001, 36, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.Y.; Jiang, Q. Tocopherols and Tocotrienols Are Bioavailable in Rats and Primarily Excreted in Feces as the Intact Forms and 13′-Carboxychromanol Metabolites. J. Nutr. 2020, 150, 222–230. [Google Scholar]
- Gupta, S.; Kesarla, R.; Omri, A. Formulation Strategies to Improve the Bioavailability of Poorly Absorbed Drugs with Special Emphasis on Self-Emulsifying Systems. ISRN Pharm. 2013, 2013, 848043. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, G.; Rai, A.; Tiwari, R. Self-emulsifying drug delivery system: An approach to enhance solubility. Syst. Rev. Pharm. 2010, 1, 133. [Google Scholar] [CrossRef]
- Elgart, A.; Cherniakov, I.; Aldouby, Y.; Domb, A.J.; Hoffman, A. Improved Oral Bioavailability of BCS Class 2 Compounds by Self Nano-Emulsifying Drug Delivery Systems (SNEDDS): The Underlying Mechanisms for Amiodarone and Talinolol. Pharm. Res. 2013, 30, 3029–3044. [Google Scholar] [CrossRef]
- Jannin, V.; Chevrier, S.; Michenaud, M.; Dumont, C.; Belotti, S.; Chavant, Y.; Demarne, F. Development of self emulsifying lipid formulations of BCS class II drugs with low to medium lipophilicity. Int. J. Pharm. 2015, 495, 385–392. [Google Scholar] [CrossRef]
- Maji, I.; Mahajan, S.; Sriram, A.; Medtiya, P.; Vasave, R.; Khatri, D.K.; Kumar, R.; Singh, S.B.; Madan, J.; Singh, P.K. Solid self emulsifying drug delivery system: Superior mode for oral delivery of hydrophobic cargos. J. Control. Release 2021, 337, 646–660. [Google Scholar] [CrossRef]
- Sakloetsakun, D.; Dünnhaupt, S.; Barthelmes, J.; Perera, G.; Bernkop-Schnürch, A. Combining two technologies: Multifunctional polymers and self-nanoemulsifying drug delivery system (SNEDDS) for oral insulin administration. Int. J. Biol. Macromol. 2013, 61, 363–372. [Google Scholar] [CrossRef]
- Bhupinder, M.; Roy, G.; Bajwa, B.; Sandeep, K. Self emulsified drug delivery system for the enhancement of oral bioavailability of poorly water soluble drugs. Int. J. Adv. Pharm. Biol. Chem. 2013, 2, 427–436. [Google Scholar]
- Nigade, P.M.; Patil, S.L.; Tiwari, S.S. Self emulsifying drug delivery system (SEDDS): A review. Int. J. Pharm. Biol. Sci. 2012, 2, 42–52. [Google Scholar]
- Rajesh, B.; Reddy, T.; Srikanth, G.; Mallikarjun, V.; Nivethithai, P. Lipid based self-emulsifying drug delivery system (SEDDS) for poorly water-soluble drugs: A review. J. Glob. Pharma. Technol. 2010, 2, 47–55. [Google Scholar]
- Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S.; Cai, Y. A review of the structure, preparation, and application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Muüller, R.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Ahmed, N.; ur Rehman, A. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Simon, L.; Astete, C.E.; Alayoubi, A.; Sylvester, P.W.; Nazzal, S.; Shen, Y.; Xu, Z.; Kaddoumi, A.; Sabliov, C.M. Cellular uptake, antioxidant and antiproliferative activity of entrapped α-tocopherol and γ-tocotrienol in poly (lactic-co-glycolic) acid (PLGA) and chitosan covered PLGA nanoparticles (PLGA-Chi). J. Colloid Interface Sci. 2015, 445, 243–251. [Google Scholar] [CrossRef]
- Parthasarathi, S.; Muthukumar, S.; Anandharamakrishnan, C. The influence of droplet size on the stability, in vivo digestion, and oral bioavailability of vitamin E emulsions. Food Funct. 2016, 7, 2294–2302. [Google Scholar] [CrossRef]
- Pouton, C.W.; Porter, C.J.H. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef]
- Jannin, V.; Musakhanian, J.; Marchaud, D. Approaches for the development of solid and semi-solid lipid-based formulations. Adv. Drug Deliv. Rev. 2008, 60, 734–746. [Google Scholar] [CrossRef]
- Rani, S.; Rana, R.; Saraogi, G.K.; Kumar, V.; Gupta, U. Self-Emulsifying Oral Lipid Drug Delivery Systems: Advances and Challenges. AAPS PharmSciTech 2019, 20, 129. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, K.; Raghavan, C.V.; Abdu, S. Self nanoemulsifying drug delivery system (SNEDDS) of Rosuvastatin calcium: Design, formulation, bioavailability and pharmacokinetic evaluation. Colloids Surf. B Biointerfaces 2013, 112, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.; Lee, B.-J.; Oh, D.H.; Kim, J.O.; Hong, M.J.; Jee, J.-P.; Kim, J.A.; Yoo, B.K.; Woo, J.S.; Yong, C.S.; et al. Enhanced oral bioavailability of dexibuprofen by a novel solid Self-emulsifying drug delivery system (SEDDS). Eur. J. Pharm. Biopharm. 2009, 72, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.J.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef]
- Shah, N.D.; Limketkai, B.N. The use of medium-chain triglycerides in gastrointestinal disorders. Pract. Gastroenterol. 2017, 41, 20–28. [Google Scholar]
- Ledeboer, M.; Masclee, A.; Jansen, J.; Lamers, C. Effect of Equimolar Amounts of Long-Chain Triglycerides and Medium-Chain Triglycerides on Small-Bowel Transit Time in Humans. J. Parenter. Enter. Nutr. 1995, 19, 5–8. [Google Scholar] [CrossRef]
- Caliph, S.M.; Charman, W.N.; Porter, C.J. Effect of short-, medium-, and long-chain fatty acid-based vehicles on the absolute oral bioavailability and intestinal lymphatic transport of halofantrine and assessment of mass balance in lymph-cannulated and non-cannulated rats. J. Pharm. Sci. 2000, 89, 1073–1084. [Google Scholar] [CrossRef]
- Almgren, M. Mixed micelles and other structures in the solubilization of bilayer lipid membranes by surfactants. Biochim. Biophys. Acta. 2000, 1508, 146–163. [Google Scholar] [CrossRef]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Gadadare, R.; Mandpe, L.; Pokharkar, V. Ultra Rapidly Dissolving Repaglinide Nanosized Crystals Prepared via Bottom-Up and Top-Down Approach: Influence of Food on Pharmacokinetics Behavior. AAPS PharmSciTech 2015, 16, 787–799. [Google Scholar] [CrossRef]
- Bittner, B.; González, R.B.; Bohrmann, B.; Kuentz, M.; Huwyler, J. Drug-excipient interactions by Vitamin E-TPGS: In vitro studies on inhibition of P-glycoprotein and colonic drug absorption. J. Drug Deliv. Sci. Technol. 2008, 18, 145–148. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, J.; Ding, R.; Fu, Y.; Gong, T.; Zhang, Z. Improved oral bioavailability and therapeutic efficacy of dabigatran etexilate via Soluplus-TPGS binary mixed micelles system. Drug Dev. Ind. Pharm. 2017, 43, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Zuccari, G.; Alfei, S.; Zorzoli, A.; Marimpietri, D.; Turrini, F.; Baldassari, S.; Marchitto, L.; Caviglioli, G. Increased Water-Solubility and Maintained Antioxidant Power of Resveratrol by Its Encapsulation in Vitamin E TPGS Micelles: A Potential Nutritional Supplement for Chronic Liver Disease. Pharmaceutics 2021, 13, 1128. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Uppal, S.; Mansi, K.; Das, J.; Pandey, S.K.; Kaur, K.; Mehta, S. Ultrasonication induced synthesis of TPGS stabilized clove oil nanoemulsions and their synergistic effect against breast cancer cells and harmful bacteria. J. Mol. Liq. 2021, 349, 118130. [Google Scholar] [CrossRef]
- Zuccari, G.; Baldassari, S.; Alfei, S.; Marengo, B.; Valenti, G.E.; Domenicotti, C.; Ailuno, G.; Villa, C.; Marchitto, L.; Caviglioli, G. D-α-Tocopherol-Based Micelles for Successful Encapsulation of Retinoic Acid. Pharmaceuticals 2021, 14, 212. [Google Scholar] [CrossRef]
- Dixit, G.R.; Mathur, V.B. Microemulsions: Platform for improvement of solubility and dissolution of poorly soluble drugs. Asian J. Pharm. Clin. Res. 2015, 8, 7–17. [Google Scholar]
- Prasad, Y.R.; Minamimoto, T.; Yoshikawa, Y.; Shibata, N.; Mori, S.; Matsuura, A.; Takada, K. In situ intestinal absorption studies on low molecular weight heparin in rats using Labrasol as absorption enhancer. Int. J. Pharm. 2004, 271, 225–232. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Kollipara, S.; Gandhi, R.K. Pharmacokinetic aspects and in vitro–in vivo correlation potential for lipid-based formulations. Acta Pharm. Sin. B 2014, 4, 333–349. [Google Scholar] [CrossRef]
- Kale, A.A.; Patravale, V.B. Design and Evaluation of Self-Emulsifying Drug Delivery Systems (SEDDS) of Nimodipine. AAPS PharmSciTech 2008, 9, 191–196. [Google Scholar] [CrossRef]
- Dash, R.N.; Mohammed, H.; Humaira, T.; Reddy, A.V. Solid supersaturatable self-nanoemulsifying drug delivery systems for improved dissolution, absorption and pharmacodynamic effects of glipizide. J. Drug Deliv. Sci. Technol. 2015, 28, 28–36. [Google Scholar] [CrossRef]
- Pavlović, N.; Goločorbin-Kon, S.; Ðanić, M.; Stanimirov, B.; Al-Salami, H.; Stankov, K.; Mikov, M. Bile Acids and Their Derivatives as Potential Modifiers of Drug Release and Pharmacokinetic Profiles. Front. Pharmacol. 2018, 9, 1283. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Si, L.; Zhai, X.; Fan, Z.; Ma, Y.; Zhang, R.; Yang, X. The influence of co-solvents on the stability and bioavailability of rapamycin formulated in self-microemulsifying drug delivery systems. Drug Dev. Ind. Pharm. 2011, 37, 986–994. [Google Scholar] [CrossRef]
- AboulFotouh, K.; Allam, A.A.; El-Badry, M.; El-Sayed, A.M. Self-emulsifying drug–delivery systems modulate P-glycoprotein activity: Role of excipients and formulation aspects. Nanomedicine 2018, 13, 1813–1834. [Google Scholar] [CrossRef]
- Gupta, S.; Chavhan, S.; Sawant, K.K. Self-nanoemulsifying drug delivery system for adefovir dipivoxil: Design, characterization, in vitro and ex vivo evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 145–155. [Google Scholar] [CrossRef]
- Wang, L.; Dong, J.; Chen, J.; Eastoe, J.; Li, X. Design and optimization of a new self-nanoemulsifying drug delivery system. J. Colloid Interface Sci. 2009, 330, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Gershanik, T.; Benita, S. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur. J. Pharm. Biopharm. 2000, 50, 179–188. [Google Scholar] [CrossRef]
- Chong, W.-T.; Tan, C.-P.; Cheah, Y.-K.B.; Lajis, A.F.; Habi Mat Dian, N.L.; Kanagaratnam, S.; Lai, O.-M. Optimization of process parameters in preparation of tocotrienol-rich red palm oil-based nanoemulsion stabilized by Tween80-Span 80 using response surface methodology. PloS ONE 2018, 13, e0202771. [Google Scholar] [CrossRef]
- San Ho, D.H.S.; Yuen, Y.K.H.; Yap, Y.S.P. Drug delivery system: Formulation for fat-soluble drugs. US6596306B1, 22 July 2003. [Google Scholar]
- Alayoubi, A.Y.; Anderson, J.F.; Satyanarayanajois, S.D.; Sylvester, P.W.; Nazzal, S. Concurrent delivery of tocotrienols and simvastatin by lipid nanoemulsions potentiates their antitumor activity against human mammary adenocarcenoma cells. Eur. J. Pharm. Sci. 2013, 48, 385–392. [Google Scholar] [CrossRef]
- Alqahtani, S.; Alayoubi, A.; Nazzal, S.; Sylvester, P.W.; Kaddoumi, A. Enhanced Solubility and Oral Bioavailability of γ-Tocotrienol Using a Self-Emulsifying Drug Delivery System (SEDDS). Lipids 2014, 49, 819–829. [Google Scholar] [CrossRef]
- Mohamad, N.-V.; Ima-Nirwana, S.; Chin, K.-Y. Therapeutic potential of annatto tocotrienol with self-emulsifying drug delivery system in a rat model of postmenopausal bone loss. Biomed. Pharmacother. 2021, 137, 111368. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.P.; Yuen, K.H. Influence of lipolysis and droplet size on tocotrienol absorption from self-emulsifying formulations. Int. J. Pharm. 2004, 281, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Khosla, P.; Patel, V.; Whinter, J.M.; Khanna, S.; Rakhkovskaya, M.; Roy, S.; Sen, C.K. Postprandial Levels of the Natural Vitamin E Tocotrienol in Human Circulation. Antioxidants Redox Signal. 2006, 8, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Rasool, A.H.; Rahman, A.R.; Yuen, K.H.; Wong, A.R. Arterial compliance and vitamin E blood levels with a self emulsifying preparation of tocotrienol rich vitamin E. Arch. Pharmacal Res. 2008, 31, 1212–1217. [Google Scholar] [CrossRef]
- Patel, V.; Rink, C.; Gordillo, G.M.; Khanna, S.; Gnyawali, U.; Roy, S.; Shneker, B.; Ganesh, K.; Phillips, G.; More, J.L.; et al. Oral Tocotrienols Are Transported to Human Tissues and Delay the Progression of the Model for End-Stage Liver Disease Score in Patients4. J. Nutr. 2012, 142, 513–519. [Google Scholar] [CrossRef]
- Yuen, K.H.; Wong, J.W.; Lim, A.B.; Ng, B.H.; Choy, W.P. Effect of Mixed-Tocotrienols in Hypercholesterolemic Subjects. Funct. Foods Health Dis. 2011, 1, 106. [Google Scholar] [CrossRef]
- Magosso, E.; Ansari, M.A.; Gopalan, Y.; Shuaib, I.L.; Wong, J.-W.; Khan, N.A.; Abu Bakar, M.R.; Ng, B.-H.; Yuen, K.-H. Tocotrienols for normalisation of hepatic echogenic response in nonalcoholic fatty liver: A randomised placebo-controlled clinical trial. Nutr. J. 2013, 12, 166. [Google Scholar] [CrossRef]
- Mahalingam, D.; Radhakrishnan, A.K.; Amom, Z.; Ibrahim, N.; Nesaretnam, K. Effects of supplementation with tocotrienol-rich fraction on immune response to tetanus toxoid immunization in normal healthy volunteers. Eur. J. Clin. Nutr. 2011, 65, 63–69. [Google Scholar] [CrossRef]
- Lindenberg, M.; Kopp, S.; Dressman, J.B. Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system. Eur. J. Pharm. Biopharm. 2004, 58, 265–278. [Google Scholar] [CrossRef]
- Hauss, D.J. Oral Lipid-based Formulations: Enhancing the Bioavailability of Poorly Water-Soluble Drugs; CRC Press: Boca Raton, FL, USA, 2007; Volume 170. [Google Scholar]
- Cserháti, T.; Forgács, E.; Oros, G. Biological activity and environmental impact of anionic surfactants. Environ. Int. 2002, 28, 337–348. [Google Scholar] [CrossRef]
- U.S Food & Drug Administration. Available online: https://www.fda.gov/food/food-ingredients-packaging (accessed on 6 June 2022).
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Dumont, C.; Bourgeois, S.; Fessi, H.; Jannin, V. Lipid-based nanosuspensions for oral delivery of peptides, a critical review. Int. J. Pharm. 2018, 541, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Huang, J.; Fishelson, Z.; Wang, C.; Zhang, S. Cell-Penetrating Peptide-Based Delivery of Macromolecular Drugs: Development, Strategies, and Progress. Biomedicines 2023, 11, 1971. [Google Scholar] [CrossRef] [PubMed]
- Malkawi, A.; Alrabadi, N.; Kennedy, R.A. Dual-Acting Zeta-Potential-Changing Micelles for Optimal Mucus Diffusion and Enhanced Cellular Uptake after Oral Delivery. Pharmaceutics 2021, 13, 974. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamad, N.-V. Strategies to Enhance the Solubility and Bioavailability of Tocotrienols Using Self-Emulsifying Drug Delivery System. Pharmaceuticals 2023, 16, 1403. https://doi.org/10.3390/ph16101403
Mohamad N-V. Strategies to Enhance the Solubility and Bioavailability of Tocotrienols Using Self-Emulsifying Drug Delivery System. Pharmaceuticals. 2023; 16(10):1403. https://doi.org/10.3390/ph16101403
Chicago/Turabian StyleMohamad, Nur-Vaizura. 2023. "Strategies to Enhance the Solubility and Bioavailability of Tocotrienols Using Self-Emulsifying Drug Delivery System" Pharmaceuticals 16, no. 10: 1403. https://doi.org/10.3390/ph16101403
APA StyleMohamad, N.-V. (2023). Strategies to Enhance the Solubility and Bioavailability of Tocotrienols Using Self-Emulsifying Drug Delivery System. Pharmaceuticals, 16(10), 1403. https://doi.org/10.3390/ph16101403