Acetylsalicylic Acid and Mood Disorders: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources, Search Strategy and Selection Process
2.3. Data Collection Process
2.4. Study Risk of Bias Assessment
2.5. Data Synthesis and Analysis
3. Results
3.1. Study Characteristics
3.2. Quality Assessment
3.3. Preclinical Studies
3.3.1. Effects of ASA on Depressive Symptoms in Animal Models
3.3.2. Effects of ASA on Manic Symptoms in Animal Model
3.3.3. Adverse Effects of ASA in Rodents
3.3.4. Effects of ASA on Inflammatory Parameters in Rodents
3.4. Observational Studies
3.4.1. Observational Studies–Effects of ASA on Depressive Episodes
3.4.2. Observational Studies-Effects of ASA on Bipolar Disorder
3.5. Interventional Studies
3.5.1. Interventional Studies-Effects of ASA on Depressive Episodes
3.5.2. Interventional Studies—Effects of ASA on Bipolar Depression
3.5.3. Interventional Studies—Adverse Effects of ASA
3.5.4. Interventional Studies-Effects of ASA on Inflammatory Parameters
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clemente, A.S.; Diniz, B.S.; Nicolato, R.; Kapczinski, F.P.; Soares, J.C.; Firmo, J.O.; Castro-Costa, É. Bipolar Disorder Prevalence: A Systematic Review and Meta-Analysis of the Literature. Rev. Bras. Psiquiatr. 2015, 37, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.J.; Stockings, E.; Khoo, J.-P.; Erskine, H.E.; Degenhardt, L.; Vos, T.; Whiteford, H.A. The Prevalence and Burden of Bipolar Disorder: Findings from the Global Burden of Disease Study 2013. Bipolar Disord. 2016, 18, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rojas, L.; Porras-Segovia, A.; Dunne, H.; Andrade-González, N.; Cervilla, J.A. Prevalence and Correlates of Major Depressive Disorder: A Systematic Review. Rev. Bras. Psiquiatr. 2020, 42, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.; Compton, W.M.; Saha, T.D.; Goldstein, B.I.; Ruan, W.J.; Huang, B.; Grant, B.F. Epidemiology of DSM-5 Bipolar I Disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions—III. J. Psychiatr. Res. 2017, 84, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.J.; Norman, R.E.; Freedman, G.; Baxter, A.J.; Pirkis, J.E.; Harris, M.G.; Page, A.; Carnahan, E.; Degenhardt, L.; Vos, T.; et al. The Burden Attributable to Mental and Substance Use Disorders as Risk Factors for Suicide: Findings from the Global Burden of Disease Study 2010. PLoS ONE 2014, 9, e91936. [Google Scholar] [CrossRef]
- Kessing, L.V.; Vradi, E.; McIntyre, R.S.; Andersen, P.K. Causes of Decreased Life Expectancy over the Life Span in Bipolar Disorder. J. Affect. Disord. 2015, 180, 142–147. [Google Scholar] [CrossRef]
- Laursen, T.M.; Musliner, K.L.; Benros, M.E.; Vestergaard, M.; Munk-Olsen, T. Mortality and Life Expectancy in Persons with Severe Unipolar Depression. J. Affect. Disord. 2016, 193, 203–207. [Google Scholar] [CrossRef]
- Sivertsen, H.; Bjørkløf, G.H.; Engedal, K.; Selbæk, G.; Helvik, A.-S. Depression and Quality of Life in Older Persons: A Review. Dement. Geriatr. Cogn. Disord. 2015, 40, 311–339. [Google Scholar] [CrossRef]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Fries, G.R.; Walss-Bass, C.; Bauer, M.E.; Teixeira, A.L. Revisiting Inflammation in Bipolar Disorder. Pharmacol. Biochem. Behav. 2019, 177, 12–19. [Google Scholar] [CrossRef]
- Gałecki, P.; Talarowska, M. Inflammatory Theory of Depression. Psychiatr. Pol. 2018, 52, 437–447. [Google Scholar] [CrossRef]
- Jones, G.H.; Vecera, C.M.; Pinjari, O.F.; Machado-Vieira, R. Inflammatory Signaling Mechanisms in Bipolar Disorder. J. Biomed. Sci. 2021, 28, 45. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, Q.; Zhang, L. The Prevalence of Bipolar Disorder in Autoimmune Disease: A Systematic Review and Meta-Analysis. Ann. Palliat. Med. 2021, 10, 350–361. [Google Scholar] [CrossRef]
- Pryce, C.R.; Fontana, A. Depression in Autoimmune Diseases. Curr. Top. Behav. Neurosci. 2017, 31, 139–154. [Google Scholar] [CrossRef]
- SayuriYamagata, A.; Brietzke, E.; Rosenblat, J.D.; Kakar, R.; McIntyre, R.S. Medical Comorbidity in Bipolar Disorder: The Link with Metabolic-Inflammatory Systems. J. Affect. Disord. 2017, 211, 99–106. [Google Scholar] [CrossRef]
- Wiltink, J.; Beutel, M.E.; Till, Y.; Ojeda, F.M.; Wild, P.S.; Münzel, T.; Blankenberg, S.; Michal, M. Prevalence of Distress, Comorbid Conditions and Well Being in the General Population. J. Affect. Disord. 2011, 130, 429–437. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral Cytokine and Chemokine Alterations in Depression: A Meta-Analysis of 82 Studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef]
- Liu, J.J.; Wei, Y.B.; Strawbridge, R.; Bao, Y.; Chang, S.; Shi, L.; Que, J.; Gadad, B.S.; Trivedi, M.H.; Kelsoe, J.R.; et al. Peripheral Cytokine Levels and Response to Antidepressant Treatment in Depression: A Systematic Review and Meta-Analysis. Mol. Psychiatry 2020, 25, 339–350. [Google Scholar] [CrossRef]
- Yang, C.; Wardenaar, K.J.; Bosker, F.J.; Li, J.; Schoevers, R.A. Inflammatory Markers and Treatment Outcome in Treatment Resistant Depression: A Systematic Review. J. Affect. Disord. 2019, 257, 640–649. [Google Scholar] [CrossRef]
- Solmi, M.; Suresh Sharma, M.; Osimo, E.F.; Fornaro, M.; Bortolato, B.; Croatto, G.; Miola, A.; Vieta, E.; Pariante, C.M.; Smith, L.; et al. Peripheral Levels of C-Reactive Protein, Tumor Necrosis Factor-α, Interleukin-6, and Interleukin-1β across the Mood Spectrum in Bipolar Disorder: A Meta-Analysis of Mean Differences and Variability. Brain. Behav. Immun. 2021, 97, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Sayana, P.; Colpo, G.D.; Simões, L.R.; Giridharan, V.V.; Teixeira, A.L.; Quevedo, J.; Barichello, T. A Systematic Review of Evidence for the Role of Inflammatory Biomarkers in Bipolar Patients. J. Psychiatr. Res. 2017, 92, 160–182. [Google Scholar] [CrossRef] [PubMed]
- Enache, D.; Pariante, C.M.; Mondelli, V. Markers of Central Inflammation in Major Depressive Disorder: A Systematic Review and Meta-Analysis of Studies Examining Cerebrospinal Fluid, Positron Emission Tomography and Post-Mortem Brain Tissue. Brain. Behav. Immun. 2019, 81, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, V.V.; Sayana, P.; Pinjari, O.F.; Ahmad, N.; da Rosa, M.I.; Quevedo, J.; Barichello, T. Postmortem Evidence of Brain Inflammatory Markers in Bipolar Disorder: A Systematic Review. Mol. Psychiatry 2020, 25, 94–113. [Google Scholar] [CrossRef]
- Ascoli, B.M.; Géa, L.P.; Colombo, R.; Barbé-Tuana, F.M.; Kapczinski, F.; Rosa, A.R. The Role of Macrophage Polarization on Bipolar Disorder: Identifying New Therapeutic Targets. Aust. N. Z. J. Psychiatry 2016, 50, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Gritti, D.; Delvecchio, G.; Ferro, A.; Bressi, C.; Brambilla, P. Neuroinflammation in Major Depressive Disorder: A Review of PET Imaging Studies Examining the 18-KDa Translocator Protein. J. Affect. Disord. 2021, 292, 642–651. [Google Scholar] [CrossRef]
- Haarman, B.C.M.B.; Riemersma-Van der Lek, R.F.; de Groot, J.C.; Ruhé, H.G.E.; Klein, H.C.; Zandstra, T.E.; Burger, H.; Schoevers, R.A.; de Vries, E.F.J.; Drexhage, H.A.; et al. Neuroinflammation in Bipolar Disorder—A [(11)C]-(R)-PK11195 Positron Emission Tomography Study. Brain. Behav. Immun. 2014, 40, 219–225. [Google Scholar] [CrossRef]
- Han, K.M.; Ham, B.J. How Inflammation Affects the Brain in Depression: A Review of Functional and Structural MRI Studies. J. Clin. Neurol. 2021, 17, 503–515. [Google Scholar] [CrossRef]
- Doney, E.; Cadoret, A.; Dion-Albert, L.; Lebel, M.; Menard, C. Inflammation-Driven Brain and Gut Barrier Dysfunction in Stress and Mood Disorders. Eur. J. Neurosci. 2022, 55, 2851–2894. [Google Scholar] [CrossRef]
- Bai, S.; Guo, W.; Feng, Y.; Deng, H.; Li, G.; Nie, H.; Guo, G.; Yu, H.; Ma, Y.; Wang, J.; et al. Efficacy and Safety of Anti-Inflammatory Agents for the Treatment of Major Depressive Disorder: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. J. Neurol. Neurosurg. Psychiatry 2020, 91, 21–32. [Google Scholar] [CrossRef]
- Pereira, A.C.; Oliveira, J.; Silva, S.; Madeira, N.; Pereira, C.M.F.; Cruz, M.T. Inflammation in Bipolar Disorder (BD): Identification of New Therapeutic Targets. Pharmacol. Res. 2021, 163, 105325. [Google Scholar] [CrossRef]
- Dai, Y.; Ge, J. Clinical Use of Aspirin in Treatment and Prevention of Cardiovascular Disease. Thrombosis 2012, 2012, 245037. [Google Scholar] [CrossRef]
- Desborough, M.J.R.; Keeling, D.M. The Aspirin Story—From Willow to Wonder Drug. Br. J. Haematol. 2017, 177, 674–683. [Google Scholar] [CrossRef]
- Elwood, P.C.; Morgan, G.; Delon, C.; Protty, M.; Galante, J.; Pickering, J.; Watkins, J.; Weightman, A.; Morris, D. Aspirin and Cancer Survival: A Systematic Review and Meta-Analyses of 118 Observational Studies of Aspirin and 18 Cancers. Ecancermedicalscience 2021, 15, 1258. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Wilson, M.; Price, J.F.; Belch, J.F.F.; Meade, T.W.; Mehta, Z. Effect of Daily Aspirin on Risk of Cancer Metastasis: A Study of Incident Cancers during Randomised Controlled Trials. Lancet 2012, 379, 1591–1601. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, R.; Yu, L.; Xiao, J.; Zhou, X.; Li, X.; Song, P.; Li, X. Aspirin Use and Common Cancer Risk: A Meta-Analysis of Cohort Studies and Randomized Controlled Trials. Front. Oncology 2021, 11, 690219. [Google Scholar] [CrossRef]
- Pasco, J.A.; Jacka, F.N.; Williams, L.J.; Henry, M.J.; Nicholson, G.C.; Kotowicz, M.A.; Berk, M. Clinical Implications of the Cytokine Hypothesis of Depression: The Association between Use of Statins and Aspirin and the Risk of Major Depression. Psychother. Psychosom. 2010, 79, 323–325. [Google Scholar] [CrossRef]
- Stolk, P.; Souverein, P.C.; Wilting, I.; Leufkens, H.G.M.; Klein, D.F.; Rapoport, S.I.; Heerdink, E.R. Is Aspirin Useful in Patients on Lithium? A Pharmacoepidemiological Study Related to Bipolar Disorder. Prostaglandins. Leukot. Essent. Fatty Acids 2010, 82, 9–14. [Google Scholar] [CrossRef]
- Kessing, L.V.; Rytgaard, H.C.; Gerds, T.A.; Berk, M.; Ekstrøm, C.T.; Andersen, P.K. New Drug Candidates for Depression—A Nationwide Population-Based Study. Acta Psychiatr. Scand. 2019, 139, 68–77. [Google Scholar] [CrossRef]
- Kessing, L.V.; Rytgaard, H.C.; Gerds, T.A.; Berk, M.; Ekstrøm, C.T.; Andersen, P.K. New Drug Candidates for Bipolar Disorder-A Nation-Wide Population-Based Study. Bipolar Disord. 2019, 21, 410–418. [Google Scholar] [CrossRef]
- Ghazanfari, N.; van Waarde, A.; Dierckx, R.A.J.O.; Doorduin, J.; de Vries, E.F.J. Is Cyclooxygenase-1 Involved in Neuroinflammation? J. Neurosci. Res. 2021, 99, 2976–2998. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, J.; Molotkov, A.; Mintz, A.; Mann, J.J. Progress in PET Imaging of Neuroinflammation Targeting COX-2 Enzyme. Molecules 2021, 26, 3208. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Prorok, T.; Roy, A.; Patel, D.; Dasarathi, S.; Pahan, K. Upregulation of IL-1 Receptor Antagonist by Aspirin in Glial Cells via Peroxisome Proliferator-Activated Receptor-Alpha. J. Alzheimer’s Dis. Rep. 2021, 5, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Chen, D.; Wu, X.; Chen, X.; Zhang, X.; Niu, J.; Shen, H.-Y.; Xiao, L. Aspirin promotes oligodendroglial differentiation through inhibition of Wnt signaling pathway. Mol. Neurobiol. 2016, 53, 3258–3266. [Google Scholar] [CrossRef] [PubMed]
- Neumann, N.R.; Thompson, D.C.; Vasiliou, V. AMPK Activators for the Prevention and Treatment of Neurodegenerative Diseases. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1199–1210. [Google Scholar] [CrossRef]
- World Health Organization. Model List of Essential Medicines—22nd List, 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Management Sciences for Health. International Drug Price Indicator Guide, 2014th ed.; Management Sciences for Health: Medford, MA, USA, 2015. [Google Scholar]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions: Online Version 5.1.0; Cochrane: London, UK, 2011. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.; Higgins, J.; Sterne, J.A.C.; Higgins, J.P.T.; Reeves, B.C. A Cochrane Risk of Bias Assessment Tool: For Non-Randomized Studies of Interventions (ACROBAT-NRSI), Version 1.0.0. 24 September 2014. Available online: https://www.bristol.ac.uk/media-library/sites/social-community-medicine/images/centres/cresyda/ACROBAT-NRSI%20Version%201_0_0.pdf (accessed on 1 December 2022).
- Higgins, J.; Morgan, R.; Rooney, A.; Taylor, K.; Thayer, K.; Silva, R.; Lemeris, C.; Akl, A.; Arroyave, W.; Bateson, T. Risk of Bias in Non-Randomized Studies—Of Exposure (ROBINS-E). Launch Version, 1 June 2022. Available online: https://www.riskofbias.info/welcome/robins-e-tool (accessed on 1 December 2022).
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-Bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-Bias Assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef]
- Glaus, J.; Vandeleur, C.L.; Lasserre, A.M.; Strippoli, M.-P.F.; Castelao, E.; Gholam-Rezaee, M.; Waeber, G.; Aubry, J.-M.; Vollenweider, P.; Preisig, M. Aspirin and Statin Use and the Subsequent Development of Depression in Men and Women: Results from a Longitudinal Population-Based Study. J. Affect. Disord. 2015, 182, 126–131. [Google Scholar] [CrossRef]
- Williams, L.J.; Pasco, J.A.; Mohebbi, M.; Jacka, F.N.; Stuart, A.L.; Venugopal, K.; O’Neil, A.; Berk, M. Statin and Aspirin Use and the Risk of Mood Disorders among Men. Int. J. Neuropsychopharmacol. 2016, 19, pyw008. [Google Scholar] [CrossRef][Green Version]
- Veronese, N.; Koyanagi, A.; Stubbs, B.; Solmi, M.; Fornaro, M.; Fernandes, B.S.; Mueller, C.; Thompson, T.; Carvalho, A.F.; Maggi, S. Aspirin and Incident Depressive Symptoms: A Longitudinal Cohort Study over 8 Years. Int. J. Geriatr. Psychiatry 2018, 33, e193–e198. [Google Scholar] [CrossRef]
- Saroukhani, S.; Emami-Parsa, M.; Modabbernia, A.; Ashrafi, M.; Farokhnia, M.; Hajiaghaee, R.; Akhondzadeh, S. Aspirin for Treatment of Lithium-Associated Sexual Dysfunction in Men: Randomized Double-Blind Placebo-Controlled Study. Bipolar Disord. 2013, 15, 650–656. [Google Scholar] [CrossRef]
- Sepehrmanesh, Z.; Fahimi, H.; Akasheh, G.; Davoudi, M.; Gilasi, H.; Ghaderi, A. The Effects of Combined Sertraline and Aspirin Therapy on Depression Severity among Patients with Major Depressive Disorder: A Randomized Clinical Trial. Electron. Physician 2017, 9, 5770–5777. [Google Scholar] [CrossRef]
- Bauer, I.E.; Green, C.; Colpo, G.D.; Teixeira, A.L.; Selvaraj, S.; Durkin, K.; Zunta-Soares, G.B.; Soares, J.C. A Double-Blind, Randomized, Placebo-Controlled Study of Aspirin and N-Acetylcysteine as Adjunctive Treatments for Bipolar Depression. J. Clin. Psychiatry 2018, 80, 459. [Google Scholar] [CrossRef]
- Savitz, J.B.; Teague, T.K.; Misaki, M.; Macaluso, M.; Wurfel, B.E.; Meyer, M.; Drevets, D.; Yates, W.; Gleason, O.; Drevets, W.C.; et al. Treatment of Bipolar Depression with Minocycline and/or Aspirin: An Adaptive, 2×2 Double-Blind, Randomized, Placebo-Controlled, Phase IIA Clinical Trial. Transl. Psychiatry 2018, 8, 27. [Google Scholar] [CrossRef]
- Zdanowicz, N.; Reynaert, C.; Jacques, D.; Lepiece, B.; Dubois, T. Selective Serotonergic (SSRI) Versus Noradrenergic (SNRI) Reuptake Inhibitors with and without Acetylsalicylic Acid in Major Depressive Disorder. Psychiatr. Danub. 2017, 29 (Suppl. 3), 270–273. [Google Scholar]
- Mendlewicz, J.; Kriwin, P.; Oswald, P.; Souery, D.; Alboni, S.; Brunello, N. Shortened Onset of Action of Antidepressants in Major Depression Using Acetylsalicylic Acid Augmentation: A Pilot Open-Label Study. Int. Clin. Psychopharmacol. 2006, 21, 227–231. [Google Scholar] [CrossRef]
- Alboni, S.; Benatti, C.; Capone, G.; Tascedda, F.; Brunello, N. Neither All Anti-Inflammatory Drugs nor All Doses Are Effective in Accelerating the Antidepressant-like Effect of Fluoxetine in an Animal Model of Depression. J. Affect. Disord. 2018, 235, 124–128. [Google Scholar] [CrossRef]
- Brunello, N.; Alboni, S.; Capone, G.; Benatti, C.; Blom, J.M.C.; Tascedda, F.; Kriwin, P.; Mendlewicz, J. Acetylsalicylic Acid Accelerates the Antidepressant Effect of Fluoxetine in the Chronic Escape Deficit Model of Depression. Int. Clin. Psychopharmacol. 2006, 21, 219–225. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Liu, Y.-F.; Gao, F.; Jiang, W. Acetylsalicylic Acid as an Augmentation Agent in Fluoxetine Treatment Resistant Depressive Rats. Neurosci. Lett. 2011, 499, 74–79. [Google Scholar] [CrossRef]
- Shvartsur, R.; Agam, G.; Uzzan, S.; Azab, A.N. Low-Dose Aspirin Augments the Anti-Inflammatory Effects of Low-Dose Lithium in Lipopolysaccharide-Treated Rats. Pharmaceutics 2022, 14, 901. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Shao, F.; Xie, X.; Chen, L.; Wang, W. Effects of Aspirin on Immobile Behavior and Endocrine and Immune Changes in the Forced Swimming Test: Comparison to Fluoxetine and Imipramine. Pharmacol. Biochem. Behav. 2014, 124, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Warner-Schmidt, J.L.; Vanover, K.E.; Chen, E.Y.; Marshall, J.J.; Greengard, P. Antidepressant Effects of Selective Serotonin Reuptake Inhibitors (SSRIs) Are Attenuated by Antiinflammatory Drugs in Mice and Humans. Proc. Natl. Acad. Sci. USA. 2011, 108, 9262–9267. [Google Scholar] [CrossRef]
- Gambarana, C.; Scheggi, S.; Tagliamonte, A.; Tolu, P.; De Montis, M.G. Animal Models for the Study of Antidepressant Activity. Brain Res. Brain Res. Protoc. 2001, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Planchez, B.; Surget, A.; Belzung, C. Animal Models of Major Depression: Drawbacks and Challenges. J. Neural Transm. 2019, 126, 1383–1408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; An, L.-T.; Qiu, Y.; Shan, X.-X.; Zhao, W.-L.; Zhao, J.-P.; Li, L.-H.; Lang, B.; Wu, R.-R. Effects of Aspirin in Rats with Ouabain Intracerebral Treatment-Possible Involvement of Inflammatory Modulation? Front. Psychiatry 2019, 10, 497. [Google Scholar] [CrossRef]
- Shvartsur, R.; Agam, G.; Shnaider, A.; Uzzan, S.; Nassar, A.; Jabarin, A.; Abu-Freha, N.; Meir, K.; Azab, A.N. Safety and Efficacy of Combined Low-Dose Lithium and Low-Dose Aspirin: A Pharmacological and Behavioral Proof-of-Concept Study in Rats. Pharmaceutics 2021, 13, 1827. [Google Scholar] [CrossRef]
- Ng, Q.X.; Ramamoorthy, K.; Loke, W.; Lee, M.W.L.; Yeo, W.S.; Lim, D.Y.; Sivalingam, V. Clinical Role of Aspirin in Mood Disorders: A Systematic Review. Brain Sci. 2019, 9, 296. [Google Scholar] [CrossRef]
- Azab, A.N.; Shnaider, A.; Osher, Y.; Wang, D.; Bersudsky, Y.; Belmaker, R.H. Lithium Nephrotoxicity. Int. J. Bipolar Disord. 2015, 3, 28. [Google Scholar] [CrossRef]
- Rej, S.; Herrmann, N.; Shulman, K.; Fischer, H.D.; Fung, K.; Harel, Z.; Gruneir, A. Lithium Use, but Not Valproate Use, Is Associated with a Higher Risk of Chronic Kidney Disease in Older Adults with Mental Illness. J. Clin. Psychiatry 2017, 78, e980–e985. [Google Scholar] [CrossRef]
- Davis, J.; Desmond, M.; Berk, M. Lithium and Nephrotoxicity: A Literature Review of Approaches to Clinical Management and Risk Stratification. BMC Nephrol. 2018, 19, 305. [Google Scholar] [CrossRef]
- Minhas, S.; Cartledge, J.J.; Eardley, I.; Joyce, A.D.; Morrison, J.F. The Interaction of Nitric Oxide and Prostaglandins in the Control of Corporal Smooth Muscle Tone: Evidence for Production of a Cyclooxygenase-Derived Endothelium-Contracting Factor. BJU Int. 2001, 87, 882–888. [Google Scholar] [CrossRef]
- Sadeghipour, H.; Ghasemi, M.; Nobakht, M.; Ebrahimi, F.; Dehpour, A.R. Effect of Chronic Lithium Administration on Endothelium-Dependent Relaxation of Rat Corpus Cavernosum: The Role of Nitric Oxide and Cyclooxygenase Pathways. BJU Int. 2007, 99, 177–182. [Google Scholar] [CrossRef]
- Bayraktar, Z.; Albayrak, S. Antiplatelet (Aspirin) Therapy as a New Option in the Treatment of Vasculogenic Erectile Dysfunction: A Prospective Randomized Double-Blind Placebo-Controlled Study. Int. Urol. Nephrol. 2018, 50, 411–418. [Google Scholar] [CrossRef]
- Irfan, M.; Ismail, S.B.; Noor, N.M.; Hussain, N.H.N. Efficacy of Aspirin for Vasculogenic Erectile Dysfunction in Men: A Meta-Analysis of Randomized Control Trials. Am. J. Mens. Health 2020, 14, 1557988320969082. [Google Scholar] [CrossRef]
- Li, T.; Wu, C.; Fu, F.; Qin, F.; Wei, Q.; Yuan, J. Association between Use of Aspirin or Non-Aspirin Non-Steroidal Anti-Inflammatory Drugs and Erectile Dysfunction: A Systematic Review. Medicine 2018, 97, e11367. [Google Scholar] [CrossRef]
- Li, T.; Wu, C.; Fu, F.; Xiong, W.; Qin, F.; Yuan, J. Long-Term Aspirin Administration Has No Effect on Erectile Function: Evidence from Adult Rats and Ageing Rat Model. Sci. Rep. 2019, 9, 7941. [Google Scholar] [CrossRef]
- Prieto, M.L.; Schenck, L.A.; Kruse, J.L.; Klaas, J.P.; Chamberlain, A.M.; Bobo, W.V.; Bellivier, F.; Leboyer, M.; Roger, V.L.; Brown, R.D.J.; et al. Long-Term Risk of Myocardial Infarction and Stroke in Bipolar I Disorder: A Population-Based Cohort Study. J. Affect. Disord. 2016, 194, 120–127. [Google Scholar] [CrossRef]
- Foroughi, M.; Medina Inojosa, J.R.; Lopez-Jimenez, F.; Saeidifard, F.; Suarez, L.; Stokin, G.B.; Prieto, M.L.; Rocca, W.A.; Frye, M.A.; Morgan, R.J. Association of Bipolar Disorder with Major Adverse Cardiovascular Events: A Population-Based Historical Cohort Study. Psychosom. Med. 2022, 84, 97–103. [Google Scholar] [CrossRef]
- Lambert, A.M.; Parretti, H.M.; Pearce, E.; Price, M.J.; Riley, M.; Ryan, R.; Tyldesley-Marshall, N.; Avşar, T.S.; Matthewman, G.; Lee, A.; et al. Temporal Trends in Associations between Severe Mental Illness and Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis. PLoS Med. 2022, 19, e1003960. [Google Scholar] [CrossRef]
- Palleria, C.; Di Paolo, A.; Giofrè, C.; Caglioti, C.; Leuzzi, G.; Siniscalchi, A.; De Sarro, G.; Gallelli, L. Pharmacokinetic Drug-Drug Interaction and Their Implication in Clinical Management. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2013, 18, 601–610. [Google Scholar]
- Moradi, S.; Farhadian, N.; Balaei, F.; Ansari, M.; Shahlaei, M. Multi Spectroscopy and Molecular Modeling Aspects Related to Drug Interaction of Aspirin and Warfarin with Pepsin; Structural Change and Protease Activity. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2020, 228, 117813. [Google Scholar] [CrossRef] [PubMed]
- Russo, N.W.; Petrucci, G.; Rocca, B. Aspirin, Stroke and Drug-Drug Interactions. Vascul. Pharmacol. 2016, 87, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.W.; Chee, S.X.; Wong, W.J.; He, Q.L.; Lau, T.C. Traditional Chinese Medicine: Herb-Drug Interactions with Aspirin. Singapore Med. J. 2018, 59, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, Q.; Wang, Q. Effect of Celecoxib on Improving Depression: A Systematic Review and Meta-Analysis. World J. Clin. Cases 2022, 10, 7872–7882. [Google Scholar] [CrossRef]
- Kanda, H.; Kobayashi, K.; Yamanaka, H.; Noguchi, K. COX-1-Dependent Prostaglandin D2 in Microglia Contributes to Neuropathic Pain via DP2 Receptor in Spinal Neurons. Glia 2013, 61, 943–956. [Google Scholar] [CrossRef]
- Pinto, J.V.; Passos, I.C.; Librenza-Garcia, D.; Marcon, G.; Schneider, M.A.; Conte, J.H.; da Silva, J.P.A.; Lima, L.P.; Quincozes-Santos, A.; Kauer-Sant Anna, M.; et al. Neuron-Glia Interaction as a Possible Pathophysiological Mechanism of Bipolar Disorder. Curr. Neuropharmacol. 2018, 16, 519–532. [Google Scholar] [CrossRef]
- Stertz, L.; Magalhães, P.V.S.; Kapczinski, F. Is Bipolar Disorder an Inflammatory Condition? The Relevance of Microglial Activation. Curr. Opin. Psychiatry 2013, 26, 19–26. [Google Scholar] [CrossRef]
- Rapoport, S.I. Lithium and the Other Mood Stabilizers Effective in Bipolar Disorder Target the Rat Brain Arachidonic Acid Cascade. ACS Chem. Neurosci. 2014, 5, 459–467. [Google Scholar] [CrossRef]
- Gariepy, H.; Zhao, J.; Levy, D. Differential Contribution of COX-1 and COX-2 Derived Prostanoids to Cortical Spreading Depression-Evoked Cerebral Oligemia. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2017, 37, 1060–1068. [Google Scholar] [CrossRef]
- Kishi, T.; Miyake, N.; Okuya, M.; Sakuma, K.; Iwata, N. N-Acetylcysteine as an Adjunctive Treatment for Bipolar Depression and Major Depressive Disorder: A Systematic Review and Meta-Analysis of Double-Blind, Randomized Placebo-Controlled Trials. Psychopharmacology 2020, 237, 3481–3487. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; McIntyre, R.S. Efficacy and Tolerability of Minocycline for Depression: A Systematic Review and Meta-Analysis of Clinical Trials. J. Affect. Disord. 2018, 227, 219–225. [Google Scholar] [CrossRef]
- Berk, M.; Woods, R.L.; Nelson, M.R.; Shah, R.C.; Reid, C.M.; Storey, E.; Fitzgerald, S.; Lockery, J.E.; Wolfe, R.; Mohebbi, M.; et al. Effect of Aspirin vs Placebo on the Prevention of Depression in Older People: A Randomized Clinical Trial. JAMA Psychiatry 2020, 77, 1012–1020. [Google Scholar] [CrossRef]
- Berk, M.; Mohebbi, M.; Dean, O.M.; Cotton, S.M.; Chanen, A.M.; Dodd, S.; Ratheesh, A.; Amminger, G.P.; Phelan, M.; Weller, A.; et al. Youth Depression Alleviation with Anti-Inflammatory Agents (YoDA-A): A Randomised Clinical Trial of Rosuvastatin and Aspirin. BMC Med. 2020, 18, 16. [Google Scholar] [CrossRef]
- Berk, M.; Agustini, B.; Woods, R.L.; Nelson, M.R.; Shah, R.C.; Reid, C.M.; Storey, E.; Fitzgerald, S.M.; Lockery, J.E.; Wolfe, R.; et al. Effects of Aspirin on the Long-Term Management of Depression in Older People: A Double-Blind Randomised Placebo-Controlled Trial. Mol. Psychiatry 2021, 26, 5161–5170. [Google Scholar] [CrossRef]







| Preclinical Studies | Observational Studies | Interventional Studies | |
|---|---|---|---|
| Patients/Subjects | Studies on mania or depression animal models. Animals may or may not be treated with mood stabilizers or antidepressants | Patients receiving chronic low-dose of ASA, with or without a diagnosis of major depression (MD) or bipolar disorder (BD), under 65 years of age and over 18 years of age | Patients diagnosed with BD or MD; under 65 years of age and over 18 years of age |
| Intervention | Use of ASA alone or as an add-on treatment | Patients receiving chronic low-dose of ASA | Treated in combination with ASA |
| Comparison | No use of ASA | Patients not chronically taking a low dose of ASA | No use of ASA; placebo |
| Outcome | Impact on behavioral tests; histological and biochemical analysis | Episodes of depression, mania: hazard ratio (HR); incident density (ID); odds ratio (OR) | Impact on affective episodes (depressive/manic/mixed)–clinical scales; adverse effects |
| Author, Year, Country | Species Sample size (n) Sex (M/F) | Drugs Tested | Procedure | Results |
|---|---|---|---|---|
| Alboni et al., 2018, Italy | Sprague-Dawley rats n = 5–10/group, M | Fluoxetine 5 mg/kg+ ASA 11.25–45 mg/kg, fluoxetine 5 mg/kg + flurbiprofen 5 mg/kg, fluoxetine 5 mg/kg + celecoxib 5 mg/kg; 7 days of treatment | Chronic escape deficit | Co-administration of ASA with fluoxetine was able to revert the stress induced condition of escape deficit. The effect of ASA was dose dependent |
| Brunello et al., 2006, Italy | Sprague-Dawley rats n = 7–8/group, M | ASA 45 mg/kg or 22.5 mg/kg, fluoxetine 5 mg/kg | Chronic escape deficit | Fluoxetine alone needs to be administered for three weeks to revert the stress induced escape deficit. Combined treatment of ASA and fluoxetine reverted the condition in seven days. ASA alone was ineffective. |
| Guan et al., 2014, China | Sprague-Dawley rats n = 7/group, M | ASA 6, 12, 25 and 50 mg/kg subacute treatment | Forced Swimming Test, | ASA dose dependently decreased immobility in FST and diminished FST-induced increase in IL-6 and TNF-α |
| Shvartsur et al., 2021, Israel | Sprague-Dawley rats n = 16/group, M | ASA IP for 42 days; 1 mg/kg alone or together with Li | Elevated Plus-Maze Test; assessment of toxicity (water consumption, urinary output, plasma Li, creatinine, urea, cystatin c levels; nephrin and podocin levels, renal tubulointerstitial damage, gastric mucosal damage and bleeding | The combination of ASA with LLD-Li enhances hedonic behavior. ASA alone did not increase them. Co-administration of low-dose ASA with low-dose Li mitigates typical renal side-effects of standard-dose Li while retaining the beneficial behavioral effects of this enigmatic cation |
| Shvartsur et al., 2022, Israel | Sprague-Dawley rats n = 16/group, M | ASA for 42 days; 1 mg/kg alone or together with Li | Amphetamine-induced Hyperactivity Test, Forced Swimming Test, Lipopolysaccharide (LPS)-Treated Rats | Chronic co-treatment of ASA with low-dose Li had no effect on amphetamine-induced hyperactivity, decreased immobility time in FST, attenuated LPS-induced hypothermia and plasma and brain cytokine level elevation. |
| Wang et al., 2011, China | Sprague-Dawley rats n = 7/group, M | ASA 20 mg/kg in conjunction with fluoxetine 10 mg/kg for 3 weeks | Chronic Unpredictable Mild Stress (CUMS) rats resistant to fluoxetine Forced Swimming Test | 20–30% CUMS-induced depressive rats were resistant to fluoxetine. ASA adjunctive treatment showed improved sucrose preference and decreased immobility time in FST, as well as inhibition of COX-2 and PGE2 concentration. |
| Warner-Schmidt et al., 2011, USA | C57BL/6 mice n = 8–16/group, M | ASA 210 mg/kg, Ibuprofen 70 mg/kg, Naproxen 140 mg/kg | Tail Suspension Test, Forced Swimming Test, | Anti-inflammatory drugs antagonized biochemical and behavioral responses to SSRIs |
| Zhang et al., 2019, China | Sprague-Dawley rats n not given, M | ASA IG for 1 week; 50 mg/kg | Ouabaine- induced hyperlocomotion | ASA decreased ouabaine induced hyperlocomotion and stereotypic behavior. |
| Author, Year, Country | Study Design | Sample Size (n), Control Sample Size, Duration | Characteristics of the Study Group | Conclusions |
|---|---|---|---|---|
| Kessing et al. 2019a, Denmark | Retrospective study | n = 1,605,365, including n = 315,542-taking low-dose ASA Control group-random sample, 30% of the Danish population (1,710,000) 10 years (2005–2015) | Patients receiving studied drugs (ASA—low (75–150 mg/d) and high dose (>500 mg/d), statins, allopurinol, and NSAIDs, drugs acting through angiotensin Age: median of 57 years | Chronic low-dose ASA (75–150 mg/d), statins, and medications that act via angiotensin were associated with a reduced risk of experiencing an episode of mania, another affective episode in BD, or taking lithium (for low-dose ASA: HR 0.92, 95% CI: 0.89 to 0.95; p < 0.001) after adjusting for age, sex, employment status, year, and somatic disorders. High-dose ASA (500 mg/d) and NSAID administration was associated with an increased risk of a depressive episode or antidepressant discharge (for high-dose ASA: HR 1.12, 95% CI: 1.03 to 1.22; p < 0.001) after adjusting for age, gender, employment, year, and somatic diseases |
| Stolk et al. 2010, Netherlands | Retrospective study | n = 5145 10-year observation period (1996–2005) | Patients with at least five lithium prescriptions (presumed BD patient population); Mean age 48 years | Low-dose ASA (up to 80 mg/day) reduced the risk of clinical deterioration in patients taking lithium, as well as the adjusted incidence of deterioration—ID (incidence density) (incident dose increase or drug change)—0.84, 95% CI: 0.75 to 0.94; p < 0.05) |
| Kessing et al. 2019b, Denmark | Retrospective study | n = 1,576,253, including n = 315,542-taking low-dose ASA Control group-random sample, 30% of the Danish population (1,710,000) 10 years (2005–2015) | Patients receiving studied drugs (ASA—low (75–150 mg/d and high dose (>500 mg/d) statins, allopurinol, and NSAIDs, drugs acting through angiotensin Age: median of 65 years | Those receiving chronic low-dose ASA (75–150 mg/d), statins, allopurinol, and drugs acting through angiotensin were associated with a reduced risk of a depressive episode or antidepressant discharge (for low-dose ASA: HR 0.93, 95% CI: 0.93 to 0.94; p < 0. 001) after adjusting for age, gender, employment status, year, and somatic disorders. High-dose ASA (500 mg/d) and NSAID intake was associated with an increased risk of a depressive episode or antidepressant discharge (for high-dose ASA: HR 1.03, 95% CI: 1.01 to 1.05; p < 0.001) after adjusting for age, gender, employment status, year, and somatic disorders |
| Pasco et al. 2010, Australia | Study 1: Controlled trial | Study group: n = 104 (taking ASA for an average of 7 years); Control group n = 282 Time period-10 years | Female only, over 50 years old, population derived from the Geelong osteoporosis study | The use of ASA in women with both a positive and negative history of major depression was associated with a lower chance of a major depressive episode (OR 0.18, 95% CI: 0.02 to 1.39) after adjustment for age. ASA dose: no data available |
| Study 2: Retrospective cohort | Study group: n = 161 Control group: n = 184; Time period-10 years | Taking ASA and statins in women with a negative prior history of depression was associated with a reduced risk of a depressive episode (HR 0.20, 95% CI: 0.04 to 0.85, p = 0.03). ASA dose: no data available | ||
| Veronese et al. 2018, USA | Cohort of the larger study | Study group: n = 137 (taking ASA); Control group: n = 4003; 8 years | Population from a multi-center, Osteoarthritis Initiative (OAI) study; Average age: 65 years old | Observation of the occurrence of depression in subjects without a prior diagnosis of depression. ASA use did not reduce the risk of depressive symptoms (HR 1.12; 95% CI: 0.78 to 1.62, p = 0.54). ASA dose: no data available |
| Glaus et al. 2015, Switzerland | Prospective study | Study group: n = 132 (receiving ASA); Control group: n = 1499; Mean follow-up 5.2 years | Female and male patients, Average age 51 years old | ASA administration did not reduce the risk of MDD (HR 1.19; 95% CI: 0.68 to 2.08, p > 0.05). The study and control groups differed significantly in age (mean age was higher in the study group) (p < 0.0001) and in the prevalence of being overweight, diabetes, hypertension, and heart disease, all of which were significantly more common in the study group (all p < 0.0001). ASA dose: no data available |
| Williams et al. 2016, Australia | Study 1: Controlled trial | Study group: n = 8 (receiving ASA for an average of 8.4 years); Control group: n = 140 5 years (2006–2011) | Male patients, mean age of 50 years, observed for 5 years; population derived from the Geelong osteoporosis study | Observation of major depression in individuals without a prior diagnosis of depression. After adjusting for age and antidepressant use, ASA exposure was associated with a lower chance of depression (OR 0.4, 95% CI: 0.2 to 0.9, p = 0.03) |
| Study 2: Retrospective cohort | Study group: n = 210 (receiving ASA or statins); Control group: n = 626 | Reduced risk of major depression in patients with a history of ASA and statin use (HR 0.55, 95% CI: 0.23 to 1.32, p = 0.18). ASA dose: no data available |
| Author, Year, Country | Study Design | Sample Size (n), Duration | Characteristics of the Research Group (Mean Age, Female Proportion); Medication; Comorbidities | Conclusions |
|---|---|---|---|---|
| Saroukhani et al. 2013, Iran | Double-blind, randomized placebo-controlled two-arm trial | n = 32 6 weeks | Male patients with stable BD (euthymia) aged 20–45 treated with lithium; ASA group: (35.9 ± 9.0; 0%) Placebo group: (39.6 ± 9.7; 0%); Medications (lithium); Comorbidities–data not given | The purpose of the study—to evaluate the effect of ASA on sexual dysfunction in patients with BD treated with lithium (1500–1800 mg/day). Safety—patients who received ASA (240 mg/day) reported improvements in overall sexual function scores. Good tolerability of treatment, no differences between groups, no effect of ASA on lithium blood levels. |
| Bauer et al. 2018, USA | Double-blind, randomized placebo and N-acetylcysteine (NAC)-controlled four-arms trial | n = 25 16 weeks | Patients with bipolar depression aged 16–65 years; ASA group: (49 ± 15.21; 75%) ASA+NAC group: (40 ± 17.64; 25%) NAC group: (36.38 ± 7.05; 62.5%) Placebo group: (39.13 ± 9.99; 75%); Medications (lithium, anticonvulsants, antidepressants, antipsychotics, benzodiazepines), with no signifiant differences between groups; Comorbidities (agoraphobia, GAD, panic disorder, PTSD, OCD, alcohol abuse, substance use, bulimia, BED), with no signifiant differences between groups | Four groups: ASA (1 g/day), NAC (1 g/d), ASA+NAC (1 g/d + 1 g/d), placebo. The probability of response at week 16 was: 0.67 (95% CI, 0.54 to 0.81) for ASA + NAC, 0.57 (95% CI, 0.45 to 0.7) for NAC + placebo, 0.55 (95% CI, 0.44 to 0.67) for placebo and 0.33 (95% CI, 0.2 to 0.45) for ASA + placebo. The ASA group (1 g/d) was not significantly different from the placebo group. Safety—no severe adverse effects were noted |
| Savitz et al. 2018, USA | Multi-center randomized placebo and minocycline-controlled four-arms trial | n = 99 6 weeks | Patients with moderate bipolar depression, 18–65 years; ASA group: (40.6 ± 10.2; 68%) ASA+minocycline: (40.8 ± 9.7; 84%) Minocycline group: (44.8 ± 8.7; 68%) Placebo group: (40.8 ± 10.4; 73%); Medications (mood stabilizers, antidepressants, antipsychotics, anxiolytics), with no signifiant differences between groups; Comorbidities–data not given | Four groups: ASA (162 mg/day), minocycline (200 mg/d), ASA+placebo (162 mg/d), minocycline+placebo (200 mg/d). There was a significant main effect of ASA on clinical response rates (χ2 = 5.52, p = 0.019). The NNT for response rate was 4.2 NNT for achieving remission. 6.5. Safety–no severe adverse effects were noted |
| Sepehrmanesh et al. 2017, Iran | Double-blind, randomized placebo-controlled two-arm trial | n = 100 8 weeks | Patients with major depression; ASA group: (48.9 ± 7.5; 58%) Placebo group: (47.8 ± 7.3; 64%); Medications (sertraline); Comorbidities (anxiety), with no signifiant differences between groups; | Two groups: patients treated with sertraline (50–200 mg/day)+ASA (dose 160 mg/d) and patients treated with sertraline+placebo. Patients in the group taking additional ASA (160 mg/d) had a greater reduction in depressive symptoms after eight weeks compared to the placebo group (p < 0.008). Safety—side effects among patients who received ASA were as similar as patients who received placebo |
| Zdanowicz et al. 2017, Belgium | Open label, randomized placebo-controlled four-arms trial | n = 40 2 years | Patients with major depression, aged 18–63; ASA group: (46.4; 82.5% in all patients) Placebo group: (34.25; 82.5% in all patients); Medications (duloxetine, escitalopram); Comorbidities–data not given | Four groups: ASA (100 mg/day), +duloxetine, duloxetine+placebo, escitalopram + ASA (100 mg/day), escitalopram + placebo. No difference was observed between groups taking ASA and placebo. A difference was found in additional subgroup analyses—between the duloxetine+ASA and escitalopram+placebo groups—faster reduction of depressive symptoms on the HAMD scale in the duloxetine+ASA group (t = −3.114, p = 0.01) and higher remission rates (chi2 = 6.296, p = 0.012). Safety—no report about adverse effects |
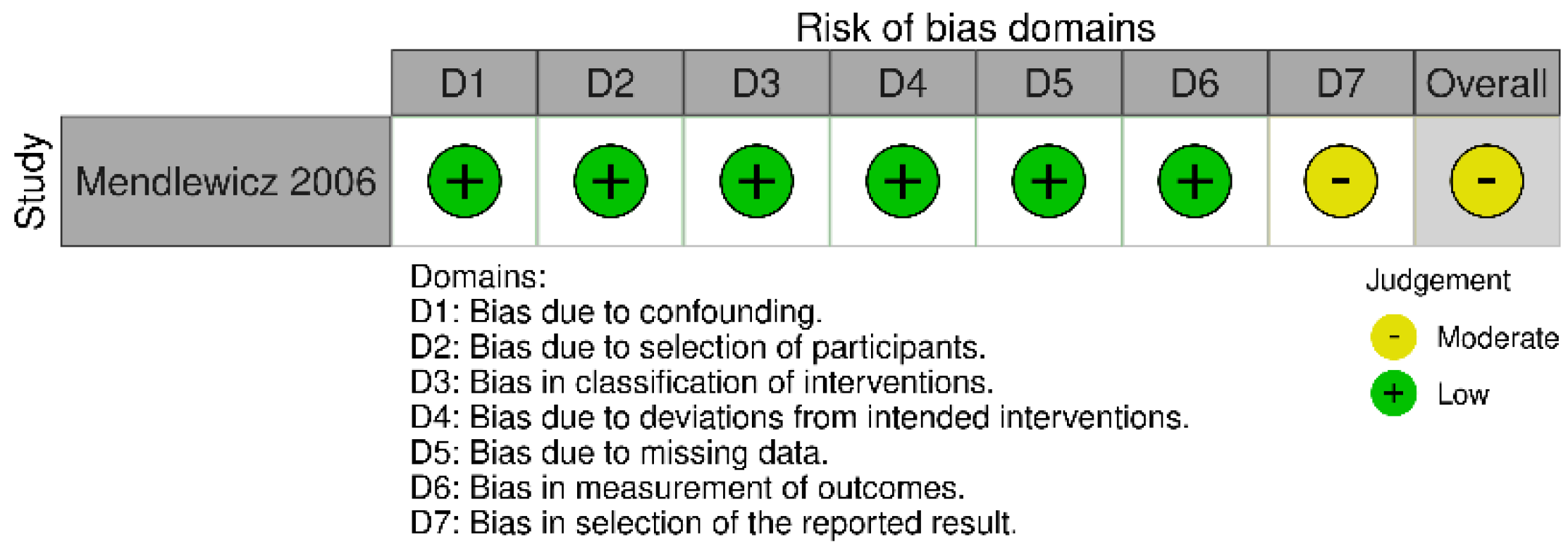
| Mendlewicz et al. 2006, Belgium | Clinical trial: Pilot open-label study | n = 21 4 weeks | 24 patients with bipolar (n = 4) or unipolar depression (n =17), treated for four weeks with SSRIs without response to treatment, aged 29–62; ASA group: (46.1±9.7; 61.9%); Medications—various SSRI drugs were used as the main treatment (citalopram, fluoxetine, paroxetine, sertraline, fluvoxamine, escitalopram), as well as mood stabilizers Comorbidities–data not given | ASA (dose 160 mg/d) was added to the SSRI treatment. Response to treatment was achieved in 47% of patients with unipolar and 75% of patients with bipolar depression, and remission was achieved in 41% of patients with unipolar and 50% of patients with bipolar depression (measured using the HAMD-21 scale). The observed improvement occurred after one week of treatment with added ASA (HAMD baseline=29.3 ± 4.5, at day 7 = 14.0 ± 4.1; p < 0.0001); Safety–no report about adverse effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominiak, M.; Gędek, A.; Sikorska, M.; Mierzejewski, P.; Wojnar, M.; Antosik-Wójcińska, A.Z. Acetylsalicylic Acid and Mood Disorders: A Systematic Review. Pharmaceuticals 2023, 16, 67. https://doi.org/10.3390/ph16010067
Dominiak M, Gędek A, Sikorska M, Mierzejewski P, Wojnar M, Antosik-Wójcińska AZ. Acetylsalicylic Acid and Mood Disorders: A Systematic Review. Pharmaceuticals. 2023; 16(1):67. https://doi.org/10.3390/ph16010067
Chicago/Turabian StyleDominiak, Monika, Adam Gędek, Michalina Sikorska, Paweł Mierzejewski, Marcin Wojnar, and Anna Z. Antosik-Wójcińska. 2023. "Acetylsalicylic Acid and Mood Disorders: A Systematic Review" Pharmaceuticals 16, no. 1: 67. https://doi.org/10.3390/ph16010067
APA StyleDominiak, M., Gędek, A., Sikorska, M., Mierzejewski, P., Wojnar, M., & Antosik-Wójcińska, A. Z. (2023). Acetylsalicylic Acid and Mood Disorders: A Systematic Review. Pharmaceuticals, 16(1), 67. https://doi.org/10.3390/ph16010067






