First-Row Transition 7-Oxo-5-phenyl-1,2,4-triazolo[1,5-a]pyrimidine Metal Complexes: Antiparasitic Activity and Release Studies
Abstract
1. Introduction
2. Results
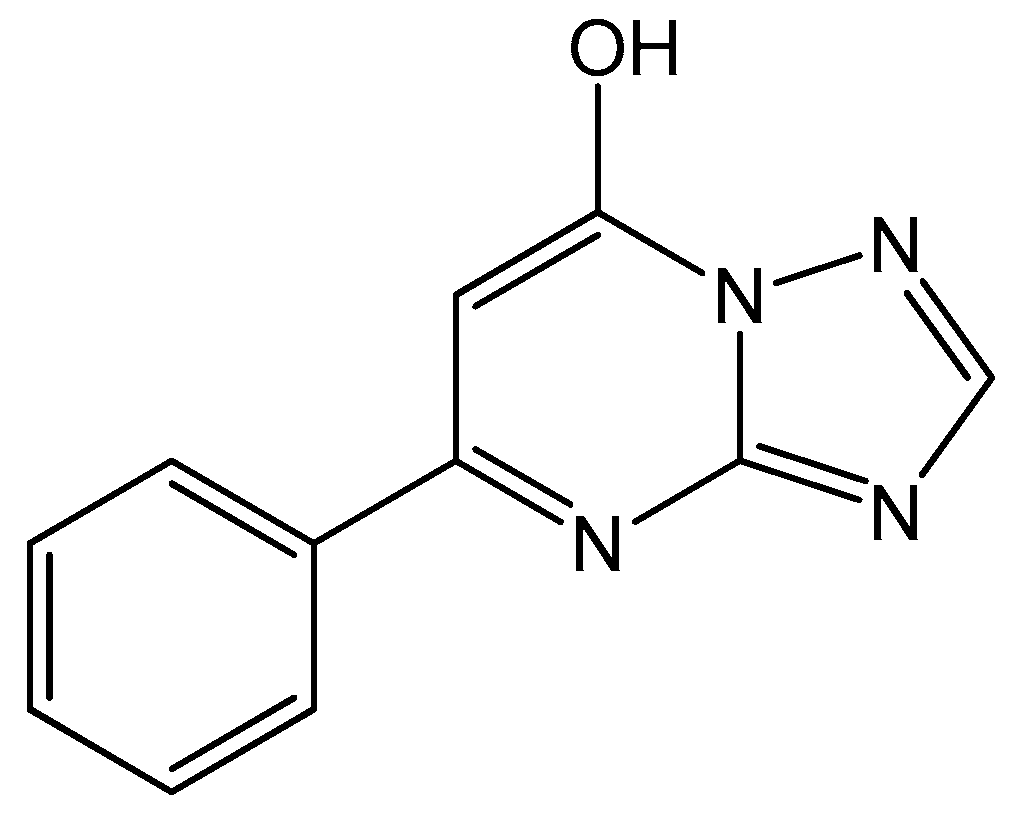
2.1. Synthesis and Characterization of Triazolopyrimidine Complexes
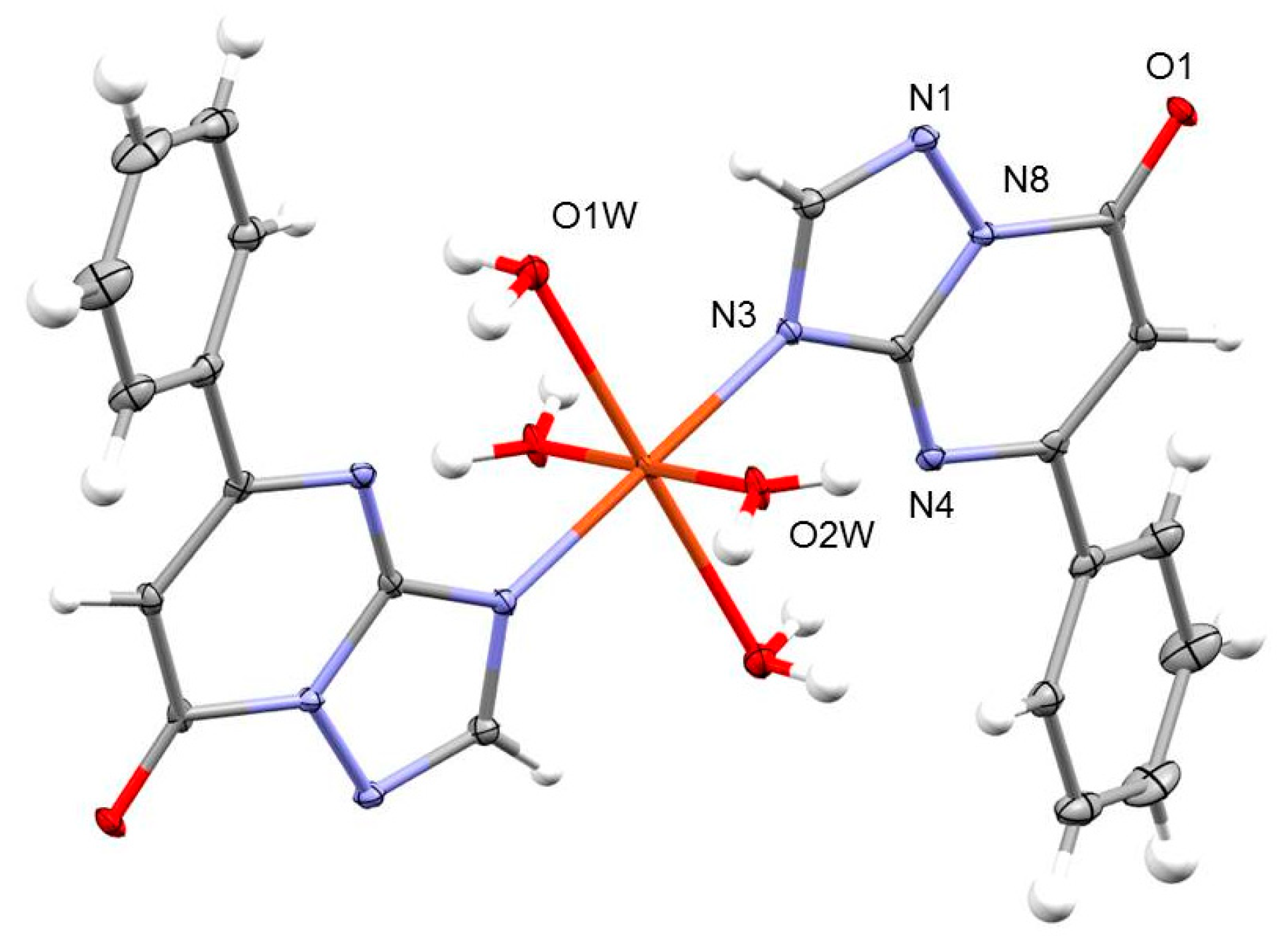
2.1.1. Crystal Structures of [M(ftpO)2(H2O)4] (1–4)
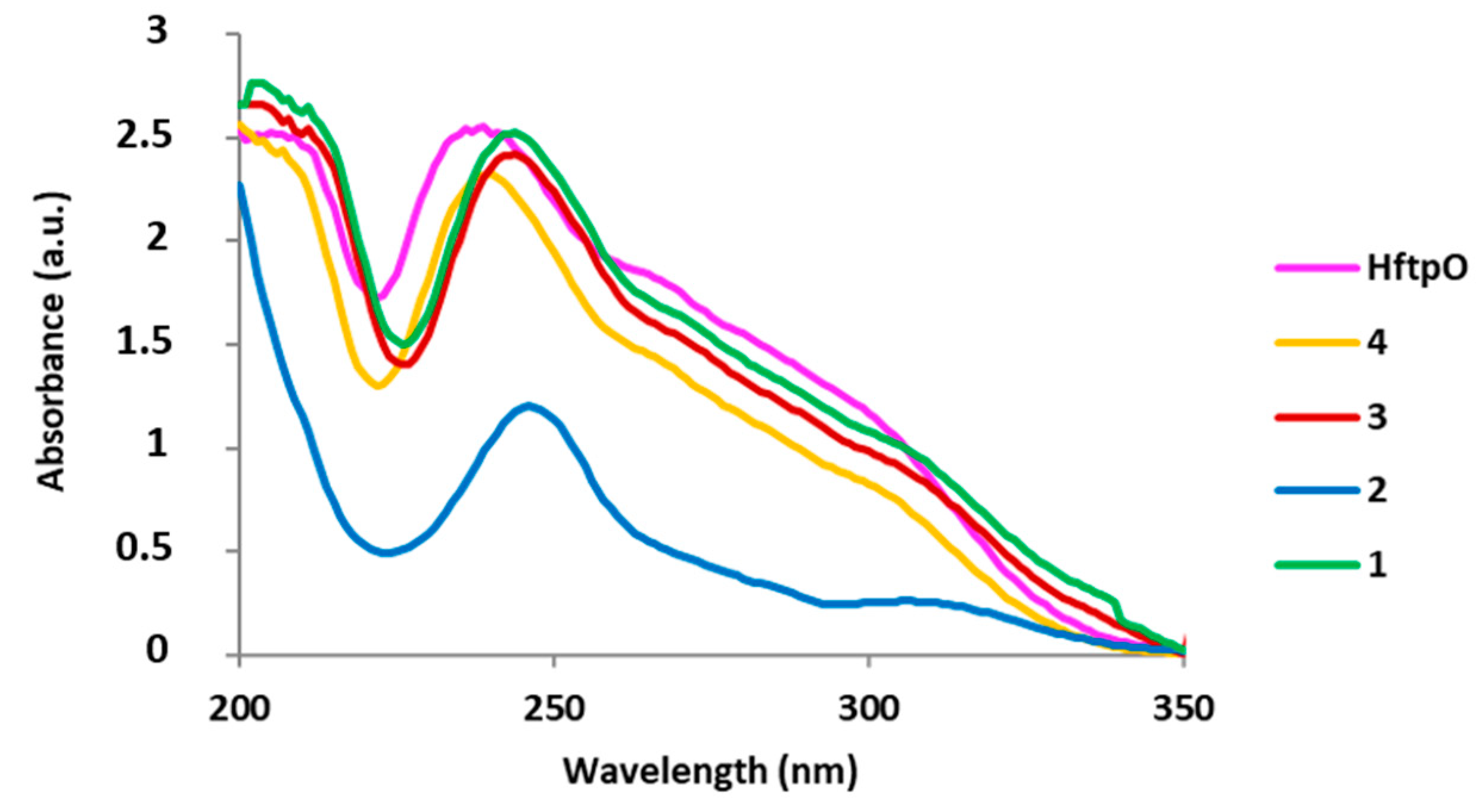
2.1.2. Spectroscopic Properties
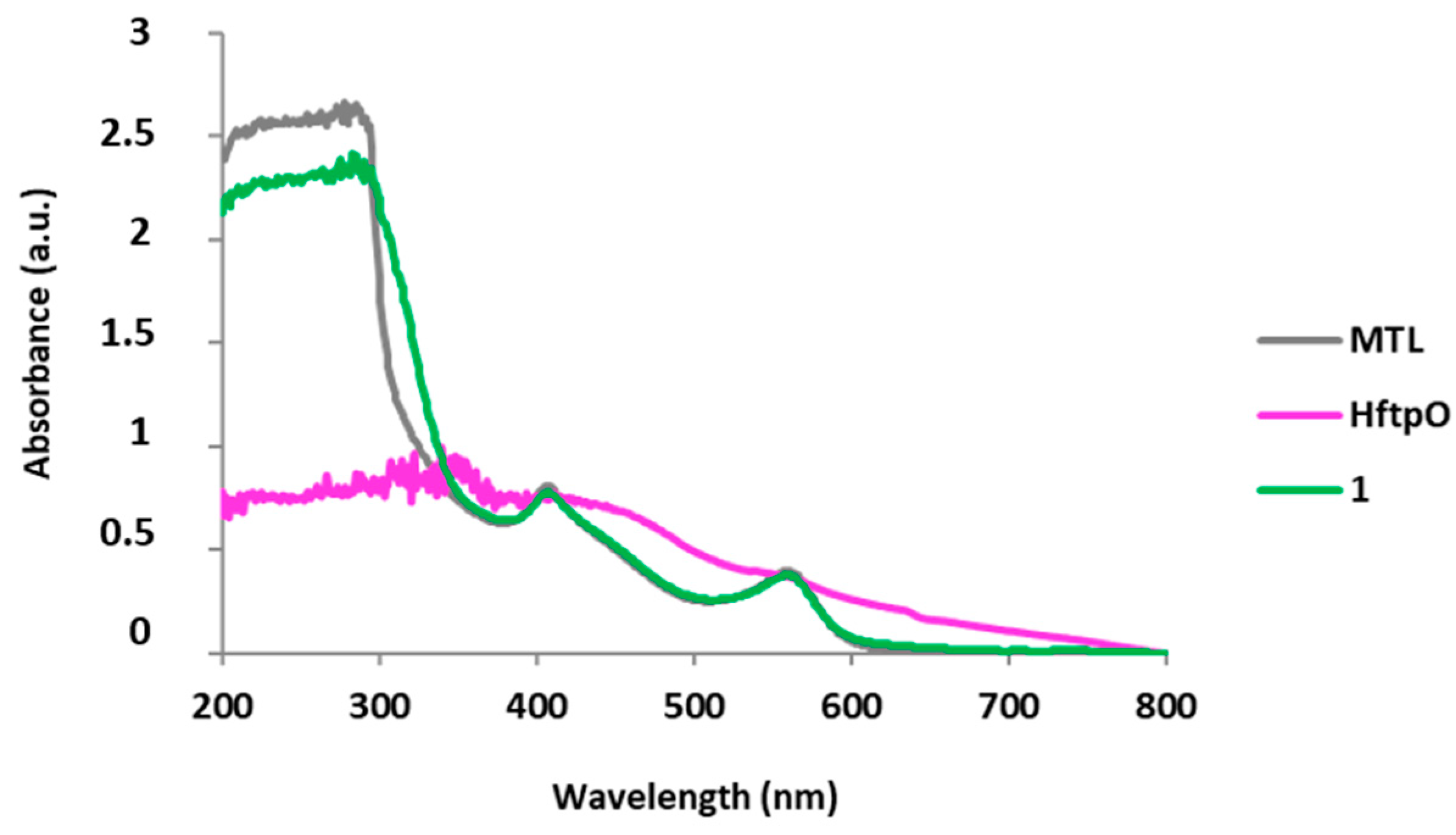
2.1.3. Luminescent Properties
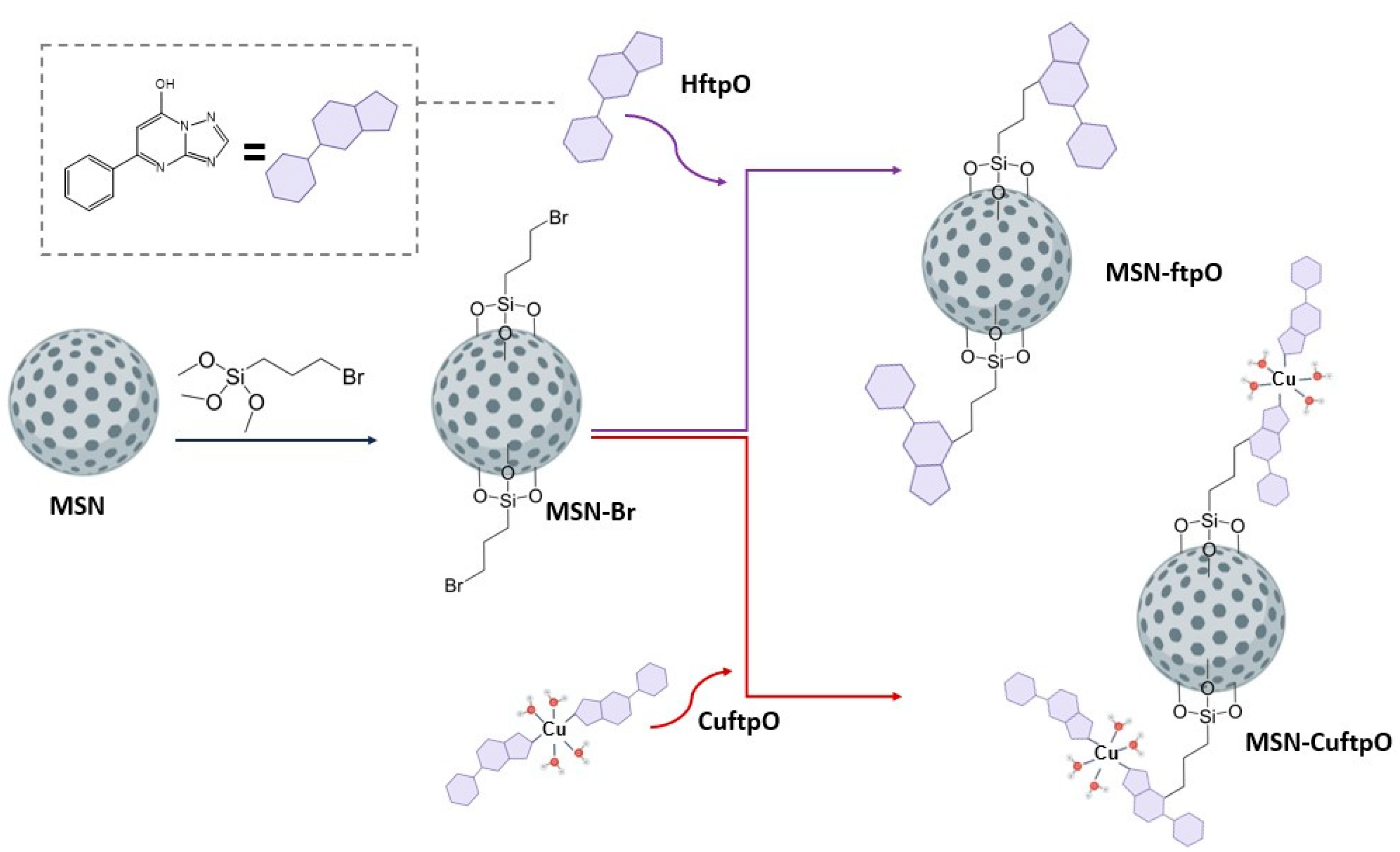
2.2. Synthesis and Characterization of Functionalized Silica Nanoparticles
2.2.1. Transmission Electron Microscopy (TEM)
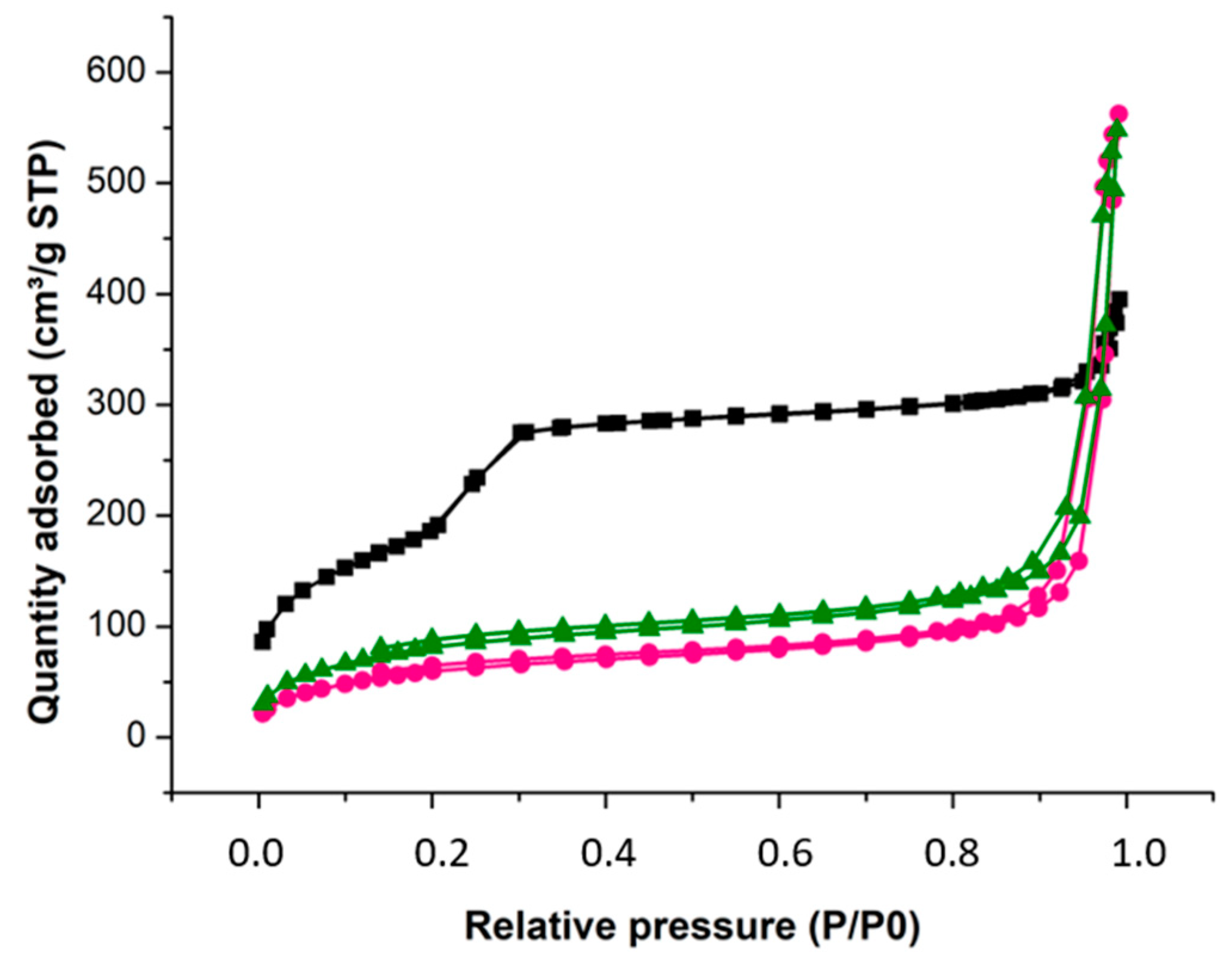
2.2.2. Nitrogen Adsorption/Desorption Isotherms
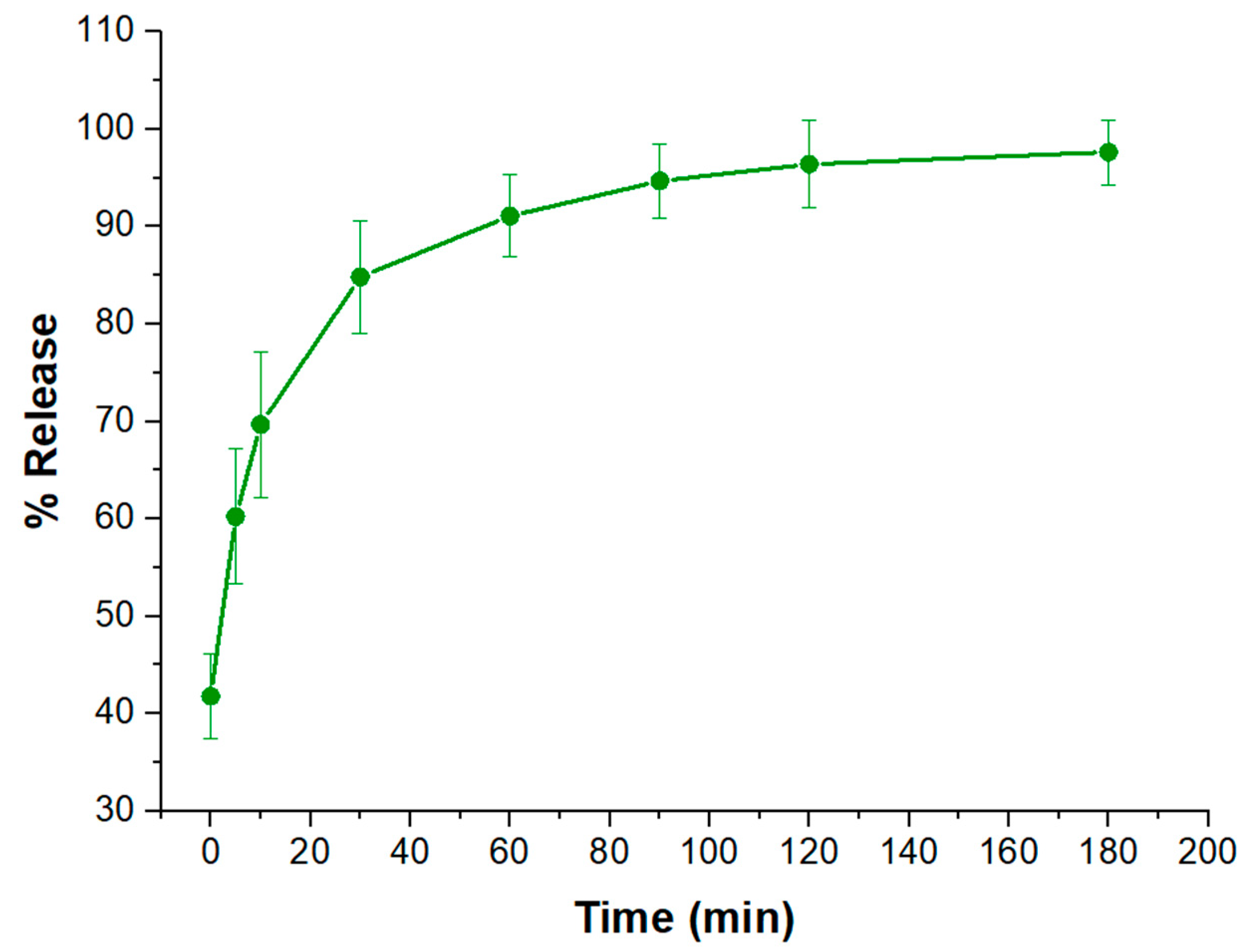
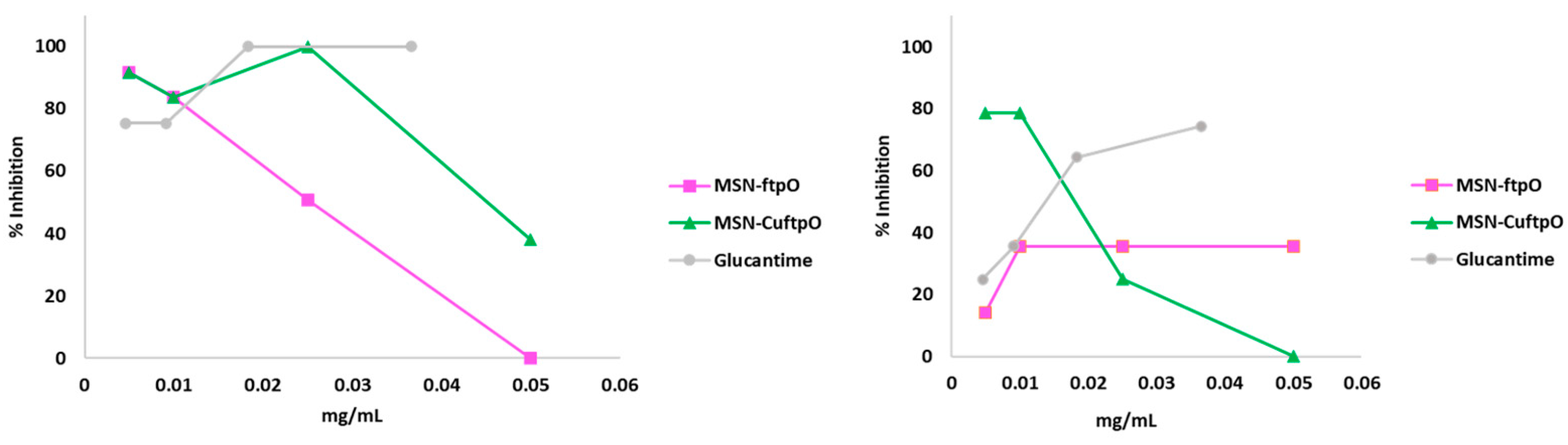
2.3. Release Studies
2.4. In Vitro Antiparasitic Activity and Toxicity
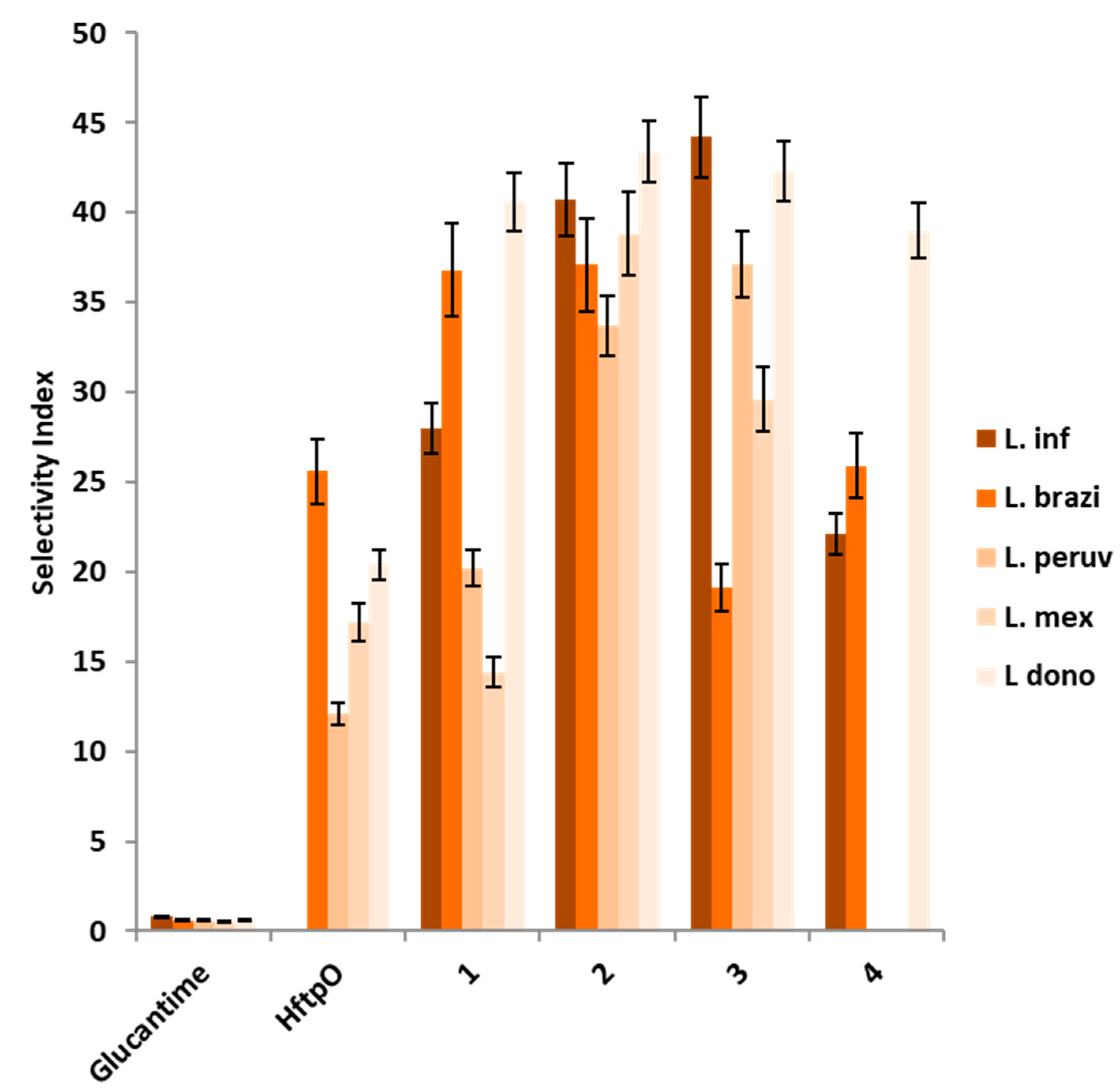
2.4.1. In Vitro Efficacy of HftpO and Its Complexes (1–4)
2.4.2. In Vitro Screening of MSN-ftpO and MSN-CuftpO
3. Discussion
4. Materials and Methods
4.1. General
4.2. Synthesis of HftpO and [M(ftpO)2(H2O)4] where M(II) = Cu (1), Co (2), Ni (3) and Zn (4)
4.3. Synthesis of Mesoporous Silica Nanoparticles
4.4. Functionalization of Mesoporous Silica Nanoparticles with (3-Bromopropyl)triethoxysilane (MSN-Br)
4.5. Ligand and Complex Coordination (MSN-ftpO and MSN-CuftpO)
4.6. Physical Measurements
4.7. Parasite Strain Culture
4.8. In Vitro Activity Assays
4.9. Cell Culture and Cytotoxicity Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bülow, C.; Haas, K. Synthetische Versuche Zur Darstellung von Derivaten Des Heterokondensierten, Heterocyclischen 1.3-Triazo-7.0′-Pyrimidins. Berichte Dtsch. Chem. Ges. 1909, 42, 4638–4644. [Google Scholar] [CrossRef]
- Birr, E.J. Azo-indolizine als photochemisch interessante Substanzen. Z. Wiss. Phot. 1952, 47, 1755–1764. [Google Scholar]
- Rodríguez Arce, E.; Sarniguet, C.; Moraes, T.S.; Vieites, M.; Tomaz, A.I.; Medeiros, A.; Comini, M.A.; Varela, J.; Cerecetto, H.; González, M.; et al. A New Ruthenium Cyclopentadienyl Azole Compound with Activity on Tumor Cell Lines and Trypanosomatid Parasites. J. Coord. Chem. 2015, 68, 2923–2937. [Google Scholar] [CrossRef]
- Ruiz, J.; Villa, M.D.; Cutillas, N.; López, G.; de Haro, C.; Bautista, D.; Moreno, V.; Valencia, L. Palladium(II) and Platinum(II) Organometallic Complexes with 4,7-Dihydro-5-Methyl-7-Oxo[1,2,4]Triazolo[1,5-a]Pyrimidine. Antitumor Activity of the Platinum Compounds. Inorg. Chem. 2008, 47, 4490–4505. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G. Recent Progress in 1,2,4-Triazolo[1,5-a]Pyrimidine Chemistry. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Academic Press: Cambridge, MA, USA, 2007; Volume 95, pp. 143–219. [Google Scholar]
- Romero, M.A.; Salas, J.M.; Quiros, M.; Sanchez, M.P.; Romero, J.; Martin, D. Structural and Magnetic Studies on a Bromine-Bridged Copper(II) Dimer with 5,7-Dimethyl[1,2,4]Triazolo[1,5-a]Pyrimidine. Inorg. Chem. 1994, 33, 5477–5481. [Google Scholar] [CrossRef]
- Salas, J.M.; Angustias Romero, M.; Purificación Sánchez, M.; Quirós, M. Metal Complexes of [1,2,4]Triazolo-[1,5-a]Pyrimidine Derivatives. Coord. Chem. Rev. 1999, 193–195, 1119–1142. [Google Scholar] [CrossRef]
- Hoffmann, K.; Wiśniewska, J.; Wojtczak, A.; Sitkowski, J.; Denslow, A.; Wietrzyk, J.; Jakubowski, M.; Łakomska, I. Rational Design of Dicarboxylato Platinum(II) Complexes with Purine-Mimetic Ligands as Novel Anticancer Agents. J. Inorg. Biochem. 2017, 172, 34–45. [Google Scholar] [CrossRef]
- Pinheiro, S.; Pinheiro, E.M.C.; Muri, E.M.F.; Pessôa, J.C.; Cadorini, M.A.; Greco, S.J. Biological Activities of [1,2,4]Triazolo[1,5-a]Pyrimidines and Analogs. Med. Chem. Res. 2020, 29, 1751–1776. [Google Scholar] [CrossRef]
- Hibot, A.; Oumessaoud, A.; Hafid, A.; Khouili, M.; Pujol, M.D. Different Synthetic Methods for the Preparation of Triazolopyrimidines and Their Biological Profile. ChemistrySelect 2023, 8, e202301654. [Google Scholar] [CrossRef]
- Caballero, A.B.; Rodríguez-Diéguez, A.; Quirós, M.; Salas, J.M.; Huertas, Ó.; Ramírez-Macías, I.; Olmo, F.; Marín, C.; Chaves-Lemaur, G.; Gutierrez-Sánchez, R.; et al. Triazolopyrimidine Compounds Containing First-Row Transition Metals and Their Activity against the Neglected Infectious Chagas Disease and Leishmaniasis. Eur. J. Med. Chem. 2014, 85, 526–534. [Google Scholar] [CrossRef]
- Astakhov, A.V.; Sokolov, A.N.; Pyatakov, D.A.; Shishkina, S.V.; Shishkin, O.V.; Chernyshev, V.M. Reactivity of 2-Amino[1,2,4]Triazolo[1,5-a]-Pyrimidines with Various Saturation of the Pyrimidine Ring towards Electrophiles. Chem. Heterocycl. Compd. 2015, 51, 1039–1047. [Google Scholar] [CrossRef]
- Wang, H.; Lee, M.; Peng, Z.; Blázquez, B.; Lastochkin, E.; Kumarasiri, M.; Bouley, R.; Chang, M.; Mobashery, S. Synthesis and Evaluation of 1,2,4-Triazolo[1,5-a]Pyrimidines as Antibacterial Agents Against Enterococcus Faecium. J. Med. Chem. 2015, 58, 4194–4203. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Arriaga, J.M.; Rubio-Mirallas, E.; Quirós, M.; Sánchez-Moreno, M. Zinc 1,2,4-Triazolo[1,5-a]Pyrimidine Complexes: Synthesis, Structural Characterization and Their Effect Against Chagas Disease. Med. Chem. 2022, 18, 444–451. [Google Scholar] [CrossRef]
- Fandzloch, M.; Dobrzańska, L.; Jędrzejewski, T.; Jezierska, J.; Wiśniewska, J.; Łakomska, I. Synthesis, Structure and Biological Evaluation of Ruthenium(III) Complexes of Triazolopyrimidines with Anticancer Properties. JBIC J. Biol. Inorg. Chem. 2020, 25, 109–124. [Google Scholar] [CrossRef]
- Maldonado, C.R.; Quirós, M.; Salas, J.M. 1,2,4-Triazolo[1,5-a]Pyrimidin-3-Ium Chloride. Acta Crystallogr. Sect. E 2007, 63, o1509–o1510. [Google Scholar] [CrossRef]
- Méndez-Arriaga, J.M.; Rodríguez-Diéguez, A.; Sánchez-Moreno, M. In Vitro Leishmanicidal Activity of Copper (II) 5,7-Dimethyl-1,2,4-Triazolo[1,5-a]Pyrimidine Complex and Analogous Transition Metal Series. Polyhedron 2020, 176, 114272. [Google Scholar] [CrossRef]
- Méndez-Arriaga, J.M.; Oyarzabal, I.; Escolano, G.; Rodríguez-Diéguez, A.; Sánchez-Moreno, M.; Salas, J.M. In Vitro Leishmanicidal and Trypanocidal Evaluation and Magnetic Properties of 7-Amino-1,2,4-Triazolo[1,5-a]Pyrimidine Cu(II) Complexes. J. Inorg. Biochem. 2018, 180, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Abul Haj, M.; Salas, J.M.; Quirós, M.; Molina, J.; Faure, R. 5-Oxo and 7-Oxo Derivatives of [1,2,4]Triazolo-[1,5-a]Pyrimidine: Characterization and Theoretical Study. J. Mol. Struct. 2000, 519, 165–172. [Google Scholar] [CrossRef]
- Navarro, J.A.R.; Romero, M.A.; Salas, J.M.; Faure, R.; Solans, X. Polymeric Silver(I) Complexes of the Multinucleating Ligand4,7-Dihydro-5-Methyl-7-Oxo[1,2,4]Triazolo[1,5-a]Pyrimidine.Analogous Hydrogen-Bonded Structures in the Crystal and Vapour Phases Ofthe Ligand. J. Chem. Soc. Dalton Trans. 1997, 1, 2321–2326. [Google Scholar] [CrossRef]
- Méndez-Arriaga, J.M.; Esteban-Parra, G.M.; Juárez, M.J.; Rodríguez-Diéguez, A.; Sánchez-Moreno, M.; Isac-García, J.; Salas, J.M. Antiparasitic Activity against Trypanosomatid Diseases and Novel Metal Complexes Derived from the First Time Characterized 5-Phenyl-1,2,4-Triazolo[1,5-a]Pyrimidi-7(4H)-One. J. Inorg. Biochem. 2017, 175, 217–224. [Google Scholar] [CrossRef]
- Steverding, D. The History of Leishmaniasis. Parasit. Vectors 2017, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Coombs, G.H. Leishmaniasis Current Chemotherapy and Recent Advances in the Search for Novel Drugs. Trends Parasitol. 2003, 19, 502–508. [Google Scholar] [CrossRef]
- Uliana, S.R.B.; Trinconi, C.T.; Coelho, A.C. Chemotherapy of Leishmaniasis: Present Challenges. Parasitology 2018, 145, 464–480. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A. Plant-Derived Compounds in Treatment of Leishmaniasis. Iran. J. Vet. Res. 2015, 16, 1–19. [Google Scholar] [CrossRef]
- Mohammadzadeh, M.; Behnaz, F.; Golshan, Z. Efficacy of Glucantime for Treatment of Cutaneous Leishmaniasis in Central Iran. J. Infect. Public Health 2013, 6, 120–124. [Google Scholar] [CrossRef]
- Alborzi, A.; Pouladfar, G.; Attar, A.; Falahi, F.; Jafarpour, Z.; Karimi, A.; Kadivar, M.R. Effectiveness of Short-Course Meglumine Antimoniate (Glucantime®) for Treatment of Visceral Leishmaniasis: A 13-Year, Multistage, Non-Inferiority Study in Iran. Am. J. Trop. Med. Hyg. 2017, 96, 182–189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, Y.; Messier, N.; Ouellette, M.; Rosen, B.P.; Mukhopadhyay, R. Leishmania major LmACR2 Is a Pentavalent Antimony Reductase That Confers Sensitivity to the Drug Pentostam. J. Biol. Chem. 2004, 279, 37445–37451. [Google Scholar] [CrossRef]
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas Disease: From Discovery to a Worldwide Health Problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef]
- Hotez, P.J.; Molyneux, D.H.; Fenwick, A.; Kumaresan, J.; Sachs, S.E.; Sachs, J.D.; Savioli, L. Control of Neglected Tropical Diseases. N. Engl. J. Med. 2007, 357, 1018–1027. [Google Scholar] [CrossRef]
- Córdoba-Aguilar, A. Chagas Bugs and Trypanosoma cruzi: Puppets and Puppeteer? Acta Trop. 2020, 211, 105600. [Google Scholar] [CrossRef]
- Abbott, A. Characteristics and Adverse Events of Patients for Whom Nifurtimox Was Released Through CDC-Sponsored Investigational New Drug Program for Treatment of Chagas Disease—United States, 2001–2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Norman, F.F.; Pérez-Molina, J.A.; López-Vélez, R. Benznidazole Shortage Makes Chagas Disease a Neglected Tropical Disease in Developed Countries: Data from Spain. Am. J. Trop. Med. Hyg. 2012, 87, 489–490. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crespillo-Andújar, C.; Comeche, B.; Hamer, D.H.; Arevalo-Rodriguez, I.; Alvarez-Díaz, N.; Zamora, J.; Pérez-Molina, J.A. Use of Benznidazole to Treat Chronic Chagas Disease: An Updated Systematic Review with a Meta-Analysis. PLoS Negl. Trop. Dis. 2022, 16, e0010386. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.D.; Gallalee, J.V.; Best, J.M. Sodium Stibogluconate (Pentostam) Inhibition of Glucose Catabolism via the Glycolytic Pathway, and Fatty Acid β-Oxidation in Leishmania mexicana Amastigotes. Biochem. Pharmacol. 1987, 36, 197–201. [Google Scholar] [CrossRef]
- Baneth, G.; Shaw, S.E. Chemotherapy of Canine Leishmaniosis. Vet. Parasitol. 2002, 106, 315–324. [Google Scholar] [CrossRef]
- Oliveira, L.F.; Schubach, A.O.; Martins, M.M.; Passos, S.L.; Oliveira, R.V.; Marzochi, M.C.; Andrade, C.A. Systematic Review of the Adverse Effects of Cutaneous Leishmaniasis Treatment in the New World. Acta Trop. 2011, 118, 87–96. [Google Scholar] [CrossRef]
- Moreno-Viguri, E.; Jiménez-Montes, C.; Martín-Escolano, R.; Santivañez-Veliz, M.; Martin-Montes, A.; Azqueta, A.; Jimenez-Lopez, M.; Zamora Ledesma, S.; Cirauqui, N.; López de Ceráin, A.; et al. In Vitro and in Vivo Anti-Trypanosoma cruzi Activity of New Arylamine Mannich Base-Type Derivatives. J. Med. Chem. 2016, 59, 10929–10945. [Google Scholar] [CrossRef]
- Baquedano, Y.; Alcolea, V.; Toro, M.Á.; Gutiérrez, K.J.; Nguewa, P.; Font, M.; Moreno, E.; Espuelas, S.; Jiménez-Ruiz, A.; Palop, J.A.; et al. Novel Heteroaryl Selenocyanates and Diselenides as Potent Antileishmanial Agents. Antimicrob. Agents Chemother. 2016, 60, 3802–3812. [Google Scholar] [CrossRef]
- Beltran-Hortelano, I.; Perez-Silanes, S.; Galiano, S. Trypanothione Reductase and Superoxide Dismutase as Current Drug Targets for Trypanosoma cruzi: An Overview of Compounds with Activity against Chagas Disease. Curr. Med. Chem. 2017, 24, 1066–1138. [Google Scholar] [CrossRef]
- Frézard, F.; Demicheli, C.; Ribeiro, R.R. Pentavalent Antimonials: New Perspectives for Old Drugs. Molecules 2009, 14, 2317–2336. [Google Scholar] [CrossRef]
- Marques, S.A.; Merlotto, M.R.; Ramos, P.M.; Marques, M.E.A. American Tegumentary Leishmaniasis: Severe Side Effects of Pentavalent Antimonial in a Patient with Chronic Renal Failure. An. Bras. Dermatol. 2019, 94, 355–357. [Google Scholar] [CrossRef]
- An, I.; Harman, M.; Esen, M.; Çelik, H. The Effect of Pentavalent Antimonial Compounds Used in the Treatment of Cutaneous Leishmaniasis on Hemogram and Biochemical Parameters. Cutan. Ocul. Toxicol. 2019, 38, 294–297. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. Liposomal Amphotericin B and Leishmaniasis: Dose and Response. J. Glob. Infect. Dis. 2010, 2, 159. [Google Scholar] [CrossRef]
- Diego, V.L.J.; Cristina, C.C.D. Leishmaniasis cutánea y anfotericina B liposomal: Reporte de caso. Infectio 2013, 17, 201–204. [Google Scholar] [CrossRef]
- Alpizar-Sosa, E.A.; Ithnin, N.R.B.; Wei, W.; Pountain, A.W.; Weidt, S.K.; Donachie, A.M.; Ritchie, R.; Dickie, E.A.; Burchmore, R.J.S.; Denny, P.W.; et al. Amphotericin B Resistance in Leishmania mexicana: Alterations to Sterol Metabolism and Oxidative Stress Response. PLoS Negl. Trop. Dis. 2022, 16, e0010779. [Google Scholar] [CrossRef]
- Palumbo, E. Current Treatment for Cutaneous Leishmaniasis: A Review. Am. J. Ther. 2009, 16, 178. [Google Scholar] [CrossRef]
- Natera, S.; Machuca, C.; Padrón-Nieves, M.; Romero, A.; Díaz, E.; Ponte-Sucre, A. Leishmania spp.: Proficiency of Drug-Resistant Parasites. Int. J. Antimicrob. Agents 2007, 29, 637–642. [Google Scholar] [CrossRef]
- Wijnant, G.-J.; Dumetz, F.; Dirkx, L.; Bulté, D.; Cuypers, B.; Van Bocxlaer, K.; Hendrickx, S. Tackling Drug Resistance and Other Causes of Treatment Failure in Leishmaniasis. Front. Trop. Dis. 2022, 3, 837460. [Google Scholar] [CrossRef]
- Rice, D.R.; Vacchina, P.; Norris-Mullins, B.; Morales, M.A.; Smith, B.D. Zinc(II)-Dipicolylamine Coordination Complexes as Targeting and Chemotherapeutic Agents for Leishmania Major. Antimicrob. Agents Chemother. 2016, 60, 2932–2940. [Google Scholar] [CrossRef]
- Soldera, P.d.F.; Chagas, A.F.d.S.; Brasil, A.M.V.; Comandolli-Wyrepkowski, C.D.; Porchia, M.; Pereira, A.M.R.F. In Vitro and in Vivo Anti-Leishmanial Potential of [Ag(PTA)4]BF4 and [Ag(HBPz3)(PPh3)] Silver Complexes. Rev. Soc. Bras. Med. Trop. 2022, 55, e0478. [Google Scholar] [CrossRef]
- Fernández, M.; Arce, E.R.; Sarniguet, C.; Morais, T.S.; Tomaz, A.I.; Azar, C.O.; Figueroa, R.; Diego Maya, J.; Medeiros, A.; Comini, M.; et al. Novel Ruthenium(II) Cyclopentadienyl Thiosemicarbazone Compounds with Antiproliferative Activity on Pathogenic Trypanosomatid Parasites. J. Inorg. Biochem. 2015, 153, 306–314. [Google Scholar] [CrossRef]
- Fandzloch, M.; Arriaga, J.M.M.; Sánchez-Moreno, M.; Wojtczak, A.; Jezierska, J.; Sitkowski, J.; Wiśniewska, J.; Salas, J.M.; Łakomska, I. Strategies for Overcoming Tropical Disease by Ruthenium Complexes with Purine Analog: Application against Leishmania Spp. and Trypanosoma cruzi. J. Inorg. Biochem. 2017, 176, 144–155. [Google Scholar] [CrossRef]
- Rodríguez Arce, E.; Machado, I.; Rodríguez, B.; Lapier, M.; Zúñiga, M.C.; Maya, J.D.; Olea Azar, C.; Otero, L.; Gambino, D. Rhenium(I) Tricarbonyl Compounds of Bioactive Thiosemicarbazones: Synthesis, Characterization and Activity against Trypanosoma cruzi. J. Inorg. Biochem. 2017, 170, 125–133. [Google Scholar] [CrossRef]
- do Nascimento, N.R.F.; de Aguiar, F.L.N.; Santos, C.F.; Costa, A.M.L.; Hardoim, D.d.J.; Calabrese, K.d.S.; Almeida-Souza, F.; de Sousa, E.H.S.; Lopes, L.G.d.F.; Teixeira, M.J.; et al. In Vitro and in Vivo Leishmanicidal Activity of a Ruthenium Nitrosyl Complex against Leishmania (Viannia) braziliensis. Acta Trop. 2019, 192, 61–65. [Google Scholar] [CrossRef]
- Braga, S.S. Ruthenium Complexes, an Emerging Class of Leishmanicidal Drug Candidates. Appl. Biosci. 2022, 1, 129–142. [Google Scholar] [CrossRef]
- Caballero, A.B.; Rodríguez-Diéguez, A.; Vidal, I.; Dobado, J.A.; Castillo, Ó.; Lezama, L.; Salas, J.M. Insights on the Binding Ability of a New Adenine Analog: 7-Amine-1,2,4-Triazolo[1,5-a]Pyrimidine. Synthesis and Magnetic Study of the First Copper(II) Complexes. Dalton Trans. 2012, 41, 1755–1764. [Google Scholar] [CrossRef]
- Caballero, A.B.; Rodríguez-Diéguez, A.; Salas, J.M.; Sánchez-Moreno, M.; Marín, C.; Ramírez-Macías, I.; Santamaría-Díaz, N.; Gutiérrez-Sánchez, R. Lanthanide Complexes Containing 5-Methyl-1,2,4-Triazolo[1,5-a] Pyrimidin-7(4H)-One and Their Therapeutic Potential to Fight Leishmaniasis and Chagas Disease. J. Inorg. Biochem. 2014, 138, 39–46. [Google Scholar] [CrossRef]
- Esteban-Parra, G.M.; Méndez-Arriaga, J.M.; Rodríguez-Diéguez, A.; Quirós, M.; Salas, J.M.; Sánchez-Moreno, M. High Antiparasitic Activity of Silver Complexes of 5,7-Dimethyl-1,2,4-Triazolo[1,5 a]Pyrimidine. J. Inorg. Biochem. 2019, 201, 110810. [Google Scholar] [CrossRef]
- Méndez-Arriaga, J.M.; Oyarzabal, I.; Martín-Montes, Á.; García-Rodríguez, J.; Quirós, M.; Sánchez-Moreno, M. First Example of Antiparasitic Activity Influenced by Thermochromism: Leishmanicidal Evaluation of 5,7-Dimethyl-1,2,4-Triazolo[1,5-a]Pyrimidine Metal Complexes. Med. Chem. 2020, 16, 422–430. [Google Scholar] [CrossRef]
- García-García, A.; Méndez-Arriaga, J.M.; Martín-Escolano, R.; Cepeda, J.; Gómez-Ruiz, S.; Salinas-Castillo, A.; Seco, J.M.; Sánchez-Moreno, M.; Choquesillo-Lazarte, D.; Ruiz-Muelle, A.B.; et al. In Vitro Evaluation of Leishmanicidal Properties of a New Family of Monodimensional Coordination Polymers Based on Diclofenac Ligand. Polyhedron 2020, 184, 114570. [Google Scholar] [CrossRef]
- Sumithaa, C.; Ganeshpandian, M. Half-Sandwich Ruthenium Arene Complexes Bearing Clinically Approved Drugs as Ligands: The Importance of Metal–Drug Synergism in Metallodrug Design. Mol. Pharm. 2023, 20, 1453–1479. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.C.; de Azevedo-França, J.A.; Barrias, E.; Cavalcante, S.C.F.; Vieira, E.G.; Ferreira, A.M.D.C.; de Souza, W.; Navarro, M. Silver and Copper-Benznidazole Derivatives as Potential Antiparasitic Metallodrugs: Synthesis, Characterization, and Biological Evaluation. J. Inorg. Biochem. 2023, 239, 112047. [Google Scholar] [CrossRef] [PubMed]
- Łakomska, I.; Fandzloch, M. Application of 1,2,4-Triazolo[1,5-a]Pyrimidines for the Design of Coordination Compounds with Interesting Structures and New Biological Properties. Coord. Chem. Rev. 2016, 327–328, 221–241. [Google Scholar] [CrossRef]
- Salas, J.M.; Caballero, A.B.; Esteban-Parra, G.M.; Méndez-Arriaga, J.M. Leishmanicidal and Trypanocidal Activity of Metal Complexes with 1,2,4-Triazolo[1,5-a]Pyrimidines: Insights on Their Therapeutic Potential against Leishmaniasis and Chagas Disease. Curr. Med. Chem. 2017, 24, 2796–2806. [Google Scholar] [CrossRef] [PubMed]
- Scalese, G.; Kostenkova, K.; Crans, D.C.; Gambino, D. Metallomics and Other Omics Approaches in Antiparasitic Metal-Based Drug Research. Curr. Opin. Chem. Biol. 2022, 67, 102127. [Google Scholar] [CrossRef] [PubMed]
- Damodharan, J. Nanomaterials in Medicine—An Overview. Mater. Today Proc. 2021, 37, 383–385. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, D.; Fischer-Fodor, E.; Vlad, C.I.; Méndez-Arriaga, J.M.; Prashar, S.; Gómez-Ruiz, S. Study of Cancer Cell Cytotoxicity, Internalization and Modulation of Growth Factors Induced by Transferrin-Conjugated Formulations of Metallodrug-Functionalized Mesoporous Silica Nanoparticles. Microporous Mesoporous Mater. 2021, 323, 111238. [Google Scholar] [CrossRef]
- Jimenez-Falcao, S.; Torres, D.; Martínez-Ruiz, P.; Vilela, D.; Martínez-Máñez, R.; Villalonga, R. Sucrose-Responsive Intercommunicated Janus Nanoparticles Network. Nanomaterials 2021, 11, 2492. [Google Scholar] [CrossRef]
- Madej, M.; Kurowska, N.; Strzalka-Mrozik, B. Polymeric Nanoparticles—Tools in a Drug Delivery System in Selected Cancer Therapies. Appl. Sci. 2022, 12, 9479. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Falcao, S.; Méndez-Arriaga, J.M.; García-Almodóvar, V.; García-Valdivia, A.A.; Gómez-Ruiz, S. Gold Nanozymes: Smart Hybrids with Outstanding Applications. Catalysts 2023, 13, 13. [Google Scholar] [CrossRef]
- Montiel Schneider, M.G.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef]
- Crisan, M.C.; Teodora, M.; Lucian, M. Copper Nanoparticles: Synthesis and Characterization, Physiology, Toxicity and Antimicrobial Applications. Appl. Sci. 2022, 12, 141. [Google Scholar] [CrossRef]
- del Hierro, I.; Pérez, Y.; Fajardo, M. Supported Choline Hydroxide (Ionic Liquid) on Mesoporous Silica as Heterogeneous Catalyst for Knoevenagel Condensation Reactions. Microporous Mesoporous Mater. 2018, 263, 173–180. [Google Scholar] [CrossRef]
- Carvalho, G.C.; Sábio, R.M.; de Cássia Ribeiro, T.; Monteiro, A.S.; Pereira, D.V.; Ribeiro, S.J.L.; Chorilli, M. Highlights in Mesoporous Silica Nanoparticles as a Multifunctional Controlled Drug Delivery Nanoplatform for Infectious Diseases Treatment. Pharm. Res. 2020, 37, 191. [Google Scholar] [CrossRef]
- Díaz-García, D.; Ferrer-Donato, Á.; Méndez-Arriaga, J.M.; Cabrera-Pinto, M.; Díaz-Sánchez, M.; Prashar, S.; Fernandez-Martos, C.M.; Gómez-Ruiz, S. Design of Mesoporous Silica Nanoparticles for the Treatment of Amyotrophic Lateral Sclerosis (ALS) with a Therapeutic Cocktail Based on Leptin and Pioglitazone. ACS Biomater. Sci. Eng. 2022, 8, 4838–4849. [Google Scholar] [CrossRef]
- Tessarolo, L.D.; de Menezes, R.R.P.P.B.; Mello, C.P.; Lima, D.B.; Magalhães, E.P.; Bezerra, E.M.; Sales, F.A.M.; Neto, I.L.B.; Oliveira, M.d.F.; dos Santos, R.P.; et al. Nanoencapsulation of Benznidazole in Calcium Carbonate Increases Its Selectivity to Trypanosoma cruzi. Parasitology 2018, 145, 1191–1198. [Google Scholar] [CrossRef]
- Arrúa, E.C.; Seremeta, K.P.; Bedogni, G.R.; Okulik, N.B.; Salomon, C.J. Nanocarriers for Effective Delivery of Benznidazole and Nifurtimox in the Treatment of Chagas Disease: A Review. Acta Trop. 2019, 198, 105080. [Google Scholar] [CrossRef]
- Arrua, E.C.; Hartwig, O.; Loretz, B.; Goicoechea, H.; Murgia, X.; Lehr, C.-M.; Salomon, C.J. Improving the Oral Delivery of Benznidazole Nanoparticles by Optimizing the Formulation Parameters through a Design of Experiment and Optimization Strategy. Colloids Surf. B Biointerfaces 2022, 217, 112678. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Konar, S.; Jana, A.; Das, K.; Dhara, A.; Chatterjee, S.; Kar, S.K. Syntheses, Crystal Structure, Spectroscopic and Photoluminescence Studies of Mononuclear Copper(II), Manganese(II), Cadmium(II), and a 1D Polymeric Cu(II) Complexes with a Pyrimidine Derived Schiff Base Ligand. J. Mol. Struct. 2014, 1058, 213–220. [Google Scholar] [CrossRef]
- Zhao, Y.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.-Y. Mesoporous Silica Nanoparticle-Based Double Drug Delivery System for Glucose-Responsive Controlled Release of Insulin and Cyclic AMP. J. Am. Chem. Soc. 2009, 131, 8398–8400. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M.; Balas, F.; Colilla, M.; Manzano, M. Drug Confinement and Delivery in Ceramic Implants. Drug Metab. Lett. 2007, 1, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Nastase, S.; Bajenaru, L.; Berger, D.; Matei, C.; Moisescu, M.; Constantin, D.; Savopol, T. Mesostructured Silica Matrix for Irinotecan Delivery Systems. Open Chem. 2014, 12, 813–820. [Google Scholar] [CrossRef]
- In Vitro Screening of Antileishmanial Activity of Natural Product Compounds: Determination of IC50, CC50 and SI Values. Available online: https://en.bio-protocol.org/en/bpdetail?id=3410&type=0 (accessed on 18 September 2023).
- Caballero, A.B.; Marín, C.; Ramírez-Macías, I.; Rodríguez-Diéguez, A.; Quirós, M.; Salas, J.M.; Sánchez-Moreno, M. Structural Consequences of the Introduction of 2,2′-Bipyrimidine as Auxiliary Ligand in Triazolopyrimidine-Based Transition Metal Complexes. In Vitro Antiparasitic Activity. Polyhedron 2012, 33, 137–144. [Google Scholar] [CrossRef]
- Champagne, E.T.; Fisher, M.S. Binding Differences of Zn(II) and Cu(II) Ions with Phytate. J. Inorg. Biochem. 1990, 38, 217–223. [Google Scholar] [CrossRef]
- Côrte-Real, L.; Pósa, V.; Martins, M.; Colucas, R.; May, N.V.; Fontrodona, X.; Romero, I.; Mendes, F.; Pinto Reis, C.; Gaspar, M.M.; et al. Cu(II) and Zn(II) Complexes of New 8-Hydroxyquinoline Schiff Bases: Investigating Their Structure, Solution Speciation, and Anticancer Potential. Inorg. Chem. 2023, 62, 11466–11486. [Google Scholar] [CrossRef]
- Bollu, V.S.; Barui, A.K.; Mondal, S.K.; Prashar, S.; Fajardo, M.; Briones, D.; Rodríguez-Diéguez, A.; Patra, C.R.; Gómez-Ruiz, S. Curcumin-Loaded Silica-Based Mesoporous Materials: Synthesis, Characterization and Cytotoxic Properties against Cancer Cells. Mater. Sci. Eng. C 2016, 63, 393–410. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Aykul, S.; Martinez-Hackert, E. Determination of Half-Maximal Inhibitory Concentration Using Biosensor-Based Protein Interaction Analysis. Anal. Biochem. 2016, 508, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, J.C.; Naesens, L.; Montoya, J. Chapter 19—Treating HHV-6 Infections: The Laboratory Efficacy and Clinical Use of Anti-HHV-6 Agents. In Human Herpesviruses HHV-6A, HHV-6B & HHV-7, 3rd ed.; Flamand, L., Lautenschlager, I., Krueger, G.R.F., Ablashi, D.V., Eds.; Elsevier: Boston, MA, USA, 2014; pp. 311–331. ISBN 978-0-444-62703-2. [Google Scholar]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Chapter Six—Validation of in-Vitro Bioassay Methods: Application in Herbal Drug Research. In Profiles of Drug Substances, Excipients and Related Methodology; Al-Majed, A.A., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 46, pp. 273–307. [Google Scholar]


| Leishmania Species | Infection | Geographical Location | Reservoir | Vector |
|---|---|---|---|---|
| L. infantum | VL, CL, ML | New World for VL Old World for CL and ML | Rodents, dogs, foxes, jackals | L. longipalpis P. pcrniciosufi, P. arias |
| L. braziliensis | CL, MCL | New World | Forest rodents, anteaters | Lutzomyia spp. |
| L. peruviana | MCL | New World | Dogs | L. verrucarum, L. pvmenis |
| L. mexicana | CL, DCL, MCL | New World | Forest rodents | L. olmeca |
| L. donovani | VL, PKDL | Old World | Humans, rodents | P. argentipes, P. orinntalis, P. martini |
| Compound | Wavelength (nm) |
|---|---|
| HftpO | 238 |
| 1 | 245 |
| 2 | 244 |
| 3 | 246 |
| 4 | 240 |
| Material | SBET (m2g−1) | Total Pore Volume (cm3g−1) | Pore Diameter (nm) |
|---|---|---|---|
| MSN | 682 | 0.52 | 3.2 |
| MSN-ftpO | 227 | 0.47 | <2.0 |
| MSN-CuftpO | 305 | 0.49 | <2.0 |
| Compound | IC50 (µM) a ± SD | SI b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L. inf. | L. brazi. | L. peruv | L. mex. | L. dono. | J774.2 macro. | L. inf. | L. brazi. | L. peruv. | L. mex. | L. dono. | |
| Glucantime | 18.0 ± 3.1 | 25.6 ± 1.7 | 25.0 ± 2.0 | 31.0 ± 2.3 | 26.6 ± 5.4 | 15.2 ± 1.0 | 0.8 | 0.6 | 0.6 | 0.5 | 0.6 |
| HftpO | >200 | 37.5 ± 2.9 | 79.0 ± 6.7 | 116.6 ± 10.2 | 46.9 ± 3.8 | 958.5 ± 74.2 | - | 25.6 (42) | 12.1 (20) | 17.2 (34) | 20.4 (34) |
| 1 | 71.5 ± 5.8 | 54.4 ± 3.6 | 99.2 ± 8.7 | 139.3 ± 11.6 | 49.3 ± 3.9 | 1827.0 ± 138.6 | 25.6 (32) | 33.6 (56) | 18.4 (31) | 13.1 (26) | 37.1 (62) |
| 2 | 49.1 ± 4.4 | 53.9 ± 4.1 | 59.3 ± 6.6 | 51.6 ± 4.5 | 46.1 ± 3.3 | 1200.8 ± 111.4 | 24.5 (31) | 22.3 (37) | 20.2 (34) | 23.3 (47) | 26.0 (43) |
| 3 | 45.2 ± 3.8 | 104.8 ± 7.4 | 53.9 ± 5.1 | 67.6 ± 5.4 | 47.2 ± 3.7 | 2438.6 ± 156.3 | 53.9 (67) | 23.3 (39) | 45.2 (57) | 36.1 (72) | 51.7 (86) |
| 4 | 90.3 ± 6.1 | 77.3 ± 4.0 | >200 | >200 | 51.3 ± 3.2 | 1026.9 ± 89.4 | 11.4 (14) | 13.3 (22) | - | - | 20.0 (33) |
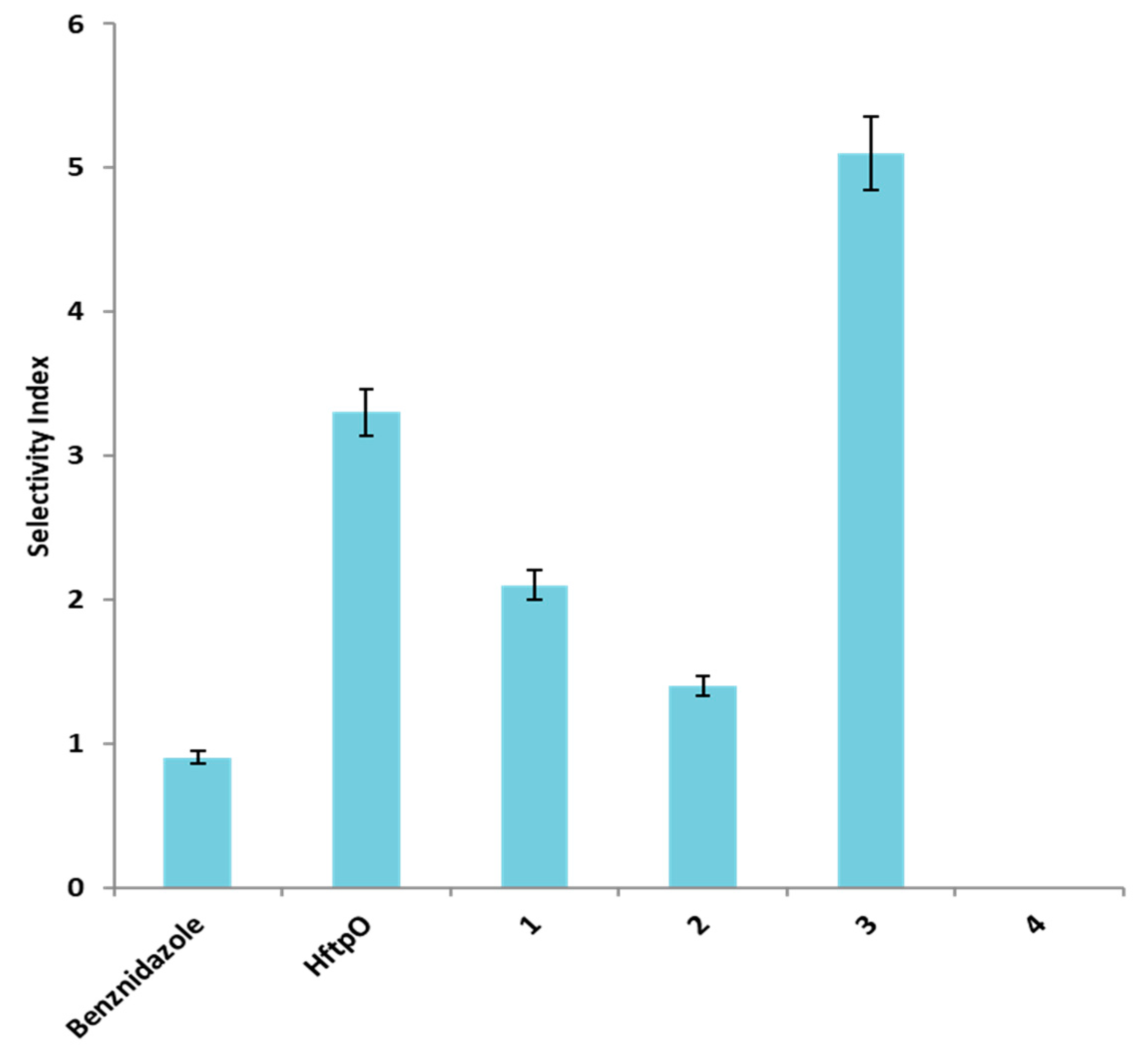
| Compound | IC50 (µM) a ± SD | SI b | |
|---|---|---|---|
| T. cruzi | Vero Cell | T. cruzi | |
| Benznidazole | 15.5 ± 1.4 | 13.6 ± 0.9 | 0.9 |
| HftpO | 173.0 ± 16.7 | 573.0 ± 43.8 | 3.3 (4) |
| 1 | 153.1 ± 13.2 | 322.8 ± 26.8 | 2.1 (2) |
| 2 | 112.5 ± 9.8 | 156.9 ± 11.4 | 1.4 (1) |
| 3 | 73.5 ± 6.8 | 375.8 ± 32.7 | 5.1 (6) |
| 4 | >200 | - | - |
| Compound | IC50 (µg/mL) a ± SD | SI b | |||
|---|---|---|---|---|---|
| L. inf. | L. brazi. | J774.2 macro. | L. inf. | L. brazi. | |
| Glucantime | 14.0 ± 1.4 | 6.0 ± 0.7 | 17.2 ± 1.0 | 1.2 | 2.9 |
| MSN | - | - | >1000 | - | - |
| MSN-Br | - | - | 54.6 ± 4.9 | - | - |
| MSN-ftpO | 25.0 ± 3.1 | 33.0 ± 3.8 | 55.4 ± 5.1 | 2.1 (2) | 1.7 (0) |
| MSN-CuftpO | 16.0 ± 0.8 | 19.0 ± 1.0 | 162 ± 18.6 | 8.7 (7) | 8.5 (3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Montes, Á.; Jimenez-Falcao, S.; Gómez-Ruiz, S.; Marín, C.; Mendez-Arriaga, J.M. First-Row Transition 7-Oxo-5-phenyl-1,2,4-triazolo[1,5-a]pyrimidine Metal Complexes: Antiparasitic Activity and Release Studies. Pharmaceuticals 2023, 16, 1380. https://doi.org/10.3390/ph16101380
Martín-Montes Á, Jimenez-Falcao S, Gómez-Ruiz S, Marín C, Mendez-Arriaga JM. First-Row Transition 7-Oxo-5-phenyl-1,2,4-triazolo[1,5-a]pyrimidine Metal Complexes: Antiparasitic Activity and Release Studies. Pharmaceuticals. 2023; 16(10):1380. https://doi.org/10.3390/ph16101380
Chicago/Turabian StyleMartín-Montes, Álvaro, Sandra Jimenez-Falcao, Santiago Gómez-Ruiz, Clotilde Marín, and José M. Mendez-Arriaga. 2023. "First-Row Transition 7-Oxo-5-phenyl-1,2,4-triazolo[1,5-a]pyrimidine Metal Complexes: Antiparasitic Activity and Release Studies" Pharmaceuticals 16, no. 10: 1380. https://doi.org/10.3390/ph16101380
APA StyleMartín-Montes, Á., Jimenez-Falcao, S., Gómez-Ruiz, S., Marín, C., & Mendez-Arriaga, J. M. (2023). First-Row Transition 7-Oxo-5-phenyl-1,2,4-triazolo[1,5-a]pyrimidine Metal Complexes: Antiparasitic Activity and Release Studies. Pharmaceuticals, 16(10), 1380. https://doi.org/10.3390/ph16101380












