Drug Distribution and Penetration of Foam-Based Intraperitoneal Chemotherapy (FBIC)
Abstract
:1. Introduction
2. Results
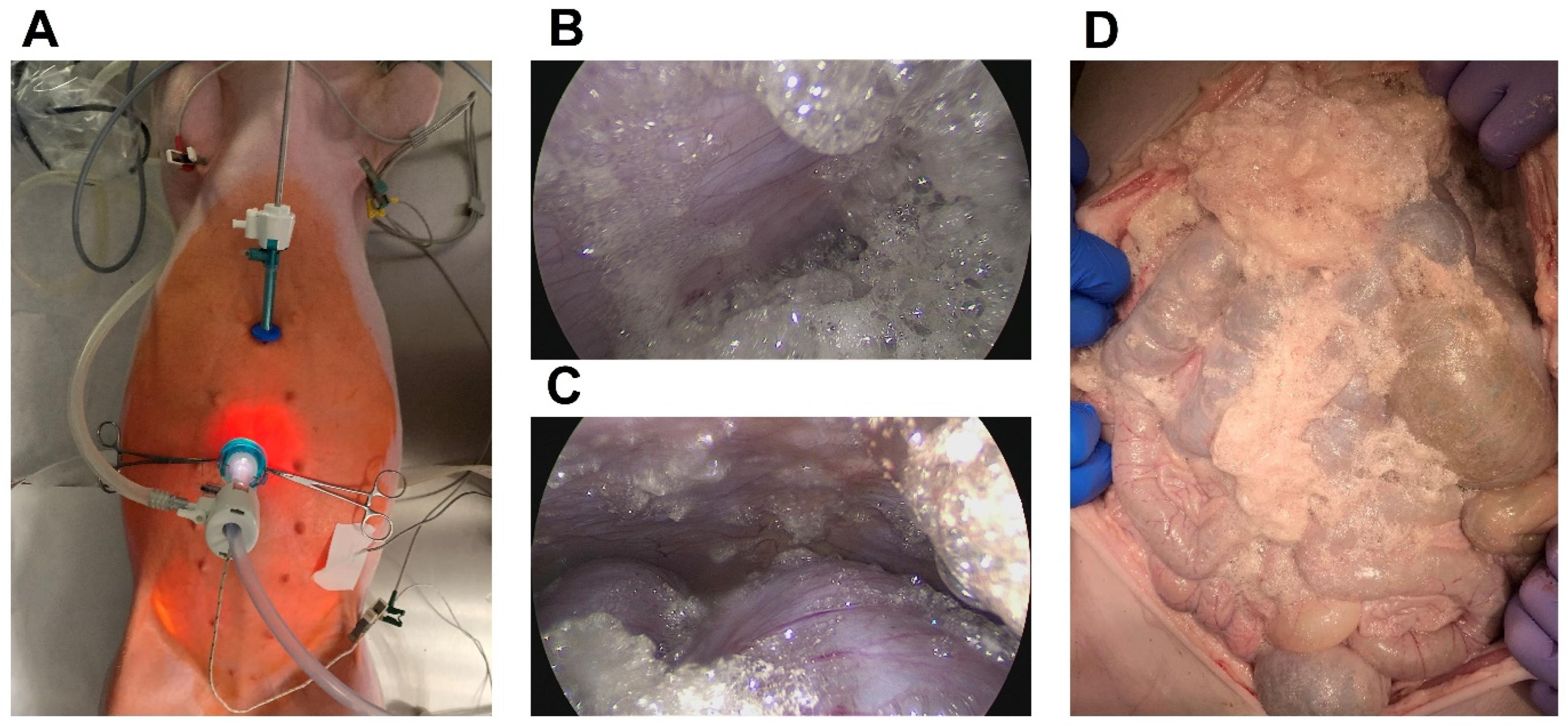
2.1. Feasibility and General Safety Concerns Regarding the Intraperitoneal Foam Application
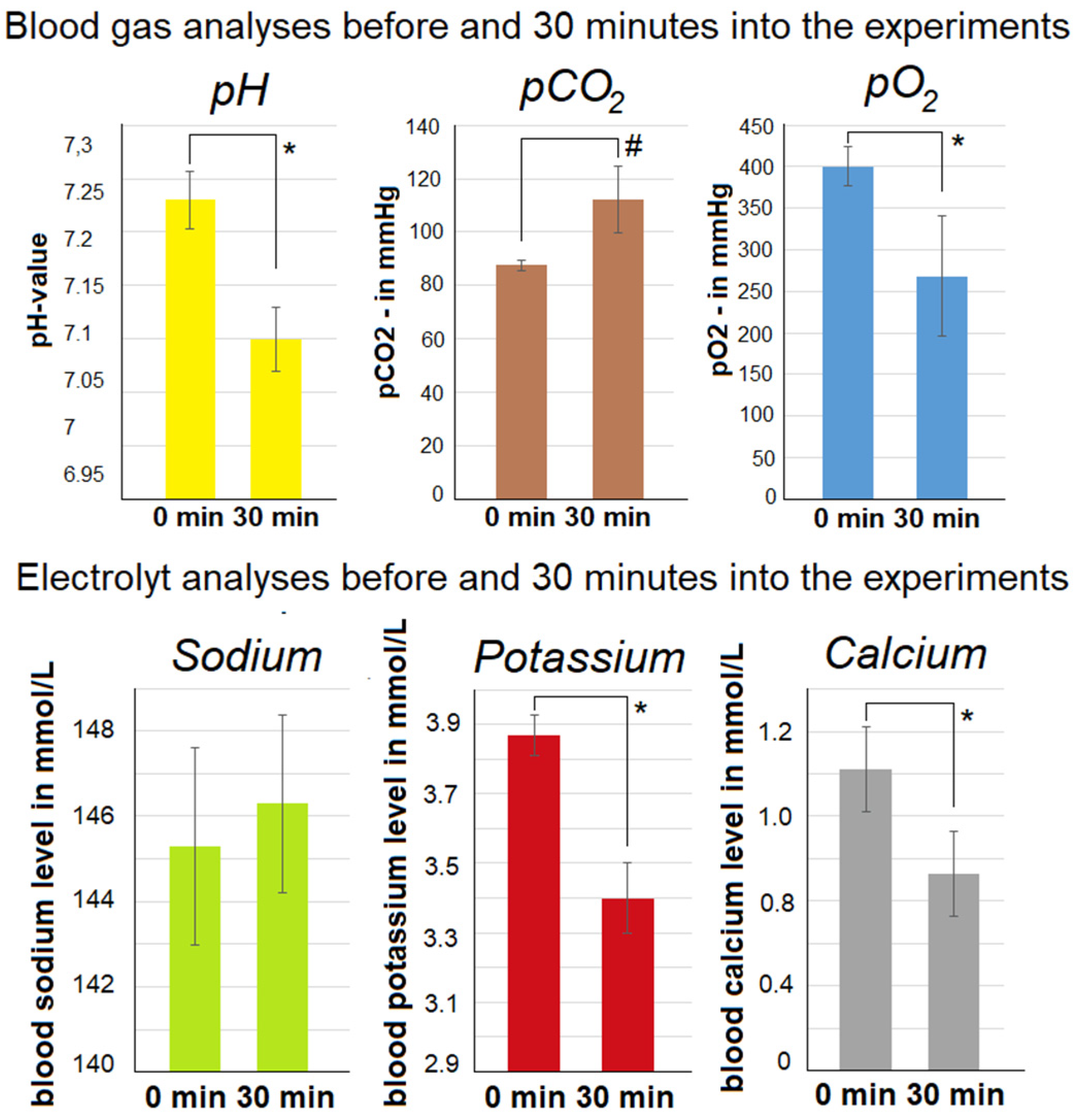
2.2. Abdominal Extension and Intraoperative Blood Gas Analyses
2.3. Development of Intraoperative Gasometric Parameters and Electrolytes
2.4. Evaluation of Computer Tomography
2.5. Evaluation of Histology
3. Discussion
4. Materials and Methods
4.1. Sequence of Procedures
4.2. The Laparoscopic In Vivo Swine Model
4.3. Euthanization
4.4. Histopathological Examination via Light Microscopy
4.5. Histological Examination via Fluorescence Microscopy
4.6. The Bicarbonate-Based Foam Carrier
4.7. Statistical Analyses of Data
4.8. Ethical Approval and Regulations
4.9. Graphic Design
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sadeghi, B.; Arvieux, C.; Glehen, O.; Beaujard, A.C.; Rivoire, M.; Baulieux, J.; Fontaumard, E.; Brachet, A.; Caillot, J.L.; Faure, J.L.; et al. Peritoneal carcinomatosis from non-gynecologic malignancies: Results of the EVOCAPE 1 multicentric prospective study. Cancer 2000, 88, 358–363. [Google Scholar] [CrossRef]
- Goéré, D.; Passot, G.; Gelli, M.; Levine, E.A.; Bartlett, D.L.; Sugarbaker, P.H.; Glehen, O. Complete cytoreductive surgery plus HIPEC for peritoneal metastases from unusual cancer sites of origin: Results from a worldwide analysis issue of the Peritoneal Surface Oncology Group International (PSOGI). Int. J. Hyperth. 2017, 33, 520–527. [Google Scholar] [CrossRef]
- Gelli, M.; Huguenin, J.F.L.; de Baere, T.; Benhaim, L.; Mariani, A.; Boige, V.; Malka, D.; Sourouille, I.; Ducreux, M.; Elias, D.; et al. Peritoneal and extraperitoneal relapse after previous curative treatment of peritoneal metastases from colorectal cancer: What survival can we expect? Eur. J. Cancer 2018, 100, 94–103. [Google Scholar] [CrossRef]
- Cheng, J.; Zhao, L.; Zhang, Y.; Qin, Y.; Guan, Y.; Zhang, T.; Liu, C.; Zhou, J. Understanding the Mechanisms of Resistance to CAR T-Cell Therapy in Malignancies. Front. Oncol. 2019, 9, 1237. [Google Scholar] [CrossRef]
- Shao, M.M.; Pei, X.B.; Chen, Q.Y.; Wang, F.; Wang, Z.; Zhai, K. Macrophage-derived exosome promotes regulatory T cell differentiation in malignant pleural effusion. Front. Immunol. 2023, 14, 1161375. [Google Scholar] [CrossRef]
- Mikolajczyk, A.; Khosrawipour, V.; Lau, H.; Li, S.; Migdal, P.; Labbé, M.K.; Kielan, W.; Nicpon, J.; Stieglitz, S.; Khosrawipour, T. Exploring the potential of taurolidine in inducing mobilization and detachment of colon cancer cells: A preliminary in-vitro study. BMC Pharmacol. Toxicol. 2022, 23, 38. [Google Scholar] [CrossRef]
- Schubert, J.; Khosrawipour, T.; Pigazzi, A.; Kulas, J.; Bania, J.; Migdal, P.; Arafkas, M.; Khosrawipour, V. Evaluation of Cell-detaching Effect of EDTA in Combination with Oxaliplatin for a Possible Application in HIPEC After Cytoreductive Surgery: A Preliminary in-vitro Study. Curr. Pharm. Des. 2019, 25, 4813–4819. [Google Scholar] [CrossRef]
- Souadka, A.; Essangri, H.; Majbar, M.A.; Benkabbou, A.; Boutayeb, S.; You, B.; Glehen, O.; Mohsine, R.; Bakrin, N. Hyperthermic Intraperitoneal Chemotherapy and Cytoreductive Surgery in Ovarian Cancer: An Umbrella Review of Meta-Analyses. Front. Oncol. 2022, 12, 809773. [Google Scholar] [CrossRef]
- Patel, M.; Arora, A.; Mukherjee, D.; Mukherjee, S. Effect of hyperthermic intraperitoneal chemotherapy (HIPEC) on survival and recurrence rates in advanced gastric cancer- a systematic review and meta-analysis. Int. J. Surg. 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Khosrawipour, V.; Mikolajczyk, A.; Schubert, J.; Khosrawipour, T. Pressurized Intra-peritoneal Aerosol Chemotherapy (PIPAC) via Endoscopical Microcatheter System. Anticancer Res. 2018, 38, 3447–3452. [Google Scholar] [CrossRef]
- Khosrawipour, T.; Schubert, J.; Khosrawipour, V.; Chaudhry, H.; Grzesiak, J.; Arafkas, M.; Mikolajczyk, A. Particle stability and structure on the peritoneal surface in pressurized intra-peritoneal aerosol chemotherapy (PIPAC) analysed by electron microscopy: First evidence of a new physical concept for PIPAC. Oncol. Lett. 2019, 17, 4921–4927. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk, A.; Khosrawipour, V.; Schubert, J.; Chaudhry, H.; Pigazzi, A.; Khosrawipour, T. Particle Stability During Pressurized Intra-peritoneal Aerosol Chemotherapy (PIPAC). Anticancer Res. 2018, 38, 4645–4649. [Google Scholar] [CrossRef]
- Schubert, J.; Khosrawipour, T.; Reinhard, S.; Arafkas, M.; Martino, A.; Bania, J.; Pieczka, M.; Pigazzi, A.; Khosrawipour, V. The concept of foam as a drug carrier for intraperitoneal chemotherapy, feasibility, cytotoxicity and characteristics. Sci. Rep. 2020, 10, 10341. [Google Scholar] [CrossRef] [PubMed]
- Khosrawipour, V.; Mikolajczyk, A.; Paslawski, R.; Plociennik, M.; Nowak, K.; Kulas, J.; Arafkas, M.; Khosrawipour, T. Intrathoracic aerosol chemotherapy via spray-catheter. Mol. Clin. Oncol. 2020, 12, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.; Khosrawipour, T.; Mikolajczyk, A.; Frelkiewicz, P.; Nicpon, J.; Arafkas, M.; Pigazzi, A.; Knoefel, W.T.; Khosrawipour, V. Intraperitoneal chemotherapy of the peritoneal surface using high-intensity ultrasound (HIUS): Investigation of technical feasibility, safety and possible limitations. J. Cancer 2020, 11, 7209–7215. [Google Scholar] [CrossRef]
- Diakun, A.; Khosrawipour, T.; Mikolajczyk-Martinez, A.; Kuropka, P.; Nicpoń, J.; Kiełbowicz, Z.; Prządka, P.; Liszka, B.; Li, S.; Lau, H.; et al. In-vivo thermodynamic exploration of gas-based intraperitoneal hyperthermia. Front. Oncol. 2022, 12, 925724. [Google Scholar] [CrossRef]
- Diakun, A.; Khosrawipour, T.; Mikolajczyk-Martinez, A.; Nicpoń, J.; Kiełbowicz, Z.; Prządka, P.; Liszka, B.; Kielan, W.; Zielinski, K.; Migdal, P.; et al. The Onset of In-Vivo Dehydration in Gas -Based Intraperitoneal Hyperthermia and Its Cytotoxic Effects on Colon Cancer Cells. Front. Oncol. 2022, 12, 927714. [Google Scholar] [CrossRef]
- Garber, J.C.; Barbee, R.W.; Bielitzko, J.T.; Clayton, L.A.; Donovan, J.C.; Hendriksen, C.F.M.; Kohn, D.F.; Lipman, P.A.; Locke, P.A.; Melcher, J.; et al. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; Available online: http://www.nap.edu/catalog/12910 (accessed on 18 June 2023).
- Jakob, S.M.; Knuesel, R.; Tenhunen, J.J.; Pradl, R.; Takala, J. Increasing abdominal pressure with and without PEEP: Effects on intra-peritoneal, intra-organ and intra-vascular pressures. BMC Gastroenterol. 2010, 10, 70. [Google Scholar] [CrossRef]
- Sugerman, H.; Windsor, A.; Bessos, M.; Wolfe, L. Intra-abdominal pressure, sagittal abdominal diameter and obesity comorbidity. J. Intern. Med. 1997, 241, 71–79. [Google Scholar] [CrossRef]
- Petrenko, A.P.; Castelo-Branco, C.; Marshalov, D.V.; Kuligin, A.V.; Mysovskaya, Y.S.; Shifman, E.M.; Abdulaev, A.M.R. Physiology of intra-abdominal volume during pregnancy. J. Obstet. Gynaecol. 2021, 41, 1016–1022. [Google Scholar] [CrossRef]
- Alijani, A.; Hanna, G.B.; Band, M.; Struthers, A.D.; Cuschieri, A. Cardiovascular autonomic function in patients with hemodynamic instability at induction of capnoperitoneum: A case-control study. Surg. Endosc. 2004, 18, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Beazley, S.G.; Cosford, K.; Duke-Novakovski, T. Cardiopulmonary effects of using carbon dioxide for laparoscopic surgery in cats. Can. Vet. J. 2011, 52, 973–978. [Google Scholar] [PubMed]
- Diakun, A.; Khosrawipour, T.; Mikolajczyk-Martinez, A.; Nicpoń, J.; Thelen, S.; Kiełbowicz, Z.; Prządka, P.; Liszka, B.; Kulas, J.; Zielinski, K.; et al. Safety, feasibility, and application of intraperitoneal gas-based hyperthermia beyond 43 °C in the treatment of peritoneal metastasis: An in-vivo pilot study. Front. Oncol. 2022, 12, 953920. [Google Scholar] [CrossRef] [PubMed]
- Schluermann, C.N.; Hoeppner, J.; Benk, C.; Schmidt, R.; Loop, T.; Kalbhenn, J. Intra-abdominal pressure, Cardiac Index and vascular resistance during hyperthermic intraperitoneal chemotherapy: A prospective observational study. Minerva Anestesiol. 2016, 82, 160–169. [Google Scholar] [PubMed]
- Bloomfield, G.; Saggi, B.; Blocher, C.; Sugerman, H. Physiologic effects of externally applied continuous negative abdominal pressure for intra-abdominal hypertension. J. Trauma. 1999, 46, 1009–1014, discussion 1014–1016. [Google Scholar] [CrossRef] [PubMed]
- Blobner, M.; Felber, A.R.; Hösl, P.; Gögler, S.; Schneck, H.J.; Jelen-Esselborn, S. Auswirkungen des Kapnoperitoneums auf den postoperativen Kohlendioxidhaushalt [Effect of capnoperitoneum on postoperative carbon dioxide homeostasis]. Anaesthesist 1994, 43, 718–722. (In Germany) [Google Scholar] [CrossRef] [PubMed]
- Martin, H.E.; Wertman, M. Clinical potassium problems. Calif. Med. 1950, 72, 133–141. [Google Scholar]
- Aronson, P.S.; Giebisch, G. Effects of pH on potassium: New explanations for old observations. J. Am. Soc. Nephrol. 2011, 22, 1981–1989. [Google Scholar] [CrossRef]
- Perez, G.O.; Oster, J.R.; Vaamonde, C.A. Serum potassium concentration in acidemic states. Nephron 1981, 27, 233–243. [Google Scholar] [CrossRef]
- Monchi, M. Citrate pathophysiology and metabolism. Transfus. Apher. Sci. 2017, 56, 28–30. [Google Scholar] [CrossRef]
- Kozik-Jaromin, J.; Nier, V.; Heemann, U.; Kreymann, B.; Böhler, J. Citrate pharmacokinetics and calcium levels during high-flux dialysis with regional citrate anticoagulation. Nephrol. Dial. Transpl. 2009, 24, 2244–2251. [Google Scholar] [CrossRef]
- Pepe, J.; Colangelo, L.; Biamonte, F.; Sonato, C.; Danese, V.C.; Cecchetti, V.; Occhiuto, M.; Piazzolla, V.; De Martino, V.; Ferrone, F.; et al. Diagnosis and management of hypocalcemia. Endocrine 2020, 69, 485–495. [Google Scholar] [CrossRef]
- Calpena Martínez, S.; Arapiles Muñoz, A. The value of the classic signs: Hypocalcemia. Med. Clin. 2021, 157, 554. [Google Scholar] [CrossRef] [PubMed]
- Isik, A.; Wysocki, A.P.; Memiş, U.; Sezgin, E.; Yezhikova, A.; Islambekov, Y. Factors Associated with the Occurrence and Healing of Umbilical Pilonidal Sinus: A Rare Clinical Entity. Adv. Skin Wound Care 2022, 35, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Arzideh, F.; Brandhorst, G.; Gurr, E.; Hinsch, W.; Hoff, T.; Roggenbuck, L.; Rothe, G.; Schumann, G.; Wolters, B.; Wosniok, W.; et al. An improved indirect approach for determining reference limits from intra-laboratory data bases exemplified by concentrations of electrolytes. J. Lab. Med. 2009, 33, 52–66. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khosrawipour, C.; Nicpoń, J.; Kiełbowicz, Z.; Prządka, P.; Liszka, B.; Khosrawipour, V.; Al-Jundi, S.; Li, S.; Lau, H.; Kulas, J.; et al. Drug Distribution and Penetration of Foam-Based Intraperitoneal Chemotherapy (FBIC). Pharmaceuticals 2023, 16, 1393. https://doi.org/10.3390/ph16101393
Khosrawipour C, Nicpoń J, Kiełbowicz Z, Prządka P, Liszka B, Khosrawipour V, Al-Jundi S, Li S, Lau H, Kulas J, et al. Drug Distribution and Penetration of Foam-Based Intraperitoneal Chemotherapy (FBIC). Pharmaceuticals. 2023; 16(10):1393. https://doi.org/10.3390/ph16101393
Chicago/Turabian StyleKhosrawipour, Carolina, Jakub Nicpoń, Zdzisław Kiełbowicz, Przemysław Prządka, Bartłomiej Liszka, Veria Khosrawipour, Said Al-Jundi, Shiri Li, Hien Lau, Joanna Kulas, and et al. 2023. "Drug Distribution and Penetration of Foam-Based Intraperitoneal Chemotherapy (FBIC)" Pharmaceuticals 16, no. 10: 1393. https://doi.org/10.3390/ph16101393
APA StyleKhosrawipour, C., Nicpoń, J., Kiełbowicz, Z., Prządka, P., Liszka, B., Khosrawipour, V., Al-Jundi, S., Li, S., Lau, H., Kulas, J., Kuropka, P., Diakun, A., Kielan, W., Chabowski, M., & Mikolajczyk-Martinez, A. (2023). Drug Distribution and Penetration of Foam-Based Intraperitoneal Chemotherapy (FBIC). Pharmaceuticals, 16(10), 1393. https://doi.org/10.3390/ph16101393







