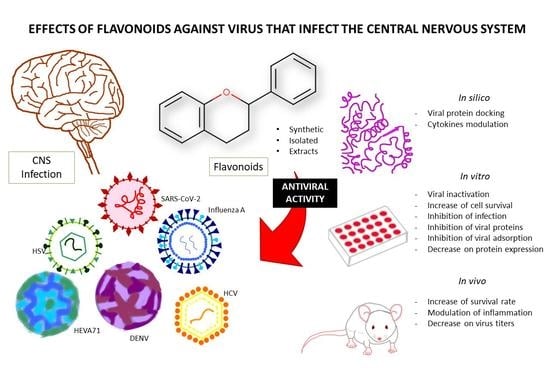
Pharmacological Potential of Flavonoids against Neurotropic Viruses
Abstract
:1. Introduction
2. Methodology
3. Results
4. Discussion
4.1. Anti-Influenza Virus Activities
4.2. Anti-Coronaviruses Activities
4.3. Anti-Herpes Virus Activities
4.4. Anti-Enteroviruses Activities
4.5. Anti-Arbovirus Activities
4.5.1. Anti-Dengue Virus (DENV) Activities
4.5.2. Anti-Zika Virus (ZIKV) Activities
4.5.3. Anti-Chikungunya Virus (CHIKV) Activities
5. Pharmacokinetic Properties of the Flavonoids and the BBB
6. Limitations of the Present Study
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stamatovic, S.M.; Johnson, A.M.; Keep, R.F.; Andjelkovic, A.V. Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers 2016, 4, e1154641. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Jo, M.; Kim, J.H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.P. Central Nervous System Opportunistic Infections. Semin. Neurol. 2019, 39, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Berhanu, G.; Desalegn, C.; Kandi, V. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): An Update. Cureus 2020, 12, e7423. [Google Scholar] [CrossRef] [PubMed]
- Radzišauskienė, D.; Vitkauskaitė, M.; Žvinytė, K.; Mameniškienė, R. Neurological complications of pandemic A(H1N1)2009pdm, postpandemic A(H1N1)v, and seasonal influenza A. Brain Behav. 2020, 11, e01916. [Google Scholar] [CrossRef]
- Soung, A.; Klein, R.S. Viral Encephalitis and Neurologic Diseases: Focus on Astrocytes. Trends Mol. Med. 2018, 24, 950–962. [Google Scholar] [CrossRef]
- Kaewpoowat, Q.; Salazar, L.; Aguilera, E.; Wootton, S.H.; Hasbun, R. Herpes simplex and varicella zoster CNS infections: Clinical presentations, treatments and outcomes. Infection 2016, 44, 337–345. [Google Scholar] [CrossRef]
- Van Riel, D.; Verdijk, R.; Kuiken, T. The olfactory nerve: A shortcut for influenza and other viral diseases into the central nervous system. J. Pathol. 2015, 235, 277–287. [Google Scholar] [CrossRef]
- Wang, T.; Li, Q.; Bi, K. shun Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Costa, S.L.; Silva, V.D.A.; Dos Santos Souza, C.; Santos, C.C.; Paris, I.; Muñoz, P.; Segura-Aguilar, J. Impact of Plant-Derived Flavonoids on Neurodegenerative Diseases. Neurotox. Res. 2016, 30, 41–52. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K.; Lu, K.P.; Sastre, J. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef] [PubMed]
- De Boer, A.G.; Gaillard, P.J. Drug Targeting to the Brain. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 323–355. [Google Scholar] [CrossRef] [PubMed]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.K.; Agrawal, A.S.; Bose, S.; Naskar, S.; Bhowmick, R.; Chakrabarti, S.; Sarkar, S.; Chawla-Sarkar, M. Antiviral activity of baicalin against influenza virus H1N1-pdm09 is due to modulation of NS1-mediated cellular innate immune responses. J. Antimicrob. Chemother. 2014, 69, 1298–1310. [Google Scholar] [CrossRef]
- Chu, M.; Xu, L.; Zhang, M.-B.; Chu, Z.-Y.; Wang, Y.-D. Role of Baicalin in Anti-Influenza Virus A as a Potent Inducer of IFN-Gamma. BioMed Res. Int. 2015, 2015, 263630. [Google Scholar] [CrossRef]
- Li, R.; Wang, L. Baicalin inhibits influenza virus A replication via activation of type I IFN signaling by reducing miR-146a. Mol. Med. Rep. 2019, 20, 5041–5049. [Google Scholar] [CrossRef]
- Moghaddam, E.; Teoh, B.-T.; Sam, S.-S.; Lani, R.; Hassandarvish, P.; Chik, Z.; Yueh, A.; Abubakar, S.; Zandi, K. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci. Rep. 2015, 4, 5452. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wu, T.; Jin, Y.; Cheng, J.; Wan, C.; Qian, W.; Xing, F.; Shi, W. The antiviral effect of baicalin on enterovirus 71 in vitro. Viruses 2015, 7, 4756–4771. [Google Scholar] [CrossRef]
- Oo, A.; Rausalu, K.; Merits, A.; Higgs, S.; Vanlandingham, D.; Bakar, S.A.; Zandi, K. Deciphering the potential of baicalin as an antiviral agent for Chikungunya virus infection. Antiviral Res. 2018, 150, 101–111. [Google Scholar] [CrossRef]
- Jo, S.; Kim, S.; Shin, D.H.; Kim, M.-S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzyme Inhib. Med. Chem. 2020, 35, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Dayem, A.A.; Choi, H.Y.; Kim, Y.B.; Cho, S.G. Antiviral effect of methylated flavonol isorhamnetin against influenza. PLoS ONE 2015, 10, e0121610. [Google Scholar] [CrossRef] [PubMed]
- Sadati, S.M.; Gheibi, N.; Ranjbar, S.; Hashemzadeh, M.S. Docking study of flavonoid derivatives as potent inhibitors of influenza H1N1 virus neuraminidas. Biomed. Rep. 2019, 10, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.Y.; Ho, B.C.; Lee, S.Y.; Chang, S.Y.; Kao, C.L.; Lee, S.S.; Lee, C.N. Houttuynia cordata targets the beginning stage of herpes simplex virus infection. PLoS ONE 2015, 10, e0115475. [Google Scholar] [CrossRef]
- Sarwar, M.W.; Riaz, A.; Dilshad, S.M.R.; Al-Qahtani, A.; Nawaz-Ul-Rehman, M.S.; Mubin, M. Structure activity relationship (SAR) and quantitative structure activity relationship (QSAR) studies showed plant flavonoids as potential inhibitors of dengue NS2B-NS3 protease. BMC Struct. Biol. 2018, 18, 6. [Google Scholar] [CrossRef]
- Zou, M.; Liu, H.; Li, J.; Yao, X.; Chen, Y.; Ke, C.; Liu, S. Structure-activity relationship of flavonoid bifunctional inhibitors against Zika virus infection. Biochem. Pharmacol. 2020, 177, 113962. [Google Scholar] [CrossRef]
- Weber, C.; Sliva, K.; Von Rhein, C.; Kümmerer, B.M.; Schnierle, B.S. The green tea catechin, epigallocatechin gallate inhibits chikungunya virus infection. Antiviral Res. 2015, 113, 1–3. [Google Scholar] [CrossRef]
- Carneiro, B.M.; Batista, M.N.; Braga, A.C.S.; Nogueira, M.L.; Rahal, P. The green tea molecule EGCG inhibits Zika virus entry. Virology 2016, 496, 215–218. [Google Scholar] [CrossRef]
- Lim, H.; Nguyen, T.T.H.; Kim, N.M.; Park, J.S.; Jang, T.S.; Kim, D. Inhibitory effect of flavonoids against NS2B-NS3 protease of ZIKA virus and their structure activity relationship. Biotechnol. Lett. 2017, 39, 415–421. [Google Scholar] [CrossRef]
- Henss, L.; Auste, A.; Schürmann, C.; Schmidt, C.; von Rhein, C.; Mühlebach, M.D.; Schnierle, B.S. The green tea catechin epigallocatechin gallate inhibits SARS-CoV-2 infection. J. Gen. Virol. 2021, 102, 1574. [Google Scholar] [CrossRef]
- Keramagi, A.R.; Skariyachan, S. Prediction of binding potential of natural leads against the prioritized drug targets of chikungunya and dengue viruses by computational screening. 3 Biotech 2018, 8, 274. [Google Scholar] [CrossRef]
- Anusuya, S.; Gromiha, M.M. Structural basis of flavonoids as dengue polymerase inhibitors: Insights from QSAR and docking studies. J. Biomol. Struct. Dyn. 2019, 37, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Care, C.; Sornjai, W.; Jaratsittisin, J.; Hitakarun, A.; Wikan, N.; Triwitayakorn, K.; Smith, D.R. Discordant activity of kaempferol towards dengue virus and Japanese encephalitis virus. Molecules 2020, 25, 1246. [Google Scholar] [CrossRef]
- Li, W.; Xu, C.; Hao, C.; Zhang, Y.; Wang, Z.; Wang, S.; Wang, W. Inhibition of herpes simplex virus by myricetin through targeting viral gD protein and cellular EGFR/PI3K/Akt pathway. Antiviral Res. 2020, 177, 104714. [Google Scholar] [CrossRef]
- Ling, L.; Lu, Y.; Zhang, Y.; Zhu, H.; Tu, P.; Li, H.; Chen, D. Flavonoids from Houttuynia cordata attenuate H1N1-induced acute lung injury in mice via inhibition of influenza virus and Toll-like receptor signalling. Phytomedicine 2020, 67, 153150. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Chen, C.; Hu, Y.; Zhan, Z.; Pan, W.; Li, R.; Li, E.; Ge, H.M.; Yang, G. Antiviral activity of Paulownia tomentosa against enterovirus 71 of hand, foot, and mouth disease. Biol. Pharm. Bull. 2015, 38, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Bi, J.; Li, F.; Wang, S.; Huang, X.; Meng, X.; Sun, B.; Wang, D.; Kong, W.; Jiang, C.; et al. Antiviral Efficacy of Flavonoids against Enterovirus 71 Infection in Vitro and in Newborn Mice. Viruses 2019, 11, 625. [Google Scholar] [CrossRef]
- Pohjala, L.; Utt, A.; Varjak, M.; Lulla, A.; Merits, A.; Ahola, T.; Tammela, P. Inhibitors of alphavirus entry and replication identified with a stable Chikungunya replicon cell line and virus-based assays. PLoS ONE 2011, 6, e28923. [Google Scholar] [CrossRef]
- Kim, S.R.; Jeong, M.S.; Mun, S.H.; Cho, J.; Seo, M.D.; Kim, H.; Lee, J.; Song, J.H.; Ko, H.J. Antiviral activity of chrysin against influenza virus replication via inhibition of autophagy. Viruses 2021, 13, 1350. [Google Scholar] [CrossRef]
- Hutchinson, E.C. Influenza Virus. Trends Microbiol. 2018, 26, 809–810. [Google Scholar] [CrossRef]
- Krammer, F. Emerging influenza viruses and the prospect of a universal influenza virus vaccine. Biotechnol. J. 2015, 10, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Van Riel, D.; Leijten, L.M.; Verdijk, R.M.; GeurtsvanKessel, C.; Van Der Vries, E.; Van Rossum, A.M.C.; Osterhaus, A.D.M.E.; Kuiken, T. Evidence for influenza virus CNS invasion along the olfactory route in an immunocompromised infant. J. Infect. Dis. 2014, 210, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Murhekar, M.; Mehendale, S. The 2015 influenza A (H1N1) pdm09 outbreak in India. Indian J. Med. Res. 2016, 143, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Rewar, S.; Mirdha, D.; Rewar, P. Treatment and Prevention of Pandemic H1N1 Influenza. Ann. Glob. Health 2015, 81, 645–653. [Google Scholar] [CrossRef]
- Enkhtaivan, G.; Maria John, K.M.; Ayyanar, M.; Sekar, T.; Jin, K.J.; Kim, D.H. Anti-influenza (H1N1) potential of leaf and stem bark extracts of selected medicinal plants of South India. Saudi J. Biol. Sci. 2015, 22, 532–538. [Google Scholar] [CrossRef]
- Li, B.; Ni, Y.; Zhu, L.-J.; Wu, F.-B.; Yan, F.; Zhang, X.; Yao, X.-S. Flavonoids from Matteuccia struthiopteris and Their Anti-influenza Virus (H1N1) Activity. J. Nat. Prod. 2015, 78, 987–995. [Google Scholar] [CrossRef]
- Traboulsi, H.; Cloutier, A.; Boyapelly, K.; Bonin, M.A.; Marsault, É.; Cantin, A.M.; Richter, M.V. The flavonoid isoliquiritigenin reduces lung inflammation and mouse morbidity during influenza virus infection. Antimicrob. Agents Chemother. 2015, 59, 6317–6327. [Google Scholar] [CrossRef]
- Chen, L.; Dou, J.; Su, Z.; Zhou, H.; Wang, H.; Zhou, W.; Guo, Q.; Zhou, C. Synergistic activity of baicalein with ribavirin against influenza A (H1N1) virus infections in cell culture and in mice. Antiviral Res. 2011, 91, 314–320. [Google Scholar] [CrossRef]
- Nair, M.S.; Huang, Y.; Fidock, D.A.; Polyak, S.J.; Wagoner, J.; Towler, M.J.; Weathers, P.J. Artemisia annua L. extracts inhibit the in vitro replication of SARS-CoV-2 and two of its variants. J. Ethnopharmacol. 2021, 274, 114016. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Ahmed, F.A.; Kurimoto, S.I.; Kim, S.Y.; Shibata, H.; Fujioka, T.; Takaishi, Y. New α-glucosides of caffeoyl quinic acid from the leaves of Moringa oleifera Lam. J. Nat. Med. 2012, 66, 217–221. [Google Scholar] [CrossRef]
- Reiberger, R.; Radilová, K.; Kráľ, M.; Zima, V.; Majer, P.; Brynda, J.; Dračínský, M.; Konvalinka, J.; Kožíšek, M.; Machara, A. Synthesis and In Vitro Evaluation of C-7 and C-8 Luteolin Derivatives as Influenza Endonuclease Inhibitors. Int. J. Mol. Sci. 2021, 22, 7735. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-M.; Lee, K.-H.; Seong, B.-L. Antiviral effect of catechins in green tea on influenza virus. Antiviral Res. 2005, 68, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Cheong, Y.; Kim, M.; Ahn, J.; Oh, H.; Lim, J.; Chae, W.; Yang, S.W.; Kim, M.S.; Yu, J.E.; Byun, S.; et al. Epigallocatechin-3-Gallate as a Novel Vaccine Adjuvant. Front. Immunol. 2021, 12, 769088. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Jin, J.; Dou, J.; Guo, Q.; Ke, X.; Zhou, C.; Guo, M. Inhibitory Activity of Honeysuckle Extracts against Influenza A Virus In Vitro and In Vivo. Virol. Sin. 2021, 36, 490–500. [Google Scholar] [CrossRef]
- Li, H.; Xue, Q.; Xu, X. Involvement of the Nervous System in SARS-CoV-2 Infection. Neurotox. Res. 2020, 38, 1–7. [Google Scholar] [CrossRef]
- Paybast, S.; Emami, A.; Koosha, M.; Baghalha, F. Novel coronavirus disease (COVID-19) and central nervous system complications: What neurologists need to know. Acta Neurol. Taiwan. 2020, 29, 24–31. [Google Scholar]
- Cohen, M.E.; Eichel, R.; Steiner-Birmanns, B.; Janah, A.; Ioshpa, M.; Bar-Shalom, R.; Paul, J.J.; Gaber, H.; Skrahina, V.; Bornstein, N.M.; et al. A case of probable Parkinson’s disease after SARS-CoV-2 infection. Lancet Neurol. 2020, 19, 804–805. [Google Scholar] [CrossRef]
- Sulzer, D.; Antonini, A.; Leta, V.; Nordvig, A.; Smeyne, R.J.; Goldman, J.E.; Al-Dalahmah, O.; Zecca, L.; Sette, A.; Bubacco, L.; et al. COVID-19 and possible links with Parkinson’s disease and parkinsonism: From bench to bedside. npj Parkinson’s Dis. 2020, 6, 18. [Google Scholar] [CrossRef]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, F.; Hou, W.; Silver, Z.; Wong, C.Y.; Chang, O.; Huang, E.; Zuo, Q.K. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: A meta-analysis. Ann. N. Y. Acad. Sci. 2021, 1486, 90–111. [Google Scholar] [CrossRef]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized COVID-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Yoon, H.; Kim, M.K.; Lee, S.D.; Chong, Y. Synthesis and antiviral evaluation of 7-O-arylmethylquercetin derivatives against SARS-associated coronavirus (SCV) and hepatitis C virus (HCV). Arch. Pharm. Res. 2012, 35, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.R.; Yen, C.T.; Ei-Shazly, M.; Lin, W.H.; Yen, M.H.; Lin, K.H.; Wu, Y.C. Anti-human coronavirus (anti-HCoV) triterpenoids from the leaves of Euphorbia neriifolia. Nat. Prod. Commun. 2012, 7, 1415–1417. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, H.; Kim, S.; Shin, D.H.; Kim, M.S. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem. Biol. Drug Des. 2019, 94, 2023–2030. [Google Scholar] [CrossRef]
- Jo, S.; Kim, S.; Kim, D.Y.; Kim, M.S.; Shin, D.H. Flavonoids with inhibitory activity against SARS-CoV-2 3CLpro. J. Enzyme Inhib. Med. Chem. 2020, 35, 1539–1544. [Google Scholar] [CrossRef]
- Basu, A.; Sarkar, A.; Maulik, U. Molecular docking study of potential phytochemicals and their effects on the complex of SARS-CoV2 spike protein and human ACE2. Sci. Rep. 2020, 10, 17699. [Google Scholar] [CrossRef]
- Song, J.; Zhang, L.; Xu, Y.; Yang, D.; Yang, S.; Zhang, W.; Wang, J.; Tian, S.; Yang, S.; Yuan, T.; et al. The comprehensive study on the therapeutic effects of baicalein for the treatment of COVID-19 in vivo and in vitro. Biochem. Pharmacol. 2021, 183, 114302. [Google Scholar] [CrossRef]
- Liu, H.; Ye, F.; Sun, Q.; Liang, H.; Li, C.; Li, S.; Lu, R.; Huang, B.; Tan, W.; Lai, L. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J. Enzyme Inhib. Med. Chem. 2021, 36, 497–503. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, R.; Lee, I.; Zhang, W.; Sun, J.; Wang, W.; Meng, X. Characterization of the SARS-CoV-2 E Protein: Sequence, Structure, Viroporin, and Inhibitors. Protein Sci. 2021, 30, 1114–1130. [Google Scholar] [CrossRef]
- Breitinger, U.; Ali, N.K.M.; Sticht, H.; Breitinger, H.G. Inhibition of SARS CoV Envelope Protein by Flavonoids and Classical Viroporin Inhibitors. Front. Microbiol. 2021, 12, 692423. [Google Scholar] [CrossRef]
- Kornfeind, E.M.; Visalli, R.J. Human herpesvirus portal proteins: Structure, function, and antiviral prospects. Rev. Med. Virol. 2018, 28, e1972. [Google Scholar] [CrossRef] [PubMed]
- Kukhanova, M.K.; Korovina, A.N.; Kochetkov, S.N. Human herpes simplex virus: Life cycle and development of inhibitors. Biochemistry 2014, 79, 1635–1652. [Google Scholar] [CrossRef]
- Whitley, R.J. Herpes Simplex Virus Infections of the Central Nervous System. Contin. Lifelong Learn. Neurol. 2015, 21, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, M.P.; Proença, J.T.; Efstathiou, S. The molecular basis of herpes simplex virus latency. FEMS Microbiol. Rev. 2012, 36, 684–705. [Google Scholar] [CrossRef]
- Vere Hodge, R.A.; Field, H.J. Antiviral agents for herpes simplex virus. In Advances in Pharmacology; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 67, pp. 1–38. [Google Scholar]
- Chen, X.; Wang, Z.; Yang, Z.; Wang, J.; Xu, Y.; Tan, R.; Li, E. Houttuynia cordata blocks HSV infection through inhibition of NF-κB activation. Antiviral Res. 2011, 92, 341–345. [Google Scholar] [CrossRef]
- Li, T.; Liu, L.; Wu, H.; Chen, S.; Zhu, Q.; Gao, H.; Yu, X.; Wang, Y.; Su, W.; Yao, X.; et al. Anti-herpes simplex virus type 1 activity of Houttuynoid A, a flavonoid from Houttuynia cordata Thunb. Antiviral Res. 2017, 144, 273–280. [Google Scholar] [CrossRef]
- Fukuchi, K.; Okudaira, N.; Adachi, K.; Odai-Ide, R.; Watanabe, S.; Ohno, H.; Yamamoto, M.; Kanamoto, T.; Terakubo, S.; Nakashima, H.; et al. Antiviral and antitumor activity of licorice root extracts. In Vivo 2016, 30, 777–785. [Google Scholar] [CrossRef]
- Hu, S.; Sheng, W.S.; Schachtele, S.J.; Lokensgard, J.R. Reactive oxygen species drive herpes simplex virus (HSV)-1-induced proinflammatory cytokine production by murine microglia. J. Neuroinflamm. 2011, 8, 123. [Google Scholar] [CrossRef]
- Kahrs, C.R.; Chuda, K.; Tapia, G.; Stene, L.C.; Mårild, K.; Rasmussen, T.; Rønningen, K.S.; Lundin, K.E.A.; Kramna, L.; Cinek, O.; et al. Enterovirus as trigger of coeliac disease: Nested case-control study within prospective birth cohort. BMJ 2019, 364, 1231. [Google Scholar] [CrossRef]
- Royston, L.; Tapparel, C. Rhinoviruses and respiratory enteroviruses: Not as simple as ABC. Viruses 2016, 8, 16. [Google Scholar] [CrossRef]
- Goksugur, N.; Goksugur, S. Images in clinical medicine. Hand, foot, and mouth disease. N. Engl. J. Med. 2010, 362, e49. [Google Scholar] [CrossRef]
- Wang, S.M.; Liu, C.C.; Tseng, H.W.; Wang, J.R.; Huang, C.C.; Chen, Y.J.; Yang, Y.J.; Lin, S.J.; Yeh, T.F. Clinical spectrum of enterovirus 71 infection in children in southern Taiwan, with an emphasis on neurological complications. Clin. Infect. Dis. 1999, 29, 184–190. [Google Scholar] [CrossRef]
- Chang, L.Y.; Lin, H.Y.; Gau, S.S.F.; Lu, C.Y.; Hsia, S.H.; Huang, Y.C.; Huang, L.M.; Lin, T.Y. Enterovirus A71 neurologic complications and long-term sequelae. J. Biomed. Sci. 2019, 26, 57. [Google Scholar] [CrossRef]
- Maloney, J.A.; Mirsky, D.M.; Messacar, K.; Dominguez, S.R.; Schreiner, T.; Stence, N.V. MRI Findings in Children with Acute Flaccid Paralysis and Cranial Nerve Dysfunction Occurring during the 2014 Enterovirus D68 Outbreak. AJNR Am. J. Neuroradiol. 2015, 36, 245–250. [Google Scholar] [CrossRef]
- Majer, A.; McGreevy, A.; Booth, T.F. Molecular Pathogenicity of Enteroviruses Causing Neurological Disease. Front. Microbiol. 2020, 11, 540. [Google Scholar] [CrossRef]
- Wang, J.; Su, H.; Zhang, T.; Du, J.; Cui, S.; Yang, F.; Jin, Q. Inhibition of Enterovirus 71 Replication by 7-Hydroxyflavone and Diisopropyl-Flavon7-yl Phosphate. PLoS ONE 2014, 9, e92565. [Google Scholar] [CrossRef]
- Galochkina, A.V.; Anikin, V.B.; Babkin, V.A.; Ostrouhova, L.A.; Zarubaev, V.V. Virus-inhibiting activity of dihydroquercetin, a flavonoid from Larix sibirica, against coxsackievirus B4 in a model of viral pancreatitis. Arch. Virol. 2016, 161, 929–938. [Google Scholar] [CrossRef]
- Davis, L.E.; Beckham, J.D.; Tyler, K.L. North American Encephalitic Arboviruses. Neurol. Clin. 2008, 26, 727–757. [Google Scholar] [CrossRef]
- Ganesan, K.; Diwan, A.; Shankar, S.K.; Desai, S.B.; Sainani, G.S.; Katrak, S.M. Chikungunya encephalomyeloradiculitis: Report of 2 cases with neuroimaging and 1 case with autopsy findings. Am. J. Neuroradiol. 2008, 29, 1636–1637. [Google Scholar] [CrossRef]
- Mehta, R.; Gerardin, P.; de Brito, C.A.A.; Soares, C.N.; Ferreira, M.L.B.; Solomon, T. The neurological complications of chikungunya virus: A systematic review. Rev. Med. Virol. 2018, 28, e1978. [Google Scholar] [CrossRef]
- Nelson, J.; Waggoner, J.J.; Sahoo, M.K.; Grant, P.M.; Pinsky, B.A. Encephalitis caused by Chikungunya virus in a traveler from the Kingdom of Tonga. J. Clin. Microbiol. 2014, 52, 3459–3461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wielanek, A.C.; De Monredon, J.; El Amrani, M.; Roger, J.C.; Serveaux, J.P. Guillain-Barré syndrome complicating a Chikungunya virus infection. Neurology 2007, 69, 2105–2107. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.T.d.O.; Estofolete, C.F.; Zini, N.; Terzian, A.C.B.; Gongora, D.V.N.; Maia, I.L.; Nogueira, M.L. Transverse Myelitis as an Unusual Complication of Dengue Fever. Am. J. Trop. Med. Hyg. 2017, 96, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J. Zika Virus and Other Emerging Arboviral Central Nervous System Infections. Contin. Lifelong Learn. Neurol. 2018, 24, 1512–1534. [Google Scholar] [CrossRef] [PubMed]
- Bollati, M.; Alvarez, K.; Assenberg, R.; Baronti, C.; Canard, B.; Cook, S.; Coutard, B.; Decroly, E.; de Lamballerie, X.; Gould, E.A.; et al. Structure and functionality in flavivirus NS-proteins: Perspectives for drug design. Antiviral Res. 2010, 87, 125–148. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Z.; Wang, M.; Cheng, A. Innate immune evasion mediated by flaviviridae non-structural proteins. Viruses 2017, 9, 291. [Google Scholar] [CrossRef]
- Bakar, F.A.; Ng, L.F.P. Nonstructural proteins of alphavirus—Potential targets for drug development. Viruses 2018, 10, 71. [Google Scholar] [CrossRef]
- Narwal, M.; Singh, H.; Pratap, S.; Malik, A.; Kuhn, R.J.; Kumar, P.; Tomar, S. Crystal structure of chikungunya virus nsP2 cysteine protease reveals a putative flexible loop blocking its active site. Int. J. Biol. Macromol. 2018, 116, 451–462. [Google Scholar] [CrossRef]
- Bastos, M.d.S.; Martins, V.d.C.A.; da Silva, N.L.; Jezine, S.; Pinto, S.; Aprigio, V.; Monte, R.L.; Fragoso, S.; Puccioni-Sohler, M. Importance of cerebrospinal fluid investigation during dengue infection in Brazilian Amazonia Region. Mem. Inst. Oswaldo Cruz 2019, 114, e180450. [Google Scholar] [CrossRef]
- Kiat, T.S.; Pippen, R.; Yusof, R.; Ibrahim, H.; Khalid, N.; Rahman, N.A. Inhibitory activity of cyclohexenyl chalcone derivatives and flavonoids of fingerroot, Boesenbergia rotunda (L.), towards dengue-2 virus NS3 protease. Bioorg. Med. Chem. Lett. 2006, 16, 3337–3340. [Google Scholar] [CrossRef]
- Frabasile, S.; Koishi, A.C.; Kuczera, D.; Silveira, G.F.; Verri, W.A.; Dos Santos, C.N.D.; Bordignon, J. The citrus flavanone naringenin impairs dengue virus replication in human cells. Sci. Rep. 2017, 7, 41864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zandi, K.; Lim, T.-H.; Rahim, N.-A.; Shu, M.-H.; Teoh, B.-T.; Sam, S.-S.; Danlami, M.-B.; Tan, K.-K.; Abubakar, S. Extract of Scutellaria baicalensis inhibits dengue virus replication. BMC Complement. Altern. Med. 2013, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Senthilvel, P.; Lavanya, P.; Kumar, K.M.; Swetha, R.; Anitha, P.; Bag, S.; Sarveswari, S.; Vijayakumar, V.; Ramaiah, S.; Anbarasu, A. Flavonoid from Carica papaya inhibits NS2B-NS3 protease and prevents Dengue 2 viral assembly. Bioinformation 2013, 9, 889–895. [Google Scholar] [CrossRef]
- Boonyasuppayakorn, S.; Saelee, T.; Visitchanakun, P.; Leelahavanichkul, A.; Hengphasatporn, K.; Shigeta, Y.; Thanh Huynh, T.N.; Hann Chu, J.J.; Rungrotmongkol, T.; Chavasiri, W. Dibromopinocembrin and Dibromopinostrobin Are Potential Anti-Dengue Leads with Mild Animal Toxicity. Molecules 2020, 25, 4154. [Google Scholar] [CrossRef] [PubMed]
- Naccache, S.N.; Thézé, J.; Sardi, S.I.; Somasekar, S.; Greninger, A.L.; Bandeira, A.C.; Campos, G.S.; Tauro, L.B.; Faria, N.R.; Pybus, O.G.; et al. Distinct zika virus lineage in Salvador, Bahia, Brazil. Emerg. Infect. Dis. 2016, 22, 1788–1792. [Google Scholar] [CrossRef]
- Chiu, C.F.; Chu, L.W.; Liao, I.C.; Simanjuntak, Y.; Lin, Y.L.; Juan, C.C.; Ping, Y.H. The Mechanism of the Zika Virus Crossing the Placental Barrier and the Blood-Brain Barrier. Front. Microbiol. 2020, 11, 214. [Google Scholar] [CrossRef]
- Gladwyn-Ng, I.; Cordón-Barris, L.; Alfano, C.; Creppe, C.; Couderc, T.; Morelli, G.; Thelen, N.; America, M.; Bessières, B.; Encha-Razavi, F.; et al. Stress-induced unfolded protein response contributes to Zika virus–associated microcephaly. Nat. Neurosci. 2017, 21, 63–71. [Google Scholar] [CrossRef]
- Jun, S.R.; Wassenaar, T.M.; Wanchai, V.; Patumcharoenpol, P.; Nookaew, I.; Ussery, D.W. Suggested mechanisms for Zika virus causing microcephaly: What do the genomes tell us? BMC Bioinform. 2017, 18, 471. [Google Scholar] [CrossRef]
- Cataneo, A.H.D.; Kuczera, D.; Koishi, A.C.; Zanluca, C.; Silveira, G.F.; de Arruda, T.B.; Suzukawa, A.A.; Bortot, L.O.; Dias-Baruffi, M.; Verri, W.A.; et al. The citrus flavonoid naringenin impairs the in vitro infection of human cells by Zika virus. Sci. Rep. 2019, 9, 16348. [Google Scholar] [CrossRef]
- Mahar Doan, K.M.; Humphreys, J.E.; Webster, L.O.; Wring, S.A.; Shampine, L.J.; Serabjit-Singh, C.J.; Adkison, K.K.; Polli, J.W. Passive permeability and P-glycoprotein-mediated efflux differentiate central nervous system (CNS) and non-CNS marketed drugs. J. Pharmacol. Exp. Ther. 2002, 303, 1029–1037. [Google Scholar] [CrossRef]
- Jäger, A.; Saaby, L. Flavonoids and the CNS. Molecules 2011, 16, 1471–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, M.; Dudonné, S.; Calon, F. Can Natural Products Exert Neuroprotection without Crossing the Blood–Brain Barrier? Int. J. Mol. Sci. 2021, 22, 3356. [Google Scholar] [CrossRef]
- Solbrig, M.V. Animal Models of CNS Viral Disease: Examples from Borna Disease Virus Models. Interdiscip. Perspect. Infect. Dis. 2010, 2010, 709791. [Google Scholar] [CrossRef] [PubMed]
- Kamitani, W.; Ono, E.; Yoshino, S.; Kobayashi, T.; Taharaguchi, S.; Lee, B.J.; Yamashita, M.; Kobayashi, T.; Okamoto, M.; Taniyama, H.; et al. Glial expression of Borna disease virus phosphoprotein induces behavioral and neurological abnormalities in transgenic mice. Proc. Natl. Acad. Sci. USA 2003, 100, 8969–8974. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Cho, W.H.; Barcelon, E.; Kim, K.H.; Hong, J.; Lee, S.J. SARS-CoV-2 spike protein induces cognitive deficit and anxiety-like behavior in mouse via non-cell autonomous hippocampal neuronal death. Sci. Rep. 2022, 12, 5496. [Google Scholar] [CrossRef]
| Flavonoid | Antiviral Activity | Source | Study Model | Reference |
|---|---|---|---|---|
Baicalin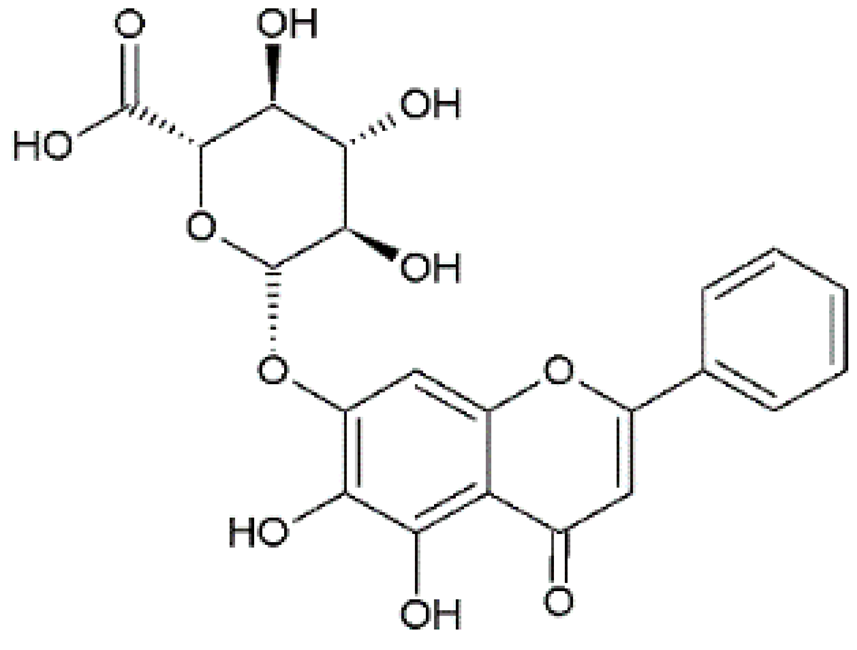 | Anti-influenza A H1N1 | Synthetic | In vitro/in vivo/in silico | Nayak et al., 2014 [15] |
| Isolated from Radix scutellariae | In vitro/in vivo | Chu et al., 2015 [16] | ||
| Synthetic | In vitro/in vivo | Li & Wang, 2019 [17] | ||
| Anti-dengue virus (DENV) | Synthetic | In vitro | Moghaddam et al., 2014 [18] | |
| Anti-enteroviruses | Synthetic | In vitro | Li et al., 2015 [19] | |
| Anti-Chikungunya virus (CHIKV) | Synthetic | In vitro | Oo et al., 2018 [20] | |
| Anti-coronaviruses | Synthetic and Bioinformatics | In vitro/In silico | Jo et al., 2020 [21] | |
Quercetin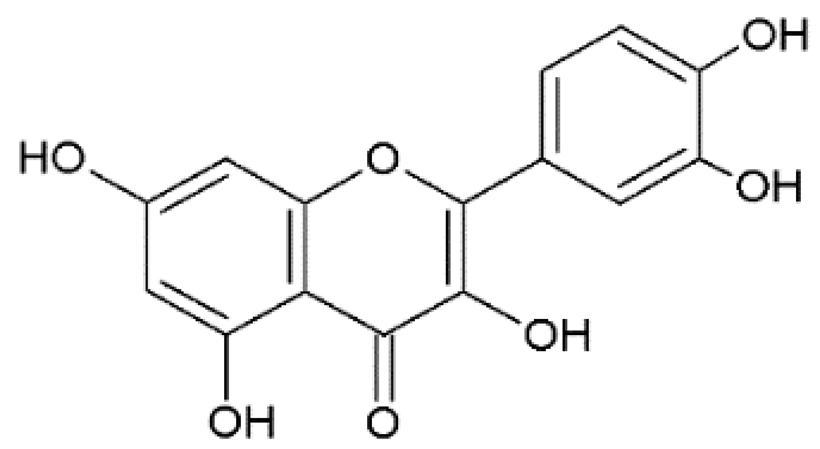 | Anti-influenza A H1N1 | Synthetic | In vitro | Dayem et al., 2015 [22] |
| Bioinformatics | In silico | Sadati et al., 2019 [23] | ||
| Anti-herpes viruses | Synthetic | In vitro | Hung et al., 2015 [24] | |
| Anti-dengue virus (DENV) | Bioinformatics | In silico | Sarwar et al., 2018 [25] | |
| Anti-Zika virus (ZIKV) | Synthetic | In vitro | Zou et al., 2020 [26] | |
ECGC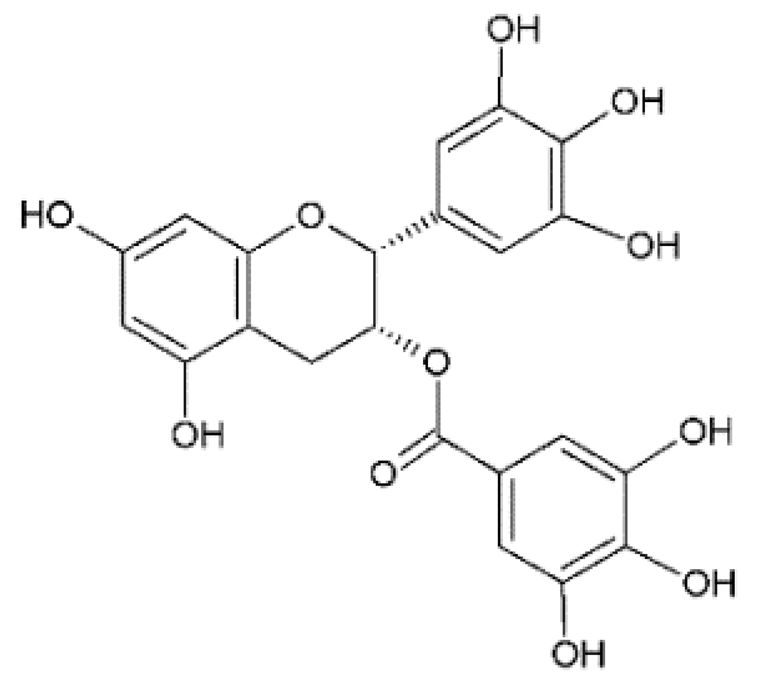 | Anti-Chikungunya virus (CHIKV) | Synthetic | In vitro | Weber et al., 2015 [27] |
| Anti-Zika virus (ZIKV) | Synthetic | In vitro | Carneiro et al., 2016 [28] | |
| Synthetic | In vitro | Lim et al., 2017 [29] | ||
| Synthetic | In vitro | Zou et al., 2020 [26] | ||
| Anti-coronaviruses | Synthetic | In vitro | Henss et al., 2021 [30] | |
Kaempferol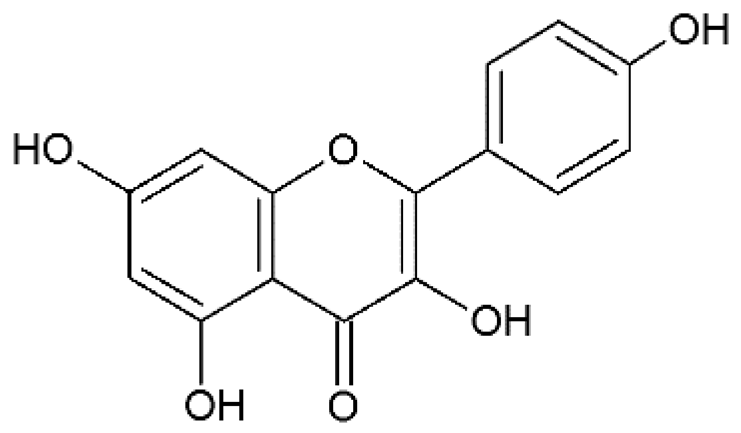 | Anti-influenza A H1N1 | Synthetic | In vitro | Dayem et al., 2015 [22] |
| Bioinformatics | In silico | Sadati et al., 2019 [23] | ||
| Anti-Chikungunya virus (CHIKV) and Anti-dengue virus (DENV) | Bioinformatics | In silico | Keramagi & Skariyachan, 2018 [31] | |
| Anti-dengue virus (DENV) | Bioinformatics | In silico | Anusuya & Gromiha, 2019 [32] | |
| Anti-dengue virus (DENV) and Anti-Japanese encephalitis virus (JEV) | Synthetic | In vitro | Care et al., 2020 [33] | |
Myricetin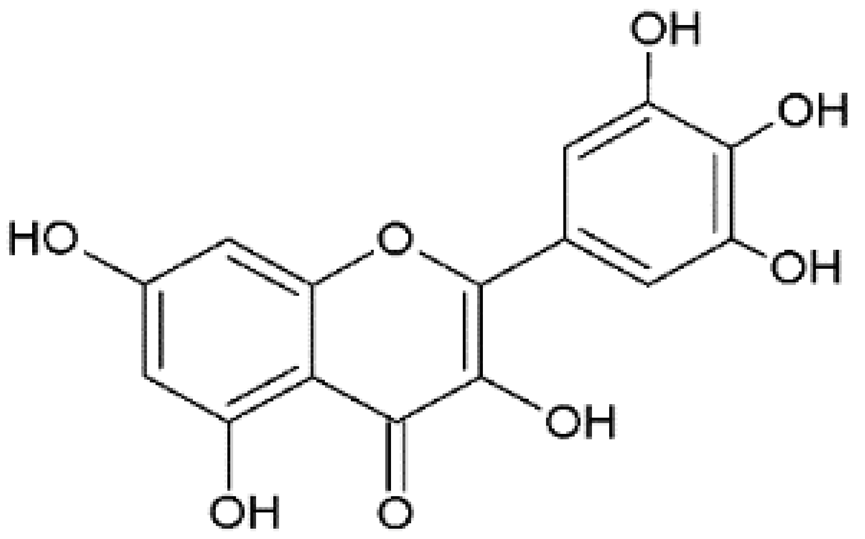 | Anti-dengue virus (DENV) | Bioinformatics | In silico | Sarwar et al., 2018 [25] |
| Anti-herpes viruses (HSV) | Synthetic | In vitro/in vivo/in silico | Li et al., 2020 [34] | |
| Anti-Zika virus (ZIKV) | Synthetic | In vitro | Lim et al., 2017 [29] | |
| Synthetic | In vitro | Zou et al., 2020 [26] | ||
Rutin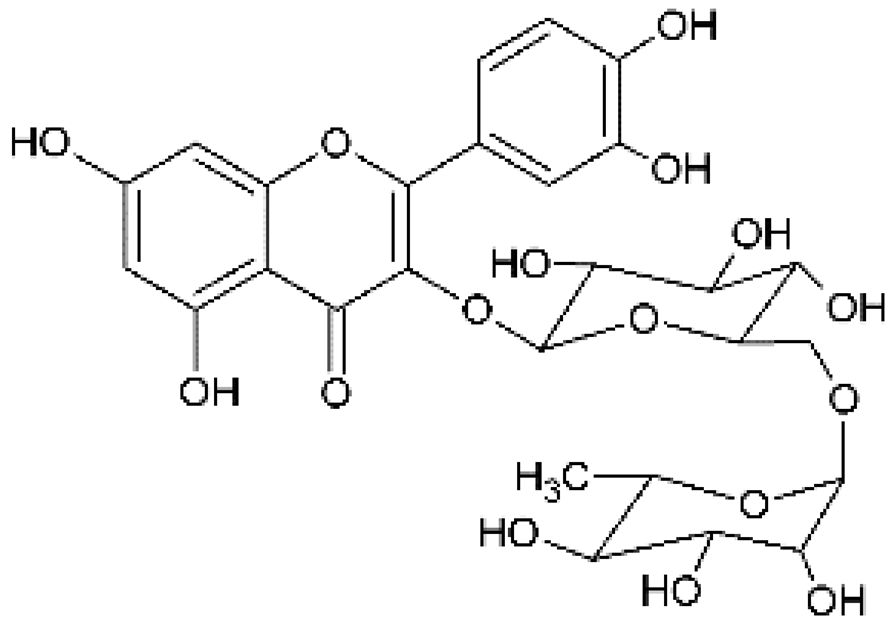 | Anti-herpes viruses (HSV) | Synthetic | In vitro | Hung et al., 2015 [24] |
| Anti-Zika virus (ZIKV) | Synthetic | In vitro | Lim et al., 2017 [29] | |
| Anti-influenza A H1N1 | Synthetic | In vitro/in vivo | Ling et al., 2020 [35] | |
Apigenin | Anti-enteroviruses | Isolated from Paulownia tomentosa Steud. | In vitro | Ji et al., 2015 [36] |
| Synthetic | In vitro/in vivo | Dai et al., 2019 [37] | ||
| Anti-Chikungunya virus (CHIKV) | Synthetic | In vitro | Pohjala et al., 2011 [38] | |
Chrysin | Anti-influenza A H1N1 | Bioinformatics | In silico | Sadati et al., 2019 [23] |
| Synthetic | In vitro | Kim et al., 2021 [39] | ||
| Anti-Chikungunya virus (CHIKV) | Synthetic | In vitro | Pohjala et al., 2011 [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro e Silva, J.H.; Souza, J.T.; Schitine, C.; Júnior, A.d.F.S.; Bastos, E.M.S.; Costa, S.L. Pharmacological Potential of Flavonoids against Neurotropic Viruses. Pharmaceuticals 2022, 15, 1149. https://doi.org/10.3390/ph15091149
Castro e Silva JH, Souza JT, Schitine C, Júnior AdFS, Bastos EMS, Costa SL. Pharmacological Potential of Flavonoids against Neurotropic Viruses. Pharmaceuticals. 2022; 15(9):1149. https://doi.org/10.3390/ph15091149
Chicago/Turabian StyleCastro e Silva, Juliana Helena, Jéssica Teles Souza, Clarissa Schitine, Aníbal de Freitas Santos Júnior, Eduardo Muniz Santana Bastos, and Silvia Lima Costa. 2022. "Pharmacological Potential of Flavonoids against Neurotropic Viruses" Pharmaceuticals 15, no. 9: 1149. https://doi.org/10.3390/ph15091149
APA StyleCastro e Silva, J. H., Souza, J. T., Schitine, C., Júnior, A. d. F. S., Bastos, E. M. S., & Costa, S. L. (2022). Pharmacological Potential of Flavonoids against Neurotropic Viruses. Pharmaceuticals, 15(9), 1149. https://doi.org/10.3390/ph15091149