Polyalthic Acid from Copaifera lucens Demonstrates Anticariogenic and Antiparasitic Properties for Safe Use
Abstract
1. Introduction
2. Results
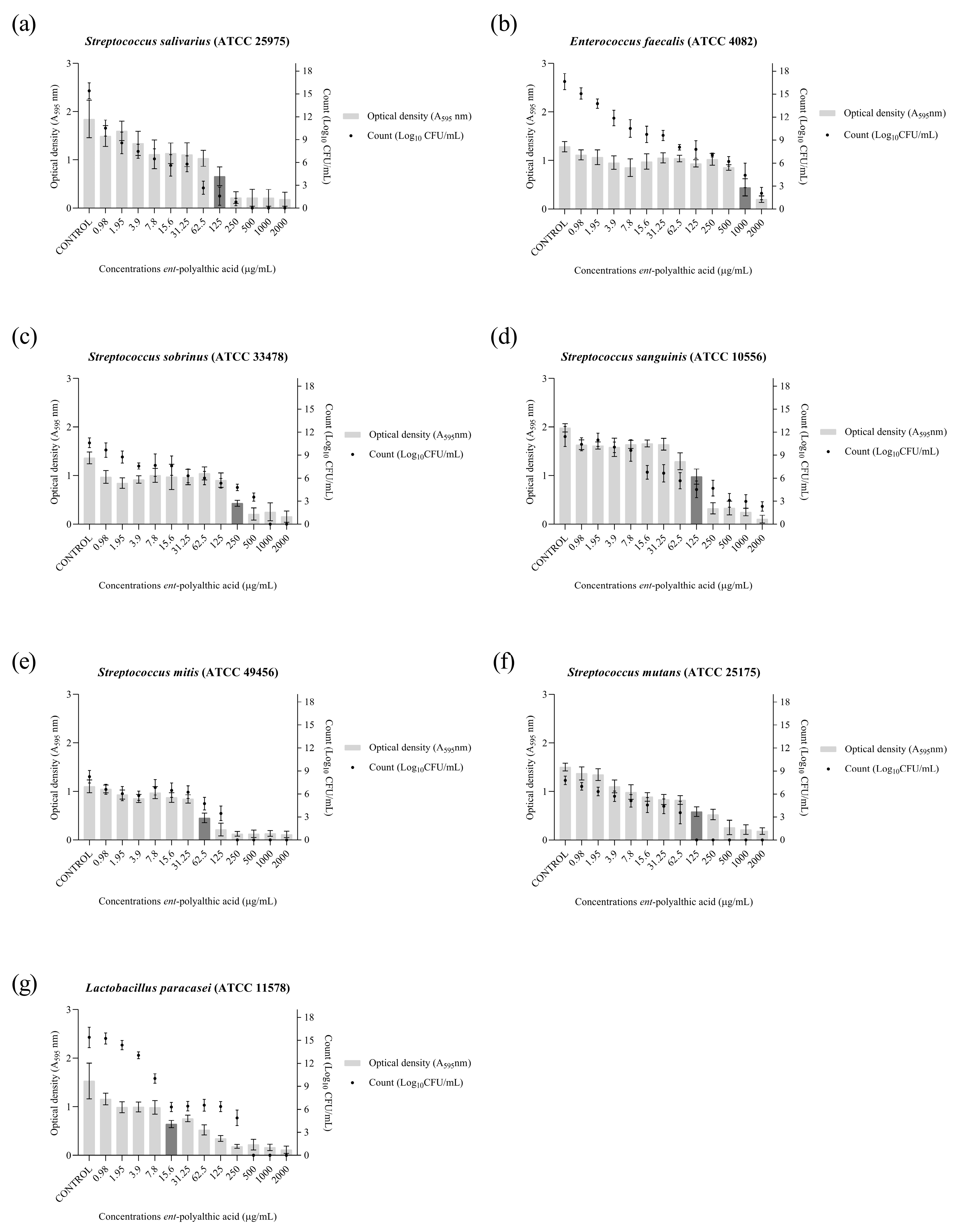
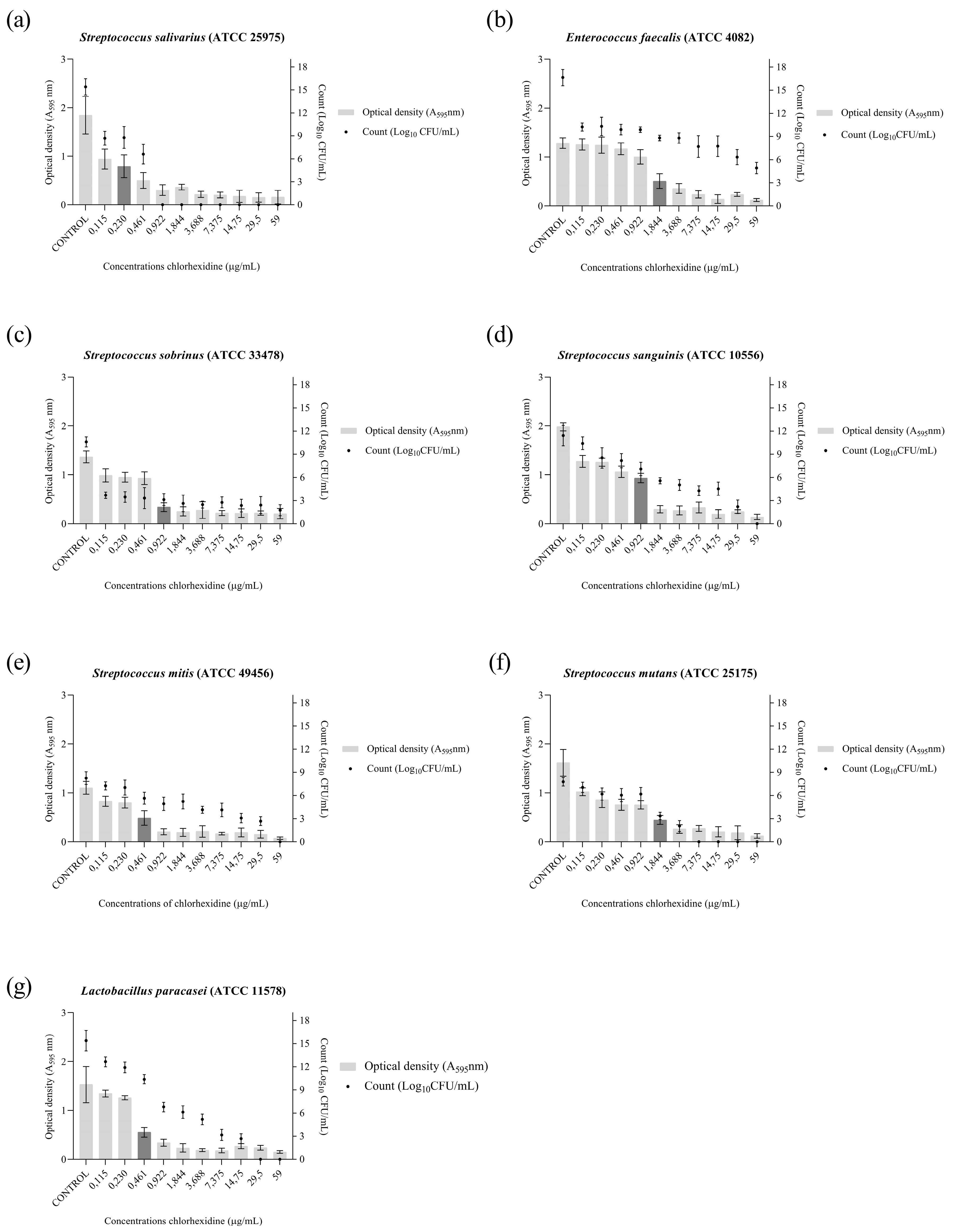
2.1. Anticariogenic Activity
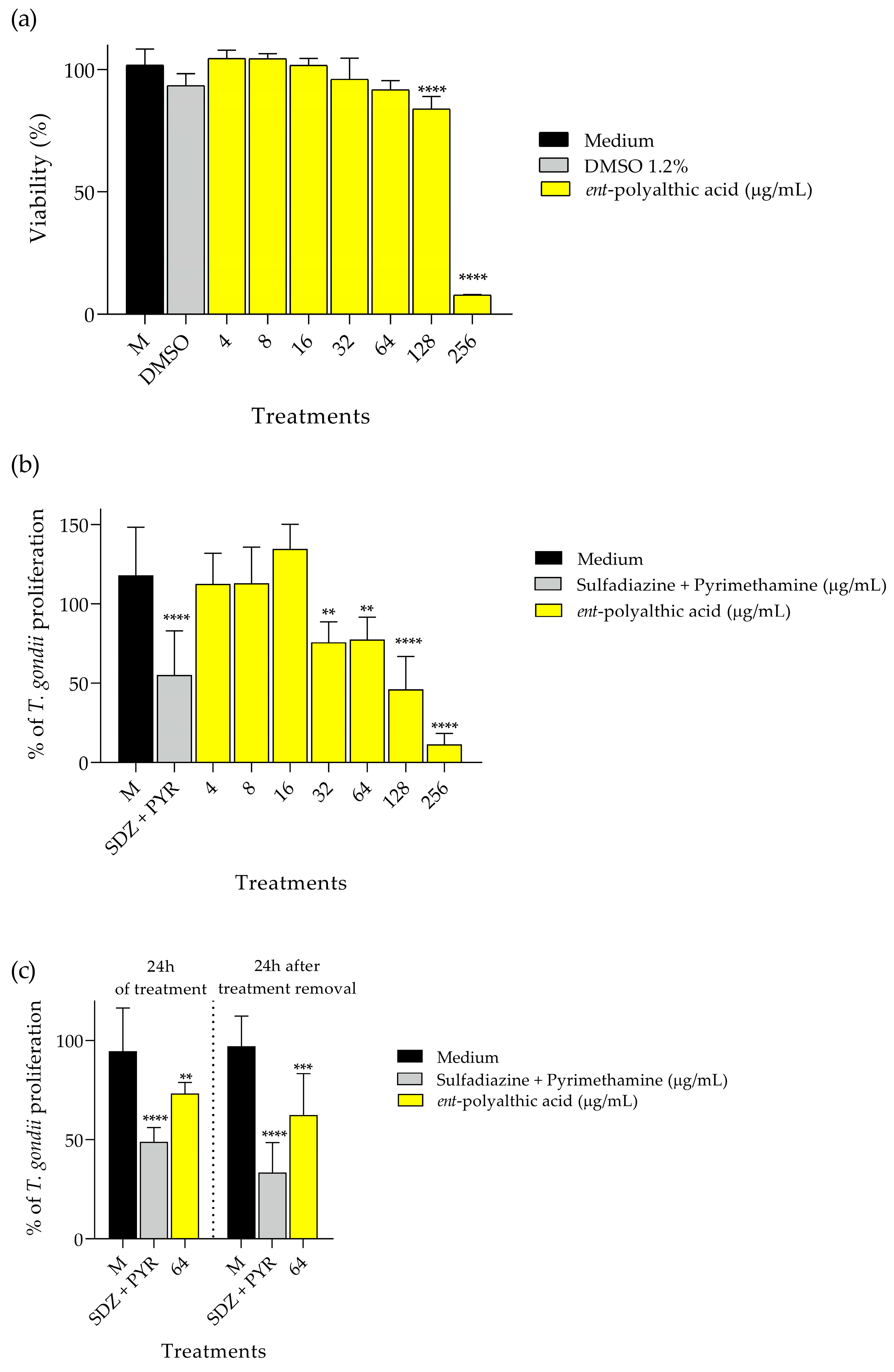
2.2. Antiparasitic Activity
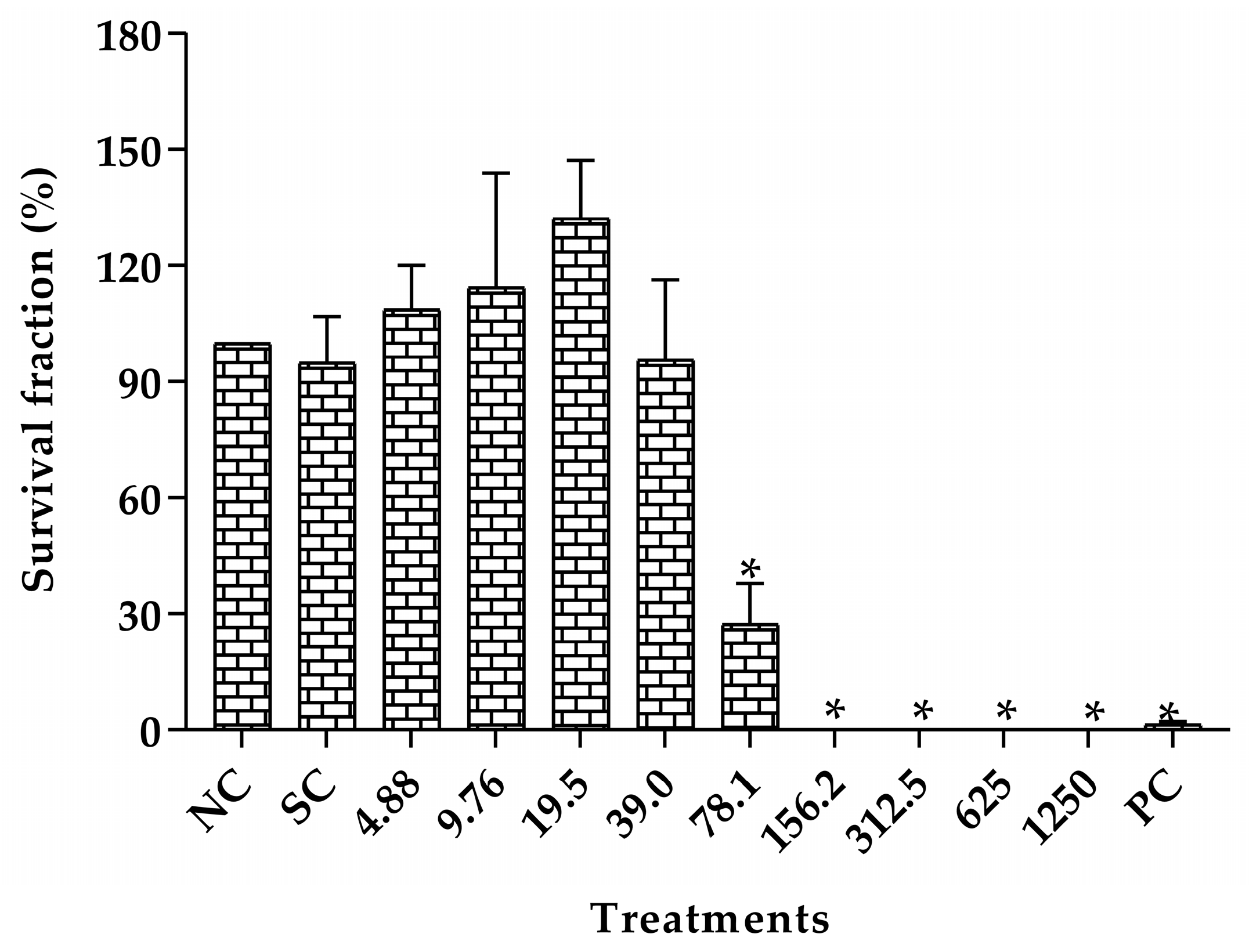
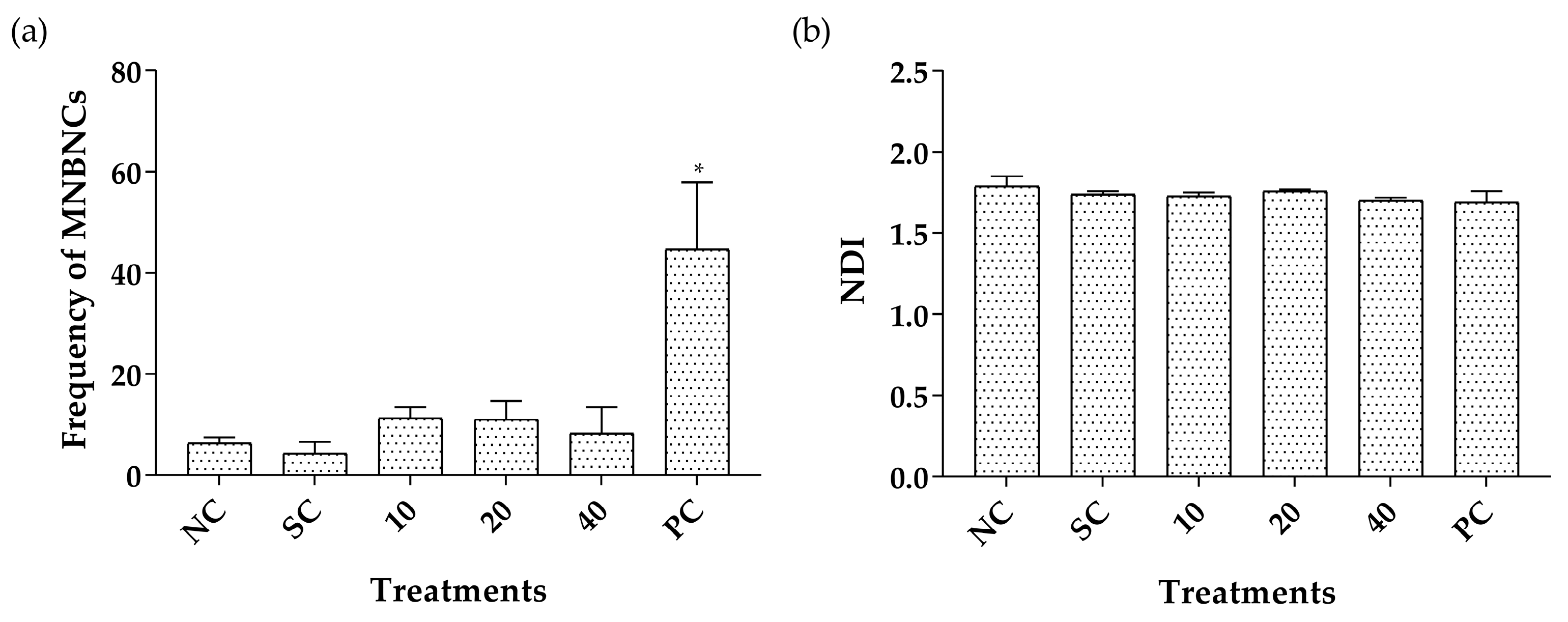
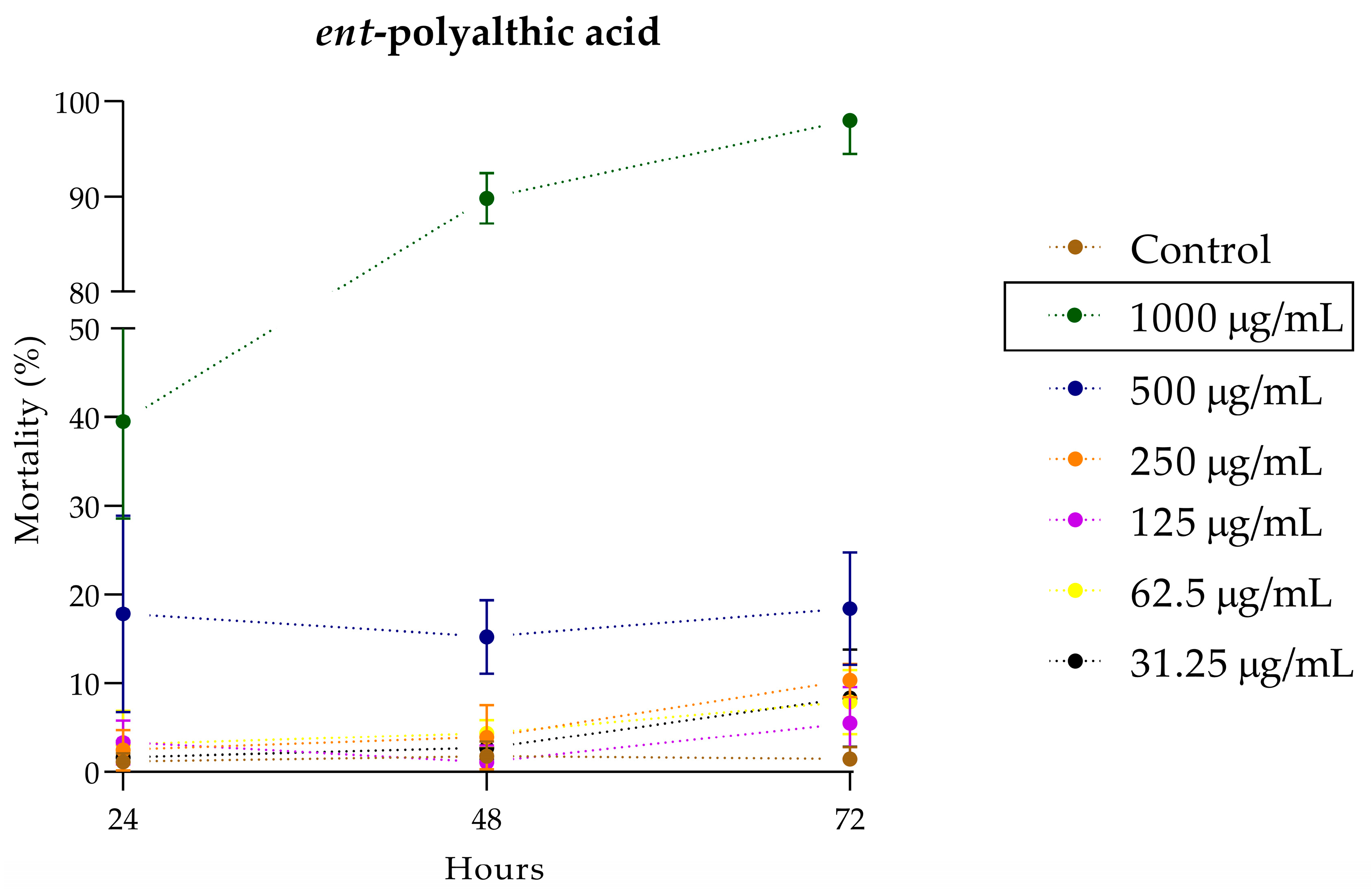
2.3. Toxicity Assessment
3. Discussion
4. Materials and Methods
4.1. Plant Material and Polyaltic Acid
4.1.1. Obtainment of CECL
4.1.2. Obtainment of CLO and Isolation of PA
4.2. Anticariogenic Activity
4.2.1. Bacteria Used
4.2.2. Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC)
4.2.3. Antibiofilm Activity
4.3. Antiparasitic Activity
4.3.1. Cell Culture and Parasite Maintenance
4.3.2. Viability of the Host Cell
4.3.3. Evaluation of Intracellular Proliferation of Toxoplasma gondii: β-Galactosidase Activity
4.3.4. Reversibility Assay
4.4. Toxicity Assessment
4.4.1. In Vitro System-Test
4.4.2. In Vivo System-Test
4.4.3. Toxicity Assessment in Caenorhabditis elegans
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arweiler, N.B.; Netuschil, L. The Oral Microbiota. Adv. Exp. Med. Biol. 2016, 902, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Valm, A.M. The Structure of Dental Plaque Microbial Communities in the Transition from Health to Dental Caries and Periodontal Disease. J. Mol. Biol. 2019, 431, 2957–2969. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lin, Y.; Li, M.; He, J. Roles of Streptococcus mutans-Candida albicans interaction in early childhood caries: A literature review. Front. Cell. Infect. Microbiol. 2023, 13, 1151532. [Google Scholar] [CrossRef]
- Wen, P.Y.F.; Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M. Global Burden and Inequality of Dental Caries, 1990 to 2019. J. Dent. Res. 2022, 101, 392–399. [Google Scholar] [CrossRef]
- Choo, A.; Delac, D.M.; Messer, L.B. Oral hygiene measures and promotion: Review and considerations. Aust. Dent. J. 2001, 46, 166–173. [Google Scholar] [CrossRef]
- Dubey, J.P.; Lago, E.G.; Gennari, S.M.; Su, C.; Jones, J.L. Toxoplasmosis in humans and animals in Brazil: High prevalence, high burden of disease, and epidemiology. Parasitology 2012, 139, 1375–1424. [Google Scholar] [CrossRef]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cezar, C.K.; Kwok, O.C.H.; Villena, I. Congenital toxoplasmosis in humans: An update of worldwide rate of congenital infections. Parasitology 2021, 148, 1406–1416. [Google Scholar] [CrossRef]
- Uddin, A.; Hossain, D.; Ahsan, M.I.; Atikuzzaman, M.; Karim, M.R. Review on diagnosis and molecular characterization of Toxoplasma gondii in humans and animals. Trop. Biomed. 2021, 38, 511–539. [Google Scholar] [CrossRef]
- Abdelbaset, A.E.; Abushahba, M.F.N.; Igarashi, M. Toxoplasma gondii in humans and animals in Japan: An epidemiological overview. Parasitol. Int. 2022, 87, 102533. [Google Scholar] [CrossRef]
- Robert-Gangneux, F.; Darde, M.L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef] [PubMed]
- Mrzljak, A.; Novak, R.; Pandak, N.; Tabain, I.; Franusic, L.; Barbic, L.; Bogdanic, M.; Savic, V.; Mikulic, D.; Pavicic-Saric, J.; et al. Emerging and neglected zoonoses in transplant population. World J. Transplant. 2020, 10, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.; Webster, J.P.; Walker, M. Toxoplasma gondii: AnUnderestimated Threat? Trends Parasitol. 2020, 36, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Arruda, C.; Aldana Mejía, J.A.; Ribeiro, V.P.; Gambeta Borges, C.H.; Martins, C.H.G.; Sola Veneziani, R.C.; Ambrósio, S.R.; Bastos, J.K. Occurrence, chemical composition, biological activities and analytical methods on Copaifera genus—A review. Biomed. Pharmacother. 2019, 109, 1–20. [Google Scholar] [CrossRef]
- Da Trindade, R.; da Silva, J.K.; Setzer, W.N. Copaifera of the Neotropics: A Review of the Phytochemistry and Pharmacology. Int. J. Mol. Sci. 2018, 19, 1511. [Google Scholar] [CrossRef]
- Mohd-Fuat, A.R.; Kofi, E.A.; Allan, G.G.C. Mutagenic and cytotoxic properties of three herbal plants from Southeast Asia. Trop. Biomed. 2007, 24, 49–59. [Google Scholar]
- Lemos, M.; Santin, J.R.; Mizuno, C.S.; Boeing, T.; de Sousa, J.P.B.; Nanayakkara, D.; Bastos, J.K.; de Andrade, S.F. Copaifera langsdorffii: Evaluation of potential gastroprotective of extract and isolated compounds obtained from leaves. Rev. Bras. Farmacogn. 2015, 25, 238–245. [Google Scholar] [CrossRef]
- Veiga Junior, V.F.; Pinto, A.C. O GÊNERO Copaifera L. Química Nova 2002, 25, 273–286. [Google Scholar] [CrossRef]
- Pieri, F.A.; Mussi, M.C.; Moreira, M.A.S. Óleo de copaíba (Copaifera sp.): Histórico, extração, aplicações industriais e propriedades medicinais. Rev. Bras. Plantas Med. 2009, 11, 465–472. [Google Scholar] [CrossRef]
- De Souza, P.A.; Rangel, L.P.; Oigman, S.S.; Elias, M.M.; Ferreira-Pereira, A.; De Lucas, N.C.; Leitão, G.G. Isolation of two bioactive diterpenic acids from Copaifera glycycarpa oleoresin by high-speed counter-current chromatography. Phytochem. Anal. 2010, 21, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Leandro, L.M.; Vargas, F.d.S.; Barbosa, P.C.S.; Neves, J.K.O.; da Silva, J.A.; da Veiga-Junior, V.F. Chemistry and biological activities of terpenoids from copaiba (Copaifera spp.) oleoresins. Molecules 2012, 17, 3866–3889. [Google Scholar] [CrossRef]
- Santos, A.O.; Ueda-Nakamura, T.; Dias Filho, B.P.; Veiga Junior, V.F.; Pinto, A.C.; Nakamura, C.V. Effect of Brazilian copaiba oils on Leishmania amazonensis. J. Ethnopharmacol. 2008, 120, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Trejo, B.; Sanchez-Mendoza, M.E.; Becerra-Garcia, A.A.; Cedillo-Portugal, E.; Castillo-Henkel, C.; Arrieta, J. Bioassay-guided isolation of an anti-ulcer diterpenoid from Croton reflexifolius: Role of nitric oxide, prostaglandins and sulfhydryls. J. Pharm. Pharmacol. 2008, 60, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Silverio, J.; Sanchez-Mendoza, M.E.; Rocha-Gonzalez, H.I.; Reyes-Garcia, J.G.; Flores-Murrieta, F.J.; Lopez-Lorenzo, Y.; Quinonez-Bastidas, G.N.; Arrieta, J. Evaluation of the Antinociceptive, Antiallodynic, Antihyperalgesic and Anti-Inflammatory Effect of Polyalthic Acid. Molecules 2021, 26, 2921. [Google Scholar] [CrossRef] [PubMed]
- Cicek, S.S.; Wenzel-Storjohann, A.; Girreser, U.; Tasdemir, D. Biological Activities of Two Major Copaiba Diterpenoids and Their Semi-synthetic Derivatives. Rev. Bras. Farmacogn. 2020, 30, 18–27. [Google Scholar] [CrossRef]
- Pfeifer Barbosa, A.L.; Wenzel-Storjohann, A.; Barbosa, J.D.; Zidorn, C.; Peifer, C.; Tasdemir, D.; Cicek, S.S. Antimicrobial and cytotoxic effects of the Copaifera reticulata oleoresin and its main diterpene acids. J. Ethnopharmacol. 2019, 233, 94–100. [Google Scholar] [CrossRef]
- Huang, D.; Qing, S.; Zeng, G.; Wang, Y.; Guo, H.; Tan, J.; Zhou, Y. Lipophilic components from Fructus Viticis Negundo and their anti-tumor activities. Fitoterapia 2013, 86, 144–148. [Google Scholar] [CrossRef]
- Sánchez-Mendoza, M.E.; Reyes-Trejo, B.; de la Rosa, L.; Rodríguez-Silverio, J.; Castillo-Henkel, C.; Arrieta, J. Polyalthic Acid Isolated from Croton reflexifoliushas Relaxing Effect in Guinea Pig Tracheal Smooth Muscle. Pharm. Biol. 2009, 46, 800–807. [Google Scholar] [CrossRef][Green Version]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.; Nakamura, C.V.; Filho, B.P. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem. Inst. Oswaldo Cruz 2002, 97, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Abrão, F.; Alves, J.A.; Andrade, G.; de Oliveira, P.F.; Ambrosio, S.R.; Veneziani, R.C.S.; Tavares, D.C.; Bastos, J.K.; Martins, C.H.G. Antibacterial Effect of Copaifera duckei Dwyer Oleoresin and Its Main Diterpenes against Oral Pathogens and Their Cytotoxic Effect. Front. Microbiol. 2018, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.C.; Cascon, V.; Possebon, L.S.; Morimoto, M.S.; Cardoso, L.G.; Kaplan, M.A.; Gilbert, B. Topical antiinflammatory and analgesic activities of Copaifera duckei dwyer. Phytother. Res. 2005, 19, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Cascon, V.; Gilbert, B. Characterization of the chemical composition of oleoresins of Copaifera guianensis Desf., Copaifera duckei Dwyer and Copaifera multijuga Hayne. Phytochemistry 2000, 55, 773–778. [Google Scholar] [CrossRef]
- Carneiro, L.; Bianchi, T.; da Silva, J.; Oliveira, L.; Borges, C.; Lemes, D.; Bastos, J.; Veneziani, R.; Ambrósio, S. Development and Validation of a Rapid and Reliable RP-HPLC-PDA Method for the Quantification of Six Diterpenes in Copaifera duckei, Copaifera reticulata and Copaifera multijuga Oleoresins. J. Braz. Chem. Soc. 2017, 29, 729–737. [Google Scholar] [CrossRef]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Fux, C.A.; Stoodley, P.; Hall-Stoodley, L.; Costerton, J.W. Bacterial biofilms: A diagnostic and therapeutic challenge. Expert. Rev. Anti Infect. Ther. 2003, 1, 667–683. [Google Scholar] [CrossRef]
- Richards, J.J.; Melander, C. Controlling bacterial biofilms. Chembiochem 2009, 10, 2287–2294. [Google Scholar] [CrossRef]
- Mooney, J.A.; Pridgen, E.M.; Manasherob, R.; Suh, G.; Blackwell, H.E.; Barron, A.E.; Bollyky, P.L.; Goodman, S.B.; Amanatullah, D.F. Periprosthetic bacterial biofilm and quorum sensing. J. Orthop. Res. 2018, 36, 2331–2339. [Google Scholar] [CrossRef]
- Izumi, E.; Ueda-Nakamura, T.; Veiga-Junior, V.F.; Nakamura, C.V. Toxicity of oleoresins from the genus Copaifera in Trypanosoma cruzi: A comparative study. Planta Med. 2013, 79, 952–958. [Google Scholar] [CrossRef]
- Mizuno, C.S.; Souza, A.B.; Tekwani, B.L.; Ambrosio, S.R.; Veneziani, R.C. Synthesis and biological evaluation of polyalthic acid derivatives for the treatment of neglected diseases. Bioorg. Med. Chem. Lett. 2015, 25, 5529–5531. [Google Scholar] [CrossRef]
- Teixeira, S.C.; Rosini, A.M.; de Souza, G.; Martinez, A.F.; Silva, R.J.; Ambrosio, S.R.; Veneziani, R.C.; Bastos, J.K.; Martins, C.H.; Barbosa, B.F.; et al. Polyalthic acid and oleoresin from Copaifera trapezifolia Hayne reduce Toxoplasma gondii growth in human villous explants, even triggering an anti-inflammatory profile. Exp. Parasitol. 2023, 250, 108534. [Google Scholar] [CrossRef] [PubMed]
- Furtado, R.A.; de Oliveira, P.F.; Senedese, J.M.; Ozelin, S.D.; de Souza, L.D.R.; Leandro, L.F.; de Oliveira, W.L.; da Silva, J.J.M.; Oliveira, L.C.; Rogez, H.; et al. Assessment of genotoxic activity of oleoresins and leaves extracts of six Copaifera species for prediction of potential human risks. J. Ethnopharmacol. 2018, 221, 119–125. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 474: Mammalian Erythrocyte Micronucleus Test; OECD: Paris, France, 2014. [Google Scholar]
- Sanchez-Me, M.E.; Garcia-Tap, M.; Garcia San, J.R.; Arrieta, J.; Olivares-C, I.M.; Santiago-C, J.A.; Ahumada-An, A.K. Effects of Polyalthic Acid from Croton reflexifolius on Viability of Cancerous Cells. Int. J. Pharmacol. 2017, 13, 286–291. [Google Scholar] [CrossRef]
- Miyazawa, M.; Shimamura, H.; Nakamura, S.-I.; Kameoka, H. Antimutagenic Activity of (+)-Polyalthic Acid from Vitex rotundiforia. J. Agric. Food Chem. 1995, 43, 3012–3015. [Google Scholar] [CrossRef]
- Ruszkiewicz, J.A.; Pinkas, A.; Miah, M.R.; Weitz, R.L.; Lawes, M.J.A.; Akinyemi, A.J.; Ijomone, O.M.; Aschner, M. C. elegans as a model in developmental neurotoxicology. Toxicol. Appl. Pharmacol. 2018, 354, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Carreras, C.R.; Rossomando, P.C.; Giordano, O.S. Ent-labdanes in eupatorium buniifolium. Phytochemistry 1998, 48, 1031–1034. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Document, 9th ed.; M7-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Nayak, A.; Sowmya, B.R.; Gandla, H.; Kottrashetti, V.; Ingalagi, P.; Srinivas, V.S.C. Determination and comparison of antimicrobial activity of aqueous and ethanolic extracts of Amorphophallus paeoniifolius on periodontal pathogens: An in vitro study. J. Indian Soc. Periodontol. 2023, 27, 40–44. [Google Scholar] [CrossRef]
- Wei, G.X.; Campagna, A.N.; Bobek, L.A. Effect of MUC7 peptides on the growth of bacteria and on Streptococcus mutans biofilm. J. Antimicrob. Chemother. 2006, 57, 1100–1109. [Google Scholar] [CrossRef]
- Sandberg, M.; Maattanen, A.; Peltonen, J.; Vuorela, P.M.; Fallarero, A. Automating a 96-well microtitre plate model for Staphylococcus aureus biofilms: An approach to screening of natural antimicrobial compounds. Int. J. Antimicrob. Agents 2008, 32, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Drewlo, S.; Baczyk, D.; Dunk, C.; Kingdom, J. Fusion assays and models for the trophoblast. Methods Mol. Biol. 2008, 475, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.W.; Dong, K.; Qin, H.X.; Yang, Y.K.; He, J.L.; Li, J.; Zheng, Z.W.; Chen, D.L.; Chen, J.P. Direct and Indirect Inhibition Effects of Resveratrol against Toxoplasma gondii Tachyzoites In Vitro. Antimicrob. Agents Chemother. 2019, 63, e01233-18. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Teo, C.F.; Zhou, X.W.; Bogyo, M.; Carruthers, V.B. Cysteine protease inhibitors block Toxoplasma gondii microneme secretion and cell invasion. Antimicrob. Agents Chemother. 2007, 51, 679–688. [Google Scholar] [CrossRef]
- Da Silva, R.J.; Gomes, A.O.; Franco, P.S.; Pereira, A.S.; Milian, I.C.B.; Ribeiro, M.; Fiorenzani, P.; Dos Santos, M.C.; Mineo, J.R.; da Silva, N.M.; et al. Enrofloxacin and Toltrazuril Are Able to Reduce Toxoplasma gondii Growth in Human BeWo Trophoblastic Cells and Villous Explants from Human Third Trimester Pregnancy. Front. Cell. Infect. Microbiol. 2017, 7, 340. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Murata, Y.; Sugi, T.; Kato, K. Inorganic nanoparticles kill Toxoplasma gondii via changes in redox status and mitochondrial membrane potential. Int. J. Nanomed. 2017, 12, 1647–1661. [Google Scholar] [CrossRef]
- Kamau, E.T.; Srinivasan, A.R.; Brown, M.J.; Fair, M.G.; Caraher, E.J.; Boyle, J.P. A focused small-molecule screen identifies 14 compounds with distinct effects on Toxoplasma gondii. Antimicrob. Agents Chemother. 2012, 56, 5581–5590. [Google Scholar] [CrossRef]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- MacGregor, J.T.; Heddle, J.A.; Hite, M.; Margolin, B.H.; Ramel, C.; Salamone, M.F.; Tice, R.R.; Wild, D. Guidelines for the conduct of micronucleus assays in mammalian bone marrow erythrocytes. Mutat. Res. 1987, 189, 103–112. [Google Scholar] [CrossRef]
- Singulani, J.L.; Scorzoni, L.; Gomes, P.C.; Nazare, A.C.; Polaquini, C.R.; Regasini, L.O.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Activity of gallic acid and its ester derivatives in Caenorhabditis elegans and zebrafish (Danio rerio) models. Future Med. Chem. 2017, 9, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
| Cariogenic Strains | CLO (µg/mL) | CECL (µg/mL) | PA (µg/mL) | Chlorhexidine (µg/mL) | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Enterococcus faecalis (ATCC 4082) | 25 | 25 | >400 | >400 | 25 | 25 | 7.37 | 7.37 |
| Lactobacillus paracasei (ATCC 11578) | 25 | 50 | >400 | >400 | 25 | 50 | 3.68 | 3.68 |
| Streptococcus mitis (ATCC 49456) | 25 | 50 | 400 | 400 | 25 | 50 | 3.68 | 3.68 |
| Streptococcus mutans (ATCC 25175) | 25 | 50 | >400 | >400 | 50 | 50 | 0.92 | 0.92 |
| Streptococcus salivarius (ATCC 25975) | 25 | 50 | 400 | 400 | 50 | 50 | 0.92 | 0.92 |
| Streptococcus sanguinis (ATCC 10556) | 25 | 50 | 400 | 400 | 25 | 50 | 7.37 | 7.37 |
| Streptococcus sobrinus (ATCC 33478) | 25 | 25 | 400 | >400 | 25 | 25 | 0.92 | 0.92 |
| Treatments (mg/kg) | MNPCEs | PCE/(PCE + NCE) |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| Negative control | 2.60 ± 1.34 | 0.59 ± 0.13 |
| Tween 80 | 2.40 ± 1.14 | 0.72 ± 0.10 |
| DMSO | 1.60 ± 0.55 | 0.69 ± 0.02 |
| CLO 125 | 5.00 ± 4.58 | 0.54 ± 0.04 |
| CLO 250 | 9.60 ± 2.88 | 0.57 ± 0.07 |
| CLO 500 | 6.80 ± 3.56 | 0.53 ± 0.07 |
| PA 1 | 4.00 ± 0.55 | 0.56 ± 0.05 |
| PA 10 | 3.60 ± 0.89 | 0.54 ± 0.02 |
| PA 20 | 2.80 ± 1.79 | 0.56 ± 0.03 |
| Positive control | 34.60 ± 1.82 * | 0.67 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago, M.B.; dos Santos, V.C.O.; Teixeira, S.C.; Silva, N.B.S.; de Oliveira, P.F.; Ozelin, S.D.; Furtado, R.A.; Tavares, D.C.; Ambrósio, S.R.; Veneziani, R.C.S.; et al. Polyalthic Acid from Copaifera lucens Demonstrates Anticariogenic and Antiparasitic Properties for Safe Use. Pharmaceuticals 2023, 16, 1357. https://doi.org/10.3390/ph16101357
Santiago MB, dos Santos VCO, Teixeira SC, Silva NBS, de Oliveira PF, Ozelin SD, Furtado RA, Tavares DC, Ambrósio SR, Veneziani RCS, et al. Polyalthic Acid from Copaifera lucens Demonstrates Anticariogenic and Antiparasitic Properties for Safe Use. Pharmaceuticals. 2023; 16(10):1357. https://doi.org/10.3390/ph16101357
Chicago/Turabian StyleSantiago, Mariana B., Vinicius Cristian O. dos Santos, Samuel C. Teixeira, Nagela B. S. Silva, Pollyanna F. de Oliveira, Saulo D. Ozelin, Ricardo A. Furtado, Denise C. Tavares, Sergio Ricardo Ambrósio, Rodrigo Cassio S. Veneziani, and et al. 2023. "Polyalthic Acid from Copaifera lucens Demonstrates Anticariogenic and Antiparasitic Properties for Safe Use" Pharmaceuticals 16, no. 10: 1357. https://doi.org/10.3390/ph16101357
APA StyleSantiago, M. B., dos Santos, V. C. O., Teixeira, S. C., Silva, N. B. S., de Oliveira, P. F., Ozelin, S. D., Furtado, R. A., Tavares, D. C., Ambrósio, S. R., Veneziani, R. C. S., Ferro, E. A. V., Bastos, J. K., & Martins, C. H. G. (2023). Polyalthic Acid from Copaifera lucens Demonstrates Anticariogenic and Antiparasitic Properties for Safe Use. Pharmaceuticals, 16(10), 1357. https://doi.org/10.3390/ph16101357









