Research Progress of Bioinspired Nanostructured Systems for the Treatment of Ocular Disorders
Abstract
1. Introduction
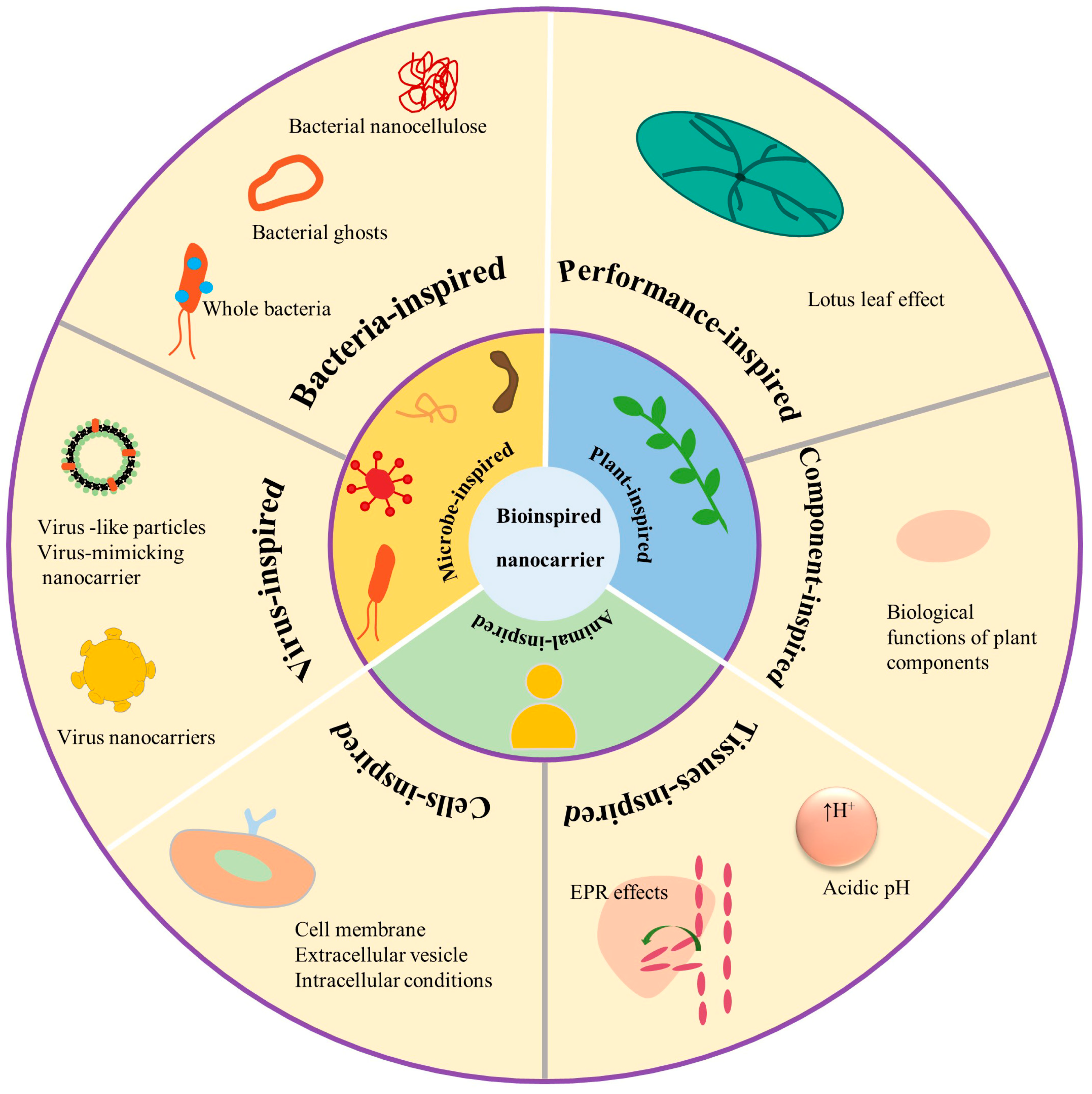
2. The Classification of Bioinspired Nanostructured Systems
2.1. Microbe-Inspired Drug Delivery Systems
2.1.1. Bacteria-Inspired Drug Delivery Systems
2.1.2. Virus-Inspired Drug Delivery Systems
2.2. Plant-Inspired Drug Delivery Systems
2.3. Animal-Inspired Drug Delivery Systems
2.3.1. Cell-Inspired Drug Delivery Systems
2.3.2. Tissues-Inspired Drug Delivery Systems
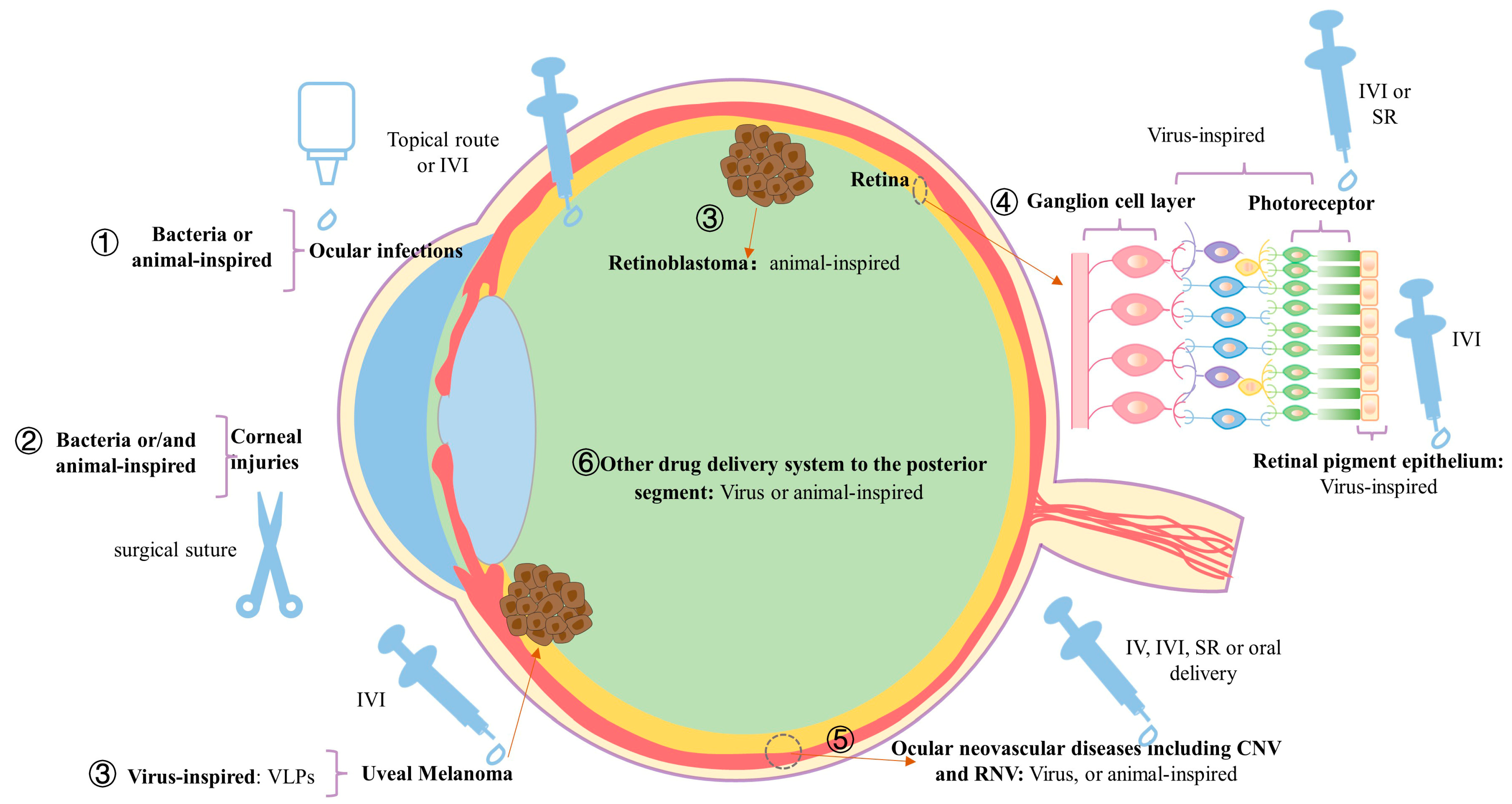
3. The Classification of Bioinspired Nanostructured Systems
3.1. Bioinspired Nanostructured Systems for the Treatment of Anterior Segment Ocular Disorders
3.2. Bioinspired Nanostructured Systems for the Treatment of Posterior Segment Ocular Disorders
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Awwad, S.; Ahmed, A.H.A.M.; Sharma, G.; Heng, J.S.; Khaw, P.T.; Brocchini, S.; Lockwood, A. Principles of Pharmacology in the Eye. Br. J. Pharmacol. 2017, 174, 4205–4223. [Google Scholar] [CrossRef] [PubMed]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-Álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tan, C.S. Safety and Complications of Intravitreal Injections Performed in an AsianPopulation in Singapore. Int. Ophthalmol. 2017, 37, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Chawla, R.; Shaikh, N.; Kandasamy, S.; Azad, S.V.; Sundar, M.D. A Comprehensive Review of Intravitreal Immunosuppressants and Biologicals used in Ophthalmology. Ther. Adv. Ophthalmol. 2022, 14, 25158414221097418. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Salamanna, F.; Gambardella, A.; Contartese, D.; Visani, A.; Fini, M. Nano-Based Biomaterials as Drug Delivery Systems Against Osteoporosis: A Systematic Review of Preclinical and Clinical Evidence. Nanomaterials 2021, 11, 530. [Google Scholar] [CrossRef]
- Fuloria, S.; Subramaniyan, V.; Karupiah, S.; Kumari, U.; Sathasivam, K.; Meenakshi, D.; Wu, Y.; Guad, R.; Udupa, K.; Fuloria, N. A Comprehensive Review on Source, Types, Effects, Nanotechnology, Detection, and Therapeutic Management of Reactive Carbonyl Species Associated with Various Chronic Diseases. Antioxidants 2020, 9, 1075. [Google Scholar] [CrossRef]
- Afarid, M.; Mahmoodi, S.; Baghban, R. Recent Achievements in Nano-based Technologies for Ocular Disease Diagnosis and Treatment, Review and Update. J. Nanobiotechnol. 2022, 20, 361. [Google Scholar] [CrossRef]
- Yang, C.; Yang, J.; Lu, A.; Gong, J.; Yang, Y.; Lin, X.; Li, M.; Xu, H. Nanoparticles in Ocular Applications and Their Potential Toxicity. Front. Mol. Biosci. 2022, 9, 931759. [Google Scholar] [CrossRef]
- Johnson, A.P.; Sabu, C.; Nivitha, K.; Sankar, R.; Shirin, V.A.; Henna, T.; Raphey, V.; Gangadharappa, H.; Kotta, S.; Pramod, K. Bioinspired and Biomimetic Micro- and Nanostructures in Biomedicine. J. Control. Release 2022, 343, 724–754. [Google Scholar] [CrossRef]
- Ahmad, F.J.; Beg, S.; Barkat, A. Bioinspired Smart Nanosystems in Advanced Therapeutic Applications. Pharm. Nanotechnol. 2019, 7, 179–180. [Google Scholar] [CrossRef]
- Mishra, S.; Sharma, S.; Javed, N.; Pottoo, F.H.; Barkat, A.; Harshita; Alam, M.S.; Amir, M.; Sarafroz, M. Bioinspired Nanocomposites: Applications in Disease Diagnosis and Treatment. Pharm. Nanotechnol. 2019, 7, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Holay, M.; Guo, Z.; Pihl, J.; Heo, J.; Park, J.H.; Fang, R.H.; Zhang, L. Bacteria-Inspired Nanomedicine. ACS Appl. Bio Mater. 2021, 4, 3830–3848. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Bao, F.; Li, L.; Yin, X.; Hua, Z. Bacterially Mediated Drug Delivery and Therapeutics: Strategies and Advancements. Adv. Drug Deliv. Rev. 2022, 187, 114363. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, Q.; Lv, Q.; Cai, B.; Xiaohalati, X.; Wang, G.; Wang, Z.; Wang, L. Oxygen-Generating Cyanobacteria Powered by Upconversion-Nanoparticles-Converted Near-Infrared Light for Ischemic Stroke Treatment. Nano Lett. 2021, 21, 4654–4665. [Google Scholar] [CrossRef]
- Chen, H.; Ji, H.; Kong, X.; Lei, P.; Yang, Q.; Wu, W.; Jin, L.; Sun, D. Bacterial Ghosts-Based Vaccine and Drug Delivery Systems. Pharmaceutics 2021, 13, 1892. [Google Scholar] [CrossRef]
- Go, G.; Lee, J.; Choi, D.S.; Kim, S.S.; Gho, Y.S. Extracellular Vesicle–Mimetic Ghost Nanovesicles for Delivering Anti-Inflammatory Drugs to Mitigate Gram-Negative Bacterial Outer Membrane Vesicle–Induced Systemic Inflammatory Response Syndrome. Adv. Healthc. Mater. 2019, 8, e1801082. [Google Scholar] [CrossRef]
- Zikmundova, M.; Vereshaka, M.; Kolarova, K.; Pajorova, J.; Svorcik, V.; Bacakova, L. Effects of Bacterial Nanocellulose Loaded with Curcumin and Its Degradation Products on Human Dermal Fibroblasts. Materials 2020, 13, 4759. [Google Scholar] [CrossRef]
- Pola, C.C.; Moraes, A.R.; Medeiros, E.A.; Teófilo, R.F.; Soares, N.F.; Gomes, C.L. Development and Optimization of pH-Responsive PLGA-Chitosan Nanoparticles for Triggered Release of Antimicrobials. Food Chem. 2019, 295, 671–679. [Google Scholar] [CrossRef]
- Chen, H.; Jin, Y.; Wang, J.; Wang, Y.; Jiang, W.; Dai, H.; Pang, S.; Lei, L.; Ji, J.; Wang, B. Design of Smart Targeted and Responsive Drug Delivery Systems with Enhanced Antibacterial Properties. Nanoscale 2018, 10, 20946–20962. [Google Scholar] [CrossRef]
- Ali, J.; Cheang, U.K.; Martindale, J.D.; Jabbarzadeh, M.; Fu, H.C.; Kim, M.J. Bacteria-inspired nanorobots with flagellar polymorphic transformations and bundling. Sci. Rep. 2017, 7, 14098. [Google Scholar] [CrossRef]
- Nehru, S.; Misra, R.; Bhaswant, M. Multifaceted Engineered Biomimetic Nanorobots Toward Cancer Management. ACS Biomater. Sci. Eng. 2022, 8, 444–459. [Google Scholar] [CrossRef]
- Suh, S.; Jo, A.; Traore, M.A.; Zhan, Y.; Coutermarsh-Ott, S.L.; Ringel-Scaia, V.M.; Allen, I.C.; Davis, R.M.; Behkam, B. Nanoscale Bacteria-Enabled Autonomous Drug Delivery System (NanoBEADS) Enhances Intratumoral Transport of Nanomedicine. Adv. Sci. 2019, 6, 1801309. [Google Scholar] [CrossRef]
- Wang, W.; Xu, H.; Ye, Q.; Tao, F.; Wheeldon, I.; Yuan, A.; Hu, Y.; Wu, J. Systemic Immune Responses to Irradiated Tumours via the Transport of Antigens to the Tumour Periphery by Injected Flagellate Bacteria. Nat. Biomed. Eng. 2022, 6, 44–53. [Google Scholar] [CrossRef]
- Chen, W.; Guo, Z.; Zhu, Y.; Qiao, N.; Zhang, Z.; Sun, X. Combination of Bacterial-Photothermal Therapy with an Anti-PD-1 Peptide Depot for Enhanced Immunity against Advanced Cancer. Adv. Funct. Mater. 2020, 30, 1906623. [Google Scholar] [CrossRef]
- Chen, F.; Zang, Z.; Chen, Z.; Cui, L.; Chang, Z.; Ma, A.; Yin, T.; Liang, R.; Han, Y.; Wu, Z.; et al. Nanophotosensitizer-Engineered Salmonella Bacteria with Hypoxia Targeting and Photothermal-Assisted Mutual Bioaccumulation for Solid Tumor Therapy. Biomaterials 2019, 214, 119226. [Google Scholar] [CrossRef]
- Ektate, K.; Munteanu, M.C.; Ashar, H.; Malayer, J.; Ranjan, A. Chemo-Immunotherapy of Colon Cancer with Focused Ultrasound and Salmonella-Laden Temperature Sensitive Liposomes (Thermobots). Sci. Rep. 2018, 8, 13062. [Google Scholar] [CrossRef]
- Moreno, V.M.; Álvarez, E.; Izquierdo-Barba, I.; Baeza, A.; Serrano-López, J.; Vallet-Regí, M. Bacteria as Nanoparticles Carrier for Enhancing Penetration in a Tumoral Matrix Model. Adv. Mater. Interfaces 2020, 7, 1901942. [Google Scholar] [CrossRef]
- Park, W.; Cho, S.; Kang, D.; Han, J.-H.; Park, J.-H.; Lee, B.; Lee, J.; Kim, D.-H. Tumor Microenvironment Targeting Nano–Bio Emulsion for Synergistic Combinational X-Ray PDT with Oncolytic Bacteria Therapy. Adv. Health Mater. 2020, 9, 1901812. [Google Scholar] [CrossRef]
- Reghu, S.; Miyako, E. Nanoengineered Bifidobacterium bifidum with Optical Activity for Photothermal Cancer Immunotheranostics. Nano Lett. 2022, 22, 1880–1888. [Google Scholar] [CrossRef]
- Ojha, S.K.; Pattnaik, R.; Singh, P.K.; Dixit, S.; Mishra, S.; Pal, S.; Kumar, S. Virus as Nanocarrier for Drug Delivery Redefining Medical Therapeutics—A Status Report. Comb. Chem. High Throughput Screen. 2022, 25, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Lam, E.; Tome-Amat, J.; Segrelles, C.; Yuste-Calvo, C.; Asensio, S.; Peral, J.; Ponz, F.; Lorz, C. Antitumor Applications of Polyphenol-Conjugated Turnip Mosaic Virus-Derived Nanoparticles. Nanomedicine 2022, 17, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Lam, E.; Imperial, J.; Ponz, F. Polyphenol-Functionalized Plant Viral-Derived Nanoparticles Exhibit Strong Antimicrobial and Antibiofilm Formation Activities. ACS Appl. Bio Mater. 2020, 3, 2040–2047. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, G.; Wang, M.; Lin, T.; Liu, W.; Wang, Y. Phage Nanoparticle as a Carrier for Controlling Fungal Infection. Appl. Microbiol. Biotechnol. 2022, 106, 3397–3403. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Y.; Inoue, N.; Oo, C.W.; Kawasaki, H.; Lim, T.S. One-Step Synthesis of M13 Phage-Based Nanoparticles and Their Fluorescence Properties. RSC Adv. 2021, 11, 1367–1375. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-Like Particles: Preparation, Immunogenicity and Their Roles as Nanovaccines and Drug Nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Le, D.; Müller, K. In Vitro Assembly of Virus-Like Particles and Their Applications. Life 2021, 11, 334. [Google Scholar] [CrossRef]
- Tan, F.; Kong, J.; Ng, J.; Alitheen, N.; Wong, C.; Yong, C.; Lee, K. Recombinant Turnip Yellow Mosaic Virus Coat Protein as a Potential Nanocarrier. J. Appl. Microbiol. 2021, 131, 2072–2080. [Google Scholar] [CrossRef]
- Akiyama, H.; Ramirez, N.-G.P.; Gibson, G.; Kline, C.; Watkins, S.; Ambrose, Z.; Gummuluru, S. Interferon-Inducible CD169/Siglec1 Attenuates Anti-HIV-1 Effects of Alpha Interferon. J. Virol. 2017, 91, e00972-17. [Google Scholar] [CrossRef]
- Izquierdo-Useros, N.; Lorizate, M.; McLaren, P.J.; Telenti, A.; Kräusslich, H.-G.; Martinez-Picado, J. HIV-1 Capture and Transmission by Dendritic Cells: The Role of Viral Glycolipids and the Cellular Receptor Siglec-1. PLoS Pathog. 2014, 10, e1004146. [Google Scholar] [CrossRef]
- Eshaghi, B.; Fofana, J.; Nodder, S.B.; Gummuluru, S.; Reinhard, B.M. Virus-Mimicking Polymer Nanoparticles Targeting CD169+ Macrophages as Long-Acting Nanocarriers for Combination Antiretrovirals. ACS Appl. Mater. Interfaces 2022, 14, 2488–2500. [Google Scholar] [CrossRef]
- Song, B. Lotus Leaf-Inspired Design of Calcium Alginate Particles with Superhigh Drug Encapsulation Efficiency and pH Responsive Release. Colloids Surf. B Biointerfaces 2018, 172, 464–470. [Google Scholar] [CrossRef]
- Kim, J.; Ju, J.; Kim, S.D.; Shin, M. Plant-inspired Pluronic–gallol micelles with low critical micelle concentration, high colloidal stability, and protein affinity. Biomater. Sci. 2022, 10, 3739–3746. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.-W.; Zhu, A.-Q.; Huang, L.-Q.; Peng, L.-H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Zaid, N.A.M.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Lum, P.T.; Begum, M.Y.; Rani, N.N.I.M.; Vaijanathappa, J.; Wu, Y.S.; Subramaniyan, V.; et al. Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug Des. Dev. Ther. 2022, 16, 23–66. [Google Scholar] [CrossRef]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Faivre, V. Targeted Drug Delivery Therapies Inspired by Natural Taxes. J. Control. Release 2020, 322, 439–456. [Google Scholar] [CrossRef]
- Zhang, M.; Du, Y.; Wang, S.; Chen, B. A Review of Biomimetic Nanoparticle Drug Delivery Systems Based on Cell Membranes. Drug Des. Dev. Ther. 2020, 14, 5495–5503. [Google Scholar] [CrossRef]
- Wang, H.; Shi, W.; Zeng, D.; Huang, Q.; Xie, J.; Wen, H.; Li, J.; Yu, X.; Qin, L.; Zhou, Y. pH-Activated, Mitochondria-Targeted, and Redox-Responsive Delivery of Paclitaxel Nanomicelles to Overcome Drug Resistance and Suppress Metastasis in Lung Cancer. J. Nanobiotechnol. 2021, 19, 152. [Google Scholar] [CrossRef]
- Battogtokh, G.; Cho, Y.-Y.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondrial-Targeting Anticancer Agent Conjugates and Nanocarrier Systems for Cancer Treatment. Front. Pharmacol. 2018, 9, 922. [Google Scholar] [CrossRef]
- Chandra, A.; Prasad, S.; Alemanno, F.; De Luca, M.; Rizzo, R.; Romano, R.; Gigli, G.; Bucci, C.; Barra, A.; del Mercato, L.L. Fully Automated Computational Approach for Precisely Measuring Organelle Acidification with Optical pH Sensors. ACS Appl. Mater. Interfaces 2022, 14, 18133–18149. [Google Scholar] [CrossRef] [PubMed]
- Balmert, S.; Little, S.R. Biomimetic Delivery with Micro- and Nanoparticles. Adv. Mater. 2012, 24, 3757–3778. [Google Scholar] [CrossRef] [PubMed]
- Rampado, R.; Caliceti, P.; Agostini, M. Latest Advances in Biomimetic Cell Membrane-Coated and Membrane-Derived Nanovectors for Biomedical Applications. Nanomaterials 2022, 12, 1543. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Xu, X.; Wang, L.; Mo, R. Advances in Living Cell-Based Anticancer Therapeutics. Biomater. Sci. 2020, 8, 2344–2365. [Google Scholar] [CrossRef] [PubMed]
- Brenner, J.S.; Mitragotri, S.; Muzykantov, V.R. Red Blood Cell Hitchhiking: A Novel Approach for Vascular Delivery of Nanocarriers. Annu. Rev. Biomed. Eng. 2021, 23, 225–248. [Google Scholar] [CrossRef]
- Bouwstra, R.; van Meerten, T.; Bremer, E. CD47-SIRPα blocking-based immunotherapy: Current and prospective therapeutic strategies. Clin. Transl. Med. 2022, 12, e943. [Google Scholar] [CrossRef]
- Ayi, K.; Lu, Z.; Serghides, L.; Ho, J.M.; Finney, C.; Wang, J.C.Y.; Liles, W.C.; Kain, K.C. CD47-SIRPα Interactions Regulate Macrophage Uptake of Plasmodium Falciparum-Infected Erythrocytes and Clearance of Malaria In Vivo. Infect. Immun. 2016, 84, 2002–2011. [Google Scholar] [CrossRef]
- Ding, Y.; Lv, B.; Zheng, J.; Lu, C.; Liu, J.; Lei, Y.; Yang, M.; Wang, Y.; Li, Z.; Yang, Y.; et al. RBC-Hitchhiking Chitosan Nanoparticles Loading Methylprednisolone for Lung-Targeting Delivery. J. Control. Release 2022, 341, 702–715. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte Membrane-Camouflaged Polymeric Nanoparticles as a Biomimetic Delivery Platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef]
- Chiangjong, W.; Netsirisawan, P.; Hongeng, S.; Chutipongtanate, S. Red Blood Cell Extracellular Vesicle-Based Drug Delivery: Challenges and Opportunities. Front. Med. 2021, 8, 761362. [Google Scholar] [CrossRef]
- Peng, B.; Nguyen, T.M.; Jayasinghe, M.K.; Gao, C.; Pham, T.T.; Vu, L.T.; Yeo, E.Y.M.; Yap, G.; Wang, L.; Goh, B.C.; et al. Robust Delivery of RIG-I Agonists Using Extracellular Vesicles for Anti-Cancer Immunotherapy. J. Extracell. Vesicles 2022, 11, e12187. [Google Scholar] [CrossRef]
- Chen, Z.; Kankala, R.K.; Yang, Z.; Li, W.; Xie, S.; Li, H.; Chen, A.-Z.; Zou, L. Antibody-Based Drug Delivery Systems for Cancer Therapy: Mechanisms, Challenges, and Prospects. Theranostics 2022, 12, 3719–3746. [Google Scholar] [CrossRef]
- Marko, A.J.; Borah, B.M.; Siters, K.E.; Missert, J.R.; Gupta, A.; Pera, P.; Isaac-Lam, M.F.; Pandey, R.K. Targeted Nanoparticles for Fluorescence Imaging of Folate Receptor Positive Tumors. Biomolecules 2020, 10, 1651. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, J.; Deng, L.; Hu, H.; Hu, J.; Zheng, G. Galactose-Modified PH-Sensitive Niosomes for Controlled Release and Hepatocellular Carcinoma Target Delivery of Tanshinone IIA. AAPS PharmSciTech 2021, 22, 96. [Google Scholar] [CrossRef]
- Long, P.; Zhang, Q.; Xue, M.; Cao, G.; Li, C.; Chen, W.; Jin, F.; Li, Z.; Li, R.; Wang, X.; et al. Tomato Lectin-Modified Nanoemulsion-Encapsulated MAGE1-HSP70/SEA Complex Protein Vaccine: Targeting Intestinal M Cells Following Peroral Administration. Biomed. Pharmacother. 2019, 115, 108886. [Google Scholar] [CrossRef]
- Chiarpotti, M.V.; Longo, G.S.; Del Pópolo, M.G. Nanoparticles Modified with Cell Penetrating Peptides: Assessing Adsorption on Membranes Containing Acidic Lipids. Colloids Surf. B Biointerfaces 2021, 197, 111373. [Google Scholar] [CrossRef]
- Kesharwani, P.; Chadar, R.; Sheikh, A.; Rizg, W.Y.; Safhi, A.Y. CD44-Targeted Nanocarrier for Cancer Therapy. Front. Pharmacol. 2021, 12, 800481. [Google Scholar] [CrossRef]
- Cheng, K.; Zhou, J.; Zhao, Y.; Chen, Y.; Ming, L.; Huang, D.; Yang, R.; Lin, Z.; Chen, D. pH-Responsive and CD44-Targeting Polymer Micelles Based on CD44p-Conjugated Amphiphilic Block Copolymer PEG-b-HES-b-PLA for Delivery of Emodin to Breast Cancer Cells. Nanotechnology 2022, 33, 275604. [Google Scholar] [CrossRef]
- Yang, R.; Lu, M.; Ming, L.; Chen, Y.; Cheng, K.; Zhou, J.; Jiang, S.; Lin, Z.; Chen, D. 89Zr-Labeled Multifunctional Liposomes Conjugate Chitosan for PET-Trackable Triple-Negative Breast Cancer Stem Cell Targeted Therapy. Int. J. Nanomed. 2020, 15, 9061–9074. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, R.; Yu, W.; Hu, C.; Zhang, Z.; Liu, D.; An, Y.; Wang, X.; He, C.; Liu, P.; et al. Chitosan Oligosaccharide Decorated Liposomes Combined with TH302 for Photodynamic Therapy in Triple Negative Breast Cancer. J. Nanobiotechnol. 2021, 19, 147. [Google Scholar] [CrossRef]
- Zhang, S.; Lv, J.; Gao, P.; Feng, Q.; Wang, H.; Cheng, Y. A pH-Responsive Phase-Transition Polymer with High Serum Stability in Cytosolic Protein Delivery. Nano Lett. 2021, 21, 7855–7861. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- George, T.A.; Hsu, C.-C.; Meeson, A.; Lundy, D.J. Nanocarrier-Based Targeted Therapies for Myocardial Infarction. Pharmaceutics 2022, 14, 930. [Google Scholar] [CrossRef] [PubMed]
- Kanthi, Y.; de la Zerda, A.; Smith, B.R. Nanotherapeutic Shots through the Heart of Plaque. ACS Nano 2020, 14, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Li, J.; He, J.; Luo, F.; Yu, T.; Dai, Q.; Chen, Y.; Xu, J.; Yang, X.; Dong, S. Bone-Targeted pH-Responsive Cerium Nanoparticles for Anabolic Therapy in Osteoporosis. Bioact. Mater. 2021, 6, 4697–4706. [Google Scholar] [CrossRef]
- AlSawaftah, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. pH-Responsive Nanocarriers in Cancer Therapy. Polymers 2022, 14, 936. [Google Scholar] [CrossRef]
- Mofidfar, M.; Abdi, B.; Ahadian, S.; Mostafavi, E.; Desai, T.A.; Abbasi, F.; Sun, Y.; Manche, E.E.; Ta, C.N.; Flowers, C.W. Drug Delivery to the Anterior Segment of the Eye: A Review of Current and Future Treatment Strategies. Int. J. Pharm. 2021, 607, 120924. [Google Scholar] [CrossRef]
- Wang, R.; Gao, Y.; Liu, A.; Zhai, G. A Review of Nanocarrier-Mediated Drug Delivery Systems for Posterior Segment Eye Disease: Challenges Analysis and Recent Advances. J. Drug Target. 2021, 29, 687–702. [Google Scholar] [CrossRef]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef]
- Zhu, K.; Qian, S.; Guo, H.; Wang, Q.; Chu, X.; Wang, X.; Lu, S.; Peng, Y.; Guo, Y.; Zhu, Z.; et al. pH-Activatable Organic Nanoparticles for Efficient Low-Temperature Photothermal Therapy of Ocular Bacterial Infection. ACS Nano 2022, 16, 11136–11151. [Google Scholar] [CrossRef]
- Chen, H.; Yang, J.; Sun, L.; Zhang, H.; Guo, Y.; Qu, J.; Jiang, W.; Chen, W.; Ji, J.; Yang, Y.; et al. Synergistic Chemotherapy and Photodynamic Therapy of Endophthalmitis Mediated by Zeolitic Imidazolate Framework-Based Drug Delivery Systems. Small 2019, 15, e1903880. [Google Scholar] [CrossRef]
- Shankar, S.; Shah, S.G.; Yadav, S.; Chugh, A. Novel Corneal Targeting Cell Penetrating Peptide as an Efficient Nanocarrier with an Effective Antimicrobial Activity. Eur. J. Pharm. Biopharm. 2021, 166, 216–226. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Rao, C.M. Condition Responsive Nanoparticles for Managing Infection and Inflammation in Keratitis. Nanoscale 2017, 9, 9946–9959. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Chang, Y.-F.; Ko, Y.-C.; Liu, C.J.-L. Development of a Dual Delivery of Levofloxacin and Prednisolone Acetate via PLGA Nanoparticles/Thermosensitive Chitosan-Based Hydrogel for Postoperative Management: An in-vitro and ex-vivo Study. Int. J. Biol. Macromol. 2021, 180, 365–374. [Google Scholar] [CrossRef]
- Tavakoli, N.; Taymouri, S.; Saeidi, A.; Akbari, V. Thermosensitive Hydrogel Containing Sertaconazole Loaded Nanostructured Lipid Carriers for Potential Treatment of Fungal Keratitis. Pharm. Dev. Technol. 2019, 24, 891–901. [Google Scholar] [CrossRef]
- Anton-Sales, I.; D’Antin, J.C.; Fernández-Engroba, J.; Charoenrook, V.; Laromaine, A.; Roig, A.; Michael, R. Bacterial Nanocellulose as a Corneal Bandage Material: A Comparison with Amniotic Membrane. Biomater. Sci. 2020, 8, 2921–2930. [Google Scholar] [CrossRef]
- Anton-Sales, I.; Koivusalo, L.; Skottman, H.; Laromaine, A.; Roig, A. Limbal Stem Cells on Bacterial Nanocellulose Carriers for Ocular Surface Regeneration. Small 2021, 17, e2003937. [Google Scholar] [CrossRef]
- Walther, G.; Stasch, S.; Kaerger, K.; Hamprecht, A.; Roth, M.; Cornely, O.A.; Geerling, G.; Mackenzie, C.R.; Kurzai, O.; von Lilienfeld-Toal, M. Fusarium Keratitis in Germany. J. Clin. Microbiol. 2017, 55, 2983–2995. [Google Scholar] [CrossRef]
- Song, A.; Deshmukh, R.; Lin, H.; Ang, M.; Mehta, J.S.; Chodosh, J.; Said, D.G.; Dua, H.S.; Ting, D.S.J. Post-keratoplasty Infectious Keratitis: Epidemiology, Risk Factors, Management, and Outcomes. Front. Med. 2021, 8, 707242. [Google Scholar] [CrossRef]
- Nagai, N.; Isaka, T.; Deguchi, S.; Minami, M.; Yamaguchi, M.; Otake, H.; Okamoto, N.; Nakazawa, Y. In Situ Gelling Systems Using Pluronic F127 Enhance Corneal Permeability of Indomethacin Nanocrystals. Int. J. Mol. Sci. 2020, 21, 7083. [Google Scholar] [CrossRef]
- Shastri, D.; Patel, L.; Parikh, R. Studies on in situ Hydrogel: A Smart Way for Safe and Sustained Ocular Drug Delivery. J. Young Pharm. 2010, 2, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Eltaher, H.M.; Ferreras, L.A.B.; Jalal, A.R.; Dixon, J.E. Direct Contact-Mediated Non-Viral Gene Therapy Using Thermo-Sensitive Hydrogel-Coated Dressings. Biomater. Adv. 2022, 143, 213177. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.S.; Abdekhodaie, M.J.; Mashayekhan, S.; Baradaran-Rafii, A.; Djalilian, A.R. Bioengineering Approaches for Corneal Regenerative Medicine. Tissue Eng. Regen. Med. 2020, 17, 567–593. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Yun, H.; Funderburgh, M.L.; Du, Y. Regenerative Therapy for the Cornea. Prog. Retin. Eye Res. 2022, 87, 101011. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, H.; Ong, H.S.; Riau, A.K.; Stanzel, T.P.; Mehta, J.S.; Yam, G.H.-F. Current Trends and Future Perspective of Mesenchymal Stem Cells and Exosomes in Corneal Diseases. Int. J. Mol. Sci. 2019, 20, 2853. [Google Scholar] [CrossRef]
- Widyaningrum, R.; Wu, Y.-W.; Delila, L.; Lee, D.-Y.; Wang, T.-J.; Burnouf, T. In Vitro Evaluation of Platelet Extracellular Vesicles (PEVs) for Corneal Endothelial Regeneration. Platelets 2022, 33, 1237–1250. [Google Scholar] [CrossRef]
- Escandon, P.; Liu, A.; Nicholas, S.E.; Khan, A.; Riaz, K.M.; Karamichos, D. Unravelling Novel Roles of Salivary Exosomes in the Regulation of Human Corneal Stromal Cell Migration and Wound Healing. Int. J. Mol. Sci. 2022, 23, 4330. [Google Scholar] [CrossRef]
- Kines, R.C.; Varsavsky, I.; Choudhary, S.; Bhattacharya, D.; Spring, S.; McLaughlin, R.; Kang, S.J.; Grossniklaus, H.E.; Vavvas, D.; Monks, S.; et al. An Infrared Dye–Conjugated Virus-like Particle for the Treatment of Primary Uveal Melanoma. Mol. Cancer Ther. 2018, 17, 565–574. [Google Scholar] [CrossRef]
- Narayana, R.V.L.; Jana, P.; Tomar, N.; Prabhu, V.; Nair, R.M.; Manukonda, R.; Kaliki, S.; Coupland, S.E.; Alexander, J.; Kalirai, H.; et al. Carboplatin- and Etoposide-Loaded Lactoferrin Protein Nanoparticles for Targeting Cancer Stem Cells in Retinoblastoma In Vitro. Investig. Opthalmology Vis. Sci. 2021, 62, 13. [Google Scholar] [CrossRef]
- Bennett, J.; Wellman, J.; Marshall, K.A.; McCague, S.; Ashtari, M.; DiStefano-Pappas, J.; Elci, O.U.; Chung, D.C.; Sun, J.; Wright, J.F.; et al. Safety and Durability of Effect of Contralateral-Eye Administration of AAV2 Gene Therapy in Patients with Childhood-Onset Blindness Caused by RPE65 Mutations: A Follow-on Phase 1 Trial. Lancet 2016, 388, 661–672. [Google Scholar] [CrossRef]
- Pavlou, M.; Schön, C.; Occelli, L.M.; Rossi, A.; Meumann, N.; Boyd, R.F.; Bartoe, J.T.; Siedlecki, J.; Gerhardt, M.J.; Babutzka, S.; et al. Novel AAV Capsids for Intravitreal Gene Therapy of Photoreceptor Disorders. EMBO Mol. Med. 2021, 13, e13392. [Google Scholar] [CrossRef]
- Ross, A.G.; Chaqour, B.; McDougald, D.S.; Dine, K.E.; Duong, T.T.; Shindler, R.E.; Yue, J.; Liu, T.; Shindler, K.S. Selective Upregulation of SIRT1 Expression in Retinal Ganglion Cells by AAV-Mediated Gene Delivery Increases Neuronal Cell Survival and Alleviates Axon Demyelination Associated with Optic Neuritis. Biomolecules 2022, 12, 830. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, F.; Qiu, Y.; Wang, S.; Li, F.; Zhao, J.; Pan, C.; Tao, Y.; Di Yu, D.; Wei, W. Reduction of Choroidal Neovascularization via Cleavable VEGF Antibodies Conjugated to Exosomes Derived from Regulatory T cells. Nat. Biomed. Eng. 2021, 5, 968–982. [Google Scholar] [CrossRef]
- Holmgaard, A.; Alsing, S.; Askou, A.L.; Corydon, T.J. CRISPR Gene Therapy of the Eye: Targeted Knockout of Vegfa in Mouse Retina by Lentiviral Delivery. CRISPR Gene Ed. 2019, 1961, 307–328. [Google Scholar] [CrossRef]
- Xia, W.; Li, C.; Chen, Q.; Huang, J.; Zhao, Z.; Liu, P.; Xu, K.; Li, L.; Hu, F.; Zhang, S.; et al. Intravenous Route to Choroidal Neovascularization by Macrophage-Disguised Nanocarriers for mTOR Modulation. Acta Pharm. Sin. B 2022, 12, 2506–2521. [Google Scholar] [CrossRef]
- Li, M.; Xu, Z.; Zhang, L.; Cui, M.; Zhu, M.; Guo, Y.; Sun, R.; Han, J.; Song, E.; He, Y.; et al. Targeted Noninvasive Treatment of Choroidal Neovascularization by Hybrid Cell-Membrane-Cloaked Biomimetic Nanoparticles. ACS Nano 2021, 15, 9808–9819. [Google Scholar] [CrossRef]
- Wang, J.; Tan, J.; Luo, J.; Huang, P.; Zhou, W.; Chen, L.; Long, L.; Zhang, L.-M.; Zhu, B.; Yang, L.; et al. Enhancement of Scutellarin Oral Delivery Efficacy by Vitamin B12-Modified Amphiphilic Chitosan Derivatives to Treat Type II Diabetes Induced-Retinopathy. J. Nanobiotechnol. 2017, 15, 18. [Google Scholar] [CrossRef]
- Behroozi, F.; Abdkhodaie, M.-J.; Abandansari, H.S.; Satarian, L.; Ashtiani, M.K.; Jaafari, M.R.; Baharvand, H. Smart Liposomal Drug Delivery for Treatment of Oxidative Stress Model in Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells. Int. J. Pharm. 2018, 548, 62–72. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.; Fang, L.; Cao, F. Multifunctional Carboxymethyl Chitosan Derivatives-Layered Double Hydroxide Hybrid Nanocomposites for Efficient Drug Delivery to the Posterior Segment of the Eye. Acta Biomater. 2020, 104, 104–114. [Google Scholar] [CrossRef]
- Schefler, A.C.; Kim, R.S. Recent Advancements in the Management of Retinoblastoma and Uveal Melanoma. Fac. Rev. 2021, 10, 1–15. [Google Scholar] [CrossRef]
- Lane, A.M.; Kim, I.; Gragoudas, E.S. Survival Rates in Patients After Treatment for Metastasis from Uveal Melanoma. JAMA Ophthalmol. 2018, 136, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Mitra, R.N.; Zheng, M.; Wang, K.; Dahringer, J.C.; Han, Z. Developing Nanoceria-Based pH-Dependent Cancer-Directed Drug Delivery System for Retinoblastoma. Adv. Funct. Mater. 2018, 28, 1806248. [Google Scholar] [CrossRef] [PubMed]
- Schneider, N.; Sundaresan, Y.; Gopalakrishnan, P.; Beryozkin, A.; Hanany, M.; Levanon, E.Y.; Banin, E.; Ben-Aroya, S.; Sharon, D. Inherited Retinal Diseases: Linking Genes, Disease-Causing Variants, and Relevant Therapeutic Modalities. Prog. Retin. Eye Res. 2022, 89, 101029. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A. Ocular Neovascularization. J. Mol. Med. 2013, 91, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Malsy, J.; Alvarado, A.C.; Lamontagne, J.O.; Strittmatter, K.; Marneros, A.G. Distinct Effects of Complement and of NLRP3- and non-NLRP3 Inflammasomes for Choroidal Neovascularization. eLife 2020, 9, e60194. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Zhu, L.; Du, S.; Wang, Z.; Zhang, Y.; Guo, Y.; Tu, Y.; Song, E. Crosstalk Between RPE Cells and Choroidal Endothelial Cells via the ANXA1/FPR2/SHP2/NLRP3 Inflammasome/Pyroptosis Axis Promotes Choroidal Neovascularization. Inflammation 2022, 45, 414–427. [Google Scholar] [CrossRef]
| Bacterial Strain | Coating | Modifications | Therapeutic Agents | Study Goals | Ref. |
|---|---|---|---|---|---|
| S. typhimurium VNP20009 | Poly(lactic-co-glycolic acid) nanoparticles | Streptavidin-biotin interaction | Immunotherapy | 4T1 mammary tumors | [23] |
| S. typhimurium VNP20009 | Polyamidoamine dendrimer | Electrostatic interactions | Immunotherapy | 4T1 or CT26 mammary tumors | [24] |
| S. typhimurium VNP20009 | Polydopamine | Electrostatic interactions | Immunotherapy | Melanoma | [25] |
| S. typhimurium Strain YB1 | Indocyanine green-loaded nanoparticles | Covalent chemical conjugation | Photothermal therapy | MB49 bladder cancer | [26] |
| Typhimurium strain YS1646 | Low-temperature sensitive Liposomes | Streptavidin-biotin interaction | Chemo-immunotherapy | C26 colon tumor model | [27] |
| E. Coli strain Seattle 1946 | Mesoporous silica nanoparticles | Covalent chemical conjugation | Chemotherapy | HT1080 human fibrosarcoma cells in a 3D tumoral matrix model | [28] |
| Clostridium novyi-NT | Photosensitizer coated core/shell-structured lanthanide-doped nanoscintillators | Emulsification | Bacteriolytic and photodynamic therapy | PC3 prostate cancer tumor model | [29] |
| Bifidobacterium bifidum | - | Incubation and washing processes | Photothermal cancer immunotheranostics | Colon26-bearing immunocompetent mice | [30] |
| Disease | Inspirations from Nature Material or Functionals | Kingdom | Nanocomposites | Administration | Application Models |
|---|---|---|---|---|---|
| Ocular infections | Acid microenvironment in a bacterial infection lesion or biofilm | Microbe | EtNBSC nanoassemblies | IVI | Ocular bacterial infections in rats [80] |
| Acid microenvironment in a bacterial infection lesion or biofilm | Microbe | ZIF-8-PAA-MB@AgNPs@Van-PEG | IVI | Mice endophthalmitis models [81] | |
| anterior ocular tissues in particular receptors, transporters, and GAGs | Human | CorTS 1 nanoparticles | Ex vivo corneas issues | Freshly excised goat eyes [82] | |
| TLR4 on the corneal epithelial cells | Human | Anti-TLR4 antibodies conjugated, ketoconazole-encapsulated gelatin nanoparticles | Topical route | Rat model of keratitis [83] | |
| Temperature of eye tissues | Human | PA and levofloxacin-loaded thermosensitive chitosan/gelatin-based hydrogel nanoparticles | Ex vivo t corneas issues | Rabbit model of Staphylococcus aureus keratitis [84] | |
| Temperature of eye tissues | Human | Thermosensitive gel containing sertaconazole-loaded NLCs | Ex vivo t corneas issues | Potential treatment of fungal keratitis [85] | |
| Regenerative ophthalmology | BNC | Microbe | BNC hydrogels | Surgical suture | BNC hydrogels were sutured to pig eyes suture [86] |
| BNC | Microbe | hESC-LSC loaded BNC | Surgical suture | Potential application on ocular surface regeneration [87] |
| Disease | Inspirations from Nature Material or Functionals | Kingdom | Nanocomposites | Administration | Application Models |
|---|---|---|---|---|---|
| Eye tumors | VLPs | Microbe | Phthalocyanine photosensitizer conjugated VLPs | IVI | Uveal melanoma model in rabbit [98] |
| Lf | Human | Carboplatin- and etoposide-loaded Lf protein nanoparticles | In vitro cell culture | Rb Y79 CSCs [99] | |
| Retina-targeting gene therapy | AAV2 | Microbe | AAV2-hRPE65v2 | SR | Childhood-onset blindness caused by RPE65 mutations [100] |
| AAV2 | Microbe | Engineered capsid variants | IVI | Cnga3−/− mouse model of achromatopsia [101] | |
| AAV7 | Microbe | AAV7m8.SNCG. SIRT1 | IVI | Mice with EAE [102] | |
| Ocular neovascular disease | Exosomes derived from regulatory T cells | Human | VEGF antibody conjugated-exosomes | IVI | CNV mouse model [103] |
| LVs | Microbe | CRISPR/Cas9 loaded lentiviral vectors | SR | Healthy mice [104] | |
| Membrane derived from macrophages | Human | MRaNPs | IV | Laser-induced CNV mouse model [105] | |
| Cell-membrane fusion by RBC and REC membrane | Human | Hybrid cell-membrane-cloaked PLGA nanoparticles | IV | Laser-induced CNV mouse model [106] | |
| IF on the receptors located in the luminal surface of the intestine | Human | VB12 modified, scutellarin loaded amphiphilic chitosan derivatives | Oral delivery | Type II diabetes-induced retinopathy [107] | |
| Other drug delivery to the posterior segment | Oxidative stress in AMD | Human | Diselenide containing liposome | In vitro cell culture | hESC-RPE cells [108] |
| PepT-1 on the ocular surface | Human | Multifunctional carboxymethyl chitosan derivatives-layered double hydroxide hybrid nanocomposites | Topical route | Healthy rabbits [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yang, R.; Shen, J.; Huang, Q.; Wu, Z. Research Progress of Bioinspired Nanostructured Systems for the Treatment of Ocular Disorders. Pharmaceuticals 2023, 16, 96. https://doi.org/10.3390/ph16010096
Chen X, Yang R, Shen J, Huang Q, Wu Z. Research Progress of Bioinspired Nanostructured Systems for the Treatment of Ocular Disorders. Pharmaceuticals. 2023; 16(1):96. https://doi.org/10.3390/ph16010096
Chicago/Turabian StyleChen, Xuan, Rui Yang, Jinyan Shen, Qingyu Huang, and Zhifeng Wu. 2023. "Research Progress of Bioinspired Nanostructured Systems for the Treatment of Ocular Disorders" Pharmaceuticals 16, no. 1: 96. https://doi.org/10.3390/ph16010096
APA StyleChen, X., Yang, R., Shen, J., Huang, Q., & Wu, Z. (2023). Research Progress of Bioinspired Nanostructured Systems for the Treatment of Ocular Disorders. Pharmaceuticals, 16(1), 96. https://doi.org/10.3390/ph16010096






