Antioxidant Therapy in Inflammatory Bowel Disease: A Systematic Review and a Meta-Analysis of Randomized Clinical Trials
Abstract
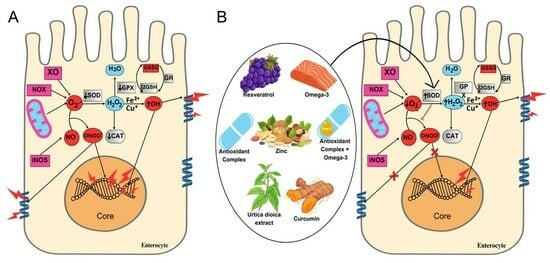
1. Introduction
2. Methods
2.1. Search Strategy and Selection of Studies
2.2. Eligibility of Clinical Research
2.2.1. Clinical Studies
2.2.2. Meta-Analysis
2.3. Data Extraction
2.3.1. Clinical Studies
2.3.2. Meta-Analysis
2.3.3. Assessment of the Risk of Bias
2.3.4. Statistical Analysis
3. Results
3.1. Search Results
3.2. Risk of Bias
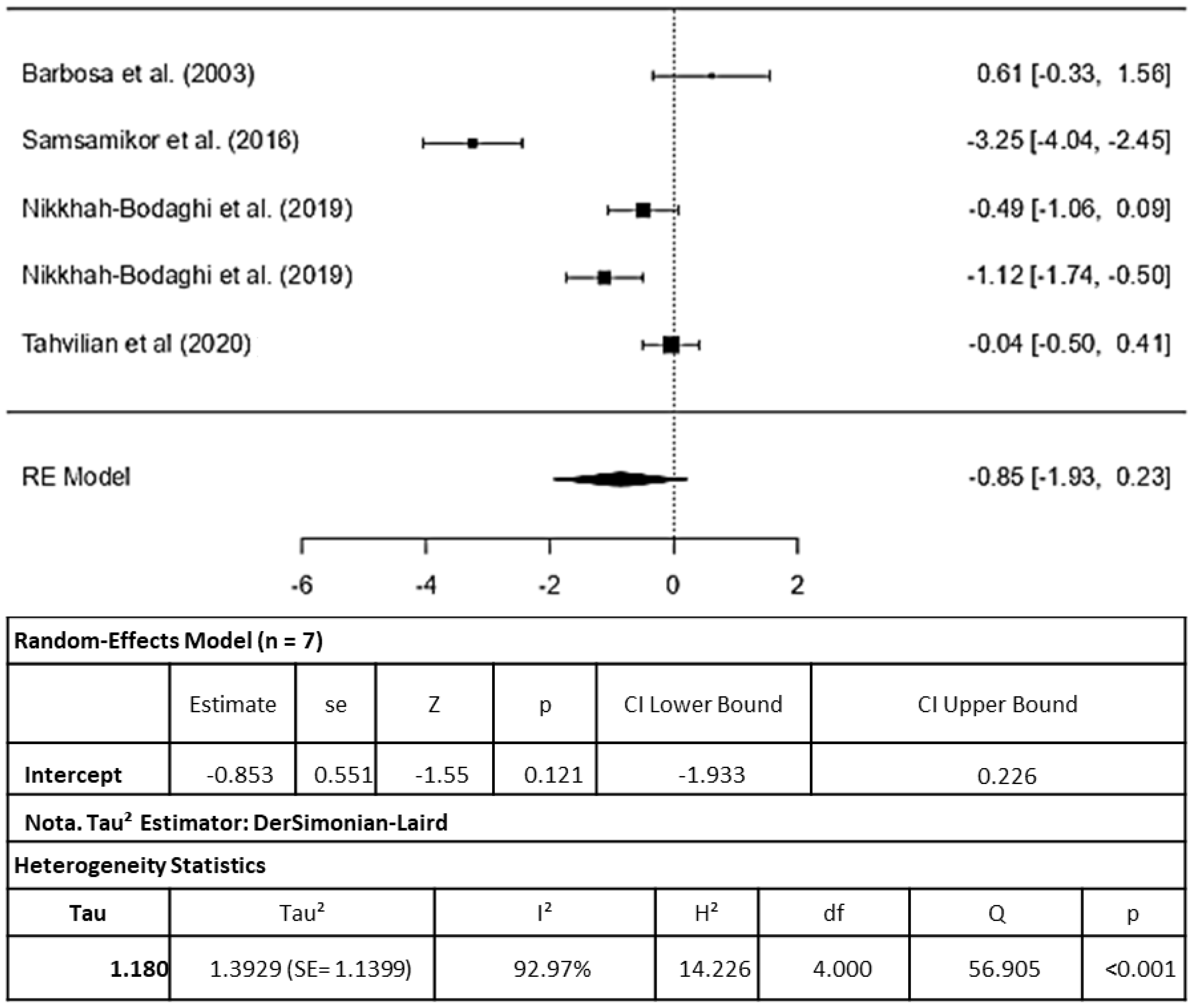
3.3. Randomized Clinical Trial: Meta-Analysis
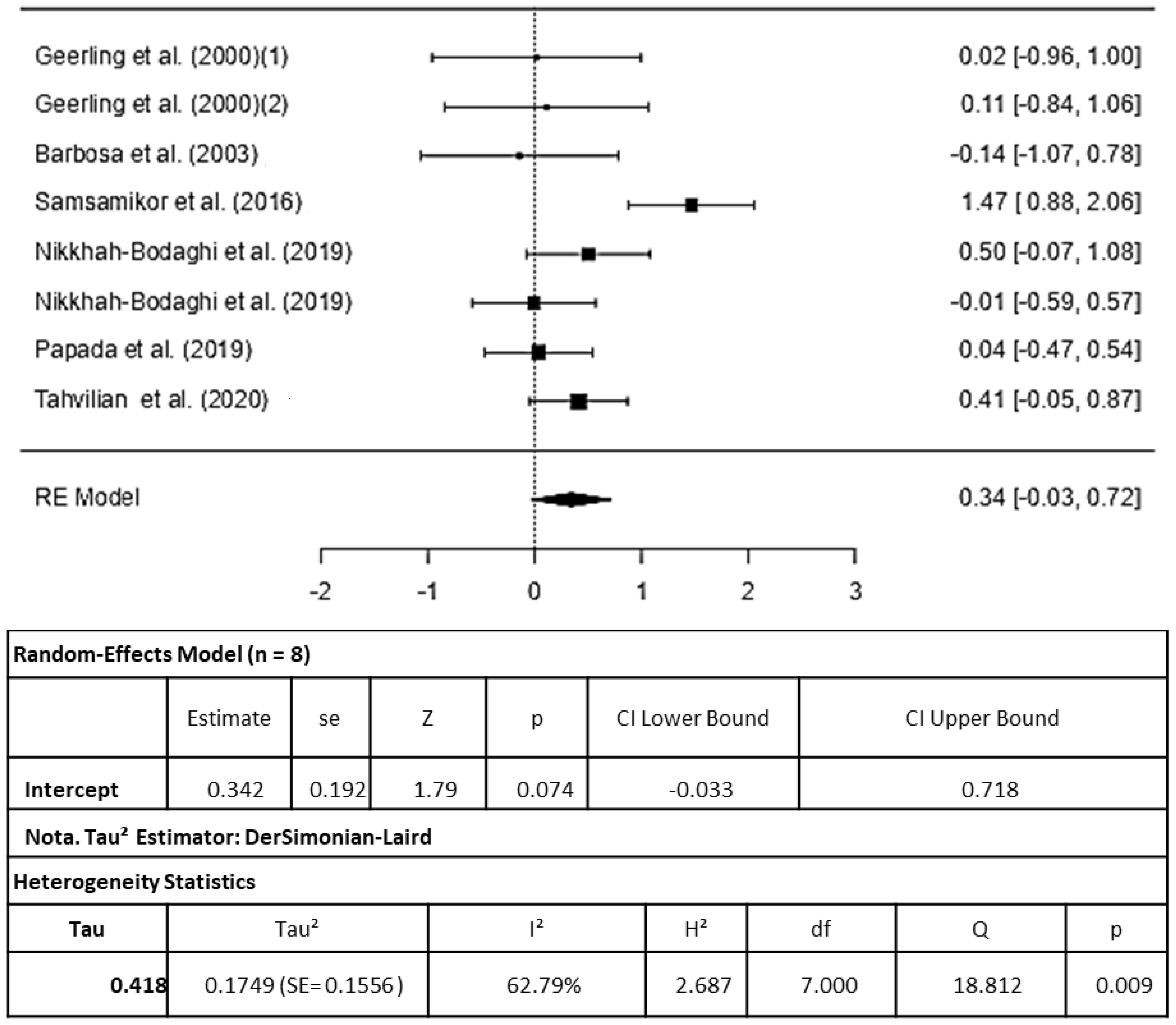
3.3.1. Antioxidant Capacity
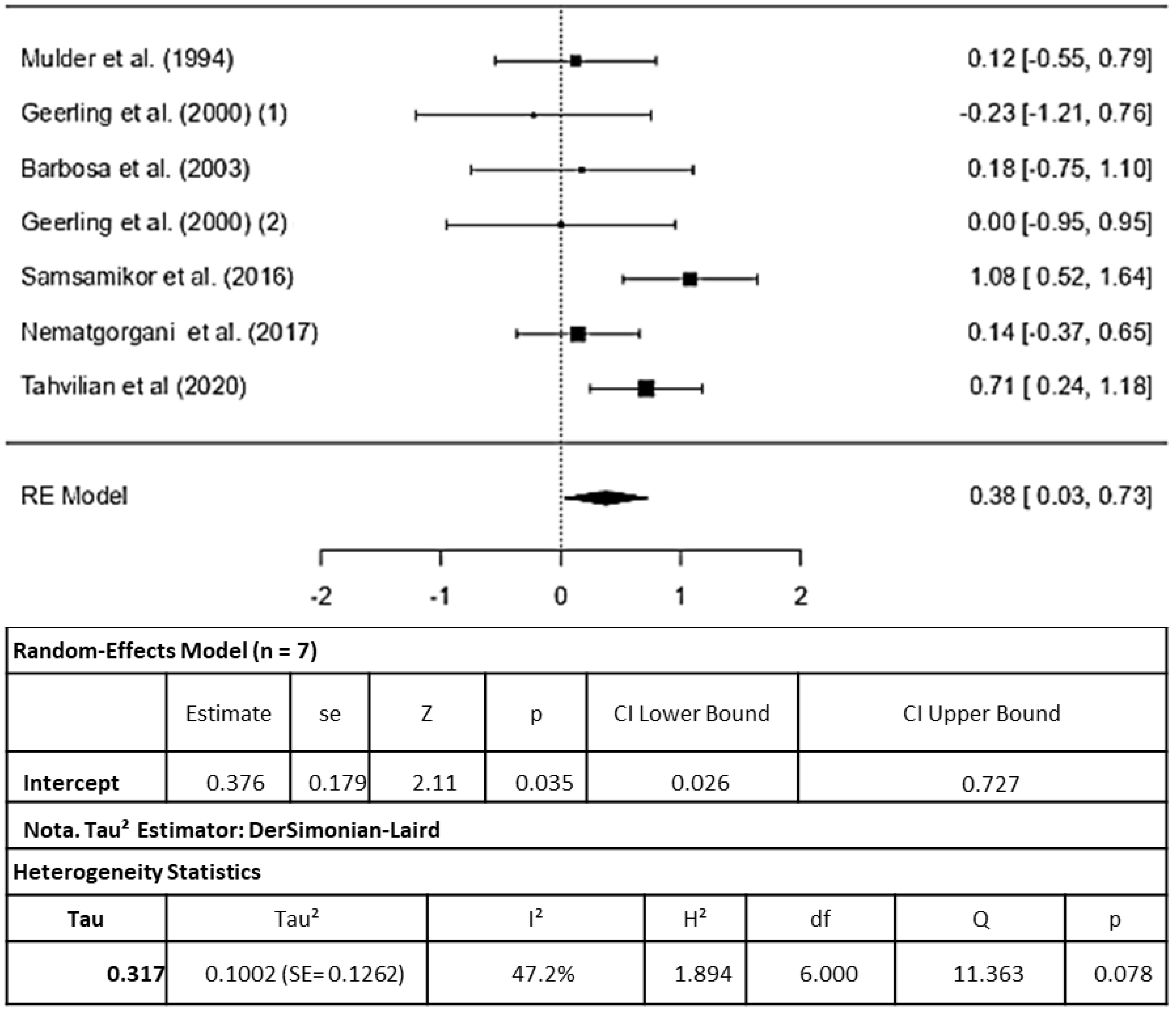
3.3.2. Superoxido Dismutase
3.3.3. Malondialdehyde (MDA)
4. Discussion
4.1. Antioxidant Capacity
4.2. Superoxide Dismutase
4.3. Malondialdehyde
4.4. Oxidative Stress and Inflammation Mediated by Cytokines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jeong, G.H.; Song, M.; Yon, D.K.; Lee, S.W.; Koyanagi, A.; Jacob, L.; Kostev, K.; Dragioti, E.; Radua, J.; et al. The global, regional, and national burden of inflammatory bowel diseases, 1990–2019: A systematic analysis for the global burden of disease study 2019. Dig. Liver Dis. 2023. [Google Scholar] [CrossRef] [PubMed]
- Moura, F.A.; de Andrade, K.Q.; dos Santos, J.C.; Araujo, O.R.; Goulart, M.O. Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biol. 2015, 6, 617–639. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Moura, F.A.; Goulart, M.O.F.; Campos, S.B.G.; da Paz Martins, A.S. The Close Interplay of Nitro-Oxidative Stress, Advanced Glycation end Products and Inflammation in Inflammatory Bowel Diseases. Curr. Med. Chem. 2020, 27, 2059–2076. [Google Scholar] [CrossRef]
- Collawn, C.; Rubin, P.; Perez, N.; Bobadilla, J.; Cabrera, G.; Reyes, E.; Borovoy, J.; Kershenobich, D. Phase II study of the safety and efficacy of a 5-lipoxygenase inhibitor in patients with ulcerative colitis. Am. J. Gastroenterol. 1992, 87, 342–346. [Google Scholar]
- Rastegarpanah, M.; Malekzadeh, R.; Vahedi, H.; Mohammadi, M.; Elahi, E.; Chaharmahali, M.; Safarnavadeh, T.; Abdollahi, M. A randomized, double blinded, placebo-controlled clinical trial of silymarin in ulcerative colitis. Chin. J. Integr. Med. 2015, 21, 902–906. [Google Scholar] [CrossRef]
- Yilmaz, I.; Dolar, M.E.; Ozpinar, H. Effect of administering kefir on the changes in fecal microbiota and symptoms of inflammatory bowel disease: A randomized controlled trial. Turk. J. Gastroenterol. 2019, 30, 242–253. [Google Scholar] [CrossRef]
- Brennan Laing, B.; Cavadino, A.; Ellett, S.; Ferguson, L.R. Effects of an Omega-3 and Vitamin D Supplement on Fatty Acids and Vitamin D Serum Levels in Double-Blinded, Randomized, Controlled Trials in Healthy and Crohn’s Disease Populations. Nutrients 2020, 12, 1139. [Google Scholar] [CrossRef]
- Alves, M.-D.-C.; Santos, M.-O.; Bueno, N.-B.; Araújo, O.-R.-P.-D.; Goulart, M.-O.-F.; Moura, F.-A. Efficacy of oral consumption of curcumin/for symptom improvement in inflammatory bowel disease: A systematic review of animal models and a meta-analysis of randomized clinical trials. Biocell 2022, 46, 2015–2047. [Google Scholar] [CrossRef]
- Barbosa, D.S.; Cecchini, R.; El Kadri, M.Z.; Rodriguez, M.A.; Burini, R.C.; Dichi, I. Decreased oxidative stress in patients with ulcerative colitis supplemented with fish oil omega-3 fatty acids. Nutrition 2003, 19, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Samsamikor, M.; Daryani, N.E.; Asl, P.R.; Hekmatdoost, A. Resveratrol Supplementation and Oxidative/Anti-Oxidative Status in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch. Med. Res. 2016, 47, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Tabataba-Vakili, S.; Yari, Z.; Alborzi, F.; Hedayati, M.; Ebrahimi-Daryani, N.; Hekmatdoost, A. The effects of two vitamin D regimens on ulcerative colitis activity index, quality of life and oxidant/anti-oxidant status. Nutr. J. 2019, 18, 16. [Google Scholar] [CrossRef] [PubMed]
- Morshedzadeh, N.; Shahrokh, S.; Aghdaei, H.A.; Pourhoseingholi, M.A.; Chaleshi, V.; Hekmatdoost, A.; Karimi, S.; Zali, M.R.; Mirmiran, P. Effects of flaxseed and flaxseed oil supplement on serum levels of inflammatory markers, metabolic parameters and severity of disease in patients with ulcerative colitis. Complement. Ther. Med. 2019, 46, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah-Bodaghi, M.; Darabi, Z.; Agah, S.; Hekmatdoost, A. The effects of Nigella sativa on quality of life, disease activity index, and some of inflammatory and oxidative stress factors in patients with ulcerative colitis. Phytother. Res. PTR 2019, 33, 1027–1032. [Google Scholar] [CrossRef]
- Nikkhah-Bodaghi, M.; Maleki, I.; Agah, S.; Hekmatdoost, A. Zingiber officinale and oxidative stress in patients with ulcerative colitis: A randomized, placebo-controlled, clinical trial. Complement. Ther. Med. 2019, 43, 1–6. [Google Scholar] [CrossRef]
- Biglari Abhari, M.; Farokhnezhad Afshar, P.; Alimoradzadeh, R.; Mirmiranpour, H. Comparing the effect of including omega-3 to treatment regimen in elderly patients with ulcerative colitis with placebo: A randomized clinical tria. Immunopathol. Persa 2020, 6, e10. [Google Scholar] [CrossRef]
- Farsi, F.; Ebrahimi-Daryani, N.; Golab, F.; Akbari, A.; Janani, L.; Karimi, M.Y.; Irandoost, P.; Alamdari, N.M.; Agah, S.; Vafa, M. A randomized controlled trial on the coloprotective effect of coenzyme Q10 on immune-inflammatory cytokines, oxidative status, antimicrobial peptides, and microRNA-146a expression in patients with mild-to-moderate ulcerative colitis. Eur. J. Nutr. 2021, 60, 3397–3410, Retracted in Eur. J. Nutr. 2022, 61, 3313. [Google Scholar] [CrossRef]
- Tahvilian, N.; Masoodi, M.; Kashani, A.F.; Vafa, M.; Aryaeian, N.; Heydarian, A.; Hosseini, A.; Moradi, N.; Farsi, F. Effects of saffron supplementation on oxidative/antioxidant status and severity of disease in ulcerative colitis patients: A randomized, double-blind, placebo-controlled study. Phytother. Res. PTR 2021, 35, 946–953. [Google Scholar] [CrossRef]
- Mulder, T.P.J.; Veer, A.V.D.S.; Verspaget, H.W.; Griffioen, G.; Peña, A.S.; Janssens, A.R.; Lamers, C.B.H.W. Effect of oral zinc supplementation on metallothionein and superoxide dismutase concentrations in patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. 1994, 9, 472–477. [Google Scholar] [CrossRef]
- Geerling, B.J.; Badart-Smook, A.; Van Deursen, C.; Van Houwelingen, A.C.; Russel, M.G.; Stockbrügger, R.W.; Brummer, R.J.M. Nutritional supplementation with N-3 fatty acids and antioxidants in patients with Crohn’s disease in remission: Effects on antioxidant status and fatty acid profile. Inflamm. Bowel Dis. 2000, 6, 77–84. [Google Scholar] [CrossRef]
- Aghdassi, E.; Wendland, B.E.; Steinhart, A.H.; Wolman, S.L.; Jeejeebhoy, K.; Allard, J.P. Antioxidant vitamin supplementation in Crohn’s disease decreases oxidative stress: A randomized controlled trial. Am. J. Gastroenterol. 2003, 98, 348–353. [Google Scholar][Green Version]
- Akobeng, A.K.; Thomas, A.G. Enteral nutrition for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2007, CD005984. [Google Scholar] [CrossRef]
- Koláček, M.; Muchová, J.; Dvořáková, M.; Paduchová, Z.; Žitňanová, I.; Čierna, I.; Országhová, Z.; Székyová, D.; Jajcaiová-Zedníčková, N.; Kovács, L.; et al. Effect of natural polyphenols (Pycnogenol) on oxidative stress markers in children suffering from Crohn’s disease—A pilot study. Free Radic. Res. 2013, 47, 624–634. [Google Scholar] [CrossRef]
- Tavassolifar, M.J.; Changaei, M.; Salehi, Z.; Ghasemi, F.; Javidan, M.; Nicknam, M.H.; Pourmand, M.R. Redox imbalance in Crohn’s disease patients is modulated by Azathioprine. Redox Rep. 2021, 26, 80–84. [Google Scholar] [CrossRef]
- Nematgorgani, S.; Agah, S.; Shidfar, F.; Gohari, M.; Faghihi, A. Effects of Urtica dioica leaf extract on inflammation, oxidative stress, ESR, blood cell count and quality of life in patients with inflammatory bowel disease. J. Herb. Med. 2017, 9, 32–41. [Google Scholar] [CrossRef]
- Papada, E.; Forbes, A.; Amerikanou, C.; Torović, L.; Kalogeropoulos, N.; Tzavara, C.; Triantafillidis, J.K.; Kaliora, A.C. Antioxidative Efficacy of a Pistacia Lentiscus Supplement and Its Effect on the Plasma Amino Acid Profile in Inflammatory Bowel Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 1779. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Santacroce, L.; Cantore, S.; Bottalico, L.; Dipalma, G.; Topi, S.; Saini, R.; De Vito, D.; Inchingolo, F. Probiotics Efficacy on Oxidative Stress Values in Inflammatory Bowel Disease: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Von Martels, J.Z.; Bourgonje, A.R.; Klaassen, M.A.; Alkhalifah, H.A.; Sadaghian Sadabad, M.; Vich Vila, A.; Gacesa, R.; Gabriëls, R.Y.; Steinert, R.E.; Jansen, B.H.; et al. Riboflavin Supplementation in Patients with Crohn’s Disease [the RISE-UP study]. J. Crohn’s Colitis 2020, 14, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Khazdouz, M.; Daryani, N.E.; Cheraghpour, M.; Alborzi, F.; Hasani, M.; Ghavami, S.B.; Shidfar, F. The effect of selenium supplementation on disease activity and immune-inflammatory biomarkers in patients with mild-to-moderate ulcerative colitis: A randomized, double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2023. [Google Scholar] [CrossRef]
- Kaliora, A.C.; Stathopoulou, M.G.; Triantafillidis, J.K.; Dedoussis, G.V.; Andrikopoulos, N.K. Chios mastic treatment of patients with active Crohn’s disease. World J. Gastroenterol. 2007, 13, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Biological redox systems and oxidative stress. Cell. Mol. Life Sci. CMLS 2007, 64, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, N.; Bakovic, M.; Paliyath, G. Molecular Mechanisms and Pathways as Targets for Cancer Prevention and Progression with Dietary Compounds. Int. J. Mol. Sci. 2017, 18, 2050. [Google Scholar] [CrossRef] [PubMed]
- Alemany-Cosme, E.; Sáez-González, E.; Moret, I.; Mateos, B.; Iborra, M.; Nos, P.; Sandoval, J.; Beltrán, B. Oxidative Stress in the Pathogenesis of Crohn’s Disease and the Interconnection with Immunological Response, Microbiota, External Environmental Factors, and Epigenetics. Antioxidants 2021, 10, 64. [Google Scholar] [CrossRef]
- Costantini, D.; Verhulst, S. Does high antioxidant capacity indicate low oxidative stress? Funct. Ecol. 2009, 23, 506–509. [Google Scholar] [CrossRef]
- Pavlick, K.P.; Laroux, F.S.; Fuseler, J.; Wolf, R.E.; Gray, L.; Hoffman, J.; Grisham, M.B. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol. Med. 2002, 33, 311–322. [Google Scholar] [CrossRef]
- Tirosh, O.; Shilo, S.; Aronis, A.; Sen, C.K. Redox regulation of mitochondrial permeability transition: Effects of uncoupler, lipoic acid and its positively charged analog LA-plus and selenium (Reprinted from Thiol Metabolism and Redox Regulation of Cellular Functions). Biofactors 2003, 17, 297–306. [Google Scholar] [CrossRef]
- Tesoriere, L.; Attanzio, A.; Allegra, M.; Gentile, C.; Livrea, M. Indicaxanthin inhibits NADPH oxidase (NOX)-1 activation and NF-κB-dependent release of inflammatory mediators and prevents the increase of epithelial permeability in IL-1β-exposed Caco-2 cells. Br. J. Nutr. 2014, 111, 415–423. [Google Scholar] [CrossRef]
- Buscarinu, M.C.; Romano, S.; Mechelli, R.; Umeton, R.P.; Ferraldeschi, M.; Fornasiero, A.; Reniè, R.; Cerasoli, B.; Morena, E.; Romano, C.; et al. Intestinal Permeability in Relapsing-Remitting Multiple Sclerosis. Neurotherapeutics 2018, 15, 68–74. [Google Scholar] [CrossRef]
- Abraham, B.P.; Ahmed, T.; Ali, T. Inflammatory Bowel Disease: Pathophysiology and Current Therapeutic Approaches. Handb. Exp. Pharmacol. 2017, 239, 115–146. [Google Scholar] [PubMed]
- Burge, K.; Gunasekaran, A.; Eckert, J.; Chaaban, H. Curcumin and Intestinal Inflammatory Diseases: Molecular Mechanisms of Protection. Int. J. Mol. Sci. 2019, 20, 1912. [Google Scholar] [CrossRef] [PubMed]
- Sottero, B.; Rossin, D.; Poli, G.; Biasi, F. Lipid Oxidation Products in the Pathogenesis of Inflammation-related Gut Diseases. Curr. Med. Chem. 2018, 25, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Zhang, J.; Decker, E.A.; Zhang, G. Roles of Lipid Peroxidation-Derived Electrophiles in Pathogenesis of Colonic Inflammation and Colon Cancer. Front. Cell Dev. Biol. 2021, 9, 665591. [Google Scholar] [CrossRef]
- Niki, E. Lipid peroxidation: Physiological levels and dual biological effects. Free Radic. Biol. Med. 2009, 47, 469–484. [Google Scholar] [CrossRef]
- Mudter, J.; Neurath, M.F. IL-6 Signaling in Inflammatory Bowel Disease: Pathophysiological Role and Clinical Relevance. Inflamm. Bowel Dis. 2007, 13, 1016–1023. [Google Scholar] [CrossRef]
- Wei, H.X.; Wang, B.; Li, B. IL-10 and IL-22 in Mucosal Immunity: Driving Protection and Pathology. Front. Immunol. 2020, 11, 1315. [Google Scholar] [CrossRef]
- Sands, B.E. Inflammatory bowel disease: Past, present, and future. J. Gastroenterol. 2007, 42, 16–25. [Google Scholar] [CrossRef]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Neurath, M.F.; Schürmann, G. Immunopathogenesis of inflammatory bowel diseases. Der Chir. 2000, 71, 30–40. [Google Scholar]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-kappaB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Adamina, M.; Bonovas, S.; Raine, T.; Spinelli, A.; Warusavitarne, J.; Armuzzi, A.; Bachmann, O.; Bager, P.; Biancone, L.; Bokemeyer, B.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Surgical Treatment. J. Crohn’s Colitis 2020, 14, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lago, I.; Del Hoyo, J.; Pérez-Girbés, A.; Garrido-Marín, A.; Casanova, M.J.; Chaparro, M.; Fernández-Clotet, A.; Castro-Poceiro, J.; García, M.J.; Sánchez, S.; et al. Early treatment with anti-tumor necrosis factor agents improves long-term effectiveness in symptomatic stricturing Crohn’s disease. United Eur. Gastroenterol. J. 2020, 8, 1056–1066. [Google Scholar] [CrossRef]
- Hart, A.L.; Ng, S.C. Review article: The optimal medical management of acute severe ulcerative colitis. Aliment. Pharmacol. Ther. 2010, 32, 615–627. [Google Scholar] [CrossRef]
- Jain, S.; Ahuja, V.; Limdi, J.K. Optimal management of acute severe ulcerative colitis. Postgrad. Med. J. 2019, 95, 32–40. [Google Scholar] [CrossRef]
| Authors, Year | IBD | Study | Intervention | Dose and Time of Intervention | Group Subjects (n) and Age [Mean ± SD/SEM or Median (IQ)] | Oxidative Stress Markers | Cytokines | General Effects | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SOD | GPX | AOC | LP | Others | ||||||||
| Mulder et al. (1994) [20] | Inactive to moderately activeCD | Randomized, double blind, placebo control | Zinc aspartate | 300 mg For 4 weeks | Placebo: n = 22; age = 38 y (23–55) Intervention: n = 14; age = 42 y (22–47) | NS | No changes were found in the plasma and erythrocyte Metallothionein | |||||
| Geerling et al. (2000) [21] | Remission CD | Randomized, double blind, placebo control | Intervention 1 (I1): Antioxidants (AO) complex intervention 2 (I2): AO complex + omega 3 (n-3) | For 12 weeks | Placebo: n = 8; age = 38 y (30–61) I1: n = 8; age = 43 y (33–52) I2: n = 9; age = 41 y (31–56) | I1 (↑) I2 (↑) | I2(↓) | NS | AO + n-3 – decreased the proportion of arachidonic acid, and increased the proportion of eicosapentanoic acid and docosahexanoic acid in both plasma phospholipids and adipose tissue | |||
| Aghdassi et al. (2003) [22] | Remission CD | Randomized double blind, placebo control | Vit C + Vit E | Vit C: 1000 mg/d + Vit E: 800 UI/d For 4 weeks | Placebo: n = 29; age = 36.5 y ± 1.7 61) Intervention: n = 28; age = 38.3 y ± 2.9 | ↓ | Did not alter disease activity | |||||
| Barbosa et al. (2003) [11] | Mild or moderate active UC | Randomized, cross-over, placebo control | Ômega 3 | 4.5 g/d (90 mg of EPA + 60 mg of DHA) For 8 weeks | Placebo: n = 9; age = not informed Intervention: n = 9; age = 40 y ± 11 | NS | ↑ | NS | Catalase: NS | Did not alter laboratory indicator or sigmoidoscopy or histology scores; | ||
| Ballini et al. (2019) [28] | DC or UC | Randomized, double blind, placebo control | Hyperbiotics Pro-15 Probiotics | 12 weeks | Placebo: n = 20; age = 30–60 y Intervention: n = 20; age = 30–60 y | D-rom: ↓ | ↑ antioxidant defense | |||||
| Akobeng et al. (2006) [23] | Active CD | Randomized, double blind, placebo control | Glutamine enriched polymeric diet | Placebo: Polymeric diet;Treatment: glutamine-enriched polymeric diet (42% of amino acid composition) For 4 weeks | Placebo: n = 8; age = 10.5 y ± 2.7 Intervention: n = 7; age = 12.2 y ± 2.8 | NS | Did not alter plasma antioxidant concentrations | |||||
| Kolacek et al. (2013) [24] | Remission CD | Pilot | Pycnogenol | 2 mg/d For 12 weeks | Healthy control: n = 15; age = 13.9 y ± 2.0 CD patients: n = 14; age = 16.3 y ± 1.5 | NS | NS | NS | Serum AOC negatively correlated with disease activity and with CRP and fecal calprotectin | |||
| Samsamikor et al. (2016) [12] | Active mild to moderate UC | Randomized double blind, placebo control | Resveratrol | 500 mg/d For 6 weeks | Placebo: n = 28; age = 38.8 ± 11.6 Intervention: n = 28; age = 37.4 y ± 16.5 | ↑ | ↑ | ↓ | ↓ severity of disease activity and ↑ the quality of life | |||
| Nematgorgani et al. (2017) [26] | Mild or moderate DC and UC | Randomized double blind, placebo control | Urtica dioica leaf extract | 400 mg For 12 weeks | Placebo: n = 29; age = 38.3 y ± 13.3 Intervention: n = 30; age = 36.6 y ± 10.9 | ↑ | ↓ hs-CRP and platelet count; ↑ the quality of life; Did not alter levels of WBC and ESR | |||||
| Papada et al. (2018) [27] | Remission DC and UC | Randomized double blind, placebo control | Pistacia lentiscus | 2800 mg/d For 12 weeks | Placebo: n = 27; age = 45 y ± 17.4 Intervention: n = 33; age = 38.2 y ± 11.9 | ↑ | Ox-LDL: ↓ | ↓ oxLDL/HDL, oxLDL/LDL and oxLDL/LDL | ||||
| Karimi et al. (2019) [13] | Active mild to moderate UC | Randomized double blind | Vitamin D | Intervention 1: 1000 UI/d (I1) Intervention 2: 2000 UI (I2) For 12 weeks | I1: n = 22; age = 39.7 y ± 15.6 I2: n = 24; age = 34 y ± 12.5 | NS | High dose group: ↑ the quality of life and ↓ severity of disease activity | |||||
| Morshedzadeha et al. (2019) [14] | UC | Randomized double blind, placebo control | Intervention 1: Grounded flaxseed (GF) Intervention 2: Flaxseed oil (FO) | GF: 30,000 mg/d FO: 10,000 g/d For 2 weeks | Placebo: n = 25; age = 35.2 y ± 10.6 GF: n = 25; age = 29.9 y ± 9.1 FO: n = 25; age = 32.2 y ± 9.9 | IL-6 and IFN-γ: GF and FO (↓) | GF and FO: ↑ TGF-β and the quality of life; ↓ fecal calprotectin, Mayo score, ESR, waist circumference, diastolic and systolic blood pressure | |||||
| Nikkhah-Bodaghi et al. (2019) [15] | Active mild to moderate UC | Randomized double blind, placebo control | Nigella sativa | 2000 mg/d For 6 weeks | Placebo: n = 24; age = 39.2 y ± 11.8; Intervention: n = 24; age = 34.8 y ± 11.2 | NS | ↓ | NFκB: NS | TNF-α: NS | ↓ stool frequency score; Did not alter severity of disease activity and the quality of life | ||
| Nikkhah-Bodaghi et al. (2019) [16] | Active mild to moderate UC | Randomized double blind, placebo control | Zingiber | 2000 mg/d For 12 weeks | Placebo: n = 24; age = 39.2 y ± 11.8 Intervention: n = 22; age = 41.4 y ± 11.4 | NS | ↓ | ↓ severity of disease activity; ↑ the quality of life | ||||
| Abhari et al. (2020) [17] | Active mild to moderate UC | Randomized double blind, placebo control | Omega 3 | 4300 mg/d For 8 weeks | Placebo: n = 35; age = 69.7 y ± 5.0. Intervention: n = 35; age = 69.7 y ±5.5 | ↑ | ↑ | ↓ |
| IL-6, IL-2, IL-1α and IL-1β: ↓ | Did not alter BMI, waist circumference, diastolic and systolic blood pressure | |
| von Martels et al. (2020) [29] | DC and UC | Prospective | Riboflavin | 100 mg/d For 3 weeks | Group 1 (Fecal Calprotectin < 200 µg/g): n = 40; age = 44.2 y ± 11.6 Group 2 (Fecal Calprotectin > 200 µg/g): n = 30; age = 38.8 y ± 13.6 | Free thiols: ↑ |
| ↓ severity disease activity, CRP and Enterobacteriaceae; No effects on diversity, taxonomy, or metabolic pathways of the fecal microbiome. | ||||
| Farsi et al. (2021) [18] | Varying disease activity UC | Randomized double blind, placebo control | Coenzyme Q10 | 200 mg/d For 8 weeks | Placebo: n = 43; age = 40.2 y ± 11.5 Intervention: n = 43; age = 38.4 y ± 8.8 |
| ↓ severity disease activity; ↑ the quality of life and serum levels of cathelicidin LL-37; Did not alter β-defensin 2 | |||||
| Tahvilian et al. (2021) [19] | Active mild to moderate UC | Randomized double blind, placebo control | Saffron | 100 mg/d For 8 weeks | Placebo: n = 35; age = 41.0 y ± 11.3 Intervention: n = 40; age = 40.5 y ± 12.7 | ↑ | ↑ | ↑ | NS | |||
| Tavassolifar et al. (2021) [25] | Active mild to moderate CD | Longitudinal | Azatioprine | 50 mg/d For 12 weeks | Healthy control: n = 15; age = 33.6 y ± 1.2 CD patients: n = 15; age = 31.5 y ± 1.8 | Normalized * | GP91PHOX, NrF2, Catalase—normalized * | ↓ severity disease activity | ||||
| Khazdouz et al. (2023) [30] | Active mild to moderate UC | Randomized double blind, placebo control | Selenium | 200 mcg/d 10 weeks | Placebo: n = 50; age = 37.9 ± 10.8 Intervention: n = 50; age = 34.5 ± 11.2 | IL-17 ↓ IL-10 (NS) | ↓ severity disease activity; ↑ the quality of life | |||||
| DOM 1 | DOM 2 | DOM 3 | DOM 4 | DOM 5 | DOM 6 | Overall | |
|---|---|---|---|---|---|---|---|
| Mulder, et al. (1994) [20] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Geerling et al. (2000) [21] | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Barbosa et al. (2003) [11] | Unclear | Unclear | Unclear | Low | High | Unclear | Unclear |
| Aghdassi et al. (2003) [22] | Low | Unclear | Unclear | Unclear | High | High | Unclear |
| Akobeng et al. (2007) [23] | Low | Low | Low | Low | Low | High | Low |
| Samsamikor et al. (2016) [12] | Unclear | Unclear | Low | Low | Low | High | Low |
| Nematgorgani et al. (2017) [26] | Unclear | Low | Low | Low | Low | High | Low |
| Papada et al. (2018) [27] | Low | Low | Low | Low | Low | Low | Low |
| Ballini et al. (2019) [28] | Low | Low | Unclear | Unclear | Low | Low | Low |
| Nikkhah-Bodaghi et al. (2019) [15] | Low | Low | Low | Low | High | Unclear | Low |
| Nikkhah-Bodaghi et al. (2019) [16] | Low | Low | Low | Low | High | Unclear | Low |
| Karimi et al. (2019) [13] | Unclear | Low | Low | Unclear | Low | Unclear | Unclear |
| Morshedzadeh et al. (2019) [14] | Unclear | Unclear | High | Unclear | Low | Low | Unclear |
| Tahvilian et al. (2020) [19] | Low | Low | Low | Unclear | Low | Low | Low |
| Abhari et al. (2020) [17] | Unclear | Unclear | High | High | High | High | High |
| Farsi et al. (2021) [18] | Low | Low | Low | Low | Low | Low | Low |
| Khazdouz et al. (2023) [30] | Low | Low | Low | Low | Low | Low | Low |
| DOM 1 | DOM 2 | DOM 3 | DOM 4 | DOM 5 | DOM 6 | DOM 7 | Overall | |
|---|---|---|---|---|---|---|---|---|
| von Martels et al. (2020) [29] | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Koláček et al. (2013) [24] | Low | Low | Low | Low | Low | Low | Low | Low |
| Tavassolifar et al. (2021) [25] | Moderate | Low | Low | Low | Low | Low | Low | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues Junior, J.I.; Vasconcelos, J.K.G.d.; Xavier, L.E.M.d.S.; Gomes, A.d.S.; Santos, J.C.d.F.; Campos, S.B.G.; Martins, A.S.d.P.; Goulart, M.O.F.; Moura, F.A. Antioxidant Therapy in Inflammatory Bowel Disease: A Systematic Review and a Meta-Analysis of Randomized Clinical Trials. Pharmaceuticals 2023, 16, 1374. https://doi.org/10.3390/ph16101374
Rodrigues Junior JI, Vasconcelos JKGd, Xavier LEMdS, Gomes AdS, Santos JCdF, Campos SBG, Martins ASdP, Goulart MOF, Moura FA. Antioxidant Therapy in Inflammatory Bowel Disease: A Systematic Review and a Meta-Analysis of Randomized Clinical Trials. Pharmaceuticals. 2023; 16(10):1374. https://doi.org/10.3390/ph16101374
Chicago/Turabian StyleRodrigues Junior, José Israel, Joice Kelly Gomes de Vasconcelos, Lylian Ellen Militão dos Santos Xavier, Amanda da Silva Gomes, Juliana Célia de Farias Santos, Samara Bomfim Gomes Campos, Amylly Sanuelly da Paz Martins, Marília Oliveira Fonseca Goulart, and Fabiana Andréa Moura. 2023. "Antioxidant Therapy in Inflammatory Bowel Disease: A Systematic Review and a Meta-Analysis of Randomized Clinical Trials" Pharmaceuticals 16, no. 10: 1374. https://doi.org/10.3390/ph16101374
APA StyleRodrigues Junior, J. I., Vasconcelos, J. K. G. d., Xavier, L. E. M. d. S., Gomes, A. d. S., Santos, J. C. d. F., Campos, S. B. G., Martins, A. S. d. P., Goulart, M. O. F., & Moura, F. A. (2023). Antioxidant Therapy in Inflammatory Bowel Disease: A Systematic Review and a Meta-Analysis of Randomized Clinical Trials. Pharmaceuticals, 16(10), 1374. https://doi.org/10.3390/ph16101374